Acute lung injury (ALI) is a severe respiratory
disease caused by uncontrolled acute inflammation of the lungs
caused by various direct factors (such as severe lung infection,
pulmonary embolism and lung injury) and indirect factors (such as
sepsis, trauma and massive blood transfusions), resulting in
impaired lung function. It can further develop into acute
respiratory distress syndrome (ARDS) characterized by progressive
respiratory distress and refractory hypoxemia (1). According to a study involving 459
intensive care units (ICUs) from 50 countries on 5 continents
during 4 consecutive weeks in winter 2014, the prevalence of ARDS
accounted for 10.4% of ICU admissions. This survey found that a
total of 2,377 patients developed ARDS within the first 48 h of
acute hypoxic respiratory failure, with 30.0% of patients
presenting with mild ARDS, 46.6% of patients with moderate ARDS and
23.4% of patients with severe ARDS. The in-hospital mortality rate
for ARDS was 34.9% in mild patients, 40.3% in moderate patients and
46.1% in patients with severe ARDS (2). Pathological features of ALI include
diffuse alveolar damage and large aggregates of neutrophils in lung
tissue, which produce and secrete pro-inflammatory cytokines
(3). This causes an uncontrolled
inflammatory response, extensive apoptosis of lung epithelial
cells, alveolar defects, impaired barrier function of capillary
membranes and alveoli, and invasion of proteolytic edema fluid into
the alveoli, which destroys surface cellular structures (4,5).
However, the pathogenesis of ALI/ARDS is complex, and there is
currently no effective, specific treatment in clinical practice.
Therefore, innovative mechanisms and therapies are urgently needed
to alter the onset and outcome of ALI/ARDS.
Numerous immune cells, such as neutrophils, lung
macrophages, alveolar epithelial cells (AECs) and T cells, are
involved in the development of ALI/ARDS, which involves the
interaction between lung structures and a complex immune cell
microenvironment that is essential for ALI/ARDS (6). Macrophages are the main cell type in
innate immunity (7). They are
widely distributed in the lung microenvironment, have a wide range
of plasticity and respond to different stimuli to convert
phenotypes (8). Therefore, lung
macrophages, as the main effector cells in the lungs and play
different roles at different stages of ALI/ARDS (9). Macrophages exacerbate ALI/ARDS by
promoting the polarization of pro-inflammatory macrophages and the
release of their products, decreasing the release of
anti-inflammatory macrophages and their products, and inducing
macrophage pyroptosis (10,11).
In addition, by decreasing the phagocytosis of macrophages,
increasing apoptosis can also promote ALI/ARDS (11). A relevant article has summarized
existing and new drugs that have been tested or are currently being
clinically tested for the treatment of ALI and ARDS, and different
data may support the selective use of neuromuscular blocking
agents, corticosteroids and neutrophil elastase inhibitors for the
treatment of ARDS (12). However,
these are not yet universally available (12). Data from patients with coronavirus
disease (COVID) associated ALI /ARDS support using IL-6 monoclonal
antibodies, corticosteroids and Janus kinase (JAK) inhibitors to
treat this condition (12).
Relatively few drugs that target macrophages are available for the
treatment of ALI/ARDS (13). IL-6
and granulocyte-macrophage colony-stimulating factor (GM-CSF) are
important cytokines involved in the activation of monocytes and the
induction of their differentiation into macrophages (14). In clinical practice, lung
inflammation in patients with COVID-19 can be reduced using GM-CSF
inhibitors (such as sargramostim and mavrilimumab), which directly
target GM-CSF and block its interaction with macrophage surface
receptors (15). However, this is
only a preventive strategy and has no therapeutic effect on ARDS
(16). Homeostatic effects of
GM-CSF in the lungs and the blockade of GM-CSF in patients with
COVID-19 have the potential risk of impairing alveolar macrophage
function and impeding pathogen clearance (17). In previous years, preclinical
studies have found that the use of natural compounds isolated from
herbal medicine can effectively improve ALI/ARDS by regulating
macrophage polarization and pyroptosis (18–20).
Furthermore, several natural products also function by regulating
phagocytosis and autophagy (21–23).
In the present review, the natural products that have been shown to
regulate macrophage abnormalities in ALI/ARDS within the past five
years and their mechanisms of action are introduced. These products
may undergo clinical trials and become potential drugs that treat
ALI/ARDS by targeting macrophages, providing new ideas and further
research directions for the development of ALI/ARDS therapeutic
drugs.
Macrophages are heterogeneous and highly plastic
immune cells that recognize pathogen-associated molecular patterns
and trigger innate immune responses to activate host defenses and
play a regulatory role in inflammatory responses (24). Under different stimuli, macrophages
can polarize into two different subtypes, specifically the M1
macrophages (possessing pro-inflammatory characteristics) and the
M2 macrophages (possessing properties that counter inflammation)
(25). An imbalance in the
polarization of the macrophages leads to an inflammatory response
(26). Studies have found that
balancing M1/M2 macrophages is beneficial to eliminate the
inflammatory storm that occurs in ALI/ARDS, which enhances recovery
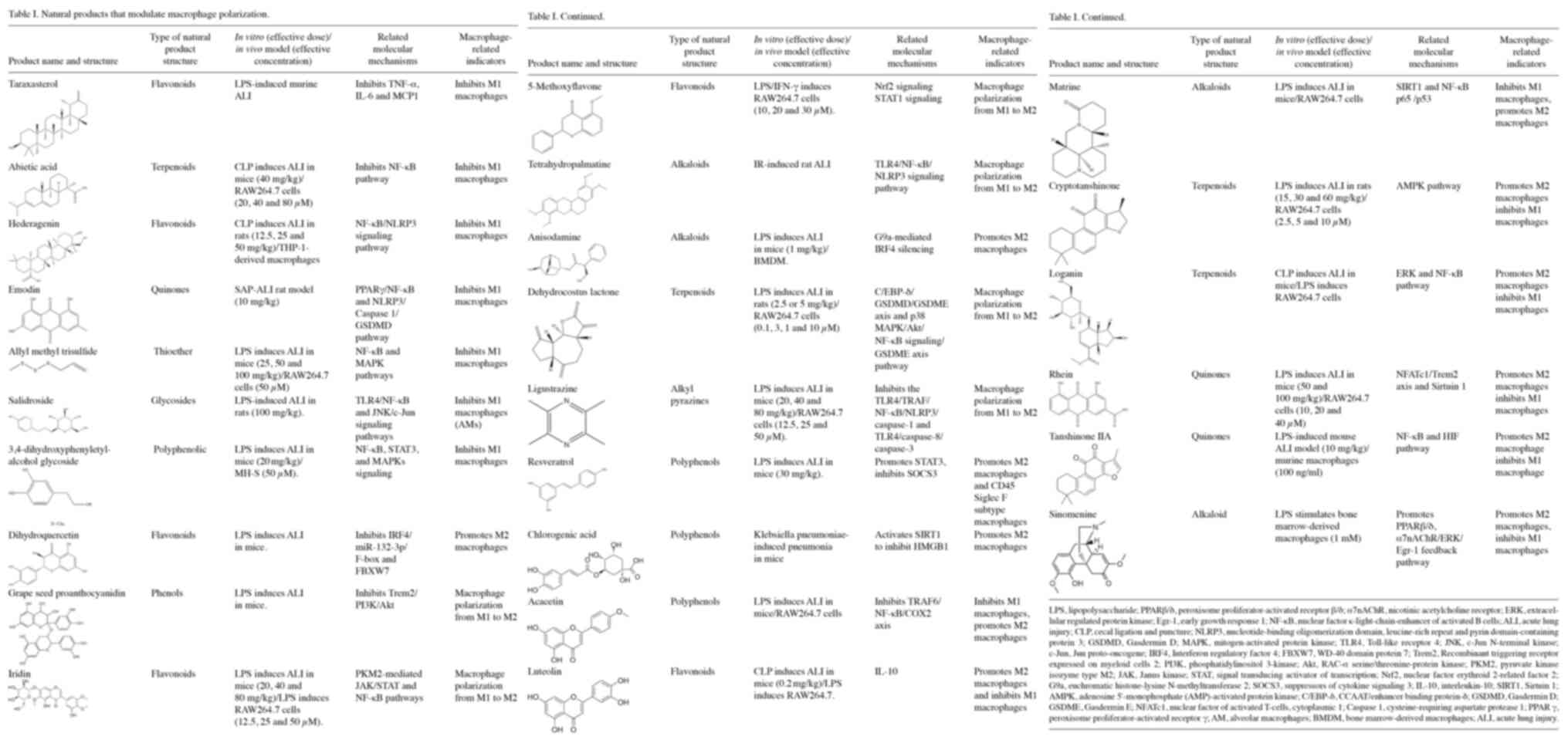
(27–29). The natural products that may be
used for the treatment of ALI/ARDS, which modulate macrophage
polarization, are summarized in Table
I and Fig. 1.
Certain natural products have been found to
attenuate ALI/ARDS by inhibiting the polarization of M1 macrophages
(28). 5-Methoxyflavone activates
nuclear factor erythroid 2-related factor 2 (Nrf2) signaling, which
reduces the activation of signal transducer and activator of
transcription (STAT)1 signaling, and blocks the LPS/IFN-γ-induced
M1 polarization and M2 repolarization with an M1 phenotype in
RAW264.7 cells; this protects RAW264.7 cells stimulated by LPS
(37). Taraxasterol protects
LPS-induced ALI in rats by decreasing the polarization of M1
macrophages and decreasing the levels of inflammatory cytokines
(38). Abietic acid improves
survival and attenuates sepsis-induced lung injury in mice; both
in vitro and in vivo studies have suggested that
abietic acid inhibits inflammation and M1 macrophage polarization,
and activates the NF-κB pathway (39). This may be the mechanism through
which abietic acid attenuates sepsis-induced lung injury (39). Hederagenin exerts anti-inflammatory
effects in ALI by inhibiting the NF-κB signaling pathway in
vivo and in vitro, thereby inhibiting M1 macrophage
polarization (40). Salidroside
can reduce the expression of the inflammatory cytokines,
high-mobility group box 1 (HMGB1) and keratin 14, and has a
significant therapeutic effect on the ALI/ARDS rat model (41). In addition, it is involved in the
regulation of LPS-induced alveolar macrophage (AM) inflammatory
activation by AECs (42). A
further study found that salidroside attenuated lung inflammation
by inhibiting M1 polarization of JNK/c-Jun-attenuated AMs (43). In LPS-induced ALI/ARDS, Rhein
significantly attenuated tissue inflammatory responses and promoted
macrophage M2 polarization shift (44). In vitro, Rhein
(4,5-dihydroxy-anthraquinone-2-carboxylic acid) reduced
intracellular ROS levels and activated P65, thereby attenuating M1
polarization in macrophages (44).
