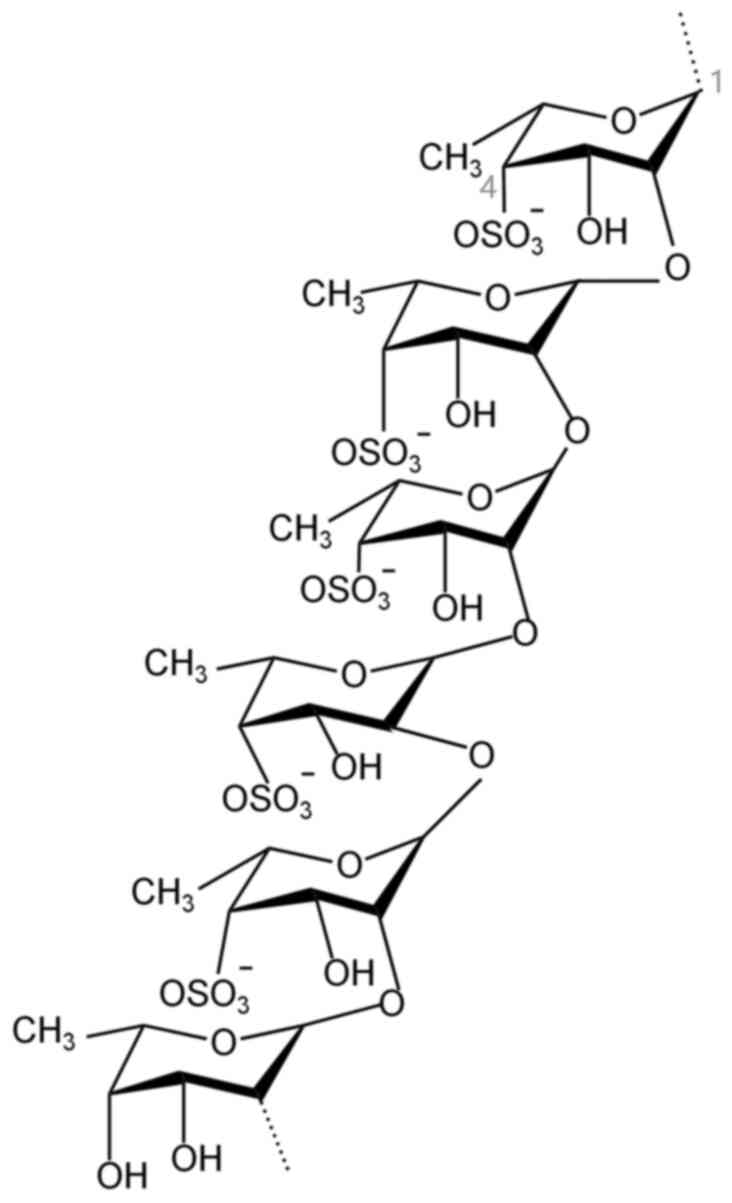
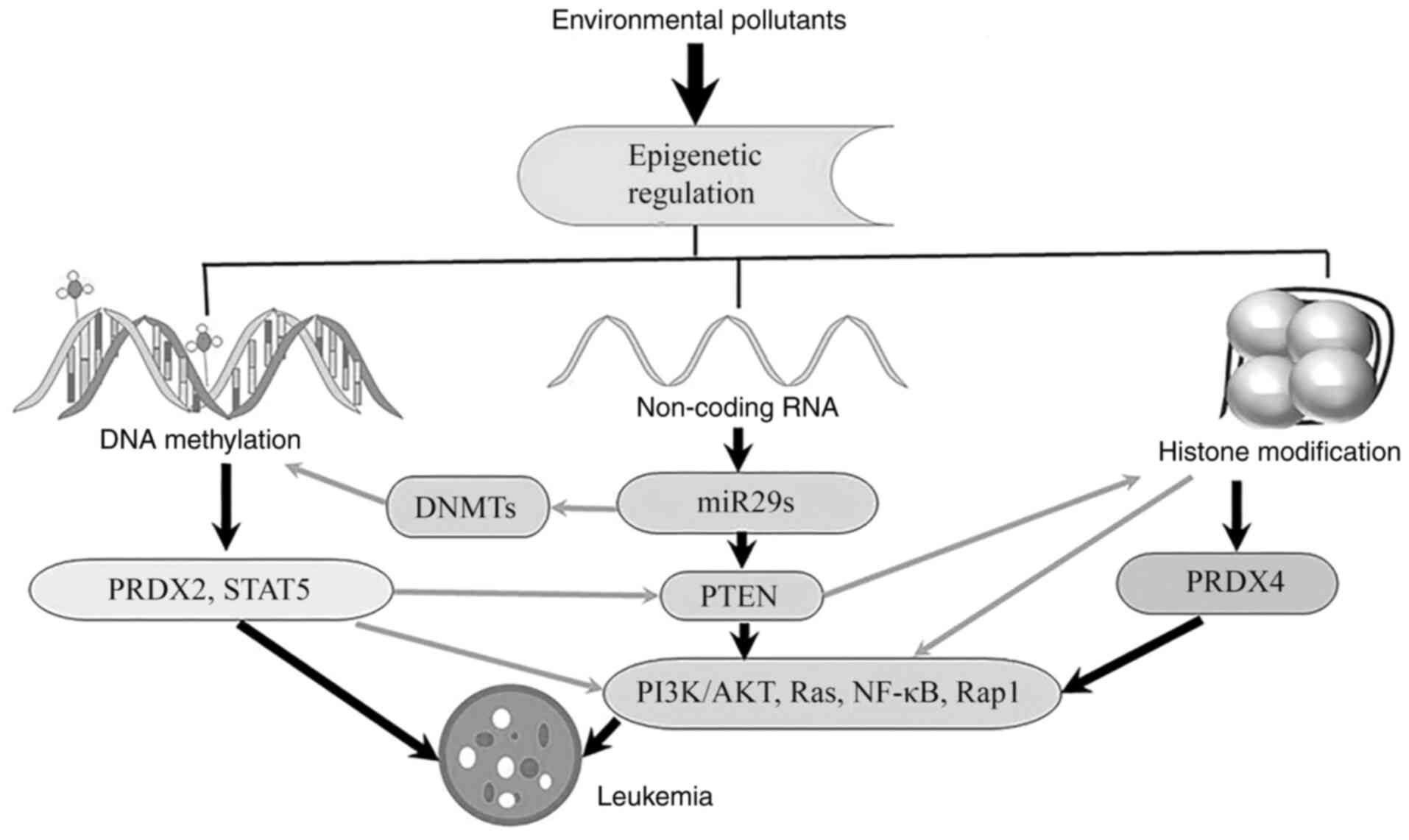
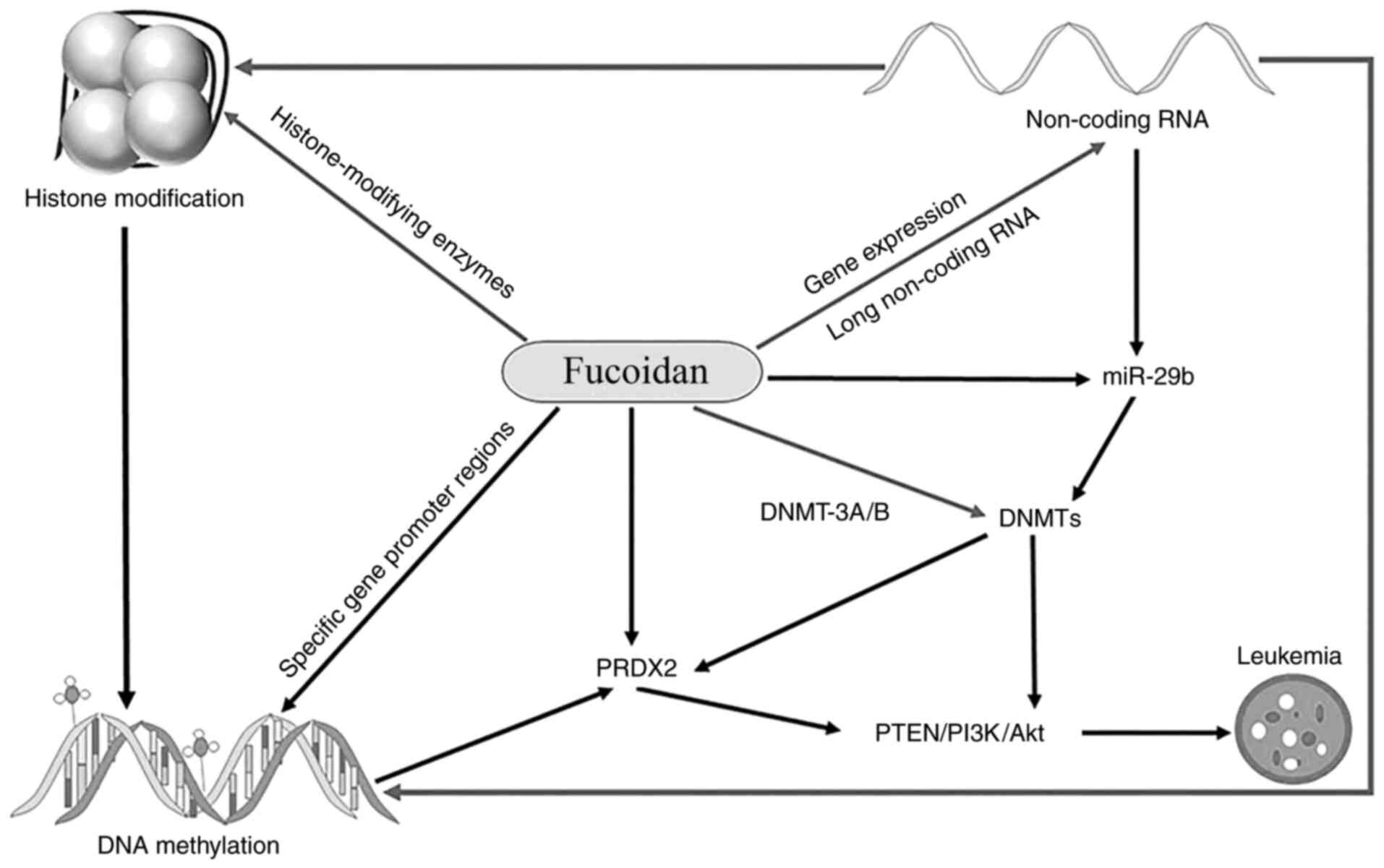
|
1
|
Zheng Z, Wang L, Cheng S, Wang Y and Zhao
W: Autophagy and Leukemia. Adv Exp Med Biol. 1207:601–613. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang X, Wong MPM and Ng RK: Aberrant DNA
methylation in acute myeloid leukemia and its clinical
implications. Int J Mol Sci. 20:45762019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu H, Yu H, Jin R, Wu X and Chen H:
Genetic and epigenetic targeting therapy for pediatric acute
lymphoblastic leukemia. Cells. 10:33492021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gowda C, Song C, Ding Y, Iyer S,
Dhanyamraju PK, McGrath M, Bamme Y, Soliman M, Kane S, Payne JL and
Dovat S: Cellular signaling and epigenetic regulation of gene
expression in leukemia. Adv Biol Regul. 75:1006652020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang L, Rau R and Goodell MA: DNMT3A in
haematological malignancies. Nat Rev Cancer. 15:152–165. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ley TJ, Ding L, Walter MJ, McLellan MD,
Lamprecht T, Larson DE, Kandoth C, Payton JE, Baty J, Welch J, et
al: DNMT3A mutations in acute myeloid leukemia. N Engl J Med.
363:2424–2433. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Walter MJ, Ding L, Shen D, Shao J, Grillot
M, McLellan M, Fulton R, Schmidt H, Kalicki-Veizer J, O'Laughlin M,
et al: Recurrent DNMT3A mutations in patients with myelodysplastic
