Introduction
Endometriosis is a prevalent gynecological disorder
associated with female infertility. It affects approximately 10% of
women of reproductive age and 25–50% of women experiencing
infertility. This disorder is characterized by the growth of
endometrial tissue outside the uterine cavity, leading to symptoms
such as inflammation, dysmenorrhea, infertility, and dyspareunia.
These symptoms significantly impair the quality of life for women
and are often accompanied by psychological burdens (1,2).
However, effective long-term management of
endometriosis remains challenging despite the availability of
treatment options that include both surgical and non-surgical
approaches. Surgical approaches for endometriosis include minimally
invasive techniques such as laparoscopy, laser ablation, and
electrocoagulation for early-stage cases, whereas advanced surgical
procedures such as laparotomy, oophorectomy, and colorectal surgery
are required for deep infiltrating endometriosis. However, these
interventions carry risks, including recurrence due to incomplete
excision, ovarian dysfunction, voiding impairment, and tissue
damage (3–5). Several non-surgical treatment options
are currently available for managing endometriosis, with the most
common approaches being hormone therapy and pain management.
Low-dose oral contraceptives, which contain estrogen and
progesterone, are widely used and are shown to alleviate menstrual
pain, ameliorate infertility, reduce dyspareunia, and shrink
endometriotic lesions. However, these treatments are associated
with adverse effects, such as nausea, headache, and breakthrough
bleeding, and an increased risk of thrombosis with long-term use.
Additionally, they are contraindicated in women who wish to
conceive (3–6). Other treatment options include
aromatase inhibitors and progestin-based medications. Aromatase
inhibitors are drugs that inhibit the enzyme aromatase, which
converts androgens, the precursors of estrogen, into estrogen,
thereby suppressing estrogen production. Although aromatase
inhibitors reduce dysmenorrhea and shrink lesions, they are
expensive and decrease bone mineral density (7,8).
Progestin-based medications, which suppress estrogen activity and
inhibit endometrial proliferation, also cause adverse effects such
as breakthrough bleeding, and their efficacy is typically lower
than that of low-dose oral contraceptives (9). Other treatment options, such as
nonsteroidal anti-inflammatory drugs (such as ibuprofen), carry
cardiovascular risks with long-term use, whereas neuromodulators
and antidepressants (such as gabapentinoids) used for chronic pain
management may lead to dependency (10,11).
Hence, effective and safe treatment options must be explored for
endometriosis.
Recently, considerable progress has been made in
understanding the pathophysiology of endometriosis; genetic and
immunological factors involved in the ectopic occurrence,
proliferation, and invasion of endometrial tissue are gradually
being elucidated. For instance, interleukin (IL)-8 and endothelin,
secreted by ectopic endometrial tissue, promote inflammatory
responses mediated by immune cells and contribute to symptoms such
as pain and complications such as adhesions (12–14).
These advances in understanding have spurred efforts to develop
alternative treatment methods. Subsequently, natural compounds are
being researched as effective therapeutic options with minimal side
effects for endometriosis, and the potential of resveratrol, an
anti-inflammatory and antioxidant compound, for the treatment of
endometriosis has been demonstrated (15–17).
However, the therapeutic utility of resveratrol is limited by its
low absorption rates. In contrast, ε-viniferin, a dimer of
resveratrol, exhibits increased lipophilicity, which enhances
membrane permeability and absorption in the gastrointestinal tract
and liver (Fig. 1).
Pharmacokinetic studies have also demonstrated high bioaccumulation
of ε-viniferin in white adipose tissue, suggesting that these
tissues may serve as a reservoir for its native form, enabling slow
release and prolonged systemic presence (18–20).
ε-viniferin is the main functional compound in wild
grapes (Ampelopsis brevipedunculata), with antioxidant,
anti-inflammatory, and anti-tumor effects (21–24).
It also has neuroprotective effects, which may be beneficial in the
treatment of neurodegenerative diseases such as Alzheimer's and
Parkinson's diseases (25), and
preventive effects against obesity-related morbidities, such as
type 2 diabetes, dyslipidemia, hypertension, and fatty liver
(26). ε-viniferin inhibits
TGF-β1-induced epithelial-mesenchymal transition, migration, and
invasion in lung cancer cells by downregulating vimentin (27). Elevated levels of TGF-β1 have also
been implicated in the development of endometriosis by promoting
cell migration and invasiveness (28). Given these shared mechanistic
pathways, we hypothesized that ε-viniferin exerts beneficial
effects in endometriosis by inhibiting key processes involved in
the disease. However, its potential effects on endometrial cells
remain unclear.
In this study, we aimed to investigate the
anti-inflammatory effects of ε-viniferin and wild-grape extract,
along with their inhibitory effects on the migration and invasion
of human endometrial stromal cells (HESCs). We believe that our
findings would contribute to the development of ε-viniferin as a
potential therapeutic option for the effective management of
endometriosis.
Materials and methods
Materials
Wild-grape extract (cat. no. 30811033) and
ε-viniferin (cat. no. NS3013) were purchased from Maruzen
Pharmaceuticals Co., Ltd. and Nagara Science Co., Ltd.,
respectively.
Cell culture
Mouse macrophage-like cell line RAW264.7
(RRID:CVCL_0493, cat. no. TIB-71; American Type Culture Collection)
and human monocytic leukemia cell line THP-1 (RRID:CVCL_0006, cat.
no. RCB3686; Riken Bioresource Center) were cultured in RPMI1640
medium (cat. no. 189-02025; FUJIFILM Wako Pure Chemical
Corporation) supplemented with 10% inactivated fetal bovine serum
(cat. no. F7524, non-USA origin; Sigma-Aldrich; Merck KGaA) and 1%
(v/v) penicillin-streptomycin (cat. no. 15140122; Gibco; Thermo
Fisher Scientific, Inc.) at 37°C in a humidified incubator with 5%
CO2. THP-1 cells (1.5×104 cells/well) were
seeded into 96-well plates and differentiated into macrophage-like
cells using 0.1 µg/ml phorbol 12-myristate 13-acetate (PMA; cat.
no. P1585; Sigma-Aldrich; Merck KGaA) for 72 h.
