Introduction
Upper gastrointestinal (UGI) tumors, characterized
by high morbidity and mortality rates, present significant global
health challenges, especially esophageal cancer (EC; 510,716 new
cases in 2022) and gastric cancer (GC; ~1 million new cases in
2022) (1). The prognosis for
patients with UGI cancer remains poor in multiple countries,
primarily because of insufficient screening initiatives (2,3).
Elucidating the mechanisms that initiate and advance UGI cancers is
essential to develop successful prevention and therapeutic
approaches. Growing evidence shows that metabolites, small
molecules intermediately produced during multiple cellular
metabolic reactions, are implicated in the pathways leading to UGI
tumors. Progress in molecular biology and the introduction of
diverse omics techniques have markedly advanced epidemiological
studies at the molecular level in this field (4).
Metabolic imbalance is increasingly recognized as a
pivotal factor in the development of UGI tumors (5). Beyond changes in glucose metabolism,
exemplified by the well-documented Warburg effect, disrupted
metabolism has been reported in nucleotides, lipids and amino acids
in both laboratory and clinical studies (6–8).
Metabolites are a group of end products arising from complex
interactions between inherent metabolism, genetic predispositions
and environmental influences. High-throughput metabolomics enables
comprehensive identification and quantification of a vast array of
low molecular weight metabolites (<1,000 Da) within a single
sample. This method is instrumental in identifying novel biomarkers
and providing insights into the mechanisms of cancer causation
(9,10). In addition, high-throughput
metabolomics facilitates the discovery of new preventive measures
and therapeutic targets (11).
Previous research has analyzed a wide range of metabolites in UGI
tumors using human samples such as urine, plasma and tissue
(12,13). Although substantial efforts have
been made in the field of UGI cancer metabolomics (14,15),
these studies are predominantly descriptive. With the increase in
research over recent years, there is a critical need for a
comprehensive analysis to enhance our understanding of metabolomic
profiles in UGI cancer, aiming to identify specific metabolites and
consistently involved pathways.
Numerous cross-sectional, cohort, or retrospective
studies have examined the association between metabolites and UGI
tumors. However, due to their observational nature, these studies
were restricted to identifying the correlations rather than the
causations (16,17). While randomized controlled trials
(RCTs), could potentially establish causality, interventions
designed to manipulate metabolites are generally neither feasible
nor ethical, thus limiting their ability to determine causal
relationships. Given the constraints of both observational and
interventional studies, Mendelian randomization (MR) in human
genetics offers a powerful tool for rigorously investigating
potential causal associations between elevated metabolite levels
and UGI tumors (16,17). Traditional observational
epidemiological approaches are susceptible to biases, rendering the
association results from these studies prone to confounding factors
(such as sex and age) and reverse causality (such as lifestyle
changes due to UGI cancer), which lead to unreliable causal
inferences (18). MR has become a
primary genetic epidemiological research method, using genetic
variations such as single nucleotide polymorphisms (SNPs) as
instrumental variables to examine exposure factors and conclude
causal relationships between exposures and outcomes. Due to the
principle of randomly distributing alleles to offspring, the causal
association estimates obtained from MR studies are not influenced
by confounding factors. Additionally, since genes are determined
before birth and cannot be altered by diseases, MR research
effectively controls for the effects of reverse causality (19).
In the present study, MR was employed to explore the
roles of metabolites in both histophysiological and
pathophysiological processes leading to UGI tumors, using insights
from a recent statistical analysis based on metabolite-focused
Genome-Wide Association Study (GWAS) data (20). The present study aimed to
investigate the causal association between 1,400 metabolites and
UGI cancer, particularly focusing on their roles in tumor
initiation, progression and treatment resistance. Moreover,
functional experiments were conducted to further validate the
findings from the MR analysis. The present study sought to provide
insights that could enhance future metabolomic methodologies and
advance etiological research, thereby supporting the development of
precise prevention and innovative therapeutic strategies. It is
anticipated that the findings will contribute to the creation of
personalized treatment plans that target the specific metabolic
vulnerabilities of UGI tumors.
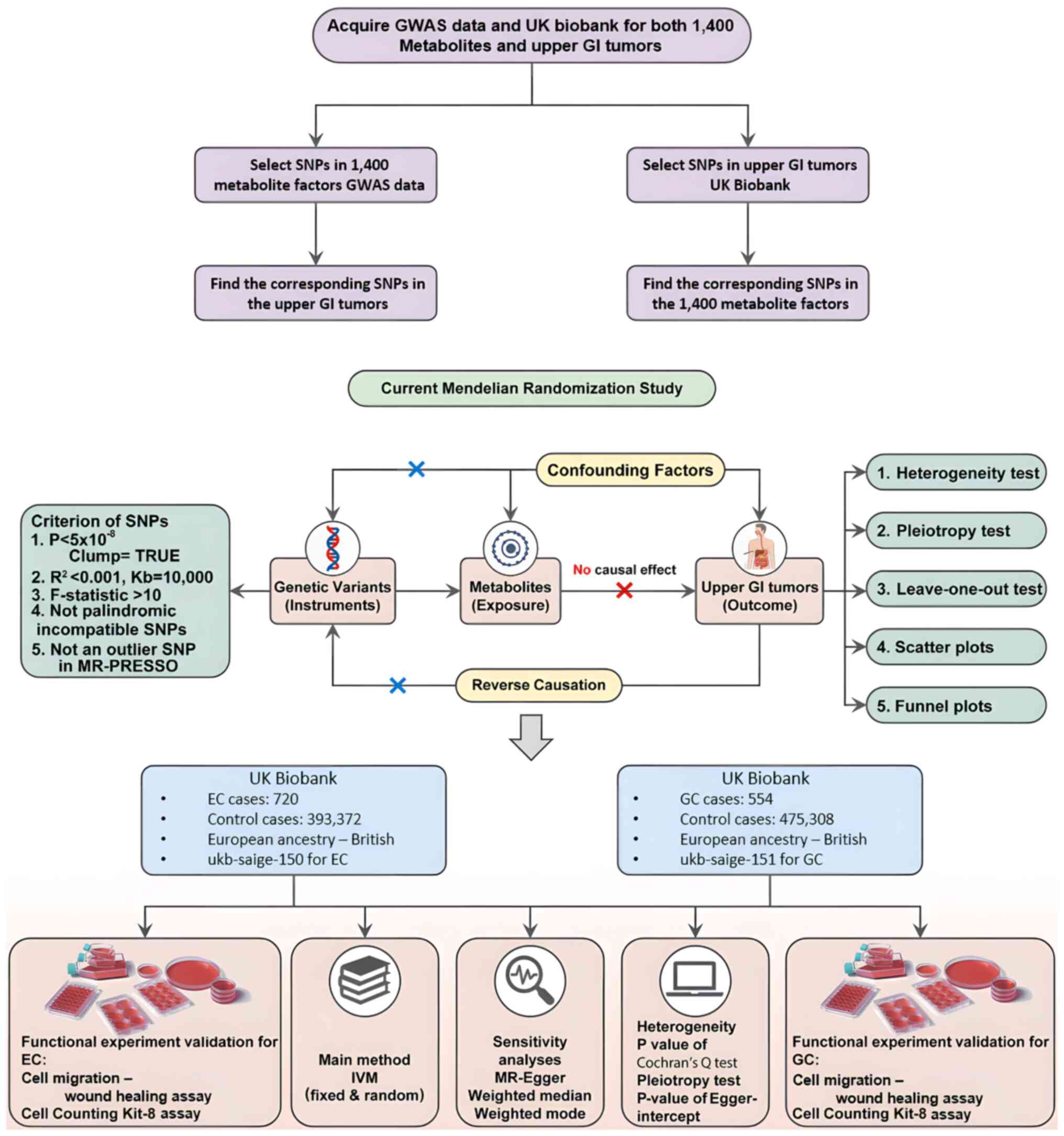
Materials and methods
Study design
The cause-and-effect association between 1,400
metabolites and UGI tumors was assessed using two-sample MR
analyses. MR utilized genetic variations as proxies for metabolic
risk factors. To ensure reliable causal inference, the instrumental
variables (IVs) used in MR were required to satisfy three critical
assumptions: i) Genetic variation must be directly associated with
the exposure (metabolites); ii) genetic variant should not be
linked to any confounders that might affect the exposure and the
outcome; and iii) the genetic variation should affect the outcome
solely through the exposure, without any alternative pathways
involved. The present study excluded points with
P<1×105 from the outcome when conducting MR analysis
to satisfy the second assumption. In addition, it used
methodologies such as MR Egger (21) and MR-pleiotropy residual sum and
outlier (PRESSO) to test and found no pleiotropy in the results for
the third assumption. Furthermore, before conducting MR analysis,
the present study had already searched for the corresponding SNPs
on the GWAS catalog and removed the sites with pleiotropy. Although
a number of measures had been taken to avoid environmental and
genetic factors, it was hypothesized that the external environment
can still have an influence. Finally, an experiment was performed
to validate the present results (Fig.
1).
Data sources for exposure and
outcome
The statistical summary of GWAS data for each
metabolite was taken from the European GWAS website (http://www.ebi.ac.uk/gwas/; accession no.
GCST90199621-90201020) (20).
Cancer-specific keywords were applied to search relevant data for
each cancer type (https://gwas.mrcieu.ac.uk/). Specifically,
ukb-saige-150 was selected for EC identification, while
ukb-saige-151 was used for GC screening. The UK Biobank, used for
this data retrieval, is a substantial biomedical database and
research resource aimed at enabling the exploration of genetic,
environmental and lifestyle factors influencing various diseases
and health outcomes (22). This
database includes health and genetic information from >500,000
participants in the UK, making it one of the most comprehensive
biomedical resources of its type.
GWAS studies aim to identify common genetic variants
linked to complex disorders, ultimately guiding the development of
translational prevention and treatment strategies. Given their
extensive coverage of common SNPs and relative cost-effectiveness,
GWAS has emerged as a valuable tool for clinical and commercial
genetic testing. Essentially, GWAS acts as a potent tool that has
greatly enhanced our knowledge of the genetic foundations of
complex traits and diseases, establishing a robust foundation for
future research and medical innovations. GWAS studies typically
involve collecting and analyzing DNA samples from individuals to
identify genetic variations associated with particular traits or
diseases. The biological material collected for GWAS usually
comprises DNA extracted from blood, saliva, or other tissues from
participants. Researchers extract DNA from these samples and employ
genotyping techniques to detect genetic variations, such as SNPs
that might be linked to the trait or disease of interest. In the
current study, data were obtained from the UK Biobank (www.ukbiobank.ac.uk) using specific ICD-10
(International Classification of Diseases 10th revision; accession
nos. ukb-saige-150), and (GC: ukb-saige-151) IDs for each type of
cancer. This involved analyzing the association between 1,400 types
of metabolites and UGI tumors. Specifically, data included 394,092
European individuals (720 case patients and 393,372 control
participants) for EC and 393,926 European individuals (554 case
patients and 475,308 control participants) for GC. The association
between metabolites and each cancer type was subsequently analyzed
according to these IDs.
Instrument selection
Considering the extensive number of SNPs
demonstrating genome-wide significance (P<5×10−8) for
metabolite traits, stricter correlation thresholds
(P<5×10−9) were implemented for selecting genetic
IVs. These IVs were categorized based on the reference panel of
Linkage Disequilibrium from the 1,000 Genomes Project (23,24),
with a threshold of R2<0.001 at a distance of 1,000
kb. Due to the relatively small size of the GWAS dataset for
metabolites, a cutoff (P=5×10−8) and a less stringent
clustering threshold (R2<0.001 at a distance of 1,000
kb) were employed (19). To ensure
the reliability of the tools used, IVs with F>10 were selected
and identified as strong elements for subsequent analyses. Next,
these IVs were obtained from the summary statistics pertaining to
UGI cancer outcomes, excluding any that displayed potential
pleiotropic effects (P<10−5) on UGI cancer, in line
with methodologies from previous research (25). To maintain consistency in this
analysis, discrepancies in SNPs between the exposure and outcome
datasets were synchronized to ensure uniform effect estimates for
the same effect allele (26).
Cell lines
The esophageal cancer cell line (KYSE150) and
gastric cancer cell line (HGC27) selected for the experiment were
purchased from the cell bank of the Chinese Academy of
Sciences.
