Introduction
In China, spontaneous abortion (SA), commonly occurs
in women of child-bearing age. SA is defined as the termination of
a pregnancy before 28 weeks of gestation with a fetal weight of
<1,000 g (1). Of clinically
diagnosed pregnancies ~15% undergo SA and ~11% of women with a
history of one abortion experience SA (2). SA not only jeopardizes the physical
and mental health of patients, but also imposes a serious burden on
families and society (2). The
causes of SA are complex, including chromosomal defects in the
embryo, abnormalities in the decidualization process, maternal
immunological abnormalities, thrombotic tendencies, endocrine
abnormalities, anatomical abnormalities of the female reproductive
tract and infections (2–4). Modern medical treatment mainly
involves correcting endocrine abnormalities, improving luteal
function, balancing immunity, anticoagulant therapy and vitamin
supplementation. However, the effectiveness and safety of these
treatments are still under investigation.
Chinese traditional medicine has a unique
theoretical basis and experience in tranquilizing fetus and is
effective in SA treatment with fewer toxic and side effects
(5–7). Cuscuta and Herba
Taxilli, as the main medicines in the basic formula of Shoutai
Pill, are commonly used in Chinese traditional medicine to what is
termed ‘tonifying the kidneys’ and ‘stabilizing the fetus’. Modern
pharmacological studies have revealed that quercetin is one of the
primary active ingredients of Cuscuta and Herba
Taxilli (8–10). As a natural polyphenol, quercetin
is found in a number of edible and medicinal plants and exhibits a
diverse array of pharmacological activities such as
anti-inflammatory, antioxidant, antiviral, antitumor,
immunomodulatory and estrogen-like functions (11–16).
However, current research on quercetin is mainly focused on
cardiovascular, endocrine, tumor and chronic pain areas (17–20)
and the specific role of quercetin in preventing and treating SA
and its related mechanisms still need to be further
investigated.
Network pharmacology emphasizes that the development
of disease is a long-term and complex dynamic process and suggests
that the essence of disease is the imbalance of complex biological
networks (21). Network
pharmacology breaks the traditional thinking paradigm of ‘one
disease, one target, one drug’ and elaborates the pathogenesis of
complex diseases through multi-targets and multi-pathways, which
has a positive predictive role in exploring the unknown
pharmacological effects of multi-target natural products (22,23).
The present study used a bacterial lipopolysaccharide (LPS)-induced
abortion model in pregnant mice to simulate the pathology of
maternal-fetal immune imbalance caused by increased blood endotoxin
levels due to reproductive tract infection and intestinal
inflammation (24). The effect of
quercetin on the embryonic loss rate was evaluated. The relevant
signaling pathways of quercetin for SA treatment were analyzed by
network pharmacology methods, which provides ideas for further
application of quercetin in reproductive medicine.
Materials and methods
Experimental animals
A total of 78 specific pathogen free (SPF) grade
female Institute of Cancer Research (ICR) mice (6–8 weeks old, body
mass 25–35 g) and 30 SPF grade male ICR mice (8–10 weeks old, body
mass 30–35 g) were purchased from Shanghai Sippe-Bk Lab Animal Co.,
Ltd. [Laboratory Animal License No. SCXK (Shanghai) 2018-0006]. The
mice were housed in Zhejiang Chinese Medical University Laboratory
Animal Research Center, SPF grade animal laboratory: 22–24°C,
relative humidity 55–65%, 12-h light/dark cycle and food and drink
supplied ad libitum.
The present study was approved by the Animal Ethical
and Welfare Committee of Zhejiang Chinese Medical University
(ethics approval no. IACUC-20220919-25).
Main drugs and reagents
Lipopolysaccharide (LPS; MilliporeSigma; cat. no.
L2630) was diluted using pH 7.2–7.4 phosphate-buffered saline
(PBS), which had been filtered through a membrane to remove
bacteria. The LPS solution was prepared at a concentration of 50
µg/ml. Quercetin (MilliporeSigma; cat. no. Q4951; purity ≥95%) was
initially dissolved in a small quantity of anhydrous ethanol. It
was subsequently diluted with PBS to create concentrations of 0.25,
1.25 and 2.5 mg/ml (25,26).
Animal modeling, grouping and drug
administration
Mice were acclimated for 1 week and were included in
the experiment after observing a normal estrous cycle. Females and
males were mated 2:1 and cages were combined at 17:00 pm. Females
were checked for vaginal plugs at 8:00 am the following morning.
Those without vaginal plugs were recorded and continued to mate
with males, while those with vaginal plugs were designated as the
1st day of gestation (D1). Pregnant mice confirmed by vaginal plugs
were randomly divided into 6 groups, including control group, model
group, quercetin + PBS group, low-dose quercetin group, medium-dose
quercetin group and high-dose quercetin group, with 13 mice in each
group.
On days 3–8 of gestation, mice in the control and
model groups were gavaged with PBS; mice in the quercetin + PBS
group and the high-dose quercetin group were gavaged with high-dose
quercetin (2.5 mg/ml); mice in the low-dose and medium-dose
quercetin group were gavaged with low-dose (0.25 mg/ml) and
medium-dose (1.25 mg/ml) quercetin, respectively. Gavage was
performed twice at 09:00 and 15:00, with each time 0.2 ml and a
total of 0.4 ml each day. On day 7 of gestation, mice in both the
control group and quercetin + PBS group received with a 0.2 ml
injection of PBS into the tail vein and mice in the model group,
low-dose quercetin group, medium-dose quercetin group and high-dose
quercetin group were injected with 0.2 ml of LPS (50 µg/ml). The
specific procedure was shown in Fig.
1.
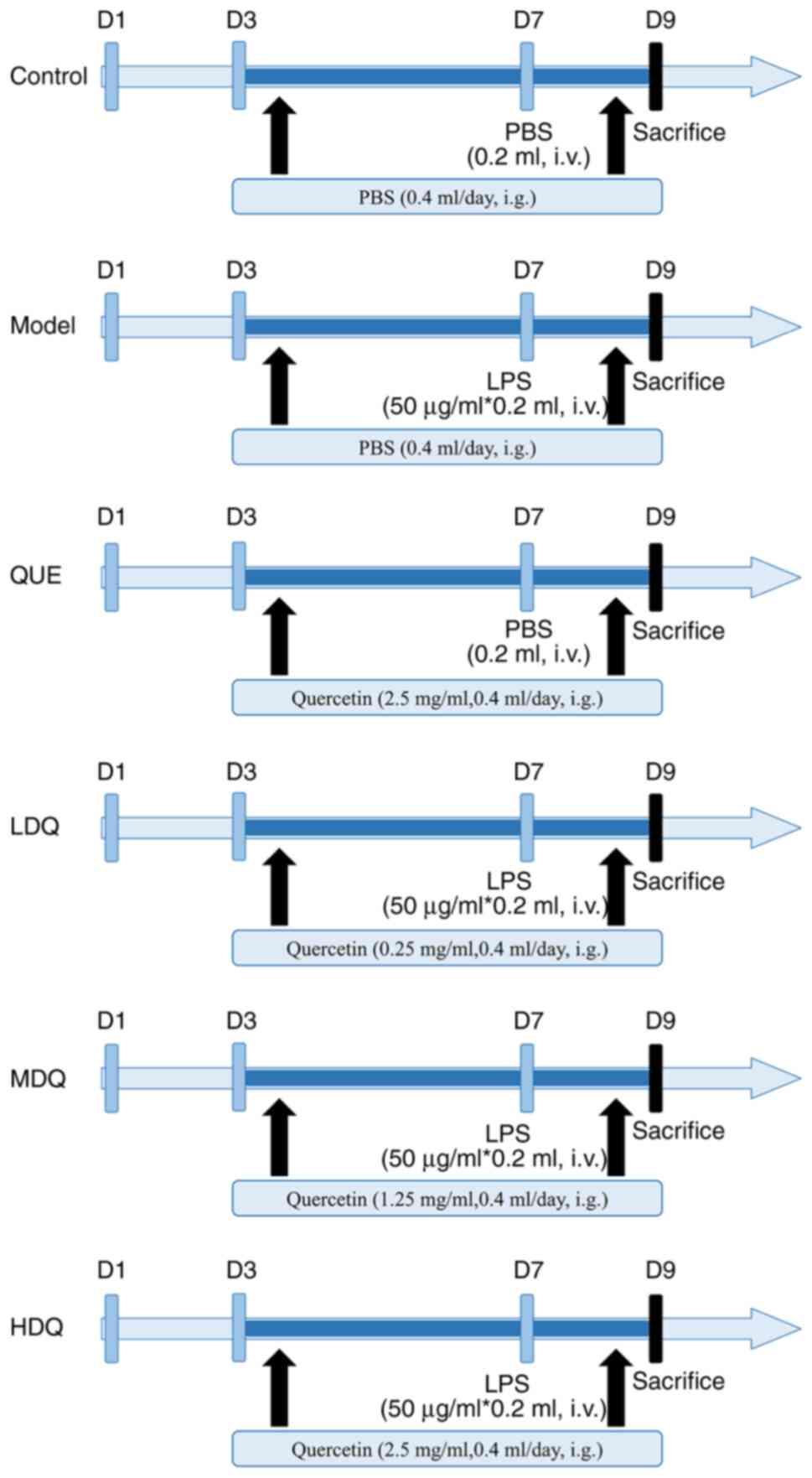 | Figure 1.Diagram of the workflow of the animal
research. MDQ, the medium-dose quercetin group; Control, the
control group; Model, the model group; QUE, the quercetin + PBS
group; LDQ, the low-dose quercetin group; MDQ, the medium-dose
quercetin group; HDQ, the high-dose quercetin group; D1, the 1st
day of pregnancy; PBS, phosphate buffered saline; LPS,
lipopolysaccharide; i.v., intravenous injection; i.g., oral
gavage. |
Humane endpoints were as follows: Inability to eat
or drink without anesthesia or sedation or stand for up to 24 h;
mice in the absence of anesthesia or sedation exhibits poor
condition including hypothermia (body temperature <37°C); mice
show the appearance of central nervous system depression, tremor,
paralysis, pain that does not respond to analgesics. 5 female mice
were sacrificed prematurely according to the humane endpoints.
