Introduction
Allergic rhinitis (AR) is a non-infectious
inflammatory condition of the nasal mucosa, which is driven by
immunoglobulin E (IgE), and impacts 10–30% of the global population
(1). When exposed to allergens,
such as pollen, dust mites and pet dander, the immune system
generates IgE antibodies that attach to mast cells, prompting the
release of inflammatory mediators, including histamine. This
cascade leads to nasal mucosal inflammation and distinct symptoms,
such as nasal congestion, rhinorrhea, frequent sneezing and ocular
discomfort (2).
The impact of AR extends beyond physical symptoms,
significantly affecting quality of life through sleep disturbances,
decreased work productivity and restricted social interactions
(3). Additionally, individuals
with AR face elevated risks of anxiety, depression and comorbid
conditions, such as asthma (4,5).
Despite its widespread prevalence, the treatment options for AR
remain limited, with allergen-specific immunotherapy standing as
the sole recognized cure. While this approach gradually diminishes
allergen sensitivity, it underscores the pressing need for
innovative therapeutic strategies (6).
Short-chain fatty acids (SCFAs) are synthesized by
gut microbiota via dietary fiber fermentation. These SCFAs are
pivotal for immune modulation and inflammation management, which
are crucial in maintaining host well-being, particularly through
their anti-inflammatory properties (6). Research has highlighted the
significance of SCFAs in treating AR, since individuals with AR
often exhibit an altered gut microbiota composition characterized
by diminished SCFA-producing bacteria (7,8).
This dysbiosis may increase susceptibility to and the severity of
AR (9). Consequently,
supplementation with SCFAs has emerged as a promising prevention
and treatment strategy for AR. Indolepropionic acid (IPA), a
derivative of SCFAs, has garnered considerable attention due to its
notable anti-inflammatory and immunomodulatory abilities (10). IPA is synthesized by specific flora
in the gut, such as Clostridium spp., via the tryptophan
metabolic pathway. It not only possesses strong antioxidant
properties, but also serves a crucial role in inflammatory
regulation by activating the aryl hydrocarbon receptor (AhR) and
modulating the function of immune cells (11). Studies have shown that IPA
effectively alleviates IL-1β-induced chondrocyte inflammation, and
attenuates the toxicity of organophosphorus pesticide exposure to
the liver and kidney through the AhR/NF-κB regulatory axis, as
demonstrated by a significant decrease in pro-inflammatory cytokine
levels (12,13). Our prior investigation demonstrated
significantly lower serum propionic acid (PA) levels in patients
with AR compared with those in healthy controls, alongside a
declining trend in IL-10 levels (14). It was thus hypothesized that IPA,
as a derivative of SCFAs, may have a similar function, i.e., the
ability to elevate the levels of IL-10.
The AKT protein is pivotal in cell signaling across
diverse cell types, and is closely associated with regulating
immune responses and inflammation (15). CCAAT enhancer binding protein β
(CEBPB) belongs to the CEBP family, which includes α-ζ members and
is characterized by a conserved leucine zipper (bZIP) domain at its
carboxyl terminus that aids in dimerization and DNA binding
(16,17). CEBPB is widely expressed in various
tissues and governs a range of biological processes, such as immune
responses (18,19), aging (20) and the development of different
types of cancer (21). In innate
immune cells, CEBPB helps suppress the production of inflammatory
cytokines while enhancing IL-10 expression (22). Notably, it has been demonstrated
that increased CEBPB levels can enhance activation of the IL-10
promoter (23), serving a crucial
role in IL-10 expression in macrophages (22). These findings suggest that IPA may
mitigate the symptoms of AR by upregulating IL-10 levels through
the AKT/CEBPB/IL-10 signaling pathway.
Despite exploring the immunomodulatory impacts of
IPA and PA, its precise role in AR remains uncertain. The present
study aimed to evaluate the therapeutic potential of IPA in
alleviating AR symptoms with a mechanism that partially coincides
with PA. The study also comprehensively investigated how IPA and PA
mitigate the inflammatory response in AR via the AKT/CEBPB/IL-10
signaling pathway.
Materials and methods
Cells and reagents
Human nasal mucosal epithelial cells (HNEpCs) were
purchased from Shanghai Huzhen Industrial Co., Ltd. at passage 4.
IPA, PA and ovalbumin (OVA) were purchased from Sigma-Aldrich;
Merck KGaA. The solvent used for the aluminum hydroxide solution
was saline.
Establishment of a mouse model of
AR
The experimental procedures followed the guidelines
set by the Ethical Review Committee for Laboratory Animal Welfare
of The First Hospital of Shanxi Medical University (approval no.
DWLL-2024-015; Taiyuan, China). The experiment followed the Guide
for the Care and Use of Laboratory Animals (24). Female BALB/c mice (n=30; weight, 20
g; age, 6–8 weeks) were obtained from Nanjing Junke Bioengineering
Co., Ltd. The mice were housed in a controlled environment with a
temperature of 25±1°C and a relative humidity of 45–55%, under a
12-h light-dark cycle, with ad libitum access to food and
water. Mice were thoroughly examined and observed twice daily at
8:30 a.m. and 5:30 p.m. to ensure that they received adequate
attention and care during the experiment. Prior to the commencement
of the experiment, the mice were acclimated for 1 week following
standard procedures to ensure that they were familiar with and
adapted to the new living environment. Subsequently, a 29-day
experimental intervention phase was initiated. During this period,
the mice were provided with ample feed and maintained a comfortable
living environment to ensure the smooth progression of the
experiment and data reliability. The entire experimental procedure,
including the feeding adaptation period and the experimental
intervention period, spanned a total of 36 days.
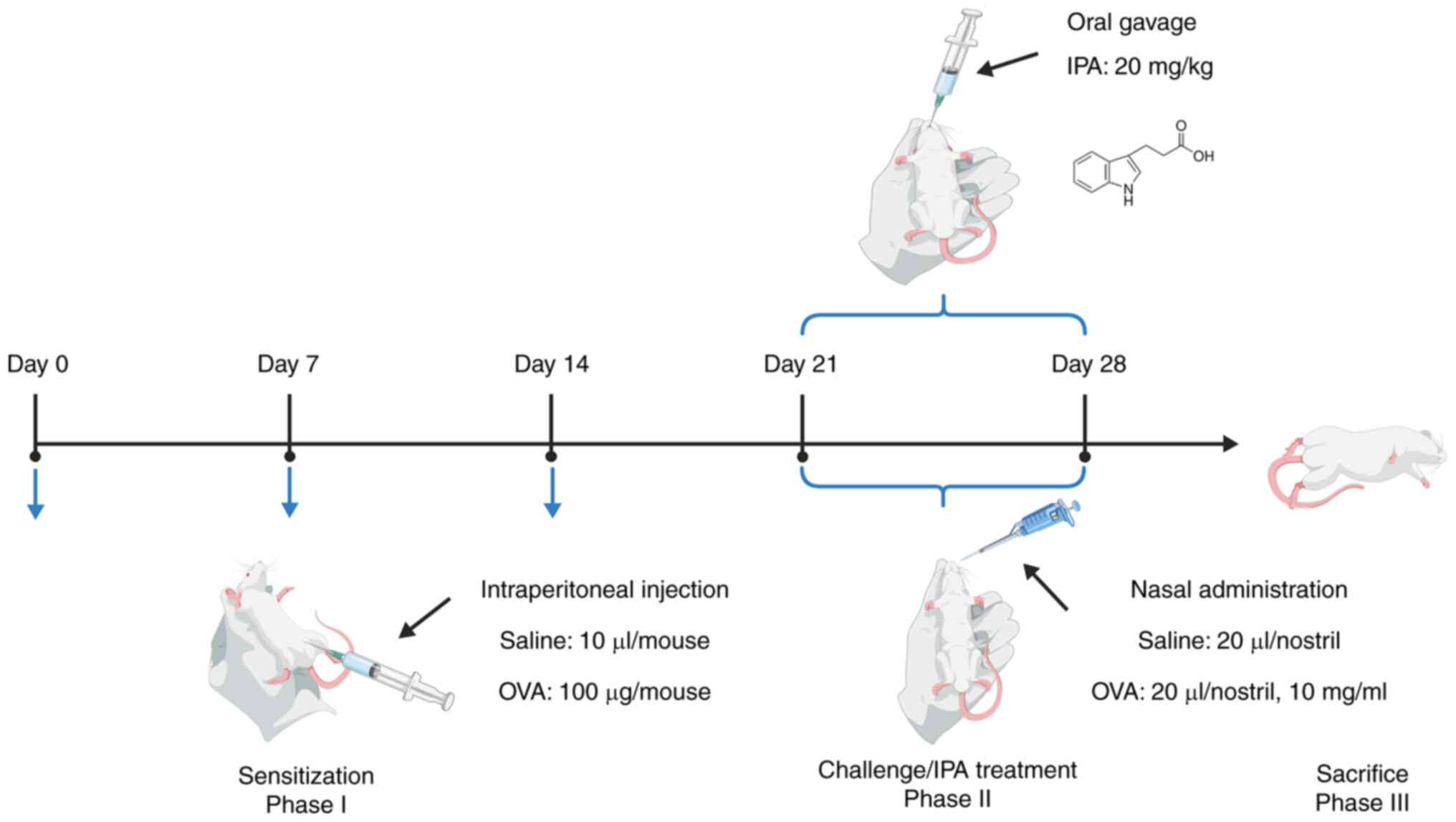
The literature relevant to OVA-sensitized mice was
extensively reviewed (25,26) and our expertise was drawn upon in
developing the mouse models of AR. The mice were randomly assigned
to the following three groups: i) A blank control group (Control
group), ii) an OVA sensitization group (OVA group) and iii) an IPA
treatment group (OVA + IPA group). Sensitization involved
intraperitoneal injections of OVA (100 µg/mouse in 0.1 ml 1 mg/ml
aluminum hydroxide solution) on days 0, 7 and 14, followed by daily
nasal administration of OVA (20 µl/nostril, 10 mg/ml) between days
21 and 28. The concentration of IPA and the intervention duration
were established based on the preliminary investigation by Zhou
et al (14) and our
pre-experiment. The OVA + IPA group was also administered a daily
oral gavage of IPA (20 mg/kg) throughout the same timeframe. The
control group received saline injections and nasal stimulation. The
volume of saline used for the control group during both the
sensitization and challenge phases was identical to that used for
the mice in the OVA group. Furthermore, during the nasal
stimulation experiment, a pre-prepared OVA solution was employed.
For the specific procedure, mice were placed in a comfortable
position, and a 20-µl pipette was used to slowly administer the
solution intranasally, to minimize the discomfort of the mice and
prevent asphyxiation (Fig. 1). On
the final day, after deeply anesthetizing the mice via
intraperitoneal injection with 50 mg/kg sodium pentobarbital, the
mice were euthanized by cervical dislocation, and nasal mucosa and
blood samples were collected. Animal euthanasia was confirmed by
observing respiration and heartbeat, examining pupil reactions and
using the touch method, which specifically entailed checking the
skin temperature by touching, and assessing heartbeat and pulse
through palpation. Throughout the experiment, the health status of
the mice was closely monitored and no unexpected deaths occurred.