Main reagents and instruments
Cell culture medium and reagents included:
Serum-free DMEM (cat. no. PM150210; Procell Life Science &
Technology Co., Ltd.), RPMI 1640 basic medium (cat. no. PM150110;
Procell Life Science & Technology Co., Ltd.), fetal bovine
serum (FBS; cat. no. 164210-50; Procell Life Science &
Technology Co., Ltd.), pancreatic enzyme (cat. no. PB180226;
Procell Life Science & Technology Co., Ltd.), fructosyllysine
(cat. no. HY-129380; MedChemExpress), 2′-deoxyuridine (cat. no.
HY-D0186; MedChemExpress), cytidine (cat. no. HY-D0158;
MedChemExpress), carnitine (cat. no. HY-B0399A; MedChemExpress),
Cell Counting Kit-8 (CCK-8; Shanghai Biyuntian Biotechnology Co.,
Ltd.), ELISA reader (BioTek; Agilent Technologies, Inc.) and an
inverted microscope (Nikon Corporation).
Cell culture
KYSE150 cells and HGC27 cells were incubated in DMEM
and RPMI 1640 medium, respectively. The media were supplemented
with 10% FBS and 100 U/ml penicillin and streptomycin (Gibco;
Thermo Fisher Scientific, Inc.). The cells were maintained in an
incubator set at 37°C with a 5% CO2 atmosphere. Upon
reaching 80–90% confluence, the cells were enzymatically
dissociated for subculturing.
Detection of cell proliferation
ability
Cells were digested with 0.25% trypsin and
resuspended for counting when entering the logarithmic growth
phase. The cells were then seeded in a 96-well plate at a density
of 3,000 cells/well. After attachment and morphological expansion,
four common metabolites identified from MR results, namely
fructosyllysine, 2′-deoxyuridine, cytidine and carnitine, were
added for treatment at 37°C for 1.5 h in darkness. The
concentrations of the metabolites were 0, 50 and 100 µM, with
triplicate wells for each concentration. Subsequently, each well
received 10 µl of CCK-8 reagent and was incubated at 37°C for 1.5 h
in darkness. The optical density was measured at 450 nm using an
ELISA reader at time intervals of 0, 24, and 48 h following the
introduction of the metabolites.
Cell scratch assay
Cells were digested with 0.25% trypsin and counted
during the logarithmic growth phase. The cells were then plated in
a 6-well plate and each well contained 1.0×105 cells.
Once the cells adhered and expanded morphologically, reaching ~80%
confluence, a scratch was made on the cell monolayer using a 10-µl
pipette tip. After making the scratch, the wells were rinsed twice
with PBS to remove any detached cells and then a serum-free medium
and varying concentrations of metabolites was added for continued
incubation. Images of the scratch were captured at 0, 24 and 48 h
after adding the metabolites using a microscope (Nikon Ts2FL
inverted microscope; Nikon Corporation). The area of the scratch
was quantified using ImageJ software (version 2023; National
Institutes of Health). To assess the rate of cell migration and
healing, the formula used for calculating wound healing rate was
[(Original scratch area-Final scratch area)/Original scratch area]
×100%.
Statistical analysis
After extracting the data concerning SNPs associated
with metabolites, including details such as effect alleles and
their corresponding β values. The formula established previously
(27) was used to calculate the
genetic variance for each metabolite. All data were presented and
the number of replicates performed following the reported formula
(20). In the present study, a
range of genetic variants were employed as IVs, rather than relying
solely on an allele score. This approach was selected to thoroughly
examine key assumptions, uncover potential pleiotropy and enhance
the sensitivity efficacy of the multivariable MR analyses (28). A total of four distinct MR
methodologies, including the inverse variance weighted (IVW;
random-effects model), weighted median, MR-Egger and MR-PRESSO,
were used to evaluate the consistency of the current findings under
different assumptions about heterogeneity and pleiotropy. The IVW
method, employing a random-effects model, served as the primary
analysis framework for all four sets of IVs. Heterogeneity was
quantified using Cochran's Q statistic.
The present study also included analyses with more
stringent conditions. Assuming that all genetic variants are valid,
the IVW method might be subject to bias if a considerable number of
SNPs are influenced by horizontal pleiotropy (29). By contrast, the weighted median
approach, effective when <50% of variants exhibit horizontal
pleiotropy, operated under the assumption that the majority of
genetic variants were valid (30).
For situations where >50% of variants were affected by
horizontal pleiotropy, the strength of the present genetic tools
was evaluated through F statistics, considering a mean F<10 to
be indicative of weak IVs (31).
Furthermore, the MR-Egger method was applied to
assess potential directional pleiotropy, where a significant
intercept would indicate a violation of IV assumptions, suggesting
directional pleiotropy (32). In
addition, the MR-PRESSO method was implemented to minimize
heterogeneity in causal effect estimates by excluding
disproportionately influential SNPs (NbDistribution=1,500)
(33). In addition,
Steiger-filtering was used to identify and exclude genetic variants
more strongly linked to the outcome than to the exposure,
indicative of potential reverse causality (34).
All statistical analyses in the present study were
performed using R (R Foundation version 4.3.1; 2023 version
R-project.org/) and specific R packages (‘TwoSampleMR’ and ‘MR’)
(35) designed for MR analysis
(36). The TwoSampleMR package
facilitated the provision of causal estimates across the four MR
models: IVW, weighted median, MR-Egger and MR-PRESSO. Analysis of
functional experiment data was conducted using the software ImageJ
(version 2023; National Institutes of Health) and GraphPad Prism 8
(GraphPad; Dotmatics). Unpaired Student's t-tests were used to
calculate differences between the two groups. For group
comparisons, one-way ANOVA and Bonferroni post hoc analysis were
used. Data are expressed as the mean ± SD. P<0.05 was considered
to indicate a statistically significant difference.
Results
Causal estimation between metabolites
and UGI cancer
Causal estimation between metabolites and EC
The present study first assessed the causal impact
of various metabolites on EC using the IVW method for a two-sample
MR analysis. The assessment revealed that 57 metabolites were
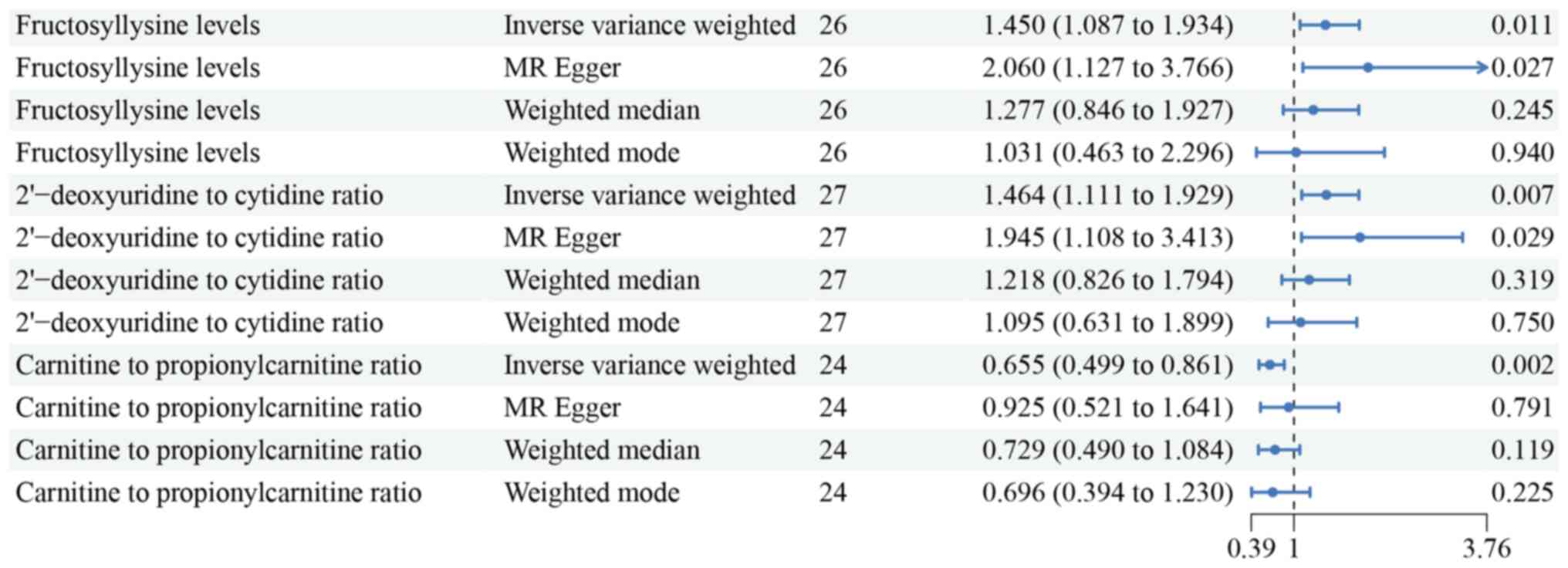
significantly associated with EC (Table I). Key findings highlighted notable
associations with hub metabolites. Specifically, fructosyllysine
levels showed a strong association with increased EC risk [odds
ratio (OR)=1.450, 95% confidence interval (CI)=1.087–1.934,
P=0.011]. Additionally, the ratio of 2′-deoxyuridine to cytidine
(OR=1.464, 95% CI=1.111–1.929, P=0.007) and carnitine to
protonylcarnitine (C3) (OR=0.655, 95% CI=0.499–0.861, P=0.002) were
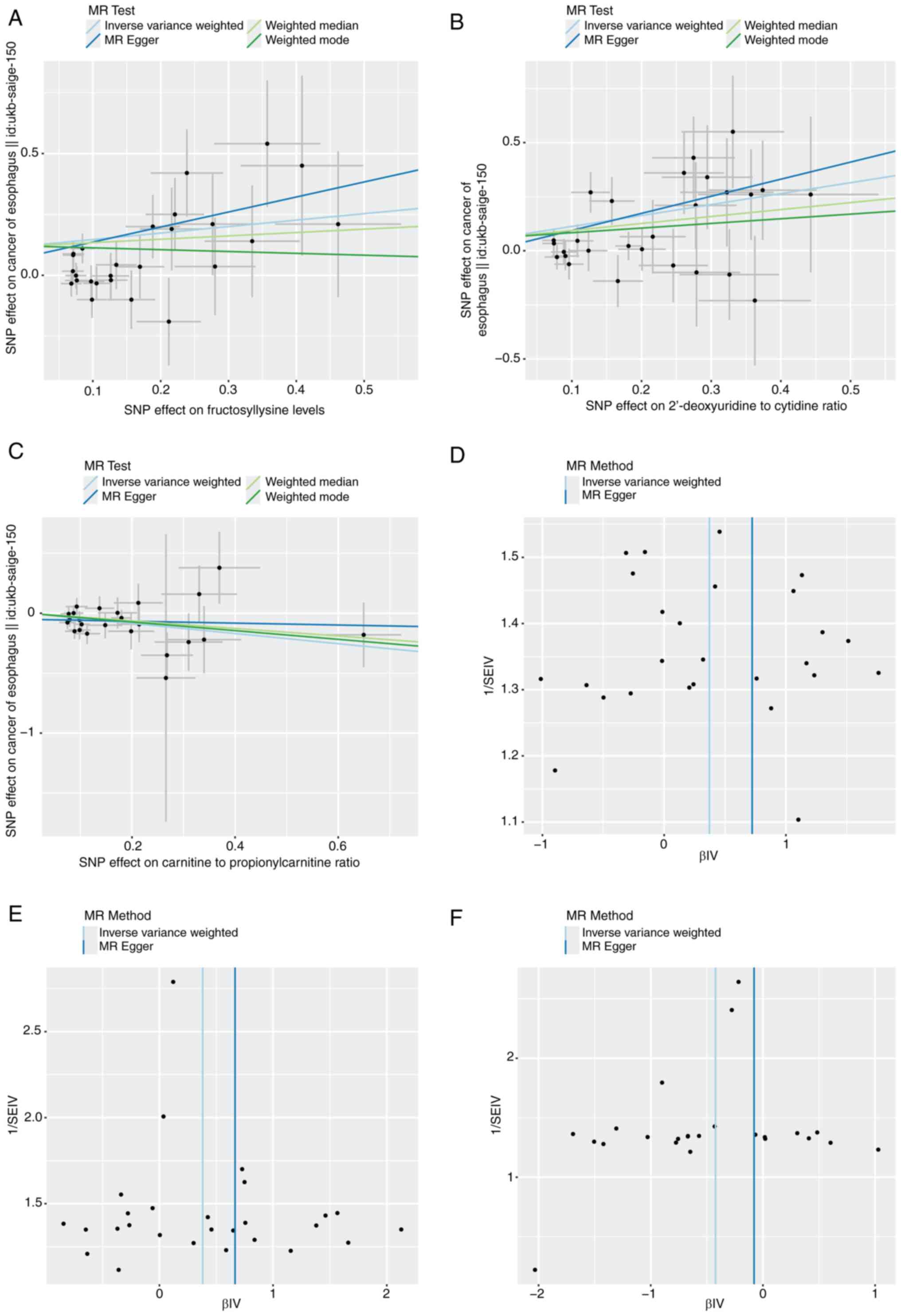
also significantly linked to EC risk (Fig. 2). Furthermore, a sensitivity
analysis was conducted. Despite observing some heterogeneity and
significant results from Cochran's Q test (P<0.05), the causal
estimates remained robust under the random-effects IVW model. The
P-values for the MR-Egger intercept were greater than 0.05,
suggesting that no significant pleiotropy effects were found
(Tables SI and SII). Additionally, the data were further
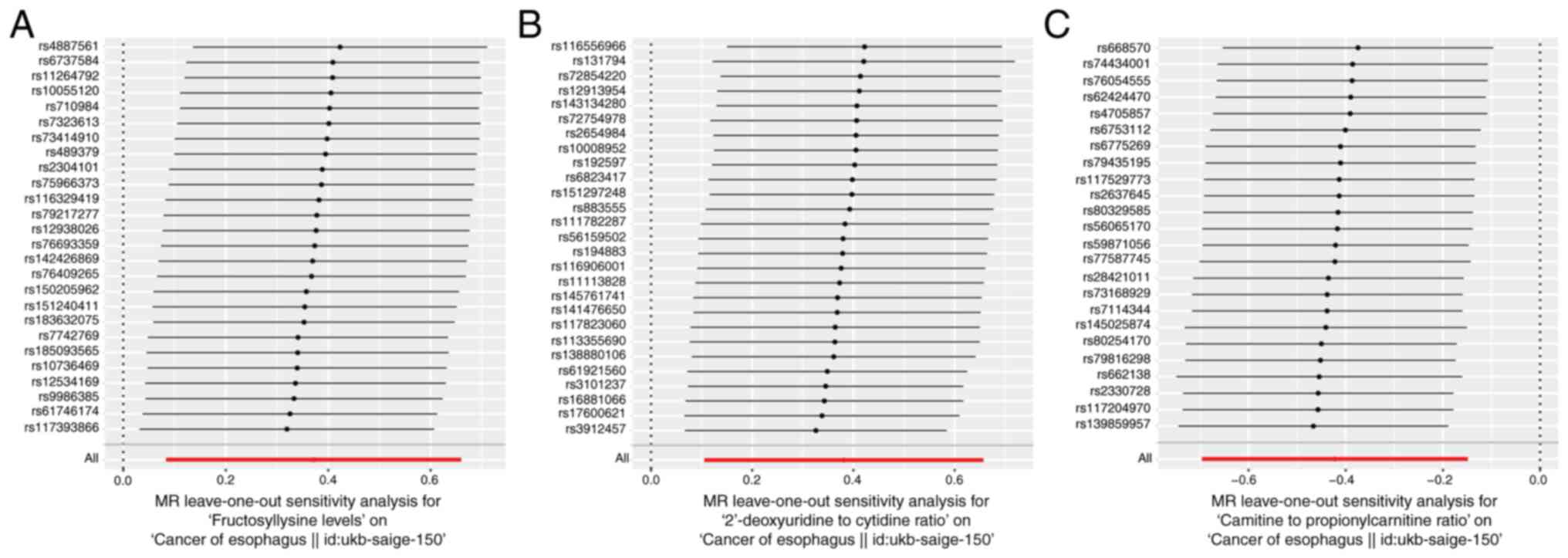
assessed using scatter (Fig.
