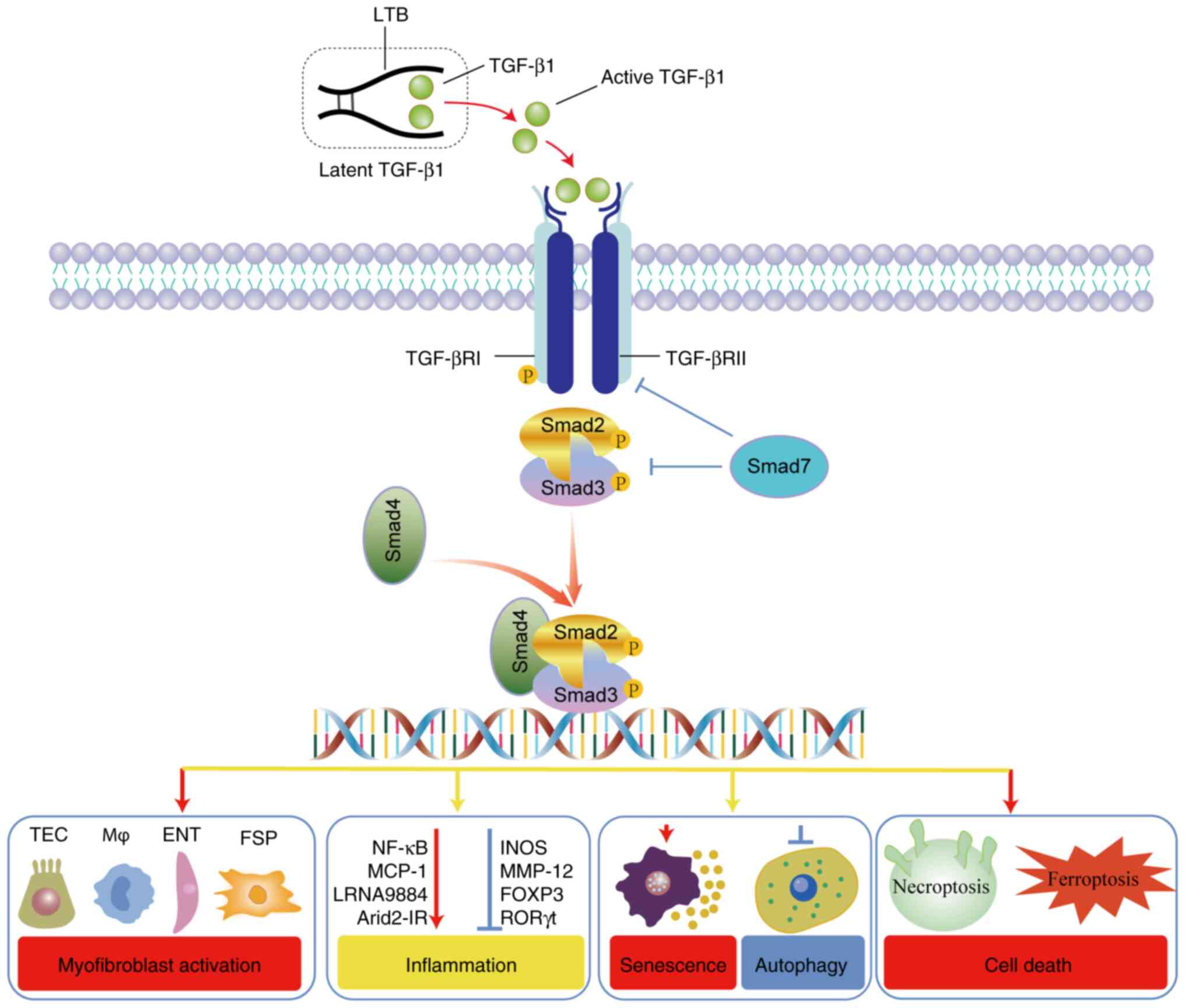
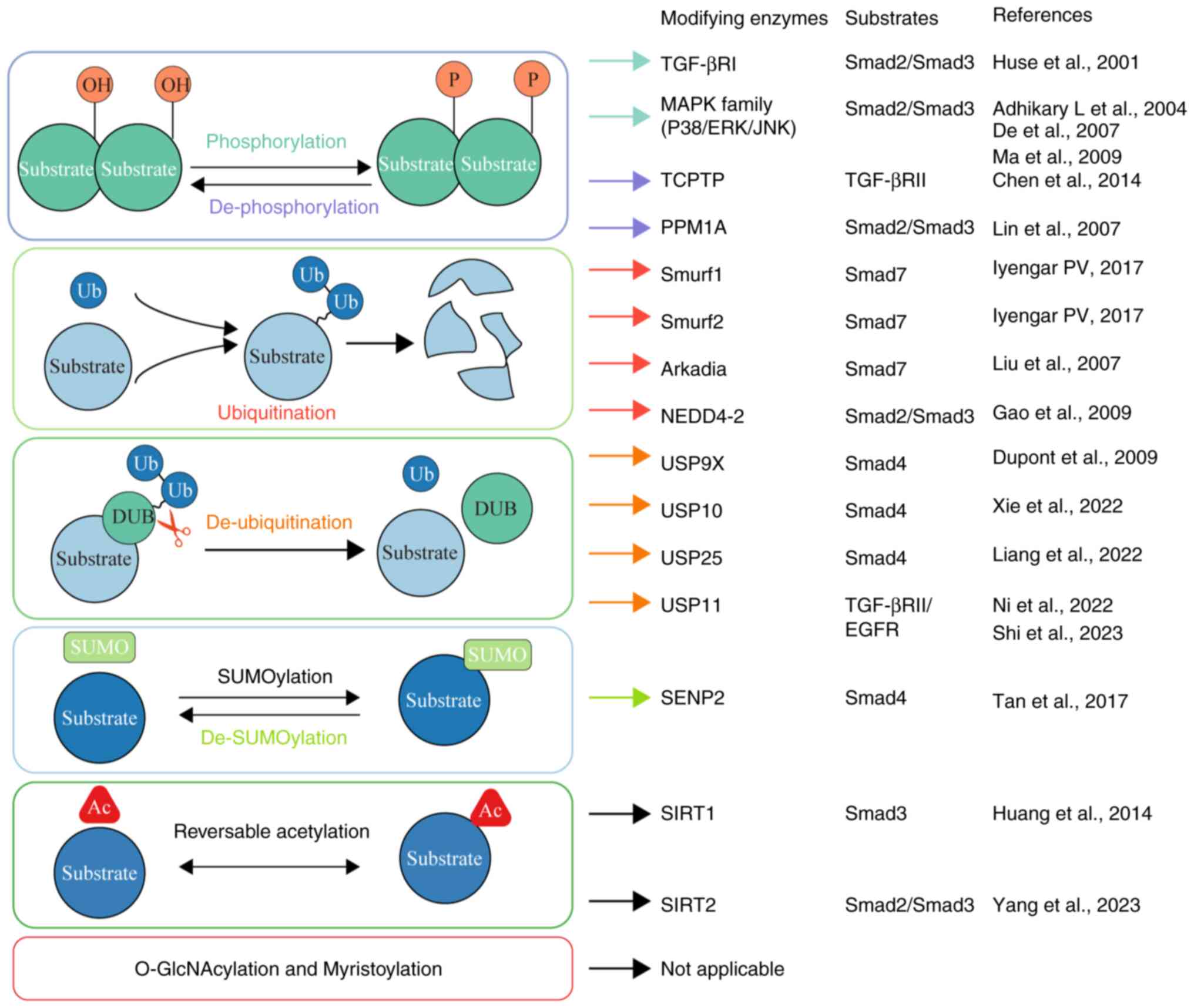
|
1
|
Webster AC, Nagler EV, Morton RL and
Masson P: Chronic kidney disease. Lancet. 389:1238–1252. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
GBD Chronic Kidney Disease Collaboration,
. Global, regional, and national burden of chronic kidney disease,
1990-2017: A systematic analysis for the Global Burden of Disease
Study 2017. Lancet. 395:709–733. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen TK, Knicely DH and Grams ME: Chronic
kidney disease diagnosis and management: A review. JAMA.
322:1294–1304. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Y: Cellular and molecular mechanisms
of renal fibrosis. Nat Rev Nephrol. 7:684–696. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meng X, Nikolic-Paterson DJ and Lan HY:
TGF-β: The master regulator of fibrosis. Nat Rev Nephrol.
12:325–338. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ruiz-Ortega M, Rayego-Mateos S, Lamas S,
Ortiz A and Rodrigues-Diez RR: Targeting the progression of chronic
kidney disease. Nat Rev Nephrol. 16:269–288. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gu YY, Liu XS, Huang XR, Yu XQ and Lan HY:
TGF-β in renal fibrosis: Triumphs and challenges. Future Med Chem.
12:853–866. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu W, Wang X, Yu X and Lan HY: Smad3
Signatures in Renal Inflammation and Fibrosis. Int J Biol Sci.
18:2795–2806. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meng XM, Tang PM, Li J and Lan HY:
TGF-β/Smad signaling in renal fibrosis. Front Physiol. 6:822015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu L, Border WA, Huang Y and Noble NA:
TGF-beta isoforms in renal fibrogenesis. Kidney Int. 64:844–856.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weiss A and Attisano L: The TGFbeta
superfamily signaling pathway. Wiley Interdiscip Rev Dev Biol.
2:47–63. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Annes JP, Munger JS and Rifkin DB: Making
sense of latent TGFbeta activation. J Cell Sci. 116:217–224. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Robertson IB, Horiguchi M, Zilberberg L,