General observation
The body mass of each group of pregnant mice was
measured in the morning at gestation day 1, 3, 7 and 9. After tail
vein administration, the general state [including appearance,
activity, autonomic activities, response to stimuli, degree of eye
closure (27), and eye secretions;
Table SI], diarrhea, vaginal
bleeding and pregnancy discharge of pregnant mice in each group
were observed and recorded in time. The body weight loss in D9
(compared with that in D7) was calculated as: Degree of weight loss
(%)=[body weight (D9)-body weight (D7)]/body weight (D7) ×100%.
Embryo loss rate and mean weight of
surviving embryos
Mice were sacrificed by cervical dislocation under
anesthesia on the morning of day 9 of gestation and complete
uterine tissues were removed by dissecting the uterus. The death
was confirmed by cessation of heartbeat, respiration, congestion
and temperature of the skin. Embryos were observed and the gross
weight and net weight of the uterus, the number of normal embryos
and the number of dead embryos were recorded (or the number of
implantation sites if the embryos were resorbed or expelled prior
to the execution).
Criteria for determining dead embryos were uterus
with ‘bamboo-like’ changes and embryos of different sizes with
black or purplish-brown color. Following the intrauterine death,
the size of the embryo does not continue to grow but shrinks,
resulting in a bamboo-like appearance of the uterus, section by
section without excessive expansion and with a knot between each
section.
Embryo loss rate (%)=total number of dead
embryos/(total number of dead embryos + total number of normal
embryos) ×100%.
Mean embryo weight=(gross uterine weight-net uterine
weight)/number of surviving embryos.
Histological analysis
Implantation site tissue including embryo specimens
from the control and model group were fixed with 4%
paraformaldehyde at room temperature for 48 h. Specimens were
embedded in paraffin, sectioned at 3 mm, and routinely stained at
room temperature with hematoxylin (Solarbio, cat. no. G1150) for
3–8 min and eosin (Solarbio, cat. no. G1100) for 1–3 min. 100× and
200× light microscopes (Olympus, cat. no. BX53) were used to
observe the pathological changes of the implantation sites.
Collection of drug targets
The 2D structure diagram of quercetin (SDF format)
was achieved from PubChem (28)
and then was uploaded to PharmMapper platform (29) to predict potential targets within
Homo sapiens. Uniprot database (30) was applied to correct and normalize
all the retrieved target names.
Collection of disease targets
SA-related genes were obtained from the databases of
OMIM (31), GeneCards (32) and DisGeNet (33) using the keywords ‘spontaneous
abortion’ and ‘pregnancy loss’ within ‘Homo sapiens’. The
SA-related genes from the searches were combined and duplicates
were removed. Gene names were standardized by the Uniprot database
(30).
Venn analysis
Venn online tool (34) was utilized to generate a Venn
diagram and obtain mutual targets by intersecting the targets of
quercetin with the targets of SA.
Protein-protein interaction (PPI)
network construction and core target identification
The overlapping targets were submitted to the STRING
database (35) to retrieve the PPI
network within the context of ‘Homo sapiens’ and at a medium
confidence level (0.04). The nodes that were discrete from the main
network were hided. Next, the PPI network in TSV file format was
imported into Cytoscape (v3.7.1) (36) for visualization. The cytoNCA plugin
was employed to calculate the degree for each target and the core
targets were identified by applying a degree value exceeding the
average degree (37).
Enrichment analysis of Gene Ontology
(GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG)
pathway
The Metascape database (38) was used to analyze the enriched GO
and KEGG pathways with the intersecting targets. P<0.01 was
defined as the criteria for statistical significance. The top 20
KEGG pathways and the top 20 terms within the categories of
molecular function (MF), biological process (BP) and cellular
component (CC) were collected based on the smallest to largest
P-value. The above results were then visualized using the
Microbiome platform (http://www.bioinformatics.com.cn/).
RNA extraction and quantitative
reverse transcriptase polymerase chain reaction (RT-qPCR)
The gene expression levels were quantified using
RT-qPCR. RNA extraction, cDNA synthesis, and qPCR were performed
according to the manufacturer's protocols. Total RNA was isolated
from uterus tissues with TRIzol Plus RNA Purification Kit (Thermo
Fisher Scientific, cat. no. 12183-555). Ultraviolet
spectrophotometer and electrophoresis were used to test RNA purity
and quantification. The RNA was reverse transcribed into cDNA using
the SuperScript III First-Strand Synthesis SuperMix (Thermo Fisher
Scientific, cat. no. 11752-050). RT-qPCR was performed using the
SYBR Green PCR Master Mix (Applied Biosystems, cat. no. 4367659)
with the CFX384 Touch Real-Time PCR Detection System (Bio-Rad), the
reaction volume of which was 20 µl. PCR cycling conditions
(denaturation, annealing and extension, times and temperatures) are
as follows:
For initial denaturation: 95°C for 60 sec, followed
by 40 cycles: 95°C for 15 sec for denaturation; 63°C for 25 sec for
annealing and extension. GAPDH) was served as the internal
reference, and the 2−ΔΔCq (39) method was applied to calculate the
relative expression. The experiments were performed in biological
triplicate for each group.
All primers were purchased from Sangon Biotech Co.,
Ltd. (Shanghai, China). Primer sequences were as follows: GAPDH
were F: 5′-GAAGGTCGGTGTGAACGGATTTG-3′; R:
5′-CATGTAGACCATGTAGTTGAGGTCA-3′. Primer sequences for Akt1 were F:
5′-GAAGGTCGGTGTGAACGGATTTG-3′; R: 5′-GCATGAGGTTCTCCAGCTTCA-3′.
Primer sequences for ESR1 were F: 5′-CAGGCTTTGGGGACTTGAATC-3′; R:
5′-CCAGACGAGACCAATCATCAGA-3′. Primer sequences for JAK2 were F:
5′-GCTACCAGATGGAAACTGTGCG-3′; R: 5′-GCCTCTGTAATGTTGGTGAGATC-3′.
Primer sequences for MAPK1 were F: 5′-TCAAGCCTTCCAACCTCCTGCT-3′; R:
5′-AGCTCTGTACCAACGTGTGGCT-3′. Primer sequences for MAPK3 were F:
5′-CAACACCACCTGCGACCTT-3′; R: 5′-CCACATACTCCGTCAGAAAGC-3′. Primer
sequences for PGR were F: 5′-GTCCGAGTTATGAGAACCCTTGA-3′; R:
5′-GATTTGGTGAAAAGGTGATTCTCTGG-3′. Primer sequences for PI3K were F:
5′-GATGTGGCTGACGCAGAAAG-3′; R: 5′-GGTTGCTGCTCCCGACATT-3′. Primers
for SGK1 were F: 5′-GCTCGATTCTACGCAGCTGAA-3′; R:
5′-CCCTGGGAGTCTAGGAGAA-3′.
Statistical analysis
Microsoft Office Excel 2021 (Microsoft Corporation)
was used to create database and SPSS 26.0 statistical analysis
software (IBM Corp.) was used for data processing and analysis.
