Introduction
Nelumbo nucifera, commonly known as the
lotus, has been revered since ancient times; according to the
Chinese proverb, it emerges untainted from the mud. This plant is
widespread across Asia and Australia and is considered important in
traditional Chinese medicine. Various parts of the lotus, including
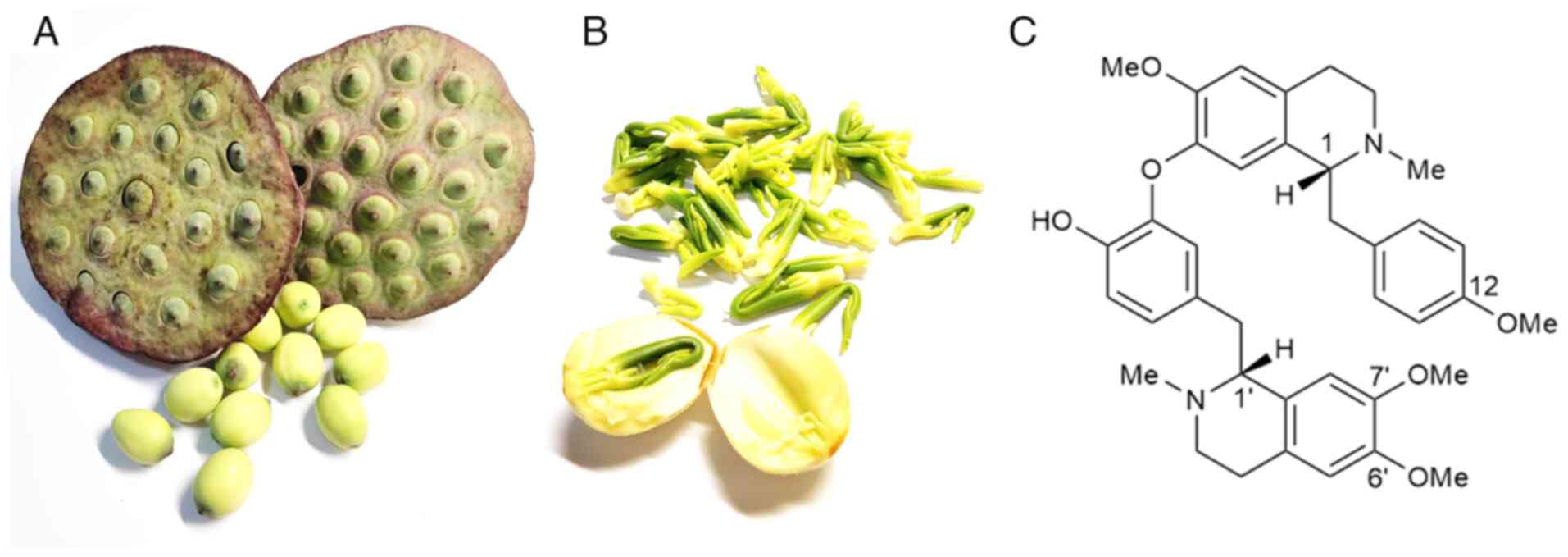
its seeds (Fig. 1A), rhizome,
leaves and flowers, have been used for medicinal purposes. The
rhizome is particularly effective in the treatment of hemorrhoids,
dysentery, chronic dyspepsia and other digestive disorders, in
addition to ameliorating diuresis and cholelithiasis. In addition,
the leaves are used to treat conditions such as hematemesis,
epistaxis, hemoptysis, hematuria, uterine bleeding and
hyperlipidemia, and the flowers are known to treat diarrhea,
cholera, fever and stomach ulcers. Furthermore, the lotus plumule
has been found to beneficial for the treatment of conditions such
as neurasthenia, insomnia, high fever with restlessness, and
cardiovascular diseases, including hypertension and arrhythmia
(1).
Advancements in medical chemistry and pharmacy have
facilitated the isolation of numerous medicinal substances from
different parts of the lotus, including alkaloids, terpenes,
flavonoids, fatty acids, carbohydrates, minerals and proteins
(2). These active compounds have
received considerable interest from researchers worldwide, who have
aimed to explore their chemical structures and pharmacological
effects, thereby laying the groundwork for the development of novel
drugs.
The lotus plumule (Fig.
1B), which comprises the radicle and young leaves of the mature
lotus seed, is traditionally used in Chinese medicine. Lotus leaf,
Euryale ferox Salisb., and lotus plumule are frequently used
together as a three-component formulation. In traditional Chinese
medicine, this formulation is considered to clear damp-heat, reduce
excess and disperse evils (3).
Similarly, Qinggong decoction (4),
which comprises lotus plumule, is traditionally believed to be
effective in clearing the heart, detoxifying, nourishing Yin and
promoting fluid production, thereby treating fever, dizziness,
insomnia and delirium (5). Key
secondary metabolites in lotus plumule include alkaloids, such as
dauricine, liensinine, isoliensinine, nuciferine, pronuciferine,
roemerine, neferine and armepavine (2,6).
Among these, neferine (Fig. 1C), a
dibenzylisoquinoline alkaloid, has attracted considerable attention
due to its wide range of pharmacological activities, including
antitumor (7), anti-inflammatory,
anti-fibrotic, anti-oxidative stress, anti-platelet aggregation and
anti-arrhythmic properties (8,9).
Inflammation is a crucial factor in the progression of various
diseases, including cancer, cardiovascular diseases, diabetes,
autoimmune diseases, obesity and ocular disorders (10). A number of studies have elucidated
the anti-inflammatory potential of neferine, demonstrating that it
exerts its biological effects via the inhibition of nuclear
factor-kB (NF-kB), mitogen-activated protein kinases (MAPKs)
(11), NOD-like receptor protein 3
(NLRP3), autophagy and the transforming growth factor-β
(TGF-β)/Smad pathway, which mediate the production of various
pro-inflammatory mediators. The present review aims to summarize
the pharmacological effects and mechanisms of neferine in various
disorders.
Anti-inflammatory action of neferine
Various factors, including pathogens, autoimmune
diseases, malignancies, metabolic disorders and certain therapeutic
interventions, provoke systemic inflammatory effects. Inflammation
is a complex, multi-stage process involving various cell types and
signaling mediators, and can be detrimental when prolonged or
chronic. A hallmark of inflammation is the excessive release of
cytokines due to the overactivation of immune cells (12), which plays a crucial role in the
progression of the inflammatory response. Thus, the inhibition of
pro-inflammatory cytokines is a key strategy for combating
inflammation. Tumor necrosis factor (TNF) is a critical cytokine in
inflammatory responses, promoting inflammation by directly inducing
inflammatory gene expression and indirectly causing cell death
(13).