When the mice appeared to have seriously deteriorated in health,
were untreatable or exhibited abnormal behavior, or when the
purpose of the experiment had been achieved and no further valuable
data could be obtained, the principles of the ethics review were
strictly followed and the animals were euthanized. This was done to
minimize the pain and discomfort suffered by the mice. No mice were
sacrificed early due to the aforementioned circumstances. All mice
died as a result of the euthanasia procedure required by the
experimental design, in full compliance with ethical review
requirements and animal welfare standards.
AR symptom scores
During the 21–28-day post-sensitization period, an
observer blinded to the groupings recorded the frequency of
sneezing and nose scratching, and the intensity of rhinorrhea in
the mice. The symptoms were assessed using a standardized scale
after OVA exposure, with a total score >5 indicating effective
sensitization (27). The score for
AR (SFAR) was calculated as the sum of the scores for sneezing,
nasal scratching and rhinorrhea; and the total scores were analyzed
using a non-parametric test. The specific scoring details are
summarized in Table I.
 | Table I.Symptom scores of AR evaluation. |
Table I.
Symptom scores of AR evaluation.
| Symptom | Slight (1
point) | Moderate (2
points) | Severe (3
points) |
|---|
| Sneezing, times/30
min | 1-3 | 4-10 | >11 |
| Nasal scratching,
times/30 min | 1-5 | 6-15 | >15 |
| Rhinorrhea | Flow to anterior
nasal foramen | Beyond the anterior
nasal foramen | Nasal discharge
flowing onto the face |
Histopathological examination of nasal
mucosa
The mouse nasal mucosa epithelium was excised and
fixed in 4% paraformaldehyde for 24 h at 4°C. Following fixation,
the samples underwent dehydration using a tissue dehydrator and
were embedded in paraffin. Each paraffin-embedded block was
pre-frozen at −20°C for 30 min, sectioned to a thickness of 5 µm
and then baked for 2 h. Subsequently, the sections were
deparaffinized using xylene and ethanol gradients, and were
subjected to hematoxylin and eosin (H&E) staining, periodic
acid-Schiff staining (PAS) and toluidine blue staining (TBS).
H&E, PAS and TBS (1%) (cat. nos. G1120, G1281
and G3668; all from Beijing Solarbio Science & Technology Co.,
Ltd.), were used at their original concentrations as produced by
the manufacturer. The staining procedures were performed at room
temperature. For H&E staining, the sections were dewaxed in
100% xylene twice (10 min each), rehydrated through a graded series
of ethanol (100, 95, 85 and 75%; 3 min each) and then immersed in
distilled water for 2 min. After staining with hematoxylin solution
for 5 min and rinsing with distilled water to remove excess stain,
the sections were differentiated with differentiation solution for
30 sec, followed by two 5-min rinses with tap water. Eosin staining
was then performed for 1 min, after which excess stain was removed
and sections were rapidly dehydrated. For dehydration, clearing and
mounting, sections were sequentially immersed in graded ethanol
(75, 85, 95 and 100% ethanol, 3 sec each; 100% ethanol for 1 min;
and 100% xylene twice, 1 min each), before being mounted with
neutral gum.
For PAS, the sections were rinsed with tap water for
3 min and then with distilled water twice, before applying periodic
acid and incubating the sections at room temperature for 5 min.
After another rinse with tap water and two rinses with distilled
water, Schiff staining solution was applied and the sections were
stained in the dark for 10 min. After a 10-min rinse with distilled
water, the sections were immersed in hematoxylin staining solution
for 1 min to stain the nuclei, differentiated with acidic
differentiation solution for 5 sec and then blued in tap water for
10 min. Finally, sections were dehydrated through a graded series
of ethanol, cleared in 100% xylene, and mounted with neutral
gum.
For TBS, paraffin-embedded sections were dewaxed in
100% xylene twice (15 min each), rehydrated through a series of
ethanol solutions (1 min each) and then rinsed with distilled water
for 2 min. TBS solution was then applied for 10 min, followed by a
1-min rinse with distilled water and blotting dry with filter
paper. Sections were differentiated with 95% ethanol until mast
cells appeared purple-blue and the background light blue, rapidly
dehydrated in 95% ethanol for 5 sec and then immersed in absolute
ethanol twice (1 min each). Finally, the sections were cleared in
100% xylene and mounted with neutral gum. Pathological changes were
examined under a light microscope (Pannoramic Scan II; 3DHISTECH,
Ltd.) and image analysis was performed using CaseViewer 2.4.0
(https://www.lumingrj.com/2024/12/18/WIN/CaseViewer-2.4.0).
ELISA
Mouse blood was collected from the eyeballs of mice
after sacrifice and incubated at room temperature for 2–3 h. The
whole blood was centrifuged at 1,800 × g for 15 min at room
temperature to obtain the serum. Subsequently, the levels of the
inflammatory factors IL-4, IL-5, IL-10, IL-13 and IgE were
quantified using ELISA-specific kits (cat. nos. JM-02448M1,
JM-02447M1, JM-02459M1, JM-02456M1 and JM-02339M1, respectively;
Jiangsu Jingmei Biological Technology Co., Ltd.) according to the
manufacturer's protocol.
Immunohistochemistry staining
The immunohistochemical assay was employed to
evaluate the expression levels of AKT, phosphorylated (P)-AKT,
CEBPB and IL-10 in the epithelial tissues of the mouse nasal
mucosa. Tissue sections underwent deparaffinization following
H&E staining, followed by immersion in water for 10 min.
Subsequently, the sections were treated with 0.01 M sodium citrate
buffer (pH 6.0) and subjected to heat treatment in a pressure
cooker. After the rotary valve popped up, which indicated that the
water temperature had reached 100°C, the sections were heated for 2
min and then cooled to room temperature naturally. Endogenous
peroxidase activity was blocked using a freshly prepared 3%
H2O2 methanol solution for 10 min in the dark
at room temperature, and the sections were subsequently washed with
PBS. The sections were then incubated with sheep-derived blocking
serum (cat. no. ZLI-9022; Beijing Zhongshan Jinqiao Biotechnology
Co., Ltd.) at room temperature for 20 min. Primary antibodies (AKT:
Cat. no. 10176-2-AP, 1:100; P-AKT (Ser473): Cat. no. 66444-1-Ig,
1:100; CEBPB: Cat. no. 23431-1-AP, 1:100; IL-10: Cat. no.
60269-1-Ig, 1:100; all from Wuhan Sanying Biotechnology) were then
used to incubate the sections overnight at 4°C. On the following
day, after the primary antibodies were removed, an appropriate
quantity of secondary antibody (enzyme-labeled goat
anti-mouse/rabbit IgG polymer: Cat. no. PV-6000, 1:100; Beijing
Zhongshan Jinqiao Biotechnology Co., Ltd.) was applied, followed by
color development using freshly prepared DAB solution (cat. no.
ZLI-9017; Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.). The
reaction was halted with tap water post-color development, and
counterstaining was performed at room temperature with hematoxylin
staining solution for 1 min, followed by rinsing with tap water for
10 min to reverse the blue coloration. Finally, the slides were
sealed with resin and images were captured using an optical
microscope. Finally, randomly selected regions were analyzed using
ImageJ-win64 software (National Institutes of Health) to determine
the expression levels of the target proteins.
Reverse transcription-quantitative PCR
(RT-qPCR)
The nasal mucosa tissue was removed from the −80°C
freezer and thawed, TriQuick (cat. no. R1100, Beijing Solarbio
Science & Technology Co., Ltd.) was added and the tissue
underwent lysis in a homogenizer. Subsequently, chloroform was
added, and the sample was mixed and centrifuged at 12,000 × g for
15 min at 4°C. The upper layer was then collected, mixed with
isopropanol and incubated at −20°C overnight. The next day, the RNA
precipitate was obtained by centrifugation at 12,000 × g for 10 min
at 4°C, and RNA was reverse transcribed into cDNA using the RT kit
(cat. no. RR036A; Takara Bio, Inc.) according to the manufacturer's
instructions, as follows: 37°C for 15 min, 85°C for 15 sec and 16°C
indefinitely. Subsequently, qPCR was performed using 2X M5 HiPer
SYBR Premix EsTaq (with Tli RNaseH) (cat. no. MF787-01; Mei5
Biotechnology, Co., Ltd). The thermocycling conditions were as
follows: Pre-denaturation at 95°C for 30 sec, followed by 40 cycles
at 95°C for 5 sec and 60°C for 30 sec, and a final melt curve
analysis. The relative mRNA expression levels were calculated using
the 2−ΔΔCq method (28)
with GAPDH as an endogenous control. The primer sequences are
listed in Table II.
 | Table II.Primer sequences. |
Table II.
Primer sequences.
| Gene | Primer
sequence |
|---|
| AKT | F:
5′-GTGGCAGGATGTGTATGAGAAGAAG-3′ |
|
| R:
5′-AGGCGGCGTGATGGTGATC-3′(Reverse) |
| CEBPB | F:
5′-GCAATCCGGATCAAACGTGG-3′ |
|
| R:
5′-GATTACTCAGGGCCCGGCTG-3′ |
| IL-10 | F:
5′-GGACAACATACTGCTAACCGACTC-3′ |
|
| R:
5′-TGGATCATTTCCGATAAGGCTTGG-3′ |
| IL-4 | F:
5′-CCATATCCACGGATGCGACA-3′ |
|
| R:
5′-CGTTGCTGTGAGGACGTTTG-3′ |
| IL-5 | F:
5′-CCACATGCTGGGCCTTACTT-3′ |
|
| R:
5′-GTAAACTGGGGGAGGCTTCT-3′ |
| IL-13 | F:
5′-ATGGCCTCTGTAACCGCAAG-3′ |
|
| R:
5′-CTCATTAGAAGGGGCCGTGG-3′ |
| GAPDH | F:
5′-GGTTGTCTCCTGCGACTTCA-3′ |
|
| R:
5′-TGGTCCAGGGTTTCTTACTCC-3′ |
HNEpC culture and intervention
HNEpCs were cultured in DMEM (Thermo Fisher
Scientific, Inc.) supplemented with 5% fetal bovine serum (cat. no.
SA301.02, Cellmax Co., Ltd.) and 1% penicillin/streptomycin
solution) (cat. no. PWL062; Dalian Meilun Biology Technology Co.,
Ltd.) at 37°C in a humidified atmosphere containing 5%
CO2. Upon reaching 80% confluence, the cells were
passaged and seeded into 6-well plates. All groups were treated at
37°C in a humidified atmosphere containing 5% CO2. The
cells were then divided into four groups for treatment: Control
group received standard medium as a control; OVA group was exposed
to 0.1% OVA (dissolved in standard medium) for 24 h; OVA + PA group
was treated with 20 µmol/l PA (dissolved in standard medium) for 24
h following 24 h of intervention with 0.1% OVA; and OVA + PA + AKT
inhibitor VIII group was pretreated with 10 mmol/l AKT inhibitor
VIII (cat. no. HY-10355; MedChemExpress) (dissolved in DMSO) for 5
h before the addition of 0.1% OVA for 24 h, followed by treatment
with 20 µmol/l PA (dissolved in standard medium) for 24 h. The
proteins were extracted at the end of the experiment and were
subsequently subjected to western blot analysis to detect related
markers.