3A-C), funnel (Fig. 3D-F) and
leave-one-out (Fig. 4A-C) plots
and the potential influence of outliers and horizontal pleiotropy
on the identified hub metabolites were excluded.
 | Table I.Causal association between all
metabolites and esophageal cancer. Inverse variance weighted was
chosen as the primary method. P<0.05 was considered to indicate
a statistically significant difference. OR >1 indicated a risk
factor. |
Table I.
Causal association between all
metabolites and esophageal cancer. Inverse variance weighted was
chosen as the primary method. P<0.05 was considered to indicate
a statistically significant difference. OR >1 indicated a risk
factor.
| Metabolite | Method | Number of single
nucleotide polymorphisms | P-value | OR | OR 95% lower
CI | OR 95% upper
CI |
|---|
| Maltotriose
levels | IVW | 24 | 0.027 | 1.28 | 1.027 | 1.603 |
| Tartarate
levels | IVW | 22 | 0.029 | 0.73 | 0.557 | 0.969 |
|
1-stearoyl-2-arachidonoyl-GPI (18:0/20:4)
levels | IVW | 24 | 0.004 | 1.34 | 1.095 | 1.654 |
| Stearidonate
(18:4n3) levels | IVW | 27 | 0.046 | 1.31 | 1.004 | 1.726 |
| Hexanoylglycine
levels | IVW | 27 | 0.036 | 1.20 | 1.011 | 1.428 |
|
Beta-hydroxyisovaleroylcarnitine
levels | IVW | 36 | 0.027 | 0.76 | 0.605 | 0.97 |
|
1-ribosyl-imidazoleacetate levels | IVW | 37 | 0.010 | 0.75 | 0.605 | 0.936 |
| Thymol sulfate
levels | IVW | 20 | 0.048 | 1.34 | 1.002 | 1.808 |
| 2-hydroxyhippurate
levels | IVW | 20 | 0.028 | 0.70 | 0.520 | 0.964 |
|
2R,3R-dihydroxybutyrate levels | IVW | 32 | 0.014 | 0.75 | 0.599 | 0.946 |
|
1-(1-enyl-palmitoyl)-GPC (p-16:0)
levels | IVW | 29 | 0.021 | 1.32 | 1.041 | 1.687 |
| Docosadioate
(C22-DC) levels | IVW | 26 | 0.044 | 0.73 | 0.547 | 0.992 |
| N-palmitoylglycine
levels | IVW | 22 | 0.021 | 1.28 | 1.036 | 1.582 |
| Fructosyllysine
levels | IVW | 27 | 0.011 | 1.45 | 1.087 | 1.934 |
| Sphingomyelin
(d18:2/14:0, d18: 1/14:1) levels | IVW | 24 | 0.019 | 1.49 | 1.067 | 2.098 |
|
Methyl-4-hydroxybenzoate sulfate
levels | IVW | 22 | 0.019 | 1.43 | 1.060 | 1.936 |
| 1,2,3-benzenetriol
sulfate (2) levels | IVW | 24 | 0.003 | 0.65 | 0.491 | 0.872 |
| 1,2-dilinoleoyl-GPC
(18:2/18:2) levels | IVW | 17 | 0.010 | 0.72 | 0.563 | 0.927 |
|
1-palmitoyl-2-stearoyl-gpc (16:0/18:0)
levels | IVW | 32 | 0.018 | 1.29 | 1.043 | 1.598 |
|
1-stearoyl-2-arachidonoyl-GPE (18:0/20:4)
levels | IVW | 32 | 0.006 | 1.24 | 1.062 | 1.450 |
|
1-(1-enyl-palmitoyl)-2-arachidonoyl-GPE
(p-16:0/20:4) levels | IVW | 22 | 0.017 | 1.40 | 1.060 | 1.850 |
|
1-palmitoyl-2-arachidonoyl-GPE (16:0/20:4)
levels | IVW | 19 | 0.044 | 1.18 | 1.003 | 1.398 |
|
Docosahexaenoylcholine levels | IVW | 20 | 0.049 | 0.72 | 0.529 | 0.998 |
| Sphingomyelin
(d18:2/21:0, d16: 2/23:0) levels | IVW | 35 | 0.022 | 1.34 | 1.043 | 1.744 |
| Sphingomyelin
(d17:1/14:0, d16: 1/15:0) levels | IVW | 26 | 0.010 | 1.43 | 1.088 | 1.889 |
|
2,2′-Methylenebis(6-tert-butyl-p-cresol)
levels | IVW | 21 | 0.048 | 0.75 | 0.566 | 0.998 |
|
3-hydroxy-2-methylpyridine sulfate
levels | IVW | 21 | 0.030 | 0.72 | 0.548 | 0.969 |
| 4-acetylcatechol
sulfate (1) levels | IVW | 19 | 0.020 | 0.68 | 0.502 | 0.943 |
|
Hydroxy-N6,N6,N6-trimethyllysine
levels | IVW | 27 | 0.029 | 1.35 | 1.031 | 1.789 |
| Alpha-tocopherol
levels | IVW | 28 | 0.016 | 1.40 | 1.063 | 1.862 |
|
Beta-hydroxyisovalerate levels | IVW | 28 | 0.033 | 1.31 | 1.022 | 1.688 |
| Eicosapentaenoate
(EPA; 20:5n3) levels | IVW | 27 | 0.0005 | 1.54 | 1.206 | 1.983 |
| Cholesterol
levels | IVW | 21 | 0.018 | 1.44 | 1.064 | 1.964 |