Mechanistically, Rhein exerted its protective effect against
LPS-induced ALI/ARDS by targeting the nuclear factor of activated T
cells 1 (NFATc1)/triggering receptor expressed on myeloid cells 2
(Trem2) axis, which was significantly attenuated in both Trem2 and
NFATc1 blockade assays (44).
Rhein intervenes in the metabolic reprogramming of macrophages in
inflammatory states via Sirtuin 1 (SIRT1), inhibits macrophage
activation into pro-inflammatory M1 macrophages, and attenuates LPS
damage to mouse lungs and RAW264.7 cells (45). Emodin (anthraquinone compound)
demonstrates its therapeutic effects on severe acute pancreatitis
(SAP)-ALI by modulating the nucleotide binding oligomerization
domain containing-leucine-rich repeat and pyrin domain-containing
protein 3 (NLRP3)/caspase 1/gasdermin D (GSDMD) signaling pathway
(46). Emodin reduces the
production of deleterious pancreatic exosomes under SAP conditions
and alters the levels of these pathological exosomes, thereby
inhibiting M1 polarization in AMs and cytokine release in the lungs
by modulating the peroxisome proliferator-activated receptor γ
(PPARγ)/NF-κB pathway (47). In
vitro experiments have confirmed that allyl methyl trisulfide
inhibits the NF-κB and MAPK pathways, reduces the expression of
cyclooxygenase-2 (COX-2) and inducible NO synthase proteins,
inhibits M1 polarization in macrophages, and reduces the
inflammatory response in LPS-induced ALI (48). 3,4-dihydroxyphenylethyl alcohol
glycoside inhibits the activation of NF-κB, STAT3 and p38 MAPK
signaling pathways, reduces the polarization of M1 macrophages, and
ameliorates CLP-induced inflammation in mice ALI and LPS-induced
MH-S cells (49).
In the pathological process of advanced pneumonia,
the causative factor is eliminated and M1 macrophages are
transformed into M2 macrophages, which are activated by Th2
cytokines (such as IL-4 and IL-13) as well as anti-inflammatory
cytokines (such as IL-10) and TGF-β (28,50,51).
Activated M2 macrophages express low IL-12 and high IL-1 receptor
antagonists, chemokine CCL18 and arginase 1 (Arg-1), and are
present in inflammatory zone 1 (31). M2 macrophages promote the repair of
lung damage by releasing anti-inflammatory cytokines, inhibiting
the production of pro-inflammatory mediators, and removing
apoptotic neutrophils from the site of inflammation (52). By promoting the activation of M2
macrophages, inflammation can be eliminated, and ALI recovery can
be favored (53–55).
A previous study has confirmed that dihydroquercetin
alleviates LPS-induced ALI inflammation and apoptosis (56). Dihydroquercetin promotes macrophage
M2 polarization through the interferon regulatory factor 4
(IRF4)/miR-132-3p/F-box and WD repeat domain containing 7 axis,
inhibits a rise in inflammatory cytokine levels and attenuates
LPS-induced lung injury (57).
Anisodamine treatment was also found to attenuate LPS-induced lung
injury and pulmonary edema by reversing LPS-induced changes in M1
and M2 polarization through the inhibition of G9a-mediated IRF4
silencing in an ALI mouse model (58). In vivo studies have
confirmed that resveratrol can reduce the severity of ALI in animal
models, while reducing the production of pro-inflammatory cytokines
and increasing anti-inflammatory cytokines (59). Resveratrol is a specific SIRT1
activator, and SIRT1 knockout reduces the anti-inflammatory effects
of resveratrol (60). In addition,
resveratrol may inhibit inflammation by inducing macrophage
pyroptosis and apoptosis (61).
Resveratrol significantly regulates macrophage activation and
polarization by modulating STAT3/suppressor of cytokine signaling 3
(SOCS3) signaling, and it enhances the polarization of
anti-inflammatory M2 and CD45+Siglec-F(−) subtype macrophages,
thereby inhibiting mouse ALI (62). A study has shown that the
activation of SIRT1 by chlorogenic acid can inhibit the acetylation
and nuclear translocation of HMGB1, thereby promoting M2
polarization in AMs and alleviating Kp-induced pneumonia (63). Acacetin can reduce LPS damage to
RAW 264.7 cells (64) and has been
found to significantly improve the survival of ALI mice. It
alleviates lung damage by reducing M1 macrophages and promoting the
polarization of M2 macrophages on the tumor necrosis factor
receptor-associated factor 6/NF-κB/COX-2 axis, thereby inhibiting
the production of TNF-α, IL-1β and IL-6 (65). Grape seed proanthocyanidin promotes
LPS-induced polarization from M1 to M2a in primary mouse lung
macrophages by inhibiting the triggering receptor expressed on
myeloid cells 2/PI3K/Akt pathway (66). Tetrahydropalmatine induces the
polarization of M1 macrophages to M2 and suppresses inflammation by
inhibiting Toll-like receptor 4 (TLR4)/NF-κB/NLRP3 signaling,
thereby attenuating ischemia-reperfusion-induced lung injury in
rats (67). Dehydrocostus lactone
can promote the polarization of M1 macrophages to the M2 phenotype
by inhibiting p38 MAPK/NF-κB signaling and activating the AMPK/Nrf2
pathway (68). Ligustrazine can
treat ALI/ARDS by inhibiting the TLR4/TRAF/NF-κB/NLRP3/caspase-1
and TLR4/caspase-8/caspase-3 signaling pathways in macrophages,
promoting macrophage polarization from M1 to M2 in macrophages, and
reducing the pyroptosis of macrophages (54).
In addition, some natural products reduce ALI by
decreasing M1 macrophages and increasing M2 macrophages. Luteolin
has a protective effect on cecal ligation puncture (CLP)-induced
mouse ALI models and LPS-induced cell models by decreasing
cytokines and IL-17A, increasing IL-10 levels, reducing M1
macrophage content, and increasing M2 macrophage number (69). Matrine restored sepsis-induced
SIRT1 downregulation, and the deacetylation of the NF-κB p65
subunit and p53, thereby inactivating the NF-κB pathway and
inhibiting the p53-induced pro-apoptotic pathway in septic lungs
(70). It inhibited infiltration
by M1 macrophages but increased infiltration by M2 macrophages,
thus decreasing the M1 to M2 macrophage ratio in septic lungs
(70). Cryptotanshinone inhibited
the accumulation of M1 macrophages and increased the accumulation
of M2 macrophages in lung tissue (71). A previous study has also suggested
that cryptotanshinone regulates the reprogramming of macrophage
metabolism by activating AMPK (72). Loganin blocks the ERK and NF-κB
pathways to inhibit M1 macrophages, induce M2 activation and
inhibit NLRP3 inflammasome-mediated caspase-1 activation by
decreasing IL-1β secretion in sepsis-induced ALI (72). Tanshinone IIA has significant
anti-inflammatory effects in LPS-stimulated RAW264.7 cell models.
It exerts these effects by inhibiting the NLRP3 inflammasome and
reducing oxidative stress (73).
In vitro assays have also confirmed that tanshinone IIA
inhibits the activation of NF-κB and hypoxia-inducible factor
pathways, thereby increasing the relative amount of the M2 isoform
and decreasing the relative amount of the M1 isoform (73). Iridin reduces glycolysis in
LPS-activated macrophages by inhibiting pyruvate kinase M2
(PKM2)-mediated JAK/STAT and NF-κB pathways, reprogramming
macrophages from the M1 polarized phenotype to the M2 phenotype and
inhibiting the production of pro-inflammatory cytokines (74). Sinomenine reduces TNF-α and IL-6 in
LPS-induced bone marrow-derived macrophages (BMDMs) by activating
peroxisome proliferator activated receptor β/δ in macrophages
(75). In addition, sinomenine
inhibits macrophage migration by downregulating Src/FAK/P130Cas
activation (76) and inhibits
LPS-induced macrophage inflammatory responses by acting on the α7
nicotinic acetylcholine receptor (α7nAChR) (77). A further study found that
sinomenine downregulates abnormally high levels of α7nAChR through
the α7nAChR/ERK/early growth response 1 feedback pathway,
attenuates the M1 phenotype and promotes the M2 phenotype in
LPS-stimulated macrophages (78).
