Introduction
Cancer is associated with high mortality rates
worldwide. Notably, DNA fragments produce non-coding (nc)RNA, which
was previously referred to genetic detritus, as they were
considered to be non-functioning (1). Following the discovery of nucleic
acids by Friedrich Miescher in 1871 (2), DNA and RNA were recognized as the
genetic code containing the information required for correct
cellular functioning. The Encyclopedia of the Elements of DNA
(ENCODE) Transcriptome Project determined that ncRNAs account for a
large fraction of nucleic acids, with protein-coding genes
accounting for ~1.2% of the genome. By contrast, >80% of the
genome is actively translated into different types of ncRNAs
(3). Results of previous studies
demonstrate that ncRNAs are crucial in numerous diseases; for
example, ncRNAs cause disruption of healthy tumor function and
control the expression of genes involved in tumor growth.
Therefore, ncRNAs may play an important role in tumors (4–7).
ncRNAs exhibit a wide range of diversity, including micro(mi)RNAs,
PIWI-interacting (pi)RNAs, transfer RNA-derived small RNA (tsRNAs),
small nucleolar RNA, small interfering (si)RNA, long ncRNAs
(lncRNAs) and circular (circ)RNAs. Different types of ncRNAs
exhibit specific regulatory roles and processes in numerous
malignancies, forming complex networks. For example, results of a
previous study showed that miRNAs may affect protein expression
through binding to mRNA; however, results of a more recent study
demonstrate that mRNAs are also found in the nucleus, suggesting
that miRNAs may directly affect DNA through miRNAs, which also
interact with other ncRNAs (8). In
addition, specific RNAs encode peptides or proteins, leading to the
development of novel therapeutic strategies for the treatment of
cancer (9). Numerous ncRNAs
exhibit high levels of stability in the bloodstream, highlighting
their suitability in clinical cancer detection. In addition,
results of a previous study showed that ncRNAs may act as targets
for tumor therapy (4).
Therefore, the present review aimed to examine the
characteristics and functions of short ncRNAs, including miRNAs,
siRNAs, tRFs and lncRNAs. It described the distinct pathways and
various functions of these ncRNAs in different types of cancer and
aimed to review the potential of liquid biopsy biomarkers for early
diagnosis or late prognosis in clinical settings. In conclusion,
the present review may provide a novel theoretical basis for the
role of ncRNAs in advanced cancer therapies and diagnostics.
Function of ncRNAs in tumors
Research has focused on the specific role of ncRNAs
in numerous cancers, and results have demonstrated that these may
promote or inhibit cancer through various modes of action (Table I). Therefore, ncRNAs may exhibit
potential as therapeutic options for the treatment of cancer.
 | Table I.Mechanisms of non-coding RNAs in
different types of cancer. |
Table I.
Mechanisms of non-coding RNAs in
different types of cancer.
| First author/s,
year | ncRNA | ncRNA type | Type of tumor | Function | Mechanism | (Refs.) |
|---|
| Su et al,
2019 | miRNA-199 | miRNA | LC | Tumor
inhibitors | Inhibits cell
proliferation and invasion | (14) |
| Cheng et al,
2019 | miRNA-129-5p | miRNA | LC | Tumor
inhibitors | Inhibits the
proliferative ability and invasiveness of LCa cells and tumor
angiogenesis by interacting with VEGF | (15) |
| Deng et al,
2020 | miRNA192, −215
miRNA | miRNA | GC | Oncogenes | Activation of
Wnt/β-catenin signaling pathway in gastric cancer by APC | (16) |
| Qiu et al,
2022 | miR-519a-3p | miRNA | HCC | Oncogenes | Promotes liver
metastasis by M2 macrophage polarization | (17) |
| Malagobadan et
al, 2020 | miR-6744-5p | miRNA | BC | Tumor
inhibitors | Inhibition of
cancer progression through NAT1 enzyme-mediated and regulated
anaerobic responses | (18) |
| Xiao et al,
2023 | miR-10527-5p | miRNA | ESCC | Tumor
inhibitors | Lymphatic
metastasis of ESCC is inhibited by Wnt/β-Catenin signaling of
Rab10 | (20) |
| Ying et al,
2024 | 3′ tiRNA
LysTTT | tsRNA | BCa | Oncogenes | Modified by m7G
modifying enzyme mettl1, it is found to bind to Annexin A2 tumor
protein and promote cancer progression | (40) |
| Xiong et al,
2024 |
tiRNA-Val-CAC-2 | tsRNA | PC | Oncogenes | Binds to FUBP1
protein, promotes the transcription of c-MYC, and promotes cell
proliferation | (41) |
| Xu et al,
2022 |
tRF-Val-CAC-016 | tsRNA | GC | Tumor
inhibitors | Binding to CACNA1d
protein mediates the classical MAPK signaling pathway and inhibits
the malignant progression of gastric cancer | (42) |
| Zhang et al,
2024 |
tRF-23-Q99P9P9NDD | tsRNA | GC | Oncogenes | Binding to the
target protein ACADSB protein promotes the development of gastric
cancer | (43) |
| Yang et al,
2022 | AS-tDR-007333 | tsRNA | NSCLC | Oncogenes | Through the
HSPB1/MED29 and ELK4/MED29 axes, the MED29 promoter is activated to
promote cancer | (46) |
| Tao et al,
2021 |
5′tiRNA-His-GTG | tsRNA | CRC | Oncogenes | In the hypoxic
microenvironment, it binds to LATS2 protein through the
HIF1α/angipoietin axis to promote cancer progression | (45) |
| Xie et al,
2022 | piRNA-14633 | piRNA | Cervical
cancer | Oncogenes | Increase m6A RNA
methylation level and METTL14 mRNA stability, promote cell
proliferation and cancer progression | (51) |
| Liu et al,
2023 | piRNA-18 | piRNA | CRC | Tumor
inhibitors | Promotes apoptosis,
cessation of the G1/S phase in the cell cycle, and thus
inhibits cancer | (54) |
| Peng et al,
2024 | piRNA-4447944 | piRNA | PCA | Oncogenes | Inhibits tumor
suppressor NEFH, prevents apoptosis and promotes cell proliferation
and migration to ultimately achieve the promoting effect | (55) |
| Ben et al,