To investigate the potential application of
ε-viniferin for its anti-endometriotic effects, we used HESCs,
which are derived from ectopic endometrial tissues associated with
endometriosis and drive the abnormal migration and invasion of
endometrial tissue.
Immortalized HESCs (cat. no. T0533; Applied
Biological Materials Inc., Richmond, BC, Canada, http://www.abmgood.com/immortalized-human-endometrial-stromal-cells-hesc.html)
were cultured in Prigrow IV medium (cat. no. TM004; Applied
Biological Materials, Inc.) supplemented with 2 mM L-Glutamine
(cat. no. G275; Applied Biological Materials, Inc.), 10%
charcoal-stripped fetal bovine serum (cat. no. 12676-029; Gibco),
and 1% (v/v) penicillin-streptomycin at 37°C in a humidified
incubator containing 5% CO2.
Cell viability
RAW264.7 cells (3×104 cells/well) were
seeded into 96-well plates and incubated for 1 h. Differentiated
THP-1 cells were rinsed with phosphate-buffered saline (PBS) and
fresh medium before overnight incubation. HESCs (1.5×104
cells/well) were seeded into 96-well plates and incubated
overnight. The test chemicals, namely, wild-grape extract at 0.03,
0.1, and 0.3 mg/ml and ε-viniferin at 3, 10, and 30 µg/ml, were
added, and the cells were incubated for an additional 24 h at 37°C.
Dimethyl sulfoxide (DMSO) was used as the control. MTT reagent
(working concentration: 0.5 mg/ml; cat. no. 10009591; Cayman
Chemical) was added to each well and incubated for 2 h at 37°C. The
supernatant was replaced with 100 µl of DMSO to dissolve the
formazan crystals. Absorbance was measured at 570 nm using a
microplate reader (Bio-Rad Laboratories).
Nitric oxide (NO) production
RAW264.7 cells (3×104 cells/well) were
seeded into 96-well plates and incubated for 1 h. Test chemicals
were added to each well and incubated for an additional hour,
followed by exposure to lipopolysaccharide (LPS, 100 ng/ml; cat.
no. L5293; Sigma-Aldrich; Merck KGaA) for 24 h. The supernatant was
collected, and 50 µl aliquots were mixed with an equal volume of
Griess reagent in a 96-well plate. Absorbance was measured at 570
nm using a microplate reader (Bio-Rad Laboratories).
Measurement of IL-6 production
RAW264.7 cells (3×104 cells/well) were seeded into
96-well plates and incubated for 1 h
Differentiated THP-1 cells were washed with PBS and
fresh medium before overnight incubation. The test chemicals,
namely, wild-grape extract at 0.1, 0.2, and 0.3 mg/ml and
ε-viniferin at 10, 20, and 30 µg/ml, were added to each well and
incubated for 1 h, followed by exposure to LPS (100 ng/ml) for 24
h. IL-6 concentration in the supernatants were measured using Mouse
IL-6 ELISA Kit (cat. no. M6000B; R&D Systems) for RAW264.7
cells and Human IL-6 ELISA Kit (cat. no. D6050; R&D Systems)
for THP-1 cells, following the manufacturer's protocol. Plates were
washed four times, and horseradish peroxidase-conjugated IL-6 was
added, followed by a 2 h incubation at 23–25°C. Substrate solutions
of tetramethylbenzidine (TMB) and hydrogen peroxide were added to
each well after washing four times, and plates were incubated for
20 min at room temperature in the dark. The reaction was quenched
using diluted hydrochloric acid. Absorbance was measured at 450 nm
using a microplate reader (Bio-Rad Laboratories).
Measurement of reactive oxygen species
(ROS) production
RAW264.7 cells (3×104 cells/well) were
seeded into 96-well plates and incubated overnight. Test chemicals
at various concentrations were added, and the cells were incubated
for 1 h, followed by exposure to LPS (100 ng/ml) for 24 h at 37°C.
ROS production was determined using DCFH-DA (20 µM; cat. no. 35845;
Sigma-Aldrich; Merck KGaA), an oxidant-sensitive fluorescent probe.
The medium was removed from each well, and the cells were washed
twice with Ca2+, Mg2+-free PBS (PBS-) and
incubated with DCFH-DA for 30 min. After removing the supernatant
and washing twice with PBS-, 200 µl PBS- was added to each well.
Fluorescence was measured with excitation and emission wavelengths
of 485 nm and 535 nm, respectively, using a fluorescence plate
reader (SpectraMax M5; Molecular Devices).
Measurement of DPPH radical scavenging
ratio
The scavenging effect was assessed using the DPPH
Antioxidant Assay Kit (cat. no. D678; Dojindo). Briefly, 20 µl of
the sample solution was added to each well of a 96-well microplate,
followed by 80 µl of assay buffer and 100 µl of
2,2-diphenyl-1-picrylhydrazyl (DPPH) working solution. Wild-grape
extract solutions (at 0.1, 0.3, and 1 mg/ml) and ε-viniferin (at 3,
10, and 30 µg/ml) were used as samples. Trolox at 80 µg/ml was used
as a positive control. The plate was incubated for 30 min at room
temperature in the dark. Absorbance was measured at 517 nm using a
microplate reader (SpectraMax M5).