syndromes. Leukemia. 25:1153–1158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Plass C, Pfister SM, Lindroth AM,
Bogatyrova O, Claus R and Lichter P: Mutations in regulators of the
epigenome and their connections to global chromatin patterns in
cancer. Nat Rev Genet. 14:765–780. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang Y, Hatzi K and Shaknovich R:
Mechanisms of epigenetic deregulation in lymphoid neoplasms. Blood.
121:4271–4279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ghazimoradi MH, Karimpour-Fard N and
Babashah S: The promising role of Non-coding RNAs as biomarkers and
therapeutic targets for leukemia. Genes (Basel). 14:1312023.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li T, Hong J, Ma Y, Yang B, Wang G, Wang
S, Chen J and Chi X: Regulatory mechanism of long noncoding RNA in
the occurrence and development of leukemia: A review. Sheng Wu Gong
Cheng Xue Bao. 37:3933–3944. 2021.(In Chinese). PubMed/NCBI
|
|
12
|
Cruz-Miranda GM, Hidalgo-Miranda A,
Bárcenas-López DA, Núñez-Enríquez JC, Ramírez-Bello J,
Mejía-Aranguré JM and Jiménez-Morales S: Long Non-coding RNA and
acute leukemia. Int J Mol Sci. 20:7352019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen Y and Shen YQ: Role of reactive
oxygen species in regulating epigenetic modifications. Cell Signal.
125:1115022025. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alimohammadi M, Abolghasemi H, Cho WC,
Reiter RJ, Mafi A, Aghagolzadeh M and Hushmandi K: Interplay
between LncRNAs and autophagy-related pathways in leukemia:
