|
1
|
Raza S, Rajak S, Upadhyay A, Tewari A and
Anthony Sinha R: Current treatment paradigms and emerging therapies
for NAFLD/NASH. Front Biosci (Landmark Ed). 26:206–237. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eslam M, Newsome PN, Sarin SK, Anstee QM,
Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour
JF, Schattenberg JM, et al: A new definition for metabolic
dysfunction-associated fatty liver disease: An international expert
consensus statement. J Hepatol. 73:202–209. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rinella ME, Lazarus JV, Ratziu V, Francque
SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab
JP, et al: A multisociety Delphi consensus statement on new fatty
liver disease nomenclature. J Hepatol. 79:1542–1556. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Younossi ZM, Golabi P, Paik JM, Henry A,
Van Dongen C and Henry L: The global epidemiology of nonalcoholic
fatty liver disease (NAFLD) and nonalcoholic steatohepatitis
(NASH): A systematic review. Hepatology. 77:1335–1347. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu R, Liu Y and Hong T: Epidemiological
characteristics and management of nonalcoholic fatty liver
disease/nonalcoholic steatohepatitis in China: A narrative review.
Diabetes Obes Metab. 25 (Suppl 1):S13–S26. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wei S, Wang L, Evans PC and Xu S: NAFLD
and NASH: Etiology, targets and emerging therapies. Drug Discov
Today. 29:1039102024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rong L, Zou J, Ran W, Qi X, Chen Y, Cui H
and Guo J: Advancements in the treatment of non-alcoholic fatty
liver disease (NAFLD). Front Endocrinol (Lausanne). 13:10872602023.
View Article : Google Scholar : PubMed/NCBI
|
|
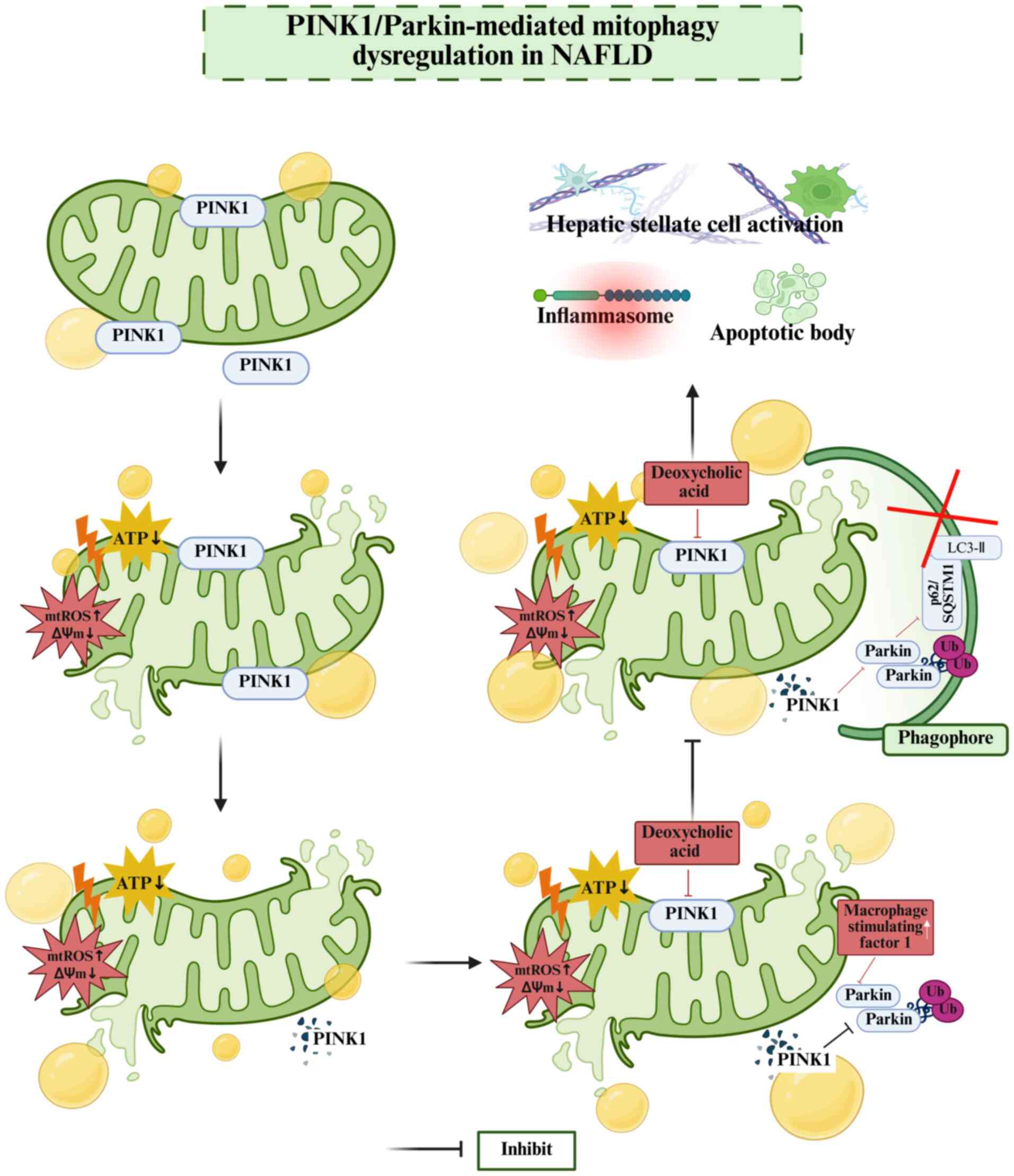
8
|
Qian H, Chao X, Williams J, Fulte S, Li T,
Yang L and Ding WX: Autophagy in liver diseases: A review. Mol
Aspects Med. 82:1009732021. View Article : Google Scholar : PubMed/NCBI
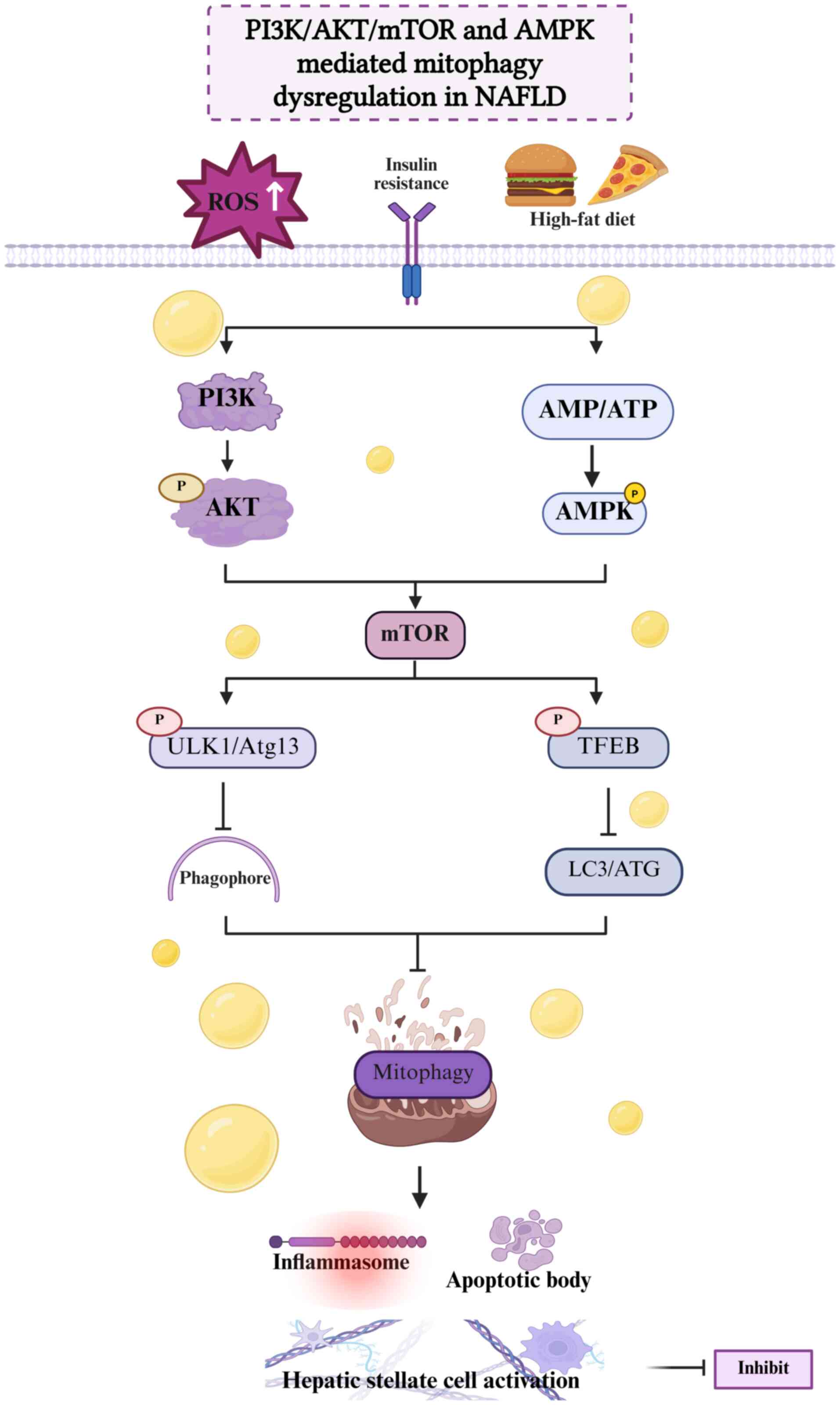
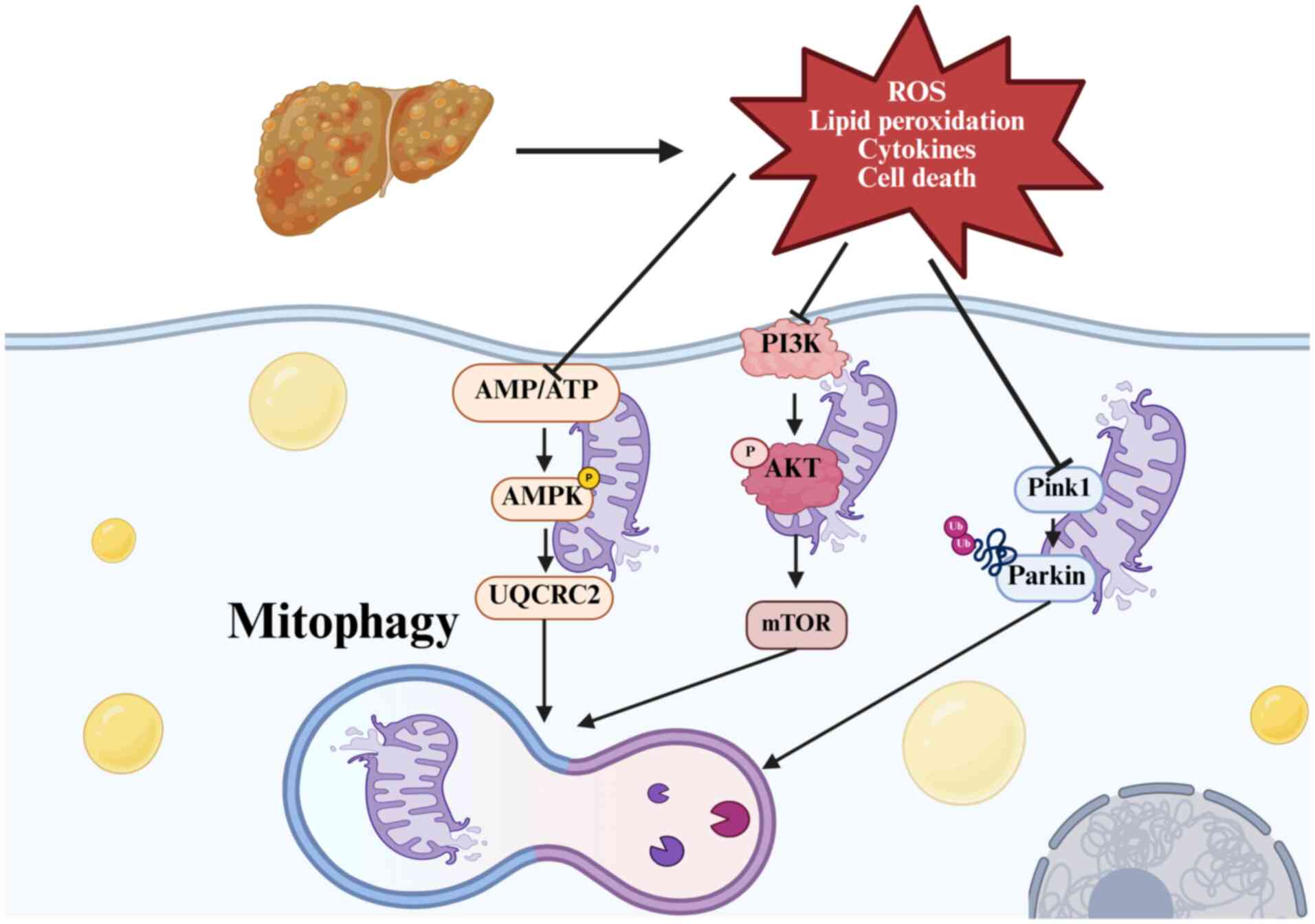
|
|
9
|
Ma X, McKeen T, Zhang J and Ding WX: Role