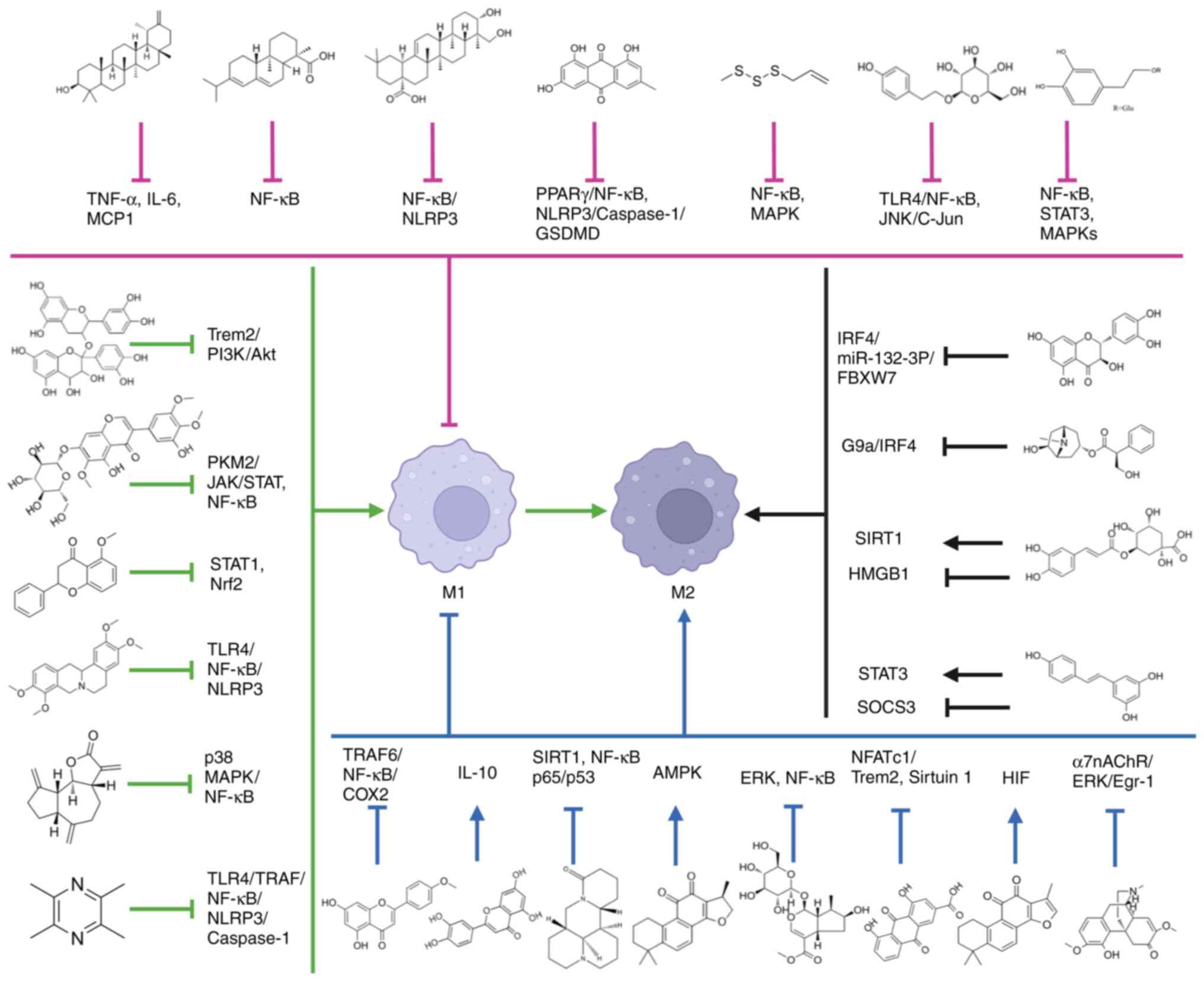
In conclusion, in terms of the mechanism of natural
products on cell polarization, natural products mainly inhibit the
polarization of pro-inflammatory M1 macrophages and promote the
transition from M1 to M2 by inhibiting NF-κB, NLRP3, Caspase-1,
GSDMD and other signaling pathways, and promoting Nrf2 and AMPK
signaling pathways. In addition, it promotes the polarization of
anti-inflammatory M2 macrophages by inhibiting STAT1 and SOCS3, and
promoting the STAT3 and HIF signaling pathways. In terms of the
effects of different types of natural products on macrophage
polarization, flavonoids, alkaloids, terpenoids and quinones affect
macrophage polarization by inhibiting NF-κB and NLRP3, and
activating major signaling pathways such as AMPK.
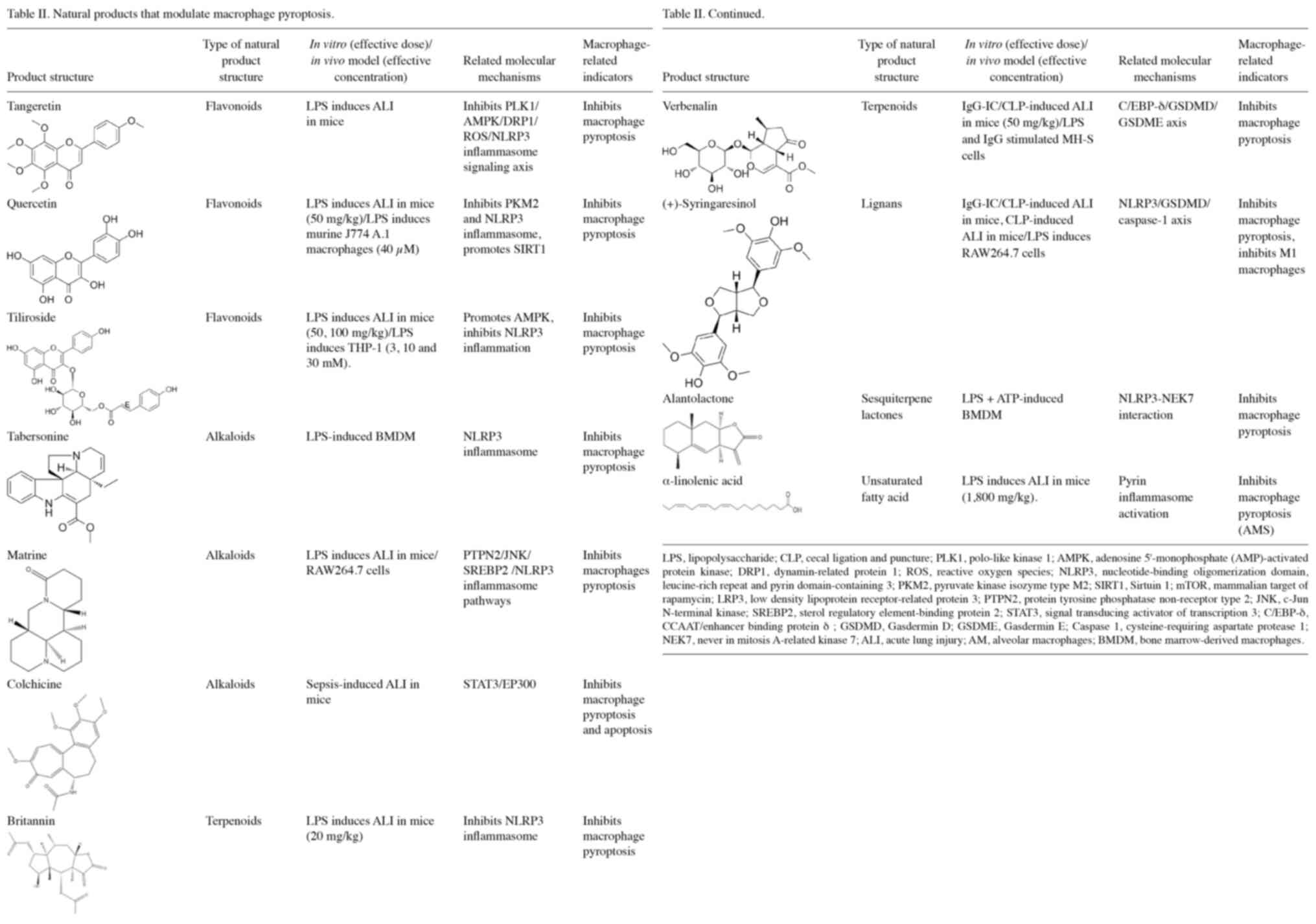
Tangeretin attenuates acute lung injury in sepsis
mice by modulating the PLK1/AMPK/DRP1 signaling axis by inhibiting
ROS-mediated NLRP3 inflammasome activation and reducing pyroptosis
of macrophages (87). Matrine
inhibits NLRP3 inflammasome activation by modulating the protein
tyrosine phosphatase non-receptor type 2/JNK/sterol regulatory
element-binding protein 2 pathway, reduces macrophage pyroptosis
and decreases the CLP-induced invasion of ALI- and LPS-stimulated
macrophages in mice (88).
Inhibition of STAT3 phosphorylation by colchicine inhibits the
acetylation of the NLRP3 promoter by the STAT3/E1A binding protein
p300 (EP300) complex, reducing pyroptosis and apoptosis in mouse
alveolar macrophages, thereby attenuating sepsis-induced ALI
(89). Verbenalin attenuates acute
lung inflammation induced by pseudomonas aeruginosa by acting on
the G protein-coupled receptor 18 receptor (90). Further studies found that
verbenalin inhibits macrophage focal death and alleviates sepsis
and IgG immune complex-induced ALI by inhibiting the C/enhancer
binding protein δ (EBP-δ)/GSDMD/GSDME axis (91). Quercetin inhibits the nuclear
accumulation of PKM2, upregulates SIRT1, inhibits the activation of
NLRP3 inflammasomes and reduces the release of pyroptosis-related
cytokines (IL-1β, IL-18 and HMGB1) in macrophages (92). Tiliroside targets the AMPK pathway,
ameliorates mitochondrial damage, attenuates NLRP3 inflammasome
activation, reduces pyroptosis in macrophages and ameliorates
LPS-induced ALI in mice (93).
Tabersonine is a natural NLRP3 inhibitor that inhibits inflammasome
activation in macrophages and attenuates NLRP3-driven ALI in mice
(94). Britannin specifically
inhibits the NLRP3 inflammasome activation step in BMDMs and binds
directly to the NLRP3 NACHT domain at Arg-335 and Gly271 (95). In addition, it inhibits NLRP3
activation in an ATPase-independent manner, inhibits the cleavage
of caspase-1 and the secretion of mature IL-1β, and inhibits
NLRP3-mediated pyroptosis in mouse and human macrophages (95). Taraxasterol inhibits NLRP3
inflammatory vesicle activation and pyroptosis in macrophages by
modulating the mTOR signaling pathway, and is protective against
LPS-induced BMDMs in mice (96).
(+)-Syringaresinol activates PPARγ, thereby inhibiting the
expression of NF-κB and C/EBP and reducing the inflammatory
response (97). By targeting the
NLRP3/GSDMD/caspase-1 axis, it suppressed macrophage pyroptosis and
effectively alleviated IgG-IC-induced ALI (98). Alantolactone inhibits the
activation and assembly of NLRP3 inflammasomes, LPS-ATP-induced
IL-1β secretion and caspase-1 activation in macrophages by binding
directly to the NACHT domain of NLRP; it also reduces macrophage
pyroptosis (99). α-linolenic acid
can alleviate NET-induced AM pyroptosis and ALI/ARDS by mediating
pyrin inflammasome activation (100).