Mechanisms and clinical implications. Med Oncol. 42:1542025.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma D, Wei J, Chen S, Wang H, Ning L, Luo
SH, Liu CL, Song G and Yao Q: Fucoidan inhibits the progression of
hepatocellular carcinoma via causing lncRNA LINC00261
overexpression. Front Oncol. 11:6539022021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan MD, Lin HY and Hwang PA: The
anti-tumor activity of brown seaweed Oligo-fucoidan via lncRNA
expression modulation in HepG2 cells. Cytotechnology. 71:363–374.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Conchie J and Percival EGV: Fucoidin. Part
II. The hydrolysis of a methylated Fucoidin prepared from fucus
vesiculosus. J Chem Soc. 827–832. 1950. View Article : Google Scholar
|
|
18
|
Li Y, Zhao W, Wang L, Chen Y, Zhang H,
Wang T, Yang X, Xing F, Yan J and Fang X: Protective effects of
fucoidan against hydrogen peroxide-induced oxidative damage in
porcine intestinal epithelial cells. Animals (Basel). 9:11082019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liang Z, Liu Z, Sun X, Tao M, Xiao X, Yu G
and Wang X: The effect of fucoidan on cellular oxidative stress and
the CatD-Bax signaling axis in MN9D cells damaged by
1-methyl-4-phenypyridinium. Front Aging Neurosci. 10:4292019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoo HJ, You DJ and Lee KW:
Characterization and immunomodulatory effects of high molecular
weight fucoidan fraction from the Sporophyll of Undaria pinnatifida
in cyclophosphamide-induced immunosuppressed mice. Mar Drugs.
