Introduction
Psoriasis is a chronic systemic inflammatory skin
disorder with a global prevalence of 2–3% (1). The most prevalent form, plaque
psoriasis, accounts for ~90% of all cases (2). The condition is characterized by
erythematous plaques covered with silvery scales, typically
affecting areas such as the scalp, elbows, knees, back and
buttocks. Histopathologically, psoriasis is marked by excessive
proliferation of keratinocytes, leading to epidermal hyperplasia
and a thickened stratum corneum (3). Inflammatory cells, primarily T
lymphocytes, infiltrate the dermal papillae, while dilated
capillaries contribute to the erythema (4,5).
These features collectively define the characteristic appearance
and symptoms of psoriasis. Three main inflammatory circuits are
involved in pathogenesis of psoriatic lesions (6): i) T helper (Th) 17 and cytotoxic T
cell (Tc) 17 responses driven by interleukin (IL)-17, IL-23 and C-C
motif chemokine ligand (CCL) 20; ii) type I and II interferon
responses driven by plasmacytoid dendritic cells (pDCs) and
interferon-γ (IFN-γ)-secreting T cells, along with C-X-C motif
chemokine ligand (CXCL) 9 and 10 feedback; and iii) an IL-36 and
neutrophil axis driven by CXCL1, 2 and 8. Cytokines such as IL-12,
IL-23 and tumor necrosis factor-α (TNF-α) collectively initiate and
sustain these inflammatory circuits (6,7).
Obesity is recognized as one of the most prevalent
comorbid conditions associated with psoriasis. Of patients with
psoriasis ~25% are also affected by obesity (8), indicating a significant association
between these two conditions. Moreover, obesity is recognized as an
independent risk factor for psoriasis (9). Research has demonstrated a positive
association between obesity and the severity of the disease in both
human patients and murine models (10,11).
Additionally, obesity may lead to higher treatment failure rates
for psoriasis (12). Notably,
interventions such as medications, surgery and diet control aiming
at reducing fat can also improve psoriatic lesions (12–14).
Consequently, obesity influences both the onset and progression of
psoriasis, as well as overall prognosis.
The expansion of white adipose tissue (WAT) is a
hallmark of obesity. This expansion involves various processes,
including adipocyte hypertrophy and hyperplasia, the
differentiation of pre-adipocytes into mature adipocytes,
endothelial cell proliferation for increased vascular density,
inflammatory cell infiltration, and remodeling of the extracellular
matrix (15–18). Collectively, these changes affect
both the function and metabolic state of WAT. Given that WAT
functions as a crucial endocrine organ, its alterations can have
widespread effects on other organs and tissues, including the skin.
For instance, adipose-derived adipokines can influence
angiogenesis, immune responses and the maintenance of skin barrier
function (19,20). Understanding these mechanisms is
essential not only for elucidating the pathophysiology of skin
diseases, but also for identifying potential new therapeutic
targets for conditions, such as psoriasis, which are linked to
obesity. Studies have also increasingly emphasized the role of the
skin in modulating WAT function (21–24).
Thus, understanding the crosstalk between WAT and skin is essential
for unraveling the pathogenesis of skin diseases.
In light of the growing evidence on the interactions
between WAT and skin in psoriasis, the aim of the present narrative
review was to provide a comprehensive overview to deepen
understanding of this complex relationship. This bidirectional
communication not only helps explain the high prevalence of obesity
among patients with psoriasis, but also highlights the critical
need to address metabolic comorbidities in treatment
strategies.
Materials and methods
To investigate the crosstalk between WAT and skin in
psoriasis, a comprehensive literature search was conducted using
PubMed and Google Scholar. The search employed keywords including
‘adipose tissue’, ‘skin lesions’, ‘psoriasis’, ‘adipokines’,
‘extracellular vesicles’, ‘FFAs’ and ‘inflammation’, focusing on
studies published over the past 20 years, up to September 30, 2024.
The inclusion criterion was the focus on the interaction between
WAT and skin in relation to psoriasis. Studies not published in
English, those lacking primary data, or reviews without new
insights were excluded. Data extraction was performed independently
by at least two reviewers, and any discrepancies were resolved
through discussion to reach a consensus. Ethical approval was not
required for the present narrative review; however, all selected
studies adhered to ethical guidelines for research involving human
and/or animal subjects.
Effect of WAT on skin during psoriasis
Overview
Over the past decade, extensive research has
highlighted the role of WAT in the development of psoriatic
lesions. Specifically, WAT exacerbates the severity of these
lesions by increasing inflammatory levels, promoting epidermal
proliferation and facilitating angiogenesis through the action of
adipokines, extracellular vesicles (EVs) and free fatty acids
(FFAs; Fig. 1).
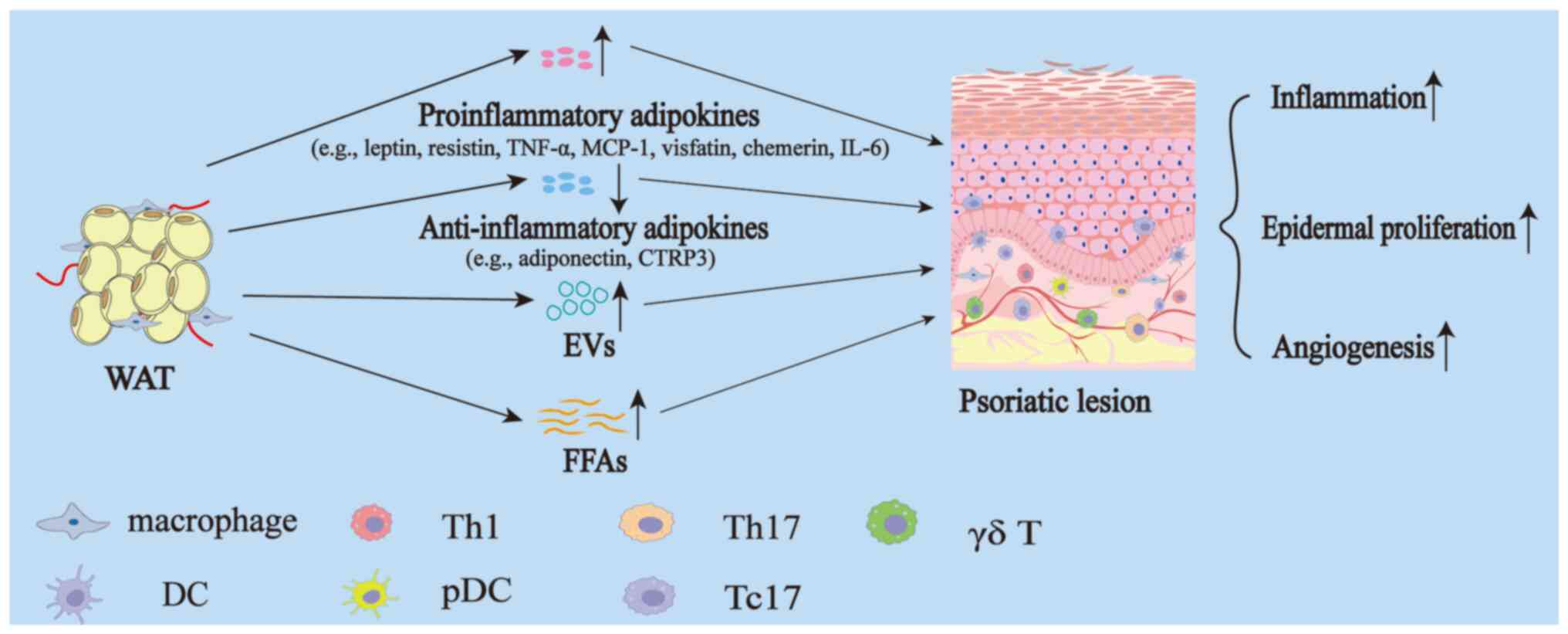 | Figure 1.Effect of WAT on skin during
psoriasis. In obesity, WAT undergoes expansion and functional
alterations, resulting in increased secretion of proinflammatory
adipokines such as leptin, resistin, TNF-α, MCP-1, visfatin,
chemerin, IL-6 and others, and decreased secretion of
anti-inflammatory adipokines such as adiponectin, CTRP3 and others.