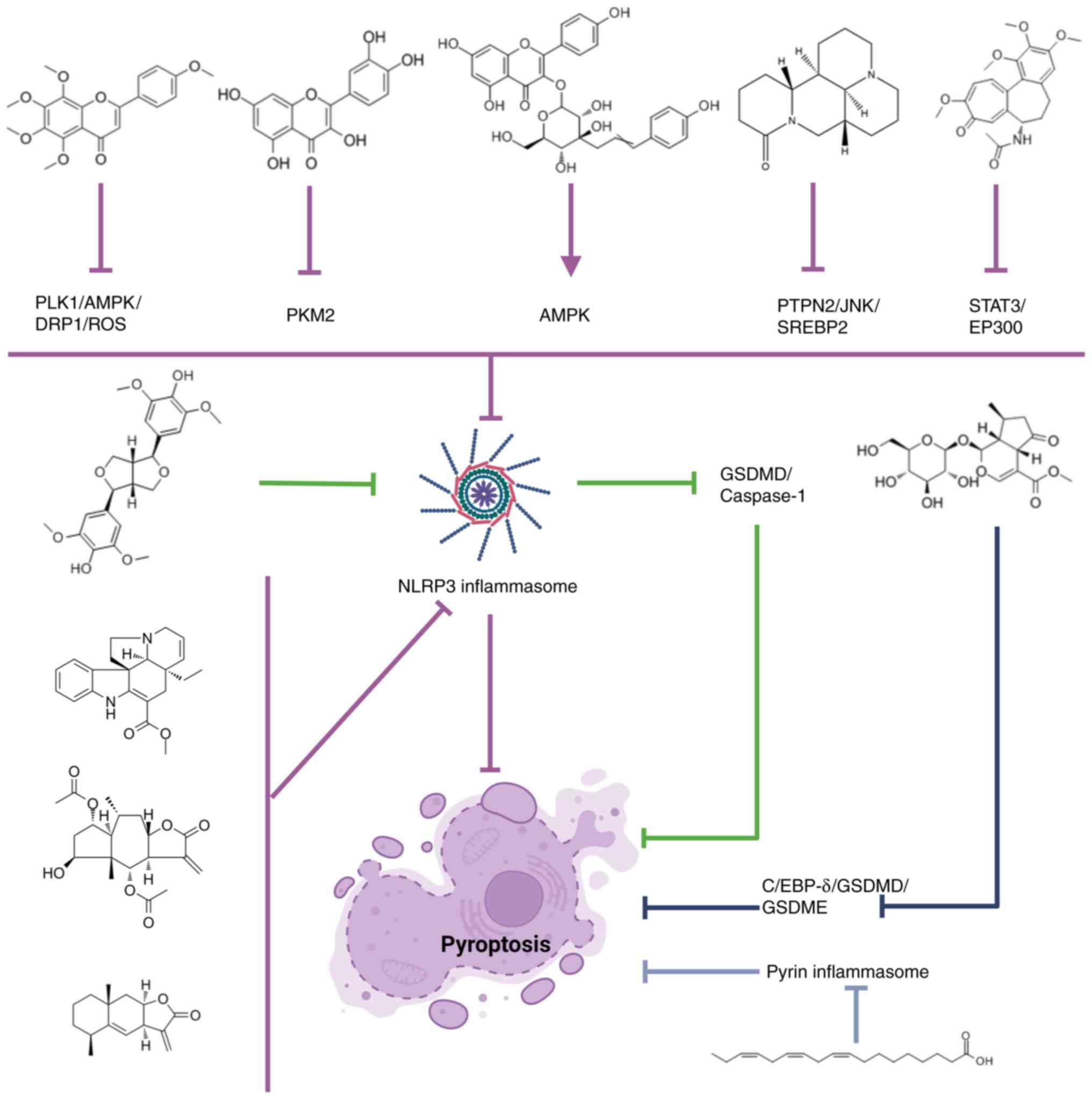
In conclusion, both flavonoids and terpenoids
(except Verbenalin) inhibit macrophage autophagy through NLRP3
inflammasomes associated with cellular pyroptosis, and play a role
in the treatment of ALI/ARDS.
Macrophages are widely recognized as one of the main
phagocytes that eliminate apoptotic cells (101). The exposed phosphatidylserine on
the surface of apoptotic cells can be recognized and cleared by
macrophages, which are subsequently activated to exert
anti-inflammatory and immune responses (102,103). However, the HMGB1 protein binds
to the receptor for advanced glycation end-products and αVβ3 on
macrophages, and inhibits endocytosis (104). A study has shown that in patients
with sepsis-associated ARDS, endocytosis by AMs is impaired,
leading to the accumulation of apoptotic neutrophils, which can
lead to long-term inflammation (105). Therefore, increasing endocytosis
by macrophages can improve ALI/ARDS. Glucocorticoids upregulate
endocytosis by macrophages through the type 1 isozyme of
3β-hydroxysteroid dehydrogenase (HSD-1), and HSD-1 deletion leads
to impaired endocytosis by AMs, resulting in their inability to
eliminate apoptotic neutrophils in model animals (106). AMs in the regression phase of ALI
increased pinocytosis by upregulating integrin αv through vascular
endothelial growth factor (VEGF)-C/VEGFR-3 signaling, thereby
digesting the majority of exogenous apoptotic neutrophils and
improving LPS-induced ALI (107).
In addition, phagocytosis of apoptotic neutrophils by AMs is
eliminated by reducing the expression of Gas6 following STAT6
(108). Macrophages have
anti-inflammatory effects after engulfing human umbilical cord
mesenchymal stem cell-derived apoptotic bodies (ABs). This is
achieved by ABs expressing programmed death-ligand 1, which binds
to PD1 on macrophages, affecting metabolic programming in
macrophages and promoting their transition to an anti-inflammatory
state (109). A Rab43 knockout
study showed that the HMGB1 protein inhibits the phagocytosis of
apoptotic cells by macrophages through inhibiting the transport of
Rab43-controlled CD91 (a key receptor for macrophage pinocytosis)
to the cell surface, which aggravates ALI/ARDS (110). Phagocytosis by macrophages is an
important way to eliminate apoptotic neutrophils. This can reduce
the release of harmful substances such as NETs, MPO and cytokines
by neutrophils following, apoptosis and can effectively alleviate
ALI/ARDS (111). The natural
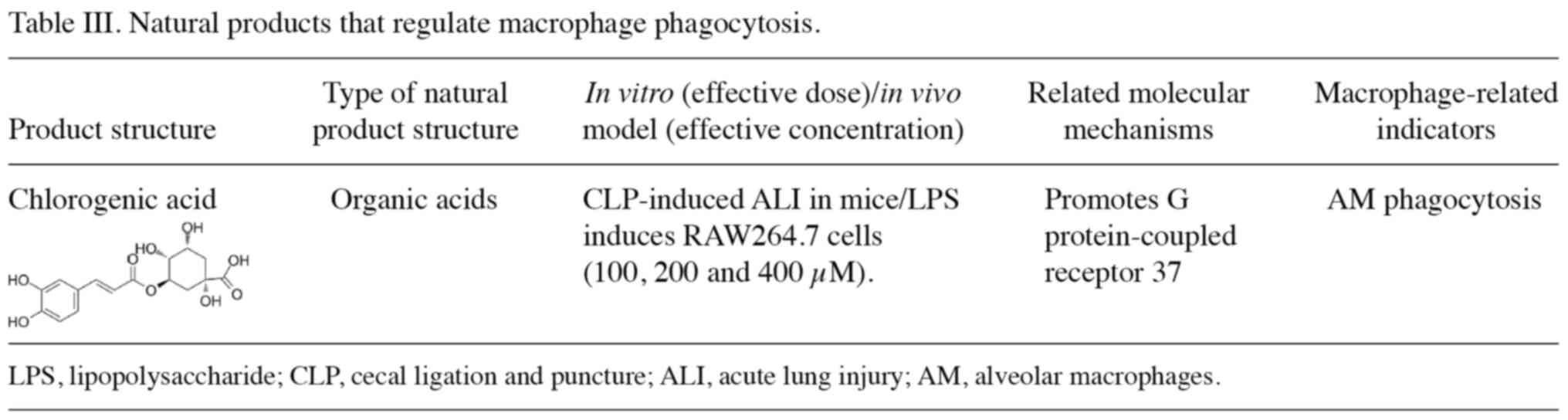
products that treat ALI/ARDS by regulating macrophage phagocytosis
are summarized in Table III. An
in vivo study has shown that chlorogenic acid significantly
improves the lipopolysaccharide-induced inflammatory response and
survival in CLP-induced ARDS mice by increasing phagocytosis by AMs
(112). In addition, chlorogenic
acid significantly upregulates the expression of GPR37 in
vivo and in vitro. In addition, the protective effect of
chlorogenic acid on ARDS was reversed after silencing GPR37
expression (112).
Autophagy is a highly conserved protein degradation
process involved in the degradation of protein cell components,
such as lipoproteins and misfolded proteins (113). Cellular autophagy includes
macroautophagy, microautophagy and chaperone-mediated autophagy
(114). In general, autophagy has
two functions, one of which is an early adaptive mechanism of the
tissue, specifically the removal of organelles or proteins to
maintain intracellular homeostasis (115). As an important component of
innate immunity, macrophages play an important role in regulating
the inflammatory response and the balance of the immune system
(116). Autophagy also has an
impact on ALI/ARDS. Studies have found that autophagy can regulate
macrophage phagocytosis, antigen presentation and polarization.
Furthermore, macrophage autophagy has a negative and positive role
in the progression of ALI/ARDS (117). On the one hand, macrophage
autophagy reduces the release of inflammatory cytokines and removes
cellular debris, thereby attenuating lung injury and providing a
protective effect (118). In a
mouse model that lacked autophagy, excessive lung inflammation and
injury occurred in the lungs of the mice, and treatment with an
autophagy inducer activated the autophagy pathway in macrophages,
resulting in a significant reduction in lung inflammation and
injury (119). On the other hand,
autophagy can exacerbate the damage and cause apoptosis, which can
aggravate lung injury (117).