Immunofluorescence staining
HNEpCs were cultured on sterile coverslips in
12-well plates until reaching 50% confluence and were treated as
aforementioned. Following fixation with 4% paraformaldehyde for 10
min at room temperature, the coverslips were subjected to
immunofluorescence staining. The harvested cells were permeabilized
using a 1% Triton X-100 solution (cat. no. T8200; Beijing Solarbio
Science & Technology Co., Ltd.) and antigenic blocking was
carried out using sheep-derived blocking serum (cat. no. ZLI-9022;
Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.) at room
temperature for 30 min. The cells were then incubated overnight at
4°C with primary antibodies against AKT (cat. no. 10176-2-AP,
1:100), P-AKT (Ser473) (cat. no. 66444-1-Ig, 1:100), CEBPB (cat.
no. 23431-1-AP, 1:100) and IL-10 (cat. no. 60269-1-Ig, 1:100) (all
from Wuhan Sanying Biotechnology). Subsequently, the samples were
incubated with the following secondary antibodies: CY3-conjugated
avidin (cat. no. BA1037, 1:200) and DyLight®
488-conjugated avidin (cat. no. BA1128, 1:200) (both from Boster
Biological Technology), for 1 h at room temperature in the dark.
After staining with DAPI solution (cat. no. AR1176; 1:000; Boster
Biological Technology) for 10 min at room temperature and washing
with PBS three times (2 min/wash), anti-fluorescence quencher (cat.
no. AR0036; Boster Biological Technology) was applied to the
slides. The immunofluorescence slides were carefully removed from
the well plates and incubated overnight at 4°C prior to observation
of the results using a fluorescence microscope. The fluorescence
microscope revealed DAPI-labeled nuclei in blue, DyLight
488-labeled proteins in green and CY3-labeled proteins in red.
Western blot analysis
Total proteins were extracted from mouse nasal
mucosa tissues and HNEpCs using RIPA lysis buffer (cat. no. AR0102;
Boster Biological Technology), and the total protein concentration
was quantified using a BCA kit (cat. no. AR1189; Boster Biological
Technology) according to the manufacturer's instructions. The
protein samples were then normalized, treated with 5X SDS-PAGE
loading buffer and denatured at 100°C for 10 min. Subsequently, the
denatured proteins were separated by SDS-PAGE on a 10% gel and
transferred to a PVDF membrane within 1.5 h. The PVDF membrane was
blocked with 5% skim milk powder at room temperature for 2 h.
Primary antibodies specific to AKT (cat. no. 10176-2-AP, 1:4,000),
P-AKT (Ser473) (cat. no. 66444-1-Ig, 1:1,000), CEBPB (cat. no.
23431-1-AP, 1:6,000), IL-10 (cat. no. 60269-1-Ig, 1:4,000) and
β-actin (cat. no. P60709, 1:10,000) from (all from Wuhan Sanying
Biotechnology) were used to incubate the membranes overnight at
4°C. After washing the membrane with TBS-0.1% Tween, the membranes
were incubated for 2 h at room temperature with HRP-goat
anti-rabbit IgG (cat. no. AS014; 1:10,000; ABclonal Biological
Technology) and HRP-goat anti-mouse IgG (cat. no. BA1050; 1:10,000;
Boster Biological Technology), before proceeding with further
washing steps. Finally, protein signals on the membranes were
detected using an ultrasensitive ECL chemiluminescence kit (cat.
no. AR1171; Boster Biological Technology), and protein analysis was
performed based on optical density measurements using ImageJ-win64
software.
Statistical analysis
Data analysis was performed using GraphPad Prism 8.0
(Dotmatics). Data are presented as the mean ± standard deviation
and were obtained from at least three independent experiments. For
comparisons involving more than two groups, a one-way analysis of
variance was performed, followed by Tukey's multiple comparisons
test to evaluate pairwise distinctions. The non-parametric
Kruskal-Wallis test and Dunn's post hoc test was employed to
analyze the SFAR and rhinorrhea score, and these data are presented
as the median (IQR). P<0.05 was considered to indicate a
statistically significant difference.
Results
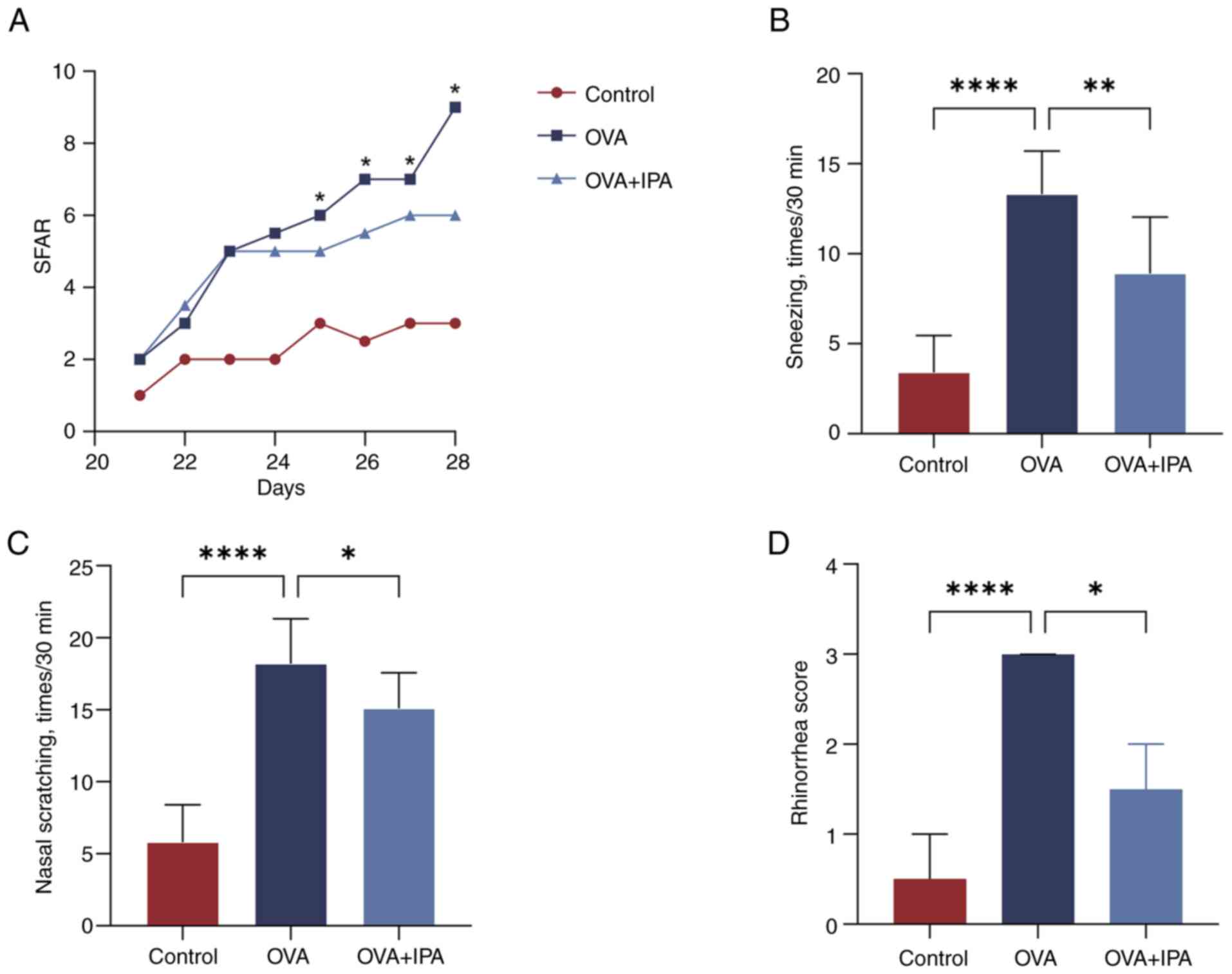
Behavioral scores of AR model
mice
On day 21 of sensitization, mice in the OVA group
began showing visible signs of sneezing, nose scratching and
rhinorrhea, with symptoms escalating as OVA stimulation increased.
Analysis of behavioral recordings revealed that by day 8 of
stimulation, scores for the blank control group remained <5, in
contrast to the OVA group, where scores for nasal symptoms >5,
confirming the successful model construction (Table III). Furthermore, mice in the OVA
+ IPA group exhibited a gradual alleviation of AR symptoms upon IPA
drug intervention, with statistical significance (P<0.05)
observed from day 5 onwards (Fig.
2A). By the final day of stimulation, significant reductions in
sneezing, nose scratching and rhinorrhea symptoms were evident in
the OVA + IPA group, with behavioral scores for nasal symptoms
significantly decreased compared with those in the OVA group
(P<0.05; Fig. 2B-D).
 | Table III.Score of nasal symptoms. |
Table III.
Score of nasal symptoms.
| Day | Control | OVA | OVA + IPA |
|---|
| Day 21 | 1.0 (1.0) | 2.0 (1.0) | 2.0 (1.0) |
| Day 22 | 2.0 (1.0) | 3.0 (1.0) | 3.5 (1.0) |
| Day 23 | 2.0 (1.0) | 5.0 (2.0) | 5.0 (2.0) |
| Day 24 | 2.0 (1.0) | 5.5 (2.0) | 5.0 (1.0) |
| Day 25 | 2.0 (1.0) | 6.0 (2.0) | 5.0 (0.5) |
| Day 26 | 2.5 (1.0) | 7.0 (2.0) | 5.5 (1.0) |
| Day 27 | 3.0 (0.5) | 7.0 (1.0) | 6.0 (1.0) |
| Day 28 | 3.0 (1.0) | 9.0 (1.0) | 6.0 (1.0) |
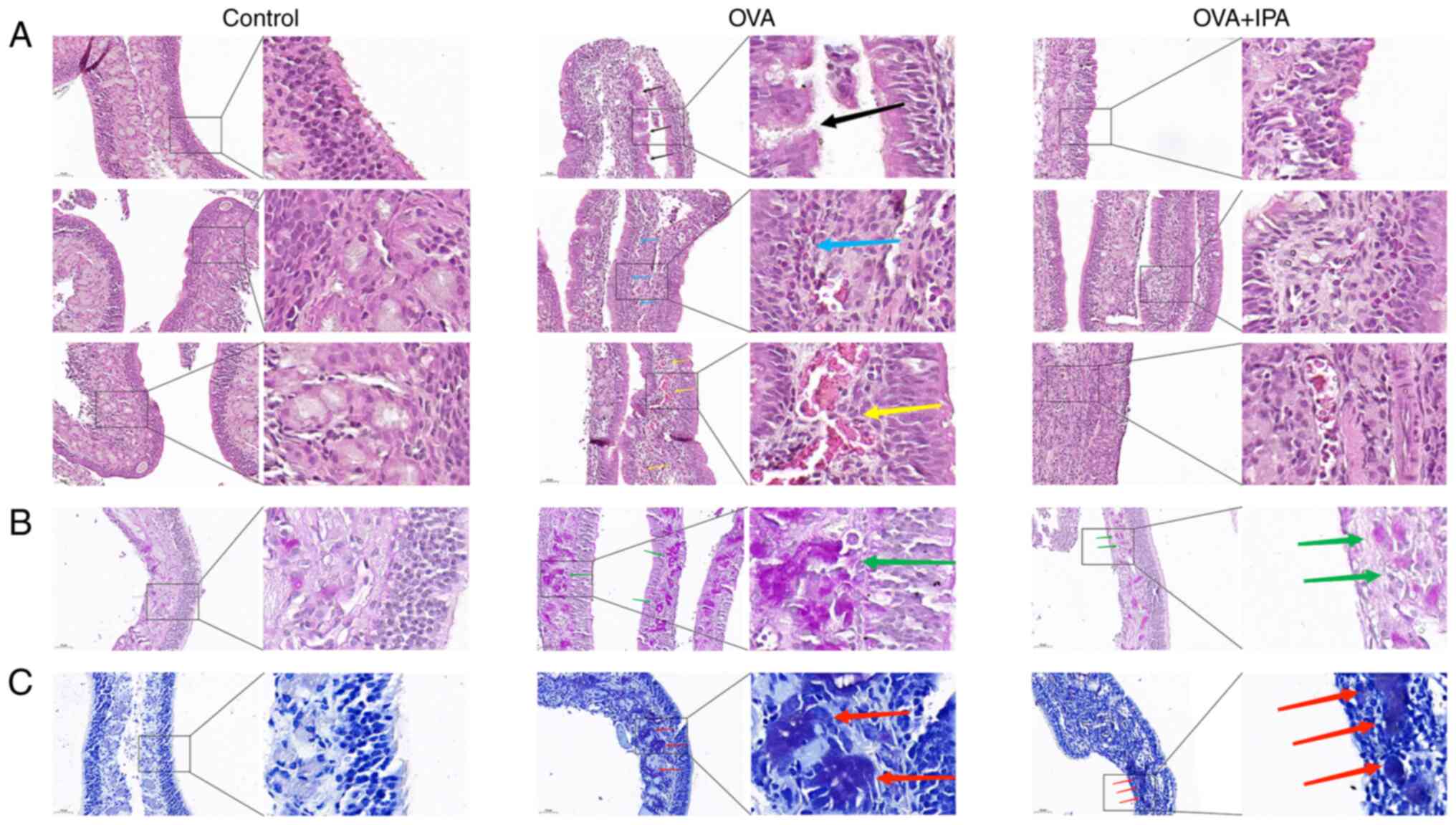
Pathologic changes of nasal mucosa in
AR model mice after IPA intervention
Histological examination using H&E staining
revealed that the nasal mucosal epithelium in the control group
exhibited structural integrity with uniformly arranged cilia,
smooth submucosal blood vessels and the absence of scattered
inflammatory cells (Fig. 3A). By
contrast, the OVA group showed discontinuous and disorganized nasal
mucosal epithelium, infiltration of numerous inflammatory cells,
mucosal edema, congested and dilated arteriolar blood vessels, and
varying degrees of nasal mucosa detachment. PAS and TBS staining
indicated the presence of proliferating cup cells and mast cells in
the OVA group, with cup cells stained purple-red and mast cells
stained purple (Fig. 3B and C).