Quantitative data that conformed to normal or near-normal
distribution were described by mean ± standard deviation. Then
t-test or one-way ANOVA was conducted and Tukey's or Scheffe's post
hoc comparison was used to evaluate differences between groups. In
case the data deviated from a normal distribution, they were
represented by the median (upper quartile, lower quartile), namely
M (P25, P75), analyzed by Kruskal-Wallis H test and multiple
comparisons were performed by Kruskal-Wallis one-way ANOVA. Count
data were described as percentages, compared using the
χ2 test or Fisher's exact probability method. Multiple
comparisons were conducted using the Bonferroni method with a
corrected test level of α'=0.008. P<0.05 was considered to
indicate a statistically significant difference.
Results
Comparison of general information and
weight between different groups
The pregnant mice in the control group and the
quercetin + PBS group were in a good health, had shiny fur and were
responsive, whereas the pregnant mice in the model group and
medium-dose quercetin group had obviously rough and dull fur and
were unresponsive, accompanied by diarrhea. About half of them had
blood stains and pregnancy residues on their vaginas. In the high-
and low-dose quercetin group, the pregnant mice showed an improved
state than those in the model group and medium-dose quercetin
group, but some of them had rough fur, diarrhea and were less
responsive, accompanied by blood stains and pregnancy residues on
their vaginas. After autopsy, it was found that 45.92% of the
embryos from abortion mice in the model group had been absorbed or
discharged. 53.57% of aborted embryos in the low-dose quercetin
group, 84.78% in the medium-dose quercetin group and 55.88% in the
high-dose quercetin group had been absorbed or discharged.
As shown in the Fig.
2B, the body weights of pregnant mice in the control group and
the model group exhibited a steady increase from day 3 to day 5 of
gestation. Conversely, pregnant mice in the medium- and high-dose
quercetin group displayed a decline pattern in their body weights
during this period. Subsequent to the tail vein injection, the body
weights of mice in the control group and quercetin + PBS group
continued to show consistent growth. In contrast, the other four
groups exhibited varying degrees of weight decline after the
injection.
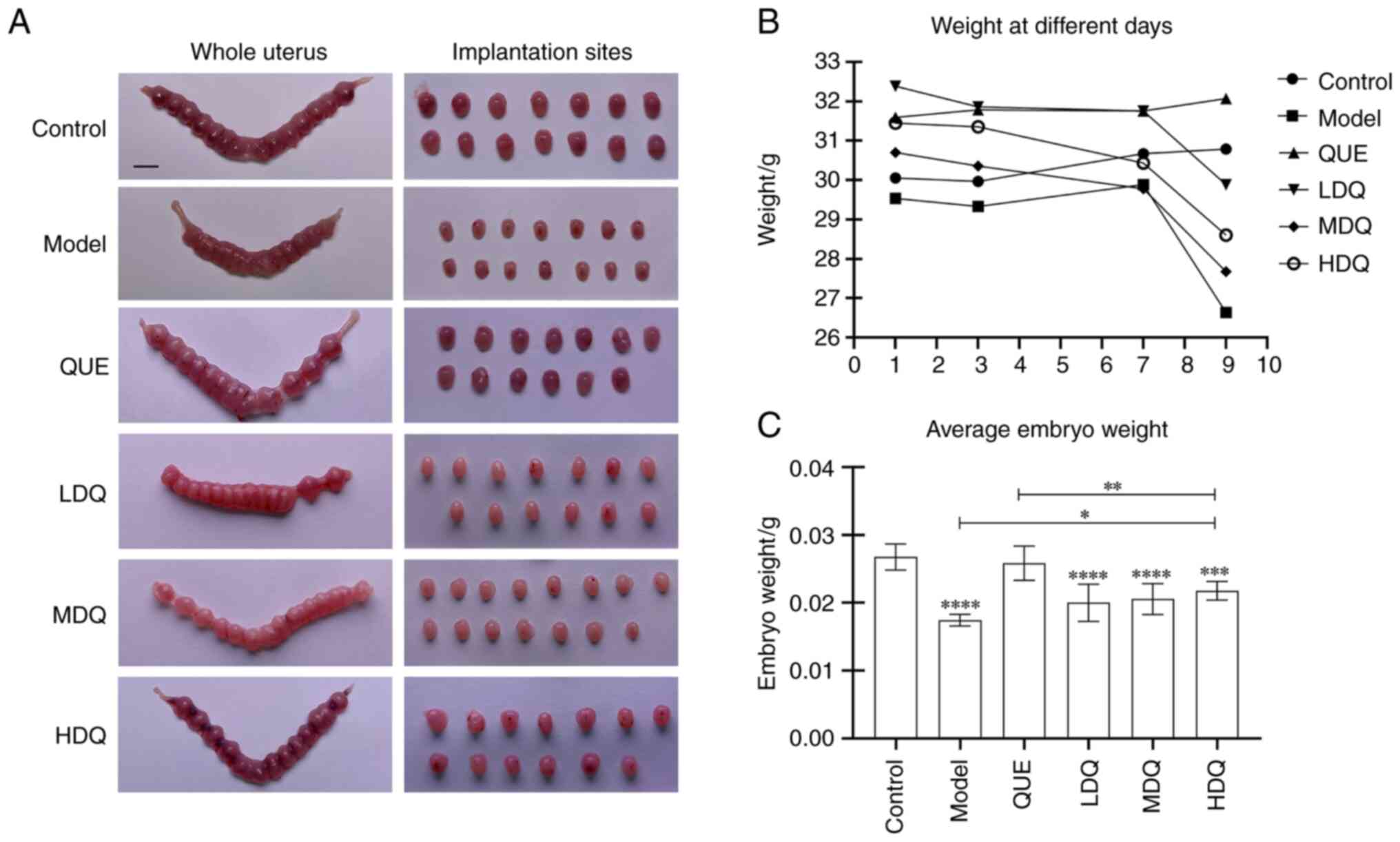 | Figure 2.Animal research of quercetin in
treating spontaneous abortion. (A) Diagram of uterus and
implantation sites of mouse. Scale bar, 5 mm. (B) Line chart of the
mouse weight of different pregnant days in the six groups. (C) Bar
chart of the average weight of surviving embryos in the six groups.
****P<0.0001, ***P<0.001 vs. the Control group, **P<0.01,
*P<0.05. Control, the control group; HDQ, the high-dose
quercetin group; LDQ, the low-dose quercetin group; MDQ, the
medium-dose quercetin group; Model, the model group; QUE, the
quercetin + PBS group. |
Following the tail vein injection on gestation day
7, the body weights of pregnant mice in low-, medium-, high-dose
quercetin and the model groups descended markedly (P<0.0001).
Notably, the model group exhibited the most substantial decrease in
body weight with the rate of 10.94±3.13%. Among three quercetin
groups, the medium-dose quercetin group experienced the greatest
reduction in body weight while the low-dose one showed the lowest
weight loss rate of 5.92±2.47%. However, the body weight growth
rate of the control group and the quercetin + PBS group remained
similar, with slight growth rates of 0.50±4.14% and 1.03±1.90%,
respectively (P=0.998; Table
I).
 | Table I.Decrease in body weight following the
tail vein injection. |
Table I.
Decrease in body weight following the
tail vein injection.
| Group | n | Body weight
(D7)/g | Body weight
(D9)/g | t | P-value | Degree of weight
loss/% |
|---|
| Control | 13 | 30.67±1.91 | 30.78±1.66 | −0.336 | 0.742 | −0.50±4.14 |
| Model | 13 | 29.88±1.42 | 26.63±1.86 | 12.857 | <0.0001 |
10.94±3.13a |
| QUE | 13 | 31.75±1.33 | 32.07±1.12 | −1.905 | 0.081 |
−1.03±1.90b |
| LDQ | 13 | 31.75±2.17 | 29.88±2.36 | 8.733 | <0.0001 |
5.92±2.47a,c,e |
| MDQ | 13 | 29.78±1.51 | 27.67±2.00 | 5.900 | <0.0001 |
7.09±4.29a,d,e |
| HDQ | 13 | 30.43±1.86 | 28.60±2.01 | 6.550 | <0.0001 |
6.01±3.18a,c,e |
Effect of quercetin on the number of
surviving and dead embryos and the mean weight of surviving
embryos
In the control group and the quercetin + PBS group,
the implantation sites were evenly distributed, with normal
embryonic development. The uterus was in red and shaped like a
bead, with a small amount of bruising in the uterine cavity. In the
model group, the development of embryos was not synchronized with
each other and the size of the implantation sites was reduced
significantly. Some disappeared or were necrotic, leaving dark
brown or dark red bamboo-like appearances of the uteri, section by
section with a knot between each section, and obvious bruising was
seen in the uterine cavity. The low- and medium-dose groups showed
shrinkage in size and a whitening color of the implantation sites,
but no bleeding spot or bruising was seen in the uterine cavity.