17:4472019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Xing M, Cao Q, Ji A, Liang H and
Song S: Biological activities of fucoidan, the factors mediating
its therapeutic effects: A review of recent studies. Mar Drugs.
17:1832019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luthuli S, Wu S, Cheng Y, Zheng X, Wu M
and Tong H: Therapeutic effects of fucoidan: A review on recent
studies. Mar Drugs. 17:4872019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim EA, Lee SH, Ko CI, Cha SH, Kang MC,
Kang SM, Ko SC, Lee WW, Ko JY, Lee JH, et al: Protective effect of
fucoidan against AAPH-induced oxidative stress in zebrafish model.
Carbohydr Polym. 102:185–191. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shiau JP, Chuang YT, Cheng YB, Tang JY,
Hou MF, Yen CY and Chang HW: Impacts of Oxidative Stress and
PI3K/AKT/mTOR on metabolism and the future direction of
investigating fucoidan-modulated metabolism. Antioxidants (Basel).
11:9112022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jin JO, Song MG, Kim YN, Park JI and Kwak
JY: The mechanism of fucoidan-induced apoptosis in leukemic cells:
Involvement of ERK1/2, JNK, glutathione, and nitric oxide. Mol
Carcinog. 49:771–782. 2010.PubMed/NCBI
|
|
26
|
Park HS, Hwang HJ, Kim GY, Cha HJ, Kim WJ,
Kim ND, Yoo YH and Choi YH: Induction of apoptosis by fucoidan in
human leukemia U937 cells through activation of p38 MAPK and
modulation of Bc-2 family. Mar Drugs. 11:2347–2364. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maruyama H, Tamauchi H, Iizuka M and
Nakano T: The role of NK cells in anti-tumor activity of dietary
fucoidan from Undaria pinnatifda sporophylls (Mekabu). Planta Med.
72:1415–1417. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liao CH, Lai IC, Kuo HC, Chuang SE, Lee
HL, Whang-Peng J, Yao CJ and Lai GM: Epigenetic modification and
differentiation induction of malignant glioma cells by
oligo-fucoidan. Mar Drugs. 17:5252019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yan MD, Yao CJ, Chow JM, Chang CL, Hwang
PA, Chuang SE, Whang-Peng J and Lai GM: Fucoidan elevates
MicroRNA-29b to regulate DNMT3B-MTSS1 axis and inhibit EMT in human
hepatocellular carcinoma cells. Mar Drugs. 13:6099–6116. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
El Asri S, Ben Mrid R, Zouaoui Z, Roussi
Z, Ennoury A, Nhiri M and Chibi F: Advances in structural
modification of fucoidans, ulvans, and carrageenans to improve
their biological functions for potential therapeutic application.
Carbohydr Res. 549:1093582025. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao A, Zhou H, Yang J, Li M and Niu T:
Epigenetic regulation in hematopoiesis and its implications in the
targeted therapy of hematologic malignancies. Signal Transduct
Target Ther. 8:712023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu H, Wen Y, Jin R and Chen H: Epigenetic
modifications and targeted therapy in pediatric acute myeloid
leukemia. Front Pediatr. 10:9758192022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mehdipour P, Santoro F and Minucci S:
Epigenetic alterations in acute myeloid leukemias. FEBS J.
282:1786–1800. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Memari F, Joneidi Z, Taheri B, Aval SF,
Roointan A and Zarghami N: Epigenetics and Epi-miRNAs: Potential
markers/therapeutics in leukemia. Biomed Pharmacother.
106:1668–1677. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cruz-Rodriguez N, Combita AL and Zabaleta
J: Epigenetics in hematological malignancies. Methods Mol Biol.
1856:87–101. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wei A and Wu H: Mammalian DNA methylome
dynamics: Mechanisms, functions and new frontiers. Development.
149:dev1826832022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lyko F: The DNA methyltransferase family:
A versatile toolkit for epigenetic regulation. Nat Rev Genet.
19:81–92. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mensah IK, Norvil AB, AlAbdi L, McGovern
S, Petell CJ, He M and Gowher H: Misregulation of the expression
and activity of DNA methyltransferases in cancer. NAR Cancer.
3:zcab0452021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kalinkova L, Sevcikova A, Stevurkova V,
Fridrichova I and Ciernikova S: Targeting DNA methylation in
leukemia, myelodysplastic syndrome, and lymphoma: A potential
diagnostic, prognostic, and therapeutic Tool. Int J Mol Sci.
24:6332022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rahmani M, Talebi M, Hagh MF, Feizi AAH
and Solali S: Aberrant DNA methylation of key genes and Acute
Lymphoblastic Leukemia. Biomed Pharmacother. 97:1493–1500. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhou L and Yin X: Clinical applications of
abnormal DNA methylation in chronic myeloid leukemia. Zhong Nan Da
Xue Xue Bao Yi Xue Ban. 49:122–127. 2024.(In English, Chinese).