Dabovic B, Hadjiolova K and Rifkin DB: Latent TGF-β-binding
proteins. Matrix Biol. 47:44–53. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Macconi D, Remuzzi G and Benigni A: Key
fibrogenic mediators: Old players. Renin-angiotensin system. Kidney
Int. Suppl (2011):4:58–64. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Loeffler I and Wolf G: Transforming growth
factor-β and the progression of renal disease. Nephrol Dial
Transplant. 29 (Suppl 1):i37–i45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Samarakoon R, Overstreet JM and Higgins
PJ: TGF-β signaling in tissue fibrosis: Redox controls, target
genes and therapeutic opportunities. Cell Signal. 25:264–268. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Samarakoon R, Overstreet JM, Higgins SP
and Higgins PJ: TGF-β1 → SMAD/p53/USF2 → PAI-1 transcriptional axis
in ureteral obstruction-induced renal fibrosis. Cell Tissue Res.
347:117–128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li J, Yang J, Zhu B, Fan J, Hu Q and Wang
L: Tectorigenin protects against unilateral ureteral obstruction by
inhibiting Smad3-mediated ferroptosis and fibrosis. Phytother Res.
36:475–487. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Q, Gao L, Hu XW, Wang JN, Zhang Y,
Dong YH, Lan HY and Meng XM: Smad3-Targeted Therapy Protects
against Cisplatin-Induced AKI by attenuating programmed cell death
and inflammation via a NOX4-dependent mechanism. Kidney Dis
(Basel). 7:372–390. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang PM, Zhou S, Li CJ, Liao J, Xiao J,
Wang QM, Lian GY, Li J, Huang XR, To KF, et al: The proto-oncogene
tyrosine protein kinase Src is essential for
macrophage-myofibroblast transition during renal scarring. Kidney
Int. 93:173–187. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liang L, Wang W, Chen J, Wu W, Huang XR,
Wei B, Zhong Y, Ma RCW, Yu X and Lan HY: SARS-CoV-2 N protein
induces acute kidney injury in diabetic mice via the
Smad3-Ripk3/MLKL necroptosis pathway. Signal Transduct Target Ther.