Autoapoptosis, caused by increased autophagy of AMs, is one of the
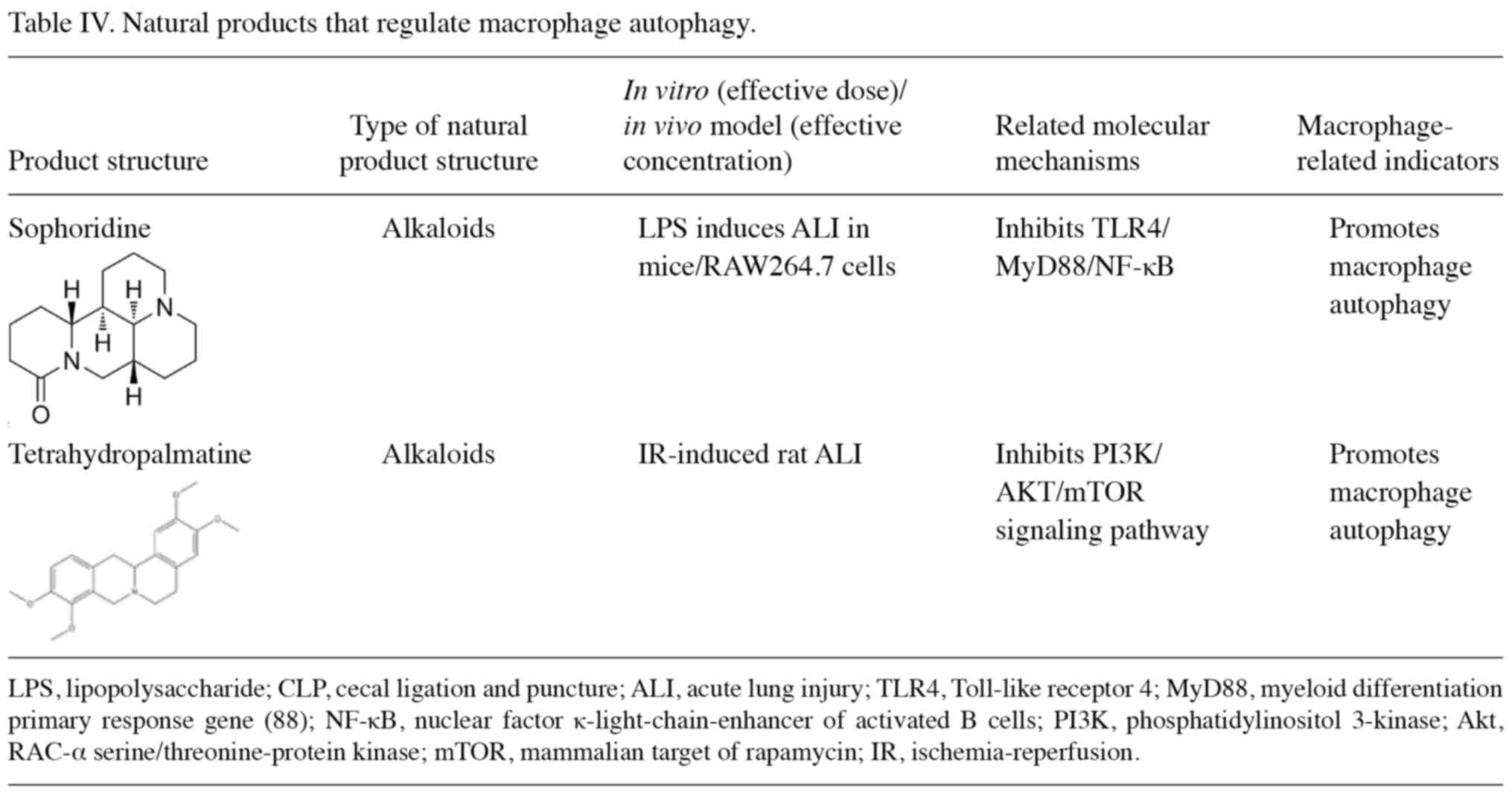
causes of LPS-induced lung injury in rats (120). The natural products that treat
ALI/ARDS by regulating macrophage autophagy are summarized in
Table IV. Sophoridine decreases
the mRNA and protein expression of TLR4/myeloid differentiation
primary response 88 (MyD88)/NF-κB and mTOR, enhances macrophage
autophagy and reduces inflammation, thereby inhibiting LPS-induced
ALI (121). Tetrahydropalmatine
attenuates limb ischemia-reperfusion-induced ALI in rats by
restoring autophagy mediated by the PI3K/AKT/mTOR pathway (122).
In conclusion, alkaloids play a role in the
prevention and treatment of ALI/ARDS by inhibiting the
TLR4/MyD88/NF-κB and PI3K/AKT/mTOR signaling pathways and
increasing macrophage autophagy.
Macrophages play an important role in ALI/ARDS, and
the regulation of macrophages may be an important means of
intervention in ALI/ARDS (123).
The present review summarizes and classifies the natural products
that may be used for the treatment of ALI/ARDS discovered within
the previous five years. These agents act by regulating macrophage
abnormalities. Their main molecular mechanisms of action are
summarized in Figs. 1 and 2. These products include flavonoids,
alkaloids, terpenoids and quinones. The majority of these products
work by regulating the polarization of macrophages. Their
mechanisms of action include reducing M1 pro-inflammatory phenotype
macrophages and increasing M2 anti-inflammatory phenotype
macrophages to achieve anti-inflammatory effects. Metabolic
programming is another factor that influences macrophage
polarization, and interfering with macrophage metabolism also
affects ALI/ARDS. M1 and M2 macrophages require different sources
of energy for proliferation and cytokine production, with M1
macrophages highly dependent on aerobic glycolysis for energy,
while M2 macrophages rely on mitochondrial oxidative
phosphorylation and fatty acid oxidation for energy (124). Therefore, ALI can be prevented
and treated by modulating metabolic programs to control macrophage
polarization. Feng et al (125) found that M2-like
immunophenotyping and metabolic reprogramming can be maintained by
modulating high Ca2+ reactivity and long-term calcium
signaling. Reprogramming glucose metabolism by activating the
mTOR/HIF-1α/glycolytic pathway in macrophages activated by Trem-1
attenuates the inflammatory response in ALI (126,127). The inhibition of macrophage
pyroptosis is also an important method for natural compounds to
affect ALI/ARDS. In addition, some natural products also protect
against alveolar damage by reducing macrophage autophagy and
apoptosis, thereby protecting lung function and reducing pulmonary
edema. Some natural products can alleviate ALI/ARDS by enhancing
phagocytosis by macrophages.
In conclusion, different compounds may interfere
with the development of ALI/ARDS by regulating polarization,
pyroptosis, autophagy and phagocytosis of lung macrophages through
the same or similar pathways or targets. Macrophage polarization
often involves the NF-κB and AMPK pathways (Fig. 1), while pyroptosis mainly involves
the NLRP3 inflammasome (Fig. 2).
In addition, macrophage autophagy is associated with the
TLR4/MyD88/NF-κB and PI3K/AKT/mTOR signaling pathways, whereas AM
phagocytosis is associated with G-protein coupled receptor 37
enhancement (128). In addition,
the main types of natural products that affect macrophage
polarization are flavonoids, alkaloids, terpenoids and quinones,
while the types of natural products that affect macrophage
pyroptosis are flavonoids, alkaloids and terpenoids; at the same
time, alkaloids can also increase macrophage autophagy. Notably,
these natural products may all regulate both macrophage
polarization and pyroptosis by affecting NF-κB, AMPK and NLRP3
inflammasomes. AMs are the first immune defenders against pathogens
and foreign particles (129),
accounting for ~95% of leukocytes in the lungs (130). AMs have a significant impact on
the development of ALI after both infectious and non-infectious
stimuli (10). Therefore,
developing AM-specific drugs may be an important measure to target
macrophages for the treatment of ALI/ARDS. However, most of the
existing research results come from animal and cell experiments,
and targeted therapy based on lung macrophages and macrophage-based
treatment of ALI are still undergoing preclinical research. The
clinical application of these findings remains a huge
challenge.
At present, numerous studies of macrophages are
systematic, and there are no further studies on the role of
different types of macrophages in ALI/ARDS. However, lung
macrophages are products of different macrophages, which may differ
in function. Secondly, it has been reported that M1 and M2
phenotype changes may be associated with changes in different
subgroups of cells during ARDS, and simply focusing on the M1 and
M2 phenotypes cannot describe the multidimensional, complex and
dynamic changes in macrophages in detail (9). The majority of current studies
investigated the effect of macrophage polarization on ALI through
cell labeling using molecules that were specific to the surface of
M1 and M2 cells. Therefore, in the future, it is necessary to
further explore the heterogeneity of macrophages with the help of
advanced technologies such as single-cell RNA sequencing, assay for
transposase-accessible chromatin with high throughput sequencing
and mass spectrometry. This will improve the understanding of the
role of different macrophages in the pathogenesis of ALI/ARDS,
clarify the molecular mechanism of natural products that target and
regulate macrophages during the treatment of ALI/ARDS, and provide
new ideas and further research directions for the development of
new drugs for the treatment of ALI/ARDS.
Not applicable.
This work was financially supported by the Yunnan Fundamental
Research Projects (grant nos. 202201AU070167 and 202301AT070258),
and the Yunnan Key Laboratory of Formulated Granules (grant no.
202105AG070014).
Not applicable.
JL and WM conceived and designed the study; ZT, YL
and RZ collected and organized data; YX wrote, reviewed and edited
the manuscript; GL conceptualized and supervised the study, and
performed project administration and funding acquisition. All
authors read and approved the final version of the manuscript. Data
authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Zhu W, Zhang Y and Wang Y: Immunotherapy
strategies and prospects for acute lung injury: Focus on immune
cells and cytokines. Front Pharmacol. 13:11033092022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bellani G, Laffey JG, Pham T, Fan E,
Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley
DF, et al: Epidemiology, patterns of care, and mortality for
patients with acute respiratory distress syndrome in intensive care
units in 50 countries. JAMA. 315:788–800. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hsieh PC, Wu YK, Yang MC, Su WL, Kuo CY
and Lan CC: Deciphering the role of damage-associated molecular
patterns and inflammatory responses in acute lung injury. Life Sci.
305:1207822022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mokrá D: Acute lung injury-from
pathophysiology to treatment. Physiol Res. 69:S353–S366.
2020.PubMed/NCBI
|
|
5
|
Mokra D, Mikolka P, Kosutova P and Mokry
J: Corticosteroids in acute lung injury: The dilemma continues. Int
J Mol Sci. 20:47652019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang F, Chen M, Ma J, Wang C, Wang J, Xia
H, Zhang D and Yao S: Integrating bulk and single-cell sequencing
reveals the phenotype-associated cell subpopulations in
sepsis-induced acute lung injury. Front Immunol. 13:9817842022.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lendeckel U, Venz S and Wolke C:
Macrophages: Shapes and functions. ChemTexts. 8:122022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Johnston LK, Rims CR, Gill SE, McGuire JK
and Manicone AM: Pulmonary macrophage subpopulations in the
induction and resolution of acute lung injury. Am J Respir Cell Mol
Biol. 47:417–426. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dang W, Tao Y, Xu X, Zhao H, Zou L and Li
Y: The role of lung macrophages in acute respiratory distress
syndrome. Inflamm Res. 71:1417–1432. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Z and Wang Z: The role of macrophages
polarization in sepsis-induced acute lung injury. Front Immunol.