Additionally, WAT exhibits elevated levels of EVs and FFAs. The
secretion of these bioactive molecules contributes to the promotion
of inflammation, epidermal hyperproliferation and angiogenesis in
psoriatic lesions. WAT, white adipose tissue; TNF-α, tumor necrosis
factor-α; MCP-1, monocyte chemoattractant protein-1; IL,
interleukin; CTRP, C1q/tumor necrosis factor-related protein; EVs,
extracellular vesicles; FFAs, free fatty acids; Th, T helper; DC,
dendritic cell; pDC, plasmacytoid dendritic cell; Tc, cytotoxic T
cell. |
Adipokines
Adipokines are bioactive molecules secreted by WAT
that are essential in modulating various physiological processes,
such as metabolism, inflammation and immune responses. To date,
>600 adipokines have been identified and they are generally
categorized into two categories, proinflammatory and
anti-inflammatory (25). During
the expansion of WAT, proinflammatory adipokines such as leptin,
resistin, TNF-α, monocyte chemoattractant protein-1 (MCP-1),
visfatin, chemerin and IL-6 are secreted in increased amounts. By
contrast, anti-inflammatory adipokines such as adiponectin and
C1q/tumor necrosis factor-related protein (CTRP) 3 are secreted in
reduced quantities (26–28). These alterations in adipokine
levels collectively contribute to increased systemic inflammation,
ultimately promoting the pathological development of inflammatory
diseases such as psoriasis (Fig.
2).
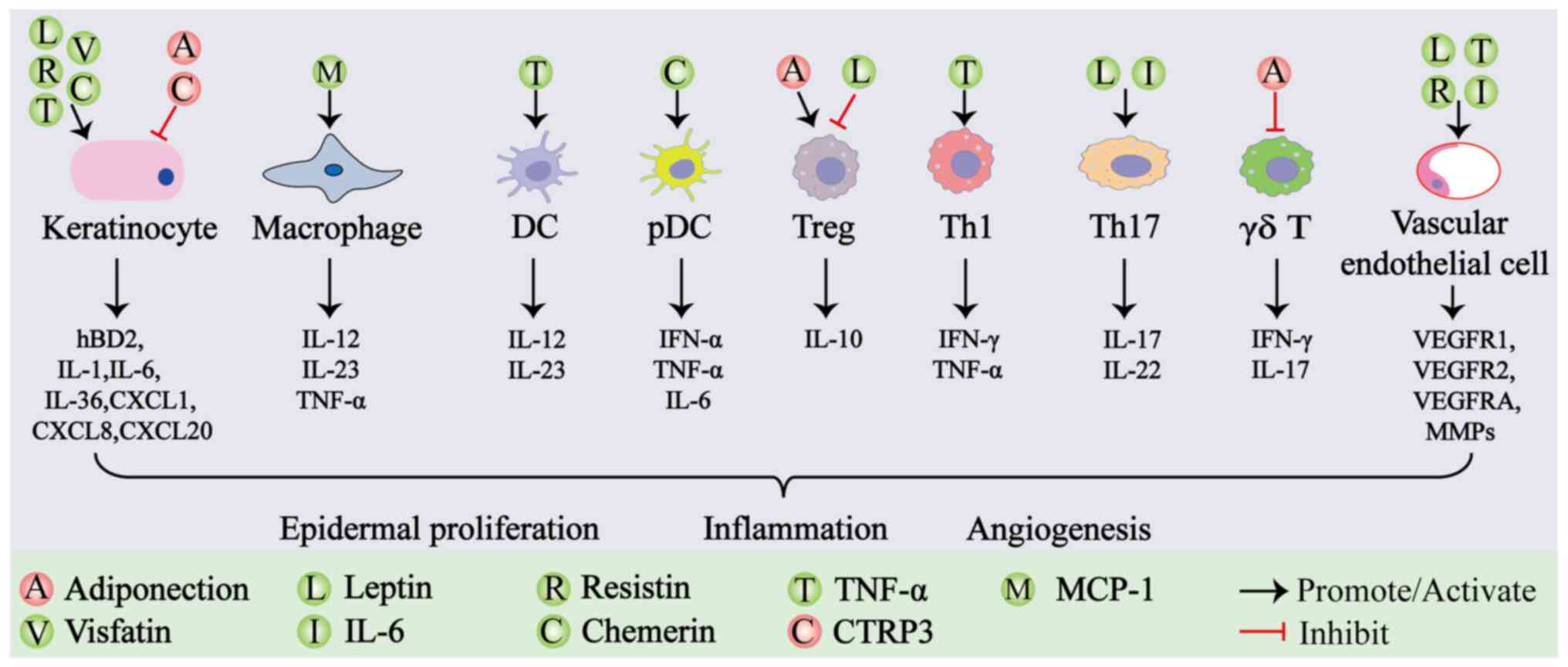 | Figure 2.Effect of adipokines on psoriatic
lesions. Different adipokines can modulate the quantity and
function of various cell types within psoriatic lesions, thereby
influencing the expression levels of cytokines. Ultimately, the
combined effects of these cytokines and dysfunctional cells lead to
the clinical manifestation of psoriatic lesions, characterized by
excessive epidermal proliferation, inflammation and angiogenesis.
Red balls labeled with different letters represent distinct
anti-inflammatory adipokines. Green balls labeled with different
letters represent distinct proinflammatory adipokines. TNF-α, tumor
necrosis factor-α; MCP-1, monocyte chemoattractant protein-1; IL,
interleukin; CTRP, C1q/tumor necrosis factor-related protein; Treg,
regulatory T cell; Th, T helper; DC, dendritic cell; pDC,
plasmacytoid dendritic cell; hBD2, human β-defensin 2; CXCL, C-X-C
motif chemokine ligand; IFN, interferon; VEGFR, vascular
endothelial growth factor receptor; VEGFA, vascular endothelial
growth factor A; MMPs, matrix metalloproteinases. |
Adiponectin
Adiponectin, a hormone secreted by WAT, exhibits
both anti-inflammatory and insulin-sensitizing effects. Its role in
psoriasis is complex, involving various mechanisms related to
immune cells and inflammatory pathways. In patients with psoriasis,
plasma, skin and subcutaneous adiponectin levels are markedly lower
than those observed in healthy individuals (29–31).
Despite these diminished levels, there is a weak association
between adiponectin concentrations and the severity of psoriasis,
as assessed by the Psoriasis Activity Score Index (PASI) (30,32).
Furthermore, clinical treatments that improve psoriasis symptoms do
not seem to normalize adiponectin levels (30). This suggests that reduced
adiponectin levels might be more closely associated with WAT
dynamics during obesity rather than with psoriasis itself.
Animal studies provide evidence that adiponectin may
play a pathogenic role in the development of psoriasis. For
instance, mice lacking adiponectin show increased susceptibility to
psoriasiform lesions (31).
Adiponectin is considered to involve the promotion of M2 macrophage
differentiation and suppression of IL-17/IL-23 production, which
could help alleviate psoriatic inflammation (19,31,33).
Notably, lower adiponectin levels are negatively associated with
IL-23 gene expression (34). In
the skin of adiponectin-deficient mice treated with imiquimod, an
increase in IL-23p19 and IL-17 production has been observed
(31). Conversely, administering
adiponectin has been shown to inhibit IL-17 production in
adiponectin-deficient mice and suppress IL-17 production from
dermal γδ T cells in vitro (31). Additionally, a peptide derived from
adiponectin, known as P5, interacts with the AdipoR1 receptor to
inhibit IL-17 and mitigate imiquimod-induced psoriasiform lesions
in mouse models (35,36).
Adiponectin also influences psoriasis by affecting
the expression of human β-defensin 2 (hBD2), a marker involved in
inflammatory pathways, and may indirectly modulate IL-23 levels
(19,37). Furthermore, adiponectin promotes
the expansion of regulatory T cell (Treg) populations (38). Adiponectin stimulates AMP-activated
protein kinase, and this activation is associated with decreased
skin thickness and reduced psoriasis severity (39,40).