While the high-dose quercetin group displayed a superior embryo
status, there was a small amount of bleeding spot or bruising in
the uterine cavity (Fig. 2A).
Compared with the model group, the number of embryo
deaths decreased in both the control group and quercetin + PBS
group, while the number of surviving embryos increased in the
quercetin + PBS group (P=0.028, P=0.028 and P=0.034, respectively).
Meanwhile, the medium-dose quercetin group exhibited a markedly
higher number of embryo deaths in comparison to the control group
(P=0.035). No significant difference was observed in the numbers of
surviving and dead embryos between the control group and quercetin
+ PBS group (P=1.000 and P=1.000, respectively; Table II).
 | Table II.Total, surviving and dead
embryos. |
Table II.
Total, surviving and dead
embryos.
| Group | n | Total
embryos/n | Surviving embryos/n
M (upper, lower quartile) | Dead embryos/n M
(P25, P75) |
|---|
| Control | 13 | 13.54±1.45 | 14.00
(13.00,14.50) | 0.00
(0.00,0.00)b |
| Model | 13 | 12.69±1.32 | 0.00
(0.00,13.00) | 11.00
(0.00,13.00) |
| QUE | 13 | 13.77±1.30 | 14.00
(12.50,15.00)b | 0.00
(0.00,0.00)b |
| LDQ | 13 | 13.69±1.23 | 13.00
(12.50,14.00) | 0.00
(0.00,0.00) |
| MDQ | 13 | 13.69±1.25 | 0.00
(0.00,14.50) | 12.00
(0.00,13.50)a,c |
| HDQ | 13 | 13.54±1.27 | 12.00
(0.00,14.00) | 0.00
(0.00,13.00) |
Regarding the mean weight of surviving embryos, both
the control group and quercetin + PBS group exhibited significantly
higher values than the model group (P<0.0001), which were
0.0267±0.0019, 0.0258±0.0025 g and 0.0174±0.0008 g, respectively.
The high-, medium- and low-dose quercetin groups all demonstrated
higher mean weight than the model group, with the high-dose
quercetin group displaying the highest mean weight of 0.0218±0.0014
g among three quercetin groups (P=0.049, P=0.376 and P=0.457,
respectively). Moreover, the mean weight in the high-, medium- and
low-dose quercetin groups were all lower than that in the quercetin
+ PBS group (P=0.0096, P=0.0011 and P<0.0001, respectively). The
results are shown in Fig. 2C and
Table SII.
Effect of quercetin on the rate of
embryo loss in SA mice
The embryo loss rate of the model group was 59.39%,
while there was no embryo loss in the control group and the
quercetin + PBS group, showing a statistically significant
difference (P<0.0001). As for the three quercetin groups, the
medium-dose quercetin group shared the highest rate of embryo loss
(51.69%), accompanied by the rates of 15.73 and 38.64% in the low-
and high-dose quercetin groups. Statistically speaking, the high-
and medium-dose quercetin groups displayed an increased number of
dead embryos and a higher rate of embryo loss in comparison to the
low-dose quercetin group (P<0.0001 and P<0.0001,
respectively). The low-dose quercetin group exhibited a reduction
in both the quantity of dead embryos and the rate of embryo loss.
Moreover, the high-dose quercetin group showed a significantly
elevated number of dead embryos and a higher rate of embryo loss
when compared with the high-dose quercetin + PBS group
(P<0.0001). Interestingly, no significant difference was found
in the rate of embryo loss between the medium-dose quercetin group
and the model group (P=0.159; Table
III).
 | Table III.Effect of quercetin on the rate of
embryo loss in SA mice. |
Table III.
Effect of quercetin on the rate of
embryo loss in SA mice.
| Group | n | Total
embryos/n | Surviving
embryos/n | Dead embryos/n | Embryo loss rate
/% |
|---|
| Control | 13 | 176 | 176 | 0 | 0.00 |
| Model | 13 | 165 | 67 | 98 | 59.39 |
| QUE | 13 | 179 | 179 | 0 | 0.00 |
| LDQ | 13 | 178 | 150 | 28 | 15.73a |
| MDQ | 13 | 178 | 86 | 92 | 51.69c,d |
| HDQ | 13 | 176 | 108 | 68 | 38.64b d |
Effect of quercetin on litter size and
birthweight of the pups
To confirm that quercetin does prevent SA, the
results of another set of experiments showed that all pregnant mice
in each group could produce offspring, and there was no significant
difference in litter size and birthweight of the pups (Table SIII and Fig. S1).
Effect of LPS on the morphology of
implantation site tissue
Fig. S2 shows the
representative hematoxylin and eosin staining for the implantation
site tissue sections of the control and model group, including the
embryo. The findings showed that the embryos of normal mice were
structurally intact with regularly and tightly arranged cells,
while those of aborted mice were characterized by irregularly and
loosely arranged cells with the significant infiltration of
inflammatory cells and red blood cells, the structures of which
were not as intact as the former.
Screening potential targets of
quercetin and SA
A total of 291 candidate quercetin targets were
obtained using PharmMapper platform and 53, 1,498 and 197
SA-related targets were estimated in OMIM, GeneCards and DisGeNet
databases respectively by using the keywords ‘spontaneous abortion’
and ‘pregnancy loss’. Finally, a total of 1,622 disease-associated
targets were determined after removing 126 duplicates.
The Venn online tool was applied to visualize the
Venn diagram. A total of 80 targets of quercetin intersection with
SA were obtained.
PPI network construction and core
target screening
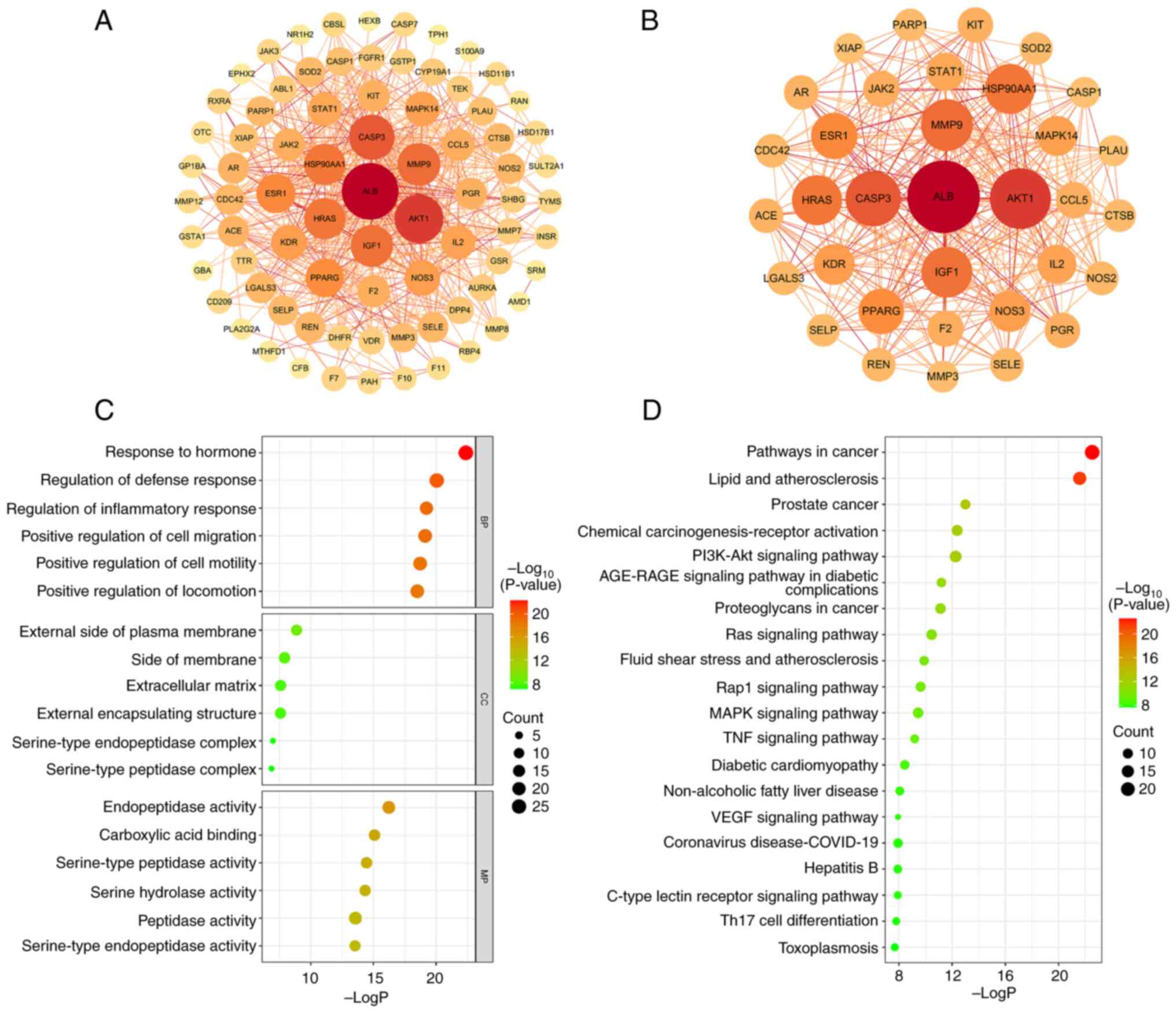
The PPI network of the 80 intersecting targets was
generated by the STRING database (Fig.