RNA isolation, reverse transcription-quantitative
polymerase chain reaction (RT-qPCR), and reverse transcription-PCR
(RT-PCR)
RAW264.7 cells (1×106 cells/well) were
seeded into 60 mm dishes and incubated for 24 h. Test chemicals
were added to each dish and incubated for 1 h, followed by LPS
treatment (100 ng/ml) for 4 h at 37°C. RNA was extracted using
TRIzol reagent (1 ml; cat. no. 15596018; Invitrogen; Thermo Fisher
Scientific, Inc.) and reverse transcribed using the High-Capacity
cDNA Reverse Transcription Kit (cat. no. 4368814; Applied
Biosystems; Thermo Fisher Scientific, Inc., Vilnius, Lithuania) at
25°C for 10 min, 37°C for 120 min, and 85°C for 5 min. For RT-qPCR,
cDNA was amplified in triplicate using KOD FX Neo PCR Buffer (14
µl) and dNTPs (cat. no. KFX-201; Toyobo Co., Ltd.). Target DNA
sequences for each primer pair were amplified in triplicate under
the following conditions: initial denaturation at 94°C for 10 sec,
followed by 40 cycles of 94°C for 10 sec, 60°C for 10 sec, and 70°C
for 20 sec, using the QuantStudio 3 system (Applied Biosystems,
Singapore). mGapdh and ACTB were used as internal
controls. Relative mRNA expression levels were calculated using the
2−ΔΔCq method (29).
For RT-PCR, PCR products were separated on a 2%
agarose gel, and the band intensities were analyzed using ImageJ
software (ImageJ 1.53e with Java 1.8.0_172; National Institutes of
Health). The following primer pairs were used: mouse iNOS,
5′- GTCTTGCAAGCTGATGGTCA-3′ (forward) and
5′-ACCACTCGTACTTGGGATGC-3′ (reverse); mouse Il1β,
5′-CGTGGACCTTCCAGGATGAG-3′ (forward) and
5′-GGAGCCTGTAGTGCAGTTGTC-3′ (reverse); mouse Il6,
5′-ACCACGGCCTTCCCTACTTC-3′ (forward) and
5′-CACAACTCTTTTCTCATTTCCACG-3′ (reverse); human IL6,
5′-AGACAGCCACTCACCTCTTCAG-3′ (forward) and 5′-
TTCTGCCAGTGCCTCTTTGCTG-3′ (reverse); mouse Gapdh,
5′-TGCACCACCAACTGCTTAG-3′ (forward) and 5′-GATGCAGGGATGATGTTC-3′
(reverse); and human ACTB, 5′-CTTCTACAATGAGCTGCGTG-3′
(forward) and 5′-TCATGAGGTAGTCAGTCAGG-3′ (reverse).
Wound healing assay
HESCs (1×105 cells/well) were seeded into
24-well plates and cultured overnight until confluence. A uniform
scratch was created across the center of the well using a 200 µl
pipette tip. Floating cells and the growth medium were removed, and
serum-free medium containing the test chemicals was added to each
well. Cells were incubated for an additional 8 h, and their
movement into the scratched area was recorded every 2 h using a
phase-contrast microscope (Nikon Eclipse TS100).
Matrigel invasion chamber assay
HESCs (5×104 cells/well) suspended in 500
µl serum-free Prigrow IV medium containing test chemicals were
seeded into commercial Matrigel-coated chambers (BD Matrigel
Basement Membrane Matrix; cat. no. 354480; Corning). The lower
chambers were filled with 750 µl of Prigrow IV medium with 10% FBS
and were incubated for 16 h at 37°C. Thereafter, non-invading cells
were removed by wiping the upper surface of the membrane with a
cotton swab, and invading cells on the lower surface of the
membrane were stained with Diff-Quick (cat. no. 16920; Sysmex)
according to the manufacturer's instruction and counted under a
phase-contrast microscope (Nikon Eclipse TS100).
PCR array
Total RNA was extracted from HESCs using the RNeasy
Mini Kit (cat. no. 74106; Qiagen, Hilden, North Rhine-Westphalia,
Germany), treated with ε-viniferin for 8 h, and reverse transcribed
using the RT2 First Strand Kit (cat. no. 330401; Qiagen,
Germantown, MD, USA). The cDNA was applied to the Human Tumor
Metastasis PCR Array (cat. no. 330231; Qiagen, Germantown, MD, USA)
using RT2 SYBR-Green ROX qPCR Mastermix (cat. no.
330520; Qiagen, Germantown, MD, USA). Data were analyzed using the
2−ΔΔCq method (29).
Measurement of NF-κB activity
RAW264.7 cells (3×106 cells/well) were
seeded into 6-well plates and incubated for 1 h. Test chemicals at
various concentrations were added to each well and incubated for 1
h, followed by LPS treatment (100 ng/ml) for 2 h at 37°C. Nuclear
extracts were prepared using the Nuclear Extract Kit (cat. no.
40010; Active Motif) according to the manufacturer's instructions.
NF-κB binding activity was measured using nuclear extract (5 µg)
with the TransAM NF-κB p65 Transcription Factor Assay Kit (cat. no.
40096; Active Motif).
Statistical analysis
Results are presented as mean±standard deviation
(SD). Statistical analysis was performed using GraphPad Prism
version 10.0 (Dotmatics). Differences between the two groups were
analyzed using the Student's t-test. One-way analysis of variance
(ANOVA) followed by Dunnett's post-hoc test was used for
comparisons among more than two groups. Statistical significance
was set at P<0.05.
Results
Wild-grape extract suppresses
inflammatory regulators and ROS in RAW264.7 cells
The anti-inflammatory effects of wild-grape extract
were evaluated in RAW264.7 cells. Wild-grape extract was not
cytotoxic to RAW264.7 cells after 24 h of treatment at
concentrations below 0.3 mg/ml (Fig.
2A). The extract suppressed LPS-induced NO and ROS production
in RAW264.7 cells (Fig. 2B and C)
and exhibited significant dose-dependent antioxidant activity in
the DPPH assay (Fig. 2D). As
inducible nitric oxide synthase (iNOS) is responsible for NO
production, we explored the effect of wild-grape extract on
LPS-induced iNOS and the expression of downstream inflammatory
factors, IL-6 and IL-1β. Although wild-grape extract did not
inhibit LPS-induced iNOS or IL-1β expression, it significantly
inhibited LPS-induced IL-6 expression (Fig. 2E). We further validated this
inhibition using RT-qPCR and ELISA in RAW264.7 cells (Fig. 2F and G). These findings indicate
that wild-grape extract suppresses ROS-activated IL-6 inflammatory
reactions in RAW264.7 cells.