Numerous studies have demonstrated the
anti-inflammatory properties of neferine, and its ability to
inhibit inflammatory mediators. For example, in one study, neferine
reduced TNF-α levels and enhanced insulin sensitivity in
insulin-resistant rats (14). In
another study, neferine modulated the hypoxia-induced inflammatory
response in human peripheral blood mononuclear cells by inhibiting
the release of TNF-α, interleukin (IL)-6 and IL-8 under hypoxic
conditions (15). Similarly,
neferine exhibited anti-inflammatory effects in a dextran sulfate
sodium-induced ulcerative colitis mouse model (16). Furthermore, neferine promoted wound
healing in diabetic rats, potentially via the downregulation of
inflammatory mediators, including NF-κB, TNF-α, IL-1β, IL-8, nitric
oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), and the
upregulation of growth factors (17).
Lipopolysaccharide (LPS) and
inflammation
LPS is a component of gram-negative bacterial
endotoxin, which elicits strong inflammatory responses in the host
(18). Neferine has been shown to
significantly reduce the production of LPS-induced inflammatory
mediators in RAW 264.7 macrophages (19), human endothelial cells (20–23),
NRK-52E cells (24) and BV-2
microglial cells (25). These
mediators include nitric oxide (NO), TNF-α, COX-2, iNOS, IL-1b,
IL-6 and IL-10. Furthermore, neferine has been shown to upregulate
B-cell lymphoma 2 expression and suppress cleaved caspase-3
activity in LPS-induced mouse heart tissue and H9c2 cells (26), highlighting its protective effects
against LPS-induced damage.
Fibrosis
Pulmonary fibrosis, a type of interstitial lung
disease, can be triggered by bacteria, viruses, smoking, autoimmune
diseases, drugs and gastroesophageal reflux. Current treatments for
pulmonary fibrosis mainly involve anti-inflammatory and
immunosuppressive therapies; however, drugs such as prednisone and
pirfenidone have limited efficacy and numerous side effects
(27). Studies have shown that
neferine can mitigate the experimental pulmonary fibrosis induced
by bleomycin (28) and amiodarone
(29). In the bleomycin-induced
pulmonary fibrosis model, the beneficial effects neferine were
attributed to its anti-inflammatory, antioxidant and
cytokine-inhibitory properties. Similarly, in the
amiodarone-induced pulmonary fibrosis model, neferine demonstrated
efficacy by exerting anti-inflammatory effects, inhibiting
surfactant protein D and modulating the T helper (Th)1/Th2
imbalance via the suppression of Th2 responses. In addition,
neferine exhibited anti-inflammatory and antioxidant activity in a
carbon tetrachloride (CCl4)-induced liver fibrosis
model, indicating its antifibrotic effects may be mediated by the
inhibition of inflammation (30).
Oxidative stress
Oxidative stress, defined as an imbalance between
the production of reactive oxygen species (ROS) and antioxidant
defenses, modulates the NF-κB pathway and the expression of
vascular cell adhesion molecule-1 and intercellular adhesion
molecule-1, all of which are critical mediators of the inflammatory
process (31). Lowering oxidant
levels inhibits NF-κB activity, whereas oxidants activate NF-κB,
leading to increased levels of proinflammatory cytokines such as
TNF-α and IL-6 (32). The
interplay between inflammation and oxidative stress is a key
contributor to the pathogenesis of numerous chronic diseases.
Neferine has demonstrated potent antioxidant
properties in numerous studies. For example Lalitha et al
(33) reported that neferine
protected rats against isoproterenol-induced oxidative stress and
myocardial infarction. In addition, other studies showed that
neferine pretreatment reduced ROS levels and mitigated changes in
superoxide dismutase (SOD) and malondialdehyde levels in high
glucose-treated human umbilical vein endothelial cells (34) and in UV-A-induced skin photoaging
(35,36). Furthermore, Priya et al
(11) evaluated the protective
effects of neferine against doxorubicin (DOX)-induced
cardiomyopathy, and found that neferine pretreatment inhibited the
NADPH oxidase system and reduced the production of ROS.
Nuclear factor erythroid 2-related factor 2 (Nrf2)
is a critical transcription factor that regulates the antioxidant
response. In conjunction with its downstream targets, heme
oxygenase-1 (HO-1) and NADPH: quinone oxidoreductase 1 (NQO1), Nrf2
plays a pivotal role in the defense against oxidative stress
(37). Neferine has been shown to
increase antioxidant activity in muscle cells under hypoxic
conditions by promoting the nuclear translocation of Nrf2 (38). In addition, neferine significantly
induced Nrf2 translocation and increased HO-1 and SOD1 expression
in a H9c2 cardiomyoblast cell model of DOX-mediated cardiotoxicity
(39). Furthermore, Liu et
al (40) demonstrated that
neferine alleviated high-glucose-induced oxidative stress injury in
NRK-52E cells by inducing HO-1 expression and increasing its
enzymatic activity. Consistent with these findings, neferine was
found to increase the nuclear levels of Nrf2 in PC12 cells treated
with tert-butyl hydroperoxide (t-BHP), accompanied by the
upregulation of HO-1 and NQO1, suggesting that neferine is
protective against t-BHP-induced neuronal injury (41). Moreover, the administration of
neferine was demonstrated to inhibit angiotensin II-induced atrial
fibrillation, atrial enlargement and atrial fibrosis in mice, with
activation of the Nrf2/HO-1 pathway and inhibition of the
TGF-β/phosphorylated (p-)Smad2/3 pathway identified as the
underlying molecular mechanisms (42). In addition, Nrf2 serves as a link
between oxidative stress and autophagy, which is further elaborated
in the subsequent section.
Potential anti-inflammatory pathways of
neferine
NF-κB pathway
The NF-κB family comprises pleiotropic transcription
factors that regulate various biological processes, including
inflammation, immunity and apoptosis (43). This pathway involves canonical and
non-canonical signaling mechanisms. The canonical NF-κB pathway is
activated by diverse external stimuli, including inflammation,
immune responses and cell proliferation. In its inactive state, the
NF-κB transcription factor, which is typically a RelA/p50
heterodimer, is retained in the cytoplasm by inhibitor of κB(IκB)
protein. Upon activation, inhibitor of κB kinase (IKK)β induces the
phosphorylation and subsequent degradation of IκB, enabling the
RelA/p50 heterodimer to translocate to the nucleus where it
regulates the expression of proinflammatory genes (44). Therefore, targeting the NF-κB
pathway represents a potential strategy for the treatment of
inflammatory diseases.