8:1472023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hayashi H, Abdollah S, Qiu Y, Cai J, Xu
YY, Grinnell BW, Richardson MA, Topper JN, Gimbrone MA Jr, Wrana JL
and Falb D: The MAD-related protein Smad7 associates with the
TGFbeta receptor and functions as an antagonist of TGFbeta
signaling. Cell. 89:1165–1173. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Feng XH and Derynck R: Smad3 and
Smad4 cooperate with c-Jun/c-Fos to mediate TGF-beta-induced
transcription. Nature. 394:909–913. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Samarakoon R, Dobberfuhl AD, Cooley C,
Overstreet JM, Patel S, Goldschmeding R, Meldrum KK and Higgins PJ:
Induction of renal fibrotic genes by TGF-β1 requires EGFR
activation, p53 and reactive oxygen species. Cell Signal.
25:2198–2209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Samarakoon R, Higgins SP, Higgins CE and
Higgins PJ: TGF-beta1-induced plasminogen activator inhibitor-1
expression in vascular smooth muscle cells requires
pp60(c-src)/EGFR(Y845) and Rho/ROCK signaling. J Mol Cell Cardiol.
44:527–538. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meng XM, Chung ACK and Lan HY: Role of the
TGF-β/BMP-7/Smad pathways in renal diseases. Clin Sci (Lond).
124:243–254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang YE: Non-Smad signaling pathways of
the TGF-β Family. Cold Spring Harb Perspect Biol. 9:a0221292017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim SI and Choi ME: TGF-β-activated
kinase-1: New insights into the mechanism of TGF-β signaling and
kidney disease. Kidney Res Clin Pract. 31:94–105. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Humphreys BD: Mechanisms of Renal
Fibrosis. Annu Rev Physiol. 80:309–326. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin SL, Kisseleva T, Brenner DA and
Duffield JS: Pericytes and perivascular fibroblasts are the primary
source of collagen-producing cells in obstructive fibrosis of the
kidney. Am J Pathol. 173:1617–1627. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakamura J, Sato Y, Kitai Y, Wajima S,
Yamamoto S, Oguchi A, Yamada R, Kaneko K, Kondo M, Uchino E, et al:
Myofibroblasts acquire retinoic acid-producing ability during
fibroblast-to-myofibroblast transition following kidney injury.
Kidney Int. 95:526–539. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen YT, Chang FC, Wu CF, Chou YH, Hsu HL,
Chiang WC, Shen J, Chen YM, Wu KD, Tsai TJ, et al: Platelet-derived
growth factor receptor signaling activates pericyte-myofibroblast
transition in obstructive and post-ischemic kidney fibrosis. Kidney
Int. 80:1170–1181. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kramann R, Schneider RK, DiRocco DP,
Machado F, Fleig S, Bondzie PA, Henderson JM, Ebert BL and
Humphreys BD: Perivascular Gli1+ progenitors are key contributors
to injury-induced organ fibrosis. Cell Stem Cell. 16:51–66. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li J, Qu X and Bertram JF:
Endothelial-myofibroblast transition contributes to the early
development of diabetic renal interstitial fibrosis in
streptozotocin-induced diabetic mice. Am J Pathol. 175:1380–1388.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Meng X, Jin J and Lan HY: Driving role of
macrophages in transition from acute kidney injury to chronic
kidney disease. Chin Med J (Engl). 135:757–766. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zeisberg M and Kalluri R: The role of
epithelial-to-mesenchymal transition in renal fibrosis. J Mol Med
(Berl). 82:175–181. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim KK, Sheppard D and Chapman HA: TGF-β1
signaling and tissue fibrosis. Cold Spring Harb Perspect Biol.
10:a0222932018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Meng XM, Huang XR, Chung AC, Qin W, Shao
X, Igarashi P, Ju W, Bottinger EP and Lan HY: Smad2 protects
against TGF-beta/Smad3-mediated renal fibrosis. J Am Soc Nephrol.
21:1477–1487. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sato M, Muragaki Y, Saika S, Roberts AB
and Ooshima A: Targeted disruption of TGF-beta1/Smad3 signaling
protects against renal tubulointerstitial fibrosis induced by
unilateral ureteral obstruction. J Clin Invest. 112:1486–1494.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zarjou A, Yang S, Abraham E, Agarwal A and
Liu G: Identification of a microRNA signature in renal fibrosis:
Role of miR-21. Am J Physiol Renal Physiol. 301:F793–F801. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhong X, Chung AC, Chen HY, Meng XM and
Lan HY: Smad3-mediated upregulation of miR-21 promotes renal
fibrosis. J Am Soc Nephrol. 22:1668–1681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang B, Komers R, Carew R, Winbanks CE, Xu
B, Herman-Edelstein M, Koh P, Thomas M, Jandeleit-Dahm K,
Gregorevic P, et al: Suppression of microRNA-29 expression by
TGF-β1 promotes collagen expression and renal fibrosis. J Am Soc
Nephrol. 23:252–265. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Meng XM, Wang S, Huang XR, Yang C, Xiao J,
Zhang Y, To KF, Nikolic-Paterson DJ and Lan HY: Inflammatory
macrophages can transdifferentiate into myofibroblasts during renal
fibrosis. Cell Death Dis. 7:e24952016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang YY, Jiang H, Pan J, Huang XR, Wang
YC, Huang HF, To KF, Nikolic-Paterson DJ, Lan HY and Chen JH:
Macrophage-to-Myofibroblast transition contributes to interstitial
fibrosis in chronic renal allograft injury. J Am Soc Nephrol.
28:2053–2067. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wei J, Xu Z and Yan X: The role of the
macrophage-to-myofibroblast transition in renal fibrosis. Front
Immunol. 13:9343772022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tang PM, Zhang YY, Xiao J, Tang PC, Chung
JY, Li J, Xue VW, Huang XR, Chong CC, Ng CF, et al: Neural
transcription factor Pou4f1 promotes renal fibrosis via
macrophage-myofibroblast transition. Proc Natl Acad Sci USA.
117:20741–20752. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang S, Meng XM, Ng YY, Ma FY, Zhou S,
Zhang Y, Yang C, Huang XR, Xiao J, Wang YY, et al: TGF-β/Smad3
signalling regulates the transition of bone marrow-derived
macrophages into myofibroblasts during tissue fibrosis. Oncotarget.
7:8809–8822. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen J, Tang Y, Zhong Y, Wei B, Huang XR,
Tang PM, Xu A and Lan HY: P2Y12 inhibitor clopidogrel inhibits
renal fibrosis by blocking macrophage-to-myofibroblast transition.