Treatment with IPA resulted in a reduction in the aforementioned
cell number of cup cells and mast cells.
Alterations in serum inflammatory
cytokines in AR model mice after IPA intervention
The levels of cellular inflammatory factors (IL-4,
IL-5, IL-13, IgE and IL-10) were detected in mouse serum samples
using ELISA. Significantly higher levels of IL-4, IL-5, IL-13 and
IgE were observed in the OVA group compared with those in the
Control and OVA + IPA groups (P<0.05; Fig. 4A, B, D and E). Conversely, IL-10
levels were significantly higher in the Control and OVA + IPA
groups than those in the OVA group (P<0.05; Fig. 4C).
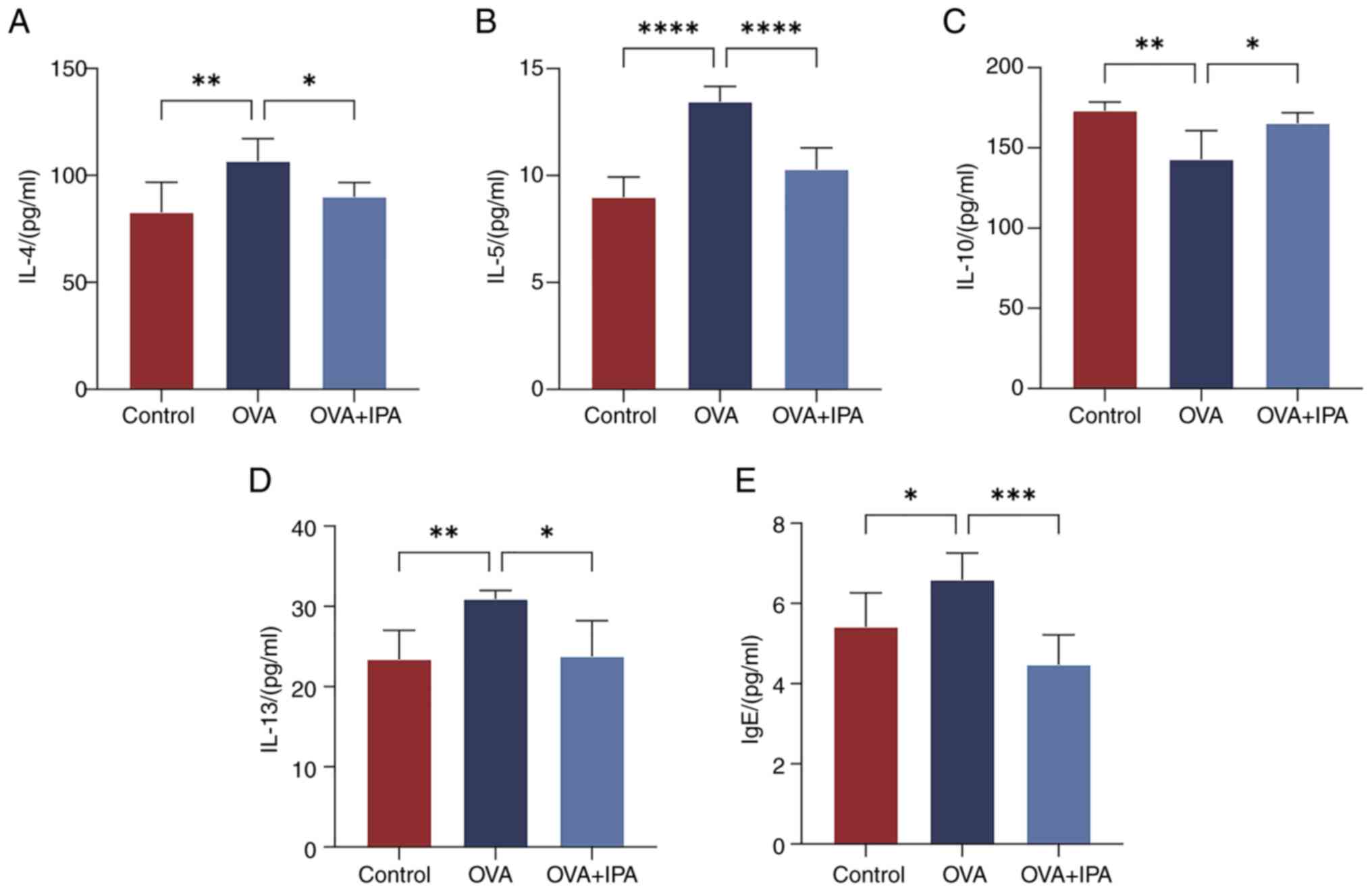 | Figure 4.Changes in serum inflammatory
cytokines in allergic rhinitis model mice following treatment with
IPA. Serum levels of (A) IL-4, (B) IL-5, (C) IL-10, (D) IL-13 and
(E) IgE were measured by ELISA. Data are presented as the mean ±
standard deviation, n=6. *P<0.05, **P<0.01, ***P<0.001,
****P<0.0001. IgE, immunoglobulin E; IPA, indolepropionic acid;
OVA, ovalbumin. |
Alterations in the AKT/CEBPB/IL-10
signaling pathway after IPA intervention
The immunohistochemical analysis of AKT, P-AKT,
CEBPB and IL-10 expression in mouse nasal mucosa tissues was
conducted using a light microscope and analyzed with ImageJ
software (Fig. 5). AKT and P-AKT
were observed in the nucleus and cytoplasm; CEBPB was predominantly
nuclear; and IL-10 was detected in the nucleus, cytoplasm and
extracellularly, with positive staining appearing brownish-yellow
(Fig. 5A). Significantly lower
levels of P-AKT and IL-10 were detected in the OVA group compared
with those in the Control group (P<0.05; Fig. 5C, D and F). Conversely, the OVA +
IPA group exhibited significantly increased expression levels of
P-AKT, CEBPB and IL-10 compared with those in the OVA group
(P<0.05; Fig. 5B-F).
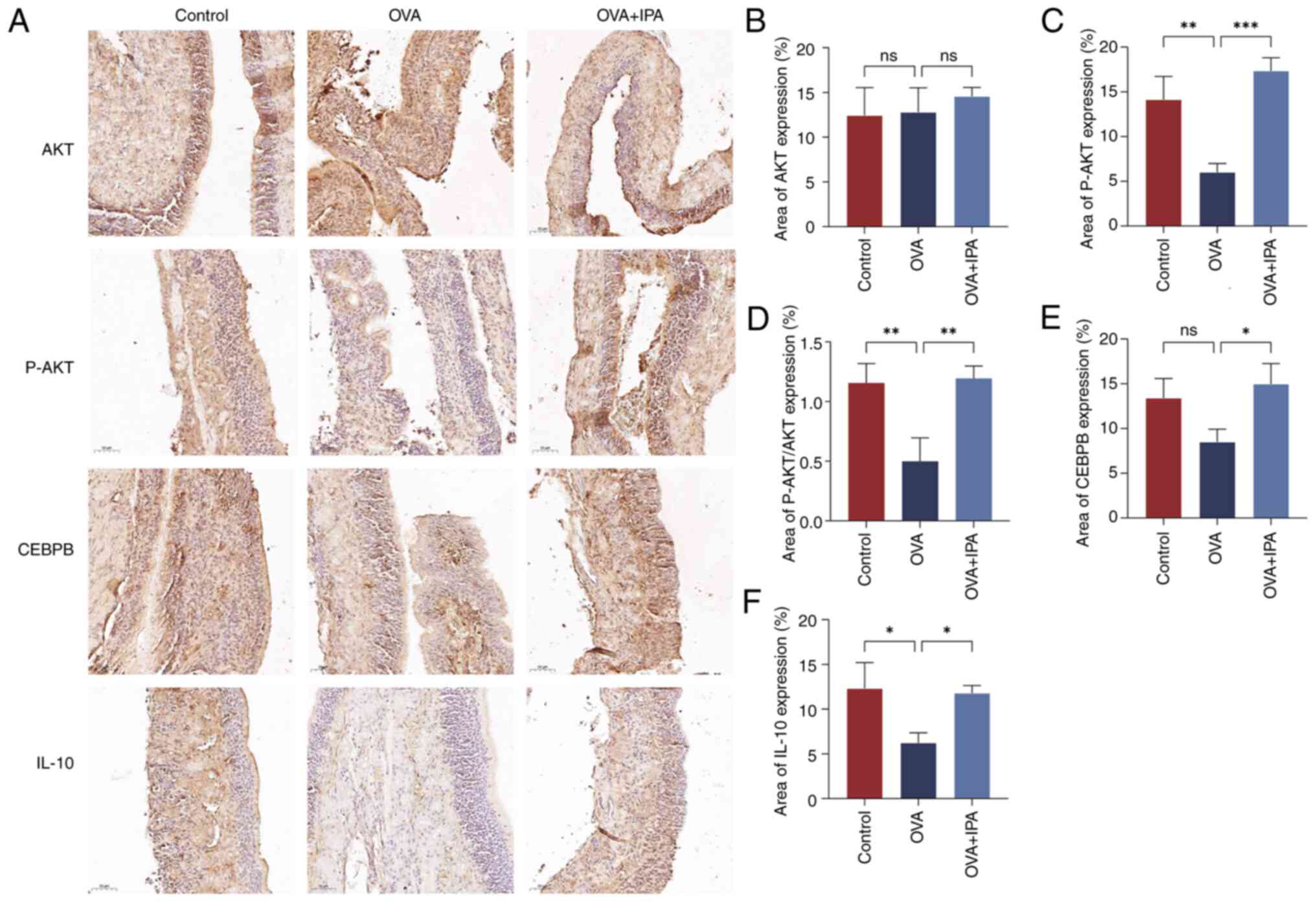 | Figure 5.Alterations in the AKT/CEBPB/IL-10
signaling pathway after IPA intervention. (A) Immunohistochemistry
staining results. Expression levels of (B) AKT, (C) P-AKT, (D)
P-AKT/AKT, (E) CEBPB and (F) IL-10. Area (%) indicates the
integrated optical density of the image/area. Data are presented as
the mean ± standard deviation, n=3. *P<0.05, **P<0.01,
***P<0.001. CEBPB, CCAAT enhancer binding protein β; IPA,
indolepropionic acid; ns, not significant; OVA, ovalbumin; P-,
phosphorylated. |
Changes in the expression levels of
genes in the inflammatory signaling pathway
Based on the immunohistochemical findings, RT-qPCR
was utilized to assess the mRNA expression levels of AKT, CEBPB,
IL-10, IL-4, IL-5 and IL-13 in the nasal mucosa of mice across
different experimental groups (Fig.