and mechanisms of mitophagy in liver diseases. Cells. 9:8372020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ramanathan R, Ali AH and Ibdah JA:
Mitochondrial dysfunction plays central role in nonalcoholic fatty
liver disease. Int J Mol Sci. 23:72802022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Petrescu M, Vlaicu SI, Ciumărnean L,
Milaciu MV, Mărginean C, Florea M, Vesa ȘC and Popa M: Chronic
inflammation-A link between nonalcoholic fatty liver disease
(NAFLD) and dysfunctional adipose tissue. Medicina (Kaunas).
58:6412022. View Article : Google Scholar : PubMed/NCBI
|
|
12
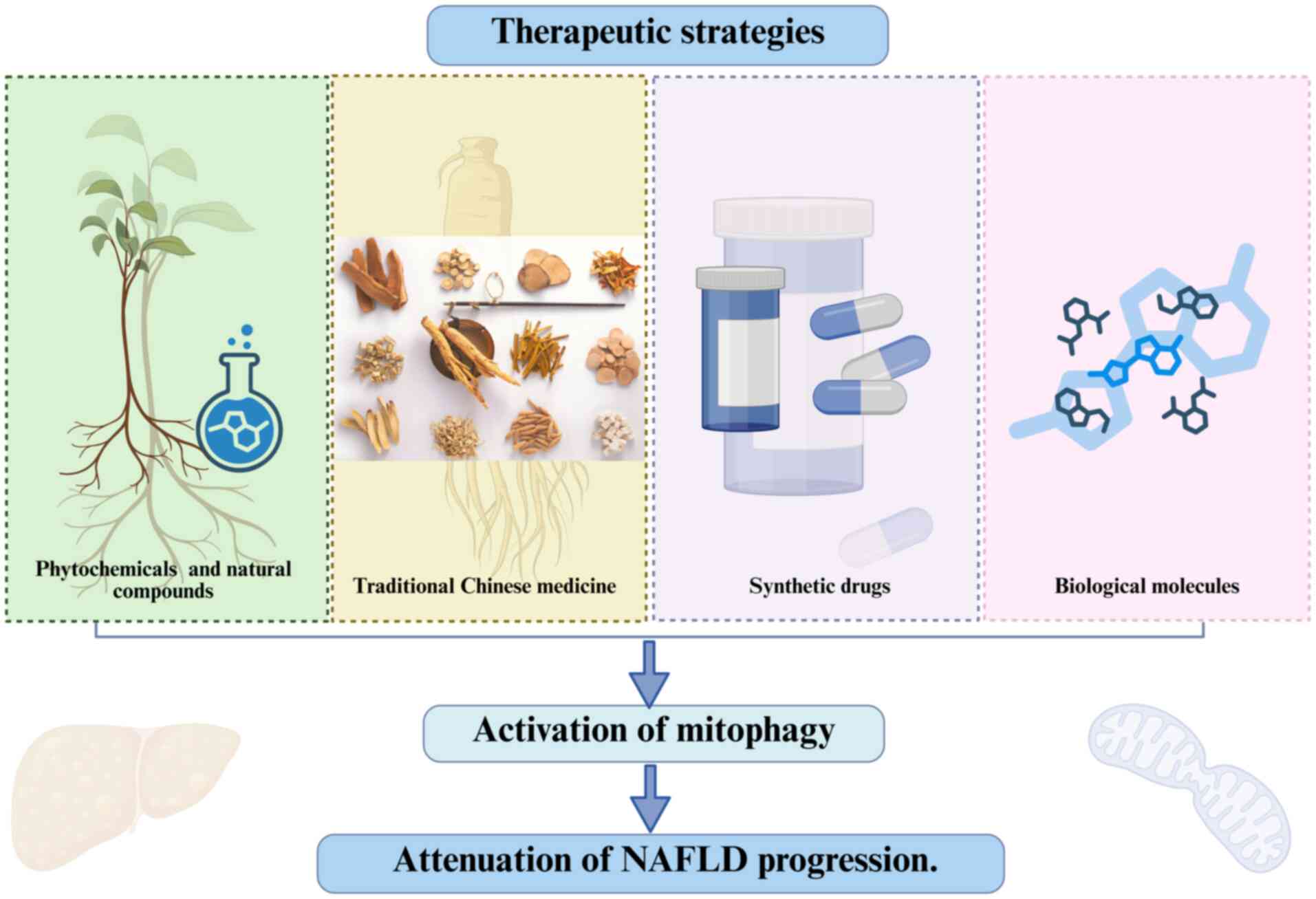
|
Tarantino G, Citro V and Balsano C:
Liver-spleen axis in nonalcoholic fatty liver disease. Expert Rev
Gastroenterol Hepatol. 15:759–769. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leung C, Rivera L, Furness JB and Angus
PW: The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol
Hepatol. 13:412–425. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mundi MS, Velapati S, Patel J, Kellogg TA,
Abu Dayyeh BK and Hurt RT: Evolution of NAFLD and its management.
