Introduction
Low-density lipoprotein cholesterol (LDL-C) has long
been recognized as a central contributor to atherosclerosis, the
underlying pathology of most cases of cardiovascular disease (CVD).
Globally, CVD is the leading cause of morbidity and mortality
(1). For several decades, elevated
LDL-C levels have provided the cornerstone of our understanding of
atherogenesis, and LDL-C has been the principal target of
lipid-lowering therapies (2).
In the management of atherosclerotic CVD (ASCVD),
European and American guidelines differ regarding the preferred
lipid markers for risk assessment and therapeutic targets. European
guidelines, particularly those from the European Society of
Cardiology (ESC) and the European Atherosclerosis Society, have
increasingly advocated for the use of non-high density lipoprotein
cholesterol (non-HDL-C) and apolipoprotein B (ApoB) as superior
indicators of atherogenic lipoprotein burden (3–5).
Non-HDL-C encompasses all atherogenic particles, including LDL,
very low-density lipoprotein (VLDL), intermediate-density
lipoprotein (IDL) and lipoprotein(a) [Lp(a)]. Therefore, non-HDL-C
is a more comprehensive marker than LDL-C, particularly in patients
with hypertriglyceridemia or metabolic syndrome. ApoB, which
directly reflects the number of atherogenic lipoprotein particles,
offers additional precision in risk stratification, especially when
discordance exists between LDL-C levels and particle number
(6). By contrast, the American
College of Cardiology (ACC) and American Heart Association
guidelines emphasize LDL-C as the primary target of lipid-lowering
therapy, largely due to its extensive historical validation in
randomized controlled trials (7,8).
Although non-HDL-C and ApoB are both acknowledged as
secondary or optional markers, they are not routinely prioritized
in clinical practice in the USA. This divergence reflects
differences in clinical emphasis: European guidelines prioritize a
comprehensive assessment of atherogenic risk, particularly in
complex cases, whereas American guidelines maintain a simpler,
population-level focus based primarily on LDL-C. However, as
evidence continues to accumulate regarding the prognostic value of
non-HDL-C and ApoB, clinical practices may converge in future
guideline updates.
Despite the widespread use of statins and aggressive
LDL-C reduction strategies, a considerable number of patients
continue to experience cardiovascular events, a phenomenon referred
to as residual risk (9,10). This has prompted researchers and
clinicians to look beyond total LDL-C and explore the heterogeneity
of LDL particles, particularly the role of small dense LDL (sdLDL),
in the pathogenesis of CVD (10,11).
sdLDL is a subfraction of LDL where the particles
are smaller in size, more compact in structure, and more
atherogenic compared with their larger, more buoyant LDL
counterparts (12–14). Although sdLDL comprises a
relatively small proportion of the total LDL mass, it has a
disproportionately large impact on vascular health (15,16).
Its unique metabolic and physicochemical characteristics, including
increased arterial wall penetration, prolonged plasma half-life and
heightened susceptibility to oxidative modification, render it
particularly potent in promoting endothelial dysfunction, foam cell
formation, inflammation and plaque instability (12). Unlike total LDL-C, which provides a
general estimate of cholesterol load, sdLDL is reflective of
vascular injury via an insidious lipid-driven mechanism (17,18).
The clinical significance of sdLDL is clearly
apparent in individuals with metabolic disorders (18). Patients with type 2 diabetes,
insulin resistance, obesity and metabolic syndrome often present
with a so-called lipid paradox, defined as having relatively normal
LDL-C levels but a high burden of sdLDL particles (19). In such cases, traditional lipid
profiles may underestimate cardiovascular risk, resulting in missed
opportunities for preventive intervention. This growing recognition
of sdLDL as a key contributor to residual cardiovascular risk has
started to challenge existing paradigms in cardiovascular risk
stratification and treatment, leading to the suggestion that a more
detailed, particle-based approach to lipidology is necessary
(20,21).
Despite the burgeoning evidence of its atherogenic
potential, sdLDL remains underutilized in routine clinical
practice. Its more widespread adoption has been restricted by a
number of barriers, including limited access to advanced lipid
testing, a lack of standardized measurement protocols and
inadequate integration into clinical guidelines (15,22).
Nevertheless, given advances in diagnostic technology, the
increasing availability of targeted therapies, and a shift towards
precision medicine, sdLDL is on the cusp of transitioning from
being primarily a research interest to recognition as a clinically
actionable biomarker.
The present review aims to provide a comprehensive
overview of sdLDL as an underestimated driver of atherosclerosis.
It explores the mechanistic underpinnings of sdLDL-induced vascular
damage, evaluates its diagnostic value and limitations in clinical
settings, and examines current and emerging treatment strategies
aimed at reducing sdLDL burden. Furthermore, the review discusses
future directions and the integration of sdLDL into risk assessment
models and personalized therapeutic pathways. Via this approach, it
highlights the importance of reconsidering how lipid-associated
cardiovascular risk should be assessed and treated.
The atherogenicity of sdLDL
sdLDL particles represent a highly atherogenic
subfraction of LDL-C that contributes disproportionately to the
initiation and progression of atherosclerosis (13,23).
Although these particles are a minor component of total LDL, their
biological behavior renders them potent drivers of vascular
pathology (24). Understanding the
atherogenic nature of sdLDL requires consideration of the
structural features of these particles, along with their metabolic
characteristics, interactions with the arterial wall, and role in
endothelial dysfunction and plaque formation (24–26).
There is currently no universally accepted cut-off
for defining an elevated sdLDL particle concentration. This lack of
standardization complicates the interpretation of sdLDL elevation
in different populations and clinical contexts (26). Metabolic remodeling of LDL results
in sdLDL particles that are cholesterol-depleted, triglyceride-rich
and prone to oxidation (24).
Critically, sdLDL particles have a prolonged half-life in plasma
due to reduced affinity for the LDL receptor. This leads to the
particles existing for a prolonged time in circulation, thereby
increasing the likelihood of them undergoing further atherogenic
modifications (27,28) (Table
I).
 | Table I.Summary of key concepts in the
atherogenicity of sdLDL. |
Table I.
Summary of key concepts in the
atherogenicity of sdLDL.
| Aspect | Key points | Advantages | Challenges | (Refs.) |
|---|
| Definition and
relevance | sdLDL is a small,
dense sub-fraction of LDL-C with disproportionately high
atherogenic potential | Recognizing sdLDL
explains residual cardiovascular risk despite normal LDL-C | Not routinely
measured in clinical practice | (14) |
| Size and
structure | 18-20.5 nm
diameter; dense, triglyceride-rich, cholesterol-poor particles with
prolonged plasma residence time | Explains enhanced
atherogenic potential compared with that of larger LDL | Requires
specialized testing, e.g., NMR or gradient gel electrophoresis | (26) |
| Metabolic
characteristics | Poor LDL receptor
affinity; more likely to remain in circulation and undergo
oxidative modification | Identifies patients
with increased oxidative risk and prolonged lipid exposure | No standardized
thresholds across populations; high variability | (24) |
| Endothelial
penetration | Easily traverses
the endothelium and binds arterial wall proteoglycans | Helps explain early
subendothelial retention in atherogenesis | Challenging to
assess or visualize directly in vivo | (24) |
| Susceptibility to
oxidation | Enriched in
polyunsaturated fats and depleted in antioxidants such as vitamin
E, making it prone to oxidation | Oxidized sdLDL
triggers immune and inflammatory responses | Not distinguished
by standard lipid panels; requires indirect evidence | (31) |
| Pathological
effects of oxLDL | Promotes
endothelial dysfunction, inflammation and foam cell formation | Target for
anti-inflammatory and antioxidant therapies | Lack of specific
inhibitors of sdLDL oxidation in current practice | (32) |
| Inflammatory and
thrombotic role | Activates NF-κB,
promotes the release of cytokines, e.g., IL-6 and TNF-α, and
promotes platelet aggregation | Links lipid
abnormalities with systemic inflammation and thrombosis | Mechanistic
complexity makes therapeutic targeting challenging | (36) |
| Endothelial
dysfunction | Reduces nitric
oxide production; increases oxidative stress and vascular tone
loss | Early marker of
vascular injury; potential target for early interventions | Few treatments
directly reverse endothelial dysfunction associated with sdLDL | (29) |
| Plaque
instability | Associated with
thin fibrous caps, large necrotic cores, and macrophage-rich
plaques, which are features of rupture-prone lesions | Provides insight
into acute coronary syndrome pathogenesis | Imaging or
detecting sdLDL-rich plaques in vivo is limited | (48) |
| Clinical
associations | Elevated in
metabolic syndrome, type 2 diabetes, insulin resistance and
obesity, even when LDL-C appears normal | Identifies patients
with hidden high-risk beyond LDL-C levels | Frequently missed
in standard risk assessments | (93) |
| Epidemiological
evidence | Independently
associated with cardiovascular events, e.g., in the Quebec
Cardiovascular study and ARIC study | Strong
justification for inclusion in future risk prediction models | Longitudinal data
required to determine whether sdLDL reduction improves
outcomes | (52) |
| Clinical
implications | Should be
considered a therapeutic and diagnostic target in modern
cardiometabolic care | Guides precision
medicine strategies and residual risk management | Integration into
guidelines, broader awareness among clinicians, and cost-effective
testing are necessary | (24) |
A defining feature of sdLDL is its enhanced ability
to penetrate the endothelial barrier and accumulate within the
subendothelial space of arteries. Beyond their smaller size, sdLDL
particles exhibit altered surface characteristics-such as reduced
sialic acid content, increased negative charge, and lower
phospholipid content-that facilitate passage across the endothelium
(14,29). Once within the arterial intima,
these modifications also confer a stronger binding affinity to
proteoglycans in the extracellular matrix, promoting their
retention and thereby amplifying their atherogenic potential.
The particles become trapped by arterial wall
proteoglycans and, once retained, are highly susceptible to
oxidative modification, a key initiating event in atherogenesis
(15). This susceptibility to
oxidation is further increased by a relative lack of endogenous
antioxidants such as vitamin E, and by a lipid core enriched in
polyunsaturated fatty acids that are prone to peroxidation
(30).
Oxidized sdLDL (oxLDL) has been shown to exert a
range of pathological effects (13,16,31)
(Table II). It promotes
endothelial dysfunction by reducing nitric oxide bioavailability
and increasing the expression of vascular adhesion molecules,
including vascular cell adhesion molecule-1 and intercellular
adhesion molecule-1 (22,30,32).
The adhesion molecules facilitate the recruitment of circulating
monocytes, which then migrate into the arterial intima and
differentiate into macrophages. These macrophages then internalize
oxLDL via scavenger receptors, becoming lipid-laden foam cells that
form the basis of early atherosclerotic plaques (32–35).
 | Table II.Summary of the cellular and molecular
mechanisms of sdLDL in atherogenesis. |
Table II.
Summary of the cellular and molecular
mechanisms of sdLDL in atherogenesis.
| Mechanism | Key features | Molecular players
and outcomes | (Refs.) |
|---|
| Structural
features | Small size (15–20
nm), dense core, triglyceride-rich, cholesterol-depleted | Increased arterial
permeability; prolonged circulation due to reduced LDL receptor
affinity | (24) |
| Endothelial
dysfunction | Enhanced
endothelial penetration via proteoglycan binding | ↑ Oxidative stress;
↓ nitric oxide bioavailability; ↑ VCAM-1/ICAM-1 expression | (51) |
| Oxidative
susceptibility | Lipid core rich in
polyunsaturated fatty acids; low antioxidant content | Rapid oxidation to
oxLDL; activation of scavenger receptors, e.g., LOX-1 and CD36 | (40) |
| Foam cell
formation | Macrophage uptake
of oxidized sdLDL via scavenger receptors | Lipid-laden foam
cells; NLRP3 inflammasome activation; ↑ IL-1β and IL-6 | (35) |
| Inflammatory
signaling | NF-κB activation;
cytokine release | ↑ TNF-α, MCP-1;
recruitment of monocytes/macrophages; vascular inflammation | (42) |
| Plaque
instability | Promotion of
necrotic core formation; thin fibrous cap | Metalloproteinase
activation; smooth muscle cell apoptosis; ↑ plaque rupture
risk | (47) |
| Prothrombotic
effects | Platelet
activation; tissue factor expression | ↑ Fibrinogen; ↑
PAI-1; thrombus formation post-plaque rupture | (41) |
| Metabolic
interactions | Association with
insulin resistance, hypertriglyceridemia | ↑ ApoC-III; ↓ LDL
receptor clearance; CETP-mediated lipid exchange | (39) |
Beyond foam cell formation, sdLDL also contributes
to a pro-inflammatory and pro-thrombotic vascular environment
(22,24,26,36)
(Table II). It activates
transcription factors such as nuclear factor-κB (NF-κB), leading to
the upregulation of inflammatory cytokines, including interleukin-6
(IL-6), tumor necrosis factor-α and monocyte chemoattractant
protein-1 (37–42). This inflammatory response drives
further immune cell infiltration, promotes smooth muscle cell
proliferation, and contributes to extracellular matrix remodelling
(37,43,44).
In addition, sdLDL induces platelet aggregation and
promotes a pro-coagulant state, thereby increasing the risk of
thrombotic events, including myocardial infarction and ischemic
stroke (37,43).
Elevated levels of sdLDL are closely associated with
endothelial dysfunction (29), an
early marker of vascular injury. sdLDL impairs vasodilation by
reducing the synthesis of nitric oxide and increasing oxidative
stress within endothelial cells (38). This disruption of vascular
homeostasis facilitates further lipoprotein infiltration and immune
cell activation, thereby sustaining a self-reinforcing cycle of
injury and repair (45).
In a study by Miceli et al (46), the histological analysis of human
atherosclerotic plaques confirmed that lesions enriched in sdLDL
are more likely to exhibit features of instability, including thin
fibrous caps, large necrotic lipid cores and extensive inflammatory
cell infiltration (47). Such
features increase the likelihood of plaque rupture, an event that
can trigger acute coronary syndrome (ACS) and sudden cardiovascular
death (48).
Clinically, sdLDL concentrations are often elevated
in individuals with metabolic syndrome, type 2 diabetes, obesity
and insulin resistance, all of which are conditions characterized
by atherogenic dyslipidemia (39,40,49–51).
Notably, patients with these metabolic disturbances may present
with normal or near-normal LDL-C levels, while harboring a
predominance of sdLDL particles, thereby evading conventional risk
stratification. Epidemiological studies, including the Quebec
Cardiovascular Study and the Atherosclerosis Risk in Communities
(ARIC) Study, have demonstrated that elevated sdLDL levels are
independently associated with an increased cardiovascular risk,
even after adjustment for total LDL-C and other traditional risk
factors (52).