6). Statistical analysis revealed no significant differences in
AKT levels among the various groups (P>0.05; Fig. 6A). Compared with those in the
Control group, the OVA group exhibited notably decreased mRNA
expression levels of CEBPB and IL-10, while showing significantly
elevated levels of IL-4, IL-5 and IL-13 mRNA (P<0.05; Fig. 6B-F). Conversely, in contrast to the
OVA group, the OVA + IPA group demonstrated significantly increased
mRNA expression levels of CEBPB and IL-10, and significantly
reduced levels of IL-4, IL-5 and IL-13 mRNA (P<0.05; Fig. 6B-F).
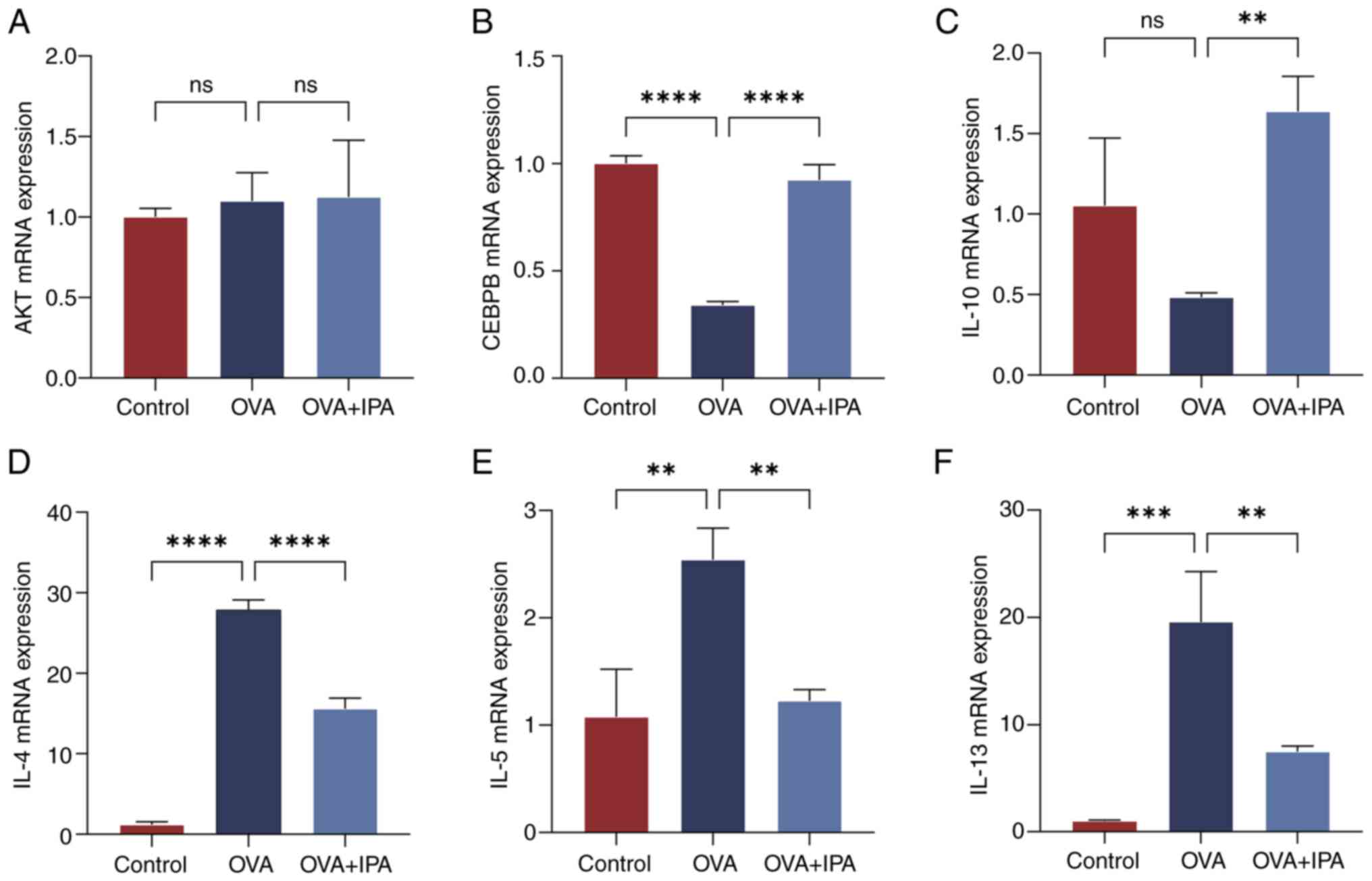 | Figure 6.Changes in the expression levels in
genes in the inflammatory signaling pathway. Comparison of the mRNA
expression levels of the inflammatory signaling pathway factors (A)
AKT, (B) CEBPB, (C) IL-10, (D) IL-4, (E) IL-5 and (F) IL-13 in mous
nasal mucosal epithelial tissues across different groups. Data are
presented as the mean ± standard deviation, n=3. **P<0.01,
***P<0.001, ****P<0.0001. CEBPB, CCAAT enhancer binding
protein β; IPA, indolepropionic acid; ns, not significant; OVA,
ovalbumin. |
Changes in the expression levels of
proteins in the inflammatory signaling pathway
The protein expression levels of AKT, P-AKT, CEBPB
and IL-10 in the nasal mucosa of mice from each group were assessed
using western blotting (Fig. 7).
Compared with those in the Control group, the OVA group exhibited a
significant decrease in the protein expression levels of CEBPB and
IL-10 (P<0.05; Fig. 7E and F).
Conversely, the OVA + IPA group displayed notably elevated protein
expression levels of P-AKT, CEBPB and IL-10 compared with those in
the OVA group (P<0.05; Fig.
7C-F).
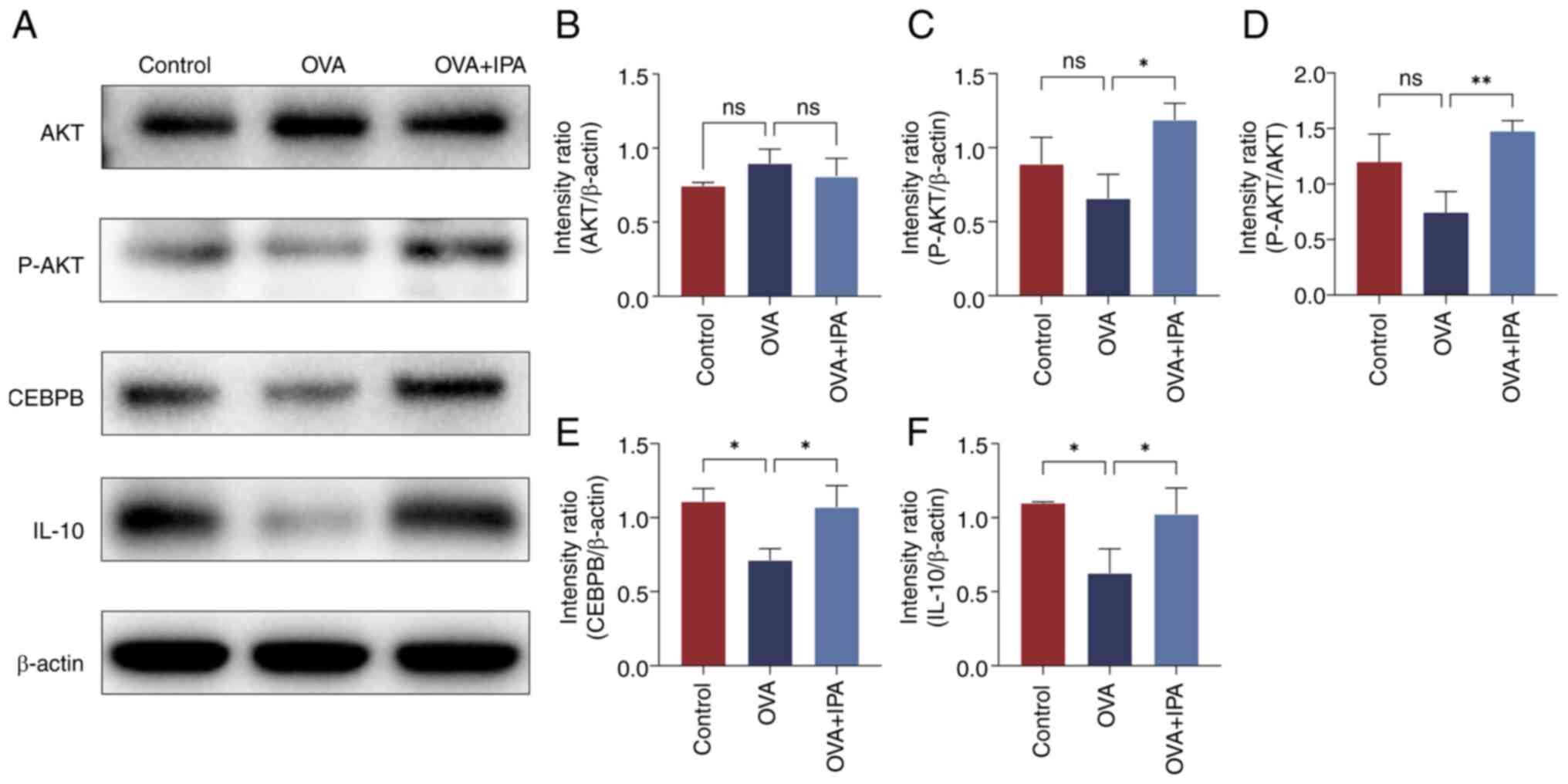 | Figure 7.Changes in the expression levels in
proteins in the inflammatory signaling pathway. (A) Western blot
analysis of (B) AKT, (C) P-AKT, (D) P-AKT/AKT, (E) CEBPB and (F)
IL-10 protein levels. Data are presented as the mean ± standard
deviation, n=3. *P<0.05, **P<0.01. CEBPB, CCAAT enhancer
binding protein β; IPA, indolepropionic acid; ns, not significant;
OVA, ovalbumin; P-, phosphorylated. |
Effect of inhibition of the
AKT/CEBPB/IL-10 signaling pathway by AKT inhibitor VIII
To elucidate the impact of PA on AR via the
AKT/CEBPB/IL-10 signaling pathway, HNEpCs were treated with AKT
inhibitor VIII, OVA and PA, followed by western blot analysis
(Fig. 8). The findings revealed a
significant decrease in the protein expression level of CEBPB in
the OVA group compared with the control group, and a significant
increase in the protein expression levels of P-AKT, CEBPB and IL-10
in the OVA + PA group compared with those in the OVA group
(P<0.05; Fig. 8C-F).