In lung injury, the NF-κB pathway is persistently
activated, driving the transcription of harmful cytokines and
promoting fibroblast proliferation. However, neferine has been
shown to reduce elevated levels of TNF-α and IL-6 in mice with
bleomycin-induced lung injury by inhibiting NF-κB activity in the
nucleus (28). In addition,
neferine has been found to ameliorate scopolamine-induced cognitive
impairment in animal models via the inhibition of lipid
peroxidation and NF-κB activation (45). It has also been shown to suppress
receptor activator of NF-κB ligand-induced osteoclast formation by
inhibiting the NF-κB signaling pathway (46,47),
and to attenuate the IL-1β-induced inflammatory response in
endothelial cells by inhibiting NF-κB nuclear translocation
(23).
In a study of human endothelial cells exposed to
LPS, neferine significantly prevented the formation of NO, TNF-α,
COX-2, iNOS and IL-1β and inhibited NF-κB pathway signaling in a
concentration-dependent manner, with reduction of the
phosphorylation of IKKα, IKKβ and IκB-α and the expression of NF-κB
p65 (20). Additionally, neferine
exhibited anti-inflammatory effects against acute kidney injury
(AKI) in in vivo and in vitro models by inhibiting
cytokine production, which was achieved through the inhibition of
IκB-α phosphorylation and NF-κB p65 nuclear translocation (24) (Fig.
2). This mechanism is similar to its action in
CCl4-induced liver fibrosis (30). However, these studies did not
investigate the mechanism of NF-κB p65 activation.
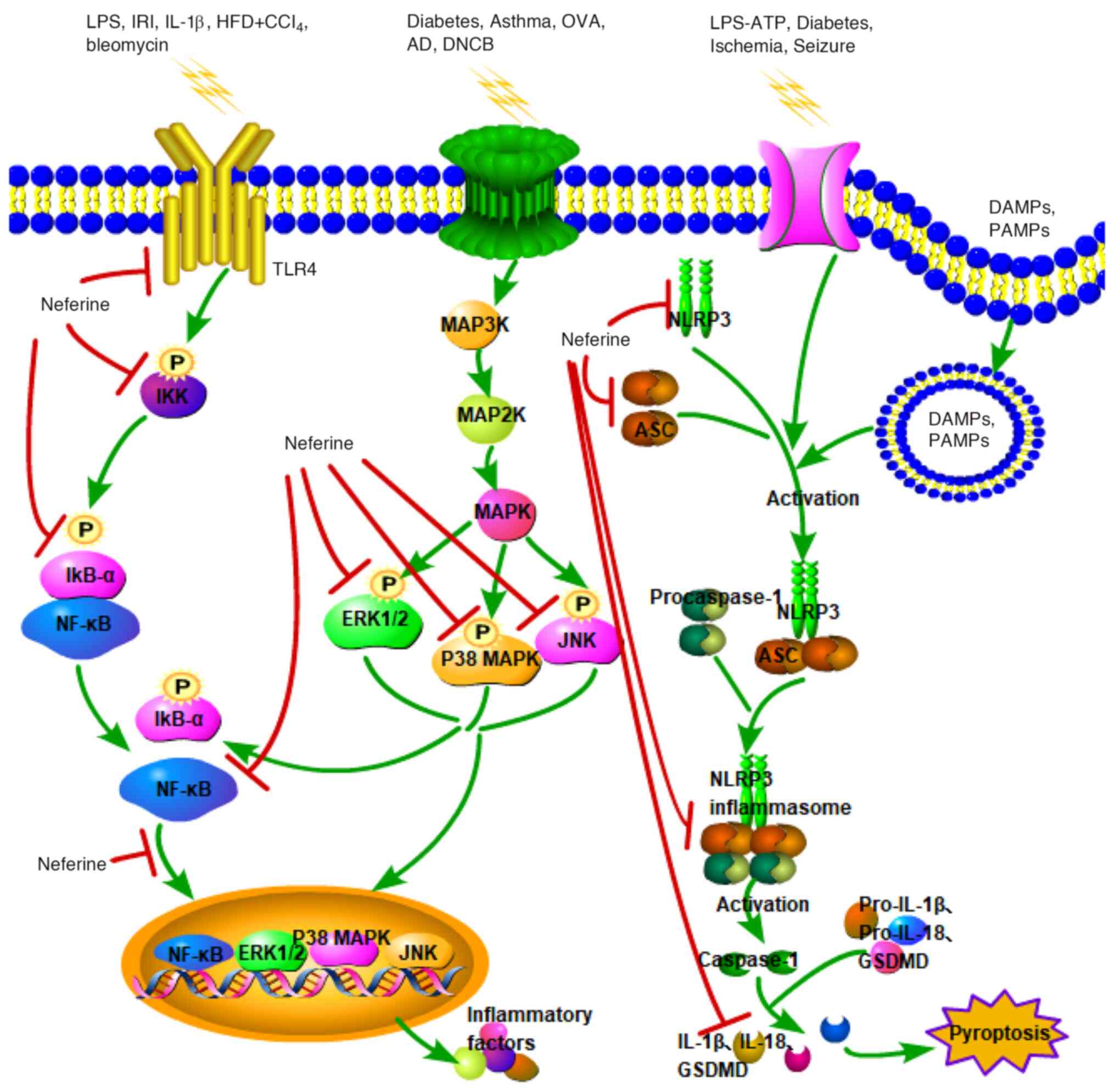 | Figure 2.Schematic presentation of the major
signaling cascades underlying the anti-inflammatory effect of
neferine. Green arrows signify the activation of signal pathways,
while red T-bars denote that neferine inhibits the activation of
these pathways. LPS, lipopolysaccharide; IRI, ischemia/reperfusion
injury; IL-1β, interleukin-1β; HFD, high-fat diet; CCl4,
carbon tetrachloride; OVA, ovalbumin; AD, atopic dermatitis; DNCB,
2,4-dinitrochlorobenzene; TLR4, Toll-like receptor 4; P,
phosphorylation; IKK, inhibitor of κB kinase; IκB-α, inhibitor of
κB a; NF-κB, nuclear factor-κB; MAPK, mitogen-activated protein
kinase; ERK1/2, extracellular-signal-regulated kinases 1/2; JNK,
c-Jun N-terminal kinases; MAP3K, MAPK kinase kinase; MAP2K, MAPK
kinase; NLRP3, NOD-like receptor protein 3; ASC,
apoptosis-associated speck-like protein containing a CARD; GSDMD,
gasdermin D; DAMPs, danger-associated molecular patterns; PAMPs,
pathogen-associated molecular patterns; IL, interleukin. |
In a study performed by Ni et al (48), neferine alleviated IL-1β-induced
inflammation in rat chondrocytes by preventing NF-κB p65
phosphorylation and nuclear translocation. Furthermore, in another
study, neferine inhibited LPS-mediated microglial activation by
preventing the phosphorylation and nuclear translocation of the
NF-κB p65 subunit (25).