Given its distinct biological behaviour and strong
association with residual cardiovascular risk, sdLDL should be
considered a critical target for both diagnostic assessment and
therapeutic intervention in modern cardio-metabolic care.
Diagnostic significance and limitations in
clinical practice
Although total LDL-C has long been a cornerstone of
cardiovascular risk assessment and lipid-lowering strategies, the
recognition of sdLDL as a highly atherogenic subfraction has
prompted a critical re-evaluation of traditional diagnostic
paradigms. Increasing evidence suggests that conventional lipid
panels, which typically measure total cholesterol, LDL-C, HDL-C and
triglycerides, may not sufficiently capture the full spectrum of
lipoprotein-associated cardiovascular risk, particularly in
individuals with metabolic dysregulation (53,54).
In this context, sdLDL has emerged as a powerful and independent
predictor of ASCVD, especially in populations that may appear to be
at low risk according to conventional criteria (55–57)
(Table III).
 | Table III.Diagnostic relevance and clinical
limitations of sdLDL. |
Table III.
Diagnostic relevance and clinical
limitations of sdLDL.
| Aspect | Key insights and
clinical relevance | Limitations and
challenges | Solutions and
opportunities | (Refs.) |
|---|
| Clinical
importance | sdLDL is a highly
atherogenic subfraction linked to residual cardiovascular risk,
even in patients with normal LDL-C | Often overlooked in
standard lipid panels and clinical guidelines | Use sdLDL to
improve risk stratification, particularly in patients with
metabolic syndrome or diabetes | (14) |
| Risk
stratification | Independently
predicts ASCVD events and coronary calcification; validated in
large cohorts, e.g., Quebec, ARIC and MESA | Predictive value
may vary by population or sdLDL threshold | Combine sdLDL with
other markers, e.g., apoB, LDL-P and/or HDL-C, for a more
comprehensive lipid risk profile | (56) |
| Measurement
methods | Techniques include
NMR, electrophoresis, precipitation, and enzymatic assays; direct
methods are becoming more accessible | High cost, low
availability, and lack of inter-laboratory standardization | Use validated,
standardized methods and maintain consistency across serial
testing | (20) |
| Cut-off values | sdLDL thresholds
differ by method and population; no universal reference ranges
exist | Lack of standard
cut-offs limits clinical decision-making | Develop and adopt
population-specific reference values; interpret results
contextually | (62) |
| Association with
diabetes | sdLDL is commonly
elevated in type 2 diabetes and contributes to cardiovascular
risk | Not all diabetic
patients show increased risk from sdLDL; context matters | Interpret alongside
glycemic control and other lipid metrics; use as an adjunct, not a
replacement | (58) |
| Therapeutic
use | Helps identify
residual risk in statin-treated patients; may inform therapy
intensification | No defined target
levels or verified benefits from lowering sdLDL directly | Use sdLDL to guide
treatment in high-risk patients pending outcome data | (78) |
| Pre-analytical
considerations | Stable in frozen
serum for up to 30 days; fasting preferred for accuracy | Hemolysis, lipemia
or icterus can compromise results; non-fasting samples reduce
accuracy | Standardize patient
preparation and reject samples that are compromised | (59) |
| Indirect
surrogates | Non-HDL-C and apoB
correlate with sdLDL and are practical for routine use | Not specific to
sdLDL and may underrepresent sdLDL burden in certain cases | Use when sdLDL
testing is unavailable; supplement with direct sdLDL if
necessary | (57) |
| Emerging tools | Tools such as the
LP-IR index and advanced sub-fraction profiling aid in precision
risk assessment | Limited clinical
validation and lack of interpretative guidelines | Investigate in
research; potential role in individualized therapy | (94) |
| Clinical
integration | sdLDL testing adds
value in selected patients with unexplained residual risk or
complex lipid profiles | Not incorporated
into current guidelines or risk calculators | Use as a
supplemental test, particularly in metabolic syndrome, lean
obesity, or discordant lipid phenotypes | (61) |
Numerous epidemiological studies have shown that
elevated sdLDL levels are strongly associated with an increased
risk of major adverse cardiovascular events, even after adjustment
for total LDL-C and other conventional risk factors (25,52,58,59).
This predictive strength is particularly notable in individuals
with metabolic syndrome, type 2 diabetes and insulin resistance, in
whom sdLDL concentrations are often elevated despite normal LDL-C
levels. In these populations, sdLDL is frequently accompanied by
high triglyceride and low HDL-C levels, forming a pro-atherogenic
lipid profile that accelerates plaque development and increases the
risk of plaque instability and cardiovascular events (20,39,60,61).
The clinical utility of sdLDL as a risk marker is
closely associated with its sensitivity and specificity. sdLDL
demonstrates a high sensitivity for detecting an increased ASCVD
risk in patients with metabolic dysfunction or residual risk, even
when LDL-C is optimally controlled (56,62).
Its small particle size, greater arterial wall penetration and
heightened susceptibility to oxidation allow sdLDL to reflect early
vascular injury and subclinical atherosclerosis that may not be
captured by standard lipid panels. However, the specificity of
sdLDL is context-dependent (61).
Although elevated sdLDL is highly specific for increased
cardiovascular risk in high-risk groups such as diabetics and
individuals with metabolic syndrome, its specificity may be lower
in the general population, where sdLDL levels can overlap between
healthy and at-risk individuals (24,60,63).
Furthermore, assay variability and lack of standardized cut-off
values can affect both sensitivity and specificity, underscoring
the requirement for harmonized measurement protocols.
The diagnostic performance of sdLDL is enhanced when
combined with other biomarkers, such as ApoB, or inflammatory
markers, such as high-sensitivity C-reactive protein (hs-CRP),
which can improve sensitivity and specificity when identifying
individuals with the highest risk (64,65).
Despite its clear prognostic value, the routine
measurement of sdLDL in clinical practice remains limited, largely
due to technical barriers, a lack of standardization and its
absence from most current guidelines. Nevertheless, in select
high-risk populations, particularly those with persistent
cardiovascular risk despite optimal LDL-C levels, the
quantification of sdLDL may provide valuable additional prognostic
information to guide personalized treatment strategies. These
factors indicate that sdLDL may be considered as a sensitive and
clinically meaningful marker for ASCVD risk, particularly in
patients with metabolic abnormalities or unexplained residual risk.
Standardizing sdLDL assays and integrating sdLDL measurement into
risk assessment algorithms may facilitate early detection and
enable more targeted interventions for those patients at the
greatest risk of cardiovascular events.
A major diagnostic implication of sdLDL is that it
can identify individuals with elevated residual cardiovascular
risk, specifically those whose total LDL-C levels fall within the
normal or near-normal range but who harbour a high concentration of
small, dense and highly atherogenic LDL particles. This situation
is frequently observed in patients with type 2 diabetes, insulin
resistance, obesity, metabolic syndrome and certain individuals
with so-called ‘lean’ metabolic obesity (66). In such cases, relying on the
standard LDL-C metric may give a false sense of security, while
elevated sdLDL levels silently contribute to subclinical
atherosclerosis (67).
Numerous epidemiological and clinical studies have
demonstrated the independent prognostic value of sdLDL (68–70).
For example, the Quebec Cardiovascular Study showed that high
concentrations of sdLDL were strongly associated with an increased
risk of ischemic heart disease, even after adjusting for LDL-C and
other conventional risk factors (71,72).
Similar associations were observed in the ARIC study and the
Multi-Ethnic Study of Atherosclerosis, where sdLDL predicted
coronary artery calcium progression and cardiovascular events
(73,74).
These findings underscore the importance of moving
beyond a simple assessment of total cholesterol, and also
considering lipoprotein quality and particle size distribution when
performing a clinical evaluation (75,76).
Importantly, sdLDL may contribute to residual
cardiovascular risk in patients with otherwise normal LDL-C levels.
Numerous studies have shown that individuals with elevated sdLDL
levels can experience major adverse cardiovascular events, even
when standard lipid metrics appear to be well controlled (77–79).
This has spurred interest in sdLDL as a potential
marker of hidden risk, particularly in patients with premature
coronary artery disease, recurrent events or a poor response to
statin therapy (78,80).
In addition, sdLDL has been associated with specific
clinical manifestations of ASCVD, including ACS and coronary artery
calcification (81,82). Higher sdLDL concentrations have
been shown to be associated with greater plaque burden, more
extensive coronary stenosis and higher levels of circulating
inflammatory markers, including hs-CFP and IL-6.
These associations suggest that sdLDL is not merely
a marker of risk but may also play an active role in the
progression and destabilization of atherosclerotic plaques. Despite
compelling evidence, the adoption of sdLDL measurement in routine
clinical practice remains limited. Current guidelines continue to
prioritize LDL-C, non-HDL-C and ApoB, partly due to the lack of
standardization in sdLDL assays and the scarcity of outcome-based
intervention trials. However, in select high-risk populations,
particularly those with metabolic dysfunction or persistent
cardiovascular risk despite optimal LDL-C control, sdLDL
quantification may offer additional prognostic value and support
more personalized treatment strategies.
As already discussed, despite its clinical
importance, the routine measurement of sdLDL remains limited in
practice (Table III). A key
barrier is the lack of standardization in analytical techniques.
Several methods are available to quantify sdLDL, including gradient
gel electrophoresis, ultracentrifugation, nuclear magnetic
resonance (NMR) spectroscopy and ion mobility analysis (83). Each technique has its own
advantages and limitations in terms of cost, accessibility,
resolution and reproducibility. Among these, gradient gel
electrophoresis and NMR spectroscopy are the most commonly applied
in research settings (84);
however, these techniques are not widely available in standard
clinical laboratories and are often cost-prohibitive for routine
use. Furthermore, there is currently no universally accepted
cut-off value for defining elevated sdLDL concentrations. This lack
of standardization complicates interpretation across different
populations and clinical contexts. In addition, sdLDL levels are
dynamic and can be influenced by diet, insulin sensitivity, weight
changes, physical activity and pharmacological interventions
(85). Consequently, interpreting
a single measurement in isolation may not reflect long-term
cardiovascular risk, unless values are monitored longitudinally or
assessed alongside other metabolic parameters.
In clinical practice, several indirect markers have
been proposed as surrogates for sdLDL burden. These include
non-HDL-C, which has gained particular attention as a more
comprehensive risk marker compared with LDL-C alone. By
encompassing the total concentration of atherogenic lipoproteins,
including LDL, VLDL, IDL and Lp(a), non-HDL-C correlates moderately
well with sdLDL levels, and has been shown to more reliably reflect
the overall atherogenic risk (86).
ApoB100 is another widely used surrogate. ApoB100 is
a structural protein present on all liver-derived atherogenic
lipoproteins, including VLDL, IDL and LDL. Since each of these
particles carries a single molecule of ApoB100, its measurement
serves as a reliable proxy for the total number of circulating
atherogenic particles. However, an important but often overlooked
limitation is that ApoB100 does not account for all atherogenic
lipoproteins, particularly those of intestinal origin (87,88).
For example, VLDL remnants, which are partially
lipolyzed derivatives obtained during VLDL metabolism, contain
ApoB100 and have well-documented atherogenic potential (88). These remnants can bind to arterial
wall proteoglycans, become retained in the subendothelial space,
and are subsequently internalized by macrophages. This process
activates Toll-like receptors 2 and 4 (TLR2 and TLR4), leading to
localized inflammation and the progression of atherosclerosis. By
contrast, chylomicron remnants, which originate from dietary fat
metabolism in the intestine, carry ApoB48 rather than ApoB100
(89). Although not detected by
ApoB100-based assays, chylomicron remnants contribute to
atherogenesis through distinct, yet equally pathogenic mechanisms.
They have been shown to activate TLR4 and the downstream NF-κB
signaling pathway, thereby promoting endothelial dysfunction,
monocyte adhesion and foam cell formation, which are key features
of early atheromatous plaque development (90,91).
This highlights a fundamental limitation of relying
solely on ApoB100 as a marker of atherogenic particle burden.
Although ApoB100 reliably quantifies liver-derived lipoproteins, it
fails to reflect the contribution of intestinally derived
particles, such as chylomicron remnants. Consequently, a more
nuanced approach to lipoprotein profiling may be necessary in
specific clinical contexts, particularly in patients with
postprandial dyslipidemia, insulin resistance or metabolic
syndrome, where chylomicron remnants may play a disproportionately
larger role in atherogenesis (92,93).
While these markers are not specific to sdLDL, they are practical
and cost-effective tools for the estimation of atherogenic risk in
settings where direct sdLDL quantification is not possible.
Emerging approaches, such as use of the Lipoprotein
Insulin Resistance Index and advanced lipoprotein subfraction
testing using ion mobility or NMR spectroscopy, may soon provide
clinicians with more nuanced insights into lipoprotein metabolism
(94). However, these technologies
must be carefully integrated into clinical workflows, with the
establishment of clear guidelines for their interpretation and
application in clinical decision-making.
The role of sdLDL in risk stratification and
treatment intensification is a critical factor requiring
consideration (95). Although
current guidelines prioritize LDL-C as the primary target for
lipid-lowering therapy, individuals with high sdLDL may benefit
from more aggressive lifestyle and pharmacological interventions,
even when their LDL-C levels appear to be acceptable. This raises
the question of whether sdLDL should be incorporated into risk
calculators or treatment algorithms, an area that remains
underexplored in current cardiovascular guidelines.
In summary, the diagnostic significance of sdLDL is
substantial and growing, particularly in the context of
cardiometabolic diseases. Its presence signifies a hidden yet
potent atherogenic threat that may be undetected by standard lipid
screening. However, limitations in measurement standardization,
cost, accessibility and clinical integration continue to hinder its
routine application. As our understanding of lipidology deepens,
and precision medicine becomes more central to cardiovascular
prevention, sdLDL is likely to gain prominence as both a biomarker
and a therapeutic target. Bridging the gap between scientific
evidence and clinical implementation is essential to harness the
full diagnostic power of sdLDL in the fight against atherosclerosis
and CVD.
Targeted treatment strategies for sdLDL
reduction
The clinical recognition of sdLDL as a highly
atherogenic lipoprotein subclass has prompted growing interest in
the development and refinement of therapeutic strategies that
specifically address elevated sdLDL concentrations. Traditional
lipid-lowering therapies have largely focused on the reduction of
total LDL-C, although a burgeoning body of evidence suggests that
not all therapies exert equivalent effects on sdLDL concentrations
or particle composition (67,96).