Mol Ther. 30:3017–3033. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xavier S, Vasko R, Matsumoto K, Zullo JA,
Chen R, Maizel J, Chander PN and Goligorsky MS: Curtailing
endothelial TGF-β signaling is sufficient to reduce
endothelial-mesenchymal transition and fibrosis in CKD. J Am Soc
Nephrol. 26:817–829. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
LeBleu VS, Taduri G, O'Connell J, Teng Y,
Cooke VG, Woda C, Sugimoto H and Kalluri R: Origin and function of
myofibroblasts in kidney fibrosis. Nat Med. 19:1047–1053. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Loeffler I, Liebisch M, Allert S, Kunisch
E, Kinne RW and Wolf G: FSP1-specific SMAD2 knockout in renal
tubular, endothelial, and interstitial cells reduces fibrosis and
epithelial-to-mesenchymal transition in murine STZ-induced diabetic
nephropathy. Cell Tissue Res. 372:115–133. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lv W, Booz GW, Wang Y, Fan F and Roman RJ:
Inflammation and renal fibrosis: Recent developments on key
signaling molecules as potential therapeutic targets. Eur J
Pharmacol. 820:65–76. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li MO and Flavell RA: TGF-beta: A master
of all T cell trades. Cell. 134:392–404. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li MO, Wan YY, Sanjabi S, Robertson AKL
and Flavell RA: Transforming growth factor-beta regulation of
immune responses. Annu Rev Immunol. 24:99–146. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kulkarni AB, Huh CG, Becker D, Geiser A,
Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM and Karlsson S:
Transforming growth factor beta 1 null mutation in mice causes
excessive inflammatory response and early death. Proc Natl Acad Sci
USA. 90:770–774. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yaswen L, Kulkarni AB, Fredrickson T,
Mittleman B, Schiffman R, Payne S, Longenecker G, Mozes E and
Karlsson S: Autoimmune manifestations in the transforming growth
factor-beta 1 knockout mouse. Blood. 87:1439–1445. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Werner F, Jain MK, Feinberg MW, Sibinga
NE, Pellacani A, Wiesel P, Chin MT, Topper JN, Perrella MA and Lee
ME: Transforming growth factor-beta 1 inhibition of macrophage
activation is mediated via Smad3. J Biol Chem. 275:36653–36658.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Martinez GJ, Zhang Z, Chung Y, Reynolds
JM, Lin X, Jetten AM, Feng XH and Dong C: Smad3 differentially
regulates the induction of regulatory and inflammatory T cell
differentiation. J Biol Chem. 284:35283–35286. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang F, Tsai S, Kato K, Yamanouchi D,
Wang C, Rafii S, Liu B and Kent KC: Transforming growth factor-beta
promotes recruitment of bone marrow cells and bone marrow-derived
mesenchymal stem cells through stimulation of MCP-1 production in
vascular smooth muscle cells. J Biol Chem. 284:17564–17574. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang YY, Tang PM, Tang PC, Xiao J, Huang
XR, Yu C, Ma RCW and Lan HY: LRNA9884, a Novel Smad3-Dependent long
noncoding rna, promotes diabetic kidney injury in db/db Mice via
Enhancing MCP-1-Dependent renal inflammation. Diabetes.
68:1485–1498. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhou Q, Huang XR, Yu J, Yu X and Lan HY:
Long Noncoding RNA Arid2-IR Is a Novel Therapeutic Target for Renal
Inflammation. Mol Ther. 23:1034–1043. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang W, Huang XR, Li AG, Liu F, Li JH,
Truong LD, Wang XJ and Lan HY: Signaling mechanism of TGF-beta1 in
prevention of renal inflammation: Role of Smad7. J Am Soc Nephrol.
16:1371–1383. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lan HY: Smad7 as a therapeutic agent for
chronic kidney diseases. Front Biosci. 13:4984–4992. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chung AC, Huang XR, Zhou L, Heuchel R, Lai
KN and Lan HY: Disruption of the Smad7 gene promotes renal fibrosis
and inflammation in unilateral ureteral obstruction (UUO) in mice.
Nephrol Dial Transplant. 24:1443–1454. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
You YK, Wu WF, Huang XR, Li HD, Ren YP,
Zeng JC, Chen H and Lan HY: Deletion of Smad3 protects against
C-reactive protein-induced renal fibrosis and inflammation in
obstructive nephropathy. Int J Biol Sci. 17:3911–3922. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Liu Z, Huang XR, Chen HY, Fung E, Liu J
and Lan HY: Deletion of angiotensin-converting enzyme-2 promotes
hypertensive nephropathy by targeting Smad7 for ubiquitin
degradation. Hypertension. 70:822–830. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wang Y, Wang Y, Yang M and Ma X:
Implication of cellular senescence in the progression of chronic
kidney disease and the treatment potencies. Biomed Pharmacother.
135:1111912021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhang JQ, Li YY, Zhang XY, Tian ZH, Liu C,
Wang ST and Zhang FR: Cellular senescence of renal tubular
epithelial cells in renal fibrosis. Front Endocrinol (Lausanne).
14:10856052023. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Li C, Shen Y, Huang L, Liu C and Wang J:
Senolytic therapy ameliorates renal fibrosis postacute kidney
injury by alleviating renal senescence. FASEB J.
35:e212292021.PubMed/NCBI
|
|
70
|
Lyu G, Guan Y, Zhang C, Zong L, Sun L,
Huang X, Huang L, Zhang L, Tian XL, Zhou Z and Tao W: TGF-β
signaling alters H4K20me3 status via miR-29 and contributes to
cellular senescence and cardiac aging. Nat Commun. 9:25602018.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ni JY, Wang X, Xie HY, Yang NH, Li JY, Sun
XA, Guo HJ, Zhou L, Zhang W, Liu J and Lu LM: Deubiquitinating
enzyme USP11 promotes renal tubular cell senescence and fibrosis
via inhibiting the ubiquitin degradation of TGF-β receptor II. Acta
Pharmacol Sin. 44:584–595. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ueda S, Tominaga T, Ochi A, Sakurai A,
Nishimura K, Shibata E, Wakino S, Tamaki M and Nagai K: TGF-β1 is
involved in senescence-related pathways in glomerular endothelial
cells via p16 translocation and p21 induction. Sci Rep.