Nevertheless, the precise mechanisms by which neferine modulates
NF-κB remain to be clarified. Toll-like receptor 4 (TLR4) has been
identified as an upstream regulator of the NF-κB pathway (49). Building on this, a study on
LPS-treated HepG2 cells and mice with nonalcoholic steatohepatitis
induced by a high-fat diet and CCl4, indicated that
neferine reduces hepatic inflammation, potentially by suppressing
the TLR4/NF-κB signaling pathway (50). Recently, findings from the present
research group suggested that the renoprotective effects of
neferine against AKI are partially mediated through the reversal of
renal peroxisome proliferator-activated receptor α (PPAR-α)
deficiency, leading to inhibition of the NF-κB pathway. This
suggests that PPAR-α may function upstream of NF-κB in the
regulatory mechanisms of neferine in the kidney (51).
MAPK pathways
MAPKs are a group of serine/threonine protein
kinases that play a crucial role in the regulation of inflammation
and various cellular processes (52). Each MAPK pathway involves a cascade
of at least three kinases: A MAPK kinase kinase (MAPKKK), a MAPK
kinase (MAPKK) and a MAPK. In mammals, the primary MAPKs include
extracellular signal-regulated kinases 1/2 (ERK1/2), p38 MAP
kinase, and c-Jun N-terminal kinases (JNK) (53). Typically, MAPKKKs activate MAPKKs
through phosphorylation, which in turn activate MAPKs, ultimately
leading to the regulation of inflammatory cytokine expression and
the initiation of inflammatory responses (54).
In vivo research has revealed that neferine
can prevent diabetes-induced cardiac fibrosis. This effect was
evidenced by a reduction of the expression levels of collagen I,
collagen III and TGF-β1 in diabetic mice treated with neferine,
which was suggested to be mediated by the inhibition of p38, ERK
and Smad 2/3 phosphorylation (55)
(Fig. 2). In addition, neferine
has been demonstrated to inhibit cell proliferation and migration
in retinal pigment epithelial cells exposed to epidermal growth
factor, potentially by reducing the phosphorylation of p38 MAPK
(56). Furthermore, in an
ovalbumin (OVA)-induced asthma model, neferine decreased various
inflammatory factors in the serum and bronchoalveolar lavage fluid
of OVA-treated animals and reduce the phosphorylation of p38, JNK,
and ERK. These findings suggest that neferine mitigates
asthma-induced inflammation via the inhibition of MAPK pathways
(57).
MAPKs are crucial signaling molecules in the
inflammatory response. Components of the MAPK pathway, including
JNK1/2, p38 MAPK and ERK1/2, can influence upstream signaling
events that modulate NF-κB activation, thereby affecting the
expression and activity of inflammatory factors (58). Studies have also highlighted the
therapeutic effects of neferine on atopic dermatitis (AD) in
various models, including HaCaT keratinocyte cells (59), mast cells (60) and mouse models. Notably, in a
2,4-dinitrochlorobenzene-induced AD model, neferine significantly
reduced cytokine expression and inhibited the phosphorylation of
p38, ERK and IκB, supporting the hypothesis that neferine exerts
anti-inflammatory effects through inhibition of the MAPK/NF-κB
pathway (59).
NLRP3 inflammasome pathway
Overactivation of the NLRP3 inflammasome pathway
contributes to various inflammatory diseases. Formation of the
NLRP3 inflammasome involves the oligomerization of NLRP3, and the
recruitment of apoptosis-associated speck-like protein containing a
CARD (ASC) and caspase-1. This complex activates caspase-1, which
subsequently cleaves pro-IL-1β, pro-IL-18 and gasdermin D (GSDMD).
Activated GSDMD then disrupts the cell membrane, leading to
pyroptosis, a form of cell death that releases proinflammatory
cellular contents (61).
Consequently, NLRP3 inflammasomes are crucial targets for
anti-inflammatory treatments (62).
Tang et al (22) were the first to investigate the
role of neferine in the prevention of endothelial cell pyroptosis.
In their study, neferine was revealed to inhibit oxidative stress
and the activation of the NLRP3 inflammasome pathway triggered by
LPS-ATP. Using NLRP3 small interfering RNA and overexpression
techniques, they also demonstrated that neferine prevented the
LPS-ATP-induced pyroptosis of endothelial cells by blocking the
ROS/NLRP3/caspase-1 signaling pathway. Furthermore, in a diabetic
db/db mouse model, neferine treatment reduced oxidative stress and
inflammation in the hippocampus (63). This was evidenced by decreased
levels of thioredoxin-interacting protein, NLRP3 inflammasomes, ASC
and IL-1β, suggesting that neferine alleviated memory and cognitive
dysfunction in diabetic mice by modulating the NLRP3 inflammasome
pathway.
Given the neuroprotective properties of neferine,
Zhu et al (64) explored
its effects on hypoxic-ischemic brain injury in neonatal rats. The
study demonstrated that neferine reduced neuroinflammation and
oxidative stress damage by inhibiting the NLRP3 inflammasome
pathway and pyroptosis (Fig. 2).
In addition, a follow-up study of ischemia/reperfusion injury in
mice revealed that neferine inhibited pyroptosis, thereby improving
the integrity of the blood-brain barrier via regulation of the
peroxisome proliferator-activated receptor γ coactivator
1α/NLRP3/GSDMD signaling pathway (65). In addition, in a kainic
acid-induced seizure rat model, neferine alleviated seizure
severity and reduced neuroinflammation in the hippocampus, likely
by inhibiting NLRP3 inflammasome activation and reducing
inflammatory cytokine levels (66).
Autophagy
Autophagy is a crucial cellular process involving
the formation of autolysosomes that degrade intracellular pathogens
and damaged organelles. Key regulators of autophagy include
AMP-activated protein kinase (AMPK) and mammalian target of
rapamycin (mTOR) (67). Neferine
has been shown to have regulatory effects on autophagy. For
example, one study demonstrated that it not only promoted the
accumulation of autophagy-related proteins, such as
microtubule-associated protein 1A/1B-light chain (LC3-II) and
p62/sequestome 1, but also hindered lysosome maturation, suggesting
that neferine inhibits macroautophagic flux (68). In hypoxic muscle cells, neferine
inhibited autophagy by downregulating beclin 1, class III PI3K and
LC3B-II while activating the Akt/mTOR pathway (38). Similarly, in H9c2 cells exposed to
DOX, neferine reduced the expression of Unc-51 like autophagy
activating kinase 1, beclin 1, autophagy-related gene 7 and LC3B,
possibly through the insulin-like growth factor 1
receptor/PI3K/Akt/mTOR pathway (39). Furthermore, neferine significantly
alleviated cerebral artery occlusion-induced cerebral ischemia in
rats by reducing the upregulation of LC3-II, beclin 1 and p62, as
well as the formation of autophagosomes, through regulation of the
Ca2+-dependent AMPK/mTOR pathway (69). By contrast, when preventing
cisplatin-induced nephrotoxicity, neferine appears to promote
autophagy via the AMPK/mTOR pathway (70). Therefore, it appears that neferine
may regulate autophagy through different mechanisms depending on
the disease context.