| Gluconate
levels | IVW | 24 | 0.007 | 0.71 | 0.553 | 0.911 |
| Deoxycholate
levels | IVW | 27 | 0.032 | 1.32 | 1.023 | 1.717 |
| Ornithine
levels | IVW | 28 | 0.013 | 0.72 | 0.563 | 0.936 |
| Dimethylglycine
levels | IVW | 33 | 0.023 | 1.25 | 1.031 | 1.525 |
| Tyrosine
levels | IVW | 29 | 0.014 | 0.71 | 0.539 | 0.934 |
|
N-stearoyl-sphinganine (d18:0/18:0)
levels | IVW | 16 | 0.003 | 0.64 | 0.478 | 0.867 |
| X-12101 levels | IVW | 25 | 0.007 | 0.74 | 0.604 | 0.926 |
| X-22834 levels | IVW | 22 | 0.041 | 0.73 | 0.549 | 0.988 |
| X-23639 levels | IVW | 23 | 0.043 | 1.44 | 1.011 | 2.057 |
| X-23648 levels | IVW | 19 | 0.024 | 1.41 | 1.045 | 1.927 |
| X-23641 levels | IVW | 30 | 0.001 | 0.71 | 0.580 | 0.872 |
| X-23665 levels | IVW | 20 | 0.048 | 0.70 | 0.495 | 0.997 |
| X-23739 levels | IVW | 28 | 0.011 | 0.75 | 0.604 | 0.938 |
| X-25810 levels | IVW | 36 | 0.029 | 0.77 | 0.619 | 0.974 |
| 3-methylcytidine
levels | IVW | 20 | 0.028 | 0.83 | 0.717 | 0.981 |
| Adenosine
5′-diphosphate to Adenosine 5′-monophosphate ratio | IVW | 26 | 0.018 | 1.27 | 1.042 | 1.557 |
| Cortisone to
cortisol ratio | IVW | 20 | 0.008 | 0.64 | 0.46 | 0.892 |
| Phosphate to
N-palmitoyl-sphingosine (d18:1 to 16:0) ratio | IVW | 24 | 0.049 | 0.75 | 0.571 | 1.000 |
| Adenosine
5′-monophosphate to arginine ratio | IVW | 17 | 0.032 | 0.65 | 0.44 | 0.964 |
| 2′-deoxyuridine to
cytidine ratio | IVW | 30 | 0.007 | 1.46 | 1.089 | 1.751 |
| Acetylcarnitine to
propionylcarnitine ratio | IVW | 19 | 0.038 | 0.71 | 0.522 | 0.982 |
| Carnitine to
propionylcarnitine ratio | IVW | 24 | 0.002 | 0.66 | 0.499 | 0.861 |
| Alpha-ketoglutarate
to trans-4-hydroxyproline ratio | IVW | 20 | 0.020 | 1.42 | 1.055 | 1.936 |
| Adenosine
5′-monophosphate to urate ratio | IVW | 27 | 0.020 | 1.41 | 1.054 | 1.896 |
Causal estimation between metabolites
and GC
Using the IVW method as the primary analytic
approach, a two-sample MR analysis was adopted to investigate the
causal effect of GC on metabolites. The results identified
associations with 58 metabolites (Table II). Notably, the GC risk was
observed to be significantly associated with elevated levels of
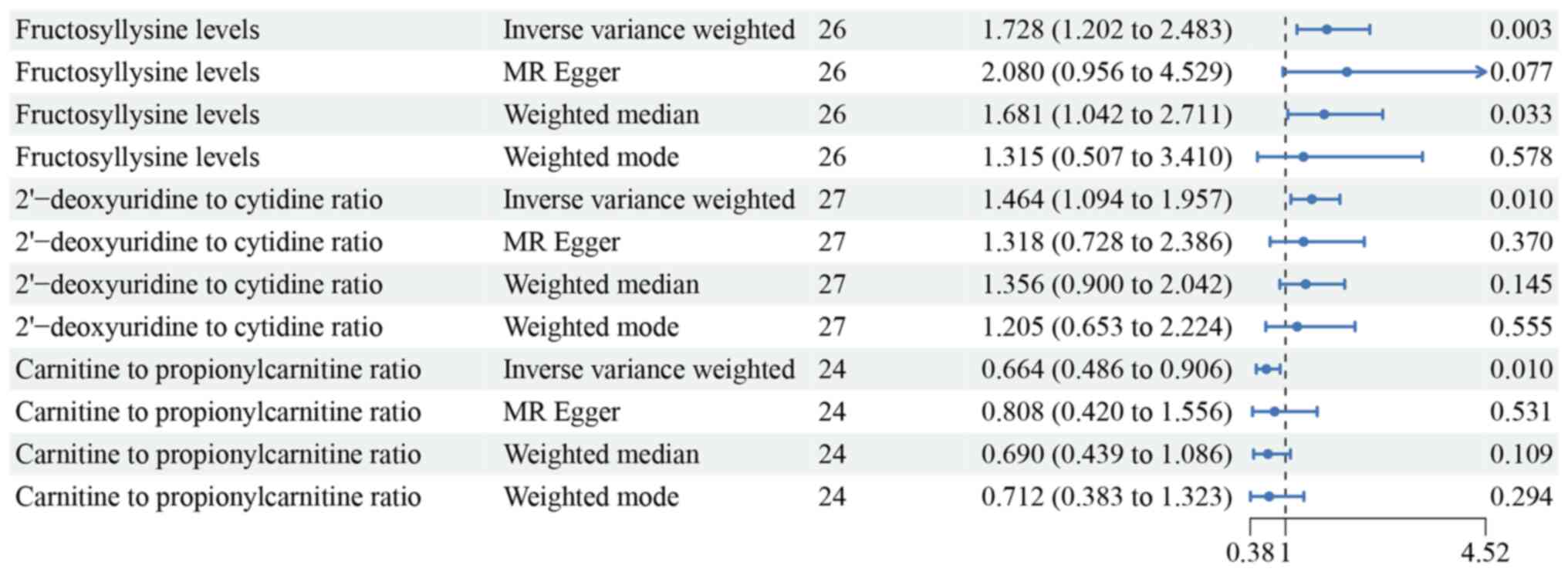
fructosyllysine (OR=1.70, 95% CI=1.240–2.346, P=0.001), the
2′-deoxyuridine to cytidine ratio (OR=1.464, 95% CI=1.094–1.957,
P=0.010) and the C3 ratio (OR=0.664, 95% CI=0.486–0.906, P=0.010;
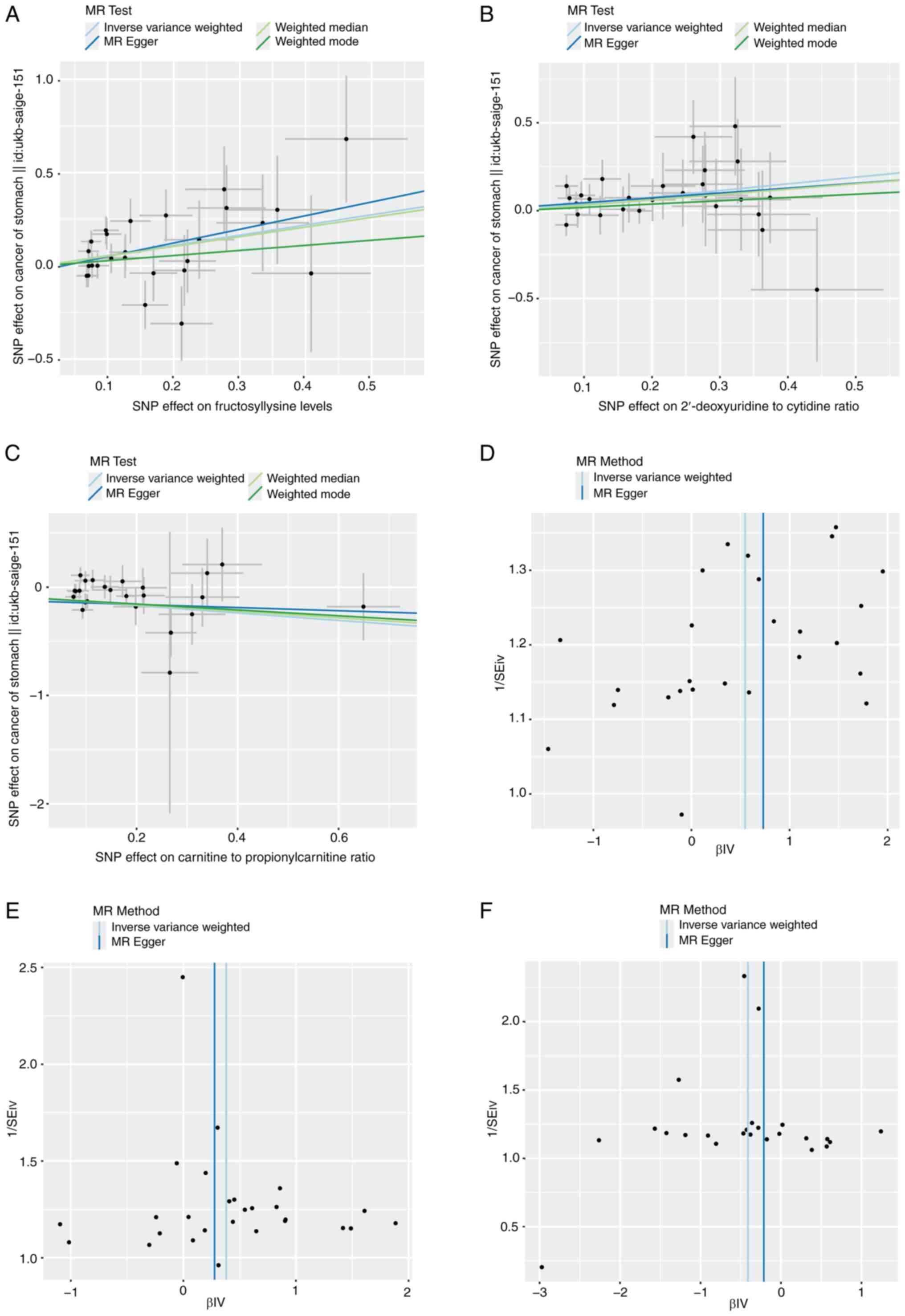
Fig. 5). Although sensitivity
analysis highlighted some heterogeneity, Cochran's Q yielded
P<0.05 and the causality estimates were satisfactory when using
a random-effects IVW approach. The P-values for the MR-Egger
intercept were >0.05, indicating that no significant pleiotropy
effects were found (Tables SIII
and SIV). Additionally, scatter
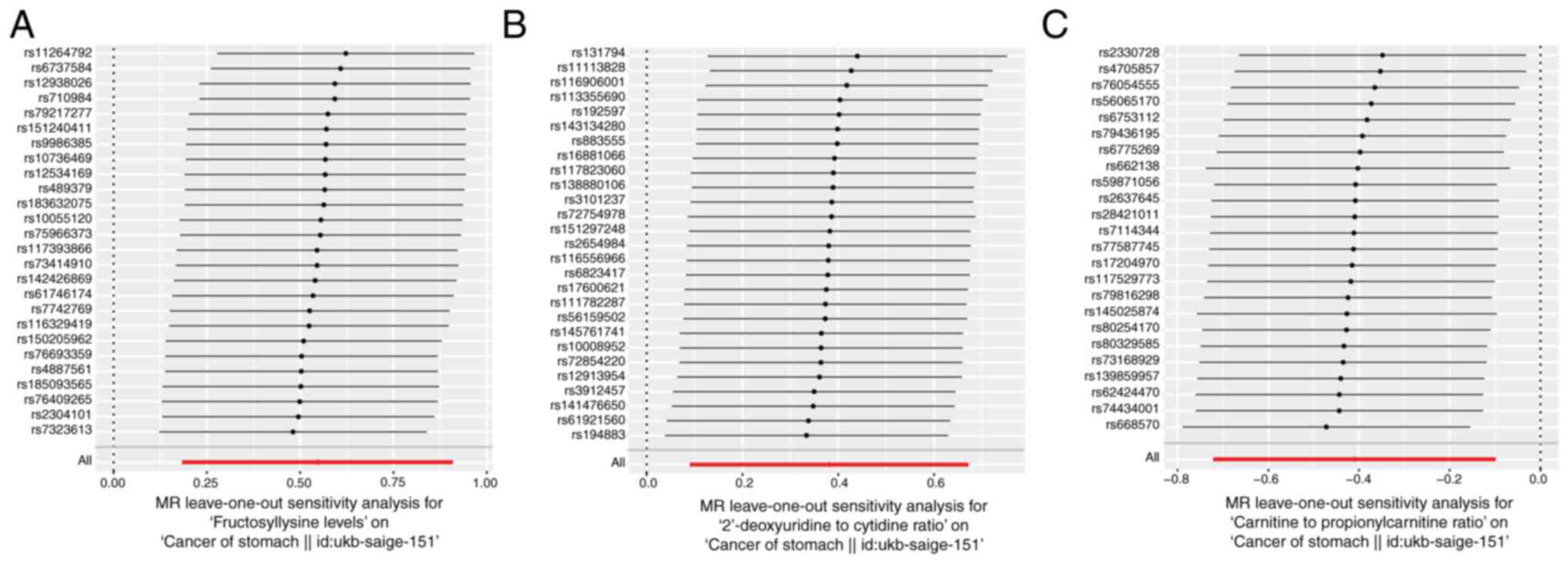
(Fig. 6A-C), funnel (Fig. 6D-F) and leave-one-out (Fig. 7A-C) plots excluded the likelihood
inflicting by potential outliers and horizontal pleiotropy on the
identified hub metabolites that were excluded.
 | Table II.The causal association between all
metabolites and gastric cancer. Inverse variance weighted was
chosen as the primary method. P<0.05 was considered to indicate
a statistically significant difference. OR >1 indicated a risk
factor. |
Table II.
The causal association between all
metabolites and gastric cancer. Inverse variance weighted was
chosen as the primary method. P<0.05 was considered to indicate
a statistically significant difference. OR >1 indicated a risk
factor.