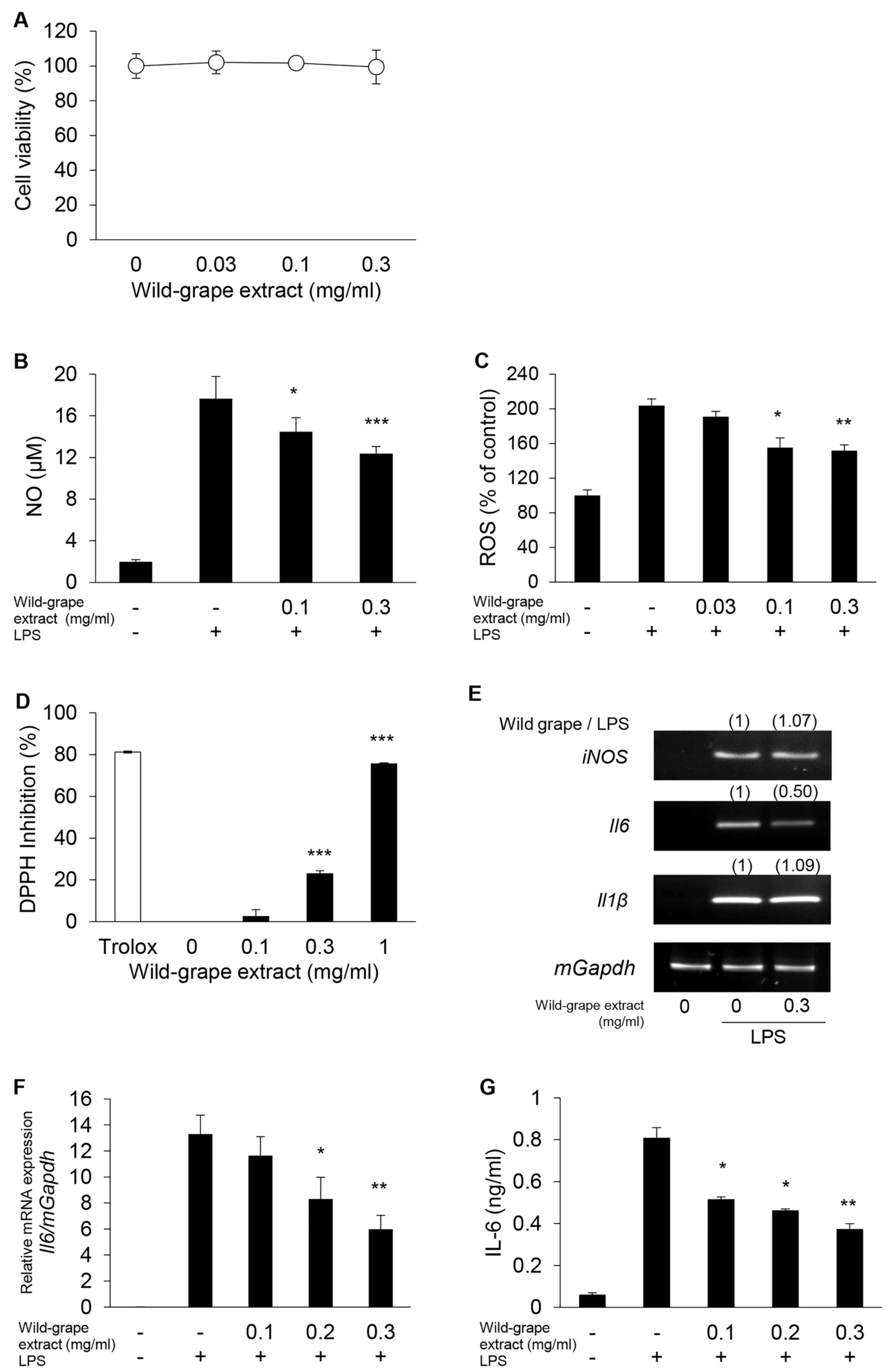 | Figure 2.Inhibition of inflammatory regulators
and ROS production by wild-grape extract in RAW264.7 cells.
(A) Effect of wild-grape extract on cell viability after 24 h of
exposure, assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5
diphenyl tetrazolium bromide (MTT) assay. (B) Inhibition of
LPS-induced NO production. (C) Reduction of LPS-induced ROS
production, measured based on fluorescent intensity. (D) In
vitro antioxidant activity determined using the DPPH
radical-scavenging assay. (E) Effect on LPS-induced expression of
iNOS, IL-6 and IL-1β. (F) Inhibition of LPS-induced IL-6
expression. (G) Inhibition of LPS-induced IL-6 secretion. RAW264.7
cells were stimulated with 100 ng/ml LPS in the presence or absence
of wild-grape extract. n=3. *P<0.05, **P<0.01, ***P<0.001
vs. control without the extract. LPS, lipopolysaccharide; NO,
nitric oxide; ROS, reactive oxygen species; DPPH,
2,2-diphenyl-1-picrylhydrazyl; iNOS, inducible NOS; IL,
interleukin; mGapdh, mouse glyceraldehyde-3-phosphate
dehydrogenase. |
ε-viniferin reduces inflammation
through ROS suppression and NF-κB inhibition in RAW264.7 cells
We investigated the anti-inflammatory action of
ε-viniferin in RAW264.7 cells. ε-viniferin was not cytotoxic to
RAW264.7 cells after 24 h of treatment at concentrations below 10
µg/ml (Fig. 3A). ε-viniferin,
similar to wild-grape extracts, showed significant dose-dependent
antioxidant activity in the DPPH assay (Fig. 3B). Although ε-viniferin did not
suppress LPS-induced NO production (Fig. 3C), it suppressed LPS-induced ROS
production at non-toxic concentrations (Fig. 3D). These results suggest that the
anti-inflammatory effects of ε-viniferin partially stem from the
suppression of ROS production.