Overall, the diverse functions of adiponectin
indicate its potential protective role in psoriasis. However, the
reduction of adiponectin due to WAT expansion diminishes its
protective effects, resulting in heightened inflammation and
worsening of psoriatic lesions. Future studies could consider
focusing on longitudinal human data and exploring therapeutic
strategies, such as AdipoR1 agonists or adiponectin mimetics, as
potential approaches to address both the metabolic and inflammatory
components of psoriasis.
Leptin
Leptin is a hormone produced by WAT that regulates
appetite and energy balance, with its levels being associated with
fat stores and involvement in inflammatory diseases such as
psoriasis. Elevated leptin levels are commonly observed in the
plasma and skin of patients with psoriasis, particularly in severe
cases, and these levels are associated with disease severity as
indicated by the PASI (29,41,42).
The primary contribution of leptin to psoriasis lies in its ability
to enhance inflammation by stimulating the production of
proinflammatory cytokines, including IL-1, IL-6, CXCL8 and TNF-α,
which are pivotal in driving the inflammatory response (19,43).
This cascade promotes a Th1/Th17 immune response, leading to
increased levels of IL-17 and IL-23, which exacerbate the
characteristic symptoms of the disease (44,45).
Leptin also modulates immune cell function by
promoting the differentiation of Th17 cells. This effect is
mediated through the upregulation of retinoic acid receptor-related
orphan receptor γ t, a key transcription factor involved in Th17
cell development, thereby contributing to the accumulation of IL-17
(46). Moreover, leptin reduces
the numbers and functionality of Tregs, diminishing their
production of IL-10 and further exacerbating inflammation (47). Anti-leptin monoclonal antibodies
have been shown to enhance Treg proliferation, while leptin
receptor antagonists can increase Foxp3 expression and inhibit
IL-17 production, highlighting the role of leptin in immune
regulation (19,48,49).
Furthermore, leptin activates the Janus kinase (JAK)/signal
transducer and activator of transcription 3 (STAT3) signaling
pathway, which amplifies the inflammatory response and promotes
angiogenesis in psoriatic lesions, thereby supporting ongoing
inflammation and disease progression (50).
In keratinocytes, leptin influences proliferation
and function by stimulating the release of amphiregulin, a growth
factor that accelerates keratinocyte proliferation, contributing to
the hyperproliferation observed in psoriatic lesions (51). Additionally, leptin upregulates the
expression of chemokines such as CXCL8, CXCL1 and CCL20, further
stimulating keratinocyte proliferation and inflammation (52). It acts synergistically with IL-17A
to enhance the expression of these chemokines and hBD2 through
mitogen-activated protein kinase (MAPK) and JAK2 pathways, leading
to impaired keratinocyte maturation and excessive expression of
disease-related genes (19,53).
In summary, leptin serves a critical mediating role
in WAT and psoriatic lesions by promoting inflammatory responses,
enhancing immune cell activity, influencing keratinocyte
proliferation and maturation, and promoting angiogenesis. These
actions collectively exacerbate the pathological progression of
psoriasis. Future research should further explore the heterogeneity
of leptin signaling pathways in specific patient subgroups, such as
obese and non-obese individuals, and assess the clinical
translational potential of targeting leptin.
Resistin
In rodents, resistin is primarily produced by WAT,
while in humans it is mainly secreted by immune and epithelial
cells. Although WAT is not the primary source of resistin in
humans, its production in WAT increases during obesity (43,54).
Elevated levels of resistin have been observed in patients with
psoriasis and are positively associated with disease severity
(29,55). The role of resistin in psoriasis
initiates through its binding to Toll-like receptor (TLR) 4 and
cysteine-rich ankle-like protein 1 receptors, activating crucial
signaling pathways such as nuclear factor κB (NF-κB) (56,57).
This activation induces the production of proinflammatory cytokines
such as TNF-α, IL-6 and IL-1β, which play a central role in the
inflammatory cascade observed in psoriasis (58). As a result, immune cell recruitment
to the skin is promoted, exacerbating inflammation (2,58).
Furthermore, resistin directly influences
keratinocyte behavior, which is crucial for the development of
psoriatic lesions. It activates the NF-κB and Phosphoinositide
3-kinase/Protein kinase B (PI3K/AKT) signaling pathways, leading to
the upregulation of genes associated with cell cycle progression
and survival, such as human telomerase reverse transcriptase and
caveolin-1 (56,59,60).
This process results in increased keratinocyte proliferation and
impaired differentiation, contributing to the formation and
persistence of psoriatic lesions. Additionally, resistin affects
cellular apoptosis, disrupting the balance necessary for
maintaining skin homeostasis. By inhibiting apoptosis pathways,
such as PI3K/AKT and MAPK/Extracellular Signal-Regulated Kinase,
resistin leads to increased keratinocyte survival and impaired
turnover, further exacerbating plaque development (56,61).
Finally, resistin promotes angiogenesis in psoriatic
lesions by activating signaling pathways that enhance endothelial
cell proliferation and modulate the expression of
angiogenesis-related genes and chemokines, including vascular
endothelial growth factor (VEGF) receptor (VEGFR) 1, VEGFR2, VEGFA
and matrix metalloproteinases (56,62).
To conclude, resistin serves a critical mediating
role in WAT and psoriatic lesions by promoting inflammatory
responses, enhancing immune cell activity, influencing keratinocyte
proliferation and maturation, and promoting angiogenesis. These
actions collectively exacerbate the pathological progression of
psoriasis. Future studies could evaluate the potential of metabolic
interventions, such as weight loss or insulin-sensitizing
treatments, in jointly regulating resistin levels and psoriasis
pathology.
TNF-α
TNF-α, a proinflammatory adipokine, is markedly
upregulated during the expansion of WAT and exerts widespread
effects on various tissues through endocrine and paracrine
mechanisms. Increased concentrations of TNF-α are frequently
observed in the plasma and skin of patients with psoriasis, and are
associated with disease severity (55,63).
TNF-α is vital in establishing the inflammatory milieu of psoriatic
lesions, initiating and sustaining the disease process through
complex interactions among various cell types and signaling
pathways. It activates DCs, resulting in increased production of
IL-12, which facilitates the differentiation of naive T cells into
Th1 cells (6,64,65).
These Th1 cells then produce notable amounts of IFN-γ and TNF-α,
amplifying the inflammatory response and creating a feedback loop
that exacerbates the condition (63).
Additionally, TNF-α directly affects keratinocytes,
inducing their hyperproliferation and increasing the production of
proinflammatory cytokines such as IL-1, IL-6 and CXCL8, along with
NF-κB (65,66). It also upregulates adhesion
molecules such as E-selectin, P-selectin and intercellular adhesion
molecule-1, facilitating the recruitment of immune cells into
psoriatic lesions (65,67). Furthermore, TNF-α collaborates with
IL-17A, stabilizing IL-17A mRNA and enhancing IL-17 receptor
expression on keratinocytes, which increases the cellular response
to IL-17A (68). This interaction
sustains an inflammatory cascade, as IL-17A also promotes the
expression of TNF receptors (6,69).
Moreover, TNF-α influences angiogenesis in psoriatic
lesions by inducing the secretion of VEGF, promoting new blood
vessel formation (66). This
contributes to the increased vascularity observed in psoriatic
lesions, further supporting the ongoing inflammatory process.
To summarize, TNF-α is a central mediator in
psoriasis, orchestrating a complex network of immune cell
activation, cytokine production, keratinocyte proliferation and
angiogenesis, all of which drive the chronic inflammation and
lesion formation characteristic of the disease. Although TNF-α
inhibitors have shown marked efficacy in the treatment of
psoriasis, the mechanisms of resistance in some patients remain
unclear. Future research should further investigate the synergistic
effects of TNF-α targeted therapy and metabolic interventions, such
as glucagon-like peptide-1 receptor agonists, to improve both skin
symptoms and systemic metabolic function and inflammation levels in
patients.