11:216432021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Tang C, Livingston MJ, Liu Z and Dong Z:
Autophagy in kidney homeostasis and disease. Nat Rev Nephrol.
16:489–508. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Yang C, Chen XC, Li ZH, Wu HL, Jing KP,
Huang XR, Ye L, Wei B, Lan HY and Liu HF: SMAD3 promotes autophagy
dysregulation by triggering lysosome depletion in tubular
epithelial cells in diabetic nephropathy. Autophagy. 17:2325–2344.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zehender A, Li YN, Lin NY, Stefanica A,
Nüchel J, Chen CW, Hsu HH, Zhu H, Ding X, Huang J, et al: TGFβ
promotes fibrosis by MYST1-dependent epigenetic regulation of
autophagy. Nat Commun. 12:44042021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Sanz AB, Sanchez-Niño MD, Ramos AM and
Ortiz A: Regulated cell death pathways in kidney disease. Nat Rev
Nephrol. 19:281–299. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Massagué J, Blain SW and Lo RS: TGFbeta
signaling in growth control, cancer, and heritable disorders. Cell.
103:295–309. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Wang W, Chen J, Hu D, Pan P, Liang L, Wu
W, Tang Y, Huang XR, Yu X, Wu J and Lan HY: SARS-CoV-2 N protein
induces acute kidney injury via Smad3-Dependent G1 cell cycle
arrest mechanism. Adv Sci (Weinh). 9:e21032482022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Fu S, Tang Y, Huang XR, Feng M, Xu AP and
Lan HY: Smad7 protects against acute kidney injury by rescuing
tubular epithelial cells from the G1 cell cycle arrest. Clin Sci
(Lond). 131:1955–1969. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhang HY, Cheng M, Zhang L and Wang YP:
Ferroptosis and renal fibrosis: A new target for the future
(Review). Exp Ther Med. 25:132022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wang J, Wang Y, Liu Y, Cai X, Huang X, Fu
W, Wang L, Qiu L, Li J and Sun L: Ferroptosis, a new target for
treatment of renal injury and fibrosis in a 5/6 nephrectomy-induced
CKD rat model. Cell Death Discov. 8:1272022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wang JN, Yang Q, Yang C, Cai YT, Xing T,
Gao L, Wang F, Chen X, Liu XQ, He XY, et al: Smad3 promotes AKI
sensitivity in diabetic mice via interaction with p53 and induction
of NOX4-dependent ROS production. Redox Biol. 32:1014792020.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Li J, Yang J, Xian Q, Su H, Ni Y and Wang
L: Kaempferitrin attenuates unilateral ureteral obstruction-induced
renal inflammation and fibrosis in mice by inhibiting NOX4-mediated
tubular ferroptosis. Phytother Res. Mar 15–2024.(Epub ahead of
print). View Article : Google Scholar
|
|
84
|
Zhu B, Ni Y, Gong Y, Kang X, Guo H, Liu X,
Li J and Wang L: Formononetin ameliorates ferroptosis-associated
fibrosis in renal tubular epithelial cells and in mice with chronic
kidney disease by suppressing the Smad3/ATF3/SLC7A11 signaling.
Life Sci. 315:1213312023. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Streets A and Ong A: Post-translational
modifications of the polycystin proteins. Cell Signal.
72:1096442020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Duan G and Walther D: The roles of
post-translational modifications in the context of protein
interaction networks. PLoS Comput Biol. 11:e10040492015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Xu P, Liu J and Derynck R:
Post-translational regulation of TGF-β receptor and Smad signaling.
FEBS Lett. 586:1871–1884. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Huse M, Muir TW, Xu L, Chen YG, Kuriyan J
and Massagué J: The TGF beta receptor activation process: An
inhibitor- to substrate-binding switch. Mol Cell. 8:671–682. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Shi Y and Massagué J: Mechanisms of
TGF-beta signaling from cell membrane to the nucleus. Cell.
113:685–700. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Xu P, Lin X and Feng XH: Posttranslational
Regulation of Smads. Cold Spring Harb Perspect Biol. 8:a0220872016.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Adhikary L, Chow F, Nikolic-Paterson DJ,
Stambe C, Dowling J, Atkins RC and Tesch GH: Abnormal p38
mitogen-activated protein kinase signalling in human and
experimental diabetic nephropathy. Diabetologia. 47:1210–1222.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
De Borst MH, Prakash J, Melenhorst WB, van
den Heuvel MC, Kok RJ, Navis G and van Goor H: Glomerular and
tubular induction of the transcription factor c-Jun in human renal
disease. J Pathol. 213:219–228. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Ma FY, Sachchithananthan M, Flanc RS and
Nikolic-Paterson DJ: Mitogen activated protein kinases in renal
fibrosis. Front Biosci (Schol Ed). 1:171–187. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Stambe C, Atkins RC, Tesch GH, Masaki T,
Schreiner GF and Nikolic-Paterson DJ: The role of p38alpha
mitogen-activated protein kinase activation in renal fibrosis. J Am
Soc Nephrol. 15:370–379. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ma FY, Flanc RS, Tesch GH, Bennett BL,
Friedman GC and Nikolic-Paterson DJ: Blockade of the c-Jun amino
terminal kinase prevents crescent formation and halts established
anti-GBM glomerulonephritis in the rat. Lab Invest. 89:470–484.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Müller R, Daniel C, Hugo C, Amann K,
Mielenz D, Endlich K, Braun T, van der Veen B, Heeringa P, Schett G
and Zwerina J: The mitogen-activated protein kinase p38α regulates
tubular damage in murine anti-glomerular basement membrane
nephritis. PLoS One. 8:e563162013. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Kamato D, Burch ML, Piva TJ, Rezaei HB,
Rostam MA, Xu S, Zheng W, Little PJ and Osman N: Transforming
growth factor-β signalling: Role and consequences of Smad linker
region phosphorylation. Cell Signal. 25:2017–2024. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Yang F, Chung ACK, Huang XR and Lan HY:
Angiotensin II induces connective tissue growth factor and collagen
I expression via transforming growth factor-beta-dependent and
-independent Smad pathways: The role of Smad3. Hypertension.