As our understanding of the role of sdLDL in atherogenesis deepens,
a more nuanced and tailored approach to treatment is likely to
become both necessary and feasible (Fig. 1).
Lifestyle modification remains the primary strategy
for managing increases in sdLDL levels. Among non-pharmacological
interventions, dietary changes, particularly those that reduce
simple carbohydrate intake and improve insulin sensitivity, have
been shown to significantly impact sdLDL particles (97). Diets low in refined sugars and rich
in monounsaturated fats, such as the Mediterranean diet, can induce
a change in the LDL particle distribution towards larger, less
atherogenic forms (98). A modest
weight loss of 5–10% body weight and increased physical activity
substantially reduce sdLDL levels, particularly in
insulin-resistant individuals (99). These findings reinforce the
suggestion that sdLDL is not merely a lipid abnormality but also a
marker of broader metabolic dysfunction.
Pharmacologically, statins remain the cornerstone of
lipid-lowering therapy, as they can effectively reduce total LDL-C
and sdLDL concentrations to a certain extent (100) (Fig.
1). However, their effect on sdLDL is variable, and often less
pronounced than their effect on larger LDL particles (101,102). Statins primarily act by
upregulating LDL receptors, which preferentially clear larger, less
dense LDL particles, leaving some sdLDL particles in circulation
(103,104). Nevertheless, when combined with
other agents or used in high-intensity regimens, statins can
contribute meaningfully to sdLDL reduction (105).
Fibrates, which activate peroxisome
proliferator-activated receptor (PPAR)-α, are among the most
effective agents for lowering sdLDL, particularly in individuals
with hypertriglyceridemia and mixed dyslipidemia (106–108). By reducing triglyceride-rich
lipoproteins and promoting lipolysis, fibrates shift the LDL
particle distribution from small dense particles to larger and more
buoyant forms. Clinical studies, such as the Veterans Affairs
High-Density Lipoprotein Intervention Trial (109) and the Fenofibrate Intervention
and Event Lowering in Diabetes trial (110) have demonstrated cardiovascular
benefits in high-risk patients, although the use of pioglitazone
must be balanced against potential side effects, including weight
gain, edema and an increased risk of heart failure (111).
Niacin, also known as vitamin B3, has historically
been effective in reducing sdLDL levels, raising HDL-C levels, and
improving overall lipoprotein particle size. However, it is no
longer frequently used due to its side-effect profile and lack of
outcome benefits in clinical trials (24,112). Its use may still be considered in
select cases of severe atherogenic dyslipidemia, particularly when
other therapeutic options are insufficient or contraindicated.
Formulations of ω-3 fatty acids, particularly
icosapent ethyl, a highly purified form of eicosapentaenoic acid
(EPA) marketed as Vazkepa, have demonstrated consistent benefits in
lowering triglyceride levels and improving lipoprotein quality
(113). Beyond triglyceride
reduction, treatment with icosapent ethyl has been shown to shift
the LDL particle distribution toward larger, more buoyant LDL
particles, thereby decreasing the proportion of small dense LDL
(sdLDL), which are strongly associated with atherogenic risk.
Subanalyses of the REDUCE-IT trial and subsequent lipidomic studies
confirmed that EPA therapy not only reduces residual cardiovascular
risk in statin-treated patients but also exerts favorable effects
on LDL particle size and composition, contributing to a less
atherogenic lipid profile (114,115). These changes in lipoprotein
remodeling may represent one of the mechanistic pathways through
which icosapent ethyl provides cardiovascular protection,
independent of triglyceride lowering (116,117).
The Reduction of Cardiovascular Events With
Icosapent Ethyl-Intervention Trial reported significant reductions
in cardiovascular events in patients treated with EPA therapy,
suggesting that modulation of sdLDL levels and inflammation may
have contributed to the observed clinical benefits (118).
Proprotein convertase subtilisin/kexin type 9
(PCSK9) inhibitors, including alirocumab and evolocumab, primarily
lower LDL-C levels by enhancing receptor-mediated clearance.
Emerging evidence suggests they may also reduce sdLDL levels,
particularly when used in combination with statins (119–121). However, their high cost is a
barrier to widespread use, although they are increasingly
recommended for high-risk patients who have persistent dyslipidemia
despite receiving statin therapy.
Antisense oligonucleotides and small interfering RNA
therapies targeting apoB or angiopoietin-like protein 3 are under
investigation for their ability to modulate atherogenic
lipoproteins, including sdLDL (122). These experimental approaches
highlight the potential of precision lipid therapy, where targeting
specific molecular pathways may enable the individualized
management of atherogenic lipoprotein profiles.
While lowering sdLDL concentrations represents a
promising strategy for the reduction of ASCVD risk, it is essential
to consider potential side effects of intensive sdLDL-lowering
therapies, particularly with regard to the risk of bleeding. Most
traditional lipid-lowering agents, including statins, fibrates and
PCSK9 inhibitors, are not strongly associated with increased
bleeding. However, the broader impact that targeting sdLDL has on
hemostasis and vascular integrity is not completely understood,
especially given that novel pharmacological agents and combination
therapies are currently undergoing development. Probucol, an
antioxidative and lipid-lowering drug, exemplifies the complexity
of this issue. Historically used to treat atherosclerosis and
xanthomas, probucol effectively reduces LDL-C and sdLDL levels,
largely through its antioxidant properties and its effects on LDL
particle size and oxidation (123,124). Notably, a recent study by Lang
et al (125) suggested
that probucol may offer unique benefits for patients at high risk
of hemorrhage. The study suggested that probucol has potential as a
novel therapeutic option for cerebral infarction in patients with a
high risk of bleeding, as it appears to exert protective effects on
the vasculature without significantly increasing hemorrhagic
complications (125). This
observation is clinically relevant, as aggressively lowering the
lipid level, particularly with agents that alter platelet function
or endothelial stability, has sometimes been associated with an
increased risk of bleeding, particularly in patients receiving
concomitant antithrombotic therapy or those with cerebrovascular
disease (126,127).
The apparent safety of probucol in this context may
be attributed to its antioxidant effects, which stabilize
endothelial function and reduce oxidative stress, potentially
counteracting pro-hemorrhagic mechanisms. Nevertheless, it is
essential to carefully evaluate the safety profile of
sdLDL-targeting therapies in diverse patient populations. Although
probucol may be a potential therapeutic option for patients with
ASCVD and a high risk of bleeding, its use is limited by concerns
such as QT prolongation and other non-hemorrhagic adverse effects.
Furthermore, is is unclear whether the protective profile of
probucol can be generalized to other sdLDL-lowering agents.
Despite these therapeutic advances, the integration
of sdLDL-focused strategies into clinical practice guidelines
remains limited. Major guidelines, such as those from the ACC and
ESC, continue to prioritize LDL-C, non-HDL-C and apoB as primary
targets of therapy (128). While
sdLDL is recognized to increase cardiovascular risk, it has not yet
been incorporated into routine risk calculators or treatment
algorithms. This may be partly due to the lack of standardization
in sdLDL measurement, as well as limited outcome data directly
associating reductions in sdLDL concentration with hard
cardiovascular endpoints.
Despite its limited integration into guidelines,
there is growing support for the consideration of sdLDL
measurements in certain clinical scenarios. These include patients
with residual cardiovascular risk despite optimal LDL-C control,
individuals with metabolic syndrome or type 2 diabetes, and those
with premature or familial atherosclerotic disease. In such cases,
the presence of an increased concentration of sdLDL may justify the
intensification of therapy or the use of combination treatment
approaches, even in the absence of overt LDL-C elevation.
Targeted reduction of sdLDL is most effective and
clinically relevant during the chronic phase of ASCVD or in
individuals at high long-term risk, such as those with metabolic
syndrome, type 2 diabetes or persistent residual risk despite
optimal LDL-C control (20,129).
Chronic management allows sufficient time for
lifestyle modifications and pharmacological therapies, including
statins, fibrates, PCSK9 inhibitors, probucol and w-3 fatty acids,
to be implemented in order to exert their full lipid-modifying and
anti-atherogenic effects.
Sustained lowering of sdLDL concentration during the
chronic phase of disease has been associated with limited
atherosclerosis progression, the stabilization of vulnerable
plaques and a reduced incidence of major adverse cardiovascular
events (25,60).
By contrast, the acute phase of ASCVD, such as acute
coronary syndrome or stroke, is not the primary window for
sdLDL-targeted interventions. Although lipid-lowering therapy is
often initiated or intensified during acute events for secondary
prevention, the specific impact of sdLDL reduction in this setting
is less well established, as acute interventions are focused on
patient stabilization, and the management of thrombosis and
immediate complications. Nevertheless, the early initiation of
sdLDL-lowering therapy during hospitalization for an acute event
may lay the foundation for effective long-term risk reduction
(130,131).
Therefore, sdLDL-lowering strategies should be
prioritized for long-term risk reduction and the prevention of
recurrent events, with their main benefits being realized during
ongoing, chronic management.
To facilitate the broader adoption of sdLDL-guided
therapy, it is necessary to integrate sdLDL measurements into the
risk stratification frameworks outlined in clinical guidelines,
supported by randomized trials and real-world data demonstrating
the predictive value and therapeutic responsiveness of sdLDL
levels. In addition, the development of cost-effective standardized
assays for sdLDL quantification is essential to ensure clinical
feasibility. Table IV summarizes
both established and emerging strategies for sdLDL reduction,
highlighting their mechanisms of action, clinical roles and other
key considerations.
 | Table IV.Targeted treatment strategies for
reducing sdLDL levels. |
Table IV.
Targeted treatment strategies for
reducing sdLDL levels.
| Treatment | Type | Effect on
sdLDL | Mechanism of
action | Notes and clinical
considerations | (Refs.) |
|---|
| Lifestyle
modification |
Non-pharmacologic | Reduces sdLDL
concentrations; shifts to larger LDL particles | Improves insulin
sensitivity; reduces refined carbohydrate intake; increases
physical activity | Foundation of all
therapies; particularly effective in metabolic syndrome | (24) |
| Statins | Pharmacologic | Modest and variable
reduction in sdLDL | Upregulate LDL
receptors, preferentially clearing larger LDL particles | Optimum results in
combination or high-intensity regimens | (60) |
| Fibrates | Pharmacologic | Significant
reduction in sdLDL | Activate PPAR-α;
reduce triglycerides; enhance lipolysis; shift LDL to larger
particles | Particularly
effective in patients with high TG and low HDL-C | (108) |
| Pioglitazone | Pharmacologic | Reduces sdLDL;
improves lipoprotein profile | Activates PPAR-γ;
enhances insulin sensitivity; reduces inflammation; lowers TG and
increases HDL-C | Benefits high-risk
patients; side effects include weight gain and edema | (54) |
| Niacin/vitamin
B3 | Pharmacologic | Reduces sdLDL;
increases HDL-C; improves LDL particle size | Decreases hepatic
VLDL production; reduces lipolysis of adipose tissue | Limited use due to
side effects and lack of outcome benefit | (24) |
| w-3 Fatty acids
(EPA) | Pharmacologic | Modulates sdLDL and
reduces TG | Reduces hepatic TG
synthesis; alters LDL particle composition | Reduced
cardiovascular events in the REDUCE-IT phase III trial | (113) |
| PCSK9
inhibitors | Pharmacologic | May reduce sdLDL
when combined with statins | Increase LDL
receptor recycling; enhance LDL clearance | High cost;
recommended for high-risk patients with residual dyslipidemia | (121) |
| Antisense/siR NA
therapies, e.g., targeting apoB or ANGPTL3 | Experimental | Potential for sdLDL
reduction | Inhibit synthesis
of apoB or ANGPTL3; reduce atherogenic lipoprotein production | Emerging tools in
precision lipid management | (141) |
The targeting of sdLDL is an important evolution in
the management of dyslipidemia and cardiovascular disease
prevention. While traditional therapies influence sdLDL levels
indirectly, emerging pharmacological agents and lifestyle
strategies provide more specific and effective approaches. Bridging
the gap between sdLDL recognition and its inclusion in clinical
practice guidelines will be a key step toward precision
cardiovascular medicine. As the evidence base expands,
incorporating sdLDL into diagnostics and treatment may enhance our
ability to address residual risk and improve outcomes for patients
at high risk of atherosclerotic disease.
Artificial intelligence (AI) and machine
learning (ML) in sdLDL research
The rapid evolution of AI and ML is profoundly
transforming cardiovascular research, particularly in the study of
sdLDL, a key but often underestimated driver of atherosclerosis.
Historically, the measurement and clinical interpretation of sdLDL
have faced substantial challenges. Standard lipid panels do not
directly quantify sdLDL, and conventional estimation equations,
such as the Friedewald or Martin equations, often yield imprecise
results, especially in patients with elevated triglyceride levels
or atypical lipid profiles (132,133). These limitations have limited the
integration of sdLDL metrics into the routine clinical risk
assessment and management of ASCVD (Table V).
 | Table V.Summary of in sdLDL research. |
Table V.
Summary of in sdLDL research.
| Domain | AI/ML
contribution | Details and
examples | Challenges and
limitations | (Refs.) |
|---|
| Measurement and
estimation | Accurate prediction
of sdLDL levels | ML models, e.g.,
gradient boosting and deep neural networks, outperform
Friedewald/Martin equations, even with high TGs | Variability in
sdLDL measurement techniques, e.g., NMR vs.
ultracentrifugation | (133) |
| Risk
stratification | Enhanced
cardiovascular risk prediction | AI integrates sdLDL
with apoB, Lp(a) and inflammation markers; outperforms Framingham
Risk Score (AUC >0.85) | Lack of
standardization and validation across population subgroups | (164) |
| Explainability | Interpretable
models via SHAP | sdLDL shown to
contribute comparably to systolic blood pressure and age in
predictive models | ‘Black box’
concerns reduce clinician trust in complex models | (154) |
| Personalized
medicine | Tailored therapy
for sdLDL reduction | AI predicts
individual responses to statins, PCSK9 inhibitors and lifestyle
changes | Requires
integration of high-resolution genetic and lipidomic data | (134) |
| Therapeutic
optimization | Adaptive treatment
planning | Reinforcement
learning guides drug/lifestyle interventions; neural nets track
plaque via coronary CT angiography | Longitudinal,
multimodal datasets are limited; high computational costs | (145) |
| Clinical trials and
interventions | AI-guided lifestyle
interventions show rapid sdLDL reduction | Customized
diet/exercise plans reduce sdLDL in weeks | Scalability and
cost of implementing AI-driven personalization in real-world
settings | (144) |
| Challenges | Data heterogeneity,
generalizability, ethical concerns | Inconsistent sdLDL
assays, bias in training data, lack of model transparency | Data privacy,
underrepresentation of certain demographic groups | (149) |
| Emerging
solutions | Federated learning,
explainable AI, digital twins | Cross-institutional
model training, transparent decision-making, virtual simulations of
sdLDL progression | Early stage of
development; limited clinical integration and regulatory
guidance | (155) |
| Future outlook | AI positions sdLDL
as central in precision cardiology | Transition from
niche biomarker to a core element in personalized atherosclerosis
prevention | Large-scale
validation studies and harmonization with clinical workflows are
necessary | (156) |
AI and ML technologies are now bridging these gaps
by leveraging large-scale, multidimensional datasets that include
clinical, biochemical, genetic and imaging data (134). ML algorithms can identify
complex, non-linear relationships among lipid parameters that are
not detected by traditional statistical methods (133). Advanced models, such as gradient
boosting machines and deep neural networks, can predict sdLDL
concentrations with high accuracy, even in the presence of
confounding factors such as elevated triglyceride levels or
metabolic syndrome. These models demonstrate substantially reduced
prediction errors compared with legacy equations, and maintain a
high performance across diverse patient subgroups (Table V).