Other adipokines
In addition to the aforementioned adipokines,
several other adipokines have been identified as being expressed at
high levels in expanded WAT and have been extensively studied for
their roles and mechanisms in psoriatic lesions.
MCP-1 levels are increased in the serum and skin of
patients with psoriasis (70,71).
MCP-1 contributes to psoriasis by promoting the migration and
activation of macrophages in the skin, thereby driving
inflammation. This process involves MCP-1 working in conjunction
with TNF-α to initiate and sustain the inflammatory environment,
influencing immune cell recruitment, and maintaining the
inflammatory cycle in psoriatic lesions (70). Visfatin levels are also increased
in the serum of patients with psoriasis, showing a positive
association with disease severity (72). Visfatin amplifies the inflammatory
responses in keratinocytes triggered by TNF-α by upregulating
CXCL8, CXCL10, CCL20 and antimicrobial peptides (73,74).
Additionally, serum chemerin concentrations are elevated in
patients with psoriasis (75).
Chemerin exacerbates psoriasis by promoting the migration of pDCs
to psoriatic lesions, activating NF-κB, and upregulating
proinflammatory cytokines such as TNF-α, IL-6 and CXCL8 (19,76).
Furthermore, chemerin increases keratin 16 expression, which is
associated with keratinocyte proliferation and contributes to
psoriasis pathogenesis (77).
Elevated levels of IL-6 have also been observed in the plasma and
skin of patients with psoriasis, and these levels are associated
with disease severity as indicated by PASI (63,78).
IL-6 contributes to psoriatic lesions by promoting Th17 cell
differentiation, inhibiting the suppressive functions of Tregs and
inducing angiogenesis through the upregulation of VEGF production
(63).
In psoriasis, reduced levels of adipocyte-derived
CTRP3 in the blood lead to decreased anti-inflammatory effects, as
CTRP3 typically exerts its protective role through the
lysosomal-associated membrane protein 1-STAT3 axis, mitigating
inflammation in the skin lesions (79).
In conclusion, various adipokines, including MCP-1,
visfatin, chemerin, IL-6 and CTRP3, play notable roles in psoriasis
by influencing inflammation, immune cell dynamics, keratinocyte
proliferation and angiogenesis. These adipokines interact within a
complex network that affects disease severity and progression.
Further investigation is required to clarify the synergistic roles
of these adipokines in the development of psoriatic lesions.
EVs
EVs are tiny membrane-bound particles released by
cells into the extracellular environment. They can be categorized
into four primary types: i) Exosomes; ii) apoptotic bodies; iii)
microvesicles; and iv) oncosomes (80). EVs are vital for intercellular
communication, transporting proteins, lipids and nucleic acids that
influence various biological processes, including immune responses,
disease progression and potential therapeutic strategies (81).
In obese patients, the levels of EVs in peripheral
blood are markedly higher compared with those in healthy
individuals, contributing to increased systemic inflammation
(82). EVs derived from WAT
primarily contain adipokines such as IL-6, MCP-1, adiponectin and
resistin (82,83). Given that these adipokines are
widely involved in the inflammatory response associated with
psoriatic lesions, it is hypothesized that adipose-derived EVs also
play a role in the pathological development of psoriasis.
Furthermore, EVs derived from WAT in obesity contain a single-exon
circular RNA known as circ_0075932, which can promote inflammation
by activating the NF-κB pathway in the skin (84).
Notably, exosomes derived from mesenchymal stem
cells of healthy WAT have been shown to suppress local inflammation
in psoriatic lesions and improve clinical symptoms (85). This finding supports the notion
that WAT can interact with the skin through EVs. The differences in
the composition of EVs released by WAT under various conditions may
represent a critical regulatory factor in the pathological changes
observed in psoriatic lesions. However, research on the role of EVs
in mediating crosstalk between WAT and skin remains limited.
Further studies are needed to elucidate the role of EVs in the
onset, progression and resolution of psoriatic lesions.
FFAs
During the expansion of WAT, FFAs increase due to
enhanced lipolysis from adipocyte, reflecting marked metabolic
changes (86). Elevated serum FFA
levels, particularly saturated FFAs, are associated with the
severity of skin lesions in both patients and mice models (87,88).
An in vitro study showed that FFAs sensitize DCs, enhancing
the secretion of cytokines during proinflammatory stimulation
(89).
Additionally, FFAs directly stimulate the secretion
of key inflammatory cytokines, including IL-1β and IL-23 from
TLR-activated myeloid cells (90).
Notably, dermal γδ T cells, which are implicated in psoriatic
lesions, exhibit higher lipid content and uptake than their
IFN-γ-producing counterparts. This suggests that FFAs not only
enhance inflammatory signaling, but also favor the differentiation
and accumulation of pathogenic T cells (91).
Moreover, the preferential uptake of elevated levels
of long-chain FFAs by Tregs leads to cellular mitochondrial
dysfunction, oxidative stress and lipotoxicity (92). This ultimately reduces the
population of Tregs and is associated with diminished regulation of
dermal γδ T cell-mediated inflammation, exacerbating the
inflammatory response associated with psoriatic lesions (92).
Collectively, the expansion of WAT can facilitate
the pathological progression of psoriatic lesions through elevated
levels of FFAs, suggesting that targeting FFA metabolism may offer
promising therapeutic avenues for managing psoriasis and its
associated inflammatory responses in the future.
Effect of skin on WAT during psoriasis
While extensive research has focused on the effects
of WAT on the skin, studies investigating the role and mechanisms
by which the skin regulates WAT remain limited. Under physiological
conditions, the skin has been shown to participate in the
development and proliferation of dermal WAT as demonstrated in
mouse models (24,47).
In psoriasis, heightened inflammation in psoriatic
lesions can provoke inflammatory responses in the underlying
subcutaneous adipose tissue (SAT), leading to increased expression
of microRNA (miR)-26b-5p in this tissue, which results in
functional defects in cholesterol efflux (21,93).
Additionally, the disruption of the skin barrier and
thermoregulatory functions caused by psoriatic lesions may promote
the browning of SAT (94).
Beyond localized effects, psoriatic lesions may also
facilitate systemic expansion of WAT, potentially contributing to
obesity. This occurs through the release of proinflammatory
cytokines into the bloodstream (94). Cytokines essential to the
inflammatory pathways in psoriasis such as IL-17, IFN-γ and IL-36
are expressed at elevated levels in the lesion (6). Studies have indicated that these
cytokines are also found in increased concentrations in the serum
of affected patients (95,96). Evidence suggests that IL-17, IFN-γ
and IL-36 can promote inflammatory cell infiltration and the
release of inflammatory factors in WAT (23,97,98).
Consequently, these cytokines, which are expressed at high levels
in psoriatic lesions, serve as a communication bridge between skin
and WAT. Conversely, a study involving transgenic mice which
overexpressed caspase-1 specific to keratin 14 and exhibited
systemic skin inflammation showed visceral WAT atrophy (22). While this model does not accurately
reflect the inflammatory changes seen in psoriasis, it highlights
the complexity of the relationship between skin inflammation and
WAT.
In brief, psoriatic lesions can induce inflammation
and dysfunction in WAT through both paracrine and endocrine
mechanisms (Fig. 3). Future
research should aim to elucidate the specific regulatory effects
and molecular mechanisms by which psoriatic lesions influence
WAT.