 | Figure 3.Inhibition of inflammatory regulator
production and NF-κB activity by ε-viniferin in RAW264.7 cells. (A)
Effect of ε-viniferin on cell viability after 24 h of exposure. (B)
In vitro antioxidant activity determined using the DPPH
radical-scavenging assay. (C) Inhibition of LPS-induced NO
production. (D) Inhibition of LPS-induced ROS production, measured
based on fluorescent intensity. (E) Effect of ε-viniferin on cell
viability after 4 h of exposure. (F) Inhibition of LPS-induced IL-6
expression. (G) Inhibition of LPS-induced IL-6 secretion. (H)
Inhibition of LPS-induced NF-κB activity. RAW264.7 cells were
stimulated with 100 ng/ml LPS in the presence or absence of
ε-viniferin. N=3. *P<0.05, **P<0.01, ***P<0.001 vs.
control without ε-viniferin and LPS. LPS, lipopolysaccharide; NO,
nitric oxide; ROS, reactive oxygen species; IL, interleukin; DPPH,
2,2-diphenyl-1-picrylhydrazyl; mGapdh, mouse
glyceraldehyde-3-phosphate dehydrogenase. |
We then explored the effects of ε-viniferin on IL-6
production in RAW264.7 cells because wild-grape extract inhibited
LPS-induced IL-6 production and IL-6 production is an early
response in LPS-induced inflammatory reaction. Within 4 h of
exposure, ε-viniferin was not cytotoxic to RAW264.7 cells at a
concentration of 30 µg/ml (Fig.
3E). However, it significantly inhibited LPS-induced IL-6
production both at gene and protein levels (Fig 3F and G).
We also tested the effect of ε-viniferin on NF-κB
activity, as this transcription factor is present upstream of IL-6,
and NF-κB activation was significantly suppressed by ε-viniferin in
a concentration-dependent manner (Fig.
3H). These results suggest that the NF-κB-mediated IL-6
signaling pathway is central to the anti-inflammatory effects of
ε-viniferin in RAW264.7 cells.
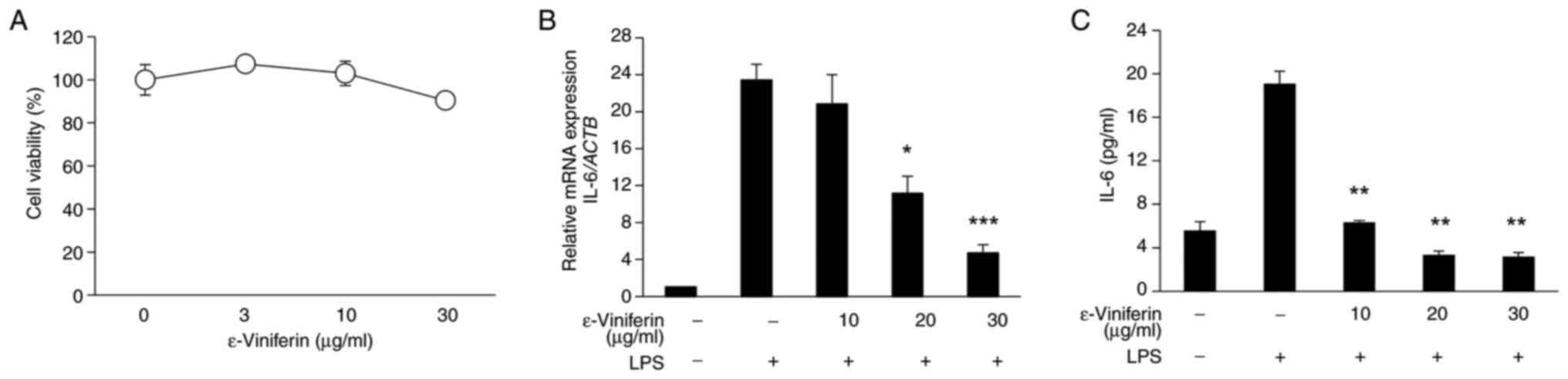
ε-viniferin exhibits anti-inflammatory
effects in human macrophage-like THP-1 cells
To further validate the anti-inflammatory effects of
ε-viniferin, we used THP-1 cells, which differentiate into
macrophage-like cells upon stimulation with phorbol esters such as
phorbol-12-myristate-13-acetate (PMA). ε-viniferin (30 µg/ml) was
not cytotoxic to THP-1 cells after 4 h of treatment (Fig. 4A). ε-viniferin significantly
suppressed LPS-induced IL6 mRNA expression at 20 µg/ml and
its protein production at 10 µg/ml (Fig. 4B and C). These results confirm that
ε-viniferin exhibits anti-inflammatory effects in both mouse- and
human-derived macrophage-like cells.