Autophagy plays a role in clearing inflammasomes,
cytokines and bacteria, and is influenced by factors including
cytokines, ROS and danger/pathogen-associated molecular patterns,
as well as pharmacological inhibitors (71), indicating a close relationship
between autophagy and inflammation. Defects in autophagy are
associated with susceptibility to several autoimmune and
inflammatory disorders (72).
Consequently, neferine may exert anti-inflammatory effects via the
regulation of autophagy, consistent with findings in Graves'
orbitopathy (GO) (73). The study
of GO showed that neferine suppressed both IL-13-induced
autophagosome formation and inflammation in GO orbital fibroblasts,
as evidenced by attenuation of the both the increased LC3-II/LC3-I
ratio and downregulated p62, and suppression of the upregulated
TNF-α, IL-1β, IL-6 and monocyte chemoattractant protein-1 levels.
In addition, the suppression of these inflammatory factors was
partially reversed by 3-methyladenine, an autophagy inhibitor,
suggesting that the anti-inflammatory effects of neferine were
mediated, at least in part, through the regulation of
autophagy.
Nrf2 serves as a link between oxidative stress and
autophagy. In the non-canonical activation of Nrf2, p62 competes
with Nrf2 to interact with Keap1, leading to the sequestration of
p62-Keap1 in autophagosomes and promoting the nuclear translocation
of Nrf2 to induce antioxidant gene expression. Experiments
involving p62 knockdown have demonstrated that p62 is crucial for
Nrf2 activation (74). Neferine
has been shown to upregulate Nrf2 expression and suppress autophagy
in diabetic wound models (17) and
in vitro models of GO (73). Future research is required to
elucidate the mechanisms underlying the effects of neferine on Nrf2
and autophagy.
TGF-β/Smad signaling pathway
TGF-β is a cytokine that regulates numerous cellular
processes, including growth, proliferation, differentiation,
senescence, apoptosis, adhesion, migration and the synthesis and
remodeling of the extracellular matrix. Smad proteins function as
downstream transcription factors in the TGF-β signaling pathway
(75,76). Disruptions in TGF-β signaling are
implicated in various conditions, such as developmental
abnormalities (77), cancers,
tissue fibrosis, cardiovascular diseases (78) and immune disorders (79).
Neferine has been investigated for its effects on
fibrotic diseases. For example, in a fibrotic endometriosis model
in mice (80), neferine
significantly reduced the expression of extracellular matrix
components, including fibronectin, collagen type I, connective
tissue growth factor and smooth muscle actin, and decreased the
levels of TGF-β and p-ERK. These results suggest that neferine
inhibited extracellular matrix deposition and fibrosis in this
model by blocking the TGF-β/ERK signaling pathway. Similarly,
neferine exhibited anti-fibrotic effects on testosterone-induced
benign prostatic hyperplasia in mice (81); the study suggested that neferine
prevented the epithelial-mesenchymal transition and prostate
enlargement caused by testosterone by modulating the TGF-β/Smad
pathway. Furthermore, neferine was shown to reduce vascular
remodeling in spontaneously hypertensive rats via the inhibition of
TGF-β1/Smad2/3 signaling (82).
Collectively, these studies highlight neferine as a promising
therapeutic agent for the management of fibrosis in various
diseases.
Side effects of neferine
Research on neferine has predominantly focused on
cell and animal experiments, with a conspicuous absence of clinical
trials. This has severely restricted comprehensive investigations
into its potential side effects. In Cell Counting Kit-8 assays,
when the concentration of neferine reached 100 µM, the
proliferation activity of NRK-52E cells remained >50% (24), indicating that neferine exhibits a
low level of toxicity. However, in a study conducted by Yu et
al (83), neferine inhibited
myocardial contractility and disrupted the calcium homeostasis in
cardiomyocytes, but had no significant effect on cell viability.
These results demonstrate the potential cardiac side effects of
neferine. Notably, in vivo data on the neurological or
reproductive toxicity of neferine are lacking. Consequently,
further research is necessary to comprehensively evaluate the
safety profile of neferine across diverse physiological
systems.
Summary and perspectives
Numerous studies have underscored that neferine
exerts anti-inflammatory activity through various signaling
pathways, establishing a foundational basis for its potential as a
novel therapeutic agent for inflammatory diseases. The wide
availability of neferine and minimal side effects further indicate
its promise for disease treatment and prevention. However,
exploration of the therapeutic efficacy of neferine has primarily
been confined to cell and animal disease models. There are several
reasons why it has not been widely used clinically. i) Lack of
sufficient clinical trial data. The translation from models to
human clinical applications requires extensive and well-designed
clinical trials to evaluate its efficacy, safety, optimal dosage
and potential side effects in humans. Without comprehensive
clinical trial results, it is challenging to determine its true
value and applicability in clinical practice. ii) Pharmacokinetic
and pharmacodynamic uncertainties. Limited research has been
performed to fully elucidate these aspects, which leads to
uncertainties in dosing regimens and potential drug-drug
interactions, hindering its immediate clinical adoption. iii)
Competition from existing treatments.
Neferine has been demonstrated to modulate multiple
cellular and molecular mediators involved in inflammation. However,
the molecular mechanisms underlying the actions of neferine are
complex, and its precise drug targets remain inadequately
understood. Future research should focus on elucidating the
detailed mechanisms of action of neferine under diverse
physiological and pathological conditions, and identifying specific
molecular targets to advance neferine as a viable therapeutic
strategy for the prevention and treatment of inflammatory diseases.
In addition, enhancing the solubility and bioavailability of
neferine through structural modifications or the development of
nanomedicine formulations may also be beneficial. Additionally, the
active components of Nelumbo nucifera should be further
explored to promote and preserve the legacy of traditional Chinese
medicine.
Acknowledgements
Not applicable.
Funding
This study was funded by the Chongqing Municipal Natural Science
Foundations (grant nos. cstc2021jcyj-msxmX0759 and
cstc2021jcyj-msxmX0895).
Availability of data and materials
Not applicable.
Authors' contributions
HL and QZho designed and conceived the review. QZha
and HL wrote the original draft of the manuscript. HL, QZha and
QZho contributed to editorial changes in the manuscript. All
authors read and approved the final version of the manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mukherjee PK, Mukherjee D, Maji AK, Rai S
and Heinrich M: The sacred lotus (Nelumbo
nucifera)-phytochemical and therapeutic profile. J Pharm
Pharmacol. 61:407–422. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sharma BR, Gautam LN, Adhikari D and Karki
R: A Comprehensive review on chemical profiling of nelumbo
nucifera: Potential for drug development. Phytother Res.