2024 | PROPER | piRNA | PCA | Oncogenes | Promote the
interaction of RNA-binding proteins between EIF2S3 and YTHDF2/YBX3,
promote DUSP1 cyclization, and ultimately lead to the malignant
occurrence of PCa | (56) |
| Hua et al,
2019 | lncRN
ALINC01123 | lncRNA | NSCLC | Oncogenes | By interacting with
miR-199a-5p sponge, it promotes cell proliferation and
glycolysis | (65) |
| Zhang et al,
2023 | SPRY4-IT1 | lncRNA | BC | Oncogenes | Promoted by
pyruvate dehydrogenase kinase 1, it promotes the expression and
stability of SPRY4-IT1 and ultimately promotes cell
proliferation | (66) |
| Zhou et al,
2022 | STEAP3-AS1 | lncRNA | CRC | Oncogenes | STEAP3-AS1 competes
with YTHDF2 to protect against m6A-mediated degradation and further
activatesWnt/β-catenin signaling to promote CRC cell proliferation
and development | (67) |
| Wang et al,
2023 | FTO-IT1 | lncRNA | HCC | Oncogenes | FTO promotes HCC
tumorigenesis by reducing the modification of m6A by glycolytic
genes GLUT1, PKM2 and c-Myc | (68) |
| Huang et al,
2021 | PSMA3-AS1 | lncRNA | GBM | Oncogenes | Effects miR-411-3p
is down-regulated in glioma cells and promotes the malignant
progression of glioma by regulating HOXA10 protein | (70) |
| Yang et al,
2024 | LINC01133 | lncRNA | PDAC | Oncogenes | The expression of
secretory phospho-protein 1 was up-regulated, resulting in
epithelial-mesenchymal transition in pancreatic ductal
adenocarcinoma and promoting malignant progression | (72) |
| Fang et al,
2022 | TP53TG1 | lncRNA | GC | Tumor
inhibitors | M6A modification
mediates interaction with cellular inhibitor phosphatase 2A | (74) |
| Kong et al,
2019 | MORT | lncRNA | HCC | Tumor
inhibitors | MORT overexpression
inhibits the expression of NOTCH1, thereby reducing cell
proliferation and invasion | (71) |
| Wang et al,
2024 | PGM5-AS1 | lncRNA | LC | Tumor
inhibitors | Acts with
miR-423-5p to promote apoptosis, resulting in
G0/G1 cell cycle arrest | (75) |
| Hu et al,
2024 | PRDM16-DT | lncRNA | CRC | Tumor
inhibitors | PRDM16-DT reduces
chemoresistance by competing with HNRNPA2B1 and binds to FOXP3 to
inhibit cell metastasis | (76) |
miRNAs
In 1993, miRNAs were initially discovered in the
cryptic nematode Hidradenitis elegans (10). However, it was not until 2002 that
genomic alterations were uncovered in the miR-15a/16 cluster in
leukemia (11), providing evidence
for the association between miRNAs and human cancers. miRNAs are a
type of small molecule RNA that are ~22 nucleotides in length, and
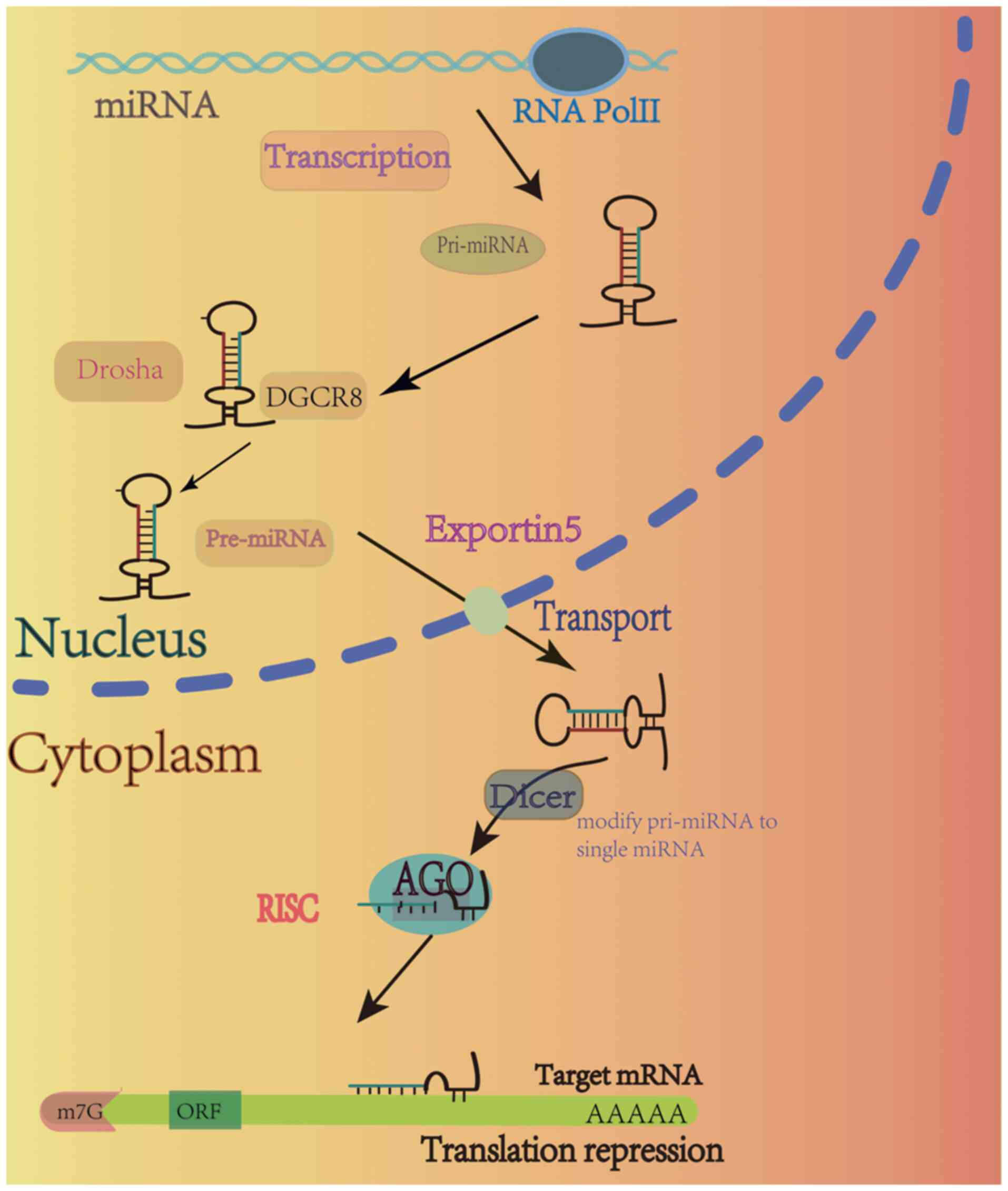
are transcribed into a primary miRNA (pri-miRNA) by polymerase II
(Pol II). Notably, pre-miRNA is processed through a complex
consisting of ribonucleic acid endonuclease III, termed Drosha, and
the protein, DGCR8. During this process, the pre-miRNA stem-loop
enzyme is created and this enters the cytoplasm via exportin-5.
Following entry into the cytoplasm, pre-miRNA is further processed
by Dicer and the associated auxiliary proteins for the generation
of miRNA duplexes, where mature miRNA binds to a member of the
Argonaute family of proteins to form the RNA-induced silencing
complex. Notably, binding to the 3′ untranslated region (3′UTR) of
mRNA may lead to degradation and translational repression (12) (Fig.
1). The genetic silencing of miRNAs plays a role in various
mechanisms (13).
miRNAs exhibit key roles in cancer, both as
oncogenes that promote tumor development and as tumor suppressors
that inhibit development. Results of a previous study demonstrated
that the expression of miRNA-199 was decreased in non-small cell
lung cancer, and this miRNA was associated with cancer stage, the
presence of distant metastasis and a negative prognosis (14). Notably, miRNA-129-5p may reduce the
growth and spread of non-small cell lung cancer cells, and inhibit
the formation of blood vessels that support tumor growth through
VEGF (15). Results of a further
previous study showed that miRNA-192 and −215 were upregulated in
gastric cancer, affecting cell proliferation and migration through
APC-mediated activation of the Wnt/β-catenin signaling pathway and
thus leading to gastric cancer progression and the discovery of
potential targets (16). The
exosome miR-519a-3p induces liver metastasis of gastric cancer
through M2 macrophage polarization and previous results showed that
miR-519a-3p expression is significantly increased in the absence of
liver metastasis. Therefore, miR-519a-3p may exhibit potential in
the treatment of gastric cancer with liver metastasis (17). The abnormal expression of miRNAs
has also been observed in breast cancer (BC). Through regulation of
the NAT1 enzyme, miR-6744-5p promotes the apoptosis of cancer
cells, inhibiting BC development by mediating the regulation of
anoikis (18). In colorectal
cancer, miR-1538 expression is reduced, leading to the suppression
of cell proliferation and cancer progression through reduced levels
of DNA methylase transferase 3A (DNMT3A) (19). Xiao et al (20) demonstrate that exosome miR-10527-5p
is reduced in the serum of patients with lymph node metastasis,
leading to reduced esophageal squamous cell carcinoma (ESCC) cell
migration and invasion, and inhibition of ESCC lymphatic
translocation through Rab10-mediated Wnt/β-catenin signaling
(20). Notably, miRNAs exhibit
diverse functions across various types of cancer. For example,
miR-200a may play a role in colorectal cancer progression,
affecting the prognosis of patients with this disease. However,
miR-200a may also contribute to cervical carcinogenesis through
regulation of the HIF-1α/VEGF signaling pathway. Results of further
previous studies show that miR-200a may inhibit gastric cancer cell
growth by targeting KLF12 (21–23).