3A). After removing the nodes that were discrete from the main
network, 79 targets and 579 edges were finally presented. The TSV
format result was downloaded and input into Cytoscape (3.7.1) for
visualization. The degree value of each target was obtained using
the cytoNCA plug-in and the average degree value of 14.658 was
calculated using Excel software. Consequently, 34 core targets that
had higher degree value than the average value were screened out
(Fig. 3B and Table SIV). Among them, the top three
targets in terms of degree value were AKT1, albumin (ALB) and
caspase-3 (CASP3).
Enrichment analysis of GO and KEGG
pathway
GO and KEGG enrichment analysis were performed by
importing the 80 intersecting targets into the Metascape
database.
The GO enrichment analysis of quercetin treating SA
yielded a total of 1,062 terms (P<0.01), including 923 entries
for BP, 44 entries for CC and 95 entries for MF. BP was mainly
involved in ‘hormone responses’, ‘regulation of defense responses’,
‘inflammatory responses’, ‘cell migration’ and
‘phosphatidylinositol 3-kinase signaling’. CC was mainly involved
in ‘outer plasma membrane’, ‘extracellular matrix’, ‘serine
peptidase complex’ and ‘serine endopeptidase complex’. MF was
mainly comprised of ‘endopeptidase activity’, ‘serine hydrolase
activity’, ‘serine peptidase activity’, ‘serine endopeptidase
activity’, ‘peptidase activity’, ‘organic acid binding’, ‘nuclear
receptor activity’ and ‘carboxylic acid binding’. The Fig. 3C shows the top six entries in BP,
CC and MF sorted from the smallest to largest P-value.
A total of 130 KEGG pathways were responsible for in
the treatment of SA (P<0.01). To unravel the basic molecular
mechanisms leading to quercetin in the treatment of SA, the top 20
pathways were selected in order of P-value from smallest to
largest. As shown in Fig. 3D,
quercetin was not only related to cancer and diabetes, but also
associated with signaling pathways of PI3K-Akt, Ras, Rap1, MAPK,
TNF, VEGF and C-type lectin receptor.
Effect of quercetin on the relative
mRNA expression of AKT1, ESR1, JAK2, MAPK1, MAPK3, PGR, PI3K and
SGK1
The relative mRNA expressions of AKT1, MAPK1, PGR
and SGK1 in the model group were lower than those in the control
group (P<0.0001, P=0.012, P=0.0002 and P=0.001, respectively),
and the expressions of the same targets in the quercetin groups
were higher than those in the model group. But the expression
changes of ESR1 and MAPK3 showed a reverse trend: the expressions
in the model group were higher than those in the control group
(P=0.013 and P=0.0004, respectively), while the ones of quercetin
groups were lower than that in the model group. There was no
statistical difference between the expressions of JAK2 in the model
and the control group, as well as PI3K. LD-QUE group shared the
highest expression of JAK2 among the six groups. Data and pictures
are shown in Table SV and
Fig. S3.
Discussion
As the most common complication of early pregnancy,
the etiology of SA is complex, with maternal immunologic
abnormalities being one of the important etiologic factors
(1). A normal pregnancy shares
similarities with a successful allogeneic hemizygous
transplantation, hinging on the intricate equilibrium between
innate and adaptive immune responses at the maternal-fetal
interface, that is, the balance of maternal-fetal immune tolerance.
Disruption in the functioning of cellular components within the
maternal-fetal interface, coupled with the excessive production of
inflammatory cytokines, can lead to maternal-fetal rejection,
thereby giving rise to miscarriage and other unfavorable pregnancy
outcomes (40). Pregnancy can be
divided into three major stages: Implantation, development and
growth and delivery (41).
Inflammation is present during implantation, but excessive
inflammation leads to an imbalance in maternal-fetal immune
tolerance, so a successful pregnancy depends on the timely
elimination of inflammation and the establishment of a balance of
maternal-fetal immune tolerance (42).
LPS is derived from gram-negative bacteria and is a
potent initiator of inflammation. The model used in the current
study was the LPS-induced SA mouse model. LPS induces macrophage
polarization towards M1 type (43), increasing the secretion of
inflammatory cytokines such as IL-1β, IL-6 and TNF-α (44), which leads to an imbalance in the
local immune microenvironment in the uterus, presenting a
pro-inflammatory microenvironment and leading to embryo loss. The
results of the present study showed that following LPS injection
through the tail vein on day 7 of gestation, all groups of pregnant
mice displayed varying degrees of poor condition including
unresponsiveness and rough hair, diarrhea and weight loss, with or
without vaginal bleeding and discharge of gestational material. The
embryo loss rate in the model group was 59.39%, markedly higher
than that of the control group (P<0.001), validating the
effectiveness of the LPS-induced SA mouse modeling.
The present study showed that quercetin could lessen
the rate of embryo loss and elevate the average weight of embryos
to a certain extent. Quercetin may play a role in promoting the
expulsion or resorption of dead embryos after miscarriage and
promote the uterus to return to the pre-pregnancy state. Quercetin
is a natural flavonoid and is the main active ingredient in the
herbs that in traditional Chinese medicine (TCM) are used to
‘tonify’ kidneys and ‘stabilize’ the fetus, such as Cuscuta
and Herba Taxilli (45–47)
that are the main components of the basic formula for the Shoutai
Pill (48). Cuscuta and
Herba Taxilli are commonly used in TCM in treating and
preventing miscarriage (48).
Studies have revealed that Shoutai Pill regulates the expression
levels of cytokines such as IL-2, IL-4, IL-6, IL-10 and
interferon-γ (IFN-γ), maintains the balance of Th1/Th2 cytokines
and improves endometrial tolerance to promote embryo implantation
(49–51). It also reduces the expression of
Th17 in the decidual tissue (52),
thus maintaining normal pregnancy. It was previously found that
Cuscuta and Herba Taxilli serve as what TCM terms
sovereign drug and assistant drug, respectively, in SA treatment
(53,54). Relevant studies have confirmed that
quercetin decreases expression of IL-1β (55), IL-6 (56) and TNF-α (57) to maintain the balance of local
immune microenvironment and therefore to contribute a favorable
environment for embryo growth and development. Liu et al
(58) investigated the mechanism
of action of quercetin by establishing a PM2.5-exposed pregnant
mouse model and found that quercetin could prevent miscarriage by
inhibiting the expression of cytokines such as IL-6 and IL-8 and
upregulating the level of heme oxygenase-1 in peripheral blood.
Quercetin has a strong antioxidant capacity and some animal
experimental findings have illustrated that quercetin can hinder
the expression of TNF-α and IL-6 in the placenta of LPS-treated
mice and rescue the abnormal oxidative stress, inflammatory
response and angiogenic factor imbalance at the placental interface
(26). Moreover, Cao et al
(59) found that quercetin
counteracted hyperglycemia-induced nitrosative stress and oxidative
stress, decreased the levels of oxidative stress markers and
increased the expression of superoxide dismutase 1 and 2 and
ultimately reduced the rate of embryonic malformations in diabetic
pregnant mice. In addition, in vitro experiments have
demonstrated that quercetin at appropriate concentrations can
improve biological function, mitochondrial membrane potential and
morphology of trophoblast cells under hypoxic conditions (60). Decidual natural killer (NK) cells
are the primary immune cell population at the maternal-fetal
interface, with low cytotoxicity (61), which enhance decidual cell invasion
as well as spiral artery remodeling through the secretion of matrix
metalloproteinases, vascular endothelial growth factor and
interleukins, regulating maternal-fetal immune tolerance and
placental developmental capacity (62–65).
Evidence has shown that cytokines such as IL-15, IL-18 and TGF-β
can convert highly cytotoxic peripheral blood NK cells into low
cytotoxic decidual NK cells (66,67).