PubMed/NCBI
|
|
42
|
Palomo L, Malinverni R, Cabezón M, Xicoy
B, Arnan M, Coll R, Pomares H, García O, Fuster-Tormo F, Grau J, et
al: DNA methylation profile in chronic myelomonocytic leukemia
associates with distinct clinical, biological and genetic features.
Epigenetics. 13:8–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu Y, Zhu H and Wu H: PTEN in regulating
hematopoiesis and leukemogenesis. Cold Spring Harb Perspect Med.
10:a0362442020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li M, Liu H, Xu ZF, Liu XR, Wang Y, Rao Q,
Wang JX and Wang M: Promoter methylation status of PTEN gene and
the effect of induced demethylation in leukemia cell lines.
Zhonghua Xue Ye Xue Za Zhi. 29:289–292. 2008.(In Chinese).
PubMed/NCBI
|
|
45
|
Zhang Y, Chen D, Shi R, Wang X, Ji X, Han
K, Tian Y and Gao Y: Chemical exposure, leukemia related DNA
methylation changes and childhood acute leukemia. Zhonghua Yu Fang
Yi Xue Za Zhi. 49:800–809. 2015.(In Chinese). PubMed/NCBI
|
|
46
|
Takeuchi A, Nishioka C, Ikezoe T, Yang J
and Yokoyama A: STAT5A regulates DNMT3A in CD34(+)/CD38(−) AML
cells. Leuk Res. 39:897–905. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bera R, Chiu MC, Huang YJ, Liang DC, Lee
YS and Shih LY: Genetic and epigenetic perturbations by DNMT3A-R882
mutants impaired apoptosis through augmentation of PRDX2 in myeloid
leukemia cells. Neoplasia. 20:1106–1120. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sanchez R and Mackenzie SA: Integrative
network analysis of differentially methylated and expressed genes
for biomarker identification in leukemia. Sci Rep. 10:21232020.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Shen N, Yan F, Pang J, Wu LC, Al-Kali A,
Litzow MR and Liu S: A nucleolin-DNMT1 regulatory axis in acute
myeloid leukemogenesis. Oncotarget. 5:5494–5509. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shen N, Yan F, Pang J, Zhao N, Gangat N,
Wu L, Bode AM, Al-Kali A, Litzow MR and Liu S: Inactivation of
receptor tyrosine kinasesreverts aberrant DNA methylation in acute
myeloid leukemia. Clin Cancer Res. 23:6254–6266. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang GH, Lu Y, Ji BQ, Ren JC, Sun P, Ding
S, Liao X, Liao K, Liu J, Cao J, et al: Do mutations in DNMT3A/3B
affect global DNA hypomethylation among benzene-exposed workers in
Southeast China?: Effects of mutations in DNMT3A/3B on global DNA
hypomethylation. Environ Mol Mutagen. 58:678–687. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Scourzic L, Couronné L, Pedersen MT, Della
Valle V, Diop M, Mylonas E, Calvo J, Mouly E, Lopez CK, Martin N,
et al: DNMT3A(R882H) mutant and Tet2 inactivation cooperate in the
deregulation of DNA methylation control to induce lymphoid
malignancies in mice. Leukemia. 30:1388–1398. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Giacopelli B, Wang M, Cleary A, Wu YZ,
Schultz AR, Schmutz M, Blachly JS, Eisfeld AK, Mundy-Bosse B,
Vosberg S, et al: DNA methylation epitypes highlight underlying
developmental and disease pathways in acute myeloid leukemia.
Genome Res. 31:747–761. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Huang J, Xie J, Wang Y, Sheng M, Sun Y,
Chen P, Rong S, Yin D, Wang Y, Zhu P, et al: STING mediates
increased self-renewal and lineage skewing in DNMT3A-mutated
hematopoietic stem/progenitor cells. Leukemia. 39:929–941. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mansouri L, Wierzbinska JA, Plass C and
Rosenquist R: Epigenetic deregulation in chronic lymphocytic
leukemia: Clinical and biological impact. Semin Cancer Biol.
51:1–11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Birch NW and Shilatifard A: The role of
histone modifications in leukemogenesis. J Biosci. 45:62020.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li Y, Ning Q, Shi J, Chen Y, Jiang M, Gao
L, Huang W, Jing Y, Huang S, Liu A, et al: A novel epigenetic
AML1-ETO/THAP10/miR-383 mini-circuitry contributes to t(8;21)
leukaemogenesis. EMBO Mol Med. 9:933–949. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Palande KK, Beekman R, van der Meeren LE,
Beverloo HB, Valk PJ and Touw IP: The antioxidant protein
peroxiredoxin 4 is epigenetically down regulated in acute
promyelocytic leukemia. PLoS One. 6:e163402011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chen ZH, Zhu M, Yang J, Liang H, He J, He
S, Wang P, Kang X, McNutt MA, Yin Y, et al: PTEN interacts with
histone H1 and controls chromatin condensation. Cell Rep.