31:3–26. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao SH, Jin D, Gu CJ and Lian FG:
Professor Tong Xiaolin's experience in using lotus leaf, gordon
euryale seed and lotus plumule for clearing and transforming
damp-heat symptoms. Jilin J Trad Chin Med. 41:336–338. 2021.(In
Chinese).
|
|
4
|
Huang XX, Xie Z, Xie MY and Li S:
Mechanism of qinggongtang against generalized anxiety disorder
based on Glu/GABA metabolic balance. Chin J Exp Trad Med Formulae.
30:28–35. 2024.(In Chinese).
|
|
5
|
Zheng ZJ, Zhu LZ, Song WC, Hu C, Chen S,
You P and Zhou Y: Pharmacological Research Progress of Nelumbinis
Plumula in the Treatment of Insomnia from the Traditional Chinese
Medicine Perspective ‘Heart Mind’. Shenzhen J Integrated Trad Chin
Western Med. 32:125–129. 2022.(In Chinese).
|
|
6
|
Wang Z, Li Y, Ma D, Zeng M, Wang Z, Qin F,
Chen J, Christian M and He Z: Alkaloids from lotus (Nelumbo
nucifera): Recent advances in biosynthesis, pharmacokinetics,
bioactivity, safety, and industrial applications. Crit Rev Food Sci
Nutr. 63:4867–4900. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bishayee A, Patel PA, Sharma P,
Thoutireddy S and Das N: Lotus (Nelumbo nucifera Gaertn.)
and its bioactive phytocompounds: A tribute to cancer prevention
and intervention. Cancers (Basel). 14:5292022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marthandam Asokan S, Mariappan R,
Muthusamy S and Velmurugan BK: Pharmacological benefits of
neferine-A comprehensive review. Life Sci. 199:60–70. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bharathi Priya L, Huang CY, Hu RM,
Balasubramanian B and Baskaran R: An updated review on
pharmacological properties of neferine-A bisbenzylisoquinoline
alkaloid from Nelumbo nucifera. J Food Biochem.
45:e139862021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arulselvan P, Fard MT, Tan WS, Gothai S,
Fakurazi S, Norhaizan ME and Kumar SS: Role of antioxidants and
natural products in inflammation. Oxid Med Cell Longev.
2016:52761302016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Priya LB, Baskaran R, Huang CY and Padma
VV: Neferine ameliorates cardiomyoblast apoptosis induced by
doxorubicin: Possible role in modulating NADPH oxidase/ROS-mediated
NFκB redox signaling cascade. Sci Rep. 7:122832017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jarczak D and Nierhaus A: Cytokine
Storm-definition, causes, and implications. Int J Mol Sci.
23:117402022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
van Loo G and Bertrand MJM: Death by TNF:
A road to inflammation. Nat Rev Immunol. 23:289–303. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pan Y, Cai B, Wang K, Wang S, Zhou S, Yu
X, Xu B and Chen L: Neferine enhances insulin sensitivity in
insulin resistant rats. J Ethnopharmacol. 124:98–102. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baskaran R, Priya LB, Kalaiselvi P,
Poornima P, Huang CY and Padma VV: Neferine from Nelumbo
nucifera modulates oxidative stress and cytokines production
during hypoxia in human peripheral blood mononuclear cells. Biomed
Pharmacother. 93:730–736. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Min X, Guo Y, Zhou Y and Chen X:
Protection against dextran sulfate Sodium-induced ulcerative
colitis in mice by neferine, a natural product from Nelumbo
nucifera gaertn. Cell J. 22:523–531. 2021.PubMed/NCBI
|
|
17
|
Li J, Chou H, Li L, Li H and Cui Z: Wound
healing activity of neferine in experimental diabetic rats through
the inhibition of inflammatory cytokines and nrf-2 pathway. Artif
Cells Nanomed Biotechnol. 48:96–106. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rietschel ET, Kirikae T, Schade FU, Mamat
U, Schmidt G, Loppnow H, Ulmer AJ, Zähringer U, Seydel U, Di Padova
F, et al: Bacterial endotoxin: Molecular relationships of structure
to activity and function. FASEB J. 8:217–225. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu X, Guo Y, Min X, Pei L and Chen X:
Neferine, a bisbenzylisoquinoline alkaloid, ameliorates dextran
sulfate Sodium-induced ulcerative colitis. Am J Chin Med.
46:1263–1279. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guolan D, Lingli W, Wenyi H, Wei Z, Baowei
C and Sen B: Anti-inflammatory effects of neferine on LPS-induced
human endothelium via MAPK, and NF-κβ pathways. Pharmazie.
73:541–544. 2018.PubMed/NCBI
|
|
21
|
Liu XY, Xu HX, Li JK, Zhang D, Ma XH,
Huang LN, Lü JH and Wang XZ: Neferine Protects endothelial
glycocalyx via mitochondrial ROS in lipopolysaccharide-Induced
acute respiratory distress syndrome. Front Physiol. 9:1022018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang YS, Zhao YH, Zhong Y, Li XZ, Pu JX,
Luo YC and Zhou QL: Neferine inhibits LPS-ATP-induced endothelial
cell pyroptosis via regulation of ROS/NLRP3/Caspase-1 signaling
pathway. Inflamm Res. 68:727–738. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhong Y, He S, Huang K and Liang M:
Neferine suppresses vascular endothelial inflammation by inhibiting
the NF-κB signaling pathway. Arch Biochem Biophys. 696:1085952020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li H, Chen W, Chen Y, Zhou Q, Xiao P, Tang
R and Xue J: Neferine attenuates acute kidney injury by inhibiting
NF-κB signaling and upregulating klotho expression. Front
Pharmacol. 10:11972019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li T, Zhai YX, Zheng T and Xu B: Neferine
exerts anti-inflammatory activity in BV-2 microglial cells and
protects mice with MPTP-induced Parkinson's disease by inhibiting
NF-κB activation. Mol Med Rep. 28:2352023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qi Z, Wang R, Liao R, Xue S and Wang Y:
Neferine ameliorates Sepsis-induced myocardial dysfunction through
Anti-apoptotic and antioxidative effects by regulating the
PI3K/AKT/mTOR signaling pathway. Front Pharmacol. 12:7062512021.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Savin IA, Zenkova MA and Sen'kova AV:
Pulmonary fibrosis as a result of acute lung inflammation:
Molecular mechanisms, relevant in vivo models, prognostic and
therapeutic approaches. Int J Mol Sci. 23:149592022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao L, Wang X, Chang Q, Xu J, Huang Y,
Guo Q, Zhang S, Wang W, Chen X and Wang J: Neferine, a
bisbenzylisoquinline alkaloid attenuates bleomycin-induced
pulmonary fibrosis. Eur J Pharmacol. 627:304–312. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Niu CH, Wang Y, Liu JD, Wang JL and Xiao
JH: Protective effects of neferine on amiodarone-induced pulmonary
fibrosis in mice. Eur J Pharmacol. 714:112–119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Y, Wang S, Wang R, Li S and Yuan Y:
Neferine exerts antioxidant and Anti-inflammatory effects on carbon
Tetrachloride-induced liver fibrosis by inhibiting the MAPK and
NF-κB/IκBα pathways. Evid Based Complement Alternat Med.