Collectively, these results highlight the complexity and diversity
of cancers and the differing roles of miRNAs in cancer
regulation.
miRNAs and therapy
miRNAs demonstrate key roles in cancer progression
and development, using a variety of mechanisms that may affect
genesis. Therefore, research has focused on the use of miRNA-based
tumor therapy in the treatment of cancer. In a previous study,
mRNA-targeted therapy was examined through the design of
oligonucleotides that bind to mRNAs, thereby altering protein
formation to affect disease processes (24). In addition, miRNAs may play key
roles as potent tumor suppressors and oncogenes, highlighting the
potential of miRNAs as novel therapeutic agents in the treatment of
disease. Notably, alterations in pathological miRNA expression may
be modified or reversed using molecule penetration, siRNA silencing
and miRNA sponging (25). A
previous clinical trial (trial no. NCT01829971) investigated the
use of MRX34, a mimic of miR-34a, in the treatment of patients with
hepatocellular carcinoma (26).
However, this trial was ended prematurely, as four patients
developed liver cancer as a result of drug dosage and severe immune
adverse reactions (27,28). At present, a clinical trial
involving the treatment of patients with gastric cancer is ongoing,
in which miRNA measurements are obtained in response to treatment
with capecitabine + cisplatin or capecitabine + oxaliplatin +/-
trastuzumab (trial no. NCT03253107). Notably, miRNA-138
demonstrates potential as a therapeutic target in ovarian cancer
and regulates pancreatic cancer cell growth by targeting FOXCI
(29,30). Results of a previous study show the
potential role of miRNA-138 as a target in the treatment of
colorectal cancer, which interacts with the 3′UTR of PD-1 (31).
tsRNA
At present, research is focused on a novel form of
ncRNA, tsRNA, due to advances in high-throughput sequencing. tsRNAs
are tRNA-derived small RNAs that are widely distributed in the
transcriptomes of eukaryotic and prokaryotic organisms. tRNAs
primarily function as carriers of amino acids, facilitating the
synthesis of proteins. They are transcribed into pre-tRNAs by Pol
III, and undergo a series of modifications to generate mature tRNA.
Notably, tRNAs are highly folded into a structure consisting of
four arms and three loops (32) as
shown in Fig. 2. tRNAs produce
different tsRNAs depending on the cleavage site at which they are
generated. There are two main categories of tsRNAs; tRNA-derived
fragments (tRFs) and tRNA-derived stress-inducible RNAs (tiRNAs).
As shown in Fig. 2, tRFs are
generated following Dicer-mediated cleavage of mature tRNA, a
process that occurs in the cytoplasm. By contrast, tiRNAs are
generated following the accumulation of angiotensin (ANG) in the
cytoplasm (33). Notably, some
tsRNAs may also be located in the mitochondria, referred to as
mt-tRNAs, and further investigations into the association between
tsRNA and mt-tRNA may lead to further understanding of the
mechanisms underlying tsRNA (33).
Results of a previous study show that tsRNA performs various
functions, including epigenetic regulation, post-transcriptional
modification (34) and
participation in RNA interference. tsRNA was initially discovered
in urine; therefore, tsRNA was considered a product of degradation.
However, further previous studies demonstrated that tsRNAs play
essential roles in numerous biological activities, including cell
proliferation, cell migration, cancer cell progression (35,36),
DNA damage (37), sperm
modification (38) and epigenetic
modifications (39). Therefore,
further investigations into the role of tsRNAs in tumors are
required.
tsRNAs exhibit potential as oncogenes and are
involved in gene regulation in a variety of types of cancer.
Results of a previous study showed that m7G-3′ tiRNA LysTTT
interacts with tumor protein ANXA2 following methyltransferase-like
protein (METTL)11-mediated modification. This interaction results
in the phosphorylation of Tyr24 in the protein, facilitating cell
proliferation and migration and the advance of bladder cancer
(40). In addition, Xiong et
al (41) demonstrated that
tiRNA-Val-CAC-2, a stress tsRNA, is highly expressed in pancreatic
cancer. Results of this study show that tiRNA-Val-CAC-2 binds to
the Far upstream element-binding protein 1 (FUBP1) protein in
pancreatic cancer cells, thereby promoting the transcription of
c-MYC, which, in turn, increases the stability of FUBP1. Notably,
metastasis is inhibited following FUBP1 knockdown, highlighting the
potential of tiRNA-Val-CAC-2 as a biomarker for pancreatic cancer
(41). In addition, results of a
previous study show that tRF-Val-CAC-016 expression is reduced in
gastric cancer and this tRF mediates the classical MAPK signaling
pathway by binding to the downstream Calcium Voltage-Gated Channel
Subunit Alpha1 D protein (42).
This study demonstrates that increased tRF-Val-CAC-016 expression
inhibits gastric cancer cell proliferation, migration and invasion,
and cell proliferation is inhibited following knockdown. Therefore,
tRF-Val-CAC-016 may inhibit gastric cancer development,
highlighting the potential of this tRF as a therapeutic target in
the treatment of gastric cancer (42). As an oncogene, tRF-23-Q99P9P9NDD is
highly expressed in gastric cancer, promoting cancer development
through binding to the Acyl-CoA dehydrogenase short/branched chain
target protein (43). Moreover,
tRF-17-79MP9PP expression is reduced in BC and tRF-17 reduces cell
invasion and migration by binding to THBS1 (44). Results of a previous study show
that stress tsRNA, 5′tiRNA-His-GTG, is differentially expressed in
colorectal cancer. Notably, this tsRNA is regulated through the
HIF1α/ANG axis in a hypoxic microenvironment and LATS2 acted as the
target gene of 5′tiRNA-His-GTG. Following binding to the protein,
cell apoptosis is suppressed, thereby promoting the progression of
colorectal cancer (45). In
addition, AS-tDR-007333 is markedly upregulated in non-small cell
lung cancer through two modes of action; the HSPB1/MED29 and the
ELK4/MED29 axes. Notably, AS-tDR-007333 activates the MED29
promoter protein in both pathways to promote non-small cell lung
cancer cell proliferation and migration (46).
piRNAs
Piwi interactors, known as piRNAs, are a group of
ncRNAs that range from 24–31 nucleotides in length. With ~20,000
different combinations, piRNAs primarily bind to the piwi protein
family, playing a crucial role in the regulation of various
biological processes. Notably, piRNAs are expressed in eukaryotes
and produced in the nucleus, where they are transcribed into
precursor piRNA. With the assistance of cofactors, precursor piRNAs
transform into piRNA intermediates containing 5′ uracil (47). These intermediates bind the
aforementioned cofactors to the Zuc-split-open piwi to generate
complexes in the cytoplasm (47).
piRNAs have been extensively studied in the field of reproductive
biomedicine and directing the silencing of transcribed genes was
considered the first functional role of these ncRNAs. Notably, this
is categorized into the silencing of transposons and other
repetitive sequences. piRNAs exhibit transcriptional and
post-transcriptional silencing (48) and may act synergistically with
other RNAs. For example, piRNAs and circRNAs play key roles in gene
immunity due to their anti-degradation properties, regulating PD-L1
expression for immunization (49).
In addition, piwil2 induces Argonaute protein, which mediates RNA
cleavage in the presence of piwi and promotes gene silencing when
expressed in miRNA precursors in human cells (50). Results of a previous study show
that piRNA-14633 was expressed at high levels in cervical cancer
and this piRNA may play a role in promoting cellular proliferation.
In addition, METTL14 knockdown reverses piRNA-mediated
proliferation and invasion (51).
Previous studies show that piRNA-651 expression is increased in BC,
leading to DNMT1-mediated phosphatase methylation of the PTEN
promoter, ultimately promoting the progression of BC and
dysregulation of non-small cell lung cancer through cyclin D1 and
CDK4 (52,53). In addition, results of a previous
study show that piRNA-18 suppresses the migration and invasion of
colorectal cancer cells, both in vivo and in vitro.
Notably, piRNA-18 may promote apoptosis and induce cell cycle
arrest in the G1/S phase. Collectively, these findings
suggested that piRNA-18 may play a crucial role in inhibiting the
progression of colorectal cancer (54).