8:2003–2014. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lund K, Adams PD and Copland M: EZH2 in
normal and malignant hematopoiesis. Leukemia. 28:44–49. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ntziachristos P, Tsirigos A, Welstead GG,
Trimarchi T, Bakogianni S, Xu L, Loizou E, Holmfeldt L, Strikoudis
A, King B, et al: Contrasting roles of histone 3 lysine 27
demethylases in acute lymphoblastic leukaemia. Nature. 514:513–517.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Psvv C, Joseph A, Ebenezer P, Sankar V,
Suravajhala R, Rao RSP and Suravajhala P: An introduction to
non-coding RNAs. Prog Mol Biol Transl Sci. 214:1–17. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yang J, Liang F, Zhang F, Zhao H, Gong Q
and Gao N: Recent advances in the reciprocal regulation of m6A
modification with non-coding RNAs and its therapeutic application
in acute myeloid leukemia. Pharmacol Ther. 259:1086712024.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Liu Y, Cheng Z, Pang Y, Cui L, Qian T,
Quan L, Zhao H, Shi J, Ke X and Fu L: Role of microRNAs, circRNAs
and long noncoding RNAs in acute myeloid leukemia. J Hematol Oncol.
12:512019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Shi H, Wei J and He C: Where, when, and
how: Context-dependent functions of RNA methylation writers,
readers, and erasers. Mol Cell. 74:640–650. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Murai M, Toyota M, Satoh A, Suzuki H,
Akino K, Mita H, Sasaki Y, Ishida T, Shen L, Garcia-Manero G, et
al: Aberrant DNA methylation associated with silencing BNIP3 gene
expression in haematopoietic tumours. Br J Cancer. 92:1165–1172.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hasegawa D, Manabe A, Kubota T, Kawasaki
H, Hirose I, Ohtsuka Y, Tsuruta T, Ebihara Y, Goto Y, Zhao XY, et
al: Methylation status of the p15 and p16 genes in paediatric
myelodysplastic syndrome and juvenile myelomonocytic leukaemia. Br
J Haematol. 128:805–812. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Schwarzer A, Emmrich S, Schmidt F, Beck D,
Ng M, Reimer C, Adams FF, Grasedieck S, Witte D, Käbler S, et al:
The non-coding RNA landscape of human hematopoiesis and leukemia.
Nat Commun. 8:2182017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Mardani R, Jafari Najaf Abadi MH, Motieian
M, Taghizadeh-Boroujeni S, Bayat A, Farsinezhad A, Gheibi Hayat SM,
Motieian M and Pourghadamyari H: MicroRNA in leukemia: Tumor
suppressors and oncogenes with prognostic potential. J Cell
Physiol. 234:8465–8486. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wallace JA and O'Connell RM: MicroRNAs and
acute myeloid leukemia: Therapeutic implications and emerging
concepts. Blood. 130:1290–1301. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yan J, Yao L, Li P, Wu G and Lv X: Long
non-coding RNA MIR17HG sponges microRNA-21 to upregulate PTEN and
regulate homoharringtonine-based chemoresistance of acute myeloid
leukemia cells. Oncol Lett. 23:242022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Amodio N, Rossi M, Raimondi L, Pitari MR,
Botta C, Tagliaferri P and Tassone P: miR-29s: A family of
epi-miRNAs with therapeutic implications in hematologic
malignancies. Oncotarget. 6:12837–12861. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wei Y, Lu W, Yu Y, Zhai Y, Guo H, Yang S,
Zhao C, Zhang Y, Liu J, Liu Y, et al: miR-29c&b2 encourage
extramedullary infiltration resulting in the poor prognosis of
acute myeloid leukemia. Oncogene. 40:3434–3448. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Schmid VK, Khadour A, Ahmed N, Brandl C,
Nitschke L, Rajewsky K, Jumaa H and Hobeika E: B-cell antigen
receptor expression and phosphatidylinositol 3-kinase signaling
regulate genesis and maintenance of mouse chronic lymphocytic
leukemia. Haematologica. 107:1796–1814. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Akbarzadeh M, Mihanfar A, Akbarzadeh S,
Yousefi B and Majidinia M: Crosstalk between miRNA and
PI3K/AKT/mTOR signaling pathway in cancer. Life Sci.