TGF-β, among the above three cytokines, is abundant in decidual
microenvironment, which is the key to drive the transformation of
placental extravillous trophoblast cells into decidual extravillous
trophoblast cells according to bioinformatic analysis (68). Hu et al (69) found that quercetin is capable to
up-regulate TGF-β expression, as well as to promote the
polarization of M2 macrophages, which is beneficial to the
maintenance of normal pregnancy.
Network pharmacological results uncovered that the
effects of quercetin on SA were mainly related to hormone response
and the modulation of defense response, inflammatory response, cell
migration and motility, involving signaling pathways such as VEGF,
MAPK and PI3K-Akt. Among them, the PI3K-Akt signaling pathway is
importantly involved in cell proliferation, apoptosis and autophagy
(70–72). It has also been suggested that the
establishment and retention of a normal pregnancy tightly associate
with PI3K-Akt pathway (73). Song
et al (74) discovered that
quercetin directly bonds to PI3K, which led to the conclusion that
PI3K was a molecular target of quercetin. VEGF is a key angiogenic
factor in the process of metamorphosis (75) and the lack of bone morphogenetic
protein receptor type 2 in mouse uterine decidualization during the
early stage of placental development inhibits VEGF signaling, which
affects the process of decidualization and leads to the abnormal
development of embryonic blood vessels (76). Relevant animal experiments have
demonstrated that a high quercetin diet increases VEGF-A and
VEGF-R2 levels in mice, enhances angiogenesis and contributes to
the reduction of systemic inflammation and insulin resistance
(77). The MAPK signaling pathway
is a classical signaling pathway in trophoblast cells and
abnormalities in this pathway impact the physiological functions of
trophoblast cells, leading to adverse pregnancy outcomes (78). Quercetin disrupts skeletal
fibrillar actin of macrophages and inhibits LPS-induced macrophage
adhesion and migration by downregulating focal adhesion
kinase–paxillin and modulating the MAPK pathway (79). In addition, it has been proposed
that the process of embryonic implantation is similar to tumor
invasion (80). For instance, the
maternal-fetal interface microenvironment in the first trimester of
pregnancy is similar to the tumor microenvironment in hypoxic,
acidic and immune features (81).
While several relevant signaling pathways identified in the present
study have been demonstrated to modulate tumorigenesis and tumor
development (82–87).
In summary, quercetin decreased the embryo loss
rate, increases the mean weight of surviving embryos and promotes
the resorption or expulsion of dead embryos in a mouse model of SA.
Network pharmacological studies revealed that quercetin can treat
SA by regulating multiple signaling pathways such as PI3K-Akt,
VEGF, MAPK and core targets such as AKT1, ALB and CASP3. RT-qPCR
showed that quercetin could upregulate AKT1, MAPK1, PGR and SGK1,
and downregulate ESR1 and MAPK3, some of which are closely related
to SA. Currently, the studies of quercetin on reproductive medicine
are scattered. Although the present study has elucidated the
influence of quercetin on embryo loss rate and mean weight of
embryos and explored its potential molecular mechanisms based on
network pharmacology, the further validation is needed. Our group
intends to select Clark classical recurrent abortion model mice to
explore and validate the specific mechanisms of quercetin in SA
treatment from the cellular and molecular perspective, which can
provide solid evidence to sustain the application of quercetin in
the field of reproductive medicine.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Zhejiang Provincial Natural
Science Foundation of China (grant no. LGF22H270021), Young Program
of National Natural Science Foundation of China (grant no.
81801475), Medical Scientific Research Foundation of Zhejiang
Province of China (grant no. 2022RC232), Key Project of Hangzhou
Health Science and Technology Program of China (grant no.
ZD20230093), Zhejiang Traditional Medicine and Technology Program
of China (grant no. 2022ZA111), Natural Science Program of Zhejiang
Chinese Medical University Scientific Research Program for Young
scholar of China (grant no. 2022JKZKTS48), Zhejiang Medicine and
Health Project for Young Scholar [Office of Zhejiang Provincial
Health Commission; grant no. 18(2020)] and First-level Talents of
Hangzhou High-level Talent Special Support Program [Hangzhou
Municipal Human Resources and Social Security Bureau; grant no.
141(2021)].
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YL, SW and YT conceived and designed the study. SW,
QZ, YT and LM conducted the animal experiments. ZF, HL and XZ
performed the network pharmacology analysis. SW analyzed the
experimental data. SW drafted the manuscript, which was reviewed
and edited by YL. YL and SW confirm the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved (ethics approval no.
IACUC-20220919-25) by the Animal Ethical and Welfare Committee of
Zhejiang Chinese Medical University (Hangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhao AM: Chinese expert consensus on
diagnosis and treatment of spontaneous abortion (2020 edition).
Chin J Pract Gynecol Obstet. 36:1082–1090. 2020.
|
|
2
|
Quenby S, Gallos ID, Dhillon-Smith RK,
Podesek M, Stephenson MD, Fisher J, Brosens JJ, Brewin J, Ramhorst
R, Lucas ES, et al: Miscarriage matters: The epidemiological,
physical, psychological, and economic costs of early pregnancy
loss. Lancet. 397:1658–1667. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dimitriadis E, Menkhorst E, Saito S,
Kutteh WH and Brosens JJ: Recurrent pregnancy loss. Nat Rev Dis
Primers. 6:982020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Larsen EC, Christiansen OB, Kolte AM and
Macklon N: New insights into mechanisms behind miscarriage. BMC
Med. 11:1542013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li HF, Shen QH, Li XQ, Feng ZF, Chen WM,
Qian JH, Shen L, Yu LY and Yang Y: The efficacy of traditional
Chinese medicine shoutai pill combined with western medicine in the
first trimester of pregnancy in women with unexplained recurrent
spontaneous abortion: A systematic review and meta-analysis. Biomed
Res Int. 2020:74951612020.PubMed/NCBI
|
|
6
|
Shi YJ, Xie JH and Li XJ: Meta-analysis of
pre-pregnancy intervention with Chinese medicinals for tonifying
kidney and activating blood in patients with recurrent spontaneous
abortion of pre-thrombotic state. Shandong J Tradit Chin Med.
41:744–752. 2022.
|
|
7
|
Wu T, Wang YZ, Li WL and Yu XH:
Meta-analysis of tonifying kidney and invigorating spleen in the
treatment of recurrent abortion. Chin J Gen Pract. 20:1056–1061.
2022.
|
|
8
|
Ahmad A, Tandon S, Xuan TD and Nooreen Z:
A review on Phytoconstituents and Biological activities of Cuscuta
species. Biomed Pharmacother. 92:772–795. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Donnapee S, Li J, Yang X, Ge AH, Donkor
PO, Gao XM and Chang YX: Cuscuta chinensis Lam.: A systematic
review on ethnopharmacology, phytochemistry and pharmacology of an
important traditional herbal medicine. J Ethnopharmacol.
157:292–308. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu X, Lin CY, Zhang WQ, Cao R, Qin WH and
Fan LL: Chemical components and pharmacological effect of Trib.
Lorantheae in China: A review. Chin J Exp Tradit Med Form.
29:209–221. 2023.
|
|
11
|
Yang D, Wang T, Long M and Li P:
Quercetin: Its main pharmacological activity and potential
application in clinical medicine. Oxid Med Cell Longev.
2020:88253872020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Sun Z, Luo G, Wang S, Cui H, Yao Z,
Xiong H, He Y, Qian Y and Fan C: Quercetin attenuates
trauma-induced heterotopic ossification by tuning immune cell
infiltration and related inflammatory insult. Front Immunol.
12:6492852021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gansukh E, Nile A, Kim DH, Oh JW and Nile
SH: New insights into antiviral and cytotoxic potential of
quercetin and its derivatives-A biochemical perspective. Food Chem.
334:1275082021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hur HJ, Jeong YH, Lee SH and Sung MJ:
Quercitrin ameliorates hyperlipidemia and hepatic steatosis in
ovariectomized mice. Life (Basel). 10:2432020.PubMed/NCBI
|
|
15
|
Huang YY, Wang ZH, Deng LH, Wang H and
Zheng Q: Oral administration of quercetin or its derivatives
inhibit bone loss in animal model of osteoporosis. Oxid Med Cell
Longev. 2020:60805972020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
van der Woude H, Ter Veld MG, Jacobs N,
van der Saag PT, Murk AJ and Rietjens IM: The stimulation of cell
proliferation by quercetin is mediated by the estrogen receptor.
Mol Nutr Food Res. 49:763–771. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Patel RV, Mistry BM, Shinde SK, Syed R,
Singh V and Shin HS: Therapeutic potential of quercetin as a
cardiovascular agent. Eur J Med Chem. 155:889–904. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reyes-Farias M and Carrasco-Pozo C: The
anti-cancer effect of quercetin: molecular implications in cancer
metabolism. Int J Mol Sci. 20:31772019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu C, Liu DQ, Tian YK, Mei W, Tian XB, Xu
AJ and Zhou YQ: The emerging role of quercetin in the treatment of
chronic pain. Curr Neuropharmacol. 20:2346–2353. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hosseini A, Razavi BM, Banach M and
Hosseinzadeh H: Quercetin and metabolic syndrome: A review.