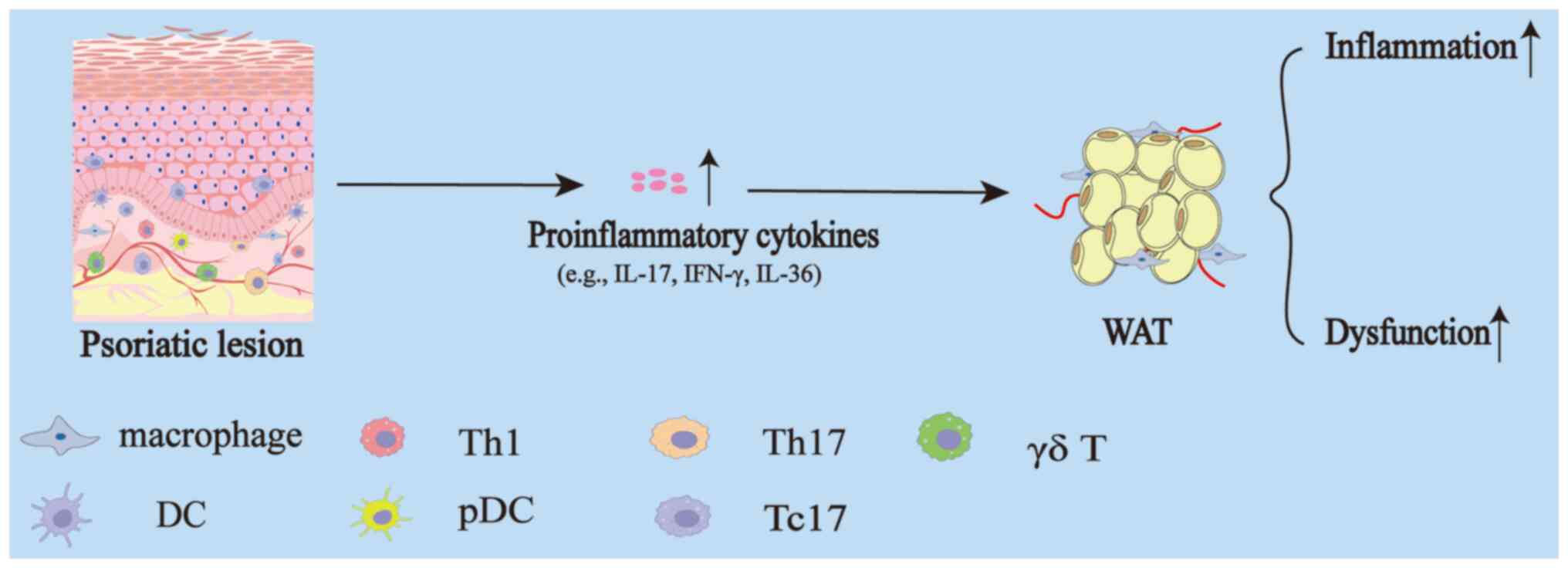 | Figure 3.Effect of skin on WAT during
psoriasis. In psoriatic lesions, there is a marked infiltration of
inflammatory cells, which produce a variety of pro-inflammatory
cytokines, such as IL-17, IFN-γ, IL-36 and others. These cytokines,
through paracrine and endocrine mechanisms, ultimately lead to
elevated levels of inflammation and functional impairment of WAT.
WAT, white adipose tissue; IL, interleukin; IFN, interferon; Th, T
helper; DC, dendritic cell; pDC, plasmacytoid dendritic cell; Tc,
cytotoxic T cell. |
Discussion and conclusions
Current research has revealed notable crosstalk
between WAT and skin lesions in psoriasis. This interaction plays a
critical role in driving inflammatory progression, epidermal
hyperproliferation and local angiogenesis in skin lesions. On the
other hand, skin lesions exacerbate inflammation and dysfunction in
WAT by upregulating inflammatory factors. Despite these insights,
several key aspects of this complex relationship remain
underexplored.
First, most existing studies have primarily focused
on the role of adipokines in mediating communication between WAT
and skin. However, the mechanisms through which EVs and lipids
influence the effects of WAT on skin lesions are largely unexplored
and require further investigation. Second, current research often
examines individual molecules or pathways in isolation, lacking a
comprehensive, integrated analysis of the way multiple molecules
interact to regulate the pathogenesis of psoriasis through
synergistic or antagonistic effects. Furthermore, research has
mainly concentrated on the unidirectional regulation of psoriatic
lesions by WAT, while the feedback effects of psoriatic lesions on
WAT remodeling and systemic metabolism lack systematic analysis.
Additionally, the differential impact of WAT from different
anatomical sites such as subcutaneous compared with visceral fat on
psoriasis has not been clearly defined in current studies. The
dynamic changes in WAT-skin interactions across different stages of
psoriasis (acute compared with chronic) also remain unclear.
Finally, while intervention strategies targeting adipokines or
lipid metabolism have shown promise in animal models, there is a
lack of large-scale clinical trials to validate their efficacy and
safety.
In the future, the field of WAT-skin crosstalk in
psoriasis still holds immense research potential, warranting
continued attention. A key area of focus should be the application
of systems biology approaches, integrating multi-omics data to
explore in greater depth the way various molecules originating from
WAT interact within complex networks to collectively drive the
development and progression of psoriasis. Furthermore, applications
of novel models, such as the transgenic zebrafish model developed
for dynamic monitoring of adipocytes (99), will enhance research into the
interactions between WAT and skin. Additionally, conducting
targeted clinical trials to evaluate the impact of obesity
treatments, such as semaglutide, on improving skin lesions in obese
patients with psoriasis is of great importance. In addition, given
the potential therapeutic value of exosomes in disease management,
developing engineered exosomes based on the secretion profile of
healthy WAT holds significant research and clinical promise.
Lastly, a promising direction for future research involves
developing novel targeted drugs based on key molecules involved in
the WAT-skin interaction mechanism, with the potential for clinical
application.
In conclusion, the present narrative review offered
a comprehensive analysis of the latest advances in understanding
the molecular mechanisms driving the bidirectional communication
between WAT and skin in psoriasis. The expansion of WAT contributes
to inflammation, epidermal proliferation, and angiogenesis in skin
lesions through the release of adipokines, EVs, and FFAs.
Conversely, psoriatic lesions induce dysregulation in the
inflammation and function of WAT. These mechanistic insights carry
significant clinical implications: Comorbidity management through
metabolic interventions could synergize with conventional
anti-psoriatic therapies to address both cutaneous and systemic
inflammation. Furthermore, lifestyle integration emphasizing weight
management and dietary interventions should be prioritized in
treatment protocols, given the cyclical relationship between
adiposity and psoriatic severity. By adopting this dual-focused
therapeutic paradigm that concurrently targets cutaneous and
metabolic pathologies, clinicians may achieve improved long-term
outcomes while mitigating metabolic risks in patients with
psoriasis.
Acknowledgements
Not applicable.
Funding
The current study was funded by the National Natural Science
Foundation of China (grant no. 82260283).
Availability of data and materials
Not applicable.
Authors' contributions
QS was responsible for data curation, funding
acquisition and writing the original draft. PX was responsible for
data curation, formal analysis, visualization and writing the
original draft. QX was responsible for investigation and writing
the original draft. CZ was responsible for methodology and writing
the original draft. YM was responsible for conceptualization,
project administration, resources, supervision, validation, writing
the original draft and writing, reviewing and editing. All authors
have read and approved the final version of the manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Greb JE, Goldminz AM, Elder JT, Lebwohl
MG, Gladman DD, Wu JJ, Mehta NN, Finlay AY and Gottlieb AB:
Psoriasis. Nat Rev Dis Primers. 2:160822016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boehncke WH and Schön MP: Psoriasis.