2021:41360192021.PubMed/NCBI
|
|
31
|
Joffre J and Hellman J: Oxidative stress
and endothelial dysfunction in sepsis and acute inflammation.
Antioxid Redox Signal. 35:1291–1307. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hussain T, Tan B, Yin Y, Blachier F,
Tossou MCB and Rahu N: Oxidative stress and inflammation: What
polyphenols can do for us? Oxid Med Cell Longev. 2016:74327972016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lalitha G, Poornima P, Archanah A and
Padma VV: Protective effect of neferine against
isoproterenol-induced cardiac toxicity. Cardiovasc Toxicol.
13:168–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guan G, Han H, Yang Y, Jin Y, Wang X and
Liu X: Neferine prevented hyperglycemia-induced endothelial cell
apoptosis through suppressing ROS/Akt/NF-κB signal. Endocrine.
47:764–771. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Khan A, Bai H, Shu M, Chen M, Khan A and
Bai Z: Antioxidative and antiphotoaging activities of neferine upon
UV-A irradiation in human dermal fibroblasts. Biosci Rep.
38:BSR201814142018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Khan A, Bai H, Khan A and Bai Z: Neferine
prevents ultraviolet radiation-induced skin photoaging. Exp Ther
Med. 19:3189–3196. 2020.PubMed/NCBI
|
|
37
|
Nguyen T, Nioi P and Pickett CB: The
Nrf2-antioxidant response element signaling pathway and its
activation by oxidative stress. J Biol Chem. 284:13291–13295. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Baskaran R, Poornima P, Priya LB, Huang CY
and Padma VV: Neferine prevents autophagy induced by hypoxia
through activation of Akt/mTOR pathway and Nrf2 in muscle cells.
Biomed Pharmacother. 83:1407–1413. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bharathi Priya L, Baskaran R, Huang CY and
Vijaya Padma V: Neferine modulates IGF-1R/Nrf2 signaling in
doxorubicin treated H9c2 cardiomyoblasts. J Cell Biochem.
119:1441–1452. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu XD, Li H, Wang CZ, Zhao HD and Xiao P:
Mechanism of neferine in antioxidant stress. China J Modern Med.
28:31–36. 2018.
|
|
41
|
Wu C, Chen J, Yang R, Duan F, Li S and
Chen X: Mitochondrial protective effect of neferine through the
modulation of nuclear factor erythroid 2-related factor 2
signalling in ischaemic stroke. Br J Pharmacol. 176:400–415. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jiang XX, Zhang R and Wang HS: Neferine
mitigates angiotensin II-induced atrial fibrillation and fibrosis
via upregulation of Nrf2/HO-1 and inhibition of TGF-β/p-Smad2/3
pathways. Aging. 16:8630–8644. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sanz AB, Sanchez-Niño MD, Ramos AM, Moreno
JA, Santamaria B, Ruiz-Ortega M, Egido J and Ortiz A: NF-kappaB in
renal inflammation. J Am Soc Nephrol. 21:1254–1262. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yu H, Lin L, Zhang Z, Zhang H and Hu H:
Targeting NF-κB pathway for the therapy of diseases: Mechanism and
clinical study. Signal Transduct Target Ther. 5:2092020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jung HA, Jin SE, Choi RJ, Kim DH, Kim YS,
Ryu JH, Kim DW, Son YK, Park JJ and Choi JS: Anti-amnesic activity
of neferine with antioxidant and anti-inflammatory capacities, as
well as inhibition of ChEs and BACE1. Life Sci. 87:420–430. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen S, Chu B, Chen Y, Cheng X, Guo D,
Chen L, Wang J, Li Z, Hong Z and Hong D: Neferine suppresses
osteoclast differentiation through suppressing NF-κB signal pathway
but not MAPKs and promote osteogenesis. J Cell Physiol.
234:22960–22971. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wu F, Wu Z, Ye Z, Niu G, Ma Z and Zhang P:
PLGA/BGP/Nef porous composite restrains osteoclasts by inhibiting
the NF-κB pathway, enhances IGF-1-mediated osteogenic
differentiation and promotes bone regeneration. J Biol Eng.
17:452023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ni B, Huang X, Xi Y, Mao Z, Chu X, Zhang
R, Ma X and You H: Neferine inhibits expression of inflammatory
mediators and matrix degrading enzymes in IL-1β-treated rat
chondrocytes via suppressing MAPK and NF-κB signaling pathways.
Inflammation. 43:1209–1221. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Luo MC, Zhou SY, Feng DY, Xiao J, Li WY,
Xu CD, Wang HY and Zhou T: Runt-related transcription factor 1
(RUNX1) binds to p50 in macrophages and enhances TLR4-triggered
inflammation and septic shock. J Biol Chem. 291:22011–22020. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang MY, Zhang SS, An MF, Xia YF, Fan MS,
Sun ZR, Zhang LJ, Zhao YL, Sheng J and Wang XJ: Neferine
ameliorates nonalcoholic steatohepatitis through regulating AMPK
pathway. Phytomedicine. 114:1547982023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xiong Y, Zhong J, Chen W, Li X, Liu H, Li
Y, Xiong W and Li H: Neferine alleviates acute kidney injury by
regulating the PPAR-α/NF-κB pathway. Clin Exp Nephrol. 28:969–987.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng
J, Li Y, Wang X and Zhao L: Inflammatory responses and
inflammation-associated diseases in organs. Oncotarget.
9:7204–7218. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kim EK and Choi EJ: Pathological roles of
MAPK signaling pathways in human diseases. Biochim Biophys Acta.
1802:396–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Pearson G, Robinson F, Beers Gibson T, Xu
BE, Karandikar M, Berman K and Cobb MH: Mitogen-activated protein
(MAP) kinase pathways: Regulation and physiological functions.
Endocr Rev. 22:153–183. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liu X, Song X, Lu J, Chen X, Liang E, Liu
X, Zhang M, Zhang Y, Du Z and Zhao Y: Neferine inhibits
proliferation and collagen synthesis induced by high glucose in
cardiac fibroblasts and reduces cardiac fibrosis in diabetic mice.