Depopulation-resistant prostate cancer (CRPC) is
associated with high mortality rates and piRNA-4447944 expression
is increased in CRPC. piR-4447944/PIWIL2 binding inhibits the tumor
suppressor NEFH, ultimately reducing apoptosis and promoting cell
proliferation and migration to promote depopulation-resistant
cancer cell growth (55).
Moreover, a specific genetic variant, rs17201241, interacts with
the piRNA PROPER to promote RNA-binding protein interactions
between EIF2S3 at the 5′UTR and YTHDF2/YBX3 at the 3′UTR. This
leads to the promotion of DUSP1 cyclization, ultimately leading to
prostate cancer progression (56).
lncRNAs
lncRNAs are often produced following Pol II-mediated
transcription and are >200 nucleotides in length. lncRNAs are
capped and polyadenylated to ensure their stability (57). According to the ENCODE project
report (3), the majority of the
genome is transcribed into lncRNAs. There are different categories
of lncRNAs based on their location in the genome, including
intergenic long-chain ncRNAs, intronic long-chain ncRNAs,
positive-strand ncRNAs and translational long-chain ncRNAs. lncRNAs
also possess pairs of isoforms, such as circular (circ)RNA and
competing endogenous (ce)RNA. Notably, lncRNAs are not highly
evolutionarily conserved and therefore exhibit a high degree of
specificity (58). lncRNAs are
highly abundant in the nucleus, with localization dictating their
function. Results of a previous study show that some lncRNAs may
shuttle between the cytoplasm and the nucleus (59). Notably, lncRNAs were initially
discovered as part of a group of genes that play a role in
modifying chromosomes. These genes, known as xist, are only
expressed in females and are responsible for silencing one of the X
chromosomes to compensate for gene expression. Results of a
previous study showed that xist contributes to a higher prevalence
of autoimmune disease in females (60). Notably, lncRNAs demonstrate a
higher level of stability in the cytoplasm, interacting with
proteins to influence mRNA translation modification and the
promotion of decay (61). In
addition, lncRNAs also exhibit direct interactions with ribosomes,
exporting them to the cytoplasm via organelles, including the Golgi
apparatus and mitochondria (61,62).
lncRNAs are complex with multiple transcription sites and these may
affect gene expression both near to and at a distance from the
transcription site (63). In
addition, lncRNAs undergo splicing, leading to the development of
multiple isoforms. However, investigations into these isoforms are
limited. A previous study shows that certain lncRNAs encode
peptides and proteins (64).
Oncogenes
lncRNAs may affect the regulation of oncogenes, such
as miRNAs, and may also exhibit potential as oncogenes (57). The glycolytic pathway plays a
crucial role in the association between lncRNAs and tumors.
Newly-discovered lncRNAC01123 exhibits distinct gene expression
patterns in both serum and tissues and is transcribed by c-Myc, a
protein that enhances cell proliferation and glycolysis. A previous
study shows that lncRNAC01123 functions through interacting with
miR-199a-5p (65). This lncRNA
also functions through enzymes involved in glycolysis, such as
pyruvate dehydrogenase kinase 1 (PDK1). A previous study shows that
increased SPRY4-IT1 expression is mediated by PDK1 in BC, leading
to increased protein stability. This leads to the promotion of cell
proliferation and inhibition of apoptosis, further contributing to
BC progression (66). Some lncRNAs
also function through RNA modification, such as m6A modification. A
previous study shows that STEAP3-AS1 is expressed at high levels in
colorectal cancer tissues, competing with YTHDF2 for binding to
protect STEAP3 from m6A-mediated degradation. This results in the
production of Fe2+ and the phosphorylation of glycogen
synthase kinase 3β. This activation further promotes the
Wnt/β-catenin signaling pathway, ultimately contributing to the
proliferation and development of colorectal cancer (67). Moreover, lncRNA FTO-IT1 interacts
with α-ketoglutarate-dependent dioxygenase FTO, a demethylase of
m6A, in hepatocellular carcinoma. This interaction leads to the
promotion of cell proliferation through glycolysis. In addition,
FTO may contribute to hepatocellular carcinoma tumorigenesis by
reducing the m6A-mediated modification of GLUT1, PKM2 and c-Myc
(68).
Results of a previous study also show that lncRNAs
play key roles as ceRNAs, where two RNAs act synergistically to
promote cellular dysregulation. For example, PSMA3-AS1, an
antisense lncRNA, is expressed at high levels in colorectal cancer,
leading to increased cell viability. Notably, miR-4429 expression
is markedly reduced by PSMA3-AS1, leading to the progression of
colorectal cancer (69). Moreover,
PSMA3-AS1 expression is reduced in glioma cells through increased
interactions with miR-411-3p and the regulation of HOXA10 (70). lncRNA-CDC6 may act as a miRNA-215
ceRNA, directly regulating CDC6 expression and promoting BC
progression (71).
LINC01133 and secreted phosphoprotein 1 (SPP1)
expression levels are increased in pancreatic ductal adenocarcinoma
and these increases are associated with malignant progression and
the induction of epithelial-mesenchymal transition (72). Moreover, lncRNAs may play key roles
in promoting cancer progression by enhancing drug metastasis and
resistance (73).
Tumor suppressors
Tumor suppressors inhibit cellular expression and
promote disease progression by binding to RNAs, enzymes and
proteins. lncRNAs may play a role in reducing the infiltration of
cancer cells (63). Notably, m6A
modification-mediated downregulation of the lncRNA TP53TG1 promotes
apoptosis and inhibits the proliferation and migration of gastric
cancer cells. In addition, cellular inhibitor of phosphatase 2A
(CIP2A) degrades TP53TG1 to stabilize expression, suggesting that
TP53TG1 may exhibit potential as a therapeutic target in the
treatment of gastric cancer (74).
A previous study shows that lncRNA MORT expression is decreased in
hepatocellular carcinoma and MORT overexpression suppresses NOTCH1
expression, leading to enhanced cell proliferation and invasion
(71). Moreover, Wang et al
(75) show that PGM5-AS1 is
overexpressed in non-small cell lung cancer, leading to reduced
cell proliferation, increased apoptosis and
G0/G1 cell cycle arrest. The results of this
study show that PGM5-AS1 is negatively associated with miR-423-5p.
In addition, miR-423-5p may interact with the SLIT2 gene to inhibit
the activity of miRNA and promote the development of cancer
(75).
Further studies show that lncRNAs may encode
proteins that affect cancer progression. For example, PRDM16-DT, a
protein encoded by LINC00982, inhibits metastasis and demonstrates
levels of chemoresistance in metastatic colorectal cancer. Notably,
CRISPR/Cas9 libraries were screened and the results show that
reduced PRDM16-DT expression promotes colorectal cancer progression
and PRDM16-DT secretes E-calmodulin to inhibit metastasis. This
process is mediated by the decreased secretion of MMP9 through
competitive interaction with HNRNPA2B1, a compound that acts
through Cimicifugoside H-1 to reduce chemoresistance and bind to
FOXP3 to inhibit cell metastasis (76).
Results of a previous study show that lncRNAs may
exhibit potential as therapeutic tools (77). Certain RNAs, despite their
potential therapeutic benefits in specific cancers, may not produce
the desired effects in cells due to various physiological factors.
This could be attributed to insufficient levels of RNAs within the
cells. Su et al (78) show
that PTENP1 may play a crucial role in enhancing PTEN expression in
prostate cancer through the utilization of ceRNAs. Results of this
study also show that ceRNAs may inhibit PTEN expression, which
exhibits potential in the prevention of tumor formation. However,
limited cellular uptake of PTEN1 may limit the effectiveness of
this approach (78). Collectively,
these results show that lncRNA may assist the uptake of therapeutic
agents in the body.
ncRNAs as biomarkers
Molecular biomarkers are crucial for early
diagnosis, providing patients with a postoperative prognosis, and
in the development of individualized treatment plans (Table II) (79).
 | Table II.Non-coding RNA as biomarkers. |
Table II.