Nutr Clin Pract. 35:72–84. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Paternostro R and Trauner M: Current
treatment of non-alcoholic fatty liver disease. J Intern Med.
292:190–204. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao Y, Zhang W, Zeng LQ, Bai H, Li J, Zhou
J, Zhou GY, Fang CW, Wang F and Qin XJ: Exercise and dietary
intervention ameliorate high-fat diet-induced NAFLD and liver aging
by inducing lipophagy. Redox Biol. 36:1016352020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao W, Li J, Yang K and Cao D: An overview
of autophagy: Mechanism, regulation and research progress. Bull
Cancer. 108:304–322. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen X, Tsvetkov AS, Shen HM, Isidoro C,
Ktistakis NT, Linkermann A, Koopman WJH, Simon HU, Galluzzi L, Luo
S, et al: International consensus guidelines for the definition,
detection, and interpretation of autophagy-dependent ferroptosis.
Autophagy. 20:1213–1246. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang H, Li X, Zhang Q, Fu C, Jiang W, Xue
J, Liu S, Meng Q, Ai L, Zhi X, et al: Autophagy in disease onset
and progression. Aging Dis. 15:1646–1671. 2024.PubMed/NCBI
|
|
20
|
Vargas J, Hamasaki M, Kawabata T, Youle RJ
and Yoshimori T: The mechanisms and roles of selective autophagy in
mammals. Nat Rev Mol Cell Bio. 24:167–185. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang K, Yan Y, Yu A, Zhang R, Zhang Y, Qiu
Z, Li Z, Zhang Q, Wu S and Li F: Mitophagy in neurodegenerative
disease pathogenesis. Neural Regen Res. 19:998–1005. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li W, He P, Huang Y, Li YF, Lu J, Li M,
Kurihara H, Luo Z, Meng T, Onishi M, et al: Selective autophagy of
intracellular organelles: Recent research advances. Theranostics.
11:222–256. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Waite KA, Burris A, Vontz G, Lang A and
Roelofs J: Proteaphagy is specifically regulated and requires
factors dispensable for general autophagy. J Biol Chem.
298:1014942022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hsiao PJ, Chiou HC, Jiang HJ, Lee MY,
Hsieh TJ and Kuo KK: Pioglitazone enhances cytosolic lipolysis,
β-oxidation and autophagy to ameliorate hepatic steatosis. Sci Rep.
7:90302017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Feng S, Sun Z, Jia X, Li L, Wu Y, Wu C,
Lin L, Liu J and Zeng B: Lipophagy: Molecular mechanisms and
implications in hepatic lipid metabolism. Front Biosci (Landmark
Ed). 28:62023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang S, Peng X, Yang S, Li X, Huang M,
Wei S, Liu J, He G, Zheng H, Yang L, et al: The regulation,
function, and role of lipophagy, a form of selective autophagy, in
metabolic disorders. Cell Death Dis. 13:1322022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Laval T and Ouimet M: A role for lipophagy
in atherosclerosis. Nat Rev Cardiol. 20:431–432. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Robichaud S, Fairman G, Vijithakumar V,
Mak E, Cook DP, Pelletier AR, Huard S, Vanderhyden BC, Figeys D,
Lavallée-Adam M, et al: Identification of novel lipid droplet
factors that regulate lipophagy and cholesterol efflux in
macrophage foam cells. Autophagy. 17:3671–3689. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pu M, Zheng W, Zhang H, Wan W, Peng C,
Chen X, Liu X, Xu Z, Zhou T, Sun Q, et al: ORP8 acts as a lipophagy
receptor to mediate lipid droplet turnover. Protein Cell.
14:653–667. 2023.PubMed/NCBI
|
|
30
|
Chung J, Park J, Lai ZW, Lambert TJ,
Richards RC, Zhang J, Walther TC and Farese RV Jr: The Troyer
syndrome protein spartin mediates selective autophagy of lipid
droplets. Nat Cell Biol. 25:1101–1110. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang M, Wang Z, Zhao Q, Yang Q, Bai J,
Yang C, Zhang ZR and Liu Y: USP20 deubiquitinates and stabilizes
the reticulophagy receptor RETREG1/FAM134B to drive reticulophagy.
Autophagy. 20:1780–1797. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gubas A and Dikic I: ER remodeling via
ER-phagy. Mol Cell. 82:1492–1500. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Reggiori F and Molinari M: ER-phagy:
Mechanisms, regulation, and diseases connected to the lysosomal
clearance of the endoplasmic reticulum. Physiol Rev. 102:1393–1448.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang S, Long H, Hou L, Feng B, Ma Z, Wu Y,
Zeng Y, Cai J, Zhang DW and Zhao G: The mitophagy pathway and its
implications in human diseases. Signal Transduct Target Ther.