54:877–884. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Chung AC, Zhang H, Kong YZ, Tan JJ, Huang
XR, Kopp JB and Lan HY: Advanced glycation end-products induce
tubular CTGF via TGF-beta-independent Smad3 signaling. J Am Soc
Nephrol. 21:249–260. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
You YK, Huang XR, Chen HY, Lyu XF, Liu HF
and Lan HY: C-Reactive protein promotes diabetic kidney disease in
db/db Mice via the CD32b-Smad3-mTOR signaling pathway. Sci Rep.
6:267402016. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Chen X, Wang H, Liao HJ, Hu W, Gewin L,
Mernaugh G, Zhang S, Zhang ZY, Vega-Montoto L, Vanacore RM, et al:
Integrin-mediated type II TGF-β receptor tyrosine dephosphorylation
controls SMAD-dependent profibrotic signaling. J Clin Invest.
124:3295–3310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Lin X, Duan X, Liang YY, Su Y, Wrighton
KH, Long J, Hu M, Davis CM, Wang J, Brunicardi F, et al: PPM1A
functions as a Smad phosphatase to terminate TGFbeta signaling.
Cell. 125:915–928. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Samarakoon R, Rehfuss A, Khakoo NS, Falke
LL, Dobberfuhl AD, Helo S, Overstreet JM, Goldschmeding R and
Higgins PJ: Loss of expression of protein phosphatase
magnesium-dependent 1A during kidney injury promotes fibrotic
maladaptive repair. FASEB J. 30:3308–3320. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Inoue K, Matsui I, Hamano T, Fujii N,
Shimomura A, Nakano C, Kusunoki Y, Takabatake Y, Hirata M,
Nishiyama A, et al: Maxacalcitol ameliorates tubulointerstitial
fibrosis in obstructed kidneys by recruiting PPM1A/VDR complex to
pSmad3. Lab Invest. 92:1686–1697. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Tang J, Goldschmeding R, Samarakoon R and
Higgins PJ: Protein phosphatase Mg2+/Mn2+ dependent-1A and PTEN
deregulation in renal fibrosis: Novel mechanisms and co-dependency
of expression. FASEB J. 34:2641–2656. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Meyer-Schwesinger C: The
ubiquitin-proteasome system in kidney physiology and disease. Nat
Rev Nephrol. 15:393–411. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Tan R, He W, Lin X, Kiss LP and Liu Y:
Smad ubiquitination regulatory factor-2 in the fibrotic kidney:
Regulation, target specificity, and functional implication. Am J
Physiol Renal Physiol. 294:F1076–F1083. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Iyengar PV: Regulation of ubiquitin
enzymes in the TGF-β Pathway. Int J Mol Sci. 18:8772017. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Bonni S, Wang HR, Causing CG, Kavsak P,
Stroschein SL, Luo K and Wrana JL: TGF-beta induces assembly of a
Smad2-Smurf2 ubiquitin ligase complex that targets SnoN for
degradation. Nat Cell Biol. 3:587–595. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Wang L, Zha H, Huang J and Shi L: Flavin
containing monooxygenase 2 regulates renal tubular cell fibrosis
and paracrine secretion via SMURF2 in AKI-CKD transformation. Int J
Mol Med. 52:1102023. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Liu FY, Li XZ, Peng YM, Liu H and Liu YH:
Arkadia-Smad7-mediated positive regulation of TGF-beta signaling in
a rat model of tubulointerstitial fibrosis. Am J Nephrol.
27:176–183. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Liu FY and Li XZ: The roles of Arkadia in
renal tubular epithelial to mesenchymal transition. Med Hypotheses.
67:1205–1207. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Wu W, Huang XR, You Y, Xue L, Wang XJ,
Meng X, Lin X, Shen J, Yu X, Lan HY and Chen H: Latent TGF-β1
protects against diabetic kidney disease via Arkadia/Smad7
signaling. Int J Biol Sci. 17:3583–3594. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Gao S, Alarcón C, Sapkota G, Rahman S,
Chen PY, Goerner N, Macias MJ, Erdjument-Bromage H, Tempst P and
Massagué J: Ubiquitin ligase Nedd4L targets activated Smad2/3 to
limit TGF-beta signaling. Mol Cell. 36:457–468. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Manning JA, Shah SS, Nikolic A, Henshall
TL, Khew-Goodall Y and Kumar S: The ubiquitin ligase NEDD4-2/NEDD4L
regulates both sodium homeostasis and fibrotic signaling to prevent
end-stage renal disease. Cell Death Dis. 12:3982021. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Henshall TL, Manning JA, Alfassy OS, Goel
P, Boase NA, Kawabe H and Kumar S: Deletion of Nedd4-2 results in
progressive kidney disease in mice. Cell Death Differ.
24:2150–2160. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Nijman SM, Luna-Vargas MP, Velds A,
Brummelkamp TR, Dirac AM, Sixma TK and Bernards R: A genomic and
functional inventory of deubiquitinating enzymes. Cell.
123:773–786. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Zhang J, Zhang X, Xie F, Zhang Z, van Dam
H, Zhang L and Zhou F: The regulation of TGF-β/SMAD signaling by
protein deubiquitination. Protein Cell. 5:503–517. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Komander D, Clague MJ and Urbé S: Breaking
the chains: structure and function of the deubiquitinases. Nat Rev
Mol Cell Biol. 10:550–563. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Soji K, Doi S, Nakashima A, Sasaki K, Doi
T and Masaki T: Deubiquitinase inhibitor PR-619 reduces Smad4
expression and suppresses renal fibrosis in mice with unilateral
ureteral obstruction. PLoS One. 13:e02024092018. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Dupont S, Mamidi A, Cordenonsi M,
Montagner M, Zacchigna L, Adorno M, Martello G, Stinchfield MJ,
Soligo S, Morsut L, et al: FAM/USP9×, a deubiquitinating enzyme
essential for TGFbeta signaling, controls Smad4 monoubiquitination.