Non-coding RNA as biomarkers.
| First author/s,
year | Name | ncRNA type | Cancer | Biomarkers | (Refs.) |
|---|
| Zhang et al,
2016 | lncRNA H19 | lncRNA | BC | Diagnosis | (80) |
| Han et al,
2023 | lncRNA H19 | lncRNA | THCA | Diagnosis,
prognosis | (81) |
| Li et al,
2023 |
ENST00000503625 | lncRNA | PCA | Prognosis | (89) |
| Wu et al,
2024 | SNHG1 | lncRNA | CRC | Prognosis | (88) |
| Li et al,
2024 | tRF-33-
RZYQHQ9M739P0J | tsRNA | GC | Diagnosis,
prognosis | (84) |
| Jin et al,
2021 | tRF-Pro-AGG-004,
tRF-Leu-CAG-002 | tsRNA | PC | Diagnosis,
prognosis | (87) |
| Mai et al,
2020 | piRNA-54265 | piRNA | CRC | Diagnosis | (82) |
| Feng et al,
2020 | piRNA-823 | piRNA | CRC | Prognosis | (91) |
| Moya et al,
2019 | miR-98-5p | miRNA | PCA | Diagnosis | (85) |
| Lian et al,
2024 | hsa-miR-449a | miRNA | PCA | Prognosis | (90) |
Diagnostic markers
The current lack of diagnostic markers in cancer is
associated with high mortality rates; therefore, the development of
novel diagnostic markers is required for early detection and the
accurate prediction of prognosis. Notably, lncRNA H19 is expressed
at high levels in the serum of patients with BC and this lncRNA is
differentially expressed in the plasma. Therefore, lncRNA H19 may
exhibit potential as a diagnostic marker in BC (80). In addition, H19 is considered a
biomarker for thyroid cancer (81).
Serum piRNA-54265 may also exhibit potential as a
diagnostic marker for the early detection of colorectal cancer,
with high levels of expression observed in the serum of patients.
Notably, these expression levels were higher in colorectal cancer
than in other cancers of the gastrointestinal tract, leading to
improved diagnostic outcomes in patients with early-stage disease
(82). In addition, serum
exosome-derived piRNAs also exhibit potential as diagnostic markers
for hepatocellular carcinoma (83). tRNA-ValTAC-3, tRNA-GlyTCC-5,
tRNA-ValAAC-5 and tRNA-GluCTC-5 exhibit higher expression levels in
patients with hepatocellular carcinoma, compared with healthy
subjects. Therefore, measurement of these tsRNAs using liquid
biopsy may exhibit potential in the diagnosis of hepatocellular
carcinoma (35). Similarly,
tRF-33-RZYQHQ9M739P0J was shown to serve as an exclusive novel
biomarker for hepatocellular carcinoma (84).
Diagnostic markers in the prostate exhibit high
levels of sensitivity; however, low levels of specificity may lead
to inaccurate diagnoses. For example, results of a previous study
showed that high miR-98-5p expression in the plasma of patients
with prostate cancer may exhibit potential for disease diagnosis;
however, miR-98-5p, miR-152-3p, miR-326 and miR-4289 expression was
dysregulated. Notably, simultaneous testing of these miRNAs showed
improved specificity and sensitivity in prostate cancer diagnosis
(85).
Prognostic markers
Investigating the expression of ncRNAs following
treatment may exhibit potential in predicting the prognosis of
patients and examinations of both prognostic and diagnostic markers
should be used in the clinic (86). Notably, tsRNAs are used as
biomarkers of pancreatic cancer. For example, tRF-Pro-AGG-004
exhibits potential as a diagnostic marker when combined with
tRF-Leu-CAG-002 examination and survival rates following surgery
have been assessed using these tsRNAs (87). Notably, intraperitoneal-free
carcinoma cells play a key role in prognosis during surgery and
lncRNA SNHG1 acts as a tumor marker in colorectal cancer. Results
of a previous study show that SNHG1 exhibits a higher potential
than CEA in the detection of IFCC (88). Moreover, lncRNA ENST00000503625 may
also exhibit potential as a prognostic marker for prostate cancer,
as cell invasion is increased following lncRNA ENST00000503625
knockdown. By contrast, increased lncRNA ENST00000503625 expression
is indicative of a more positive prognosis (89). ceRNAs may also play a role in
determining prognosis. The TRHDE-AS1/hsa-miR-449a/ADAMTS5 axis acts
as a ceRNA network, exhibiting potential as a novel prognostic
marker for prostate cancer (90).
A previous study also demonstrated that piRNA-823 may exhibit
potential as a novel diagnostic and prognostic biomarker in
colorectal cancer (91).
Conclusions
Gene editing technology, known as CRISPR/cas9, uses
a combination of CRISPR RNA and cas9 protein to precisely identify
and modify specific genetic sequences. Research has focused on the
use of CRISPR/cas9 in cancer therapeutics (92,93)
and the use of chimeric antigen receptor T-cell immunotherapy in
the treatment of cancer, which involves modifying γδ T-cells using
CRISPR/cas9 to inhibit autophagy, creating CAR-γδ TCD5-cells. These
edited cells demonstrate high levels of functionality and notable
anti-tumor effects when targeting malignant T-cell lines (94). Therefore, CRISPR/cas9 may exhibit
potential in the treatment of cancer through modification of
abnormal oncogenic ncRNAs, or as a tool for studying ncRNAs through
targeting specific genes. These methods may aid in further
understanding the functional roles of ncRNAs in cells, leading to
the development of novel therapeutic strategies.
Research has also focused on the use of ncRNAs in
the treatment of cancer (95).
ncRNA expression is often disrupted in various types of cancer;
however, the current understanding of specific underlying
mechanisms is limited. Therefore, further investigations with
improved experimental design are required to validate the function
of ncRNAs in cancer. Multidisciplinary approaches may aid in
further understanding the presence and secretion of ncRNAs in
various body fluids, leading to the development of novel
biomarkers. However, not all ncRNAs exhibit potential as
biomarkers. Therefore, further research is required to determine
the specific ncRNAs that exhibit potential as biomarkers and to
understand the factors that contribute to the lack of biomarker
function in other ncRNAs.
Moreover, the intricate structure of ncRNAs may lead
to limitations in the use of these agents in the treatment of
cancer. At present, research is focused on enhancing the accuracy
of ncRNA-mediated diagnosis. Notably, advances have been made in
optimizing RNA therapies, leading to improved delivery of
substances into specific cells. Reliable delivery systems are
crucial for the safe and effective transportation of targeted drugs
without impacting their intended targets. Expanding the current
understanding of RNA therapies and delivery systems may aid in the
use of ncRNAs in clinic. In conclusion, ncRNAs play key roles in
tumors, and may exhibit potential in diagnosis, treatment and
predicting the prognosis of patients with cancer. Further
investigations are required to overcome the aforementioned
challenges.
Acknowledgements
Not applicable.
Funding
The present review was supported by the Key Medical Research
Projects of Jiangsu Provincial Health Commission (grant no.
ZD2022008)
Availability of data and materials
Not applicable.
Authors' contributions
ZZ prepared the original manuscript draft. CM and ZZ
participated in conceptualization. ZZ, YWa and YWu participated in
guiding the preparation and design of this manuscript. HC reviewed
and edited the paper. Data authentication is not applicable. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
International Human Genome Sequencing
Consortium, . Finishing the euchromatic sequence of the human
genome. Nature. 431:931–945. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miescher F: On the chemical composition of
pus cells. Medicinisch-chemische Untersuchungen. 4:441–460.