Han et al (135) developed and validated a number of
ML models, including XGBoost, Random Forest and Logistic
Regression, to predict the likelihood of LDL-C target levels being
achieved in patients with coronary artery disease treated with
moderate-dose statins. These models, trained on a large hospital
dataset, achieved respectable area under the receiver operating
characteristic curve values of ~0.695, even following significant
dimensionality reduction. Notably, SHapley Additive exPlanations
(SHAP) analysis was included to interpret the predictions made by
the models, providing transparency and clinical insight that can
guide individualized treatment strategies.
Similarly, Olmo et al (136) applied ML-driven decision tree
algorithms to cluster patients based on LDL-C levels, family
history of coronary heart disease and age. This led to the
identification of distinct risk groups with progressively higher
levels of Lp(a). Their study of >2,300 patients from a
multicenter Spanish registry revealed that nearly 39% of patients
had elevated Lp(a) levels, defined as >50 mg/dl, highlighting
the importance of using ML to stratify patients and identify those
at higher cardiovascular risk.
In another recent application of AI in lipidomics,
Tavaglione et al (137)
leveraged neural network models to assess the contribution of
lipoproteins to liver fat accumulation and inflammation,
particularly in the context of metabolic dysfunction-associated
steatotic liver disease (MASLD; formerly known as non-alcoholic
fatty liver disease) and metabolic dysfunction-associated
steatohepatitis. Using data from the UK Biobank, the research team
found that the ability of isolated hypertriglyceridemia to predict
MASLD was stronger than that of isolated hypercholesterolemia, a
finding with potential implications for targeted screening and
early intervention in dyslipidemic populations (54).
Sezer et al (138) investigated both AI and
explainable AI (XAI) techniques, including Random Forests and
Gradient Boosting, for the prediction of LDL-C from standard lipid
panels. By applying SHAP and Local Interpretable Model-agnostic
Explanations techniques, they demonstrated how XAI can bridge the
gap between predictive accuracy and clinical interpretability.
Their findings further suggested that AI-based LDL-C predictions
may offer more reliable and cost-effective alternatives to
traditional estimation formulas, particularly in large-scale
screening scenarios.
Collectively, these studies highlight a growing
trend toward the adoption of AI and ML methods in the diagnosis and
management of lipid disorders. The integration of explainable
models with clinical variables such as LDL-C, Lp(a) and
triglycerides is supporting a shift from population-based
approaches to precision cardiovascular medicine. Notably, SHAP
analysis and XAI frameworks are enhancing the ability of clinicians
to trust and act on AI-driven outputs, a critical factor for
successful clinical adoption.
Beyond estimation methods, AI-driven approaches are
redefining cardiovascular risk stratification by incorporating
sdLDL as a dynamic, actionable biomarker. Unlike static risk
scores, ML models can account for the cumulative burden of sdLDL
exposure over time and integrate it with other risk factors,
including ApoB, Lp(a) and inflammatory markers. In large cohort
studies, these AI-enhanced risk models have consistently
outperformed traditional tools such as the Framingham Risk Score,
achieving area under the curve values >0.85 (139). Notably, XAI techniques, such as
SHAP analysis, have shown that the sdLDL percentage contributes as
much to risk prediction as do established factors such as systolic
blood pressure or age, thereby highlighting its clinical
significance (140).
The impact of AI is increasingly extending into the
realm of personalized medicine. Reinforcement learning and other
adaptive algorithms are being used to tailor interventions that
specifically target sdLDL reduction. These systems can model
individual responses to statins, PCSK9 inhibitors and lifestyle
modifications, thereby optimizing treatment regimens based on the
unique lipidomic and genetic profile of each patient (141). Clinical trials using AI-generated
dietary and exercise recommendations have already reported
significant reductions in sdLDL levels within weeks, demonstrating
the potential for rapid, individualized risk mitigation (142–144). In addition, neural networks that
analyze longitudinal imaging data, such as coronary computed
tomography angiography, can identify optimal windows for
intervention by tracking the progression of atherosclerotic plaques
in relation to sdLDL fluctuations (145,146).
Despite these advances, several challenges remain
that must be addressed before AI and ML can be fully integrated
into routine sdLDL management (147,148).
Data heterogeneity remains a major barrier, as
different measurement techniques, for example, ultracentrifugation
and NMR spectroscopy, may produce inconsistent results,
complicating model training and validation. Concerns about the
generalizability of AI models also persist, particularly when they
are trained on datasets that do not adequately represent diverse
populations. Ethical considerations, including algorithmic bias and
data privacy, also require ongoing attention to ensure equitable
and responsible deployment of these technologies. In addition, the
opaque nature of certain deep learning models may undermine the
trust of clinicians and hinder their clinical adoption, thereby
underscoring the requirement for transparent and interpretable AI
systems (149).
Looking ahead, the AI and ML field is moving towards
federated learning frameworks that enable collaborative model
development across institutions without compromising patient
privacy (150,151).
The integration of XAI should further enhance
clinician confidence by making the decision-making process clearer.
In addition, so-called ‘digital twins’ or virtual patient models
are emerging that may simulate sdLDL trajectories under various
treatment scenarios, and have the potential to revolutionize
individualized care and preventative strategies (152–155).
The convergence of AI, ML and sdLDL research
represents a paradigm shift in the fight against atherosclerosis
(156). By enabling precise
estimation, robust risk prediction and personalized intervention,
these technologies are poised to elevate sdLDL from an emerging
biomarker to a central pillar of precision cardiology. As
validation studies progress and implementation barriers are
addressed, AI-driven approaches have the potential to transform the
science and practice of CVD prevention and management.
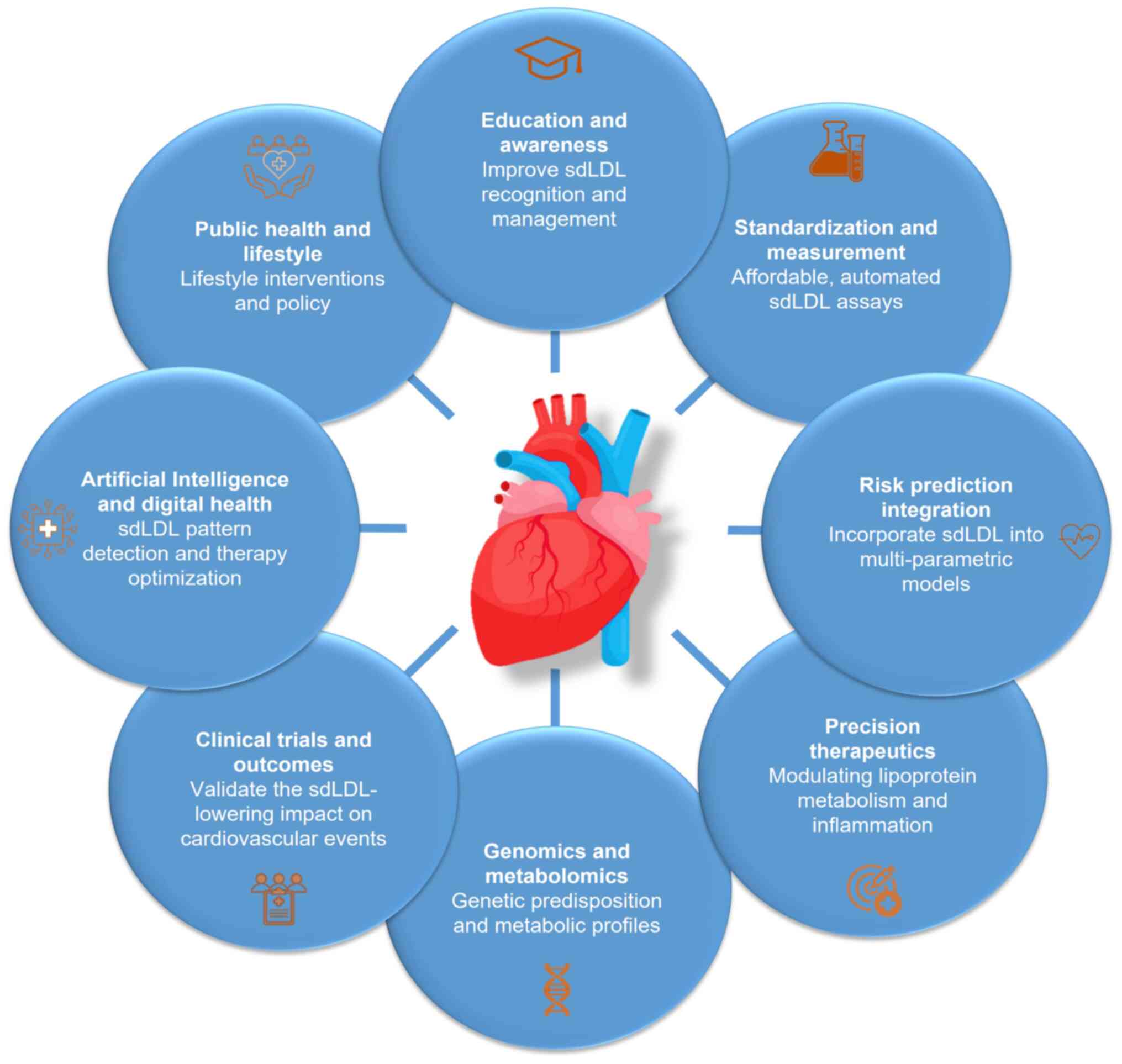
Future directions
As cardiovascular medicine enters an era
increasingly defined by precision and personalization, the
recognition of sdLDL as a critical contributor to residual
atherosclerotic risk opens numerous avenues for future research,
scientific innovation and clinical integration. The body of
evidence associating sdLDL with heightened cardiovascular morbidity
and mortality, despite standard lipid control, highlights the
necessity of redefining our understanding of lipoprotein pathology
and risk assessment (18,27). In the future, several key areas
must be addressed to fully harness the clinical potential of
sdLDL-focused strategies (Fig.
2).
First and foremost, it is essential to standardize
sdLDL measurements and increase their accessibility. Although
advanced techniques, including NMR spectroscopy, ion mobility
analysis and density gradient ultracentrifugation, have provided
invaluable insights into lipoprotein subfractions, these tools
remain largely confined to research settings (157,158). The development of affordable,
automated and reproducible assays for sdLDL detection is necessary
for widespread adoption in clinical practice (18,159). Furthermore, establishing
universally accepted cut-off values and reference ranges,
stratified by age, sex, ethnicity and comorbidities, will enhance
the interpretability and clinical relevance of sdLDL metrics.
Another critical priority is the integration of
sdLDL into existing cardiovascular risk prediction models. Current
tools, such as the ASCVD Risk Calculator and SCORE2, primarily
consider traditional lipid markers and broad demographic factors
(160). Incorporating sdLDL
concentrations into multi-parametric indices alongside ApoB,
triglycerides and inflammatory biomarkers, may substantially
improve the accuracy of risk stratification, particularly in
individuals with metabolic syndrome or type 2 diabetes, or those
with normal LDL-C levels but persistent vascular disease.
At the therapeutic level, the evolution of precision
medicine approaches is necessary. Although current lipid-lowering
therapies, including statins and fibrates, partially reduce sdLDL
levels, more selective and sdLDL-targeted interventions are
required (161,162). Future pharmacological studies
should prioritize agents that modify hepatic lipoprotein secretion,
lipolysis and receptor-mediated lipoprotein to preferentially
reduce sdLDL formation and persistence. Furthermore, combinatorial
therapies addressing both lipid and inflammatory signaling pathways
may yield synergistic benefits for patients with a high sdLDL
burden.
Personalized treatment algorithms that incorporate
genetic and metabolic profiling could further enhance the targeting
of sdLDL. Advances in genomics and metabolomics may help to
identify individuals who are genetically predisposed to overproduce
or inefficiently clear sdLDL. This information, combined with
real-time lipidomic and glycemic monitoring, may guide
individualized therapeutic regimens and dynamic risk tracking over
time.
Longitudinal and interventional studies are required
to clarify the clinical benefits of lowering sdLDL levels. Although
the atherogenicity of sdLDL has been well documented, conclusive
evidence that reducing sdLDL leads to improvements in
cardiovascular outcomes remains elusive. Large, well-powered
randomized controlled trials are necessary to determine whether
targeting sdLDL levels, either through lifestyle or pharmacological
means, leads to measurable reductions in myocardial infarction,
stroke and cardiovascular death, particularly in high-risk
populations (Fig. 2).
The integration of AI and digital health
technologies in routine sdLDL assessment and management is a
promising strategy. ML algorithms could be trained to detect
patterns of sdLDL elevation based on indirect clinical and
biochemical markers, thereby reducing dependence on direct
measurement. AI-driven platforms may also leverage patient-specific
data streams from electronic health records and wearable devices to
optimize therapy selection, adherence and monitoring (134,163,164).
Lipidomics, an emerging discipline within the
broader field of metabolomics, focuses on the comprehensive
analysis of cellular lipid profiles using advanced analytical
chemistry techniques and high-throughput technological platforms.