Conversely, following AKT inhibitor VIII intervention, there was a
notable decrease in the protein expression levels of P-AKT, CEBPB
and IL-10 (P<0.05).
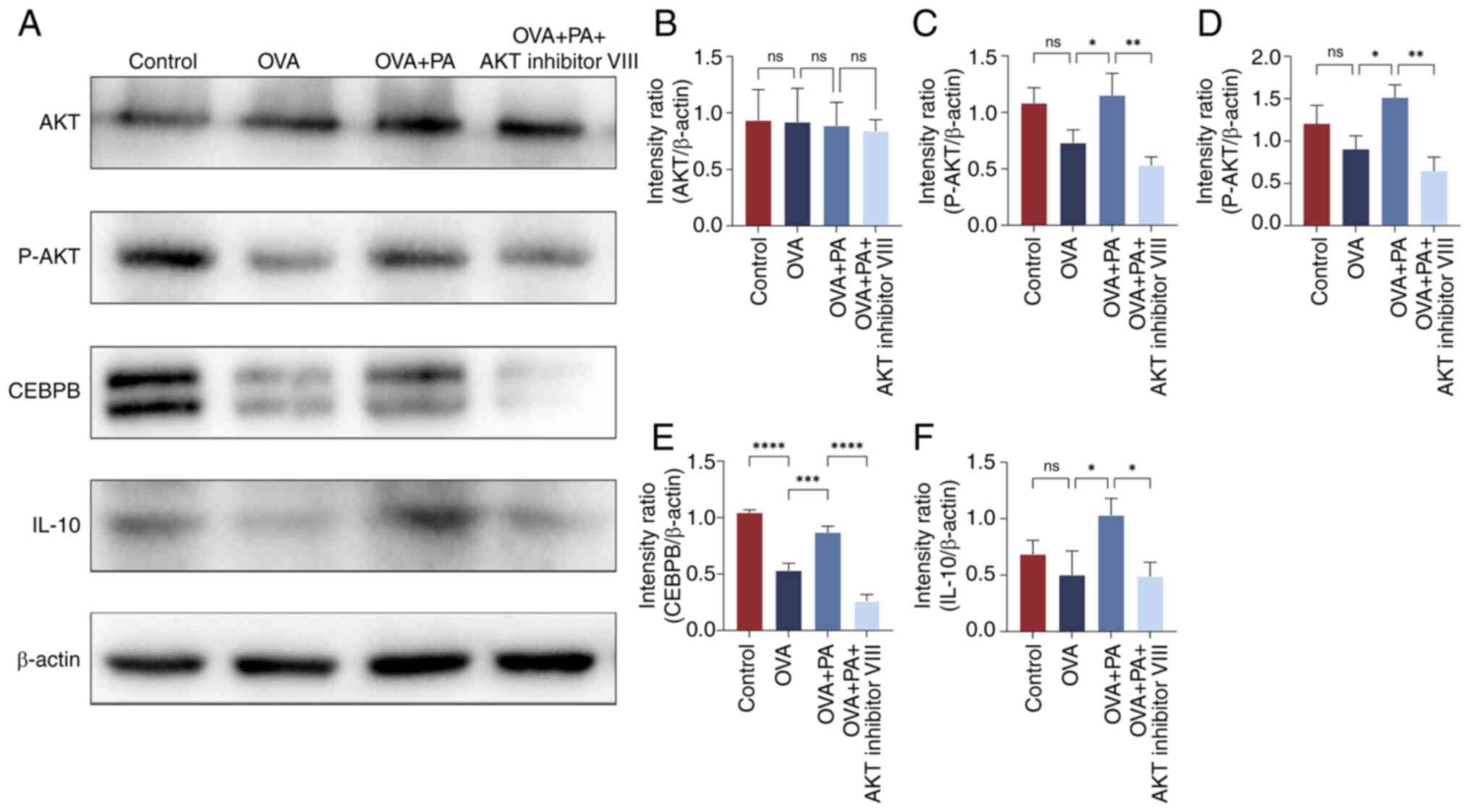 | Figure 8.Protein expression levels of AKT,
P-AKT, CEBPB and IL-10 in human nasal mucosal epithelial cells
treated with AKT inhibitor VIII, OVA and PA. (A) Western blot
analysis of (B) AKT, (C) P-AKT, (D) P-AKT/AKT, (E) CEBPB and (F)
IL-10 protein levels. Data are presented as the mean ± standard
deviation, n=3. *P<0.05, **P<0.01, ***P<0.001,
****P<0.0001. CEBPB, CCAAT enhancer binding protein β; ns, not
significant; OVA, ovalbumin; P-, phosphorylated; PA, propionic
acid. |
Effects of cotreatment with AKT
inhibitor VIII, OVA and PA on the AKT/CEBPB/IL-10 signaling
pathway
Immunofluorescence staining was utilized to detect
the protein expression levels of AKT, P-AKT, CEBPB and IL-10 in the
nucleus and cytoplasm (Fig. 9).
Significantly decreased fluorescence staining signals of P-AKT,
CEBPB and IL-10 were observed in the HNEpCs of the OVA group
compared with those in the control group, whereas significantly
increased fluorescence staining signals of P-AKT, CEBPB and IL-10
were observed in the HNEpCs of the OVA + PA group compared with
those in the OVA group (P<0.05; Fig. 9F-I). Conversely, intervention with
AKT inhibitor VIII led to a notable decrease in the fluorescence
signals of P-AKT, CEBPB and IL-10 (P<0.05).
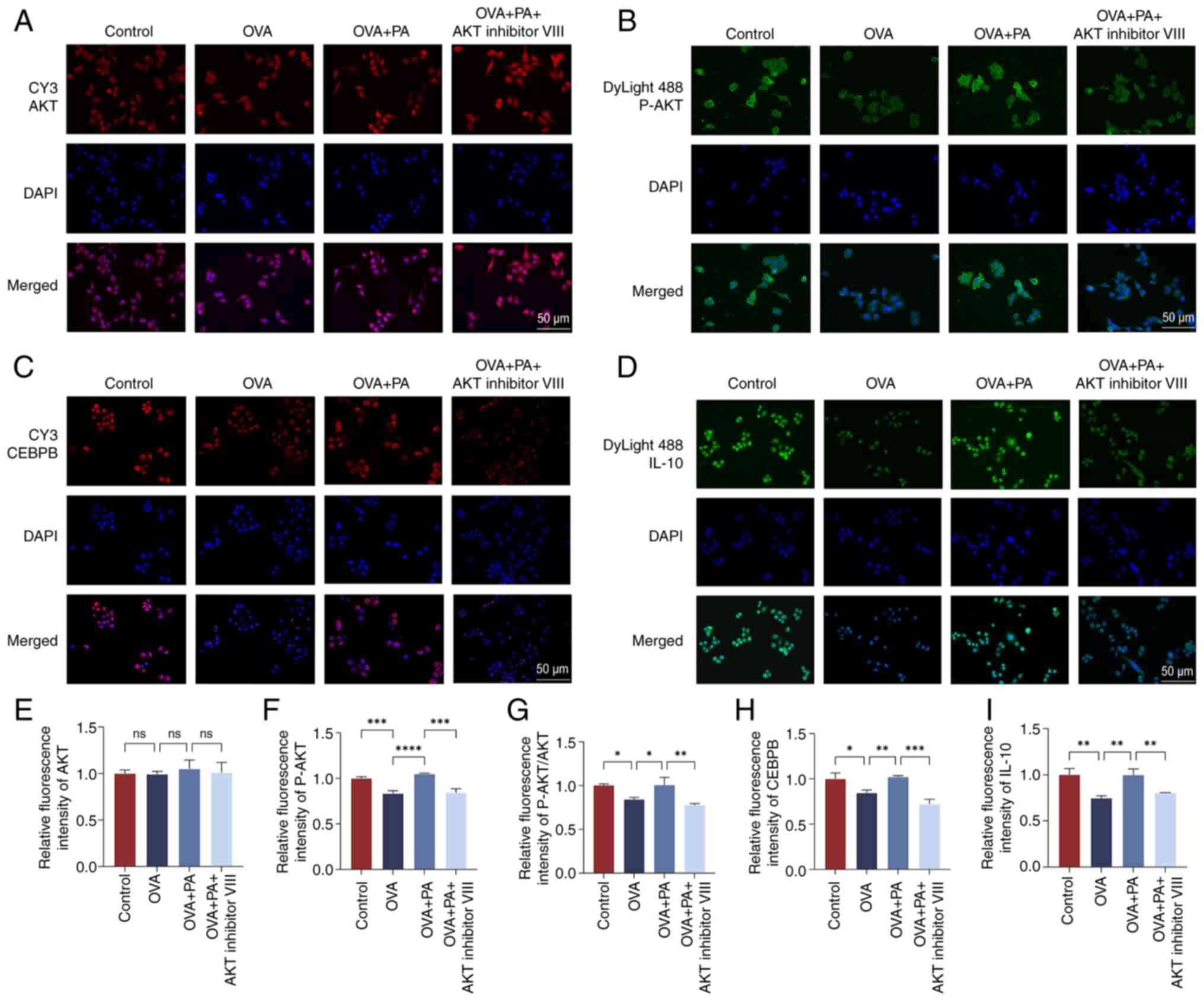 | Figure 9.Localization and expression levels of
AKT, P-AKT, CEBPB and IL-10 proteins in human nasal mucosal
epithelial cells. Cellular immunofluorescence staining of (A) AKT,
(B) P-AKT, (C) CEBPB and (D) IL-10. Relative fluorescence
intensities of (E) AKT, (F) P-AKT, (G) P-AKT/AKT, (H) CEBPB and (I)
IL-10. Data are presented as the mean ± standard deviation, n=3.
*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. CEBPB,
CCAAT enhancer binding protein β; ns, not significant; OVA,
ovalbumin; P-, phosphorylated; PA, propionic acid. |
Discussion
AR, a common allergic condition, disrupts immune
homeostasis by altering the T helper (Th)1/Th2 cell balance
(29). Upon exposure to allergens,
there is a predominance of Th2 cells that release cytokines, such
as IL-4, IL-5 and IL-13, thus stimulating IgE production,
eosinophil activation and mucus secretion (30). The sensitization to OVA imitates
the typical pathological features observed in human AR, including
IgE-mediated tachyphylaxis, eosinophilic infiltration, heightened
Th2 cytokines (IL-4, IL-5, IL-13) and nasal mucosal edema (27,31,32).