Phytother Res. 35:5352–5364. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tang F, Tang Q, Tian Y, Fan Q, Huang Y and
Tan X: Network pharmacology-based prediction of the active
ingredients and potential targets of Mahuang Fuzi Xixin decoction
for application to allergic rhinitis. J Ethnopharmacol.
176:402–412. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nogales C, Mamdouh ZM, List M, Kiel C,
Casas AI and Schmidt HHHW: Network pharmacology: Curing causal
mechanisms instead of treating symptoms. Trends Pharmacol Sci.
43:136–150. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He RP, Jin Z, Ma RY, Hu FD and Dai JY:
Network pharmacology unveils spleen-fortifying effect of Codonopsis
Radix on different gastric diseases based on theory of ‘same
treatment for different diseases’ in traditional Chinese medicine.
Chin Herb Med. 13:189–201. 2020.PubMed/NCBI
|
|
24
|
Shi QQ, Yan MQ, Yu HH, Chen QQ, Chen SH
and Lyu GY: Effect of Yunkang oral liquid on preventing LPS-induced
abortion and regulating immune tolerance in mice. Zhongguo Zhong
Yao Za Zhi. 44:1227–1232. 2019.(In Chinese). PubMed/NCBI
|
|
25
|
Chen LL, Song C, Zhang Y, Li Y, Zhao YH,
Lin FY, Han DD, Dai MH, Li W and Pan PH: Quercetin protects against
LPS-induced lung injury in mice via SIRT1-mediated suppression of
PKM2 nuclear accumulation. Eur J Pharmacol. 936:1753522022.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Q, Yin L, Si Y, Zhang C, Meng Y and
Yang W: The bioflavonoid quercetin improves pathophysiology in a
rat model of preeclampsia. Biomed Pharmacother. 127:1101222020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wierwille WW and Ellsworth LA: Evaluation
of driver drowsiness by trained raters. Accid Anal Prev.
26:571–581. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim S, Chen J, Cheng T, Gindulyte A, He J,
He S, Li Q, Shoemaker BA, Thiessen PA, Yu B, et al: PubChem 2023
update. Nucleic Acids Res. 51(D1): D1373–D1380. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang X, Shen Y, Wang S, Li S, Zhang W, Liu
X, Lai L, Pei J and Li H: PharmMapper 2017 update: a web server for
potential drug target identification with a comprehensive target
pharmacophore database. Nucleic Acids Res. 45((W1)): W356–W360.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
UniProt Consortium: UniProt: The universal
protein knowledgebase in 2023. Nucleic Acids Res. 51(D1):
D523–D531. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hamosh A, Amberger JS, Bocchini C, Scott
AF and Rasmussen SA: Online mendelian inheritance in man
(OMIM®): Victor McKusick's magnum opus. Am J Med Genet
A. 185:3259–3265. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stelzer G, Rosen N, Plaschkes I, Zimmerman
S, Twik M, Fishilevich S, Stein TI, Nudel R, Lieder I, Mazor Y, et
al: The GeneCards suite: From gene data mining to disease genome
sequence analyses. Curr Protoc Bioinformatics. 54:1.30.1–1.30.33.
2016. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Piñero J, Ramírez-Anguita JM,
Saüch-Pitarch J, Ronzano F, Centeno E, Sanz F and Furlong LI: The
DisGeNET knowledge platform for disease genomics: 2019 Update.
Nucleic Acids Res. 48(D1): D845–D855. 2020.PubMed/NCBI
|
|
34
|
Bardou P, Mariette J, Escudié F, Djemiel C
and Klopp C: Jvenn: An interactive Venn diagram viewer. BMC
Bioinformatics. 15:2932014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Szklarczyk D, Kirsch R, Koutrouli M,
Nastou K, Mehryary F, Hachilif R, Gable AL, Fang T, Doncheva NT,
Pyysalo S, et al: The STRING database in 2023: Protein-protein
association networks and functional enrichment analyses for any
sequenced genome of interest. Nucleic Acids Res. 51(D1): D638–D646.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang D and Wang XL: Mechanism of diosgenin
in treatment of atherosclerosis based on network pharmacology,
molecular docking and experimental validation. Chin Tradit Herb
Drugs. 53:7783–7794. 2022.
|
|
38
|
Zhou Y, Zhou B, Pache L, Chang M,
Khodabakhshi AH, Tanaseichuk O, Benner C and Chanda SK: Metascape
provides a biologist-oriented resource for the analysis of
systems-level datasets. Nat Commun. 10:15232019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vaitsopoulou CI, Kolibianakis EM, Bosdou
JK, Neofytou E, Lymperi S, Makedos A, Savvaidou D, Chatzimeletiou
K, Grimbizis GF, Lambropoulos A and Tarlatzis BC: Expression of
genes that regulate follicle development and maturation during
ovarian stimulation in poor responders. Reprod Biomed Online.
42:248–259. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Brown MB, von Chamier M, Allam AB and
Reyes L: M1/M2 macrophage polarity in normal and complicated
pregnancy. Front Immunol. 5:6062014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chavan AR, Griffith OW and Wagner GP: The
inflammation paradox in the evolution of mammalian pregnancy:
Turning a foe into a friend. Curr Opin Genet Dev. 47:24–32. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li Y, Zhang D, Xu L, Dong L, Zheng J, Lin
Y, Huang J, Zhang Y, Tao Y, Zang X, et al: Cell-cell contact with
proinflammatory macrophages enhances the immunotherapeutic effect
of mesenchymal stem cells in two abortion models. Cell Mol Immunol.
16:908–920. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu PS, Chen YT, Li X, Hsueh PC, Tzeng SF,
Chen H, Shi PZ, Xie X, Parik S, Planque M, et al: CD40 signal
rewires fatty acid and glutamine metabolism for stimulating
macrophage anti-tumorigenic functions. Nat Immunol. 24:452–462.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang L and He C: Nrf2-mediated
anti-inflammatory polarization of macrophages as therapeutic
targets for osteoarthritis. Front Immunol. 13:9671932022.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang W, Fu ZT, Liu CG, Wang BL, Liu RD
and Fan RH: Stimultaneous determination of six flavonoids in Semen
Cuscutae by UPLC-MS/MS. J Shenyang Med Coll. 20:377–380. 2018.
|
|
46
|
Su BW, Wang H, Li YH, Pei HH, Zhu KX and
Lu D: Contents of avicularin, quercetrin amd quercetin in Taxilli
Herba harvested from different areas and time points. Chin J Hosp
Pharm. 37:1922–1926. 2017.
|
|
47
|
Sun XM, Song H, Yan XJ, Hu Y, Xu BL, Zhao
LZ and Li WL: Screening and determination of estrogen-like quality
markers of Cuscuta chinensis. Chin Tradit Herb Drugs. 51:2671–2679.
2020.
|
|
48
|
Wu H, Hao LL, Li WL and Jin Y: Analysis of
medication rules of traditional Chinese medicine in treatment of
immunological recurrent abortion by data driven approach. Liaoning
J Tradit Chin Med. 47:52–55. 2020.
|
|
49
|
Zhang J, Chen L, Zheng CH, Wang J, Xie D
and Zhou YX: Effect of shoutai pills on Th1/Th2 cytokines in serum
and endometrium of rats with stimulated ovulation. Curr Med Sci.
39:285–290. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li YQ, Zhao F, Ji MM, Li K and Wang YH:
Efficacy of Jiawei Shoutai Pill in the treatment of threatened
miscarriage and its effect on reproductive immuno-endocrine
function. Lishizhen Med Mater Med Res. 31:2971–2973. 2020.
|
|
51
|
Zhou H, Zhen XY, Wang H, Zeng Q, Deng LW
and Ding WJ: Exploration of the mechanism differences between
Shoutaiwan and Juyuanjian in reversing the pathology of decidual of
spontaneous abortion patients based on the ‘uterine collaterals
connecting the kidney’ and ‘fetal collaterals connecting the
spleen’ theory. Chin J Exp Tradit Med Form. 28:186–200. 2022.
|
|
52
|
Hao XL, Wang DY, Gao J and Luo SP: Effect
of modified Shoutai Pills on IL-17 in mice model of spontaneous
abortion due to kidney deficiency. World J Integr Tradit West Med.