| Metabolite | Method | Number of single
nucleotide polymorphisms | P-value | OR | OR 95% lower
CI | OR 95% upper
CI |
|---|
| Gentisate
levels | IVW | 27 | 0.036 | 1.44 | 1.024 | 2.028 |
| 1,5-anhydroglucitol
(1,5-ag) levels | IVW | 32 | 0.018 | 1.35 | 1.052 | 1.742 |
| 2-linoleoylglycerol
(18:2) levels | IVW | 23 | 0.016 | 1.43 | 1.069 | 1.921 |
|
3-carboxy-4-methyl-5-propyl-2-furanpropanoate
(cmpf) levels | IVW | 17 | 0.047 | 1.48 | 1.004 | 2.201 |
| 10-nonadecenoate
(19:1n9) levels | IVW | 13 | 0.018 | 0.57 | 0.363 | 0.912 |
| Stachydrine
levels | IVW | 24 | 0.036 | 1.41 | 1.021 | 1.961 |
| 2-palmitoleoyl-GPC
(16:1) levels | IVW | 26 | 0.045 | 1.33 | 1.006 | 1.769 |
| Isobutyrylglycine
levels | IVW | 30 | 0.027 | 1.3 | 1.029 | 1.666 |
|
Glycerophosphoethanolamine levels | IVW | 25 | 0.048 | 1.34 | 1.002 | 1.807 |
| Taurolithocholate
3-sulfate levels | IVW | 23 | 0.018 | 0.69 | 0.510 | 0.939 |
| Glycolithocholate
sulfate levels | IVW | 23 | 0.0009 | 0.59 | 0.435 | 0.808 |
| Pregnenolone
sulfate levels | IVW | 36 | 0.023 | 0.72 | 0.543 | 0.955 |
| S-methylmethionine
levels | IVW | 21 | 0.018 | 0.68 | 0.500 | 0.939 |
|
2,3-dihydroxyisovalerate levels | IVW | 27 | 0.002 | 1.61 | 1.185 | 2.212 |
| N-oleoyltaurine
levels | IVW | 21 | 0.008 | 1.46 | 1.103 | 1.956 |
| 2-aminooctanoate
levels | IVW | 29 | 0.037 | 1.23 | 1.011 | 1.495 |
| 3-acetylphenol
sulfate levels | IVW | 21 | 0.037 | 1.43 | 1.020 | 2.018 |
| N-acetylalliin
levels | IVW | 30 | 0.001 | 0.69 | 0.557 | 0.872 |
| Fructosyllysine
levels | IVW | 27 | 0.001 | 1.70 | 1.200 | 2.483 |
| N-acetyltaurine
levels | IVW | 20 | 0.045 | 0.71 | 0.516 | 0.992 |
| 3-hydroxypyridine
sulfate levels | IVW | 24 | 0.014 | 1.50 | 1.084 | 2.081 |
| Arabitol/xylitol
levels | IVW | 24 | 0.049 | 0.70 | 0.493 | 0.999 |
| Tricosanoyl
sphingomyelin (d18: 1/23:0) levels | IVW | 29 | 0.006 | 0.64 | 0.469 | 0.882 |
|
1-stearoyl-2-arachidonoyl-GPE (18:0/20:4)
levels | IVW | 32 | 0.012 | 1.33 | 1.062 | 1.668 |
| 5-hydroxyindole
sulfate levels | IVW | 19 | 0.049 | 1.36 | 1.001 | 1.864 |
| Nisinate (24:6n3)
levels | IVW | 18 | 0.003 | 1.51 | 1.145 | 2.002 |
|
Arachidonoylcarnitine (C20:4) levels | IVW | 35 | 0.004 | 1.27 | 1.076 | 1.508 |
| Heptenedioate
(C7:1-DC) levels | IVW | 16 | 0.016 | 1.69 | 1.103 | 2.605 |
| Octadecenedioate
(C18:1-DC) levels | IVW | 23 | 0.013 | 0.75 | 0.606 | 0.943 |
| Perfluorooctanoate
(PFOA) levels | IVW | 23 | 0.036 | 0.69 | 0.491 | 0.977 |
|
Glyco-beta-muricholate levels | IVW | 25 | 0.017 | 0.76 | 0.617 | 0.954 |
| 8-methoxykynurenate
levels | IVW | 26 | 0.026 | 1.38 | 1.038 | 1.850 |
| 2-ketocaprylate
levels | IVW | 23 | 0.028 | 1.37 | 1.034 | 1.837 |
|
Palmitoyl-sphingosine-phosphoe-thanolamine
(d18:1/16:0) levels | IVW | 29 | 0.005 | 0.66 | 0.496 | 0.887 |
| Picolinate
levels | IVW | 23 | 0.032 | 0.74 | 0.565 | 0.975 |
| Erucate (22:1n9)
levels | IVW | 19 | 0.003 | 0.58 | 0.410 | 0.842 |
| Cys-gly, oxidized
levels | IVW | 26 | 0.028 | 1.33 | 1.030 | 1.719 |
|
1-palmitoyl-2-linoleoyl-gpc (16:0/18:2)
levels | IVW | 35 | 0.040 | 0.78 | 0.622 | 0.989 |
| Eicosapentaenoate
(20:5n3) levels | IVW | 27 | 0.040 | 1.34 | 1.012 | 1.791 |
| Stearate (18:0)
levels | IVW | 21 | 0.033 | 0.67 | 0.469 | 0.97 |
| Pseudouridine
levels | IVW | 23 | 0.004 | 0.59 | 0.418 | 0.85 |
| X-11632 levels | IVW | 26 | 0.014 | 0.68 | 0.508 | 0.928 |
| X-12701 levels | IVW | 15 | 0.006 | 0.6 | 0.426 | 0.871 |
| X-18901 levels | IVW | 30 | 0.006 | 1.5 | 1.119 | 2.018 |
| X-21283 levels | IVW | 17 | 0.007 | 0.72 | 0.58 | 0.917 |
| X-22834 levels | IVW | 22 | 0.026 | 0.71 | 0.525 | 0.961 |
| X-23648 levels | IVW | 19 | 0.028 | 1.48 | 1.043 | 2.113 |
| X-23782 levels | IVW | 20 | 0.027 | 1.57 | 1.051 | 2.365 |
| X-24344 levels | IVW | 17 | 0.006 | 1.54 | 1.129 | 2.116 |
| X-24585 levels | IVW | 19 | 0.013 | 1.58 | 1.099 | 2.29 |
|
Oleoyl-linoleoyl-glycerol (18:1 to 18:2)
to linoleoyl-arachidonoyl-glycerol (18:2 to 20:4) ratio | IVW | 25 | 0.021 | 0.80 | 0.661 | 0.968 |
| Spermidine to
adenosine 5′-diphosphate (ADP) ratio | IVW | 25 | 0.039 | 0.78 | 0.624 | 0.987 |
| Glycine to
phosphate ratio | IVW | 28 | 0.024 | 1.27 | 1.032 | 1.565 |
| Adenosine
5′-diphosphate (ADP) to glutamate ratio | IVW | 32 | 0.022 | 0.77 | 0.619 | 0.963 |
| Cortisol to
4-cholesten-3-one ratio | IVW | 21 | 0.020 | 1.53 | 1.068 | 2.192 |
| 2′-deoxyuridine to
cytidine ratio | IVW | 30 | 0.010 | 1.46 | 1.094 | 1.957 |
| Carnitine to
propionylcarnitine (C3) ratio | IVW | 24 | 0.010 | 0.66 | 0.486 | 0.906 |
| Arachidonate
(20:4n6) to linoleate (18:2n6) ratio | IVW | 26 | 0.017 | 1.40 | 1.062 | 1.865 |
Hub metabolites affect the
proliferation and migration abilities of EC and GC cells
To verify the effects of four metabolites,
fructosyllysine, 2′-deoxyuridine, cytidine and carnitine, on the
proliferation and migration abilities of EC and GC cells, the
present study established in vitro models of EC and GC using
KYSE150 and HGC27 cells, respectively. CCK-8 assay showed that
fructosyllysine and 2′-deoxyuridine promoted the proliferation of
EC and GC cells, while cytidine and carnitine exhibited inhibitory
effects on the proliferation of these two tumor cell lines. Thus,
the 2′-deoxyuridine to cytidine ratio was associated with the
proliferation promotion of EC and GC cells. Scratch assay
demonstrated that EC and GC cells treated with fructosyllysine and
2′-deoxyuridine showed significantly faster scratch healing
compared with the control group, indicating enhanced migratory
capabilities. By contrast, cells treated with cytidine or carnitine
displayed notably slower wound healing than those of the control
group, suggesting a suppressive effect on cell migration. Thus, the
scratch healing speed of EC and GC cells treated with a ratio of
2′-deoxyuridine to cytidine was significantly faster compared with
the control group. In summary, the metabolites fructosyllysine and
the 2′-deoxyuridine to cytidine ratio were found to enhance the
proliferation and migration of EC and GC cells. Conversely,
carnitine was observed to inhibit both the proliferation and
migration of these tumor cell types (Fig. 8, Fig.
9, Fig. 10, Fig. 11).
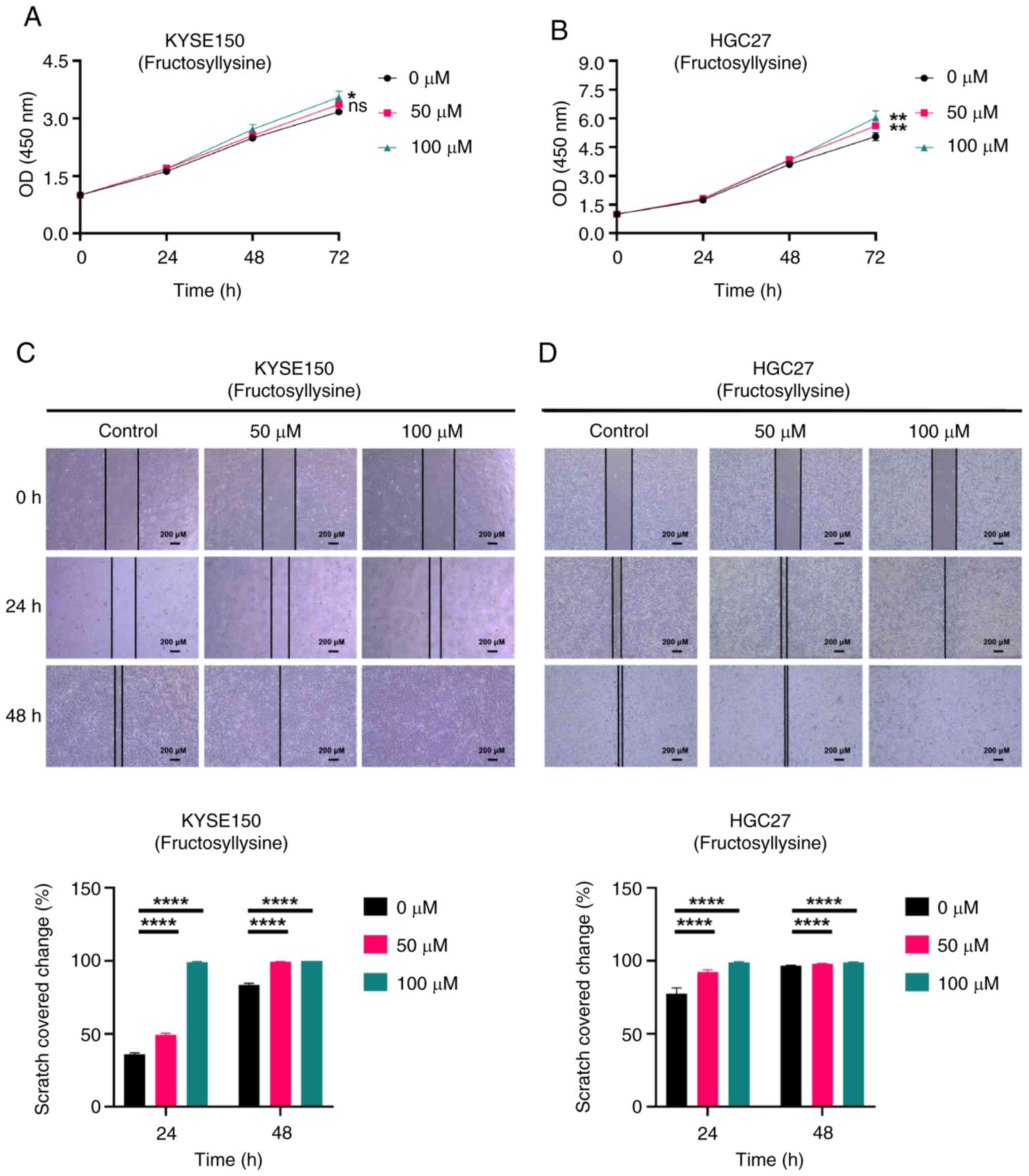 | Figure 8.Fructosyllysine promotes the
proliferation and migration of both EC (KYSE150) and GC (HGC27)
cell lines. (A) CCK-8 assay measured the effect of fructosyllysine
on the proliferation of KYSE150 cells. (B) CCK-8 assay measured the
effect of fructosyllysine on the proliferation of HGC27 cells.