Our previous study further confirmed the typical representativeness
of OVA in inducing AR in a mouse model by comparing the sensitizing
properties of OVA with Artemisia pollen. Therefore, the OVA
model was selected because it can accurately reflect the
physiological and immune response characteristics of human AR
(27). The current study
established an AR mouse model using OVA, leading to pronounced
symptoms. Histological examination demonstrated notable nasal
mucosal damage characterized by vascular congestion and
inflammatory cell infiltration, consistent with prior research
(27). Significantly, these
pathological changes were markedly alleviated with the
administration of IPA treatment.
There is a growing interest in microbial
metabolites, particularly SCFAs and their derivatives, for the
modulation of the immune response (33). IPA is a microbial metabolite
produced by gut bacteria through tryptophan decarboxylation,
facilitated by specific strains such as Clostridium
perfringens (34). Unlike PA
derived directly from dietary fiber fermentation, IPA is a
secondary metabolite linking tryptophan breakdown to SCFAs-related
pathways (35). Recent studies
have indicated that IPA possesses anti-inflammatory properties by
influencing immune cell function, enhancing epithelial barrier
integrity and regulating oxidative stress (36,37).
Experimental evidence has demonstrated that IPA can alleviate
inflammatory damage in human chondrocytes and colonic epithelial
cells by modulating the NF-κB signaling pathway (12,38).
These effects align with the reduced inflammation in AR observed in
the present study, where IPA treatment significantly suppressed Th2
cytokines and increased IL-10 levels. Despite structural
distinctions between PA and IPA, they exhibit similar
immunomodulatory effects. PA primarily activates G-protein-coupled
receptors, such as GPR41 and GPR43 (39). Our prior research suggested that
GPR41 and GPR43 serve as upstream targets for PA, indirectly
controlling the AKT pathway, which subsequently regulates IL-10
expression (14). GPR43
specifically mediates PA-induced IL-10 regulation in B cells. While
the interaction of IPA with specific G-protein-coupled receptors
remains less understood, it is conceivable that IPA may interact
with these receptors through structural or functional mechanisms.
Nonetheless, further experimental validation is required to verify
this hypothesis.
In the present study, IPA exhibited a comparable
capacity to activate the AKT/CEBPB/IL-10 signaling pathway to PA,
indicating a convergence in their downstream effects. The distinct
indole structure of IPA implies potential supplementary regulatory
roles, such as influencing AhR-dependent gene expression (11,40),
which may augment IL-10 production in B cells and macrophages. PA
and IPA, which are predominantly synthesized by the gut microbiota,
are subject to dietary and microbial influences. Dysbiosis in the
gut microbiota of patients with AR, resulting in the diminished
production of SCFAs, including IPA, could exacerbate allergic
inflammation. In the present study, supplementation with IPA was
shown to normalize IL-10 levels and mitigate inflammation,
suggesting a compensatory function for IPA in the context of PA
deficiency in AR. Moreover, IPA can facilitate PA synthesis by
intestinal bacteria, with PA reciprocally impeding the degradation
of IL-10 mRNA in regulatory B cells (Bregs) via the AKT/T-bet
pathway; this stabilization of IL-10 expression in Bregs has been
reported to effectively alleviate AR symptoms (14). Due to limitations, the changes in
Tregs or B cells after IPA treatment were not evaluated in the
present study. In the future, we aim to conduct relevant studies to
assess the changes of these cells after IPA treatment; through this
in-depth investigation, we aim to further confirm the central role
of IL-10 in AR immunomodulation, and provide a more solid
scientific basis and innovative ideas for the treatment and
prevention of AR.
IL-10, a pivotal cytokine, is essential for
maintaining immune homeostasis (41). In allergic conditions, Bregs and M2
macrophages can promote regulatory T-cell differentiation or
directly inhibit T-cell proliferation by producing IL-10 and
transforming growth factor β, thus restoring the balance between
Th1 and Th2 cells, and reducing inflammation (42). IL-10 attenuates the activation of
Th2 cells by inhibiting the expression of MHC-II molecules and
co-stimulatory molecules on antigen-presenting cells, thereby
suppressing their function (43).
In addition, IL-10 exerts a positive regulatory effect on STAT3 by
enhancing the phosphorylation of STAT3 in Th2 cells, which
contributes to the reduction of inflammatory cytokine expression
(IL-4, IL-5 and IL-13) in these cells (44). Moreover, inflammatory cytokines,
such as IL-4 and IL-13, rapidly induce IL-10 production via STAT6
activation, a mechanism that effectively prevents excessive
inflammatory responses (45,46).
In the present study, the AKT/CEBPB/IL-10 signaling pathway was
investigated, focusing on changes in IL-10 expression. The findings
revealed a significant association between IL-10 expression and AR,
with the OVA group exhibiting lower IL-10 levels than the other
experimental groups. To understand the process by which IPA
alleviates inflammation associated with AR by restoring the Th1/Th2
balance, IL-4, IL-5, IL-10 and IL-13 levels were also examined in
the nasal mucosa and serum. However, the effects of IL-10 on these
cytokines were not evaluated. ELISA detected increased levels of
IL-4, IL-5, IL-13 and IgE in the serum of OVA-treated mice.
Furthermore, the results of RT-qPCR confirmed the elevated
expression of Th2 cytokines in the nasal mucosa post-OVA
intervention, which was significantly reduced after IPA
intervention. These findings may advance the understanding of the
role of IL-10 in AR and suggest novel directions for future
research.
CEBPB, a transcriptional regulator, serves a role in
modulating immune and inflammatory responses (47,48).
Its deficiency has been associated with excessive CD4 T-cell
proliferation and the impaired function of regulatory T cells in
colitis (49). Notably, SCFAs have
been shown to downregulate DUSP6 expression via the
CEBPB/microRNA-145 pathway, reducing intestinal inflammation
(50). In the present
investigation, a notable difference was observed: CEBPB displayed a
distinct single band in the western blot analysis of animal protein
samples, whereas it exhibited double bands in cell protein samples.
A comprehensive analysis led to the proposal that this variation
could be attributed to disparities in processing and extraction
methodologies, antibody specificity, post-translational protein
modifications. and differences in sample concentrations between
animal and cellular protein samples. In addition, the animal
protein samples were derived from mouse mucosal epithelial tissue,
whereas the cell protein samples were obtained human nasal mucosal
epithelial cells; therefore, this different may also due to
species-specific differences.
AKT is a key player in immune regulation, and the
PI3K/AKT signaling pathway in dendritic cells has been shown to
suppress inflammatory responses by increasing the expression of the
anti-inflammatory cytokine IL-10 and decreasing the expression of
the pro-inflammatory cytokine IL-12 (51). In the current study, a significant
decrease in the expression levels of the pathway factors P-AKT,
CEBPB and IL-10 were detected in the nasal mucosal epithelial
tissues of mice with AR. Following intervention with IPA, these
expression levels were significantly enhanced in AR model mice.
Furthermore, intervention of OVA-sensitized HNEpCs with PA resulted
in increased expression of P-AKT, CEBPB and IL-10 proteins.
Conversely, inhibition of AKT led to a decrease in the expression
levels of these molecules. These findings confirmed the pivotal
role of PA and IPA in the pathogenesis of AR through the
AKT/CEBPB/IL-10 signaling pathway.
Notably, it is essential to recognize the
limitations of the present study. Firstly, it utilized a mouse
model with OVA induction, mimicking key aspects of AR. However,
caution is required when extrapolating the findings to humans
without additional clinical validation. Secondly, using 16S rRNA
sequencing and metabolomics analysis (52,53),
previous studies have demonstrated that the gut microbiota can
modulate immune responses by mediating IPA production. A previous
comparison of microbiota profiles between patients with AR and
healthy controls identified 37 bacterial taxa enriched in the
healthy group, suggesting a potential role of microbiota changes in
AR development (53). Although
IPA, which is primarily produced by the gut microbiota, likely
influences AR, the present study did not directly explore the
specific contribution of the microbiota to IPA-mediated immune
modulation. Future investigations, including 16S rRNA sequencing
and metabolomics analyses, are planned to elucidate this
relationship. Moreover, while IPA and PA share biological
activities, there is inadequate evidence to support the complete
substitution of PA with IPA. Further comprehensive studies are
essential to fully comprehend the specific mechanisms and potential
interchangeability between IPA and PA. Upon reviewing the
experimental design of the present study, the absence of an OVA +
AKT inhibitor VIII group constitutes an oversight. To enhance the
rigor and completeness of the experiment, an OVA + AKT inhibitor
VIII group will be included in future experiments, thereby further
consolidating the scientific validity and reliability of the
findings. Finally, the failure to consider concentration gradient
modeling is another shortcoming of the current study, as it is
critical for accurately assessing drug effects. In light of this,
the incorporation of concentration gradient factors will be
prioritized in our subsequent trial design.
In conclusion, IPA has emerged as a potential
therapeutic option for AR by utilizing mechanisms similar to PA,
including AKT/CEBPB/IL-10 activation, and by inducing unique
immunomodulatory pathways associated with its indole structure.
Future investigations should focus on exploring the combined
utilization of IPA and PA, and their translational prospects in
developing novel strategies for reinstating immune balance in
allergic disorders.
Acknowledgements
Not applicable.
Funding
The present research project was supported by the Shanxi
Scholarship Council of China (grant no. 2022-194) and the
Fundamental Research Program of Shanxi Province (grant no.
202303021221218).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LG designed the research and wrote the draft. YS and
FZ analyzed the experimental data. YZ and HH recorded animal
behavior and provided data. YF participated in the conception and
design of the research, provided resources and supervised the
entire work. LG, YS and YF confirm the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical Review
Committee for Laboratory Animal Welfare of The First Hospital of
Shanxi Medical University (approval no. DWLL-2024-015). All methods
were performed in accordance with the relevant guidelines and
regulations.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang Y, Lan F and Zhang L: Update on
pathomechanisms and treatments in allergic rhinitis. Allergy.
77:3309–3319. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Y, Lan F and Zhang L: Advances and
highlights in allergic rhinitis. Allergy. 76:3383–3389. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang T, Wang HR, Mou YK, Liu WC, Wang Y,
Song XY, Ren C and Song XC: Mutual influence between allergic
rhinitis and sleep: Factors, mechanisms, and interventions-a
narrative review. Nat Sci Sleep. 16:1451–1467. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mou YK, Wang HR, Zhang WB, Zhang Y, Ren C
and Song XC: Allergic rhinitis and depression: Profile and
proposal. Front Psychiatry. 12:8204972022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bousquet J, Schunemann HJ, Togias A,
Bachert C, Erhola M, Hellings PW, Klimek L, Pfaar O, Wallace D,
Ansotegui I, et al: Next-generation allergic rhinitis and its
impact on asthma (ARIA) guidelines for allergic rhinitis based on
grading of recommendations assessment, development and evaluation
(GRADE) and real-world evidence. J Allergy Clin Immunol.
145:70–80.e3. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shamji MH, Sharif H, Layhadi JA, Zhu R,
Kishore U and Renz H: Diverse immune mechanisms of allergen
immunotherapy for allergic rhinitis with and without asthma. J
Allergy Clin Immunol. 149:791–801. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaczynska A, Klosinska M, Chmiel P,
Janeczek K and Emeryk A: The crosstalk between the gut microbiota
composition and the clinical course of allergic rhinitis: The Use
of probiotics, prebiotics and bacterial lysates in the treatment of
allergic rhinitis. Nutrients. 14:43282022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu Y, Zhang R, Li J, Wang H, Wang M, Ren
Q, Fang Y and Tian L: Association between gut and nasal microbiota
and allergic rhinitis: A systematic review. J Asthma Allergy.
