Introduction
Cardiopulmonary resuscitation (CPR) is a life-saving
emergency technique used after cardiac arrest to restore
spontaneous circulation and maintain oxygenated blood flow to vital
organs, particularly the brain and heart (1). It involves chest compressions and
artificial ventilation; compressions simulate the heart's pumping
action, while ventilation supplies oxygen to the lungs (2).
Despite its importance, CPR faces challenges.
Survival rates for out-of-hospital cardiac arrests remain low, with
only 5–10% of patients surviving to hospital discharge (3). Several factors affect outcomes,
including delays in CPR initiation, the quality of compressions,
the cause of the arrest and reversible conditions such as pulmonary
embolism or myocardial infarction (4,5).
Inadequate advanced interventions and post-resuscitation care also
reduce success rates, driving ongoing efforts to enhance CPR
effectiveness and neurological outcomes (6).
Thrombolytic therapy, or fibrinolysis, uses drugs to
dissolve clots that block critical blood vessels (7). In cardiac arrest, thrombolysis
targets clots contributing to circulatory failure, such as those
from myocardial infarction or pulmonary embolism (8). This strategy is well-established in
acute myocardial infarction and ischemic stroke, where prompt clot
dissolution improves perfusion and limits tissue damage (9). Applying thrombolytics during CPR is
based on the premise that thrombotic occlusions may trigger or
worsen cardiac arrest (10). By
incorporating thrombolytic agents into CPR protocols, blood flow is
considered to be more effectively restored, thereby enhancing the
chances of successful resuscitation and improving overall outcomes
(8). However, this approach
carries bleeding risks, so its use requires careful risk-benefit
assessment.
Recombinant tissue plasminogen activator (rtPA) is a
bioengineered version of a naturally occurring enzyme that plays a
critical role in the breakdown of blood clots. rtPA converts
plasminogen, a blood protein, into plasmin, an enzyme that
dissolves fibrin clots (11). Due
to its targeted action on fibrin, rtPA is highly effective in
dissolving clots while minimizing systemic effects (12). In the context of thrombolytic
therapy for CPR, rtPA is considered one of the most promising
agents. Its efficacy has been well-documented in the treatment of
acute ischemic stroke and myocardial infarction, leading
researchers to explore its potential benefits in cardiac arrest
scenarios (13). rtPA presents
advantages over conventional TPA, including a more favorable
pharmacokinetic profile that enables faster thrombolysis, which is
crucial in cardiac arrest. Additionally, rtPA has a lower risk of
immunogenic reactions, enhancing its safety (14). The administration of rtPA during
CPR could potentially address the thrombotic component of certain
cardiac arrests, thus enhancing the likelihood of restoring
spontaneous circulation (15). The
present review aims to critically analyze the efficacy of
thrombolytic therapy, specifically focusing on the role of rtPA in
CPR. The present review evaluated the current evidence surrounding
rtPA administration during CPR, considering its effect on patient
outcomes, such as survival rates, neurological function and
long-term prognosis. By synthesizing available research findings
the present review provided insights into the potential benefits,
limitations and optimal protocols for implementing rtPA in CPR
protocols, ultimately contributing to informed decision-making and
advancements in resuscitative care strategies.