14:12094382023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng P, Li S and Chen H: Macrophages in
lung injury, repair, and fibrosis. Cells. 10:4362021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aribindi K, Lim M, Lakshminrusimha S and
Albertson T: Investigational pharmacological agents for the
treatment of ARDS. Expert Opin Investig Drugs. 33:243–277. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vichare R and Janjic JM:
Macrophage-targeted nanomedicines for ARDS/ALI: Promise and
potential. Inflammation. 45:2124–2141. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Booz GW, Altara R, Eid AH, Wehbe Z, Fares
S, Zaraket H, Habeichi NJ and Zouein FA: Macrophage responses
associated with COVID-19: A pharmacological perspective. Eur J.
887:1735472020.PubMed/NCBI
|
|
15
|
Panahi Y, Gorabi AM, Talaei S, Beiraghdar
F, Akbarzadeh A, Tarhriz V and Mellatyar H: An overview on the
treatments and prevention against COVID-19. Virol J. 20:232023.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matera MG, Rogliani P, Bianco A and
Cazzola M: Pharmacological management of adult patients with acute
respiratory distress syndrome. Expert Opin Pharmacother.
21:2169–2183. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lang FM, Lee KMC, Teijaro JR, Becher B and
Hamilton JA: GM-CSF-based treatments in COVID-19: Reconciling
opposing therapeutic approaches. Nat Rev Immunol. 20:507–514. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li S, Feng T, Zhang Y, Shi Q, Wang W, Ren
J, Shen G, Gu H, Luo C and Li Y: Lianhua Qingwen protects
LPS-induced acute lung injury by promoting M2 macrophage
infiltration. J Ethnopharmacol. 320:1174672024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liang X and Liu JX: Role of macrophage
polarization in pulmonary diseases and intervention of traditional
Chinese medicines. Zhongguo Zhong Yao Za Zhi. 49:334–343. 2024.(In
Chinese). PubMed/NCBI
|
|
20
|
Dong J, Liu W, Liu W, Wen Y, Liu Q, Wang
H, Xiang G, Liu Y and Hao H: Acute lung injury: A view from the
perspective of necroptosis. Inflamm Res. 73:997–1018. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qin Y, Li W, Liu J, Wang F, Zhou W, Xiao
L, Zhou P, Wu F, Chen X, Xu S, et al: Andrographolide ameliorates
sepsis-induced acute lung injury by promoting autophagy in alveolar
macrophages via the RAGE/PI3K/AKT/mTOR pathway. Int
Immunopharmacol. 139:1127192024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma J, Wang J, Wang J, Zhou J, Jiang C,
Chen W, Zhang X, Pan J, Zhu J and Chen M: Araloside A alleviates
sepsis-induced acute lung injury via PHD2/HIF-1α in macrophages.
Phytomedicine. 135:1560892024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang WT, Zhang YY, Li ZR, Li JM, Deng HS,
Li YY, Yang HY, Lau CC, Yao YJ, Pan HD, et al: Syringic acid
attenuates acute lung injury by modulating macrophage polarization
in LPS-induced mice. Phytomedicine. 129:1555912024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Helou DG, Quach C, Hurrell BP, Li X, Li M,
Akbari A, Shen S, Shafiei-Jahani P and Akbari O: LAIR-1 limits
macrophage activation in acute inflammatory lung injury. Mucosal
Immunol. 16:788–800. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Short KR, Kroeze EJBV, Fouchier RAM and
Kuiken T: Pathogenesis of influenza-induced acute respiratory
distress syndrome. Lancet Infect Dis. 14:57–69. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luo M, Zhao F, Cheng H, Su M and Wang Y:
Macrophage polarization: An important role in inflammatory
diseases. Front Immunol. 15:13529462024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu C, Xiao K and Xie L: Advances in the
regulation of macrophage polarization by mesenchymal stem cells and
implications for ALI/ARDS treatment. Front Immunol. 13:9281342022.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen X, Tang J, Shuai W, Meng J, Feng J
and Han Z: Macrophage polarization and its role in the pathogenesis
of acute lung injury/acute respiratory distress syndrome. Inflamm
Res. 69:883–895. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang L, Wang D, Zhang T, Ma Y, Tong X and
Fan H: The role of immunometabolism in macrophage polarization and
its impact on acute lung injury/acute respiratory distress
syndrome. Front Immunol. 14:11175482023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Murray PJ, Allen JE, Biswas SK, Fisher EA,
Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence
T, et al: Macrophage activation and polarization: Nomenclature and
experimental guidelines. Immunity. 41:14–20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Aggarwal NR, King LS and D'alessio FR:
Diverse macrophage populations mediate acute lung inflammation and
resolution. Am J Physiol Lung Cell Mol Physiol. 306:L709–L725.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fukui S, Iwamoto N, Takatani A, Igawa T,
Shimizu T, Umeda M, Nishino A, Horai Y, Hirai Y, Koga T, et al: M1
and M2 monocytes in rheumatoid arthritis: A contribution of
imbalance of M1/M2 monocytes to osteoclastogenesis. Front Immunol.
8:19582017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shi C and Pamer EG: Monocyte recruitment
during infection and inflammation. Nat Rev Immunol. 11:762–774.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang J, Li Q, Qiu Y and Lu H: COVID-19:
Imbalanced cell-mediated immune response drives to immunopathology.
Emerg Microbes Infect. 11:2393–2404. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jiao Y, Zhang T, Zhang C, Ji H, Tong X,
Xia R, Wang W, Ma Z and Shi X: Exosomal miR-30d-5p of neutrophils
induces M1 macrophage polarization and primes macrophage pyroptosis
in sepsis-related acute lung injury. Crit Care. 25:3562021.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li Z, Pan H, Yang J, Chen D, Wang Y, Zhang
H and Cheng Y: Xuanfei Baidu formula alleviates impaired
mitochondrial dynamics and activated NLRP3 inflammasome by
repressing NF-κB and MAPK pathways in LPS-induced ALI and
inflammation models. Phytomedicine. 108:1545452023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liang P, Wang L, Yang S, Pan X, Li J,
Zhang Y, Liang Y, Li J and Zhou B: 5-Methoxyflavone alleviates
LPS-mediated lung injury by promoting Nrf2-mediated the suppression
of NOX4/TLR4 axis in bronchial epithelial cells and M1 polarization
in macrophages. J Inflamm (Lond). 19:242022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bu C, Wang R, Wang Y, Lu B, He S and Zhao
X: Taraxasterol inhibits hyperactivation of macrophages to
alleviate the sepsis-induced inflammatory response of ARDS rats.
Cell Biochem Biophys. 80:763–770. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fang H, Chen J, Luo J, Hu J, Wang D, Lv L
and Zhang W: Abietic acid attenuates sepsis-induced lung injury by
inhibiting nuclear factor kappa-light-chain-enhancer of activated B
cells (NF-κB) pathway to inhibit M1 macrophage polarization. Exp
Anim. 71:481–490. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang L and Zhao M: Suppression of NOD-like
receptor protein 3 inflammasome activation and macrophage M1
polarization by hederagenin contributes to attenuation of
sepsis-induced acute lung injury in rats. Bioengineered.
13:7262–7276. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zheng L, Su J, Zhang Z, Jiang L, Wei J, Xu
X and Lv S: Salidroside regulates inflammatory pathway of alveolar
macrophages by influencing the secretion of miRNA-146a exosomes by
lung epithelial cells. Sci Rep. 10:207502020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cai YC, Huang Q, Wei XL, Mei RH, Sa LN and
Hu XL: Effects of salidroside on the secretion of inflammatory
mediators induced by lipopolysaccharide in the co-culture of rat
alveolar macrophages and type II alveolar epithelial cells. Sheng
Li Xue Bao. 71:575–580. 2019.(In Chinese). PubMed/NCBI
|
|
43
|
Feng H, Zhang D, Yin Y, Kang J and Zheng
R: Salidroside ameliorated the pulmonary inflammation induced by
cigarette smoke via mitigating M1 macrophage polarization by
JNK/c-Jun. Phytother Res. 37:4251–4264. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li X, Xiao C, Yuan J, Chen X, Li Q and
Shen F: Rhein-attenuates LPS-induced acute lung injury via
targeting NFATc1/Trem2 axis. Inflamm Res. 72:1237–1255. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang W, Wang Z, Yang X, Song W, Chen P,
Gao Z, Wu J and Huang F: Rhein ameliorates septic lung injury and
intervenes in macrophage metabolic reprogramming in the
inflammatory state by sirtuin 1. Life Sci. 310:1211152022.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wu X, Yao J, Hu Q, Kang H, Miao Y, Zhu L,
Li C, Zhao X, Li J, Wan M and Tang W: Emodin ameliorates acute
pancreatitis-associated lung injury through inhibiting the alveolar
macrophages pyroptosis. Front Pharmacol. 13:8730532022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hu Q, Yao J, Wu X, Li J, Li G, Tang W, Liu
J and Wan M: Emodin attenuates severe acute pancreatitis-associated
acute lung injury by suppressing pancreatic exosome-mediated
alveolar macrophage activation. Acta Pharm Sin B. 12:3986–4003.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang S, Liu J, Dong J, Fan Z, Wang F, Wu
P, Li X, Kou R and Chen F: Allyl methyl trisulfide protected
against LPS-induced acute lung injury in mice via inhibition of the
NF-κB and MAPK pathways. Front Pharmacol. 13:9198982022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhuo Y, Li D, Cui L, Li C, Zhang S, Zhang
Q, Zhang L, Wang X and Yang L: Treatment with
3,4-dihydroxyphenylethyl alcohol glycoside ameliorates
sepsis-induced ALI in mice by reducing inflammation and regulating
M1 polarization. Biomed Pharmacother. 116:1090122019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mantovani A, Biswas SK, Galdiero MR, Sica
A and Locati M: Macrophage plasticity and polarization in tissue
repair and remodelling. J Pathol. 229:176–185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yadav S, Priya A, Borade DR and
Agrawal-Rajput R: Macrophage subsets and their role: Co-relation
with colony-stimulating factor-1 receptor and clinical relevance.