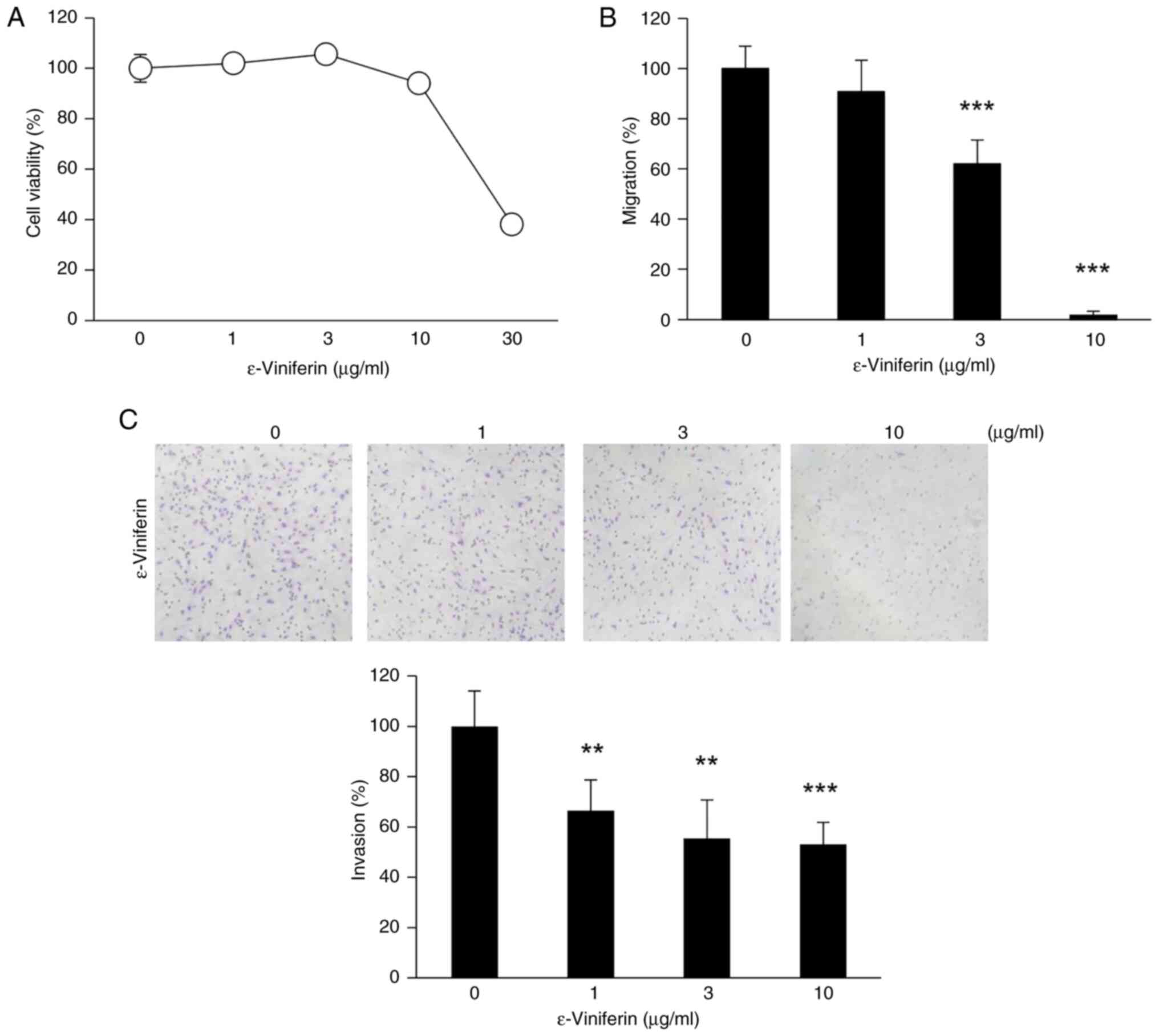
ε-viniferin inhibits migration and
invasion of HESCs and modulates inflammatory signaling
pathways
ε-viniferin was not cytotoxic to HESCs at
concentrations below 10 µg/ml (Fig.
5A). It suppressed HESC migration at a concentration of 1 µg/ml
and invasion at concentrations of 3 µg/ml and above (Fig. 5B and C). Similar inhibitory effects
on migration and invasion were observed with wild-grape extract
(Fig. S1A-C).
To further investigate the inflammatory signaling
pathways potentially involved in the anti-endometriotic effects of
ε-viniferin, a PCR array was employed (Fig. S2). Treatment of HESCs with
ε-viniferin upregulated CDKN2A, a gene that inhibits
abnormal cell growth and proliferation, and downregulated
TNFSF10, a regulator of inflammation. Additionally,
ε-viniferin suppressed the expression of metastasis-promoting genes
such as HPSE and FGFR4.
Discussion
In this study, we investigated the anti-inflammatory
and anti-endometriotic effects of wild-grape extract and purified
ε-viniferin and demonstrated that both compounds effectively
suppressed LPS-induced inflammatory mediators, including NO, ROS,
and IL-6, in mouse and human macrophage cell lines. They also
inhibited the activation of NF-κB, a transcription factor that
regulates the expression of these mediators. These findings suggest
that wild-grape extract and purified ε-viniferin exhibit
anti-inflammatory activity by inhibiting NF-κB mediated
inflammatory pathways. Furthermore, we validated the previously
demonstrated radical scavenging activity of ε-viniferin in
vitro. ε-viniferin consists of two stilbenol units linked
together, and its radical-scavenging activity is attributed to the
functional groups such as phenolic hydroxyl groups and double bonds
within this structure (30,31).
Previously, we reported the potential of NF-κB
inhibitor dehydroxymethylepoxyquinimicin (DHMEQ) in the treatment
of endometriosis (32). Based on
these findings and the observed NF-κB inhibition by ε-viniferin, we
further explored the potential application of ε-viniferin for its
anti-endometriotic effects. We demonstrated that ε-viniferin
effectively inhibited the migration and invasion of HESCs, a key
cell type implicated in endometriosis. Thus, ε-viniferin exhibited
potential for the treatment of endometriosis. Additionally, PCR
array analysis revealed that ε-viniferin suppressed the expression
of mobility-related genes, such as HPSE and FGFR4,
and the inflammation-promoting mediator TNFSF10. The
observed gene expression patterns revealed that ε-viniferin could
inhibit the progression of endometriosis by modulating inflammatory
regulators and metastasis-related pathways. To gain deeper
mechanistic insights into ε-viniferin's molecular targets, future
transcriptomic and/or proteomic analyses of HPSE and FGFR4
functions are warranted. In the present study, we focused on HESCs
to assess the direct anti-migratory and anti-inflammatory effects
of ε-viniferin. To further evaluate its therapeutic relevance in
the endometriotic microenvironment, future studies will examine
ε-viniferin's effects on other cellular components of endometriotic
lesions, including epithelial cells, immune cells, and vascular
endothelial cells.