Cells were divided into three groups and treated with various
concentrations of fructosyllysine (0, 50 and 100 µM). The
absorbance values of cells at different time points (0, 24 and 48
h) were measured by CCK-8 assay and the proliferation of cells was
evaluated by graphs. (C) Scratch assay determined the influence of
fructosyllysine on the migration of KYSE150 cells. (D) Scratch
assay determined the influence of fructosyllysine on the migration
of HGC27 cells. scale bars, 200 µM. Magnification, ×40. KYSE150 and
HGC27 cells were treated with different concentrations of
fructosyllysine for 24 and 48 h and the scratch area at different
time points was calculated to evaluate the migration ability of the
cells. All experiments were repeated three times and the results
were expressed as the mean ± SD. ns, no significant difference vs.
control. *P<0.05, **P<0.01, ****P<0.001 vs. control. EC,
esophageal cancer; GC, gastric cancer; CCK-8, Cell Counting
Kit-8. |
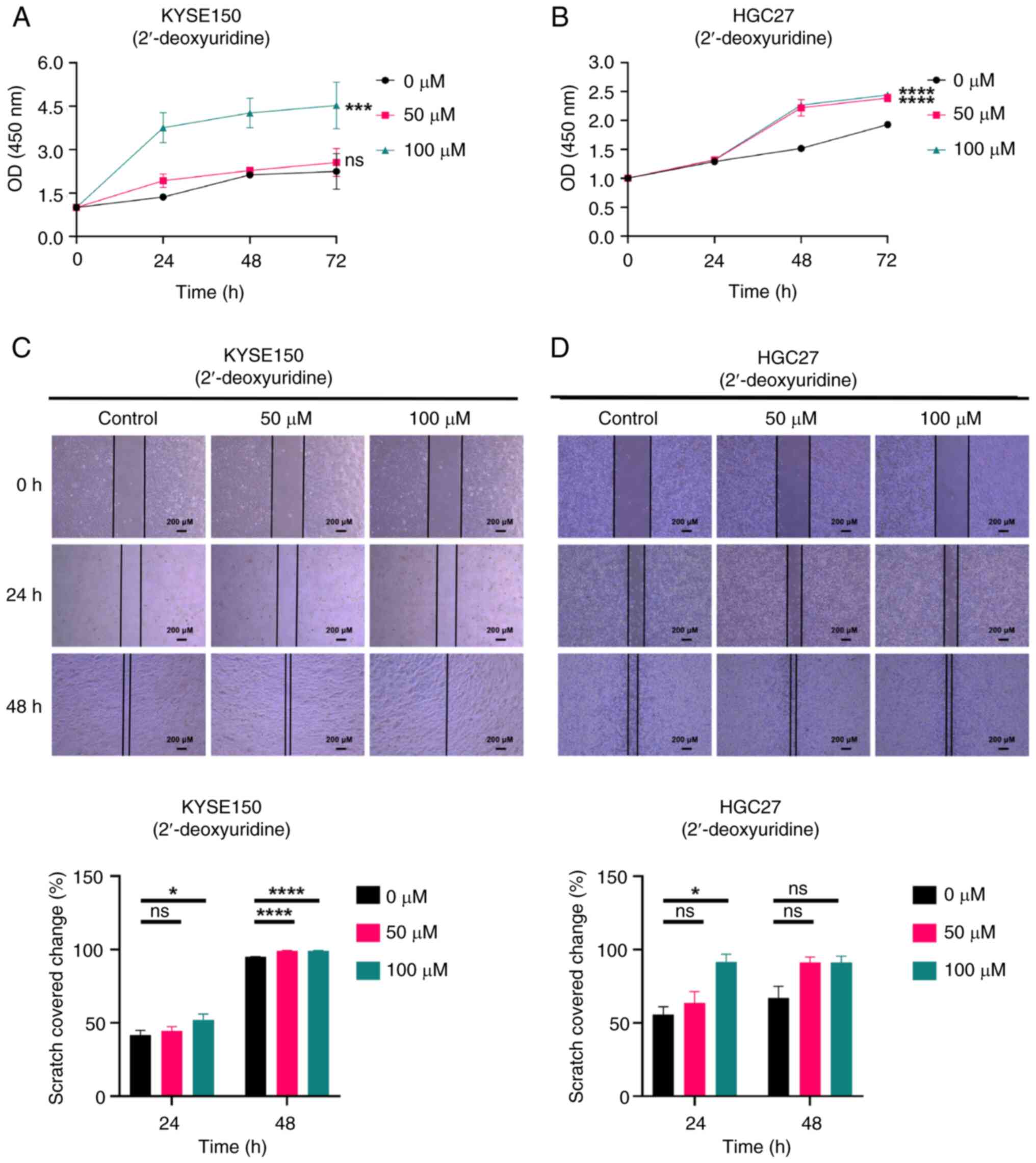 | Figure 9.The 2′-deoxyuridine promotes the
proliferation and migration of both EC (KYSE150) and GC (HGC27)
cell lines. (A) CCK-8 assay measured the effect of 2′-deoxyuridine
on the proliferation of KYSE150 cells. (B) CCK-8 assay measured the
effect of 2′-deoxyuridine on the proliferation of HGC27 cells.
Cells were divided into three groups and treated with various
concentrations of 2′-deoxyuridine (0, 50 and 100 µM). The
absorbance values of cells at different time points (0, 24 and 48
h) were measured by CCK-8 assay and the proliferation of cells was
evaluated by graphing. (C) Scratch assay determined the influence
of 2′-deoxyuridine on the migration of KYSE150 cells. (D) Scratch
assay determined the influence of 2′-deoxyuridine on the migration
of HGC27 cells. scale bars, 200 µM. Magnification, ×40. KYSE150 and
HGC27 cells were treated with different concentrations of
2′-deoxyuridine for 24 and 48 h and the scratch area at different
time points was calculated to evaluate the migration ability of the
cells. All experiments were repeated three times and the results
were expressed as the mean ± SD. ns, no significant difference vs.
control. *P<0.05, ***P<0.005, ****P<0.001 vs. control. EC,
esophageal cancer; GC, gastric cancer; CCK-8, Cell Counting
Kit-8. |
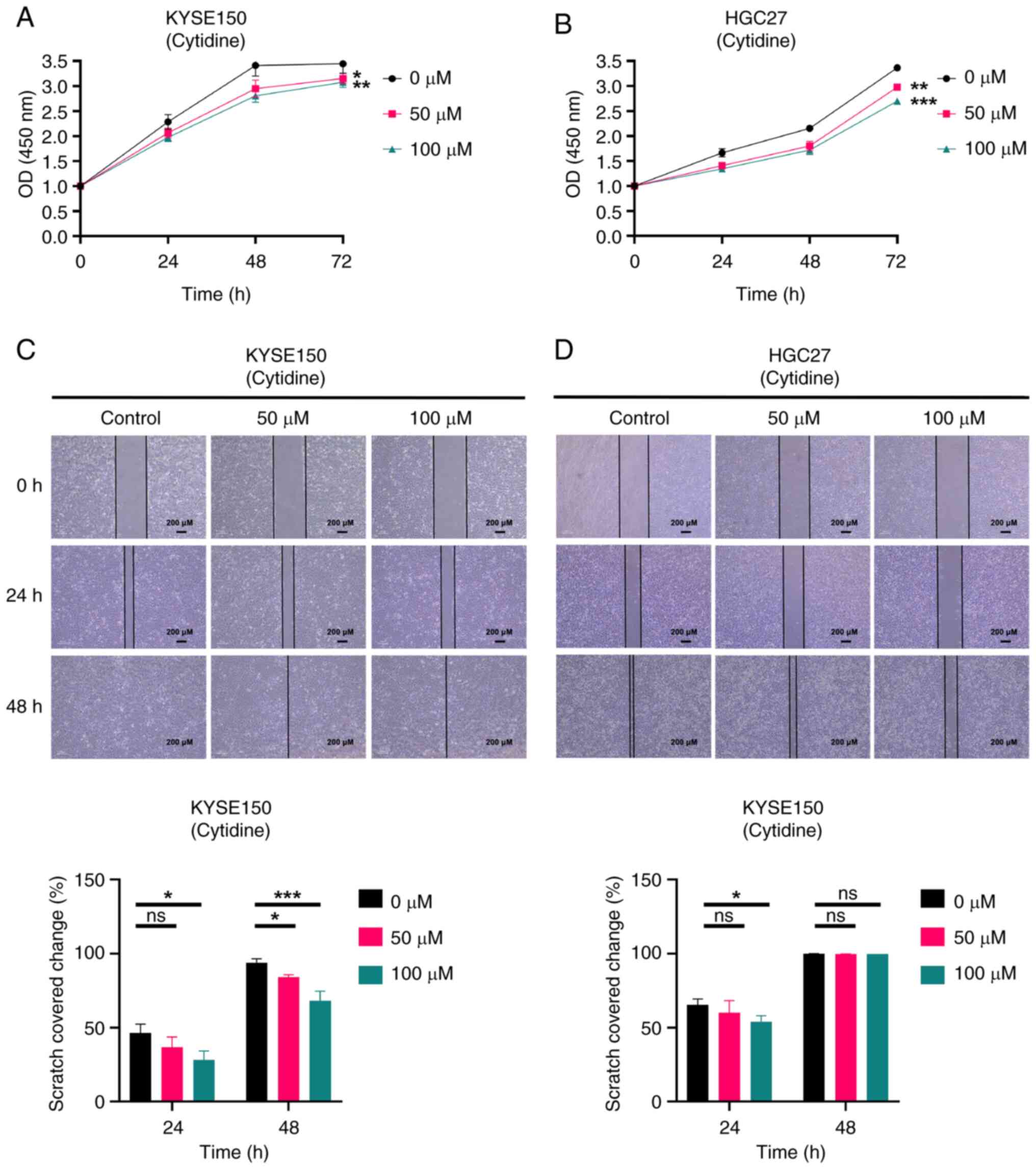 | Figure 10.Cytidine inhibits the proliferation
and migration of both EC (KYSE150 cell) and GC (HGC27 cell) cell
lines. (A) CCK-8 assay measured the effect of cytidine on the
proliferation of KYSE150 cells. (B) CCK-8 assay measured the effect
of cytidine on the proliferation of HGC27 cells. Cells were divided
into three groups and treated with various concentrations of
cytidine (0, 50 and 100 µM). The absorbance values of cells at
different time points (0, 24 and 48 h) were measured by CCK-8 assay
and the proliferation of cells was evaluated by graphing. (C)
Scratch assay determined the influence of cytidine on the migration
of KYSE150 cells. (D) Scratch assay determined the influence of
cytidine on the migration of HGC27 cells. scale bars, 200 µM.
Magnification, ×40. KYSE150 and HGC27 cells were treated with
different concentrations of cytidine for 24 and 48 h and the
scratch area at different time points was calculated to evaluate
the migration ability of the cells. All experiments were repeated 3
times and the results were expressed as the mean ± SD. ns, no
significant difference vs. control. *P<0.05, **P<0.01,
***P<0.005 vs. control. EC, esophageal cancer; GC, gastric
cancer; CCK-8, Cell Counting Kit-8. |
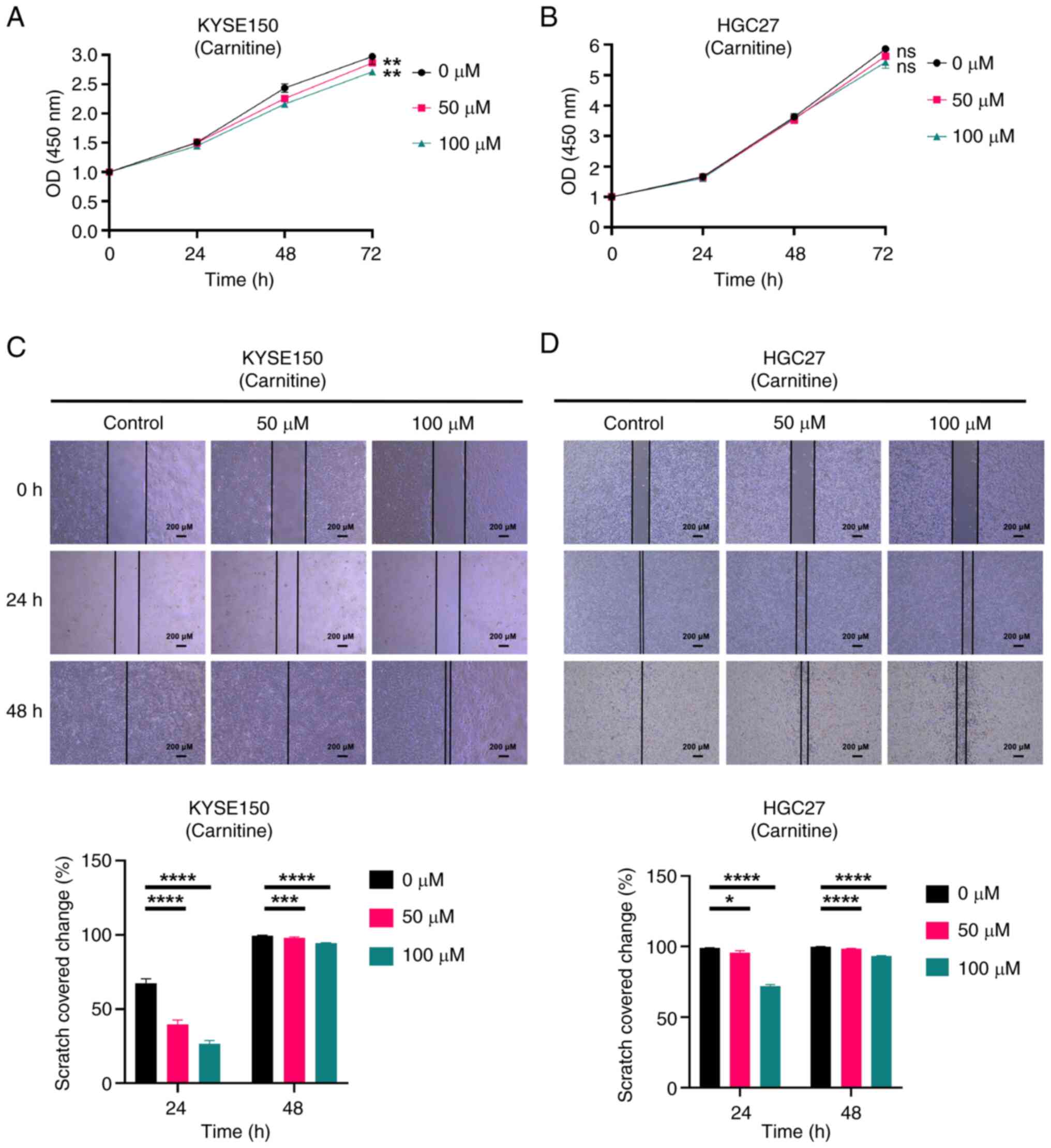 | Figure 11.Carnitine inhibits the proliferation
and migration of both EC (KYSE150) and GC (HGC27) cell lines. (A)
CCK-8 assay measured the effect of carnitine on the proliferation
of KYSE150 cells. (B) CCK-8 assay measured the effect of carnitine
on the proliferation of HGC27 cells. Cells were divided into three
groups and treated with various concentrations of carnitine (0, 50
and 100 µM). The absorbance values of cells at different time
points (0, 24 and 48 h) were measured by CCK-8 assay and the
proliferation of cells was evaluated by graphing. (C) Scratch assay
determined the influence of carnitine on the migration of KYSE150
cells. (D) Scratch assay determined the influence of carnitine on
the migration of HGC27 cells. scale bars, 200 µM. Magnification,
×40. KYSE150 and HGC27 cells were treated with different
concentrations of carnitine for 24 and 48 h and the scratch area at
different time points was calculated to evaluate the migration
ability of the cells. All experiments were repeated 3 times and the
results were expressed as the mean ± SD. ns, no significant
difference vs. control. *P<0.05, **P<0.01, ***P<0.005,
****P<0.001 vs. control. EC, esophageal cancer; GC, gastric
cancer; CCK-8, Cell Counting Kit-8. |
Discussion
MR analysis is increasingly recognized for its
ability to delineate potential causal relationships between risk
factors and diseases (19). The
present study identified 57 metabolites associated with EC and 58
with GC. Notably, fructosyllysine levels, 2′-deoxyuridine to
cytidine ratio and C3 ratio emerged as common metabolites
influencing EC and GC. Among them, fructosyllysine levels and
2′-deoxyridine to cytidine ratio were identified as risk factors
for both cancers, while the C3 ratio appeared as protective factors
for GC and EC.