Cell. 136:123–135. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Xie S, Xing Y, Shi W, Zhang M, Chen M,
Fang W, Liu S, Zhang T, Zeng X, Chen S, et al: Cardiac fibroblast
heat shock protein 47 aggravates cardiac fibrosis post myocardial
ischemia-reperfusion injury by encouraging ubiquitin specific
peptidase 10 dependent Smad4 deubiquitination. Acta Pharm Sin B.
12:4138–4153. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Liao X, Li Y, Liu J, Zhang Y, Tan J, Kass
DJ, Rojas M, Mallampalli RK, Zhao J and Zhao Y: Deubiquitinase
USP13 promotes extracellular matrix expression by stabilizing Smad4
in lung fibroblast cells. Transl Res. 223:15–24. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Song C, Liu W and Li J: USP17 is
upregulated in osteosarcoma and promotes cell proliferation,
metastasis, and epithelial-mesenchymal transition through
stabilizing SMAD4. Tumour Biol. 39:10104283177171382017. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Zhao Y, Chen X, Lin Y, Li Z, Su X, Fan S,
Chen Y, Wang X and Liang G: USP25 inhibits renal fibrosis by
regulating TGFβ-SMAD signaling pathway in Ang II-induced
hypertensive mice. Biochim Biophys Acta Mol Basis Dis.
1869:1667132023. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Sun XH, Xiao HM, Zhang M, Lin ZY, Yang Y,
Chen R, Liu PQ, Huang KP and Huang HQ: USP9X deubiquitinates
connexin43 to prevent high glucose-induced
epithelial-to-mesenchymal transition in NRK-52E cells. Biochem
Pharmacol. 188:1145622021. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Huang K and Zhao X: USP9X prevents
AGEs-induced upregulation of FN and TGF-β1 through activating
Nrf2-ARE pathway in rat glomerular mesangial cells. Exp Cell Res.
393:1121002020. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Gao F, Qian M, Liu G, Ao W, Dai D and Yin
C: USP10 alleviates sepsis-induced acute kidney injury by
regulating Sirt6-mediated Nrf2/ARE signaling pathway. J Inflamm
(Lond). 18:252021. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Huang Z, Shen S, Wang M, Li W, Wu G, Huang
W, Luo W and Liang G: Mouse endothelial OTUD1 promotes angiotensin
II-induced vascular remodeling by deubiquitinating SMAD3. EMBO Rep.
24:e561352023. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Huang YT, Cheng AC, Tang HC, Huang GC, Cai
L, Lin TH, Wu KJ, Tseng PH, Wang GG and Chen WY: USP7 facilitates
SMAD3 autoregulation to repress cancer progression in p53-deficient
lung cancer. Cell Death Dis. 12:8802021. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Wicks SJ, Haros K, Maillard M, Song L,
Cohen RE, Dijke PT and Chantry A: The deubiquitinating enzyme UCH37
interacts with Smads and regulates TGF-beta signalling. Oncogene.
24:8080–8084. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Tian Y, Liao F and Wu G, Chang D, Yang Y,
Dong X, Zhang Z, Zhang Y and Wu G: Ubiquitination and regulation of
Smad7 in the TGF-β1/Smad signaling of aristolochic acid
nephropathy. Toxicol Mech Methods. 25:645–652. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Zhao Y, Thornton AM, Kinney MC, Ma CA,
Spinner JJ, Fuss IJ, Shevach EM and Jain A: The Deubiquitinase CYLD
targets Smad7 protein to regulate transforming growth factor β
(TGF-β) signaling and the development of regulatory T cells. J Biol
Chem. 286:40520–40530. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Wang B, Xu X, Yang Z, Zhang L and Liu Y,
Ma A, Xu G, Tang M, Jing T, Wu L and Liu Y: POH1 contributes to
hyperactivation of TGF-β signaling and facilitates hepatocellular
carcinoma metastasis through deubiquitinating TGF-β receptors and
caveolin-1. EBioMedicine. 41:320–332. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Shi Y, Tao M, Chen H, Ma X, Wang Y, Hu Y,
Zhou X, Li J, Cui B, Qiu A, et al: Ubiquitin-specific protease 11
promotes partial epithelial-to-mesenchymal transition by
deubiquitinating the epidermal growth factor receptor during kidney
fibrosis. Kidney Int. 103:544–564. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Jacko AM, Nan L, Li S, Tan J, Zhao J, Kass
DJ and Zhao Y: De-ubiquitinating enzyme, USP11, promotes
transforming growth factor β-1 signaling through stabilization of
transforming growth factor β receptor II. Cell Death Dis.
7:e24742016. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Du C, Chen X, Su Q, Lu W, Wang Q, Yuan H,
Zhang Z, Wang X, Wu H and Qi Y: The function of SUMOylation and Its
critical roles in cardiovascular diseases and potential clinical
implications. Int J Mol Sci. 22:106182021. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Wang X, Liu T, Huang Y, Dai Y and Lin H:
Regulation of transforming growth factor-beta signalling by
SUMOylation and its role in fibrosis. Open Biol. 11:2100432021.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Kang JS, Saunier EF, Akhurst RJ and
Derynck R: The type I TGF-beta receptor is covalently modified and
regulated by sumoylation. Nat Cell Biol. 10:654–664. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Enserink JM: Sumo and the cellular stress
response. Cell Div. 10:42015. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Reverter D and Lima CD: A basis for SUMO
protease specificity provided by analysis of human Senp2 and a
Senp2-SUMO complex. Structure. 12:1519–1531. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Gong L and Yeh ETH: Characterization of a
family of nucleolar SUMO-specific proteases with preference for
SUMO-2 or SUMO-3. J Biol Chem. 281:15869–15877. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Tan M, Zhang D, Zhang E, Xu D, Liu Z, Qiu
J, Fan Y and Shen B: SENP2 suppresses epithelial-mesenchymal
transition of bladder cancer cells through deSUMOylation of
TGF-βRI. Mol Carcinog. 56:2332–2341. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Long J, Wang G, He D and Liu F: Repression
of Smad4 transcriptional activity by SUMO modification. Biochem J.