While our current study focused on the NF-κB
pathway, it is important to explore broader signaling networks to
fully understand the therapeutic effects of ε-viniferin. Previous
studies (22,27) reported that ε-viniferin suppresses
inflammatory cytokines such as TNF-α and TGF-β, which are
associated with the JAK/STAT and TGF-β pathways. These findings
suggest that ε-viniferin may have broader anti-inflammatory effects
beyond NF-κB inhibition. In addition, our PCR array data showed
that ε-viniferin downregulated TNFSF10 (Fig. S2), a gene regulated by TRAIL and
involved in immune and apoptotic pathways. These results support
the potential of wider regulatory effects. Future studies will
investigate the JAK/STAT and TGF-β pathways to further clarify
ε-viniferin's therapeutic mechanisms.
Overall, our results suggest that ε-viniferin is a
promising anti-endometriosis agent with potent anti-inflammatory
effects, which are crucial for managing disease progression.
ε-viniferin-based therapy may be a safer, non-hormonal alternative
to conventional hormonal therapies, which often cause side effects
such as bone loss and menstrual irregularities. While these
findings strongly support the therapeutic potential of ε-viniferin,
further in vivo and clinical studies are necessary to
confirm its efficacy and long-term safety. Moreover, previous
pharmacokinetic studies have shown that ε-viniferin accumulates in
white adipose tissue and remains in the body for extended periods
(18–20). However, critical pharmacokinetic
parameters-such as absorption rate, metabolism, and plasma
half-life-remain uncharacterized in the context of endometriosis.
Pharmacokinetic modeling and in vivo biodistribution studies
are therefore planned to optimize its therapeutic use. As a
non-hormonal agent, ε-viniferin may also be considered for
combination therapy. Although its interaction with standard
hormonal treatments remains unexplored, previous studies have shown
that resveratrol, a structurally related polyphenol, enhances the
efficacy of hormonal therapies and improves the management of
endometriosis-related pain by reducing inflammation (33). These findings raise the possibility
that ε-viniferin may offer similar combinatorial benefits. To
enhance clinical applicability, we also plan to explore combination
therapies using ε-viniferin alongside standard hormonal treatments.
Together, our findings present a novel strategy for developing
anti-endometriosis therapies by targeting both inflammation and
cell migration, paving the way for future translational
research.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Mr. Kazuyuki Ino
(General Affairs Department, Fukuyu Hospital, Fukuyu Medical
Corporation, Fukuyu, Japan) for providing support in writing and
editing the manuscript.
Funding
This work was supported by the Japan Society for the Promotion
of Science Kakenhi (grant no. 22H03062) and the Japan Agency for
Medical Research and Development (grant no. JP18fk0310118JSPS).
Additional financial support was provided by Fukuyu Medical
Corporation.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YL, KU, SK and YN contributed to the experimental
design and manuscript preparation. YL, SK, MH and HF conducted the
experiments. YH, AW and NK contributed to the experimental design.
YL, YN and SK confirmed the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The Department of Molecular Target Medicine, to
which KU belongs, is a funding-supported laboratory financially
supported by Fukuyu Medical Corporation (Nisshin, Japan); Brunaise
Co., Ltd. (Nagoya, Japan); Shenzhen Wanhe Pharmaceutical Company
(Shenzhen, China); and Meiji Seika Pharma (Tokyo, Japan). This
study is partly supported by Fukuyu Medical Corporation.
Glossary
Abbreviations
Abbreviations:
|
THP-1
|
human monocytic leukemia cell line
|
|
HESCs
|
human endometrial stromal cells
|
References
|
1
|
Zondervan KT, Becker CM and Missmer SA:
Endometriosis. N Engl J Med. 382:1244–1256. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pessoa de Farias Rodrigues M, Lima
Vilarino F, de Souza Barbeiro Munhoz A, da Silva Paiva L, de
Alcantara Sousa LV, Zaia V and Parente Barbosa C: Clinical aspects
and the quality of life among women with endometriosis and
infertility: A cross-sectional study. BMC Womens Health.
20:1242020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Johnson NP and Hummelshoj L; World
Endometriosis Society Montpellier Consortium, : Consensus on
current management of endometriosis. Hum Reprod. 28:1552–1568.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Taylor HS, Kotlyar AM and Flores VA:
Endometriosis is a chronic systemic disease: Clinical challenges
and novel innovations. Lancet. 397:839–852. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hee L, Kettner LO and Vejtorp M:
Continuous use of oral contraceptives: An overview of effects and
side-effects. Acta Obstet Gynecol Scand. 92:125–136. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
De Corte P, Milhoranca I, Mechsner S,
Oberg AS, Kurth T and Heinemann K: Unravelling the causal
relationship between endometriosis and the risk for developing
venous thromboembolism: A pooled analysis. Thromb Haemost.
125:385–394. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Polyzos NP, Fatemi HM, Zavos A, Grimbizis
G, Kyrou D, Velasco JG, Devroey P, Tarlatzis B and Papanikolaou EG:
Aromatase inhibitors in post-menopausal endometriosis. Reprod Biol
Endocrinol. 9:902011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pavone ME and Bulun SE: Clinical review:
The use of aromatase inhibitors for ovulation induction and
superovulation. J Clin Endocrinol Metab. 98:1838–1844. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takagi H, Takakura M and Sasagawa T: Risk
factors of heavy uterine bleeding in patients with endometriosis
and adenomyosis treated with dienogest. Taiwan J Obstet Gynecol.
62:852–857. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bally M, Dendukuri N, Rich B, Nadeau L,
Helin-Salmivaara A, Garbe E and Brophy JM: Risk of acute myocardial
infarction with NSAIDs in real world use: Bayesian meta-analysis of
individual patient data. BMJ. 357:j19092017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hägg S, Jönsson AK and Ahlner J: Current
evidence on abuse and misuse of gabapentinoids. Drug Saf.