Unlike traditional lipid panels that measure only a small number of
lipid classes, such as total cholesterol, LDL-C, HDL-C and
triglycerides, lipidomics enables the simultaneous identification
and quantification of hundreds to thousands of distinct lipid
species within biological samples (165). This comprehensive approach is
achieved using sophisticated tools, including mass spectrometry,
NMR spectroscopy and liquid chromatography, which together provide
high resolution and sensitivity in lipid analysis (166,167).
The application of lipidomics in cardiovascular
research has enhanced our understanding of lipid metabolism and its
role in atherosclerosis (168).
Specifically, lipidomics allows for the detailed characterization
of lipoprotein subfractions, including sdLDL (157,169). By profiling the molecular
composition of sdLDL particles, including their enrichment in
triglycerides, depletion of cholesterol, and susceptibility to
oxidative modifications that increase atherogenicity, lipidomics
can reveal mechanistic links between specific lipid species and the
pathogenesis of ASCVD (170,171).
Lipidomics offers the potential to identify novel
lipid biomarkers that may improve risk stratification beyond
conventional measures (165). For
example, recent studies have uncovered unique lipid signatures that
are associated with increased sdLDL levels in patients with
metabolic syndrome, type 2 diabetes and insulin resistance
(172–174). These signatures include specific
oxidized phospholipids, ceramides and sphingolipids, which have
been implicated in endothelial dysfunction, inflammation and plaque
instability. Integrating such lipidomic data with clinical and
genetic information may increase the precision and
individualization of risk assessment, as well as the development of
targeted therapies aimed at modulating pathogenic lipid
species.
Despite its promise, the clinical implementation of
lipidomics is hindered by a lack of standardized protocols, robust
data interpretation frameworks and cost-effective analytical
platforms. However, as technology advances and a deeper
understanding of lipid biology is obtained, lipidomics is poised to
become an indispensable tool in research and clinical practice,
particularly in the context of sdLDL and its role in residual
cardiovascular risk. Future studies leveraging lipidomic profiling
are likely to yield new insights into the molecular mechanisms of
atherogenesis, facilitating the transition towards precision
cardiovascular medicine.
Finally, promoting greater awareness and education
among clinicians and patients is paramount. Despite its clinical
significance, sdLDL remains under-recognized in numerous healthcare
settings. Medical curricula, continuing education programs and
clinical guidelines should be updated to reflect the evolving
understanding of sdLDL, ensuring that healthcare providers are
equipped to interpret, communicate and act on sdLDL-associated
information effectively.
In summary, the future of sdLDL research and
application lies in the convergence of technological innovation,
translational science and personalized care. By advancing
measurement techniques, integrating sdLDL into risk models,
validating targeted therapies and implementing health system-level
changes, insights into the pathophysiological role of sdLDL can be
translated into actionable clinical strategies. In so doing,
cardiovascular prevention may become both more comprehensive and
more precisely targeted.
Conclusion
Over the last few decades, our understanding of
atherosclerosis has evolved from a simplistic cholesterol-centric
view to a complex appreciation of lipid subfractions, inflammation,
endothelial dysfunction and metabolic interactions. Among these
factors, sdLDL has emerged as a critical, yet under-recognized,
contributor to CVD. Its unique physicochemical properties, such as
elevated arterial wall permeability, prolonged circulation time and
heightened susceptibility to oxidation, result in its
atherogenicity being greater than that of larger LDL particles. A
growing body of evidence demonstrates that sdLDL plays a central
role in promoting endothelial injury, foam cell formation,
inflammation and plaque instability, all of which are key features
of progressive atherosclerosis.
Despite these insights, sdLDL remains
insufficiently addressed in clinical diagnostics and practice
guidelines. Traditional lipid panels do not detect sdLDL, and so
potentially overlook high-risk individuals, particularly those with
metabolic syndrome, type 2 diabetes or normocholesterolemia. The
absence of routine sdLDL measurement may lead to suboptimal risk
assessment and missed opportunities for early intervention.
Integrating sdLDL into diagnostic protocols, either directly
through specialized testing or indirectly via surrogate markers
such as ApoB and non-HDL-C, could refine cardiovascular risk
prediction and guide personalized treatment strategies.
Therapeutically, although conventional
lipid-lowering agents such as statins and fibrates can reduce sdLDL
levels, emerging therapies, including w-3 fatty acid formulations,
PCSK9 inhibitors and RNA-targeting agents, have the potential to
provide more targeted and effective sdLDL modulation. Additional
clinical trials are required to determine whether specific
sdLDL-lowering strategies translate into improved long-term
cardiovascular outcomes. Alongside pharmacological innovations,
lifestyle modifications, particularly those aimed at improving
insulin sensitivity and reducing the levels of triglyceride-rich
lipoproteins, remain crucial for managing sdLDL-associated
risk.
Looking ahead, the future management of sdLDL must
be grounded in an integrative approach that combines advanced
diagnostics, precision therapeutics and population-level
interventions. Efforts should focus on standardizing measurement
techniques, educating clinicians and updating clinical guidelines
to reflect the evolving understanding of the pathophysiological
role of sdLDL.
Acknowledgements
Not applicable.
Funding
This study was supported by program-targeted funding from the
Ministry of Science and Higher Education of the Republic of
Kazakhstan: ‘Development of new screening methods, to prevent early
mortality and treatment of cardiovascular-diseases of
atherosclerotic genesis in patients with atherosclerosis’ (grant
no. BR21881970).
Availability of data and materials
Not applicable.
Authors' contributions
MB was responsible for writing, reviewing and
editing the manuscript, supervision and project administration. TS
was responsible for writing the original draft of the manuscript,
defining the scope and focus of the review, devising a literature
search strategy, formal analysis and conceptualization. TIR was
responsible for collecting data. SA was responsible for writing the
manuscript, methodology, analysis and conceptualization. AK was
responsible for reviewing and editing the manuscript, formal
analysis, methodology and conceptualization. GM was responsible for
editing the manuscript, methodology and formal analysis. Data
authentication is not applicable. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Timur Saliev: https://orcid.org/0000-0001-5696-6363.
References
|
1
|
Agnello F, Ingala S, Laterra G, Scalia L
and Barbanti M: Novel and emerging LDL-C lowering strategies: A new
era of dyslipidemia management. J Clin Med. 13:12512024. View Article : Google Scholar
|
|
2
|
Mukherjee D and Nissen SE: Lipoprotein (a)
as a biomarker for cardiovascular diseases and potential new
therapies to mitigate risk. Curr Vasc Pharmacol. 22:171–179. 2024.
View Article : Google Scholar
|
|
3
|
German CA and Shapiro MD: Assessing
atherosclerotic cardiovascular disease risk with advanced lipid
testing: State of the science. Eur Cardiol. 15:e562020. View Article : Google Scholar
|
|
4
|
Antoniades C and West HW: ESC CVD
Prevention Guidelines 2021: Improvements, controversies, and
opportunities. Cardiovasc Res. 118:e17–e19. 2022. View Article : Google Scholar
|
|
5
|
McDonagh TA, Metra M, Adamo M, Gardner RS,
Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, et
al: 2021 ESC guidelines for the diagnosis and treatment of acute
and chronic heart failure: Developed by the task force for the
diagnosis and treatment of acute and chronic heart failure of the
European Society of Cardiology (ESC) with the special contribution
of the Heart Failure Association (HFA) of the ESC. Rev Esp Cardiol
(Engl Ed). 75:5232022.
|
|
6
|
De Oliveira-Gomes D, Joshi PH, Peterson
ED, Rohatgi A, Khera A and Navar AM: Apolipoprotein B: bridging the
gap between evidence and clinical practice. Circulation. 150:62–79.
2024. View Article : Google Scholar
|
|
7
|
Arnett DK, Blumenthal RS, Albert MA,
Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A,
Lloyd-Jones D, McEvoy JW, et al: 2019 ACC/AHA guideline on the
primary prevention of cardiovascular disease: Executive summary: A
report of the American college of Cardiology/American heart
association task force on clinical practice guidelines. J Am Coll
Cardiol. 74:1376–1414. 2019. View Article : Google Scholar
|
|
8
|
Grundy SM, Stone NJ, Bailey AL, Beam C,
Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S,
Faiella-Tommasino J, Forman DE, et al: 2018
AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline
on the management of blood cholesterol: A report of the American
college of Cardiology/American heart association task force on
clinical practice guidelines. Circulation. 139:e1082–e1143. 2019.
View Article : Google Scholar
|
|
9
|
Akyol O, Yang CY, Woodside DG, Chiang HH,
Chen CH and Gotto AM: Comparative analysis of atherogenic
lipoproteins L5 and Lp(a) in atherosclerotic cardiovascular
disease. Curr Atheroscler Rep. 26:317–329. 2024. View Article : Google Scholar
|
|
10
|
Proctor SD, Wang M, Vine DF and Raggi P:
Predictive utility of remnant cholesterol in atherosclerotic
cardiovascular disease. Curr Opin Cardiol. 39:300–307. 2024.
View Article : Google Scholar
|
|
11
|
Attiq A, Afzal S, Ahmad W and Kandeel M:
Hegemony of inflammation in atherosclerosis and coronary artery
disease. Eur J Pharmacol. 966:1763382024. View Article : Google Scholar
|
|
12
|
Khosravi M, Sheikhnia F, Pashaei MR,
Karimi-Dehkordi M and Alizadeh-Fanalou S: Association between small
dense low-density lipoprotein and carotid intima-media thickness. J
Cardiovasc Thorac Res. 16:202–210. 2024. View Article : Google Scholar
|
|
13
|
Benitez S, Puig N, Camps-Renom P and
Sánchez-Quesada JL: Atherogenic circulating lipoproteins in
ischemic stroke. Front Cardiovasc Med. 11:14703642024. View Article : Google Scholar
|
|
14
|
Wang J, Zhao X, Zhao Y, Jin R, Li Y, Wang
J, Liu Y, Wu Z, Guo X and Tao L: Discordance of small dense LDL
cholesterol beyond LDL cholesterol or non-HDL cholesterol and
carotid plaque. JACC Asia. 5:1012–1028. 2025. View Article : Google Scholar
|
|
15
|
Luciani L, Pedrelli M and Parini P:
Modification of lipoprotein metabolism and function driving
atherogenesis in diabetes. Atherosclerosis. 394:1175452024.
View Article : Google Scholar
|
|
16
|
Orekhov AN: We must abandon the Myth:
Oxidized low-density lipoprotein is not a lipoprotein that plays a
key role in atherogenesis. Curr Med Chem. 32:2899–2914. 2025.
View Article : Google Scholar
|
|
17
|
Vedamurthy D, Sagheer U, Patel A, Singh G
and Kalra D: Management of dyslipidemia in chronic kidney disease.
Curr Cardiovasc Risk Rep. 19:102025. View Article : Google Scholar
|
|
18
|
Kim S and Subramanian S: Approach to lipid
management in the patient with diabetes. J Clin Endocrinol Metab.
110:1740–1755. 2025. View Article : Google Scholar
|
|
19
|
Tomlinson B and Chan P: Exploring emerging
pharmacotherapies for type 2 diabetes patients with
hypertriglyceridemia. Expert Opin Pharmacother. 26:279–289. 2025.
View Article : Google Scholar
|
|
20
|
Hirano T: Cinical significance of small
dense low-density lipoprotein cholesterol measurement in type 2
diabetes. J Diabetes Investig. 16:370–383. 2025. View Article : Google Scholar
|
|
21
|
Hernando-Redondo J, Niño OC and Fitó M:
Atherogenic low-density lipoprotein and cardiovascular risk. Curr
Opin Lipidol. 36:8–13. 2025. View Article : Google Scholar
|
|
22
|
Konieczynska M, Natorska J, Ząbczyk M and
Undas A: Lipoprotein(a) and thromboembolism: Current state of
knowledge and unsolved issues. Arch Med Sci. 20:1770–1783.
2024.
|
|
23
|
Qiao YN, Zou YL and Guo SD: Low-density
lipoprotein particles in atherosclerosis. Front Physiol.
13:9319312022. View Article : Google Scholar
|
|
24
|
Jin X, Yang S, Lu J and Wu M: Small, dense
low-density lipoprotein-cholesterol and atherosclerosis:
Relationship and therapeutic strategies. Front Cardiovasc Med.
8:8042142022. View Article : Google Scholar
|
|
25
|
Stanciulescu LA, Scafa-Udriste A and
Dorobantu M: Exploring the association between low-density
lipoprotein subfractions and major adverse cardiovascular
outcomes-a comprehensive review. Int J Mol Sci. 24:66692023.
View Article : Google Scholar
|
|
26
|
Nakayama A, Morita H, Sato T, Kawahara T,
Takeda N, Kato S, Itoh H and Komuro I: Small dense low-density
lipoprotein cholesterol is a potential marker for predicting laser
treatment for retinopathy in diabetic patients. J Atheroscler
Thromb. 29:678–691. 2022. View Article : Google Scholar
|
|
27
|
Piccirillo F, Mastroberardino S, Nusca A,
Frau L, Guarino L, Napoli N, Ussia GP and Grigioni F: Novel
antidiabetic agents and their effects on lipid profile: A single
shot for several cardiovascular targets. Int J Mol Sci.
24:101642023. View Article : Google Scholar
|
|
28
|
Chary A, Tohidi M and Hedayati M:
Association of LDL-cholesterol subfractions with cardiovascular
disorders: A systematic review. BMC Cardiovasc Disord. 23:5332023.
View Article : Google Scholar
|
|
29
|
Gluba-Sagr A, Olszewski R, Franczyk B,
Młynarska E, Rysz-Górzyńska M, Rysz J, Surma S, Sohum S, Banach M
and Toth PP: High-density lipoproteins. Part 2. Impact of disease
states on functionality. Am J Prev Cardiol. 23:1010732025.
View Article : Google Scholar
|
|
30
|
Orekhov A, Sukhorukov V and Melnichenko A:
Is oxidized low-density lipoprotein a principal actor in
atherogenesis? Curr Med Chem. 31:6909–6910. 2024. View Article : Google Scholar
|
|
31
|
Mosalmanzadeh N and Pence BD: Oxidized
low-density lipoprotein and its role in immunometabolism. Int J Mol
Sci. 25:113862024. View Article : Google Scholar
|
|
32
|
Jiang H, Zhou Y, Nabavi SM, Sahebkar A,
Little PJ, Xu S, Weng J and Ge J: Mechanisms of oxidized
LDL-mediated endothelial dysfunction and its consequences for the
development of atherosclerosis. Front Cardiovasc Med. 9:9259232022.