Lancet. 386:983–994. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fülle M, Metze D, Böer-Auer A, Osada N and
Braun SA: Psoriasis on the leg: Site-specific histopathological and
immuno-histochemical features and diagnostic difficulties. Acta
Derm Venereol. 101:adv004532021.PubMed/NCBI
|
|
4
|
Ma F, Plazyo O, Billi AC, Tsoi LC, Xing X,
Wasikowski R, Gharaee-Kermani M, Hile G, Jiang Y, Harms PW, et al:
Single cell and spatial sequencing define processes by which
keratinocytes and fibroblasts amplify inflammatory responses in
psoriasis. Nat Commun. 14:34552023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Han Q, Niu X, Hou R, Li J, Liu Y, Li X, Li
J, Li Y, Zhang K and Wu Y: Dermal mesenchymal stem cells promoted
adhesion and migration of endothelial cells by integrin in
psoriasis. Cell Biol Int. 45:358–367. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Griffiths CEM, Armstrong AW, Gudjonsson JE
and Barker JNWN: Psoriasis. Lancet. 397:1301–1315. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Singh R, Koppu S, Perche PO and Feldman
SR: The cytokine mediated molecular pathophysiology of psoriasis
and its clinical implications. Int J Mol Sci. 22:127932021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alajroush WA, Alrshid AI, Alajlan AH,
Alsalamah YB, Alhumaidan MI, Alhoumedan AI, Alrasheed MI,
Alowairdhi YA, Alowirdi F, Aljoufi AZ, et al: Psoriasis and
metabolic disorders: A comprehensive meta-analysis of million
adults worldwide. Cureus. 16:e520992024.PubMed/NCBI
|
|
9
|
Budu-Aggrey A, Brumpton B, Tyrrell J,
Watkins S, Modalsli EH, Celis-Morales C, Ferguson LD, Vie GÅ,
Palmer T, Fritsche LG, et al: Evidence of a causal relationship
between body mass index and psoriasis: A mendelian randomization
study. PLoS Med. 16:e10027392019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leite BF, Morimoto MA, Gomes C, de
Carvalho Klemz BN, de Souza Genaro P, Damasceno NRT, Szejnfeld VL
and de Medeiros Pinheiro M: Higher bodily adiposity, fat intake,
and cholesterol serum levels are associated with higher disease
activity in psoriatic arthritis patients: Is there a link among fat
and skin and joint involvement? Lipids Health Dis. 19:212020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park SH, Lee KA, Choi JH, Park S, Kim DW
and Jung SY: Impact of obesity on the IL-6 immune marker and Th17
immune cells in C57BL/6 mice models with imiquimod-induced
psoriasis. Int J Mol Sci. 24:55922023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paroutoglou K, Papadavid E,
Christodoulatos GS and Dalamaga M: Deciphering the association
between psoriasis and obesity: Current evidence and treatment
considerations. Curr Obes Rep. 9:165–178. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Malavazos AE, Meregalli C, Sorrentino F,
Vignati A, Dubini C, Scravaglieri V, Basilico S, Boniardi F,
Spagnolo P, Malagoli P, et al: Semaglutide therapy decreases
epicardial fat inflammation and improves psoriasis severity in
patients affected by abdominal obesity and type-2 diabetes.
Endocrinol Diabetes Metab Case Rep. 2023:23–0017. 2023.PubMed/NCBI
|
|
14
|
Corbetta S, Angioni R, Cattaneo A,
Beck-Peccoz P and Spada A: Effects of retinoid therapy on insulin
sensitivity, lipid profile and circulating adipocytokines. Eur J
Endocrinol. 154:83–86. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Horwitz A and Birk R: Adipose tissue
hyperplasia and hypertrophy in common and syndromic obesity-the
case of BBS obesity. Nutrients. 15:34452023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Corvera S, Solivan-Rivera J and Loureiro
ZY: Angiogenesis in adipose tissue and obesity. Angiogenesis.
25:439–453. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kawai T, Autieri MV and Scalia R: Adipose
tissue inflammation and metabolic dysfunction in obesity. Am J
Physiol Cell Physiol. 320:C375–C391. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ruiz-Ojeda FJ, Méndez-Gutiérrez A,
Aguilera CM and Plaza-Díaz J: Extracellular matrix remodeling of
adipose tissue in obesity and metabolic diseases. Int J Mol Sci.
20:48882019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kiełbowski K, Bakinowska E, Ostrowski P,
Pala B, Gromowska E, Gurazda K, Dec P, Modrzejewski A and Pawlik A:
The role of adipokines in the pathogenesis of psoriasis. Int J Mol
Sci. 24:63902023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guan J, Feng J, Xu M, Liu M, He Y and Lu
F: Adipokines-enriched adipose extract restores skin barrier and
ameliorates inflammatory dysregulation in an atopic dermatitis
mouse model. Plast Reconstr Surg. 154:701e–712e. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Caton PW, Evans EA, Philpott MP and Hannen
RF: Can the skin make you fat? A role for the skin in regulating
adipose tissue function and whole-body glucose and lipid
homeostasis. Curr Opin Pharmacol. 37:59–64. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mizutani K, Shirakami E, Ichishi M,
Matsushima Y, Umaoka A, Okada K, Yamaguchi Y, Watanabe M, Morita E
and Yamanaka K: systemic dermatitis model mice exhibit atrophy of
visceral adipose tissue and increase stromal cells via skin-derived
inflammatory cytokines. Int J Mol Sci. 21:33672020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pestel J, Chehimi M, Bonhomme M, Robert M,
Vidal H and Eljaafari A: IL-17A contributes to propagation of
inflammation but does not impair adipogenesis and/or insulin
response, in adipose tissue of obese individuals. Cytokine.
126:1548652020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ueyama T, Sakuma M, Nakatsuji M, Uebi T,
Hamada T, Aiba A and Saito N: Rac-Dependent signaling from
keratinocytes promotes differentiation of intradermal white
adipocytes. J Invest Dermatol. 140:75–84.e6. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kovács D, Fazekas F, Oláh A and Törőcsik
D: Adipokines in the skin and in dermatological diseases. Int J Mol
Sci. 21:90482020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hwang J, Yoo JA, Yoon H, Han T, Yoon J, An
S, Cho JY and Lee J: The role of leptin in the association between
obesity and psoriasis. Biomol Ther (Seoul). 29:11–21. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Soujanya KV and Jayadeep AP:
Obesity-associated biochemical markers of inflammation and the role
of grain phytochemicals. J Food Biochem. 46:e142572022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wolf RM, Steele KE, Peterson LA, Magnuson
TH, Schweitzer MA and Wong GW: Lower circulating C1q/TNF-related
protein-3 (CTRP3) levels are associated with obesity: A
cross-sectional study. PLoS One. 10:e01339552015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Słuczanowska-Głabowska S, Staniszewska M,
Marchlewicz M, Duchnik E, Łuczkowska K, Safranow K, Machaliński B
and Pawlik A: Adiponectin, leptin and resistin in patients with
psoriasis. J Clin Med. 12:6632023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gerdes S, Pinter A, Biermann M,
Papavassilis C and Reinhardt M: Adiponectin levels in a large
pooled plaque psoriasis study population. J Dermatolog Treat.
31:531–534. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shibata S, Tada Y, Hau CS, Mitsui A,
Kamata M, Asano Y, Sugaya M, Kadono T, Masamoto Y, Kurokawa M, et
al: Adiponectin regulates psoriasiform skin inflammation by
suppressing IL-17 production from γδ-T cells. Nat Commun.
6:76872015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gerdes S, Osadtschy S, Rostami-Yazdi M,
Buhles N, Weichenthal M and Mrowietz U: Leptin, adiponectin,
visfatin and retinol-binding protein-4-mediators of comorbidities
in patients with psoriasis? Exp Dermatol. 21:43–47. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oh J, Lee Y, Oh SW, Li T, Shin J, Park SH
and Lee J: The role of adiponectin in the skin. Biomol Ther
(Seoul). 30:221–231. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kochumon S, Hasan A, Al-Rashed F, Sindhu
S, Thomas R, Jacob T, Al-Sayyar A, Arefanian H, Al Madhoun A,
Al-Ozairi E, et al: Increased adipose tissue expression of IL-23
associates with inflammatory markers in people with high LDL
cholesterol. Cells. 11:30722022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ohn J, Been KW, Kim JY, Kim EJ, Park T,
Yoon HJ, Ji JS, Okada-Iwabu M, Iwabu M, Yamauchi T, et al:
Discovery of a transdermally deliverable pentapeptide for
activating AdipoR1 to promote hair growth. EMBO Mol Med.
13:e137902021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Suh JH, Lee Y, Ohn J, Kim EJ, Kim TG, Jo
SJ, Kim SJ and Chung JH: Adiponectin-derived pentapeptide
ameliorates psoriasiform skin inflammation by suppressing IL-17
production in γδT cells. J Dermatol Sci. 106:45–52. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jin T, Sun Z, Chen X, Wang Y, Li R, Ji S
and Zhao Y: Serum human beta-defensin-2 is a possible biomarker for
monitoring response to JAK inhibitor in psoriasis patients.