8:3042023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Doblado L, Lueck C, Rey C, Samhan-Arias
AK, Prieto I, Stacchiotti A and Monsalve M: Mitophagy in human
diseases. Int J Mol Sci. 22:39032021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lu Y, Li Z, Zhang S, Zhang T, Liu Y and
Zhang L: Cellular mitophagy: Mechanism, roles in diseases and small
molecule pharmacological regulation. Theranostics. 13:736–766.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Degli Esposti M: Did mitophagy follow the
origin of mitochondria? Autophagy. 20:985–993. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang M, Wei X, Yi X and Jiang DS:
Mitophagy-related regulated cell death: Molecular mechanisms and
disease implications. Cell Death Dis. 15:5052024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu B, Cao Y, Wang D, Zhou Y, Zhang P, Wu
J, Chen J, Qiu J and Zhou J: Zhen-Wu-Tang induced mitophagy to
protect mitochondrial function in chronic glomerulonephritis via
PI3K/AKT/mTOR and AMPK pathways. Front Pharmacol. 12:7776702021.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Poole LP and Macleod KF: Mitophagy in
tumorigenesis and metastasis. Cell Mol Life Sci. 78:3817–3851.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Allaire M, Rautou PE, Codogno P and
Lotersztajn S: Autophagy in liver diseases: Time for translation? J
Hepatol. 70:985–998. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
An L, Wirth U, Koch D, Schirren M, Drefs
M, Koliogiannis D, Niess H, Andrassy J, Guba M, Bazhin AV, et al:
Metabolic role of autophagy in the pathogenesis and development of
NAFLD. Metabolites. 13:1012023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
González-Rodríguez A, Mayoral R, Agra N,
Valdecantos MP, Pardo V, Miquilena-Colina ME, Vargas-Castrillón J,
Lo Iacono O, Corazzari M, Fimia GM, et al: Impaired autophagic flux
is associated with increased endoplasmic reticulum stress during
the development of NAFLD. Cell Death Dis. 5:e11792014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li Q, Lin Y, Liang G, Xiao N, Zhang H,
Yang X, Yang J and Liu A: Autophagy and senescence: The molecular
mechanisms and implications in liver diseases. Int J Mol Sci.
24:168802023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nasiri-Ansari N, Nikolopoulou C, Papoutsi
K, Kyrou I, Mantzoros CS, Kyriakopoulos G, Chatzigeorgiou A,
Kalotychou V, Randeva MS, Chatha K, et al: Empagliflozin attenuates
non-alcoholic fatty liver disease (NAFLD) in high fat diet fed
ApoE(−/-) mice by activating autophagy and reducing ER
stress and apoptosis. Int J Mol Sci. 22:8182021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen Y, Yang F, Shi Y, Sheng J, Wang Y,
Zhang L, Zhou J, Jin Y and Yan Y: RNF31 alleviates liver steatosis
by promoting p53/BNIP3-related mitophagy in hepatocytes. Free
Radical Bio Med. 219:163–179. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nazeer B, Khawar MB, Khalid MU, Hamid SE,
Rafiq M, Abbasi MH, Sheikh N, Ali A, Fatima H and Ahmad S: Emerging
role of lipophagy in liver disorders. Mol Cell Biochem. 479:1–11.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Scorletti E and Carr RM: A new perspective
on NAFLD: Focusing on lipid droplets. J Hepatol. 76:934–945. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Grefhorst A, van de Peppel IP, Larsen LE,
Jonker JW and Holleboom AG: The role of lipophagy in the
development and treatment of non-alcoholic fatty liver disease.
Front Endocrinol (Lausanne). 11:6016272021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zechner R, Madeo F and Kratky D: Cytosolic
lipolysis and lipophagy: Two sides of the same coin. Nat Rev Mol
Cell Biol. 18:671–684. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Byrnes K, Blessinger S, Bailey NT, Scaife
R, Liu G and Khambu B: Therapeutic regulation of autophagy in
hepatic metabolism. Acta Pharm Sin B. 12:33–49. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Minami Y, Hoshino A, Higuchi Y, Hamaguchi
M, Kaneko Y, Kirita Y, Taminishi S, Nishiji T, Taruno A, Fukui M,
et al: Liver lipophagy ameliorates nonalcoholic steatohepatitis
through extracellular lipid secretion. Nat Commun. 14:40842023.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gusdon AM, Song KX and Qu S: Nonalcoholic
fatty liver disease: Pathogenesis and therapeutics from a
mitochondria-centric perspective. Oxid Med Cell Longev.
2014:6370272014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Aryapour E and Kietzmann T: Mitochondria,
mitophagy, and the role of deubiquitinases as novel therapeutic
targets in liver pathology. J Cell Biochem. 123:1634–1646. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lee J, Park JS and Roh YS: Molecular
insights into the role of mitochondria in non-alcoholic fatty liver
disease. Arch Pharm Res. 42:935–946. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Moore MP, Cunningham RP, Meers GM, Johnson
SA, Wheeler AA, Ganga RR, Spencer NM, Pitt JB, Diaz-Arias A, Swi
AIA, et al: Compromised hepatic mitochondrial fatty acid oxidation
and reduced markers of mitochondrial turnover in human NAFLD.
Hepatology. 76:1452–1465. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen J, Jian L, Guo Y, Tang C, Huang Z and
Gao J: Liver cell mitophagy in metabolic dysfunction-associated
steatotic liver disease and liver fibrosis. Antioxidants (Basel).
13:7292024. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cao Y, Chen X, Pan F, Wang M, Zhuang H,
Chen J, Lu L, Wang L and Wang T: Xinmaikang-mediated mitophagy
attenuates atherosclerosis via the PINK1/Parkin signaling pathway.
Phytomedicine. 119:1549552023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Gao X, Ruan Y, Zhu X, Lin X, Xin Y, Li X,
Mai M and Guo H: Deoxycholic acid promotes pyroptosis in free fatty
acid-induced steatotic hepatocytes by inhibiting PINK1-mediated
mitophagy. Inflammation. 45:639–650. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhou T, Chang L, Luo Y, Zhou Y and Zhang
J: Mst1 inhibition attenuates non-alcoholic fatty liver disease via
reversing Parkin-related mitophagy. Redox Biol. 21:1011202019.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Edmunds LR, Xie B, Mills AM, Huckestein
BR, Undamatla R, Murali A, Pangburn MM, Martin J, Sipula I, Kaufman
BA, et al: Liver-specific Prkn knockout mice are more susceptible
to diet-induced hepatic steatosis and insulin resistance. Mol
Metab. 41:1010512020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Xu ZX, Li JZ, Li Q, Xu MY and Li HY:
CircRNA608-microRNA222-PINK1 axis regulates the mitophagy of
hepatic stellate cells in NASH related fibrosis. Biochem Biophys
Res Commun. 610:35–42. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
He H, Tang Y, Zhuang L, Zheng Y and Huang
X: PINK1/Park2-mediated mitophagy relieve non-alcoholic fatty liver
disease. Physiol Res. 73:253–263. 2024.PubMed/NCBI
|
|
64
|
Matsuda S, Kobayashi M and Kitagishi Y:
Roles for PI3K/AKT/PTEN pathway in cell signaling of nonalcoholic
fatty liver disease. ISRN Endocrinol. 2013:4724322013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Shamsan E, Almezgagi M, Gamah M, Khan N,
Qasem A, Chuanchuan L and Haining F: The role of PI3k/AKT signaling
pathway in attenuating liver fibrosis: A comprehensive review.