Immunol Res. 71:130–152. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shapouri-Moghaddam A, Mohammadian S,
Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi
A, Afshari JT and Sahebkar A: Macrophage plasticity, polarization,
and function in health and disease. J Cell Physiol. 233:6425–6440.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liang L, Xu W, Shen A, Fu X, Cen H, Wang
S, Lin Z, Zhang L, Lin F and Zhang X: Inhibition of YAP1 activity
ameliorates acute lung injury through promotion of M2 macrophage
polarization. MedComm (2020). 4:e2932023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jiang R, Xu J, Zhang Y, Zhu X, Liu J and
Tan Y: Ligustrazine alleviate acute lung injury through suppressing
pyroptosis and apoptosis of alveolar macrophages. Front Pharmacol.
12:6805122021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhou W, Hong J, Liu T, Li M, Jin H and
Wang X: Polygonatum polysaccharide regulates macrophage
polarization and improves LPS-Induced acute lung injury through
TLR4-MAPK/NF-κB pathway. Can Respir J. 2022:1–11. 2022. View Article : Google Scholar
|
|
56
|
Liu JH, Cao L, Zhang CH, Li C, Zhang ZH
and Wu Q: Dihydroquercetin attenuates lipopolysaccharide-induced
acute lung injury through modulating FOXO3-mediated NF-κB signaling
via miR-132-3p. Pulm Pharmacol Ther. 64:1019342020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li C, Liu J, Zhang C, Cao L, Zou F and
Zhang Z: Dihydroquercetin (DHQ) ameliorates LPS-induced acute lung
injury by regulating macrophage M2 polarization through
IRF4/miR-132-3p/FBXW7 axis. Pulm Pharmacol Ther. 83:1022492023.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhang Y, Song D, Peng Z, Wang R, Li K, Ren
H, Sun X, Du N and Tang SC: Anisodamine enhances macrophage M2
polarization through suppressing G9a-mediated interferon regulatory
factor 4 silencing to alleviate lipopolysaccharide-induced acute
lung injury. J Pharmacol Exp Ther. 381:247–256. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang Y, Wang X, Zhang L and Zhang R:
Alleviation of acute lung injury in rats with sepsis by resveratrol
via the phosphatidylinositol 3-Kinase/Nuclear factor-erythroid 2
related factor 2/Heme oxygenase-1 (PI3K/Nrf2/HO-1) pathway. Med Sci
Monit. 24:3604–3611. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhu X, Liu Q, Wang M, Liang M, Yang X, Xu
X, Zou H and Qiu J: Activation of Sirt1 by resveratrol inhibits
TNF-α induced inflammation in fibroblasts. PLoS One. 6:e270812011.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Misawa T, Saitoh T, Kozaki T, Park S,
Takahama M and Akira S: Resveratrol inhibits the acetylated
α-tubulin-mediated assembly of the NLRP3-inflammasome. Int Immunol.
27:425–434. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hu L, Chen Z, Li L, Jiang Z and Zhu L:
Resveratrol decreases CD45+CD206− subtype
macrophages in LPS-induced murine acute lung injury by SOCS3
signalling pathway. J Cell Mol Med. 23:8101–8113. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Li QR, Tan SR, Yang L, He W, Chen L, Shen
FX, Wang Z and Wang HF: Mechanism of chlorogenic acid in alveolar
macrophage polarization in Klebsiella pneumoniae-induced pneumonia.
J Leukoc Biol. 112:9–21. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Pan MH, Lai CS, Wang YJ and Ho CT:
Acacetin suppressed LPS-induced up-expression of iNOS and COX-2 in
murine macrophages and TPA-induced tumor promotion in mice. Biochem
Pharmacol. 72:1293–1303. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Chang B, Wang Z, Cheng H, Xu T, Chen J, Wu
W, Li Y and Zhang Y: Acacetin protects against sepsis-induced acute
lung injury by facilitating M2 macrophage polarization via
TRAF6/NF-κB/COX2 axis. Innate Immun. 30:11–20. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Qiao X, Wang H, He Y, Song D, Altawil A,
Wang Q and Yin Y: Grape seed proanthocyanidin ameliorates
LPS-induced acute lung injury by modulating M2a macrophage
polarization via the TREM2/PI3K/Akt pathway. Inflammation.
46:2147–2164. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wen H, Lu D, Chen H, Zhu Y, Xie Q, Zhang Z
and Wu Z: Tetrahydropalmatine induces the polarization of M1
macrophages to M2 to relieve limb ischemia-reperfusion-induced lung
injury via inhibiting the TLR4/NF-κB/NLRP3 signaling pathway. Drug
Dev Res. 83:1362–1372. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wu YX, Jiang FJ, Liu G, Wang YY, Gao ZQ,
Jin SH, Nie YJ, Chen D, Chen JL and Pang QF: Dehydrocostus lactone
attenuates methicillin-resistant staphylococcus aureus-induced
inflammation and acute lung injury via modulating macrophage
polarization. Int J Mol Sci. 22:97542021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Xie K, Chai YS, Lin SH, Xu F and Wang CJ:
Luteolin regulates the differentiation of regulatory T cells and
activates IL-10-dependent macrophage polarization against acute
lung injury. J Immunol Res. 2021:1–12. 2021. View Article : Google Scholar
|
|
70
|
Yang L, Zhang YM, Guo MN, Zhang H, Zhu XY,
Xu C and Liu YJ: Matrine attenuates lung injury by modulating
macrophage polarization and suppressing apoptosis. J Surg Res.
281:264–274. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ye Z, Wang P, Feng G, Wang Q, Liu C, Lu J,
Chen J and Liu P: Cryptotanshinone attenuates LPS-induced acute
lung injury by regulating metabolic reprogramming of macrophage.
Front Med (Lausanne). 9:10754652022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhang J, Wang C, Wang H, Li X, Xu J and Yu
K: Loganin alleviates sepsis-induced acute lung injury by
regulating macrophage polarization and inhibiting NLRP3
inflammasome activation. Int Immunopharmacol. 95:1075292021.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhao JY, Pu J, Fan J, Feng XY, Xu JW,
Zhang R and Shang Y: Tanshinone IIA prevents acute lung injury by
regulating macrophage polarization. J Integr Med. 20:274–280. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ying ZH, Li HM, Yu WY and Yu CH: Iridin
prevented against lipopolysaccharide-induced inflammatory responses
of macrophages via inactivation of PKM2-mediated glycolytic
pathways. J Inflamm Res. 14:341–354. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhao L, Zhang M, Liu YW, Tan Y, Yin J,
Chen Y, Chen D and Ni B: Sinomenine alleviates
lipopolysaccharide-induced acute lung injury via a
PPARβ/δ-dependent mechanism. Eur J Pharmacol. 953:1758382023.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Gao WJ, Liu JX, Xie Y, Luo P, Liu ZQ, Liu
L and Zhou H: Suppression of macrophage migration by
down-regulating Src/FAK/P130Cas activation contributed to the
anti-inflammatory activity of sinomenine. Pharmacol Res.
167:1055132021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Yi L, Luo JF, Xie BB, Liu JX, Wang JY, Liu
L, Wang PX, Zhou H and Dong Y: α7 nicotinic acetylcholine receptor
is a novel mediator of sinomenine anti-inflammation effect in
macrophages stimulated by lipopolysaccharide. Shock. 44:188–195.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zhi YK, Li J, Yi L, Zhu RL, Luo JF, Shi
QP, Bai SS, Li YW, Du Q, Cai JZ, et al: Sinomenine inhibits
macrophage M1 polarization by downregulating α7nAChR via a feedback
pathway of α7nAChR/ERK/Egr-1. Phytomedicine. 100:1540502022.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Guo R, Wang H and Cui N: Autophagy
regulation on pyroptosis: Mechanism and medical implication in
sepsis. Mediators Inflamm. 2021:99250592021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Shi J, Gao W and Shao F: Pyroptosis:
Gasdermin-mediated programmed necrotic cell death. Trends Biochem
Sci. 42:245–254. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wei T, Zhang C and Song Y: Molecular
mechanisms and roles of pyroptosis in acute lung injury. Chin Med J
(Engl). 135:2417–2426. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Tang Y, Yu Y, Li R, Tao Z, Zhang L, Wang
X, Qi X, Li Y, Meng T, Qu H, et al: Phenylalanine promotes alveolar
macrophage pyroptosis via the activation of CaSR in ARDS. Front
Immunol. 14:11141292023. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wu DD, Pan PH, Liu B, Su XL, Zhang LM, Tan
HY, Cao Z, Zhou ZR, Li HT, Li HS, et al: Inhibition of alveolar
macrophage pyroptosis reduces lipopolysaccharide-induced acute lung
injury in mice. Chin Med J (Engl). 128:2638–2645. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Liu Y, Zhang Y, Feng Q, Liu Q, Xie J, Li
H, Yang F, Liu X, Gao W, Bai X, et al: GPA peptide attenuates
sepsis-induced acute lung injury in mice via inhibiting oxidative
stress and pyroptosis of alveolar macrophage. Oxid Med Cell Longev.
2021:1–12. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Li H, Li Y, Song C, Hu Y, Dai M, Liu B and
Pan P: Neutrophil extracellular traps augmented alveolar macrophage
pyroptosis via AIM2 inflammasome activation in LPS-induced
ALI/ARDS. J Inflamm Res. 14:4839–4858. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Hsu CG, Chávez CL, Zhang C, Sowden M, Yan
C and Berk BC: The lipid peroxidation product 4-hydroxynonenal
inhibits NLRP3 inflammasome activation and macrophage pyroptosis.
Cell Death Differ. 29:1790–1803. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Liu Y, Zhang Y, You G, Zheng D, He Z, Guo
W, Antonina K, Shukhrat Z, Ding B, Zan J and Zhang Z: Tangeretin
attenuates acute lung injury in septic mice by inhibiting
ROS-mediated NLRP3 inflammasome activation via regulating
PLK1/AMPK/DRP1 signaling axis. Inflamm Res. 73:47–63. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wang X, Wu FP, Huang YR, Li HD, Cao XY,
You Y, Meng ZF, Sun KY and Shen XY: Matrine suppresses NLRP3
inflammasome activation via regulating PTPN2/JNK/SREBP2 pathway in
sepsis. Phytomedicine. 109:1545742023. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Liu Y, Yang H, Zhu F, Ouyang Y and Pan P:
Inhibition of STAT3 phosphorylation by colchicine regulates NLRP3
activation to alleviate sepsis-induced acute lung injury.