Dermatology. 233:164–169. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tsang JYS, Li D, Ho D, Peng J, Xu A, Lamb
J, Chen Y and Tam PKH: Novel immunomodulatory effects of
adiponectin on dendritic cell functions. Int Immunopharmacol.
11:604–609. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
D'Amico F, Costantino G, Salvatorelli L,
Ramondetta A, De Pasquale R, Sortino MA and Merlo S: Inverse
correlation between the expression of AMPK/SIRT1 and NAMPT in
psoriatic skin: A pilot study. Adv Med Sci. 67:262–268. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shen H, Sha Y, Huang J, Mao AQ, Zhang T,
Wu MY, Sun F, Yu YY, Cheng ZQ and Gong YT: The roles of
AMPK-mediated autophagy and mitochondrial autophagy in a mouse
model of imiquimod-induced psoriasis. Am J Transl Res.
13:12626–12637. 2021.PubMed/NCBI
|
|
41
|
Wang R, Yu C, Tang Z, Sun J, Wang Y, Zhao
Z, Lin B and Li C: Leptin induces altered differentiation of
keratinocytes by inducing insulin resistance: Implications for
metabolic syndrome-induced resistance of psoriatic therapy. J
Dermatolog Treat. 35:23093052024. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhu KJ, Zhang C, Li M, Zhu CY, Shi G and
Fan YM: Leptin levels in patients with psoriasis: A meta-analysis.
Clin Exp Dermatol. 38:478–483. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Johnston A, Arnadottir S, Gudjonsson JE,
Aphale A, Sigmarsdottir AA, Gunnarsson SI, Steinsson JT, Elder JT
and Valdimarsson H: Obesity in psoriasis: Leptin and resistin as
mediators of cutaneous inflammation. Br J Dermatol. 159:342–350.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Su X, Cheng Y and Chang D: The important
role of leptin in modulating the risk of dermatological diseases.
Front Immunol. 11:5935642020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Su X, Zhang G, Cheng Y and Wang B: Leptin
in skin disease modulation. Clin Chim Acta. 516:8–14. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yu Y, Liu Y, Shi FD, Zou H, Matarese G and
La Cava A: Cutting edge: Leptin-induced RORγt expression in CD4+ T
cells promotes Th17 responses in systemic lupus erythematosus. J
Immunol. 190:3054–3058. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Walendzik K, Kopcewicz M, Bukowska J,
Panasiewicz G, Szafranska B and Gawronska-Kozak B: The
transcription factor FOXN1 regulates skin adipogenesis and affects
susceptibility to diet-induced obesity. J Invest Dermatol.
140:1166–1175.e9. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
De Rosa V, Procaccini C, Calì G, Pirozzi
G, Fontana S, Zappacosta S, La Cava A and Matarese G: A key role of
leptin in the control of regulatory T cell proliferation. Immunity.
26:241–255. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang W, Zhang BT, Jiang QL, Zhao HQ, Xu Q,
Zeng Y, Xu JY and Jiang J: Leptin receptor antagonist attenuates
experimental autoimmune thyroiditis in mice by regulating Treg/Th17
cell differentiation. Front Endocrinol (Lausanne). 13:10425112022.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Calautti E, Avalle L and Poli V:
Psoriasis: A STAT3-centric view. Int J Mol Sci. 19:1712018.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Markaki A, Gkouskou K, Stylianou K,
Dermitzaki E, Perakis K, Margioris A and Daphnis E: Relationship
between adiposity, adipokines, inflammatory markers and lipid
profile in hemodialysis patients. Eur Rev Med Pharmacol Sci.
18:1496–1498. 2014.PubMed/NCBI
|
|
52
|
Ikeda K, Morizane S, Akagi T,
Hiramatsu-Asano S, Tachibana K, Yahagi A, Iseki M, Kaneto H, Wada
J, Ishihara K, et al: Obesity and dyslipidemia synergistically
exacerbate psoriatic skin inflammation. Int J Mol Sci. 23:43122022.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kanda N and Watanabe S: Leptin enhances
human beta-defensin-2 production in human keratinocytes.
Endocrinology. 149:5189–5198. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Khanna D, Khanna S, Khanna P, Kahar P and
Patel BM: Obesity: A chronic low-grade inflammation and its
markers. Cureus. 14:e227112022.PubMed/NCBI
|
|
55
|
Žužul K, Kunjko K, Milošević M, Tončić RJ,
Kelava T and Hadžavdić SL: The association between the severity of
psoriasis and obesity based on the analysis of visceral fat index
and serum levels of tumor necrosis factor-α, interleukin-6, and
resistin. Acta Dermatovenerol Croat. 30:8–17. 2022.PubMed/NCBI
|
|
56
|
Srikanth M and Rasool M: Resistin-A
plausible therapeutic target in the pathogenesis of psoriasis.
Immunol Invest. 53:115–159. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Shao S, Fang H, Dang E, Xue K, Zhang J, Li
B, Qiao H, Cao T, Zhuang Y, Shen S, et al: Neutrophil extracellular
traps promote inflammatory responses in psoriasis via activating
epidermal TLR4/IL-36R crosstalk. Front Immunol. 10:7462019.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Taouis M and Benomar Y: Is resistin the
master link between inflammation and inflammation-related chronic
diseases? Mol Cell Endocrinol. 533:1113412021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Mahmoud MA, Mohamed RW, Attia FM, Atwa MA
and Abdel-Moneim SM: Expression of telomerase reverse transcriptase
in psoriatic lesional skin. Dis Markers. 22:265–269. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang F, Li H, Zhou Y, Gu Y and Wang L:
Caveolin-1 expression in different types of psoriatic lesions:
Analysis of 66 cases. Indian J Dermatol. 59:225–229. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Huang TH, Lin CF, Alalaiwe A, Yang SC and
Fang JY: Apoptotic or antiproliferative activity of natural
products against keratinocytes for the treatment of psoriasis. Int
J Mol Sci. 20:25582019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chen SS, Tang CH, Chie MJ, Tsai CH, Fong
YC, Lu YC, Chen WC, Lai CT, Wei CY, Tai HC, et al: Resistin
facilitates VEGF-A-dependent angiogenesis by inhibiting miR-16-5p
in human chondrosarcoma cells. Cell Death Dis. 10:312019.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sieminska I, Pieniawska M and Grzywa TM:
The immunology of psoriasis-current concepts in pathogenesis. Clin
Rev Allergy Immunol. 66:164–191. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Jang DI, Lee AH, Shin HY, Song HR, Park
JH, Kang TB, Lee SR and Yang SH: The role of tumor necrosis factor
alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in
therapeutics. Int J Mol Sci. 22:27192021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Man AM, Orăsan MS, Hoteiuc OA,
Olănescu-Vaida-Voevod MC and Mocan T: Inflammation and psoriasis: A
comprehensive review. Int J Mol Sci. 24:160952023. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Patel AB, Tsilioni I, Weng Z and
Theoharides TC: TNF stimulates IL-6, CXCL8 and VEGF secretion from
human keratinocytes via activation of mTOR, inhibited by
tetramethoxyluteolin. Exp Dermatol. 27:135–143. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tan JK, Aphale A, Malaviya R, Sun Y and
Gottlieb AB: Mechanisms of action of etanercept in psoriasis. J
Investig Dermatol Symp Proc. 12:38–45. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Johansen C, Funding AT, Otkjaer K,
Kragballe K, Jensen UB, Madsen M, Binderup L, Skak-Nielsen T,
Fjording MS and Iversen L: Protein expression of TNF-alpha in
psoriatic skin is regulated at a posttranscriptional level by
MAPK-activated protein kinase 2. J Immunol. 176:1431–1438. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
de Alcantara CC, Reiche EMV and Simão ANC:
Cytokines in psoriasis. Adv Clin Chem. 100:171–204. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Behfar S, Hassanshahi G, Nazari A and
Khorramdelazad H: A brief look at the role of monocyte
chemoattractant protein-1 (CCL2) in the pathophysiology of
psoriasis. Cytokine. 110:226–231. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Purzycka-Bohdan D, Nedoszytko B, Zabłotna
M, Gleń J, Szczerkowska-Dobosz A and Nowicki RJ: Chemokine profile
in psoriasis patients in correlation with disease severity and
pruritus. Int J Mol Sci. 23:133302022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Chyl-Surdacka KM, Bartosińska J, Kowal M,
Przepiórka-Kosińska J, Krasowska D and Chodorowska G: Assessment of
visfatin concentrations in the serum of male psoriatic patients in
relation to metabolic abnormalities. Adv Clin Exp Med. 29:79–84.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Hau CS, Kanda N, Noda S, Tatsuta A, Kamata
M, Shibata S, Asano Y, Sato S, Watanabe S and Tada Y: Visfatin
enhances the production of cathelicidin antimicrobial peptide,
human β-defensin-2, human β-defensin-3, and S100A7 in human
keratinocytes and their orthologs in murine imiquimod-induced
psoriatic skin. Am J Pathol. 182:1705–1717. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kanda N, Hau CS, Tada Y, Tatsuta A, Sato S
and Watanabe S: Visfatin enhances CXCL8, CXCL10, and CCL20
production in human keratinocytes. Endocrinology. 152:3155–3164.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Chyl-Surdacka KM, Gerkowicz A, Bartosińska
J, Kowal M, Przepiórka-Kosińska J, Surdacki G, Krasowska D and
Chodorowska G: Analysis of serum chemerin concentrations in
psoriatic patients in relation to metabolic abnormalities. Postepy
Dermatol Alergol. 36:531–537. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wang Y, Huo J, Zhang D, Hu G and Zhang Y:
Chemerin/ChemR23 axis triggers an inflammatory response in
keratinocytes through ROS-sirt1-NF-κB signaling. J Cell Biochem.