15:292–295. 2020.
|
|
53
|
Lou YY, Wu XT and Fu P: Effect of Chinese
medicine on PI3K signaling pathway at the maternal-fetal interface
of recurrent miscarriage of kidney deficiency type. Zhejiang J
Tradit Chin Med. 54:564–566. 2019.
|
|
54
|
Zhang MY, Lou YY and Fu P: Effect of
Huatai Antai Decoction regulating PI3K signaling pathway on
endometrial decidulization in recurrent spontaneous abortion of
kidney deficiency type. Zhejiang J Tradit Chin Med. 55:892–894.
2020.
|
|
55
|
Chiang SCC, Owsley E, Panchal N,
Chaturvedi V, Terrell CE, Jordan MB, Mehta PA, Davies SM, Akeno N,
Booth C and Marsh RA: Quercetin ameliorates XIAP
deficiency-associated hyperinflammation. Blood. 140:706–715. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
da Silva AB, Cerqueira Coelho PL, das
Neves Oliveira M, Oliveira JL, Oliveira Amparo JA, da Silva KC,
Soares JRP, Pitanga BPS, Dos Santos Souza C, de Faria Lopes GP, et
al: The flavonoid rutin and its aglycone quercetin modulate the
microglia inflammatory profile improving antiglioma activity. Brain
Behav Immun. 85:170–185. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Olayinka J, Eduviere A, Adeoluwa O, Fafure
A, Adebanjo A and Ozolua R: Quercetin mitigates memory deficits in
scopolamine mice model via protection against neuroinflammation and
neurodegeneration. Life Sci. 292:1203262022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liu W, Zhang M, Feng J, Fan A, Zhou Y and
Xu Y: The influence of quercetin on maternal immunity, oxidative
stress, and inflammation in mice with exposure of fine particulate
matter during gestation. Int J Environ Res Public Health.
14:5922017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Cao L, Tan C, Meng F, Liu P, Reece EA and
Zhao Z: Amelioration of intracellular stress and reduction of
neural tube defects in embryos of diabetic mice by phytochemical
quercetin. Sci Rep. 6:214912016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhou J, Li L, Pan X, Wang J, Qi Q, Sun H,
Li C and Wang L: The effect of a traditional Chinese
quadri-combination therapy and its component quercetin on recurrent
spontaneous abortion: A clinical trial, network pharmacology and
experiments-based study. Front Pharmacol. 13:9656942022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Albini A and Noonan DM: Decidual-like NK
cell polarization: From cancer killing to cancer nurturing. Cancer
Discov. 11:28–33. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hazan AD, Smith SD, Jones RL, Whittle W,
Lye SJ and Dunk CE: Vascular-leukocyte interactions: Mechanisms of
human decidual spiral artery remodeling in vitro. Am J Pathol.
177:1017–1030. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Hanna J, Goldman-Wohl D, Hamani Y, Avraham
I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon
TI, Manaster I, et al: Decidual NK cells regulate key developmental
processes at the human fetal-maternal interface. Nat Med.
12:1065–1074. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hao F, Zhou X and Jin L: Natural killer
cells: Functional differences in recurrent spontaneous abortion†.
Biol Reprod. 102:524–531. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Gaynor LM and Colucci F: Uterine natural
killer cells: Functional distinctions and influence on pregnancy in
humans and mice. Front Immunol. 8:4672017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Bruno A, Ferlazzo G, Albini A and Noonan
DM: A think tank of TINK/TANKs: Tumor-infiltrating/tumor-associated
natural killer cells in tumor progression and angiogenesis. J Natl
Cancer Inst. 106:dju2002014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Jabrane-Ferrat N: Features of human
decidual NK cells in healthy pregnancy and during viral infection.
Front Immunol. 10:13972019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Haider S, Lackner AI, Dietrich B, Kunihs
V, Haslinger P, Meinhardt G, Maxian T, Saleh L, Fiala C, Pollheimer
J, et al: Transforming growth factor-β signaling governs the
differentiation program of extravillous trophoblasts in the
developing human placenta. Proc Natl Acad Sci USA.
119:e21206671192022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hu Y, Gui Z, Zhou Y, Xia L, Lin K and Xu
Y: Quercetin alleviates rat osteoarthritis by inhibiting
inflammation and apoptosis of chondrocytes, modulating synovial
macrophages polarization to M2 macrophages. Free Radic Biol Med.
145:146–160. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ediriweera MK, Tennekoon KH and Samarakoon
SR: Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer:
Biological and therapeutic significance. Semin Cancer Biol.
59:147–160. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Paskeh MDA, Ghadyani F, Hashemi M,
Abbaspour A, Zabolian A, Javanshir S, Razzazan M, Mirzaei S,
Entezari M, Goharrizi MASB, et al: Biological impact and
therapeutic perspective of targeting PI3K/Akt signaling in
hepatocellular carcinoma: Promises and challenges. Pharmacol Res.
187:1065532023. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ding J, Yang C, Zhang Y, Wang J, Zhang S,
Guo D, Yin T and Yang J: M2 macrophage-derived G-CSF promotes
trophoblasts EMT, invasion and migration via activating
PI3K/Akt/Erk1/2 pathway to mediate normal pregnancy. J Cell Mol
Med. 25:2136–2147. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Song NR, Chung MY, Kang NJ, Seo SG, Jang
TS, Lee HJ and Lee KW: Quercetin suppresses invasion and migration
of H-Ras-transformed MCF10A human epithelial cells by inhibiting
phosphatidylinositol 3-kinase. Food Chem. 142:66–71. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Halder JB, Zhao X, Soker S, Paria BC,
Klagsbrun M, Das SK and Dey SK: Differential expression of VEGF
isoforms and VEGF(164)-specific receptor neuropilin-1 in the mouse
uterus suggests a role for VEGF(164) in vascular permeability and
angiogenesis during implantation. Genesis. 26:213–224. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Nagashima T, Li Q, Clementi C, Lydon JP,
DeMayo FJ and Matzuk MM: BMPR2 is required for postimplantation
uterine function and pregnancy maintenance. J Clin Invest.
123:2539–2550. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Perdicaro DJ, Rodriguez Lanzi C, Gambarte
Tudela J, Miatello RM, Oteiza PI and Vazquez Prieto MA: Quercetin
attenuates adipose hypertrophy, in part through activation of
adipogenesis in rats fed a high-fat diet. J Nutr Biochem.
79:1083522020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zhang J, Liu X and Gao Y: Abnormal H3K27
histone methylation of RASA1 gene leads to unexplained recurrent
spontaneous abortion by regulating Ras-MAPK pathway in trophoblast
cells. Mol Biol Rep. 48:5109–5119. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Cui S, Wu Q, Wang J, Li M, Qian J and Li
S: Quercetin inhibits LPS-induced macrophage migration by
suppressing the iNOS/FAK/paxillin pathway and modulating the
cytoskeleton. Cell Adh Migr. 13:1–12. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Mor G, Aldo P and Alvero AB: The unique
immunological and microbial aspects of pregnancy. Nat Rev Immunol.
17:469–482. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ma LN, Huang XB, Muyayalo KP, Mor G and
Liao AH: Lactic acid: A novel signaling molecule in early
pregnancy? Front Immunol. 11:2792020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
He Y, Sun MM, Zhang GG, Yang J, Chen KS,
Xu WW and Li B: Targeting PI3K/Akt signal transduction for cancer
therapy. Signal Transduct Target Ther. 6:4252021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Downward J: Targeting RAS signalling
pathways in cancer therapy. Nat Rev Cancer. 3:11–22. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Shah S, Brock EJ, Ji K and Mattingly RR:
Ras and Rap1: A tale of two GTPases. Semin Cancer Biol. 54:29–39.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Campbell BB, Galati MA, Stone SC,
Riemenschneider AN, Edwards M, Sudhaman S, Siddaway R, Komosa M,
Nunes NM, Nobre L, et al: Mutations in the RAS/MAPK pathway drive
replication repair-deficient hypermutated tumors and confer
sensitivity to MEK inhibition. Cancer Discov. 11:1454–1467. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Endo M, Yamamoto H, Setsu N, Kohashi K,
Takahashi Y, Ishii T, Iida K, Matsumoto Y, Hakozaki M, Aoki M, et
al: Prognostic significance of AKT/mTOR and MAPK pathways and
antitumor effect of mTOR inhibitor in NF1-related and sporadic
malignant peripheral nerve sheath tumors. Clin Cancer Res.
19:450–461. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Chuai Y, Rizzuto I, Zhang X, Li Y, Dai G,
Otter SJ, Bharathan R, Stewart A and Wang A: Vascular endothelial
growth factor (VEGF) targeting therapy for persistent, recurrent,
or metastatic cervical cancer. Cochrane Database Syst Rev.
3:CD0133482021.PubMed/NCBI
|