View Article : Google Scholar
|
|
33
|
Kloc M, Uosef A, Kubiak JZ and Ghobrial
RM: Role of Macrophages and RhoA pathway in atherosclerosis. Int J
Mol Sci. 22:2162020. View Article : Google Scholar
|
|
34
|
Farahi L, Sinha SK and Lusis AJ: Roles of
macrophages in atherogenesis. Front Pharmacol. 12:7852202021.
View Article : Google Scholar
|
|
35
|
Gruber EJ, Aygun AY and Leifer CA:
Macrophage uptake of oxidized and acetylated low-density
lipoproteins and generation of reactive oxygen species are
regulated by linear stiffness of the growth surface. PLoS One.
16:e02607562021. View Article : Google Scholar
|
|
36
|
Malekmohammad K, Bezsonov EE and
Rafieian-Kopaei M: Role of lipid accumulation and inflammation in
atherosclerosis: Focus on molecular and cellular mechanisms. Front
Cardiovasc Med. 8:7075292021. View Article : Google Scholar
|
|
37
|
Chakraborty S, Verma A, Garg R, Singh J
and Verma H: Cardiometabolic risk factors associated with type 2
diabetes mellitus: A mechanistic Insight. Clin Med Insights
Endocrinol Diabetes. 16:117955142312207802023. View Article : Google Scholar
|
|
38
|
Yanai H, Adachi H, Hakoshima M and
Katsuyama H: Molecular biological and clinical understanding of the
statin residual cardiovascular disease risk and peroxisome
proliferator-activated receptor alpha agonists and ezetimibe for
its treatment. Int J Mol Sci. 23:34182022. View Article : Google Scholar
|
|
39
|
He Q, Wang L, Fang Y, Liang M, Chen X, Hu
R and Zhong J: Relationship of small dense low-density lipoprotein
cholesterol level with pre-diabetes and newly detected type 2
diabetes. Sci Rep. 15:195002025. View Article : Google Scholar
|
|
40
|
Vekic J, Stromsnes K, Mazzalai S,
Zeljkovic A, Rizzo M and Gambini J: Oxidative stress, atherogenic
dyslipidemia, and cardiovascular risk. Biomedicines. 11:28972023.
View Article : Google Scholar
|
|
41
|
Eichhorn T, Weiss R, Huber S,
Ebeyer-Masotta M, Mostageer M, Emprechtinger R, Knabl L Sr, Knabl
L, Würzner R and Weber V: Expression of tissue factor and
platelet/leukocyte markers on extracellular vesicles reflect
platelet-leukocyte interaction in severe COVID-19. Int J Mol Sci.
24:168862023. View Article : Google Scholar
|
|
42
|
Tsioufis P, Theofilis P, Tsioufis K and
Tousoulis D: The impact of cytokines in coronary atherosclerotic
plaque: Current therapeutic approaches. Int J Mol Sci.
23:159372022. View Article : Google Scholar
|
|
43
|
Sleutjes JAM, van Lennep JER, van der
Woude CJ and de Vries AC: Thromboembolic and atherosclerotic
cardiovascular events in inflammatory bowel disease: Epidemiology,
pathogenesis and clinical management. Therap Adv Gastroenterol.
2021.1417562848211032126. 2021.
|
|
44
|
Hall AE, Jade D, Shaik F,
Homer-Vanniasinkam S, Muench SP, Harrison MA and Ponnambalam S:
Modified lipid particle recognition: A link between atherosclerosis
and cancer? Biology (Basel). 14:6752025.
|
|
45
|
Therond P and Chapman MJ:
Sphingosine-1-phosphate: Metabolism, transport, atheroprotection
and effect of statin treatment. Curr Opin Lipidol. 33:199–207.
2022. View Article : Google Scholar
|
|
46
|
Miceli G, Basso MG, Pintus C, Pennacchio
AR, Cocciola E, Cuffaro M, Profita M, Rizzo G and Tuttolomondo A:
Molecular pathways of vulnerable carotid plaques at risk of
ischemic stroke: A narrative review. Int J Mol Sci. 25:43512024.
View Article : Google Scholar
|
|
47
|
Alonso-Herranz L, Albarrán-Juárez J and
Bentzon JF: Mechanisms of fibrous cap formation in atherosclerosis.
Front Cardiovasc Med. 10:12541142023. View Article : Google Scholar
|
|
48
|
Jebari-Benslaiman S, Galicia-García U,
Larrea-Sebal A, Olaetxea JR, Alloza I, Vandenbroeck K,
Benito-Vicente A and Martín C: Pathophysiology of Atherosclerosis.
Int J Mol Sci. 23:33462022. View Article : Google Scholar
|
|
49
|
Islam MS, Wei P, Suzauddula M, Nime I,
Feroz F, Acharjee M and Pan F: The interplay of factors in
metabolic syndrome: Understanding its roots and complexity. Mol
Med. 30:2792024. View Article : Google Scholar
|
|
50
|
Bays HE, Kirkpatrick CF, Maki KC, Toth PP,
Morgan RT, Tondt J, Christensen SM, Dixon DL and Jacobson TA:
Obesity, dyslipidemia, and cardiovascular disease: A joint expert
review from the obesity medicine association and the national lipid
association 2024. J Clin Lipidol. 18:e320–e350. 2024. View Article : Google Scholar
|
|
51
|
Jayaraman S, Pérez A, Miñambres I,
Sánchez-Quesada JL and Gursky O: LDL binding to cell receptors and
extracellular matrix is proatherogenic in obesity but improves
after bariatric surgery. J Lipid Res. 64:1004512023. View Article : Google Scholar
|
|
52
|
Superko H and Garrett B: Small Dense LDL:
Scientific background, clinical relevance, and recent evidence
still a risk even with ‘Normal’ LDL-C Levels. Biomedicines.
10:8292022. View Article : Google Scholar
|
|
53
|
Manemann SM, Bielinski SJ, Moser ED, St
Sauver JL, Takahashi PY, Roger VL, Olson JE, Chamberlain AM,
Remaley AT, Decker PA, et al: Variability in lipid levels and risk
for cardiovascular disease: An electronic health record-based
population cohort study. J Am Heart Assoc. 12:e0276392023.
View Article : Google Scholar
|
|
54
|
Cao X, Wang N, Yang M and Zhang C: Lipid
accumulation and insulin resistance: Bridging metabolic
dysfunction-associated fatty liver disease and chronic kidney
disease. Int J Mol Sci. 26:69622025. View Article : Google Scholar
|
|
55
|
Seehusen KE, Remaley AT, Sampson M,
Meeusen JW, Larson NB, Decker PA, Killian JM, Takahashi PY, Roger
VL, Manemann SM, et al: Discordance between very low-density
lipoprotein cholesterol and low-density lipoprotein cholesterol
increases cardiovascular disease risk in a geographically defined
cohort. J Am Heart Assoc. 13:e0318782024. View Article : Google Scholar
|
|
56
|
Farooq S, Generoso G, Bensenor IM, Santos
RD, Jones SR, Moraes E, Blaha MJ, Toth PP, Lotufo PA, Staniak HL
and Bittencourt MS: Low-density lipoprotein-cholesterol
subfractions as predictors for coronary artery calcium incidence
and progression - The Brazilian longitudinal study of Adult Health
(ELSA - Brasil). Atherosclerosis. 403:1191712025. View Article : Google Scholar
|
|
57
|
Katsi V, Argyriou N, Fragoulis C and
Tsioufis K: The role of Non-HDL cholesterol and apolipoprotein B in
cardiovascular disease: A comprehensive review. J Cardiovasc Dev
Dis. 12:2562025.
|
|
58
|
Huang J, Gu JX, Bao HZ, Li SS, Yao XQ,
Yang M, Li Y, Zhang AM, Yin Y, Zhang N, et al: Elevated serum small
dense low-density lipoprotein cholesterol may increase the risk and
severity of coronary heart disease and predict cardiovascular
events in patients with type 2 diabetes mellitus. Dis Markers.
2021:55970282021. View Article : Google Scholar
|
|
59
|
Deza S, Colina I, Beloqui O, Monreal JI,
Martínez-Chávez E, Maroto-García J, Mugueta C, González A and Varo
N: Evaluation of measured and calculated small dense low-density
lipoprotein in capillary blood and association with the metabolic
syndrome. Clin Chim Acta. 557:1178972024. View Article : Google Scholar
|
|
60
|
Stoicescu C, Vacarescu C and Cozma D: HDL
function versus small dense LDL: Cardiovascular benefits and
implications. J Clin Med. 14:49452025. View Article : Google Scholar
|
|
61
|
Płaczkowska S, Sołkiewicz K, Bednarz-Misa
I and Kratz EM: Atherogenic plasma index or non-high-density
lipoproteins as markers best reflecting age-related high
concentrations of small dense low-density lipoproteins. Int J Mol
Sci. 23:50892022. View Article : Google Scholar
|
|
62
|
Liou L and Kaptoge S: Association of
small, dense LDL-cholesterol concentration and lipoprotein particle
characteristics with coronary heart disease: A systematic review
and meta-analysis. PLoS One. 15:e02419932020. View Article : Google Scholar
|
|
63
|
Qi Y, Liu J, Wang W, Wang M, Zhao F, Sun
J, Liu J, Deng Q and Zhao D: High sdLDL cholesterol can be used to
reclassify individuals with low cardiovascular risk for early
intervention: Findings from the Chinese multi-provincial cohort
study. J Atheroscler Thromb. 27:695–710. 2020. View Article : Google Scholar
|
|
64
|
Hsu SH, Jang MH, Torng PL and Su TC:
Positive association between small dense low-density lipoprotein
cholesterol concentration and biomarkers of inflammation,
thrombosis, and prediabetes in non-diabetic adults. J Atheroscler
Thromb. 26:624–635. 2019. View Article : Google Scholar
|
|
65
|
Liu Z, Xv Y, Liu X and Zhou X:
Associations of systemic inflammatory markers with the risks of
chronic heart failure: A case-control study. Clinics (Sao Paulo).
77:1000562022. View Article : Google Scholar
|
|
66
|
Imamura T, Hori M, Narang N, Ueno H and
Kinugawa K: Prognostic implication of small dense LDL-cholesterol
levels following acute coronary syndrome. Medicina (Kaunas).
59:1582023. View Article : Google Scholar
|
|
67
|
Bashir B, Schofield J, Downie P, France M,
Ashcroft DM, Wright AK, Romeo S, Gouni-Berthold I, Maan A,
Durrington PN and Soran H: Beyond LDL-C: Unravelling the residual
atherosclerotic cardiovascular disease risk landscape-focus on
hypertriglyceridaemia. Front Cardiovasc Med. 11:13891062024.
View Article : Google Scholar
|
|
68
|
Auger N, Potter BJ, He S, Healy-Profitós
J, Schnitzer ME and Paradis G: Maternal cardiovascular disease 3
decades after preterm birth longitudinal cohort study of pregnancy
vascular disorders. Hypertension. 75:788–795. 2020. View Article : Google Scholar
|
|
69
|
Shelbaya K, Claggett B, Dorbala P, Skali
H, Solomon SD, Matsushita K, Konety S, Mosley TH and Shah AM:
Stages of valvular heart disease among older adults in the
community: The atherosclerosis risk in communities study.
Circulation. 147:638–649. 2023. View Article : Google Scholar
|
|
70
|
Jia XM, Sun C, Nambi V, Virani SS, Taffet
G, Boerwinkle E, Bressler J, Ndumele C, Windham BG, de Lemos JA, et
al: Midlife determinants of healthy cardiovascular aging: The
Atherosclerosis Risk in Communities (ARIC) study. Atherosclerosis.
350:82–89. 2022. View Article : Google Scholar
|
|
71
|
Ivensky V, Zonga P, Dallaire G, Desbiens
LC, Nadeau-Fredette AC, Rousseau G and Goupil R: Differences in
antihypertensive medication prescription profiles between 2009 and
2021: A retrospective cohort study of CARTaGENE. Can J Kidney
Health Dis. 11:205435812412347292024. View Article : Google Scholar
|
|
72
|
Bodoarca R, Yeung RO and Lau D: New
diabetes guidelines: Impact on eligibility for sodium-glucose
cotransporter-2 inhibitors and glucagon-like peptide-1 receptor
agonists in Canada. Can J Diabetes. 46:691–698. 2022. View Article : Google Scholar
|
|
73
|
Zhang P, Zhang Z, Li D, Han R, Li H, Ma J,
Xu P, Qi Z, Liu L and Zhang A: Association of remnant cholesterol
with intracranial atherosclerosis in community-based population:
The ARIC study. J Stroke Cerebrovasc Dis. 32:1072932023. View Article : Google Scholar
|
|
74
|
Blaha MJ and DeFilippis AP: Multi-Ethnic
Study of Atherosclerosis (MESA): JACC Focus Seminar 5/8. J Am Coll
Cardiol. 77:3195–3216. 2021. View Article : Google Scholar
|
|
75
|
Wolska A, Sampson M, Zubirán R, Meeusen
JW, Donato LJ, Jaffe AS and Remaley AT: An equation for estimating
low-density lipoprotein-triglyceride content and its use for
cardiovascular disease risk stratification. Front Cardiovasc Med.
11:14528692024. View Article : Google Scholar
|
|
76
|
Hussain A, Sun C, Selvin E, Nambi V,
Coresh J, Jia X, Ballantyne CM and Hoogeveen RC: Triglyceride-rich
lipoproteins, apolipoprotein C-III, angiopoietin-like protein 3,
and cardiovascular events in older adults: Atherosclerosis Risk in
Communities (ARIC) study. Eur J Prev Cardiol. 29:E53–E64. 2022.
View Article : Google Scholar
|
|
77
|
Bruggen FHV and Diamond DM: Is targeting
LDL-C levels below 70 mg/dL beneficial for cardiovascular and
overall health? A critical examination of the evidence. J Clin Med.
14:35692025. View Article : Google Scholar
|
|
78
|
Ishii J, Kashiwabara K, Ozaki Y, Takahashi
H, Kitagawa F, Nishimura H, Ishii H, Iimuro S, Kawai H, Muramatsu
T, et al: Small dense low-density lipoprotein cholesterol and
cardiovascular risk in statin-treated patients with coronary artery
disease. J Atheroscler Thromb. 29:1458–1474. 2022. View Article : Google Scholar
|
|
79
|
Wang X, Wang L, Cao R, Yang X, Xiao W,
Zhang Y and Ye P: Correlation between small and dense low-density
lipoprotein cholesterol and cardiovascular events in Beijing
community population. J Clin Hypertens (Greenwich). 23:345–351.