17:633–651. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng HY, Chan J, Yap GC, Huang CH, Kioh
DYQ, Tham EH, Loo EXL, Shek LPC, Karnani N, Goh A, et al:
Evaluation of stool short chain fatty acids profiles in the first
year of life with childhood atopy-related outcomes. Front Allergy.
3:8731682022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Konopelski P and Mogilnicka I: Biological
effects of indole-3-propionic acid, a gut microbiota-derived
metabolite, and its precursor tryptophan in mammals' health and
disease. Int J Mol Sci. 23:12222022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao Y, Liu KY, Xiao W, Xie X, Liang Q, Tu
Z, Yang L, Yu H, Guo H, Huang S, et al: Aryl hydrocarbon receptor
confers protection against macrophage pyroptosis and intestinal
inflammation through regulating polyamine biosynthesis.
Theranostics. 14:4218–4239. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhuang H, Ren X, Jiang F and Zhou P:
Indole-3-propionic acid alleviates chondrocytes inflammation and
osteoarthritis via the AhR/NF-ĸB axis. Mol Med. 29:172023.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Owumi SE, Najophe ES and Otunla MT:
3-Indolepropionic acid prevented chlorpyrifos-induced hepatorenal
toxicities in rats by improving anti-inflammatory, antioxidant, and
pro-apoptotic responses and abating DNA damage. Environ Sci Pollut
Res Int. 29:74377–74393. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou CJ, Xie BL, Han HY, Wang Y, Wang YH,
Hong JY, Wei YX, Liu ZG, Feng Y, Yang G and Yang PC: Short-Chain
fatty acids promote immunotherapy by modulating immune regulatory
property in B cells. J Immunol Res. 2021:26843612021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Manning BD and Toker A: AKT/PKB signaling:
Navigating the network. Cell. 169:381–405. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martin LJ and Nguyen HT: Basic leucine
zipper transcription factors as important regulators of leydig
cells' functions. Int J Mol Sci. 23:128872022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ramji DP and Foka P:
CCAAT/enhancer-binding proteins: Structure, function and
regulation. Biochem J. 365:561–575. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hernandez-Encinas E, Aguilar-Morante D,
Cortes-Canteli M, Morales-Garcia JA, Gine E, Santos A and
Perez-Castillo A: CCAAT/enhancer binding protein β directly
regulates the expression of the complement component 3 gene in
neural cells: Implications for the pro-inflammatory effects of this
transcription factor. J Neuroinflammation. 12:142015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cao Y, Fan M, Pei Y, Su L, Xiao W, Chen F,
Huang J, Liu X, Gu Z, Zhang Z, et al: CCAAT/enhancer-binding
protein homologous protein (CHOP) deficiency attenuates
heatstroke-induced intestinal injury. Inflammation. 45:695–711.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Niehrs C and Calkhoven CF: Emerging Role
of C/EBPβ and epigenetic DNA methylation in ageing. Trends Genet.
36:71–80. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Greene LA, Zhou Q, Siegelin MD and
Angelastro JM: Targeting transcription factors ATF5, CEBPB and
CEBPD with cell-penetrating peptides to treat brain and other
cancers. Cells. 12:5812023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Larabee JL, Hauck G and Ballard JD:
Unique, intersecting, and overlapping roles of C/EBP β and CREB in
cells of the innate immune system. Sci Rep. 8:169312018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu YW, Tseng HP, Chen LC, Chen BK and
Chang WC: Functional cooperation of simian virus 40 promoter factor
1 and CCAAT/enhancer-binding protein beta and delta in
lipopolysaccharide-induced gene activation of IL-10 in mouse
macrophages. J Immunol. 171:821–828. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Selter F, Hetzel T, Kahrass H and Mertz M:
Animal research ethics as interaction of research ethics, animal
ethics, and (animal protection) law. ALTEX. 40:541–544.
2023.PubMed/NCBI
|
|
25
|
Shi Z, Jiang W, Chen X, Xu M, Wang J, Lai
Y and Zha D: Chlorogenic acid ameliorated allergic rhinitis-related
symptoms in mice by regulating Th17 cells. Biosci Rep.
40:BSR202016432020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bui TT, Kwon DA, Choi DW, Jung SY, Lee SY,
Piao CH, Hyeon E, Fan Y, Yeon SH, Son RH, et al: Rosae multiflorae
fructus extract and its four active components alleviate
ovalbumin-induced allergic inflammatory responses via regulation of
Th1/Th2 imbalance in BALB/c rhinitis mice. Phytomedicine.
55:238–248. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang J, Gao L, Yu D, Song Y, Zhao Y and
Feng Y: Three Artemisia pollens trigger the onset of allergic
rhinitis via TLR4/MyD88 signaling pathway. Mol Biol Rep.
51:3192024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wise SK, Damask C, Roland LT, Ebert C,
Levy JM, Lin S, Luong A, Rodriguez K, Sedaghat AR, Toskala E, et
al: International consensus statement on allergy and rhinology:
Allergic rhinitis- 2023. Int Forum Allergy Rhinol. 13:293–859.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goetzl EJ: Th2 cells in rapid immune
responses and protective avoidance reactions. FASEB J.
38:e234852024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Casaro M, Souza VR, Oliveira FA and
Ferreira CM: OVA-induced allergic airway inflammation mouse model.
Methods Mol Biol. 1916:297–301. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xiao H, Tang AZ, Xu ML, Chen HL, Wang F
and Li CQ: Mycobacterium vaccae attenuates airway inflammation by
inhibiting autophagy and activating PI3K/Akt signaling pathway in
OVA-induced allergic airway inflammation mouse model. Mol Immunol.
173:30–39. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tan JK, Macia L and Mackay CR: Dietary
fiber and SCFAs in the regulation of mucosal immunity. J Allergy
Clin Immunol. 151:361–370. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li L, Yang C, Jia M, Wang Y, Zhao Y, Li Q,
Gong J, He Y, Xu K, Liu X, et al: Synbiotic therapy with
Clostridium sporogenes and xylan promotes gut-derived
indole-3-propionic acid and improves cognitive impairments in an
Alzheimer's disease mouse model. Food Funct. 15:7865–7882. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu H, Luo Y, An Y and Wu X: The mechanism
of action of indole-3-propionic acid on bone metabolism. Food
Funct. 16:406–421. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang C, Du Y, Li Q, Liu L, Zhao L, Gao C,
Tang Z, Zhang X, Zhao Y and Yang X: Fructo-oligosaccharides
alleviated ulcerative colitis via gut microbiota-dependent
tryptophan metabolism in association with aromatic hydrocarbon
receptor activation in mice. J Agric Food Chem. 72:27912–27922.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang A, Guan C, Wang T, Mu G and Tuo Y:
Lactobacillus-derived indole derivatives ameliorate intestinal
barrier damage in rat pups with complementary food administration.
Food Funct. 15:8775–8787. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen Y, Li Y, Li X, Fang Q, Li F, Chen S
and Chen W: Indole-3-propionic acid alleviates intestinal
epithelial cell injury via regulation of the TLR4/NF-ĸB pathway to
improve intestinal barrier function. Mol Med Rep. 30:1892024.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li G, Lin J, Zhang C, Gao H, Lu H, Gao X,
Zhu R, Li Z, Li M and Liu Z: Microbiota metabolite butyrate
constrains neutrophil functions and ameliorates mucosal
inflammation in inflammatory bowel disease. Gut Microbes.
13:19682572021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hezaveh K, Shinde RS, Klotgen A, Halaby
MJ, Lamorte S, Ciudad MT, Quevedo R, Neufeld L, Liu ZQ, Jin R, et
al: Tryptophan-derived microbial metabolites activate the aryl
hydrocarbon receptor in tumor-associated macrophages to suppress
anti-tumor immunity. Immunity. 55:324–340. e82022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
York AG, Skadow MH, Oh J, Qu R, Zhou QD,
Hsieh WY, Mowel WK, Brewer JR, Kaffe E, Williams KJ, et al: IL-10
constrains sphingolipid metabolism to limit inflammation. Nature.
627:628–635. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fan K, Jin L and Yu S: Roles of regulatory
B cells in the pathogenesis of allergic rhinitis. Allergol
Immunopathol (Madr). 50:7–15. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fang D and Zhu J: Molecular switches for
regulating the differentiation of inflammatory and IL-10-producing
anti-inflammatory T-helper cells. Cell Mol Life Sci. 77:289–303.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Coomes SM, Kannan Y, Pelly VS, Entwistle
LJ, Guidi R, Perez-Lloret J, Nikolov N, Müller W and Wilson MS:
CD4(+) Th2 cells are directly regulated by IL-10 during allergic
airway inflammation. Mucosal Immunol. 10:150–161. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mitchell RE, Hassan M, Burton BR, Britton
G, Hill EV, Verhagen J and Wraith DC: IL-4 enhances IL-10
production in Th1 cells: Implications for Th1 and Th2 regulation.
Sci Rep. 7:113152017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Caucheteux SM, Hu-Li J, Guo L,
Bhattacharyya N, Crank M, Collins MT and Paul WE: IL-1β enhances
inflammatory TH2 differentiation. J Allergy Clin Immunol.
138:898–901.e4. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
van der Krieken SE, Popeijus HE, Mensink
RP and Plat J: CCAAT/enhancer binding protein β in relation to ER
stress, inflammation, and metabolic disturbances. Biomed Res Int.
2015:3248152015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhou J, Li H, Xia X, Herrera A, Pollock N,
Reebye V, Sodergren MH, Dorman S, Littman BH, Doogan D, et al:
Anti-inflammatory activity of MTL-CEBPA, a small activating RNA
drug, in LPS-stimulated monocytes and humanized mice. Mol Ther.
27:999–1016. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Collins CB, Puthoor PR, Nguyen TT,
Strassheim D, Jedlicka P, Friedman JE and de Zoeten EF: C/EBPβ
deletion promotes expansion of poorly functional intestinal
regulatory T cells. J Crohns Colitis. 12:1475–1485. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu Q, Peng Z, Zhou L, Peng R, Li X, Zuo
W, Gou J, Zhou F, Yu S, Huang M and Liu H: Short-chain fatty acid
decreases the expression of CEBPB to inhibit mir-145-mediated DUSP6
and thus further suppresses intestinal inflammation. Inflammation.
45:372–386. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Weichhart T, Hengstschlager M and Linke M:
Regulation of innate immune cell function by mTOR. Nat Rev Immunol.
15:599–614. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xu J, Ye Y, Ji J, Sun J, Wang JS and Sun
X: Untargeted metabolomic profiling reveals changes in gut
microbiota and mechanisms of its regulation of allergy in
OVA-Sensitive BALB/c mice. J Agric Food Chem. 70:3344–3356. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tang Y, She Y, Chen D, Zhou Y, Xie D and
Liu Z: 16S rRNA sequencing-based evaluation of the protective
effects of key gut microbiota on inhaled allergen-induced allergic
rhinitis. Front Microbiol. 15:14972622024. View Article : Google Scholar : PubMed/NCBI
|