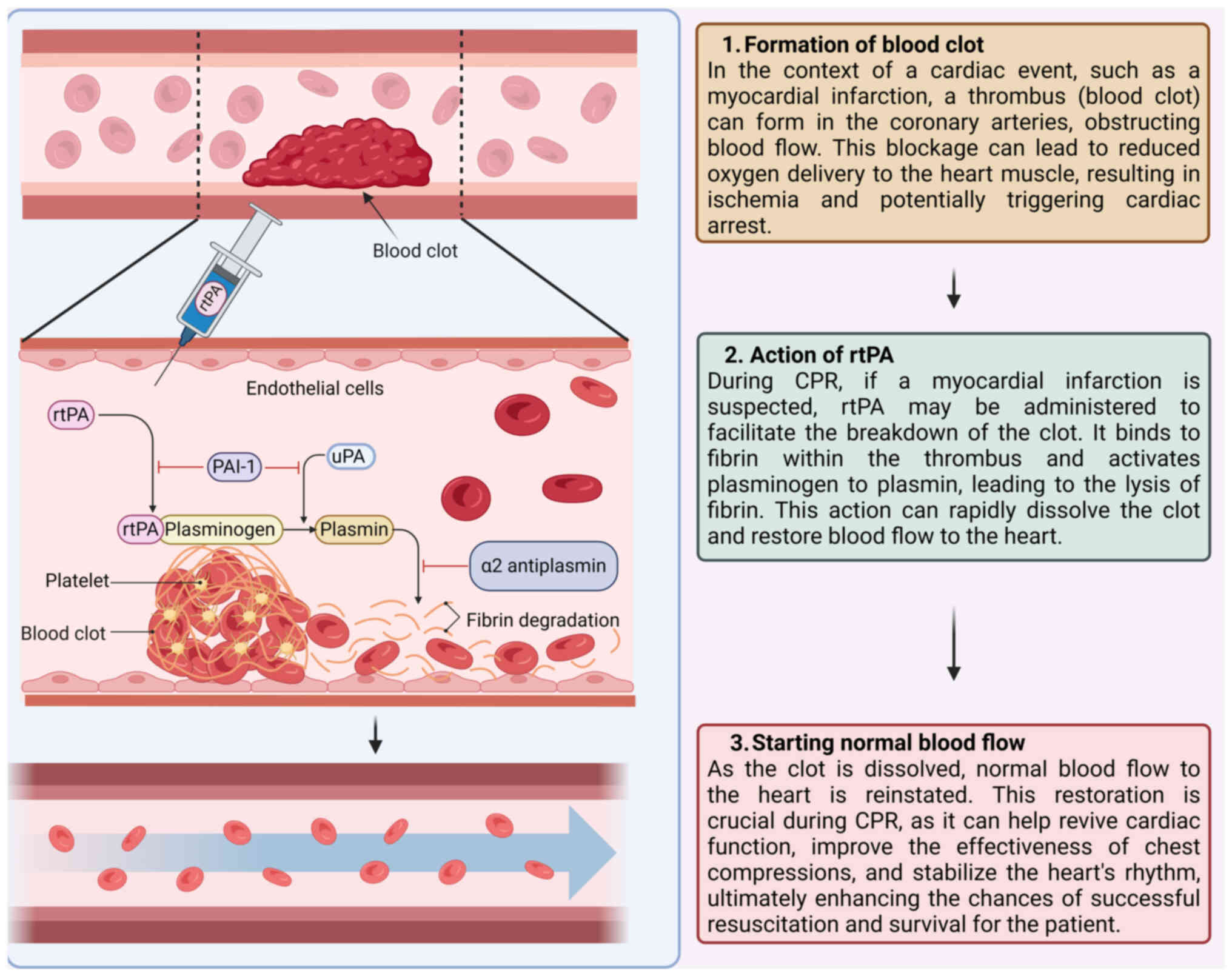
Mechanism of action of thrombolytic therapy
in CPR
The fibrinolytic pathway and clot
dissolution
The fibrinolytic pathway is a crucial biological
mechanism responsible for the breakdown of thrombi. This pathway
removes unnecessary or harmful clots, maintaining vascular patency
and normal blood flow, but it can also disrupt normal hemostasis,
increasing the risk of bleeding (16). The process begins with activating
plasminogen, a precursor glycoprotein converted into its active
form, plasmin (17). Plasmin is a
serine protease that digests fibrin, the primary protein
constituent of blood clots (18)
(Fig. 1). The fibrinolytic system
can be activated via two pathways: The intrinsic pathway, initiated
by contact with negatively charged surfaces, and the extrinsic
pathway, which is triggered by tissue plasminogen activator (tPA)
or urokinase plasminogen activator (uPA) released from endothelial
cells in response to injury or stress (17,19).