120:6459–6470. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhang X, Yin M and Zhang LJ: Keratin 6, 16
and 17-critical barrier alarmin molecules in skin wounds and
psoriasis. Cells. 8:8072019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Lynch M, Ahern T, Sweeney CM, Malara A,
Tobin AM, O'Shea D and Kirby B: Adipokines, psoriasis, systemic
inflammation, and endothelial dysfunction. Int J Dermatol.
56:1103–1118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Xue K, Shao S, Fang H, Ma L, Li C, Lu Z
and Wang G: Adipocyte-derived CTRP3 exhibits anti-inflammatory
effects via LAMP1-STAT3 axis in psoriasis. J Invest Dermatol.
142:1349–1359.e8. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wang K and Zeng C: Extracellular vesicles
and obesity. Adv Exp Med Biol. 1418:143–153. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Jeppesen DK, Zhang Q, Franklin JL and
Coffey RJ: Extracellular vesicles and nanoparticles: Emerging
complexities. Trends Cell Biol. 33:667–681. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Kumar V, Kiran S, Kumar S and Singh UP:
Extracellular vesicles in obesity and its associated inflammation.
Int Rev Immunol. 41:30–44. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Kranendonk MEG, Visseren FLJ, van
Herwaarden JA, Nolte-'t Hoen ENM, de Jager W, Wauben MHM and
Kalkhoven E: Effect of extracellular vesicles of human adipose
tissue on insulin signaling in liver and muscle cells. Obesity
(Silver Spring). 22:2216–2223. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhang X, Chen L, Xiao B, Liu H and Su Y:
Circ_0075932 in adipocyte-derived exosomes induces inflammation and
apoptosis in human dermal keratinocytes by directly binding with
PUM2 and promoting PUM2-mediated activation of AuroraA/NF-κB
pathway. Biochem Biophys Res Commun. 511:551–558. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Meybodi MA, Nilforoushzadeh MA,
KhandanDezfully N and Mansouri P: The safety and efficacy of
adipose tissue-derived exosomes in treating mild to moderate plaque
psoriasis: A clinical study. Life Sci. 353:1229152024. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Nussbaumerova B and Rosolova H: Obesity
and dyslipidemia. Curr Atheroscler Rep. 25:947–955. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Saalbach A, Seitz AT, Kohlmann J, Kalweit
L, Vogt L, Selig L, Engel KM and Simon JC: Modulation of dietary
fatty acids in an open-label study improves psoriasis and dampens
the inflammatory activation status. Nutrients. 15:16982023.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Herbert D, Franz S, Popkova Y, Anderegg U,
Schiller J, Schwede K, Lorz A, Simon JC and Saalbach A: High-Fat
diet exacerbates early psoriatic skin inflammation independent of
obesity: Saturated fatty acids as key players. J Invest Dermatol.
138:1999–2009. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Stelzner K, Herbert D, Popkova Y, Lorz A,
Schiller J, Gericke M, Klöting N, Blüher M, Franz S, Simon JC and
Saalbach A: Free fatty acids sensitize dendritic cells to amplify
TH1/TH17-immune responses. Eur J Immunol. 46:2043–2053. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Mogilenko DA, Haas JT, L'homme L, Fleury
S, Quemener S, Levavasseur M, Becquart C, Wartelle J, Bogomolova A,
Pineau L, et al: Metabolic and innate immune cues merge into a
specific inflammatory response via the UPR. Cell.
177:1201–1216.e19. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Lopes N, McIntyre C, Martin S, Raverdeau
M, Sumaria N, Kohlgruber AC, Fiala GJ, Agudelo LZ, Dyck L, Kane H,
et al: Distinct metabolic programs established in the thymus
control effector functions of γδ T cell subsets in tumor
microenvironments. Nat Immunol. 22:179–192. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Sivasami P, Elkins C, Diaz-Saldana PP,
Goss K, Peng A, Hamersky M IV, Bae J, Xu M, Pollack BP, Horwitz EM,
et al: Obesity-induced dysregulation of skin-resident PPARγ+ Treg
cells promotes IL-17A-mediated psoriatic inflammation. Immunity.
56:1844–1861.e6. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Cheung L, Fisher RM, Kuzmina N, Li D, Li
X, Werngren O, Blomqvist L, Ståhle M and Landén NX: Psoriasis skin
inflammation-induced microRNA-26b targets NCEH1 in underlying
subcutaneous adipose tissue. J Invest Dermatol. 136:640–648. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Zhu T, Yang S, Mauro TM and Man MQ:
Association of epidermal biophysical properties with obesity and
its implications. Skin Pharmacol Physiol. 36:165–173. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Arican O, Aral M, Sasmaz S and Ciragil P:
Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and
IL-18 in patients with active psoriasis and correlation with
disease severity. Mediators Inflamm. 2005:273–279. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Sehat M, Talaei R, Dadgostar E,
Nikoueinejad H and Akbari H: Evaluating serum levels of IL-33,
IL-36, IL-37 and gene expression of IL-37 in patients with
psoriasis vulgaris. Iran J Allergy Asthma Immunol. 17:179–187.
2018.PubMed/NCBI
|
|
97
|
Sharma N, Akkoyunlu M and Rabin RL:
Macrophages-common culprit in obesity and asthma. Allergy.
73:1196–1205. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Frühbeck G, Gómez-Ambrosi J, Ramírez B,
Mentxaka A, Rodríguez A, Becerril S, Reina G, Valentí V, Moncada R,
Silva C and Catalán V: Corrigendum: Increased levels of
interleukin-36 in obesity and type 2 diabetes fuels adipose tissue
inflammation by inducing its own expression and release by
adipocytes and macrophages. Front Immunol. 14:11383162023.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Mao Y, Hong KH, Liao W, Li L, Kim SJ,
Xiong Y, Nam IK, Choe SK and Kwak SA: Generation of a novel
transgenic zebrafish for studying adipocyte development and
metabolic control. Int J Mol Sci. 22:39942021. View Article : Google Scholar : PubMed/NCBI
|

