1871.(In German).
|
|
3
|
ENCODE Project Consortium, . Moore JE,
Purcaro MJ, Pratt HE, Epstein CB, Shoresh N, Adrian J, Kawli T,
Davis CA, Dobin A, et al: Expanded encyclopaedias of DNA elements
in the human and mouse genomes. Nature. 583:699–710. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Budakoti M, Panwar AS, Molpa D, Singh RK,
Büsselberg D, Mishra AP, Coutinho HDM and Nigam M: Micro-RNA: The
darkhorse of cancer. Cell Signal. 83:1099952021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen H, Xu Z and Liu D: Small non-coding
RNA and colorectal cancer. J Cell Mol Med. 23:3050–3057. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fanale D, Taverna S, Russo A and Bazan V:
Circular RNA in exosomes. Adv Exp Med Biol. 1087:109–117. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li L, Wang Y, Zhang X, Huang Q, Diao Y,
Yin H and Liu H: Long non-coding RNA HOXD-AS1 in cancer. Clin Chim
Acta. 487:197–201. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Berindan-Neagoe I, Monroig P, Pasculli B
and Calin GA: MicroRNAome genome: A treasure for cancer diagnosis
and therapy. CA Cancer J Clin. 64:311–336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao H, Zhou Q and Li X: Protein bait
hypothesis: circRNA-Encoded proteins competitively inhibit cognate
functional isoforms. Trends Genet. 37:616–624. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wightman B, Ha I and Ruvkun G:
Posttranscriptional regulation of the heterochronic gene lin-14 by
lin-4 mediates temporal pattern formation in C. elegans. Cell.
75:855–862. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Calin GA, Dumitru CD, Shimizu M, Bichi R,
Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, et al:
Frequent deletions and down-regulation of micro- RNA genes miR15
and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad
Sci USA. 99:15524–15529. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He B, Zhao Z, Cai Q, Zhang Y, Zhang P, Shi
S, Xie H, Peng X, Yin W, Tao Y and Wang X: miRNA-based biomarkers,
therapies, and resistance in cancer. Int J Biol Sci. 16:2628–2647.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao Q, Lei F, Zeng Q, Gao Z, Niu P, Junnan
Ning Li J and Zhang J: Functional passenger-Strand miRNAs in
exosomes derived from human colon cancer cells and their
heterogeneous paracrine effects. Int J Biol Sci. 16:1044–1058.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Su WZ and Ren LF: MiRNA-199 inhibits
malignant progression of lung cancer through mediating RGS17. Eur
Rev Med Pharmacol Sci. 23:3390–3400. 2019.PubMed/NCBI
|
|
15
|
Cheng XK, Lin WR, Jiang H, Su ZH, Li L and
Wang J: MicroRNA-129-5p inhibits invasiveness and metastasis of
lung cancer cells and tumor angiogenesis via targeting VEGF. Eur
Rev Med Pharmacol Sci. 23:2827–2837. 2019.PubMed/NCBI
|
|
16
|
Deng S, Zhang X, Qin Y, Chen W, Fan H,
Feng X, Wang J, Yan R, Zhao Y, Cheng Y, et al: miRNA-192 and −215
activate Wnt/β-catenin signaling pathway in gastric cancer via APC.
J Cell Physiol. 235:6218–6229. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qiu S, Xie L, Lu C, Gu C, Xia Y, Lv J,
Xuan Z, Fang L, Yang J, Zhang L, et al: Gastric cancer-derived
exosomal miR-519a-3p promotes liver metastasis by inducing
intrahepatic M2-like macrophage-mediated angiogenesis. J Exp Clin
Cancer Res. 41:2962022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Malagobadan S, Ho CS and Nagoor NH:
MicroRNA-6744-5p promotes anoikis in breast cancer and directly
targets NAT1 enzyme. Cancer Biol Med. 17:101–111. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang X, Yang Y, Zhang W, Huang K, Xu L,
Shahid N, Pan Y, Xu C, Jiao X and Yang K: Downregulation of
MiR-1538 promotes proliferation and metastasis of colorectal cancer
by targeting DNMT3A. Biochem Biophys Res Commun. 609:119–126. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiao Z, Feng X, Zhou Y, Li P, Luo J, Zhang
W, Zhou J, Zhao J, Wang D, Wang Y, et al: Exosomal miR-10527-5p
inhibits migration, invasion, lymphangiogenesis and lymphatic
metastasis by affecting Wnt/β-catenin signaling via Rab10 in
esophageal squamous cell carcinoma. Int J Nanomedicine. 18:95–114.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jia C, Zhang Y, Xie Y, Ren Y, Zhang H,
Zhou Y, Gao N, Ding S and Han S: miR-200a-3p plays tumor suppressor
roles in gastric cancer cells by targeting KLF12. Artif Cells
Nanomed Biotechnol. 47:3697–3703. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pichler M, Ress AL, Winter E, Stiegelbauer
V, Karbiener M, Schwarzenbacher D, Scheideler M, Ivan C, Jahn SW,
Kiesslich T, et al: MiR-200a regulates epithelial to mesenchymal
transition-related gene expression and determines prognosis in
colorectal cancer patients. Br J Cancer. 110:1614–1621. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Su X, Lang C, Luan A and Zhao P: MiR-200a
promotes proliferation of cervical cancer cells by regulating
HIF-1α/VEGF signaling pathway. J BUON. 25:1935–1940.
2020.PubMed/NCBI
|
|
24
|
Matsui M and Corey DR: Non-coding RNAs as
drug targets. Nat Rev Drug Discov. 16:167–179. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Diener C, Keller A and Meese E: Emerging
concepts of miRNA therapeutics: From cells to clinic. Trends Genet.
38:613–626. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bouchie A: First microRNA mimic enters
clinic. Nat Biotechnol. 31:5772013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Beg MS, Brenner AJ, Sachdev J, Borad M,
Kang YK, Stoudemire J, Smith S, Bader AG, Kim S and Hong DS: Phase
I study of MRX34, a liposomal miR-34a mimic, administered twice
weekly in patients with advanced solid tumors. Invest New Drugs.
35:180–188. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hong DS, Kang YK, Borad M, Sachdev J,
Ejadi S, Lim HY, Brenner AJ, Park K, Lee JL, Kim TY, et al: Phase 1
study of MRX34, a liposomal miR-34a mimic, in patients with
advanced solid tumours. Br J Cancer. 122:1630–1637. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yeh YM, Chuang CM, Chao KC and Wang LH:
MicroRNA-138 suppresses ovarian cancer cell invasion and metastasis
by targeting SOX4 and HIF-1α. Int J Cancer. 133:867–878. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu C, Wang M, Li Z, Xiao J, Peng F, Guo X,
Deng Y, Jiang J and Sun C: MicroRNA-138-5p regulates pancreatic
cancer cell growth through targeting FOXC1. Cell Oncol (Dordr).
38:173–181. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao L, Yu H, Yi S, Peng X, Su P, Xiao Z,
Liu R, Tang A, Li X, Liu F and Shen S: The tumor suppressor
miR-138-5p targets PD-L1 in colorectal cancer. Oncotarget.
7:45370–45384. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee S, Kim J, Valdmanis PN and Kim HK:
Emerging roles of tRNA-derived small RNAs in cancer biology. Exp
Mol Med. 55:1293–1304. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu B, Cao J, Wang X, Guo C, Liu Y and
Wang T: Deciphering the tRNA-derived small RNAs: Origin,
development, and future. Cell Death Dis. 13:242021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu D, Qiao D, Lei Y, Zhang C, Bu Y and
Zhang Y: Transfer RNA-derived small RNAs (tsRNAs): Versatile
regulators in cancer. Cancer Lett. 546:2158422022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu L, Li J, Gong Y, Wu Q, Tan S, Sun D,
Xu X, Zuo Y, Zhao Y, Wei YQ, et al: Exosomal tRNA-derived small RNA
as a promising biomarker for cancer diagnosis. Mol Cancer.