Oncotarget. 7:61703–61715. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ozal SA, Gurlu V, Turkekul K, Guclu H and
Erdogan S: Neferine inhibits epidermal growth factor-induced
proliferation and migration of retinal pigment epithelial cells
through downregulating p38 MAPK and PI3K/AKT signalling. Cutan Ocul
Toxicol. 39:97–105. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhu T, Xiao X, Dong Y and Yuan C: Neferine
alleviates ovalbumin-induced asthma via MAPK signaling pathways in
mice. Allergol Immunopathol (Madr). 51:135–142. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Han Q, Li H, Zhao F, Gao J, Liu X and Ma
B: Auricularia auricula peptides nutritional supplementation delays
H2O2-induced senescence of hepG2 cells by modulation of MAPK/NF-κB
signaling pathways. Nutrients. 15:37312023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yang CC, Hung YL, Ko WC, Tsai YJ, Chang
JF, Liang CW, Chang DC and Hung CF: Effect of neferine on
DNCB-induced atopic dermatitis in HaCaT cells and BALB/c Mice. Int
J Mol Sci. 22:82372021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chiu KM, Hung YL, Wang SJ, Tsai YJ, Wu NL,
Liang CW, Chang DC and Hung CF: Anti-allergic and Anti-inflammatory
effects of neferine on RBL-2H3 cells. Int J Mol Sci. 22:109942021.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang L and Hauenstein AV: The NLRP3
inflammasome: Mechanism of action, role in disease and therapies.
Mol Aspects Med. 76:1008892020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Coll RC, Schroder K and Pelegrín P: NLRP3
and pyroptosis blockers for treating inflammatory diseases. Trends
Pharmacol Sci. 43:653–668. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wu XL, Deng MZ, Gao ZJ, Dang YY, Li YC and
Li CW: Neferine alleviates memory and cognitive dysfunction in
diabetic mice through modulation of the NLRP3 inflammasome pathway
and alleviation of endoplasmic-reticulum stress. Int
Immunopharmacol. 84:1065592020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhu JJ, Yu BY, Huang XK, He MZ, Chen BW,
Chen TT, Fang HY, Chen SQ, Fu XQ, Li PJ, et al: Neferine protects
against Hypoxic-ischemic brain damage in neonatal rats by
suppressing NLRP3-mediated inflammasome activation. Oxid Med Cell
Longev. 2021:66549542021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zheng ZJ, Zhu LZ, Qiu H, Zheng WY, You PT,
Chen SH, Hu CL, Huang JR and Zhou YJ: Neferine inhibits BMECs
pyroptosis and maintains blood-brain barrier integrity in ischemic
stroke by triggering a cascade reaction of PGC-1α. Sci Rep.
14:144382024. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lin TY, Hung CY, Chiu KM, Lee MY, Lu CW
and Wang SJ: Neferine, an alkaloid from lotus seed embryos, exerts
antiseizure and neuroprotective effects in a kainic Acid-induced
seizure model in rats. Int J Mol Sci. 23:41302022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Deretic V: Autophagy in inflammation,
infection, and immunometabolism. Immunity. 54:437–453. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Xu T, Singh D, Liu J, Li H, Peng S,
Rizzolo LJ and Wang SB: Neferine, is not inducer but blocker for
macroautophagic flux targeting on lysosome malfunction. Biochem
Biophys Res Commun. 495:1516–1521. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Sengking J, Oka C, Wicha P, Yawoot N,
Tocharus J, Chaichompoo W, Suksamrarn A and Tocharus C: Neferine
protects against brain damage in permanent cerebral ischemic rat
associated with autophagy suppression and AMPK/mTOR regulation. Mol
Neurobiol. 58:6304–6315. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Li H, Tang Y, Wen L, Kong X, Chen X, Liu
P, Zhou Z, Chen W, Xiao C, Xiao P and Xiao X: Neferine reduces
cisplatin-induced nephrotoxicity by enhancing autophagy via the
AMPK/mTOR signaling pathway. Biochem Biophys Res Commun.
484:694–701. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Jones SA, Mills KH and Harris J: Autophagy
and inflammatory diseases. Immunol Cell Biol. 91:250–258. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yang Z, Goronzy JJ and Weyand CM:
Autophagy in autoimmune disease. J Mol Med (Berl). 93:707–717.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Li H, Gao L, Min J, Yang Y and Zhang R:
Neferine suppresses autophagy-induced inflammation, oxidative
stress and adipocyte differentiation in Graves' orbitopathy. J Cell
Mol Med. 25:1949–1957. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Jiang T, Harder B, Rojo de la Vega M, Wong
PK, Chapman E and Zhang DD: p62 links autophagy and Nrf2 signaling.
Free Radic Biol Med. 88:199–204. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Heldin CH, Miyazono K and ten Dijke P:
TGF-beta signalling from cell membrane to nucleus through SMAD
proteins. Nature. 390:465–471. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
76
|
Xu P, Liu J and Derynck R:
Post-translational regulation of TGF-β receptor and Smad signaling.
FEBS Lett. 586:1871–1884. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Dumbrava MG, Lacanlale JL, Rowan CJ and
Rosenblum ND: Transforming growth factor beta signaling functions
during mammalian kidney development. Pediatr Nephrol. 36:1663–1672.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Goumans MJ and Ten Dijke P: TGF-β
signaling in control of cardiovascular function. Cold Spring Harb
Perspect Biol. 10:a0222102018. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Laudisi F, Stolfi C, Monteleone I and
Monteleone G: TGF-β1 signaling and Smad7 control T-cell responses
in health and immune-mediated disorders. Eur J Immunol.
53:e23504602023. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Xia Y, Guo Y, Zhou J, Fan L, Xie J, Wang
Y, Du H and Ni X: Neferine mediated TGF-β/ERK signaling to inhibit
fibrosis in endometriosis. Am J Transl Res. 15:3240–3253.
2023.PubMed/NCBI
|
|
81
|
Liu CM, Shao Z, Chen X, Chen H, Su M,
Zhang Z, Wu Z, Zhang P, An L, Jiang Y and Ouyang AJ: Neferine
attenuates development of testosterone-induced benign prostatic
hyperplasia in mice by regulating androgen and TGF-β/Smad signaling
pathways. Saudi Pharm J. 31:1219–1228. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zeng W, Zhang X, Lu Y, Wen Y, Xie Q, Yang
X, He S, Guo Z, Li J, Shen A and Peng J: Neferine ameliorates
hypertensive vascular remodeling modulating multiple signaling
pathways in spontaneously hypertensive rats. Biomed Pharmacother.
158:1142032023. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yu Y, Sun S, Wang S, Zhang Q, Li M, Lan F,
Li S and Liu C: Liensinine- and Neferine-induced cardiotoxicity in
primary neonatal rat cardiomyocytes and Human-induced pluripotent
stem Cell-derived cardiomyocytes. Int J Mol Sci. 17:1862016.
View Article : Google Scholar : PubMed/NCBI
|