Front Med (Lausanne). 11:13893292024. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Tsuji A, Yoshikawa S, Ikeda Y, Taniguchi
K, Sawamura H, Morikawa S, Nakashima M, Asai T and Matsuda S:
Tactics with prebiotics for the treatment of metabolic
dysfunction-associated fatty liver disease via the improvement of
mitophagy. Int J Mol Sci. 24:54652023. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Aslam M and Ladilov Y: Emerging role of
cAMP/AMPK signaling. Cells. 11:3082022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Lu X, Xuan W and Li J, Yao H, Huang C and
Li J: AMPK protects against alcohol-induced liver injury through
UQCRC2 to up-regulate mitophagy. Autophagy. 17:3622–3643. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Desjardins EM, Smith BK, Day EA, Ducommun
S, Sanders MJ, Nederveen JP, Ford RJ, Pinkosky SL, Townsend LK,
Gutgesell RM, et al: The phosphorylation of AMPKβ1 is critical for
increasing autophagy and maintaining mitochondrial homeostasis in
response to fatty acids. Proc Natl Acad Sci USA.
119:e21198241192022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Gu M, Luo L and Fang K: Crocin inhibits
obesity via AMPK-dependent inhibition of adipocyte differentiation
and promotion of lipolysis. Biosci Trends. 12:587–594. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Herzig S and Shaw RJ: AMPK: Guardian of
metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol.
19:121–135. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Fang C, Pan J, Qu N, Lei Y, Han J, Zhang J
and Han D: The AMPK pathway in fatty liver disease. Front Physiol.
13:9702922022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Marcondes-de-Castro IA, Reis-Barbosa PH,
Marinho TS, Aguila MB and Mandarim-de-Lacerda CA: AMPK/mTOR pathway
significance in healthy liver and non-alcoholic fatty liver disease
and its progression. J Gastroenterol Hepatol. 38:1868–1876. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Chen L, Zhang Q, Meng Y, Zhao T, Mu C, Fu
C, Deng C, Feng J, Du S, Liu W, et al: Saturated fatty acids
increase LPI to reduce FUNDC1 dimerization and stability and
mitochondrial function. EMBO Rep. 24:e547312023. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lian CY, Li HJ, Xia WH, Li Y, Zhou XL,
Yang DB, Wan XM and Wang L: Insufficient FUNDC1-dependent mitophagy
due to early environmental cadmium exposure triggers mitochondrial
redox imbalance to aggravate diet-induced lipotoxicity. Environ
Pollut. 361:1247242024. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Li L, Martin-Levilain J, Jiménez-Sánchez
C, Karaca M, Foti M, Martinou JC and Maechler P: In vivo
stabilization of OPA1 in hepatocytes potentiates mitochondrial
respiration and gluconeogenesis in a prohibitin-dependent way. J
Biol Chem. 294:12581–12598. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Mucinski JM, Manrique-Acevedo C, Kasumov
T, Garrett TJ, Gaballah A and Parks EJ: Relationships between very
low-density lipoproteins-ceramides, -diacylglycerols, and
-triacylglycerols in insulin-resistant men. Lipids. 55:387–393.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Sentelle RD, Senkal CE, Jiang W, Ponnusamy
S, Gencer S, Selvam SP, Ramshesh VK, Peterson YK, Lemasters JJ,
Szulc ZM, et al: Ceramide targets autophagosomes to mitochondria
and induces lethal mitophagy. Nat Chem Biol. 8:831–838. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kim KM and Kim SG: Autophagy and microRNA
dysregulation in liver diseases. Arch Pharm Res. 37:1097–1116.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Yuan X, Li Y, Wen S, Xu C, Wang C, He Y
and Zhou L: CircLDLR acts as a sponge for miR-667-5p to regulate
SIRT1 expression in non-alcoholic fatty liver disease. Lipids
Health Dis. 21:1272022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Dong M, Zhang T, Liang X, Cheng X, Shi F,
Yuan H, Zhang F, Jiang Q and Wang X: Sesamin alleviates lipid
accumulation induced by oleic acid via PINK1/Parkin-mediated
mitophagy in HepG2 cells. Biochem Biophys Res Commun.
708:1498152024. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Li W, Cai Z, Schindler F, Afjehi-Sadat L,
Montsch B, Heffeter P, Heiss EH and Weckwerth W: Elevated
PINK1/Parkin-dependent mitophagy and boosted mitochondrial function
mediate protection of hepg2 cells from excess palmitic acid by
hesperetin. J Agric Food Chem. 72:13039–13053. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zhang H, You Y, Xu J, Jiang H, Jiang J, Su
Z, Chao Z, Du Q and He F: New sesquiterpenes and viridin
derivatives from Penicillium sp. Ameliorates NAFLD by
regulating the PINK1/Parkin mitophagy pathway. Bioorg Chem.