18:742019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Du J, Huang T, Zheng Z, Fang S, Deng H and
Liu K: Biological function and clinical application prospect of
tsRNAs in digestive system biology and pathology. Cell Commun
Signal. 21:3022023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Burger K, Schlackow M and Gullerova M:
Tyrosine kinase c-Abl couples RNA polymerase II transcription to
DNA double-strand breaks. Nucleic Acids Res. 47:3467–3484. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen Q, Yan M, Cao Z, Li X, Zhang Y, Shi
J, Feng GH, Peng H, Zhang X, Zhang Y, et al: Sperm tsRNAs
contribute to intergenerational inheritance of an acquired
metabolic disorder. Science. 351:397–400. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Park J, Ahn SH, Shin MG, Kim HK and Chang
S: tRNA-derived small RNAs: Novel epigenetic regulators. Cancers
(Basel). 12:27732020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ying X, Hu W, Huang Y, Lv Y, Ji D, Chen C,
Yang B, Zhang C, Liang Y, Zhang H, et al: A Novel tsRNA, m7G-3′
tiRNA LysTTT, promotes bladder cancer malignancy via regulating
ANXA2 phosphorylation. Adv Sci (Weinh). 18:e24001152024. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xiong Q, Zhang Y, Xu Y, Yang Y, Zhang Z,
Zhou Y, Zhang S, Zhou L, Wan X, Yang X, et al: tiRNA-Val-CAC-2
interacts with FUBP1 to promote pancreatic cancer metastasis by
activating c-MYC transcription. Oncogene. 43:1274–1287. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu W, Zheng J, Wang X, Zhou B, Chen H, Li
G and Yan F: tRF-Val-CAC-016 modulates the transduction of
CACNA1d-mediated MAPK signaling pathways to suppress the
proliferation of gastric carcinoma. Cell Commun Signal. 20:682022.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang Y, Gu X, Li Y, Li X, Huang Y and Ju
S: Transfer RNA-derived fragment tRF-23-Q99P9P9NDD promotes
progression of gastric cancer by targeting ACADSB. J
Zhejiang Univ Sci B. 25:438–450. 2024.(In English, Chinese).
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mo D, He F, Zheng J, Chen H, Tang L and
Yan F: tRNA-derived fragment tRF-17-79MP9PP attenuates cell
invasion and migration via THBS1/TGF-β1/Smad3 axis in breast
cancer. Front Oncol. 11:6560782021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tao EW, Wang HL, Cheng WY, Liu QQ, Chen YX
and Gao QY: A specific tRNA half, 5′tiRNA-His-GTG, responds to
hypoxia via the HIF1α/ANG axis and promotes colorectal cancer
progression by regulating LATS2. J Exp Clin Cancer Res. 40:672021.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang W, Gao K, Qian Y, Huang Y, Xiang Q,
Chen C, Chen Q, Wang Y, Fang F, He Q, et al: A novel tRNA-derived
fragment AS-tDR-007333 promotes the malignancy of NSCLC via the
HSPB1/MED29 and ELK4/MED29 axes. J Hematol Oncol. 15:532022.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Czech B, Munafò M, Ciabrelli F, Eastwood
EL, Fabry MH, Kneuss E and Hannon GJ: piRNA-Guided genome defense:
From biogenesis to silencing. Annu Rev Genet. 52:131–157. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu Y, Dou M, Song X, Dong Y, Liu S, Liu
H, Tao J, Li W, Yin X and Xu W: The emerging role of the piRNA/piwi
complex in cancer. Mol Cancer. 18:1232019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Han R, Rao X, Zhou H and Lu L: Synergistic
immunoregulation: Harnessing CircRNAs and PiRNAs to amplify
PD-1/PD-L1 inhibition therapy. Int J Nanomedicine. 19:4803–4834.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bastiaanssen C, Ugarte PB, Kim K,
Finocchio G, Feng Y, Anzelon TA, Köstlbacher S, Tamarit D, Ettema
TJG, Jinek M, et al: RNA-guided RNA silencing by an Asgard archaeal
Argonaute. Nat Commun. 15:54992024. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xie Q, Li Z, Luo X, Wang D, Zhou Y, Zhao
J, Gao S, Yang Y, Fu W, Kong L and Sun T: piRNA-14633 promotes
cervical cancer cell malignancy in a METTL14-dependent m6A RNA
methylation manner. J Transl Med. 20:512022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li D, Luo Y, Gao Y and Yang Y, Wang Y, Xu
Y, Tan S, Zhang Y, Duan J and Yang Y: piR-651 promotes tumor
formation in non-small cell lung carcinoma through the upregulation
of cyclin D1 and CDK4. Int J Mol Med. 38:927–936. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu T, Wang J, Sun L, Li M, He X, Jiang J
and Zhou Q: Piwi-interacting RNA-651 promotes cell proliferation
and migration and inhibits apoptosis in breast cancer by
facilitating DNMT1-mediated PTEN promoter methylation. Cell Cycle.
20:1603–1616. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu Q, Chen Q, Zhou Z, Tian Z, Zheng X and
Wang K: piRNA-18 inhibition cell proliferation, migration and
invasion in colorectal cancer. Biochem Genet. 61:1881–1897. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Peng Q, Chen Y, Xie T, Pu D, Ho VW, Sun J,
Liu K, Chan RC, Ding X, Teoh JY, et al: PiRNA-4447944 promotes
castration-resistant growth and metastasis of prostate cancer by
inhibiting NEFH expression through forming the
piRNA-4447944-PIWIL2-NEFH complex. Int J Biol Sci. 20:3638–3655.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ben S, Ding Z, Xin J, Li F, Cheng Y, Chen
S, Fan L, Zhang Q, Li S, Du M, et al: piRNA PROPER suppresses DUSP1
translation by targeting N6-methyladenosine-mediated RNA
circularization to promote oncogenesis of prostate cancer. Adv Sci
(Weinh). 4:e24029542024. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tan YT, Lin JF, Li T, Li JJ, Xu RH and Ju
HQ: LncRNA-mediated posttranslational modifications and
reprogramming of energy metabolism in cancer. Cancer Commun (Lond).
41:109–120. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bridges MC, Daulagala AC and Kourtidis A:
LNCcation: lncRNA localization and function. J Cell Biol.
220:e2020090452021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bhan A, Soleimani M and Mandal SS: Long
noncoding RNA and cancer: A new paradigm. Cancer Res. 77:3965–3981.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Dou DR, Zhao Y, Belk JA, Zhao Y, Casey KM,
Chen DC, Li R, Yu B, Srinivasan S, Abe BT, et al: Xist
ribonucleoproteins promote female sex-biased autoimmunity. Cell.
187:733–749.e16. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
van Heesch S, Witte F, Schneider-Lunitz V,
Schulz JF, Adami E, Faber AB, Kirchner M, Maatz H, Blachut S,
Sandmann CL, et al: The translational landscape of the human heart.
Cell. 178:242–260.e29. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Klinge CM: Estrogenic control of
mitochondrial function. Redox Biol. 31:1014352020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kopp F and Mendell JT: Functional
classification and experimental dissection of long noncoding RNAs.
Cell. 172:393–407. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Matsumoto A, Pasut A, Matsumoto M,
Yamashita R, Fung J, Monteleone E, Saghatelian A, Nakayama KI,
Clohessy JG and Pandolfi PP: mTORC1 and muscle regeneration are
regulated by the LINC00961-encoded SPAR polypeptide. Nature.
541:228–232. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hua Q, Jin M, Mi B, Xu F, Li T, Zhao L,
Liu J and Huang G: LINC01123, a c-Myc-activated long non-coding
RNA, promotes proliferation and aerobic glycolysis of non-small
cell lung cancer through miR-199a-5p/c-Myc axis. J Hematol Oncol.
12:912019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhang Y, Chen H, Yuan R, Wang Y, Zhang D,
Zeng Q, Jiang W, Zhang R, Chen T, Chai C and Guo B: PDK1-stabilized
LncRNA SPRY4-IT1 promotes breast cancer progression via activating
NF-κB signaling pathway. Mol Carcinog. 62:1009–1024. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhou L, Jiang J, Huang Z, Jin P, Peng L,
Luo M, Zhang Z, Chen Y, Xie N, Gao W, et al: Hypoxia-induced lncRNA
STEAP3-AS1 activates Wnt/β-catenin signaling to promote colorectal
cancer progression by preventing m6A-mediated degradation of STEAP3
mRNA. Mol Cancer. 21:1682022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wang F, Hu Y, Wang H, Hu P, Xiong H, Zeng
Z, Han S, Wang D, Wang J, Zhao Y, et al: LncRNA FTO-IT1 promotes
glycolysis and progression of hepatocellular carcinoma through
modulating FTO-mediated N6-methyladenosine modification on GLUT1
and PKM2. J Exp Clin Cancer Res. 42:2672023. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Peng P, Wang Y, Wang BL, Song YH, Fang Y,
Ji H, Huangfu CN, Wang KM and Zheng Q: LncRNA PSMA3-AS1 promotes
colorectal cancer cell migration and invasion via regulating
miR-4429. Eur Rev Med Pharmacol Sci. 24:11594–11601.