2021. View Article : Google Scholar
|
|
80
|
Chen S, Li Z, Li H, Zeng X, Yuan H and Li
Y: Novel lipid biomarkers and ratios as risk predictors for
premature coronary artery disease: A retrospective analysis of 2952
patients. J Clin Hypertens (Greenwich). 25:1172–1184. 2023.
View Article : Google Scholar
|
|
81
|
Borén J, Chapman MJ, Krauss RM, Packard
CJ, Bentzon JF, Binder CJ, Daemen MJ, Demer LL, Hegele RA, Nicholls
SJ, et al: Low-density lipoproteins cause atherosclerotic
cardiovascular disease: pathophysiological, genetic, and
therapeutic insights: A consensus statement from the European
atherosclerosis society consensus panel. Eur Heart J. 41:2313–2330.
2020. View Article : Google Scholar
|
|
82
|
Cesaro A, Acerbo V, Scialla F, Scherillo
G, De Michele G, Panico D, Porcelli G, de Sio V, Capolongo A,
Sperlongano S, et al: Role of LipoprotEin(a) in CardiovascuLar
diseases and premature acute coronary syndromes (RELACS study):
Impact of Lipoprotein(a) levels on the premature coronary event and
the severity of coronary artery disease. Nutr Metab Cardiovasc Dis.
35:1038432025. View Article : Google Scholar
|
|
83
|
Chary A and Hedayati M: Review of
laboratory methods to determine HDL and LDL subclasses and their
clinical importance. Rev Cardiovasc Med. 23:1472022. View Article : Google Scholar
|
|
84
|
Ye Y, Markussen B, Engelsen SB and
Khakimov B: The quality, uniqueness, and causality of NMR-based
prediction models for low-density lipoprotein cholesterol
subfractions in human blood plasma. Comput Biol Med.
184:1093792025. View Article : Google Scholar
|
|
85
|
Berisha H, Hattab R, Comi L, Giglione C,
Migliaccio S and Magni P: Nutrition and lifestyle interventions in
managing dyslipidemia and cardiometabolic risk. Nutrients.
17:7762025. View Article : Google Scholar
|
|
86
|
Packard CJ: Remnants, LDL, and the
quantification of lipoprotein-associated risk in atherosclerotic
cardiovascular disease. Curr Atheroscler Rep. 24:133–142. 2022.
View Article : Google Scholar
|
|
87
|
Cole J, Zubirán R, Wolska A, Jialal I and
Remaley AT: Use of apolipoprotein B in the Era of precision
medicine: time for a paradigm change? J Clin Med. 12:57372023.
View Article : Google Scholar
|
|
88
|
Kounatidis D, Vallianou NG, Poulaki A,
Evangelopoulos A, Panagopoulos F, Stratigou T, Geladari E,
Karampela I and Dalamaga M: ApoB100 and atherosclerosis: What's New
in the 21st Century? Metabolites. 14:1232024. View Article : Google Scholar
|
|
89
|
Gugliucci A: The chylomicron saga: Time to
focus on postprandial metabolism. Front Endocrinol (Lausanne).
14:13228692024. View Article : Google Scholar
|
|
90
|
Araujo G, Valencia LM, Martin-Ozimek A,
Soto Y and Proctor SD: Atherosclerosis: From lipid-lowering and
anti-inflammatory therapies to targeting arterial retention of
ApoB-containing lipoproteins. Front Immunol. 16:14858012025.
View Article : Google Scholar
|
|
91
|
Lorey MB, Öörni K and Kovanen PT: Modified
lipoproteins induce arterial wall inflammation during
atherogenesis. Front Cardiovasc Med. 9:8415452022. View Article : Google Scholar
|
|
92
|
Bekbossynova M, Ivanova-Razumova T, Kali
A, Sailybayeva A, Khamitov S, Daniyarova G, Akzholova K and Saliev
T: Apolipoprotein B and glycemic dysregulation: New predictors of
type 2 diabetes in high-cardiovascular-risk populations. J Pers
Med. 15:1632025. View Article : Google Scholar
|
|
93
|
Zhu X, Chen Y, Zhu M and Hu J: The
relationship between small dense low-density lipoprotein
cholesterol and metabolic syndrome. Diabetes Metab Syndr Obes.
17:1523–1532. 2024. View Article : Google Scholar
|
|
94
|
Fosam A, Bansal R, Ramanathan A, Sarcone
C, Iyer I, Murthy M, Remaley AT and Muniyappa R: Lipoprotein
insulin resistance index: A simple, accurate method for assessing
insulin resistance in South Asians. J Endocr Soc. 7:bvac1892023.
View Article : Google Scholar
|
|
95
|
Natale F, Franzese R, Marotta L, Mollo N,
Solimene A, Luisi E, Gentile C, Loffredo FS, Golino P and Cimmino
G: Evolving concepts of the SCORE system: Subtracting cholesterol
from risk estimation: A way for a healthy longevity? Life (Basel).
14:6792024.
|
|
96
|
Vekic J, Zeljkovic A, Stefanovic A,
Bogavac-Stanojevic N, Ilias I, Silva-Nunes J, Stoian AP, Janez A
and Rizzo M: Novel pharmaceutical and nutraceutical-based
approaches for cardiovascular diseases prevention targeting
atherogenic small dense LDL. Pharmaceutics. 14:8252022. View Article : Google Scholar
|
|
97
|
Kirkpatrick CF, Sikand G, Petersen KS,
Anderson CAM, Aspry KE, Bolick JP, Kris-Etherton PM and Maki KC:
Nutrition interventions for adults with dyslipidemia: A clinical
perspective from the National lipid association. J Clin Lipidol.
17:428–451. 2023. View Article : Google Scholar
|
|
98
|
Candás-Estébanez B, Fernández-Cidón B,
Corbella E, Tebé C, Fanlo-Maresma M, Esteve-Luque V, Salas-Salvadó
J, Fitó M, Riera-Mestre A, Ros E and Pintó X: The impact of the
mediterranean diet and lifestyle intervention on lipoprotein
subclass profiles among metabolic syndrome patients: Findings of a
randomized controlled trial. Int J Mol Sci. 25:13382024. View Article : Google Scholar
|
|
99
|
Falkenhain K, Roach LA, McCreary S,
McArthur E, Weiss EJ, Francois ME and Little JP: Effect of
carbohydrate-restricted dietary interventions on LDL particle size
and number in adults in the context of weight loss or weight
maintenance: A systematic review and meta-analysis. Am J Clin Nutr.
114:1455–1466. 2021. View Article : Google Scholar
|
|
100
|
Zubirán R, Neufeld EB, Dasseux A, Remaley
AT and Sorokin AV: Recent advances in targeted management of
inflammation in atherosclerosis: A narrative review. Cardiol Ther.
13:465–491. 2024. View Article : Google Scholar
|
|
101
|
Yang BF, Ma X, Yang L, Bian G, Qiao B, Lu
H, Wang Z, Zhang T and Cheng Y: Trends and prospects of low-density
lipoprotein cholesterol in stroke: A bibliometric analysis. Cureus.
16:e694922024.
|
|
102
|
Wilson DP and Patel M: Statin use in
children and adolescents-dos, don'ts and practical tips. Curr
Atheroscler Rep. 27:162024. View Article : Google Scholar
|
|
103
|
Ueki Y, Itagaki T and Kuwahara K:
Lipid-lowering therapy and coronary plaque regression. J
Atheroscler Thromb. 31:1479–1495. 2024. View Article : Google Scholar
|
|
104
|
Soleimani H, Mousavi A, Shojaei S,
Tavakoli K, Salabat D, Farahani Rad F, Askari MK, Nelson J, Ruzieh
M and Hosseini K: Safety and effectiveness of high-intensity
statins versus low/moderate-intensity statins plus ezetimibe in
patients with atherosclerotic cardiovascular disease for reaching
LDL-C goals: A systematic review and meta-analysis. Clin Cardiol.
47:e243342024. View Article : Google Scholar
|
|
105
|
Zeng WW and Tomlinson B: Statin
alternatives for the treatment of hypercholesterolemia-a safety
evaluation. Expert Opin Drug Saf. 24:17–24. 2025. View Article : Google Scholar
|
|
106
|
Wierzbicki AS: Advances in the
pharmacological management of hyperlipidemia through the use of
combination therapies. Expert Opin Pharmacother. 26:157–165. 2025.
View Article : Google Scholar
|
|
107
|
Canfora I and Pierno S:
Hypertriglyceridemia therapy: Past, present and future
perspectives. Int J Mol Sci. 25:97272024. View Article : Google Scholar
|
|
108
|
Fuior EV, Zvintzou E, Filippatos T,
Giannatou K, Mparnia V, Simionescu M, Gafencu AV and Kypreos KE:
Peroxisome proliferator-activated receptor α in lipoprotein
metabolism and atherosclerotic cardiovascular disease.
Biomedicines. 11:26962023. View Article : Google Scholar
|
|
109
|
Rubins HB, Robins SJ and Collins D: The
veterans affairs high-density lipoprotein intervention trial:
Baseline characteristics of normocholesterolemic men with coronary
artery disease and low levels of high-density lipoprotein
cholesterol. Am J Cardiol. 78:572–575. 1996. View Article : Google Scholar
|
|
110
|
Keech A, Simes RJ, Barter P, Best J, Scott
R, Taskinen MR, Forder P, Pillai A, Davis T, Glasziou P, et al:
Effects of long-term fenofibrate therapy on cardiovascular events
in 9795 people with type 2 diabetes mellitus (the FIELD study):
Randomised controlled trial. Lancet. 366:1849–1861. 2005.
View Article : Google Scholar
|
|
111
|
Nesti L, Tricò D, Mengozzi A and Natali A:
Rethinking pioglitazone as a cardioprotective agent: A new
perspective on an overlooked drug. Cardiovasc Diabetol. 20:1092021.
View Article : Google Scholar
|
|
112
|
Shao L, Zhi A, Li M, Zhang Y, Jiang S,
Zhang J, Yang K, Yang E, Zhu X, Cheng Y and Sun Y: Exploring the
impact of niacin intake on cardiovascular outcomes: A comprehensive
analysis using NHANES Data (2003–2018). Rev Cardiovasc Med.
25:4102024. View Article : Google Scholar
|
|
113
|
Sherratt SCR, Libby P, Budoff MJ, Bhatt DL
and Mason RP: Role of omega-3 fatty acids in cardiovascular
disease: The debate continues. Curr Atheroscler Rep. 25:1–17. 2023.
View Article : Google Scholar
|
|
114
|
Bhatt DL, Steg PG, Miller M, Brinton EA,
Jacobson TA, Ketchum SB, Doyle RT Jr, Juliano RA, Jiao L, Granowitz
C, et al: Cardiovascular risk reduction with icosapent ethyl for
hypertriglyceridemia. N Engl J Med. 380:11–22. 2019. View Article : Google Scholar
|
|
115
|
Parhofer KG, Chapman MJ and Nordestgaard
BG: Efficacy and safety of icosapent ethyl in
hypertriglyceridaemia: A recap. Eur Heart J Suppl. 22
(Supplement_J):J21–J33. 2020. View Article : Google Scholar
|
|
116
|
Basha A and Ramakrishnan S: Lipid clinical
trials with special reference to Indian population. Indian Heart J.
76 (Suppl 1):S130–S137. 2024. View Article : Google Scholar
|
|
117
|
Kaur G, Mason RP, Steg PG and Bhatt DL:
Omega-3 fatty acids for cardiovascular event lowering. Eur J Prev
Cardiol. 31:1005–1014. 2024. View Article : Google Scholar
|
|
118
|
Machado NM, Oliveira MVB, Quesada K, Haber
JFDS, José Tofano R, Rubira CJ, Zutin TLM, Direito R, Pereira ESBM,
de Oliveira CM, et al: Assessing omega-3 therapy and its
cardiovascular benefits: What about icosapent ethyl? A systematic
review and meta-analysis. Pharmaceuticals (Basel). 18:6012025.
View Article : Google Scholar
|
|
119
|
Abduljabbar MH: PCSK9 inhibitors: Focus on
evolocumab and its impact on atherosclerosis progression.
Pharmaceuticals (Basel). 17:15812024. View Article : Google Scholar
|
|
120
|
Giordano S, Ielapi J, Salerno N, Cersosimo
A, Lucchino A, Laschera A, Canino G, Di Costanzo A, De Rosa S,
Torella D and Sorrentino S: Rationale for early administration of
PCSK9 inhibitors in acute coronary syndrome. Rev Cardiovasc Med.
25:3742024. View Article : Google Scholar
|
|
121
|
Schonck WAM, Stroes ESG, Hovingh GK and
Reeskamp LF: Long-term efficacy and tolerability of PCSK9 targeted
therapy: A review of the literature. Drugs. 84:165–178. 2024.
View Article : Google Scholar
|
|
122
|
Gareri C, Polimeni A, Giordano S, Tammè L,
Curcio A and Indolfi C: Antisense oligonucleotides and small
interfering RNA for the treatment of dyslipidemias. J Clin Med.
11:38842022. View Article : Google Scholar
|
|
123
|
Sharif A, Mamo J, Lam V, Al-Salami H,
Mooranian A, Watts GF, Clarnette R, Luna G and Takechi R: The
therapeutic potential of probucol and probucol analogues in
neurodegenerative diseases. Transl Neurodegener. 13:62024.
View Article : Google Scholar
|
|
124
|
Yamashita S, Arai H, Bujo H, Masuda D,
Ohama T, Ishibashi T, Yanagi K, Doi Y, Nakagawa S, Yamashiro K, et
al: Probucol trial for secondary prevention of atherosclerotic
events in patients with coronary heart disease (PROSPECTIVE). J
Atheroscler Thromb. 28:103–123. 2021. View Article : Google Scholar
|
|
125
|
Lang L, Zhang J, Zheng D and Gao H:
Probucol will become a new model for treating cerebral infarction
with a high risk of hemorrhage: A narrative review. Brain Circ.
9:222–227. 2023. View Article : Google Scholar
|
|
126
|
Adili R, Hawley M and Holinstat M:
Regulation of platelet function and thrombosis by omega-3 and
omega-6 polyunsaturated fatty acids. Prostaglandins Other Lipid
Mediat. 139:10–18. 2018. View Article : Google Scholar
|
|
127
|
Siniscalchi C, Basaglia M, Meschi T,
Imbalzano E, Futura Bernardi F, Perrella A, Trama U, Passannanti A,
Di Micco P and Schiano C: Low LDL-cholesterol and hemorrhagic risk:
Mechanistic insights and clinical perspectives. Int J Mol Sci.