151:1076562024. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Dong Y, Yu M, Wu Y, Xia T, Wang L, Song K,
Zhang C, Lu K and Rahimnejad S: Hydroxytyrosol promotes the
mitochondrial function through activating mitophagy. Antioxidants
(Basel). 11:8932022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Li X, Shi Z, Zhu Y, Shen T, Wang H, Shui
G, Loor JJ, Fang Z, Chen M, Wang X, et al: Cyanidin-3-O-glucoside
improves non-alcoholic fatty liver disease by promoting
PINK1-mediated mitophagy in mice. Br J Pharmacol. 177:3591–3607.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Yao Z, Li X, Wang W, Ren P, Song S, Wang
H, Xie Y, Li X and Li Z: Corn peptides attenuate non-alcoholic
fatty liver disease via PINK1/Parkin-mediated mitochondrial
autophagy. Food Nutr Res. 672023.PubMed/NCBI
|
|
87
|
Wu Y, Kuang Y, Wu Y, Dai H, Bi R, Hu J and
Sun L: Yang-Gan-Jiang-Mei formula alleviates non-alcoholic
steatohepatitis by inhibiting NLRP3 inflammasome through mitophagy.
Biotechnol Genet Eng Rev. 40:1314–1333. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Dou SD, Zhang JN, Xie XL, Liu T, Hu JL,
Jiang XY, Wang MM and Jiang HD: MitoQ inhibits hepatic stellate
cell activation and liver fibrosis by enhancing
PINK1/parkin-mediated mitophagy. Open Med (Wars). 16:1718–1727.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Song YW, Zhu YH and Ma MZ: Furin inhibits
HSCs activation and ameliorates liver fibrosis by regulating
PTEN-L/PINK1/parkin mediated mitophagy in mouse. FASEB Bioadv.
7:e700092025. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Mehmood A, Zhao L, Wang Y, Pan F, Hao S,
Zhang H, Iftikhar A and Usman M: Dietary anthocyanins as potential
natural modulators for the prevention and treatment of
non-alcoholic fatty liver disease: A comprehensive review. Food Res
Int. 142:1101802021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Chen H, Yan S, Xiang Q, Liang J, Deng X,
He W, Cheng Y and Yang L: Network analysis and experimental
verification of Salvia miltiorrhiza Bunge-Reynoutria
japonica Houtt. drug pair in the treatment of non-alcoholic
fatty liver disease. BMC Complement Med Ther. 24:3052024.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Sun C, Zhang J, Hou J, Hui M, Qi H, Lei T,
Zhang X, Zhao L and Du H: Induction of autophagy via the
PI3K/Akt/mTOR signaling pathway by Pueraria flavonoids improves
non-alcoholic fatty liver disease in obese mice. Biomed
Pharmacother. 157:1140052023. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Sarin SK, Choudhury A, Sharma MK, Maiwall
R, Al Mahtab M, Rahman S, Saigal S, Saraf N, Soin AS, Devarbhavi H,
et al: Acute-on-chronic liver failure: Consensus recommendations of
the Asian Pacific association for the study of the liver (APASL):
An update. Hepatol Int. 13:353–390. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Axley P, Ahmed Z, Arora S, Haas A, Kuo YF,
Kamath PS and Singal AK: NASH is the most rapidly growing etiology
for acute-on-chronic liver failure-related hospitalization and
disease burden in the United States: A population-based study.
Liver Transpl. 25:695–705. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Li J, Huang Q, Ma W, Yi J, Zhong X, Hu R,
Sun J, Ma M, Lv M, Han Z, et al: Hepatoprotective efficacy and
interventional mechanism of JianPi LiShi YangGan formula in
acute-on-chronic liver failure. J Ethnopharmacol. 318:1168802024.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Dai X, Sun F, Deng K, Lin G, Yin W, Chen
H, Yang D, Liu K, Zhang Y and Huang L: Mallotucin D, a clerodane
diterpenoid from Croton crassifolius, suppresses HepG2 cell growth
via inducing autophagic cell death and pyroptosis. Int J Mol Sci.
23:142172022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Yan X, Inta A, Yang X, Pandith H,
Disayathanoowat T and Yang L: An investigation of the effect of the
traditional naxi herbal formula against liver cancer through
network pharmacology, molecular docking, and in vitro experiments.
Pharmaceuticals (Basel). 17:14292024. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Liu JS, Huo CY, Cao HH, Fan CL, Hu JY,
Deng LJ, Lu ZB, Yang HY, Yu LZ, Mo ZX and Yu ZL: Aloperine induces
apoptosis and G2/M cell cycle arrest in hepatocellular carcinoma
cells through the PI3K/Akt signaling pathway. Phytomedicine.
61:1528432019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Mottillo EP, Desjardins EM, Crane JD,
Smith BK, Green AE, Ducommun S, Henriksen TI, Rebalka IA, Razi A,
Sakamoto K, et al: Lack of adipocyte AMPK exacerbates insulin
resistance and hepatic steatosis through brown and beige adipose
tissue function. Cell Metab. 24:118–129. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Smith BK, Marcinko K, Desjardins EM, Lally
JS, Ford RJ and Steinberg GR: Treatment of nonalcoholic fatty liver
disease: Role of AMPK. Am J Physiol Endocrinol Metab.
311:E730–E740. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Cao P, Wang Y, Zhang C, Sullivan MA, Chen
W, Jing X, Yu H, Li F, Wang Q, Zhou Z, et al: Quercetin ameliorates
nonalcoholic fatty liver disease (NAFLD) via the promotion of
AMPK-mediated hepatic mitophagy. J Nutr Biochem. 120:1094142023.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Yao Z, Guo J, Du B, Hong L, Zhu Y, Feng X,
Hou Y and Shi A: Effects of Shenling Baizhu powder on intestinal
microflora metabolites and liver mitochondrial energy metabolism in
nonalcoholic fatty liver mice. Front Microbiol. 14:11470672023.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Lv T, Fan X, He C, Zhu S, Xiong X, Yan W,
Liu M, Xu H, Shi R and He Q: SLC7A11-ROS/αKG-AMPK axis regulates
liver inflammation through mitophagy and impairs liver fibrosis and
NASH progression. Redox Biol. 72:1031592024. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Song N, Xu H, Wu S, Luo S, Xu J, Zhao Q,
Wang R and Jiang X: Synergistic activation of AMPK by AdipoR1/2
agonist and inhibitor of EDPs-EBP interaction recover NAFLD through
enhancing mitochondrial function in mice. Acta Pharm Sin B.