2020.PubMed/NCBI
|
|
70
|
Huang T, Chen Y, Zeng Y, Xu C, Huang J, Hu
W, Chen X and Fu H: Long non-coding RNA PSMA3-AS1 promotes glioma
progression through modulating the miR-411-3p/HOXA10 pathway. BMC
Cancer. 21:8442021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kong X, Duan Y, Sang Y, Li Y, Zhang H,
Liang Y, Liu Y, Zhang N and Yang Q: LncRNA-CDC6 promotes breast
cancer progression and function as ceRNA to target CDC6 by sponging
microRNA-215. J Cell Physiol. 234:9105–9117. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yang Y, Gong Y, Ding Y, Sun S, Bai R, Zhuo
S and Zhang Z: LINC01133 promotes pancreatic ductal adenocarcinoma
epithelial-mesenchymal transition mediated by SPP1 through binding
to Arp3. Cell Death Dis. 15:4922024. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Liu C, Lu C, Yixi L, Hong J, Dong F, Ruan
S, Hu T and Zhao X: Exosomal Linc00969 induces trastuzumab
resistance in breast cancer by increasing HER-2 protein expression
and mRNA stability by binding to HUR. Breast Cancer Res.
25:1242023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Fang D, Ou X, Sun K, Zhou X, Li Y, Shi P,
Zhao Z, He Y, Peng J and Xu J: m6A modification-mediated lncRNA
TP53TG1 inhibits gastric cancer progression by regulating CIP2A
stability. Cancer Sci. 113:4135–4150. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wang J, Ye J, Dang Y and Xu S: LncRNA
PGM5-AS1 inhibits non-small cell lung cancer progression by
targeting miRNA-423-5p/SLIT2 axis. Cancer Cell Int. 24:2162024.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hu HF, Han L, Fu JY, He X, Tan JF, Chen
QP, Han JR and He QY: LINC00982-encoded protein PRDM16-DT regulates
CHEK2 splicing to suppress colorectal cancer metastasis and
chemoresistance. Theranostics. 14:3317–3338. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Hashemi M, Moosavi MS, Abed HM, Dehghani
M, Aalipour M, Heydari EA, Behroozaghdam M, Entezari M,
Salimimoghadam S, Gunduz ES, et al: Long non-coding RNA (lncRNA)
H19 in human cancer: From proliferation and metastasis to therapy.
Pharmacol Res. 184:1064182022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Su S, Liu L, Fu Q, Ma M, Yang N, Pan T,
Geng S, Yu XF and Zhu J: A black phosphorus nanosheet-based RNA
delivery system for prostate cancer therapy by increasing the
expression level of tumor suppressor gene PTEN via CeRNA mechanism.
J Nanobiotechnology. 22:3912024. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Biomarkers Definitions Working Group, :
Biomarkers and surrogate endpoints: Preferred definitions and
conceptual framework. Clin Pharmacol Ther. 69:89–95. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhang K, Luo Z, Zhang Y, Zhang L, Wu L,
Liu L, Yang J, Song X and Liu J: Circulating lncRNA H19 in plasma
as a novel biomarker for breast cancer. Cancer Biomark. 17:187–194.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Han B, Li S, Huang S, Huang J, Wu T and
Chen X: Cuproptosis-related lncRNA SNHG16 as a biomarker for the
diagnosis and prognosis of head and neck squamous cell carcinoma.
PeerJ. 11:e161972023. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Mai D, Zheng Y, Guo H, Ding P, Bai R, Li
M, Ye Y, Zhang J, Huang X, Liu D, et al: Serum piRNA-54265 is a New
Biomarker for early detection and clinical surveillance of human
colorectal cancer. Theranostics. 10:8468–8478. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Rui T, Wang K, Xiang A, Guo J, Tang N, Jin
X, Lin Y, Liu J and Zhang X: Serum exosome-derived piRNAs could be
promising biomarkers for HCC diagnosis. Int J Nanomedicine.
18:1989–2001. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Li X, Li Y, Yuan J, Zhang W, Xu T, Jing R
and Ju S: Serum tRF-33-RZYQHQ9M739P0J as a novel biomarker for
auxiliary diagnosis and disease course monitoring of hepatocellular
carcinoma. Heliyon. 10:e300842024. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Moya L, Meijer J, Schubert S, Matin F and
Batra J: Assessment of miR-98-5p, miR-152-3p, miR-326 and miR-4289
expression as biomarker for prostate cancer diagnosis. Int J Mol
Sci. 20:11542019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Weng W, Liu N, Toiyama Y, Kusunoki M,
Nagasaka T, Fujiwara T, Wei Q, Qin H, Lin H, Ma Y and Goel A: Novel
evidence for a PIWI-interacting RNA (piRNA) as an oncogenic
mediator of disease progression, and a potential prognostic
biomarker in colorectal cancer. Mol Cancer. 17:162018. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Jin F, Yang L, Wang W, Yuan N, Zhan S,
Yang P, Chen X, Ma T and Wang Y: A novel class of tsRNA signatures
as biomarkers for diagnosis and prognosis of pancreatic cancer. Mol
Cancer. 20:952021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wu Y, Liu L, He F, Zhang Y, Jiang W, Cao
Z, Xu X and Gong J: Long noncoding RNA small nucleolar RNA host
gene 1 as a potential novel biomarker for intraperitoneal free
cancer cells in colorectal cancer. iScience. 27:1102282024.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Li Y, Fang Z, Ge S, Li J, Qu L, Shi X,
Zhang W, Sun Y, Ren S and Wang L: Long non-coding RNA
ENST00000503625 is a potential prognostic biomarker and metastasis
suppressor gene in prostate cancer. J Cancer Res Clin Oncol.
149:7305–7317. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Lian X, Pang L, Zhou H and Liu S:
Identification and validation of the TRHDE-AS1/hsa-miR-449a/ADAMTS5
axis as a novel prognostic biomarker in prostate cancer.
Biofactors. 50:1251–1267. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Feng J, Yang M, Wei Q, Song F, Zhang Y,
Wang X, Liu B and Li J: Novel evidence for oncogenic piRNA-823 as a
promising prognostic biomarker and a potential therapeutic target
in colorectal cancer. J Cell Mol Med. 24:9028–9040. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Woodward EA, Wang E, Wallis C, Sharma R,
Tie AWJ, Murthy N and Blancafort P: Protocol for delivery of
CRISPR/dCas9 systems for epigenetic editing into solid tumors using
lipid nanoparticles encapsulating RNA. Methods Mol Biol.
2842:267–287. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Issa II, Due H, Brøndum RF, Veeravakaran
V, Haraldsdóttir H, Sylvester C, Brogaard A, Dhanjal S, Schmierer B
and Dybkær K: CRISPR-Cas9 knockout screens identify DNA damage
response pathways and BTK as essential for cisplatin
response in diffuse large B-Cell lymphoma. Cancers (Basel).
16:24372024. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Zhu Z, Li H, Lu Q, Zhang Z, Li J, Wang Z,
Yang N, Yu Z, Yang C, Chen Y, et al: mRNA-engineered
CD5-CAR-γδTCD5- cells for the immunotherapy of T-cell acute
lymphoblastic leukemia. Adv Sci (Weinh). 16:e24000242024.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Slack FJ and Chinnaiyan AM: The role of
non-coding RNAs in oncology. Cell. 179:1033–1055. 2019. View Article : Google Scholar : PubMed/NCBI
|