Inflammopharmacology. 31:2007–2021. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yuan Y, Liao Q, Xue M, Shi Y, Rong L, Song
Z, Tong Z, Zheng W, Zhu Q, Cui X and Tao Z: Shufeng jiedu capsules
alleviate lipopolysaccharide-induced acute lung inflammatory injury
via activation of GPR18 by verbenalin. Cell Physiol Biochem.
50:629–639. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Yang L, Liu T, Zhuo Y, Li D, Li D, Liu J,
Gao H, Zhang L, Lin J and Wang X: Verbenalin alleviates acute lung
injury induced by sepsis and IgG immune complex through GPR18
receptor. Cell Signal. 109:1107682023. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Chen LL, Song C, Zhang Y, Li Y, Zhao YH,
Lin FY, Han DD, Dai MH, Li W and Pan PH: Quercetin protects against
LPS-induced lung injury in mice via SIRT1-mediated suppression of
PKM2 nuclear accumulation. Eur J Pharmacol. 936:1753522022.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhong C, Yang J, Deng K, Lang X, Zhang J,
Li M, Qiu L, Zhong G and Yu J: Tiliroside attenuates NLRP3
inflammasome activation in macrophages and protects against acute
lung injury in mice. Molecules. 28:75272023. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Xu HW, Li WF, Hong SS, Shao JJ, Chen JH,
Chattipakorn N, Wu D, Luo W and Liang G: Tabersonine, a natural
NLRP3 inhibitor, suppresses inflammasome activation in macrophages
and attenuate NLRP3-driven diseases in mice. Acta Pharmacol Sin.
44:1252–1261. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Shao JJ, Li WF, Sun JF, Zhuang ZS, Min JL,
Long XH, Wu GJ, Xu HW and Liang G: Britannin as a novel NLRP3
inhibitor, suppresses inflammasome activation in macrophages and
alleviates NLRP3-related diseases in mice. Acta Pharmacol Sin.
45:803–814. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Yang F, Ye XJ, Chen MY, Li HC, Wang YF,
Zhong MY, Zhong CS, Zeng B, Xu LH, He XH and Ouyang DY: Inhibition
of NLRP3 inflammasome activation and pyroptosis in macrophages by
taraxasterol is associated with its regulation on mTOR signaling.
Front Immunol. 12:6326062021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Jang WY, Kim MY and Cho JY: Antioxidant,
anti-inflammatory, anti-menopausal, and anti-cancer effects of
lignans and their metabolites. Int J Mol Sci. 23:154822022.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhang S, Yang L, Hu D, He S, Cui L, Zhao
J, Zhuo Y, Zhang L and Wang X: Syringaresinol alleviates IgG immune
complex induced acute lung injury via activating PPARγ and
suppressing pyroptosis. Int Immunopharmacol 124(Pt B). 1110712023.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Li W, Xu H, Shao J, Chen J, Lin Y, Zheng
Z, Wang Y, Luo W and Liang G: Discovery of alantolactone as a
naturally occurring NLRP3 inhibitor to alleviate NLRP3-driven
inflammatory diseases in mice. Br J Pharmacol. 180:1634–1647. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Liu C, Zhou Y, Tu Q, Yao L, Li J and Yang
Z: Alpha-linolenic acid pretreatment alleviates NETs-induced
alveolar macrophage pyroptosis by inhibiting pyrin inflammasome
activation in a mouse model of sepsis-induced ALI/ARDS. Front
Immunol. 14:11466122023. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Weavers H, Evans IR, Martin P and Wood W:
Corpse engulfment generates a molecular memory that primes the
macrophage inflammatory response. Cell. 165:1658–1671. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Sun D, Zhang G, Xie M, Wang Y, Liang X, Tu
M, Su Z and Zeng R: Softness enhanced macrophage-mediated therapy
of inhaled apoptotic-cell-inspired nanosystems for acute lung
injury. J Nanobiotechnology. 21:1722023. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Leventis PA and Grinstein S: The
distribution and function of phosphatidylserine in cellular
membranes. Ann Rev Biophysics. 39:407–427. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Banerjee S, Friggeri A, Liu G and Abraham
E: The C-terminal acidic tail is responsible for the inhibitory
effects of HMGB1 on efferocytosis. J Leukoc Biol. 88:973–979. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Mahida RY, Scott A, Parekh D, Lugg ST,
Hardy RS, Lavery GG, Matthay MA, Naidu B, Perkins GD and Thickett
DR: Acute respiratory distress syndrome is associated with impaired
alveolar macrophage efferocytosis. Eur Respir J. 58:21008292021.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Mahida RY, Lax S, Bassford CR, Scott A,
Parekh D, Hardy RS, Naidu B, Matthay MA, Stewart PM, Cooper MC, et
al: Impaired alveolar macrophage 11β-hydroxysteroid dehydrogenase
type 1 reductase activity contributes to increased pulmonary
inflammation and mortality in sepsis-related ARDS. Front Immunol.
14:11598312023. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Martín-Vicente P, López-Martínez C and
Albaiceta GM: The last-minute redemption of inflammatory cells in
lung repair. Eur Respir J. 59:21030002022. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Nepal S, Tiruppathi C, Tsukasaki Y,
Farahany J, Mittal M, Rehman J, Prockop DJ and Malik AB: STAT6
induces expression of Gas6 in macrophages to clear apoptotic
neutrophils and resolve inflammation. Proc Natl Acad Sci USA.
116:16513–16518. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Jiang T, Xia Y, Wang W, Zhao J, Liu W, Liu
S, Shi S, Li B, He X and Jin Y: Apoptotic bodies inhibit
inflammation by PDL1-PD1-mediated macrophage metabolic
reprogramming. Cell Prolif. 57:e135312023. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Wang Y, Zhang W, Xu Y, Wu D, Gao Z, Zhou
J, Qian H, He B and Wang G: Extracellular HMGB1 impairs
macrophage-mediated efferocytosis by suppressing the
Rab43-controlled cell surface transport of CD91. Front Immunol.
13:7676302022. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Aderem A and Underhill DM: Mechanisms of
phagocytosis in macrophages. Annu Rev Immunol. 17:593–623. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
He F, Gao F, Cai N, Jiang M and Wu C:
Chlorogenic acid enhances alveolar macrophages phagocytosis in
acute respiratory distress syndrome by activating G protein-coupled
receptor 37 (GPR 37). Phytomedicine. 107:1544742022. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Aman Y, Schmauck-Medina T, Hansen M,
Morimoto RI, Simon AK, Bjedov I, Palikaras K, Simonsen A, Johansen
T, Tavernarakis N, et al: Autophagy in healthy aging and disease.
Nat Aging. 1:634–650. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Saha S, Panigrahi DP, Patil S and Bhutia
SK: Autophagy in health and disease: A comprehensive review. Biomed
Pharmacother. 104:485–495. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Wang K, Chen Y, Zhang P, Lin P, Xie N and
Wu M: Protective features of autophagy in pulmonary infection and
inflammatory diseases. Cells. 8:1232019. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Murray PJ: On macrophage diversity and
inflammatory metabolic timers. Nat Rev Immunol. 20:89–90. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Liu C, Xiao K and Xie L: Progress in
preclinical studies of macrophage autophagy in the regulation of
ALI/ARDS. Front Immunol. 13:9227022022. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Huang M, Yu Y, Tang X, Dong R, Li X, Li F,
Jin Y, Gong S, Wang X, Zeng Z, et al: 3-Hydroxybutyrate ameliorates
sepsis-associated acute lung injury by promoting autophagy through
the activation of GPR109α in macrophages. Biochem Pharmacol.
213:1156322023. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Quach C, Helou DG, Li M, Hurrell BP,
Howard E, Shafiei-Jahani P, Soroosh P, Ou JJ, Razani B, Rehan V and
Akbari O: Enhancing autophagy in CD11c+ antigen-presenting cells as
a therapeutic strategy for acute respiratory distress syndrome.
Cell Rep. 42:1129902023. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Qiu P, Liu Y, Chen K, Dong Y, Liu S and
Zhang J: Hydrogen-rich saline regulates the polarization and
apoptosis of alveolar macrophages and attenuates lung injury via
suppression of autophagy in septic rats. Ann Transl Med. 9:9742021.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Liang J, Liu J, Tang Y, Peng Q, Zhang L,
Ma X, Xu N, Wei J and Han H: Sophoridine inhibits endotoxin-induced
acute lung injury by enhancing autophagy of macrophage and reducing
inflammation. J Leukoc Biol. 112:115–125. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Wen H, Zhang H, Wang W and Li Y:
Tetrahydropalmatine protects against acute lung injury induced by
limb ischemia/reperfusion through restoring PI3K/AKT/mTOR-mediated
autophagy in rats. Pulm Pharmacol Ther. 64:1019472020. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Tang D, Cao F, Yan C, Fang K, Ma J, Gao L,
Sun B and Wang G: Extracellular Vesicle/Macrophage axis: Potential
targets for inflammatory disease intervention. Front Immunol.
13:7054722022. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Viola A, Munari F, Sánchez-Rodríguez R,
Scolaro T and Castegna A: The metabolic signature of macrophage
responses. Front Immunol. 10:14622019. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Feng Z, Jing Z, Li Q, Chu L, Jiang Y,
Zhang X, Yan L, Liu Y, Jiang J, Xu P, et al: Exosomal STIMATE
derived from type II alveolar epithelial cells controls metabolic
reprogramming of tissue-resident alveolar macrophages.
Theranostics. 13:991–1009. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Zhong WJ, Liu T, Yang HH, Duan JX, Yang
JT, Guan XX, Xiong JB, Zhang YF, Zhang CY, Zhou Y and Guan CX:
TREM-1 governs NLRP3 inflammasome activation of macrophages by
firing up glycolysis in acute lung injury. Int J Biol Sci.
19:242–257. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Breda CN, Davanzo GG, Basso PJ, Câmara NO
and Moraes-Vieira PMM: Mitochondria as central hub of the immune
system. Redox Biol. 26:1012552019. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Rosales C and Uribe-Querol E:
Phagocytosis: A fundamental process in immunity. Biomed Res Int.
2017:90428512017. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Meidaninikjeh S, Sabouni N, Marzouni HZ,
Bengar S, Khalili A and Jafari R: Monocytes and macrophages in
COVID-19: Friends and foes. Life Sci. 269:1190102021. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Wang Z, Li S and Huang B: Alveolar
macrophages: Achilles' heel of SARS-CoV-2 infection. Signal
Transduct Target Ther. 7:2422022. View Article : Google Scholar : PubMed/NCBI
|