43:1235–1254. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rahmioglu N, Nyholt DR, Morris AP, Missmer
SA, Montgomery GW and Zondervan KT: Genetic variants underlying
risk of endometriosis: Insights from meta-analysis of eight
genome-wide association and replication datasets. Hum Reprod
Update. 20:702–716. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nishimoto-Kakiuchi A, Sato I, Nakano K,
Ohmori H, Kayukawa Y, Tanimura H, Yamamoto S, Sakamoto Y, Nakamura
G, Maeda A, et al: A long-acting anti-IL-8 antibody improves
inflammation and fibrosis in endometriosis. Sci Transl Med.
15:eabq58582023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoshino O, Yamada-Nomoto K, Kobayashi M,
Andoh T, Hongo M, Ono Y, Hasegawa-Idemitsu A, Sakai A, Osuga Y and
Saito S: Bradykinin system is involved in endometriosis-related
pain through endothelin-1 production. Eur J Pain. 22:501–510. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bruner-Tran KL, Osteen KG, Taylor HS,
Sokalska A, Haines K and Duleba AJ: Resveratrol inhibits
development of experimental endometriosis in vivo and
reduces endometrial stromal cell invasiveness in vitro. Biol
Reprod. 84:106–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ricci AG, Olivares CN, Bilotas MA, Bastón
JI, Singla JJ, Meresman GF and Barañao RI: Natural therapies
assessment for the treatment of endometriosis. Hum Reprod.
28:178–188. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rudzitis-Auth J, Menger MD and Laschke MW:
Resveratrol is a potent inhibitor of vascularization and cell
proliferation in experimental endometriosis. Hum Reprod.
28:1339–1347. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zghonda N, Yoshida S, Araki M, Kusunoki M,
Mliki A, Ghorbel A and Miyazaki H: Greater effectiveness of
ε-viniferin in red wine than its monomer resveratrol for inhibiting
vascular smooth muscle cell proliferation and migration. Biosci
Biotechnol Biochem. 75:1259–1267. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Courtois A, Atgié C, Marchal A,
Hornedo-Ortega R, Lapèze C, Faure C, Richard T and Krisa S:
Tissular distribution and metabolism of trans-ε-viniferin
after intraperitoneal injection in rat. Nutrients. 10:16602018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim J, Min JS, Kim D, Zheng YF, Mailar K,
Choi WJ, Lee C and Bae SK: A simple and sensitive liquid
chromatography-tandem mass spectrometry method for
trans-ε-viniferin quantification in mouse plasma and its
application to a pharmacokinetic study in mice. J Pharm Biomed
Anal. 134:116–121. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiménez-Moreno N, Volpe F, Moler JA,
Esparza I and Ancín-Azpilicueta C: Impact of extraction conditions
on the phenolic composition and antioxidant capacity of grape stem
extracts. Antioxidants (Basel). 8:5972019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sergi D, Gélinas A, Beaulieu J, Renaud J,
Tardif-Pellerin E, Guillard J and Martinoli MG: Anti-apoptotic and
anti-inflammatory role of trans ε-viniferin in a neuron-glia
co-culture cellular model of Parkinson's disease. Foods.
10:5862021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Beaumont P, Amintas S, Krisa S, Courtois
A, Richard T, Eseberri I and Portillo MP: Glucuronide metabolites
of trans-ε-viniferin decrease triglycerides accumulation in an
in vitro model of hepatic steatosis. J Physiol Biochem.
80:685–696. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang C, Lin ZJ, Chen JC, Zheng HJ, Lai YH
and Huang HC: α-viniferin-induced apoptosis through downregulation
of SIRT1 in non-small cell lung cancer cells. Pharmaceuticals
(Basel). 16:7272023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang S, Ma Y and Feng J: Neuroprotective
mechanisms of ε-viniferin in a rotenone-induced cell model of
Parkinson's disease: Significance of SIRT3-mediated FOXO3
deacetylation. Neural Regen Res. 15:2143–2153. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gómez-Zorita S, Milton-Laskibar I,
Eseberri I, Beaumont P, Courtois A, Krisa S and Portillo MP:
Beneficial effects of ε-viniferin on obesity and related health
alterations. Nutrients. 15:9282023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chiou WC, Huang C, Lin ZJ, Hong LS, Lai
YH, Chen JC and Huang HC: α-viniferin and ε-viniferin inhibited
TGF-β1-induced epithelial-mesenchymal transition, migration and
invasion in lung cancer cells through downregulation of vimentin
expression. Nutrients. 14:22942022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Soni UK, Chadchan SB, Kumar V, Ubba V,
Khan MTA, Vinod BSV, Konwar R, Bora HK, Rath SK, Sharma S and Jha
RK: A high level of TGF-B1 promotes endometriosis development via
cell migration, adhesiveness, colonization, and invasiveness†. Biol
Reprod. 100:917–938. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Szewczuk LM, Lee SH, Blair IA and Penning
TM: Viniferin formation by COX-1: Evidence for radical
intermediates during co-oxidation of resveratrol. J Nat Prod.
68:36–42. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Privat C, Telo JP, Bernardes-Genisson V,
Vieira A, Souchard JP and Nepveu F: Antioxidant properties of
trans-ε-viniferin as compared to stilbene derivatives in aqueous
and nonaqueous media. J Agric Food Chem. 50:1213–1217. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin Y, Kojima S, Ishikawa A, Matsushita H,
Takeuchi Y, Mori Y, Ma J, Takeuchi K, Umezawa K and Wakatsuki A:
Inhibition of MLCK-mediated migration and invasion in human
endometriosis stromal cells by NF-κB inhibitor DHMEQ. Mol Med Rep.
28:1412023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Maia H Jr, Haddad C, Pinheiro N and Casoy
J: Advantages of the association of resveratrol with oral
contraceptives for management of endometriosis-related pain. Int J
Womens Health. 4:543–549. 2012. View Article : Google Scholar : PubMed/NCBI
|