Fructosyllysine is a precursor of
carboxymethyllysine, an advanced glycation end-product (AGE)
presenting in heated foods, which is considered potentially harmful
to human health (37). AGEs are
produced through non-enzymatic glycation reactions between amino
acids and reducing sugars, a process commonly known as the Maillard
reaction (38). AGEs exist in both
protein-bound and free forms in food, with the majority being
absorbed through the gastrointestinal tract. According to previous
research, AGEs contribute to the progression of various chronic
diseases, including diabetes-related complications, cardiovascular,
renal and neurodegenerative disorders, as well as aging (38–41).
Carboxymethyllysine, the predominant component of AGEs, is formed
through fructosyllysine oxidation (42), which is linked to inflammation and
endothelial dysfunction. Notably, chronic inflammation in the
gastrointestinal tract is closely associated with cancer (43). AGEs primarily mediate inflammation
by binding to the receptor for AGE (RAGE). Expression of RAGE has
been detected in human intestinal epithelial cells and colon
tissues, where its engagement with ligands enhances the oxidase
activity of nicotinamide adenine dinucleotide phosphate, leading to
the transcription of NF-κB and the subsequent expression of
pro-inflammatory genes, such as IL-6 and TNF-α (44). Thus, an elevated level of
fructosyllysine may trigger the inflammatory response in the
intestine, potentially inducing cancer over time. In the study by
Raupbach et al (45), the
expression of RAGE in colon cancer HCT116 cells was shown to be
comparable to that in the intestinal epithelium and the absorption
efficacy of fructosyllysine by HCT116 cells was largely related to
their structural characteristics. However, reports linking
fructosyllysine to cancer are limited. In the present study,
increased fructosyllysine levels were identified as a risk factor
for EC and GC, consistent with previous findings (44,45),
although the mechanisms require further exploration, particularly
concerning the role of inflammatory activation. Additionally,
previous studies have shown that gut microbiota can degrade AGEs
and their precursors under anaerobic conditions, reducing the
exposure to dietary fructosyllysine or carboxymethyllysine in the
gastrointestinal tract and thereby mitigating potential harm to
humans (37). Investigating the
role of gut microbiota presents a promising avenue for developing
treatments for gastrointestinal tumors.
The present study indicated an increase in the
2′-deoxyuridine to cytidine ratio as a risk factor for GC and EC.
Cytidine deaminase (CDA) is an enzyme involved in the pyrimidine
recovery pathway, catalyzing the hydrolysis and deamination of
cytidine and 2′-deoxycytidine to produce uridine and
2′-deoxyuridine (46). Research
has demonstrated that CDA expression is often lost or downregulated
in various types of cancer in breast, lung, ovarian, liver, colon
and cervix, including EC (47).
Low levels of CDA may contribute to cancer development (48) by suppressing the deoxyuridine
synthesis. Deoxyuridine plays a role in mitigating reactive oxygen
species (ROS)-induced endoplasmic reticulum stress, thereby
supporting cancer cell survival; thus, its increased synthesis
could potentially facilitate tumor development (49). In GC and EC, antitumor therapy
commonly results in the accumulation of ROS, which promotes cancer
cell apoptosis and inhibits tumor growth (50–53).
The present results suggested that the 2′-deoxyuridine to cytidine
ratio was a risk factor for EC and GC.
Carnitine serves as a potent antioxidant with
multiple regulatory functions, such as boosting cellular
respiration, promoting apoptosis and decreasing both tumor cell
growth and inflammation. L-carnitine, the active form of carnitine,
transports long-chain fatty acid acyl groups from the cytoplasm
into the mitochondrial matrix. This transport facilitates the
β-oxidation process, converting these acids into acetyl-coenzyme A,
which then enters the citric acid cycle to produce energy. However,
tumor cells proliferate rapidly and require notable energy and they
rely on a distinct metabolic pathway known as the Warburg effect
(54), but not the citric acid
cycle, to proliferate. The accumulation of carnitine can promote
the citric acid cycle, creating an environment that is harmful to
the proliferation of cancer cells and even affects their stemness
(55). Acylcarnitine is an ester
produced by the combination of fatty acid (that is. acyl) and
L-carnitine, which plays an important role in numerous cell
metabolism pathways related to energy production. Acylcarnitine has
been identified as an important indicator of multiple
metabolism-related diseases, including cardiovascular diseases,
diabetes, metabolic disorders, depression and certain tumors
(56). Short-chain acylcarnitine
is the most abundant acylcarnitine class in the human body and it
has been reported to be closely associated with mental illness
(57). Propionyl-L-carnitine is a
type of carnitine donor that increases the transport of fatty acids
across mitochondrial membranes and is used as a supplement for the
treatment of depression (56). In
the study by Zhang et al (58), a negative correlation between
propionyl-L-carnitine and lung cancer was observed. In addition,
L-carnitine has also been shown to improve cancer-related
anorexia/cachexia by reducing inflammation (59). Agreeing with previous research, an
increase in the ratio of C3 may promote the citric acid cycle,
inhibit tumor cell proliferation and serve as a co-protective
factor for EC and GC. The functional experiments in the present
study also demonstrated that carnitine inhibited tumor cell
proliferation.
The current two-sample MR analysis was designed to
evaluate the causal relationship between metabolites and UGI tumors
using large-sample GWAS and UK Biobank data. This approach overcame
some limitations of traditional observational studies by reducing
the influence of confounding factors and reverse causality. In
addition, MR mitigated the representativeness and feasibility
issues that are common in RCTs. However, the present study faced
several challenges. First, non-fasting plasma samples were used for
metabolomics profiling. Despite adjustments made for the time
elapsed since the last meal or beverage, residual variability may
still influence the results. Second, the study focused on
gene-metabolite pairs identified from current expression data and
biological understanding, specifically targeting effector genes.
Nonetheless, the potential relevance of other highly heritable
metabolites or ratios to the disease cannot be overlooked. Future
studies should expand on this by including additional expression
data and metabolic insights to identify effector genes for these
additional metabolites and ratios. Third, the MR analyses used in
the present study were constrained by the fact that most
metabolites and metabolite ratios were associated with only a
single IV. This constraint made it difficult to apply common MR
sensitivity tests such as MR-Egger, which require multiple IVs.
However, the selection of IVs that are closely linked to effector
genes influencing metabolite levels in the present study mitigated
the risk of horizontal pleiotropy. Additionally, assessments of
metabolic pleiotropy were conducted to remove IVs linked to
multiple metabolites not involved in the same metabolic pathways.
Despite these measures, biases may not be fully eliminated due to
limitations in metabolome profiling and uncertainties in
metabolite-protein interaction databases. Finally, the present
study primarily involved elderly individuals of European descent.
Future research should explore the effects of identified genetic
variations on metabolites and their ratios in a more diverse
demographic spectrum. Moreover, the current study covered a broad
range of metabolites; however, the metabolic pathway and mechanisms
of certain metabolites in disease remain unclear, which limited the
interpretability of MR findings.
In summary, this comprehensive MR analysis of
metabolites has uncovered new gene-metabolite associations,
offering an enhanced understanding of genetic regulation in human
metabolism. These discoveries are expected to facilitate the
identification of potential markers and targets that could aid in
precision prevention and lead to behavioral modifications,
innovative pharmaceutical interventions and personalized
treatments. Such strategies would be specifically designed to
address the unique metabolic vulnerabilities associated with UGI
tumors.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural Science
Foundation of Guangdong Province (grant no. 2023A1515012548) and
the Science and Technology Program of Guangzhou (grant no.
2024A04J4802).
Availability of data and materials
The data generated in the present study may be found
in the GWAS catalog and UKBiobank ICD PheWeb under accession number
(GCST90199621-GCST90201020, inclusive; ukb-saige-150;
ukb-saige-151) or at the following URL: (https://www.ebi.ac.uk/gwas/ and https://pheweb.org/UKB-SAIGE/, or https://pheweb.org/UKB-SAIGE/pheno/150
and http://pheweb.org/UKB-SAIGE/pheno/151).
Authors' contributions
PNo collected, analyzed and interpreted the data.
contributed to conception, design and drafted the manuscript. CZ
performed the cell scratch assay and the CCK-8 assay. SS, ST, SK,
PNi, QK, YL, AP, WF, KD and JL designed, revised and supervised the
study. PNo and KD confirm the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zong L, Abe M, Seto Y and Ji J: The
challenge of screening for early gastric cancer in China. Lancet.
388:26062016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang S, Guo Y, Li Z, Zhang Y, Zhou T, You
W, Pan K and Li W: A systematic review of metabolomic profiling of
gastric cancer and esophageal cancer. Cancer Biol Med. 17:181–198.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abbassi-Ghadi N, Kumar S, Huang J, Goldin
R, Takats Z and Hanna GB: Metabolomic profiling of
oesophago-gastric cancer: A systematic review. Eur J Cancer.
49:3625–3637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gu J, Hu X, Shao W, Ji T, Yang W, Zhuo H,
Jin Z, Huang H, Chen J, Huang C and Lin D: Metabolomic analysis
reveals altered metabolic pathways in a rat model of gastric
carcinogenesis. Oncotarget. 7:60053–60073. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim KB, Yang JY, Kwack SJ, Park KL, Kim
HS, Ryu DH, Kim YJ, Hwang GS and Lee BM: Toxicometabolomics of
urinary biomarkers for human gastric cancer in a mouse model. J
Toxicol Environ Health A. 73:1420–1430. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsunaga S, Nishiumi S, Tagawa R and
Yoshida M: Alterations in metabolic pathways in gastric epithelial
cells infected with Helicobacter pylori. Microb Pathog.