26:56122025. View Article : Google Scholar
|
|
128
|
Kohli-Lynch CN, Thanassoulis G, Moran AE
and Sniderman AD: The clinical utility of apoB versus
LDL-C/non-HDL-C. Clin Chim Acta. 508:103–108. 2020. View Article : Google Scholar
|
|
129
|
Chait A, Eckel RH, Vrablik M and Zambon A:
Lipid-lowering in diabetes: An update. Atherosclerosis.
394:1173132024. View Article : Google Scholar
|
|
130
|
Yang Z, Deng Q, Hao Y, Yang N, Han L, Jia
P, Zhou P, Hao Y, Wang Z, Zhao W, et al: Effectiveness of
treat-to-target cholesterol-lowering interventions on
cardiovascular disease and all-cause mortality risk in the
community-dwelling population: A target trial emulation. Nat
Commun. 15:99222024. View Article : Google Scholar
|
|
131
|
Virani SS, Aspry K, Dixon DL, Ferdinand
KC, Heidenreich PA, Jackson EJ, Jacobson TA, McAlister JL, Neff DR,
Gulati M and Ballantyne CM: The importance of low-density
lipoprotein cholesterol measurement and control as performance
measures: A joint clinical perspective from the national lipid
association and the American Society for preventive cardiology. J
Clin Lipidol. 17:208–218. 2023. View Article : Google Scholar
|
|
132
|
Alsadig REK and Morsi AN: Comparison of
multiple equations for low-density lipoprotein cholesterol
calculation against the direct homogeneous method. J Lipid
Atheroscler. 13:348–357. 2024. View Article : Google Scholar
|
|
133
|
Meng JB, An ZJ and Jiang CS: Machine
learning-based prediction of LDL cholesterol: Performance
evaluation and validation. PeerJ. 13:e192482025. View Article : Google Scholar
|
|
134
|
Ahn J and Kim B: Application of generative
artificial intelligence in dyslipidemia care. J Lipid Atheroscler.
14:77–93. 2025. View Article : Google Scholar
|
|
135
|
Han J, Kim Y, Kang HJ, Seo J, Choi H, Kim
M, Kee G, Park S, Ko S, Jung H, et al: Predicting low density
lipoprotein cholesterol target attainment using machine learning in
patients with coronary artery disease receiving moderate-dose
statin therapy. Sci Rep. 15:53462025. View Article : Google Scholar
|
|
136
|
Olmo RF, Cortez G, Toro MM, Sandín M, Mora
J, Oterino A, Bailen MC, Quiles-Granado J, Urbiola P, Ruz LF and
Cordero A: A machine learning algorithm for the identification
elevated Lp(a) in patients with, or high-risk of having, coronary
heart disease. Int J Cardiol. 418:1326122025. View Article : Google Scholar
|
|
137
|
Tavaglione F, Marafioti G, Romeo S and
Jamialahmadi O: Machine learning reveals the contribution of
lipoproteins to liver triglyceride content and inflammation. J Clin
Endocrinol Metab. 110:218–227. 2025. View Article : Google Scholar
|
|
138
|
Sezer S, Oter A, Ersoz B, Topcuoglu C,
İbrahim Bulbul H, Sagiroglu S, Akin M and Yilmaz G: Explainable
artificial intelligence for LDL cholesterol prediction and
classification. Clin Biochem. 130:1107912024. View Article : Google Scholar
|
|
139
|
Jalepalli SK, Gupta P, Dekker ALAJ,
Bermejo I and Kar S: Development and validation of multicentre
study on novel Artificial intelligence-based cardiovascular risk
score (AICVD). Fam Med Community Health. 12 (Suppl 1):e0023402024.
View Article : Google Scholar
|
|
140
|
Mohanty PK, Francis SAJ, Barik RK, Roy DS
and Saikia MJ: Leveraging shapley additive explanations for feature
selection in ensemble models for diabetes prediction.
Bioengineering (Basel). 11:12152024. View Article : Google Scholar
|
|
141
|
Bezsonov E, Chernyi N, Saruhanyan M,
Shimchenko D, Bondar N, Gavrilova D, Baig MS and Malogolovkin A:
Gene therapy approaches for atherosclerosis focusing on targeting
lipid metabolism and inflammation. Int J Mol Sci. 26:69502025.
View Article : Google Scholar
|
|
142
|
Kaya Kaçar H, Kaçar OF and Avery A: Diet
quality and caloric accuracy in ai-generated diet plans: A
comparative study across chatbots. Nutrients. 17:2062025.
View Article : Google Scholar
|
|
143
|
Kassem H, Beevi AA, Basheer S, Lutfi G,
Cheikh Ismail L and Papandreou D: Investigation and assessment of
AI's role in nutrition-an updated narrative review of the evidence.
Nutrients. 17:1902025. View Article : Google Scholar
|
|
144
|
Wang X, Sun Z, Xue H and An R: Artificial
intelligence applications to personalized dietary recommendations:
A systematic review. Healthcare (Basel). 13:14172025. View Article : Google Scholar
|
|
145
|
Lin A, Manral N, McElhinney P, Killekar A,
Matsumoto H, Kwiecinski J, Pieszko K, Razipour A, Grodecki K, Park
C, et al: Deep learning-enabled coronary CT angiography for plaque
and stenosis quantification and cardiac risk prediction: An
international multicentre study. Lancet Digit Health. 4:e256–e265.
2022. View Article : Google Scholar
|
|
146
|
Zhang LJ, Chen Q, Zhou F, Xie G, Tang CX,
Gao X, Zhang Y, Yin X and Xu H: Advances in Artificial
intelligence-assisted coronary computed tomographic angiography for
atherosclerotic plaque characterization. Rev Cardiovasc Med.
25:272024. View Article : Google Scholar
|
|
147
|
Chong PL, Vaigeshwari V, Mohammed
Reyasudin BK, Noor Hidayah BRA, Tatchanaamoorti P, Yeow JA and Kong
FY: Integrating artificial intelligence in healthcare:
Applications, challenges, and future directions. Future Sci OA.
11:25275052025. View Article : Google Scholar
|
|
148
|
Młynarska E, Bojdo K, Frankenstein H,
Kustosik N, Mstowska W, Przybylak A, Rysz J and Franczyk B:
Nanotechnology and artificial intelligence in dyslipidemia
management-cardiovascular disease: Advances, challenges, and future
perspectives. J Clin Med. 14:8872025. View Article : Google Scholar
|
|
149
|
Cross JL, Choma MA and Onofrey JA: Bias in
medical AI: Implications for clinical decision-making. PLOS Digital
Health. 3:e00006512024. View Article : Google Scholar
|
|
150
|
Almogadwy B and Alqarafi A: Fused
federated learning framework for secure and decentralized patient
monitoring in healthcare 5.0 using IoMT. Sci Rep. 15:242632025.
View Article : Google Scholar
|
|
151
|
Pati S, Kumar S, Varma A, Edwards B, Lu C,
Qu L, Wang JJ, Lakshminarayanan A, Wang SH, Sheller MJ, et al:
Privacy preservation for federated learning in health care.
Patterns (N Y). 5:1009742024. View Article : Google Scholar
|
|
152
|
Katsoulakis E, Wang Q, Wu H, Shahriyari L,
Fletcher R, Liu J, Achenie L, Liu H, Jackson P, Xiao Y, et al:
Digital twins for health: A scoping review. NPJ Digit Med.
7:772024. View Article : Google Scholar
|
|
153
|
Vallée A: Digital twin for healthcare
systems. Front Digit Health. 5:12530502023. View Article : Google Scholar
|
|
154
|
Marey A, Arjmand P, Alerab ADS, Eslami MJ,
Saad AM, Sanchez N and Umair M: Explainability, transparency and
black box challenges of AI in radiology: Impact on patient care in
cardiovascular radiology. Egypt J Radiol Nucl Med. 55:1832024.
View Article : Google Scholar
|
|
155
|
Nagaraj D, Khandelwal P, Steyaert S and
Gevaert O: Augmenting digital twins with federated learning in
medicine. Lancet Digit Health. 5:e251–e253. 2023. View Article : Google Scholar
|
|
156
|
Kolaszyńska O and Lorkowski J: Artificial
intelligence in cardiology and atherosclerosis in the context of
precision medicine: A scoping review. Appl Bionics Biomech.
2024:29912432024. View Article : Google Scholar
|
|
157
|
Saether JC, Klevjer M, Giskeødegård GF,
Bathen TF, Gigante B, Gjære S, Myhra M, Vesterbekkmo EK, Wiseth R,
Madssen E and Bye A: Small LDL subfractions are associated with
coronary atherosclerosis despite no differences in conventional
lipids. Physiol Genomics. 55:16–26. 2023. View Article : Google Scholar
|
|
158
|
Licari C, Tenori L, Di Cesare F, Luchinat
C, Giusti B, Kura A, De Cario R, Inzitari D, Piccardi B, Nesi M, et
al: Nuclear magnetic resonance-based metabolomics to predict early
and late adverse outcomes in ischemic stroke treated with
intravenous thrombolysis. J Proteome Res. 22:16–25. 2023.
View Article : Google Scholar
|
|
159
|
Kanonidou C: Small dense low-density
lipoprotein: Analytical review. Clin Chim Acta. 520:172–178. 2021.
View Article : Google Scholar
|
|
160
|
Wong ND, Budoff MJ, Ferdinand K, Graham
IM, Michos ED, Reddy T, Shapiro MD and Toth PP: Atherosclerotic
cardiovascular disease risk assessment: An American Society for
Preventive Cardiology clinical practice statement. Am J Prev
Cardiol. 10:1003352022. View Article : Google Scholar
|
|
161
|
Srichawla BS, Gopal D and Moonis M:
Association of statin therapy with functional outcomes and survival
in intracerebral and subarachnoid hemorrhage. Neurol Int.
17:272025. View Article : Google Scholar
|
|
162
|
Hafiz A, Aljohani S, Kutbi H, Fatani N,
Alkhathran L, Alyaqub M, Alhamed MS, Alhaqbani AO, Alhadlaq AA,
Alsalman MA, et al: Statin use and major adverse cardiovascular
events among patients with ischemic heart diseases: A multi-center
retrospective study. J Clin Med. 14:9082025. View Article : Google Scholar
|
|
163
|
Liao PC, Chen MS, Jhou MJ, Chen TC, Yang
CT and Lu CJ: Integrating health data-driven machine learning
algorithms to evaluate risk factors of early stage hypertension at
different levels of HDL and LDL Cholesterol. Diagnostics (Basel).
12:19652022. View Article : Google Scholar
|
|
164
|
Masson W, Corral P, Nogueira JP and
Lavalle-Cobo A: Applicability of artificial intelligence in the
field of clinical lipidology: A narrative review. J Lipid
Atheroscler. 13:111–121. 2024. View Article : Google Scholar
|
|
165
|
Guo CL, Han X, Zhang T, Zhang H, Li X,
Zhou X, Feng S, Tao T, Yin C and Xia J: Lipidomic analyses reveal
potential biomarkers for predicting death and heart failure after
acute myocardial infarction. Clin Chim Acta. 562:1198922024.
View Article : Google Scholar
|
|
166
|
Girona J, Soler O, Samino S, Junza A,
Martínez-Micaelo N, García-Altares M, Ràfols P, Esteban Y, Yanes O,
Correig X, et al: Lipidomics reveals myocardial lipid composition
in a murine model of insulin resistance induced by a high-fat diet.
Int J Mol Sci. 25:27022024. View Article : Google Scholar
|
|
167
|
Wittenhofer P, Kiesewetter L, Schmitz OJ
and Meckelmann SW: Investigation of the cholesterol biosynthesis by
heart-cut liquid chromatography and mass spectrometric detection. J
Chromatogr A. 1738:4654752024. View Article : Google Scholar
|
|
168
|
Castro C, Harshfield EL, Butterworth AS,
Wood AM, Koulman A and Griffin JL: A lipidomic dataset for
epidemiological studies of acute myocardial infarction. Data Brief.
57:1109252024. View Article : Google Scholar
|
|
169
|
Gadgil MD, Herrington DM, Singh SK,
Kandula NR and Kanaya AM: Association of lipoprotein subfractions
with incidence of type 2 diabetes among five US race and ethnic
groups: The mediators of atherosclerosis in South Asians living in
America (MASALA) and multi-ethnic study of atherosclerosis (MESA).
Diabetes Res Clin Pract. 204:1109262023. View Article : Google Scholar
|
|
170
|
Lomonosova A, Gognieva D, Suvorov A,
Silantyev A, Abasheva A, Vasina Y, Abdullaev M, Nartova A,
Eroshchenko N, Kazakova V, et al: The blood plasma lipidomic
profile in atherosclerosis of the brachiocephalic arteries.
Biomedicines. 12:12792024. View Article : Google Scholar
|
|
171
|
Miao GH, Pechlaner R, Fiehn O, Malloy KM,
Zhang Y, Umans JG, Mayr M, Willeit J, Kiechl S and Zhao J:
Longitudinal lipidomic signature of coronary heart disease in
American Indian people. J Am Heart Assoc. 13:e0318252024.
View Article : Google Scholar
|
|
172
|
Wang SJ, Han Y, Liu R, Hou M, Neumann D,
Zhang J, Wang F, Li Y, Zhao X, Schianchi F, et al:
Glycolysis-mediated activation of v-ATPase by nicotinamide
mononucleotide ameliorates lipid-induced cardiomyopathy by
repressing the CD36-TLR4 Axis. Circ Res. 134:505–525. 2024.
View Article : Google Scholar
|
|
173
|
Lui DTW and Tan KCB: High-density
lipoprotein in diabetes: Structural and functional relevance. J
Diabetes Investig. 15:805–816. 2024. View Article : Google Scholar
|
|
174
|
Hirano T, Satoh N and Ito Y: Specific
increase in small dense low-density lipoprotein-cholesterol levels
beyond triglycerides in patients with diabetes: Implications for
cardiovascular risk of MAFLD. J Atheroscler Thromb. 31:36–47. 2024.
View Article : Google Scholar
|















![Targeted treatment strategies for
sdLDL reduction. EPA, eicosapentaenoic acid; lab, laboratory;
LDL-C, low-density lipoprotein cholesterol; PCSK9, proprotein
convertase subtilisin/kexin type 9; sdLDL, small dense low-density
lipoprotein; TG, triglycerides. [167] [183]](/article_images/mmr/32/6/mmr-32-06-13693-g00.jpg)