285:1199842021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Yadav P, Bandyopadhayaya S, Ford BM and
Mandal C: Interplay between DNA Methyltransferase 1 and microRNAs
during tumorigenesis. Curr Drug Targets. 22:1129–1148. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhang TJ, Zhang LC, Xu ZJ and Zhou JD:
Expression and prognosis analysis of DNMT family in acute myeloid
leukemia. Aging (Albany NY). 12:14677–14690. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Carraway HE, Malkaram SA, Cen Y, Shatnawi
A, Fan J, Ali HEA, Abd Elmageed ZY, Buttolph T, Denvir J, Primerano
DA and Fandy TE: Activation of SIRT6 by DNA hypomethylating agents
and clinical consequences on combination therapy in leukemia. Sci
Rep. 10:103252020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Richter WF, Shah RN and Ruthenburg AJ:
Non-canonical H3K79me2-dependent pathways promote the survival of
MLL-rearranged leukemia. Elife. 10:e649602021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Huang FL, Yu SJ and Li CL: Role of
autophagy and apoptosis in acute lymphoblastic leukemia. Cancer
Control. 28:107327482110191382021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Boustani H, Khodadi E and Shahidi M:
Autophagy in hematological malignancies: Molecular aspects in
leukemia and lymphoma. Lab Med. 52:16–23. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Shu F, Xiao H, Li QN, Ren XS, Liu ZG, Hu
BW, Wang HS, Wang H and Jiang GM: Epigenetic and post-translational
modifications in autophagy: Biological functions and therapeutic
targets. Signal Transduct Target Ther. 8:322023. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Du W, Xu A, Huang Y, Cao J, Zhu H, Yang B,
Shao X, He Q and Ying M: The role of autophagy in targeted therapy
for acute myeloid leukemia. Autophagy. 17:2665–2679. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhang N, Xue M, Sun T, Yang J, Pei Z and
Qin K: Fucoidan as an autophagy regulator: Mechanisms and
therapeutic potentials for cancer and other diseases. Nutr Cancer.
74:1568–1579. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Yu H, Zhang Q, Farooqi AA, Wang J, Yue Y,
Geng L and Wu N: Opportunities and challenges of fucoidan for
tumors therapy. Carbohydr Polym. 324:1215552024. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Aanniz T, Bouyahya A, Balahbib A, El Kadri
K, Khalid A, Makeen HA, Alhazmi HA, El Omari N, Zaid Y, Wong RS, et
al: Natural bioactive compounds targeting DNA methyltransferase
enzymes in cancer: Mechanisms insights and efficiencies. Chem Biol
Interact. 392:1109072024. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ow SH, Chua PJ and Bay BH: Epigenetic
regulation of peroxiredoxins: Implications in the pathogenesis of
cancer. Exp Biol Med (Maywood). 242:140–147. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Nepstad I, Hatfield KJ, Grønningsæter IS
and Reikvam H: The PI3K-Akt-mTOR signaling pathway in human acute
myeloid leukemia (AML) cells. Int J Mol Sci. 21:29072020.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Bernardo VS, Torres FF, de Paula CP, da
Silva JPMO, de Almeida EA, da Cunha AF and da Silva DGH: Potential
cytoprotective and regulatory effects of ergothioneine on gene
expression of proteins involved in erythroid adaptation mechanisms
and redox pathways in K562 Cells. Genes (Basel). 13:23682022.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
van Weelden G, Bobiński M, Okła K, van
Weelden WJ, Romano A and Pijnenborg JMA: Fucoidan structure and
activity in relation to anti-cancer mechanisms. Mar Drugs.
17:322019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Li Y, Chen X and Lu C: The interplay
between DNA and histone methylation: Molecular mechanisms and
disease implications. EMBO Rep. 22:e518032021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Huang W, Li H, Yu Q, Xiao W and Wang DO:
LncRNA-mediated DNA methylation: An emerging mechanism in cancer
and beyond. J Exp Clin Cancer Res. 41:1002022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wang S, Wu W and Claret FX: Mutual
regulation of microRNAs and DNA methylation in human cancers.
Epigenetics. 12:187–197. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Afgar A, Ramezani Zadeh Kermani M, Pabarja
A, Afgar AR, Kavyani B, Arezoomand H, Zanganeh S, Sanaei MJ,
Sattarzadeh Bardsiri M and Vahidi R: 6-Gingerol modulates miRNAs
and PODXL gene expression via methyltransferase enzymes in NB4
cells: An in silico and in vitro study. Sci Rep. 14:183562024.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Chen YL, Zhang ZX, Shou LH and Di JY:
Regulation of DNA methylation and tumor suppression gene expression
by miR-29b in leukemia patients and related mechanisms. Eur Rev Med
Pharmacol Sci. 22:158–165. 2018.PubMed/NCBI
|
|
96
|
Yang Y, Hassan SHA, Awasthi MK, Gajendran
B, Sharma M, Ji M-K and Salama El-S: The recent progress on the
bioactive compounds from algal biomass for human health
applications. Food Bioscience. 51:1022672023. View Article : Google Scholar
|
|
97
|
Geng H, Chen M, Guo C, Wang W and Chen D:
Marine polysaccharides: Biological activities and applications in
drug delivery systems. Carbohydr Res. 538:1090712024. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Mustafa S, Pawar JS and Ghosh I: Fucoidan
induces ROS-dependent epigenetic modulation in cervical cancer HeLa
cell. Int J Biol Macromol. 181:180–192. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
DesJarlais R and Tummino PJ: Role of
histone-modifying enzymes and their complexes in regulation of
chromatin biology. Biochemistry. 55:1584–1599. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Kim J, Lee H, Yi SJ and Kim K: Gene
regulation by histone-modifying enzymes under hypoxic conditions: A
focus on histone methylation and acetylation. Exp Mol Med.