13:542–558. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Ren K, Su H, Lv LJ, Yi LT, Gong X, Dang
LS, Zhang RF and Li MH: Effects of four compounds from
Gentianella acuta (Michx.) hulten on hydrogen
peroxide-induced injury in H9c2 cells. Biomed Res Int.
2019:26929702019. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Li J, Wu J, Chen Q, Yu H, Liu M, Wang Y,
Zhang Y and Wang T: 7′-Hydroxyl substituted xanthones from
Gentianella acuta revert hepatic steatosis in obese diabetic
mice through preserving mitochondrial homeostasis. Biochem
Pharmacol. 236:1168782025. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Li W, Li Y, Siraj S, Jin H, Fan Y, Yang X,
Huang X, Wang X, Wang J, Liu L, et al: FUN14 Domain-containing
1-mediated mitophagy suppresses hepatocarcinogenesis by inhibition
of inflammasome activation in mice. Hepatology. 69:604–621. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Ma Z, Chen W, Liu Y, Yu L, Mao X, Guo X,
Jiang F, Guo Q, Lin N and Zhang Y: Artesunate Sensitizes human
hepatocellular carcinoma to sorafenib via exacerbating
AFAP1L2-SRC-FUNDC1 axis-dependent mitophagy. Autophagy. 20:541–556.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Ma Z, Zhang D, Sun J, Zhang Q, Qiao Y, Zhu
Y, Niu J, Ren Q, Zhou L, Wen A and Wang J: Formononetin inhibits
hepatic I/R-induced injury through regulating PHB2/PINK1/Parkin
pathway. Oxid Med Cell Longev. 2022:64811922022. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Kalavalapalli S, Bril F, Koelmel JP, Abdo
K, Guingab J, Andrews P, Li WY, Jose D, Yost RA, Frye RF, et al:
Pioglitazone improves hepatic mitochondrial function in a mouse
model of nonalcoholic steatohepatitis. Am J Physiol Endocrinol
Metab. 315:E163–E173. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Yang W, Zhao J, Zhao Y, Li W, Zhao L, Ren
Y, Ou R and Xu Y: Hsa_circ_0048179 attenuates free fatty
acid-induced steatosis via hsa_circ_0048179/miR-188-3p/GPX4
signaling. Aging (Albany NY). 12:23996–24008. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Chen M, Huang F, Chen B, Kang J, Yao Y,
Liua M, Li Y, Li Y, Zhou T, Peng D, et al: A classical herbal
formula alleviates high-fat diet induced nonalcoholic
steatohepatitis (NASH) via targeting mitophagy to rehabilitate
dysfunctional mitochondria, validated by UPLC-HRMS identification
combined with in vivo experiment. Biomed Pharmacother.
168:1158312023. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Deng J, Long J, Yang Y, Yang F and Wei Y:
Gentiana decoction inhibits liver fibrosis and the activation of
hepatic stellate cells via upregulating the expression of Parkin.
Fitoterapia. 178:1061702024. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Chen Y, Chen HN, Wang K, Zhang L, Huang Z,
Liu J, Zhang Z, Luo M, Lei Y, Peng Y, et al: Ketoconazole
exacerbates mitophagy to induce apoptosis by downregulating
cyclooxygenase-2 in hepatocellular carcinoma. J Hepatol. 70:66–77.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Bano A, Chaker L, Plompen EP, Hofman A,
Dehghan A, Franco OH, Janssen HL, Darwish Murad S and Peeters RP:
Thyroid function and the risk of nonalcoholic fatty liver disease:
The rotterdam study. J Clin Endocrinol Metab. 101:3204–3211. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Bruinstroop E, Dalan R, Cao Y, Bee YM,
Chandran K, Cho LW, Soh SB, Teo EK, Toh SA, Leow MKS, et al:
Low-dose levothyroxine reduces intrahepatic lipid content in
patients with type 2 diabetes mellitus and NAFLD. J Clin Endocrinol
Metab. 103:2698–2706. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Zhou J, Sinha RA and Yen PM: The roles of
autophagy and thyroid hormone in the pathogenesis and treatment of
NAFLD. Hepatoma Res. 7:722021.PubMed/NCBI
|
|
118
|
Harrison SA, Bashir MR, Guy CD, Zhou R,
Moylan CA, Frias JP, Alkhouri N, Bansal MB, Baum S,
Neuschwander-Tetri BA, et al: Resmetirom (MGL-3196) for the
treatment of non-alcoholic steatohepatitis: A multicentre,
randomised, double-blind, placebo-controlled, phase 2 trial.
Lancet. 394:2012–2024. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Keam SJ: Resmetirom: First approval.
Drugs. 84:729–735. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Lee JH, Seo KH, Yang JH, Cho SS, Kim NY,
Kim JH, Kim KM and Ki SH: CCCP induces hepatic stellate cell
activation and liver fibrogenesis via mitochondrial and lysosomal
dysfunction. Free Radic Biol Med. 225:181–192. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Wei R, Cao J and Yao S: Matrine promotes
liver cancer cell apoptosis by inhibiting mitophagy and
PINK1/Parkin pathways. Cell Stress Chaperones. 23:1295–1309. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Wu H, Wang T, Liu Y, Li X, Xu S, Wu C, Zou
H, Cao M, Jin G, Lang J, et al: Mitophagy promotes sorafenib
resistance through hypoxia-inducible ATAD3A dependent axis. J Exp
Clin Canc Res. 39:2742020. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Yu SX, Liang ZM, Wu QB, Shou L, Huang XX,
Zhu QR, Xie H, Mei RY, Zhang RN, Zhai XY, et al: A novel diagnostic
and therapeutic strategy for cancer patients by integrating Chinese
medicine syndrome differentiation and precision medicine. Chin J
Integr Med. 28:867–871. 2022. View Article : Google Scholar : PubMed/NCBI
|