379((Pt 1)): 23–29. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Zhou X, Gao C, Huang W, Yang M, Chen G,
Jiang L, Gou F, Feng H, Ai N and Xu Y: High glucose induces
sumoylation of Smad4 via SUMO2/3 in mesangial cells. Biomed Res
Int. 2014:7826252014. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Liu P, Zhang J, Wang Y, Wang C, Qiu X and
Chen DQ: Natural products against renal fibrosis via modulation of
SUMOylation. Front Pharmacol. 13:8008102022. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Liu W, Yuan Q, Cao S, Wang G, Liu X, Xia
Y, Bian Y, Xu F and Chen Y: Review: Acetylation mechanisms and
targeted therapies in cardiac fibrosis. Pharmacol Res.
193:1068152023. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Bugyei-Twum A, Advani A, Advani SL, Zhang
Y, Thai K, Kelly DJ and Connelly KA: High glucose induces Smad
activation via the transcriptional coregulator p300 and contributes
to cardiac fibrosis and hypertrophy. Cardiovasc Diabetol.
13:892014. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Inoue Y, Itoh Y, Abe K, Okamoto T, Daitoku
H, Fukamizu A, Onozaki K and Hayashi H: Smad3 is acetylated by
p300/CBP to regulate its transactivation activity. Oncogene.
26:500–508. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Rai R, Verma SK, Kim D, Ramirez V, Lux E,
Li C, Sahoo S, Wilsbacher LD, Vaughan DE, Quaggin SE and Ghosh AK:
A novel acetyltransferase p300 inhibitor ameliorates
hypertension-associated cardio-renal fibrosis. Epigenetics.
12:1004–1013. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Morigi M, Perico L and Benigni A: Sirtuins
in renal health and disease. J Am Soc Nephrol. 29:1799–1809. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Huang XZ, Wen D, Zhang M, Xie Q, Ma L,
Guan Y, Ren Y, Chen J and Hao CM: Sirt1 activation ameliorates
renal fibrosis by inhibiting the TGF-β/Smad3 pathway. J Cell
Biochem. 115:996–1005. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Chen Q, Zeng Y, Yang X, Wu Y, Zhang S,
Huang S, Zhong Y and Chen M: Resveratrol ameliorates myocardial
fibrosis by regulating Sirt1/Smad3 deacetylation pathway in rat
model with dilated cardiomyopathy. BMC Cardiovasc Disord.
22:172022. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Simic P, Williams EO, Bell EL, Gong JJ,
Bonkowski M and Guarente L: SIRT1 suppresses the
epithelial-to-mesenchymal transition in cancer metastasis and organ
fibrosis. Cell Rep. 3:1175–1186. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Yang S, Yang G, Wang X, Xiang J, Kang L
and Liang Z: SIRT2 alleviated renal fibrosis by deacetylating SMAD2
and SMAD3 in renal tubular epithelial cells. Cell Death Dis.
14:6462023. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Ma J and Hart GW: O-GlcNAc profiling: From
proteins to proteomes. Clin Proteomics. 11:82014. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Harosh-Davidovich SB and Khalaila I:
O-GlcNAcylation affects β-catenin and E-cadherin expression, cell
motility and tumorigenicity of colorectal cancer. Exp Cell Res.
364:42–49. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
He XF, Hu X, Wen GJ, Wang Z and Lin WJ:
O-GlcNAcylation in cancer development and immunotherapy. Cancer
Lett. 566:2162582023. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Ma J and Hart GW: Protein O-GlcNAcylation
in diabetes and diabetic complications. Expert Rev Proteomics.
10:365–380. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Park J, Lai MKP, Arumugam TV and Jo DG:
O-GlcNAcylation as a therapeutic target for Alzheimer's disease.
Neuromolecular Med. 22:171–193. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Feng D, Sheng-Dong L, Tong W and Zhen-Xian
D: O-GlcNAcylation of RAF1 increases its stabilization and induces
the renal fibrosis. Biochim Biophys Acta Mol Basis Dis.
1866:1655562020. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Kim YJ, Kang MJ, Kim E, Kweon TH, Park YS,
Ji S, Yang WH, Yi EC and Cho JW: O-GlcNAc stabilizes SMAD4 by
inhibiting GSK-3β-mediated proteasomal degradation. Sci Rep.
10:199082020. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Yuan M, Song ZH, Ying MD, Zhu H, He QJ,
Yang B and Cao J: N-myristoylation: From cell biology to
translational medicine. Acta Pharmacol Sin. 41:1005–1015. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Stockwell BR and Schreiber SL:
TGF-beta-signaling with small molecule FKBP12 antagonists that bind
myristoylated FKBP12-TGF-beta type I receptor fusion proteins. Chem
Biol. 5:385–395. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Zhu F, Xie N, Jiang Z, Li G, Ma L and Tong
T: The cellular senescence-inhibited gene is essential for PPM1A
myristoylation to modulate transforming growth factor β signaling.
Mol Cell Biol. 38:e00414–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Al-Salihi MA, Herhaus L, Macartney T and
Sapkota GP: USP11 augments TGFβ signalling by deubiquitylating
ALK5. Open Biol. 2:1200632012. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Siwy J, Mischak H and Zürbig P: Proteomics
and personalized medicine: A focus on kidney disease. Expert Rev
Proteomics. 16:773–782. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Giudice G and Petsalaki E: Proteomics and
phosphoproteomics in precision medicine: Applications and
challenges. Brief Bioinform. 20:767–777. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Xu H, Wu T and Huang L: Therapeutic and
delivery strategies of phytoconstituents for renal fibrosis. Adv
Drug Deliv Rev. 177:1139112021. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Trac N, Ashraf A, Giblin J, Prakash S,
Mitragotri S and Chung EJ: Spotlight on genetic kidney diseases: A
call for drug delivery and nanomedicine solutions. ACS Nano.
17:6165–6177. 2023. View Article : Google Scholar : PubMed/NCBI
|