54:878–889. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Deng Y, Cheng Q and He J: HDAC inhibitors:
Promising agents for leukemia treatment. Biochem Biophys Res
Commun. 680:61–72. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Wu YW, Chao MW, Tu HJ, Chen LC, Hsu KC,
Liou JP, Yang CR, Yen SC, HuangFu WC and Pan SL: A novel dual HDAC
and HSP90 inhibitor, MPT0G449, downregulates oncogenic pathways in
human acute leukemia in vitro and in vivo. Oncogenesis. 10:392021.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Kanna R, Choudhary G, Ramachandra N,
Steidl U, Verma A and Shastri A: STAT3 inhibition as a therapeutic
strategy for leukemia. Leuk Lymphoma. 59:2068–2074. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Tan N, Luo H, Li W, Ling G, Wei Y, Wang W
and Wang Y: The dual function of autophagy in doxorubicin-induced
cardiotoxicity: Mechanism and natural products. Semin Cancer Biol.
109:83–90. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Zhang J, Sun Z, Lin N, Lu W, Huang X, Weng
J, Sun S, Zhang C, Yang Q, Zhou G, et al: Fucoidan from Fucus
vesiculosus attenuates doxorubicin-induced acute cardiotoxicity by
regulating JAK2/STAT3-mediated apoptosis and autophagy. Biomed
Pharmacother. 130:1105342020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Kato T, Shimono Y, Hasegawa M, Jijiwa M,
Enomoto A, Asai N, Murakumo Y and Takahashi M: Characterization of
the HDAC1 complex that regulates the sensitivity of cancer cells to
oxidative stress. Cancer Res. 69:3597–3604. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Yan H and Bu P: Non-coding RNA in cancer.
Essays Biochem. 65:625–639. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Bhat AA, Younes SN, Raza SS, Zarif L,
Nisar S, Ahmed I, Mir R, Kumar S, Sharawat SK, Hashem S, et al:
Role of non-coding RNA networks in leukemia progression, metastasis
and drug resistance. Mol Cancer. 19:572020. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Peschansky VJ and Wahlestedt C: Non-coding
RNAs as direct and indirect modulators of epigenetic regulation.
Epigenetics. 9:3–12. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Farooqi AA, Fayyaz S, Poltronieri P, Calin
G and Mallardo M: Epigenetic deregulation in cancer: Enzyme players
and non-coding RNAs. Semin Cancer Biol. 83:197–207. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Chuang YT, Yen CY, Tang JY, Wu KC, Chang
FR, Tsai YH, Chien TM and Chang HW: Marine anticancer drugs in
modulating miRNAs and antioxidant signaling. Chem Biol Interact.
399:1111422024. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Pradhan B, Patra S, Nayak R, Behera C,
Dash SR, Nayak S and Sahu BB: Multifunctional role of fucoidan,
sulfated polysaccharides in human health and disease: A journey
under the sea in pursuit of potent therapeutic agents. Int J Biol
Macromol. 164:4263–4278. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Gueven N, Spring KJ, Holmes S, Ahuja K,
Eri R, Park AY and Fitton JH: Micro RNA expression after ingestion
of Fucoidan; A clinical study. Mar Drugs. 18:1432020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Cui J, Zhou B, Ross SA and Zempleni J:
Nutrition, microRNAs, and Human Health. Adv Nutr. 8:105–112. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Zhang W, Park HB, Yadav D, Hwang J, An EK,
Eom HY, Kim SJ, Kwak M, Lee PC and Jin JO: Comparison of human
peripheral blood dendritic cell activation by four fucoidans. Int J
Biol Macromol. 174:477–484. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Riether C, Schürch CM and Ochsenbein AF:
Regulation of hematopoietic and leukemic stem cells by the immune
system. Cell Death Differ. 22:187–198. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
El-Far YM, Khodir AE, Emarah ZA, Ebrahim
MA and Al-Gayyar MMH: Fucoidan ameliorates hepatocellular carcinoma
induced in rats: Effect on miR143 and inflammation. Nutr Cancer.
73:1498–1510. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Atashrazm F, Lowenthal RM, Woods GM,
Holloway AF, Karpiniec SS and Dickinson JL: Fucoidan suppresses the
growth of human acute promyelocytic leukemia cells in vitro and in
vivo. J Cell Physiol. 231:688–697. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Sanjeewa KKA, Lee JS, Kim WS and Jeon YJ:
The potential of brown-algae polysaccharides for the development of
anticancer agents: An update on anticancer effects reported for
fucoidan and laminaran. Carbohydr Polym. 177:451–459. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Zhao Y, Wang Y, Chen L, Bai L and Guan S:
Co-immobilization of natural marine polysaccharides and bioactive
peptides on ZE21B magnesium alloy to enhance hemocompatibility and
cytocompatibility. Int J Biol Macromol. 272:1327472024. View Article : Google Scholar : PubMed/NCBI
|