124:122–129. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Di Gialleonardo V, Tee SS, Aldeborgh HN,
Miloushev VZ, Cunha LS, Sukenick GD and Keshari KR: High-throughput
indirect quantitation of 13C enriched metabolites using
1H NMR. Anal Chem. 88:11147–11153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Holmes E, Wilson ID and Nicholson JK:
Metabolic phenotyping in health and disease. Cell. 134:714–717.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wishart DS: Emerging applications of
metabolomics in drug discovery and precision medicine. Nat Rev Drug
Discov. 15:473–484. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chan AW, Gill RS, Schiller D and Sawyer
MB: Potential role of metabolomics in diagnosis and surveillance of
gastric cancer. World J Gastroenterol. 20:12874–12882. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Che J, Zhao Y, Gu B, Li S, Li Y, Pan K,
Sun T, Han X, Lv J, Zhang S, et al: Untargeted serum metabolomics
reveals potential biomarkers and metabolic pathways associated with
the progression of gastroesophageal cancer. BMC Cancer.
23:12382023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tokunaga M, Kami K, Ozawa S, Oguma J,
Kazuno A, Miyachi H, Ohashi Y, Kusuhara M and Terashima M:
Metabolome analysis of esophageal cancer tissues using capillary
electrophoresis-time-of-flight mass spectrometry. Int J Oncol.
52:1947–1958. 2018.PubMed/NCBI
|
|
15
|
Wang J, Kunzke T, Prade VM, Shen J, Buck
A, Feuchtinger A, Haffner I, Luber B, Liu DHW, Langer R, et al: A
serum metabolomics analysis reveals a panel of screening metabolic
biomarkers for esophageal squamous cell carcinoma. Clin Transl Med.
11:e4192021. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li J, Li S, Yu L, Wei J, Sun H, Yang C and
Tan H: Identification of preoperative serum metabolites associated
with postoperative opioid consumption in gastric cancer patients by
extreme phenotype sampling. Pain Physician. 25:E385–E396.
2022.PubMed/NCBI
|
|
17
|
Yuan LW, Yamashita H and Seto Y: Glucose
metabolism in gastric cancer: The cutting-edge. World J
Gastroenterol. 22:2046–2059. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiao S and Zhou L: Gastric cancer:
Metabolic and metabolomics perspectives (review). Int J Oncol.
51:5–17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Davey Smith G and Hemani G: Mendelian
randomization: Genetic anchors for causal inference in
epidemiological studies. Hum Mol Genet. 23((R1)): R89–R98. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen Y, Lu T, Pettersson-Kymmer U, Stewart
ID, Butler-Laporte G, Nakanishi T, Cerani A, Liang KYH, Yoshiji S,
Willett JDS, et al: Genomic atlas of the plasma metabolome
prioritizes metabolites implicated in human diseases. Nat Genet.
55:44–53. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Burgess S and Thompson SG: Interpreting
findings from Mendelian randomization using the MR-egger method.
Eur J Epidemiol. 32:377–389. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng Q, Lacey B, Bešević J, Omiyale W,
Conroy M, Starkey F, Calvin C, Callen H, Bramley L, Welsh S, et al:
UK biobank: Enhanced assessment of the epidemiology and long-term
impact of coronavirus disease-2019. Camb Prism Precis Med.
1:e302023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang J, Yan B, Zhao B, Fan Y, He X, Yang
L, Ma Q, Zheng J, Wang W, Bai L, et al: Assessing the causal
effects of human serum metabolites on 5 major psychiatric
disorders. Schizophr Bull. 46:804–813. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Choi KW, Chen CY, Stein MB, Klimentidis
YC, Wang MJ, Koenen KC and Smoller JW; Major Depressive Disorder
Working Group of the Psychiatric Genomics Consortium, : Assessment
of bidirectional relationships between physical activity and
depression among adults: A 2-sample mendelian randomization study.
JAMA Psychiatry. 76:399–408. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun Y, Zhou J and Ye K: White blood cells
and severe COVID-19: A mendelian randomization study. J Pers Med.
11:1952021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sidore C, Busonero F, Maschio A, Porcu E,
Naitza S, Zoledziewska M, Mulas A, Pistis G, Steri M, Danjou F, et
al: Genome sequencing elucidates Sardinian genetic architecture and
augments association analyses for lipid and blood inflammatory
markers. Nat Genet. 47:1272–1281. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park JH, Wacholder S, Gail MH, Peters U,
Jacobs KB, Chanock SJ and Chatterjee N: Estimation of effect size
distribution from genome-wide association studies and implications
for future discoveries. Nat Genet. 42:570–575. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Davies NM, Holmes MV and Davey SG: Reading
Mendelian randomisation studies: A guide, glossary, and checklist
for clinicians. BMJ. 362:k6012018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hartwig FP, Davey Smith G and Bowden J:
Robust inference in summary data Mendelian randomization via the
zero modal pleiotropy assumption. Int J Epidemiol. 46:1985–1998.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bowden J, Davey Smith G, Haycock PC and
Burgess S: Consistent estimation in Mendelian randomization with
some invalid instruments using a weighted median estimator. Genet
Epidemiol. 40:304–314. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Allen RJ, Porte J, Braybrooke R, Flores C,
Fingerlin TE, Oldham JM, Guillen-Guio B, Ma SF, Okamoto T, John AE,
et al: Genetic variants associated with susceptibility to
idiopathic pulmonary fibrosis in people of European ancestry: A
genome-wide association study. Lancet Respir Med. 5:869–880. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Burgess S, Bowden J, Fall T, Ingelsson E
and Thompson SG: Sensitivity analyses for robust causal inference
from Mendelian randomization analyses with multiple genetic
variants. Epidemiology. 28:30–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Verbanck M, Chen CY, Neale B and Do R:
Detection of widespread horizontal pleiotropy in causal
relationships inferred from Mendelian randomization between complex
traits and diseases. Nat Genet. 50:693–698. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hemani G, Tilling K and Davey Smith G:
Orienting the causal relationship between imprecisely measured
traits using GWAS summary data. PLoS Genet. 13:e10070812017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yavorska OO and Burgess S:
MendelianRandomization: An R package for performing Mendelian
randomization analyses using summarized data. Int J Epidemiol.
46:1734–1739. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hemani G, Zheng J, Elsworth B, Wade KH,
Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R, et
al: The MR-base platform supports systematic causal inference
across the human phenome. Elife. 7:e344082018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
van Dongen KCW, Belzer C, Bakker W,
Rietjens IMCM and Beekmann K: Inter- and intraindividual
differences in the capacity of the human intestinal microbiome in
fecal slurries to metabolize fructoselysine and
carboxymethyllysine. J Agric Food Chem. 70:11759–11768. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sergi D, Boulestin H, Campbell FM and
Williams LM: The role of dietary advanced glycation end products in
metabolic dysfunction. Mol Nutr Food Res. 65:e19009342021.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rabbani N and Thornalley PJ: Hidden
complexities in the measurement of fructosyl-lysine and advanced
glycation end products for risk prediction of vascular
complications of diabetes. Diabetes. 64:9–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sobenin IA, Tertov VV, Koschinsky T,
Bünting CE, Slavina ES, Dedov II and Orekhov AN: Modified low
density lipoprotein from diabetic patients causes cholesterol
accumulation in human intimal aortic cells. Atherosclerosis.
100:41–54. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ahmed N, Ahmed U, Thornalley PJ, Hager K,
Fleischer G and Münch G: Protein glycation, oxidation and nitration
adduct residues and free adducts of cerebrospinal fluid in
Alzheimer's disease and link to cognitive impairment. J Neurochem.
92:255–263. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ahmed MU, Thorpe SR and Baynes JW:
Identification of N epsilon-carboxymethyllysine as a degradation
product of fructoselysine in glycated protein. J Biol Chem.
261:4889–4894. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li L, Liu H, Yu J, Sun Z, Jiang M, Yu H
and Wang C: Intestinal microbiota and metabolomics reveal the role
of auricularia delicate in regulating colitis-associated colorectal
cancer. Nutrients. 15:50112023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zen K, Chen CXJ, Chen YT, Wilton R and Liu
Y: Receptor for advanced glycation endproducts mediates neutrophil
migration across intestinal epithelium. J Immunol. 178:2483–2490.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Raupbach J, Müller SK, Schnell V,
Friedrich S, Hellwig A, Grune T and Henle T: The effect of free and
protein-bound maillard reaction products N-ε-carboxymethyllysine,
N-ε-fructosyllysine, and pyrraline on Nrf2 and NFκB in HCT 116
cells. Mol Nutr Food Res. 67:e23001372023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Urbelienė N, Tiškus M, Tamulaitienė G,
Gasparavičiūtė R, Lapinskaitė R, Jauniškis V, Sūdžius J, Meškienė
R, Tauraitė D, Skrodenytė E, et al: Cytidine deaminases catalyze
the conversion of N(S,O)4-substituted pyrimidine
nucleosides. Sci Adv. 9:eade43612023. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen Y, Ma Z, Shen X, Li L, Zhong J, Min
LS, Xu L, Li H, Zhang J and Dai L: Serum lipidomics profiling to
identify biomarkers for non-small cell lung cancer. Biomed Res Int.
2018:52762402018.PubMed/NCBI
|
|
48
|
Onclercq-Delic R, Buhagiar-Labarchède G,
Leboucher S, Larcher T, Ledevin M, Machon C, Guitton J and
Amor-Guéret M: Cytidine deaminase deficiency in mice enhances
genetic instability but limits the number of chemically induced
colon tumors. Cancer Lett. 555:2160302023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Olou AA, King RJ, Yu F and Singh PK: MUC1
oncoprotein mitigates ER stress via CDA-mediated reprogramming of
pyrimidine metabolism. Oncogene. 39:3381–3395. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Deng YQ, Gao M, Lu D, Liu QP, Zhang RJ, Ye
J, Zhao J, Feng ZH, Li QZ and Zhang H: Compound-composed Chinese
medicine of Huachansu triggers apoptosis of gastric cancer cells
through increase of reactive oxygen species levels and suppression
of proteasome activities. Phytomedicine. 123:1551692024. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sun W, Yuan Y, Chen J, Bao Q, Shang M, Sun
P and Peng H: Construction and validation of a novel
senescence-related risk score can help predict the prognosis and
tumor microenvironment of gastric cancer patients and determine
that STK40 can affect the ROS accumulation and proliferation
ability of gastric cancer cells. Front Immunol. 14:12592312023.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Javid H, Hashemy SI, Heidari MF, Esparham
A and Gorgani-Firuzjaee S: The anticancer role of cerium oxide
nanoparticles by inducing antioxidant activity in esophageal cancer
and cancer stem-like ESCC spheres. Biomed Res Int.
2022:32681972022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cannon A, Maher SG and Lynam-Lennon N:
Generation and characterization of an isogenic cell line model of
radioresistant esophageal adenocarcinoma. Methods Mol Biol.
2645:139–152. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
DeBerardinis RJ: Is cancer a disease of
abnormal cellular metabolism? New angles on an old idea. Genet Med.
10:767–777. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Farahzadi R, Sanaat Z,
Movassaghpour-Akbari AA, Fathi E and Montazersaheb S: Investigation
of L-carnitine effects on CD44+ cancer stem cells from
MDA-MB-231 breast cancer cell line as anti-cancer therapy. Regen
Ther. 24:219–226. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Dambrova M, Makrecka-Kuka M, Kuka J,
Vilskersts R, Nordberg D, Attwood MM, Smesny S, Sen ZD, Guo AC,
Oler E, et al: Acylcarnitines: Nomenclature, biomarkers,
therapeutic potential, drug targets, and clinical trials. Pharmacol
Rev. 74:506–551. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Nasca C, Bigio B, Lee FS, Young SP, Kautz
MM, Albright A, Beasley J, Millington DS, Mathé AA, Kocsis JH, et
al: Acetyl-l-carnitine deficiency in patients with major depressive
disorder. Proc Natl Acad Sci USA. 115:8627–8632. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhang X, Wang C, Li C and Zhao H:
Development and internal validation of nomograms based on plasma
metabolites to predict non-small cell lung cancer risk in smoking
and nonsmoking populations. Thorac Cancer. 14:1719–1731. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Laviano A, Molfino A, Seelaender M,
Frascaria T, Bertini G, Ramaccini C, Bollea MR, Citro G and Rossi
Fanelli F: Carnitine administration reduces cytokine levels,
improves food intake, and ameliorates body composition in
tumor-bearing rats. Cancer Invest. 29:696–700. 2011. View Article : Google Scholar : PubMed/NCBI
|