In considering thrombolytic therapy, a comparative analysis of tPA
and uPA is crucial. tPA is widely preferred for its rapid action
and specificity for fibrin-bound plasminogen, making it effective
in acute ischemic stroke and myocardial infarction. However, its
use may be restricted by bleeding risks and contraindications
(20,21). Conversely, uPA, while less commonly
used, offers advantages in patients with higher bleeding risk or
contraindications to tPA and it can be effective in various
thrombotic conditions, including pulmonary embolism. A thorough
comparison of these agents can guide clinicians in making informed
decisions tailored to individual patient needs and specific
clinical scenarios (22).
The use of tPA and uPA carries inherent bleeding
risks and specific contraindications. For tPA, the reported risk of
symptomatic intracranial hemorrhage (ICH) is ~6.4% when
administered for acute ischemic stroke, with overall bleeding risks
reaching 10–15%. Contraindications for tPA include recent
intracranial hemorrhage, active bleeding, significant head trauma,
major surgery within the past three months and severe uncontrolled
hypertension (23). By contrast,
uPA is associated with a lower overall bleeding risk, estimated at
1–2% for major events, although its specific risk profile is less
characterized compared with tPA (24). Regarding efficacy, tPA has
demonstrated 30–35% effectiveness in achieving functional
independence in ischemic stroke patients and is effective in
myocardial infarction and pulmonary embolism (25). In comparison, uPA has shown
variable efficacy rates, typically lower than tPA and is not as
widely accepted in acute stroke or myocardial infarction. tPA has
high fibrin specificity, a half-life of <5 min and is
administered via bolus and infusion over 90 min, while uPA lacks
fibrin specificity, has a half-life of 10–20 min and is given as an
infusion. The two agents have similar ICH risks, but tPA shows
higher rates of recanalization and improved long-term recovery
outcomes (26). In terms of roles
in stroke, tPA is critical for acute thrombolysis, affecting
vascular permeability and inflammation, while uPA promotes synaptic
recovery during the recovery phase. tPA disrupts the blood-brain
barrier, increasing permeability and leukocyte infiltration,
whereas uPA may enhance neovascularization (27). Overall, tPA can enhance synaptic
plasticity but may induce excitotoxicity, while uPA supports
neuronal recovery and synaptic repair. Understanding these
parameters is crucial for optimizing thrombolytic therapy and
managing associated risks effectively.
Role of rtPA in converting plasminogen
to plasmin
rtPA is a synthetic form of the naturally occurring
tPA designed for therapeutic use to enhance clot dissolution
(28). rtPA functions by binding
to fibrin within the clot and converting the adjacent plasminogen
to plasmin (29). This localized
conversion is critical as it ensures that plasmin is generated
primarily at the site of the thrombus, minimizing systemic
activation, which could lead to widespread bleeding complications
(30,31). Once administered, rtPA enhances the
conversion efficiency of plasminogen to plasmin, amplifying the
fibrinolytic activity at the site of the thrombus. This increase in
plasmin leads to the more rapid and effective breakdown of fibrin,
the structural framework of the clot. By dissolving the fibrin
mesh, rtPA facilitates the disintegration of the thrombus, thereby
restoring blood flow through the occluded vessel (32).
In comparing rTPA with conventional plasminogen
activators such as TPA, several key differences emerge that
highlight the advantages of rTPA in clinical practice. rTPA
specifically targets fibrin-bound plasminogen, which enhances
fibrinolysis directly at the site of the clot. This targeted action
increases its effectiveness in dissolving thrombi, a critical
factor in acute ischemic events (33). Furthermore, rTPA demonstrates a
more rapid onset of action compared with conventional TPA, allowing
for quicker therapeutic effects in urgent situations. This rapid
response is crucial for improving patient outcomes in acute stroke
management. The pharmacological profile of rTPA also emphasizes its
preferential action on clot-bound fibrin, which markedly boosts its
efficacy in thrombus dissolution (34). The safety profile of rTPA is more
thoroughly characterized in the literature, with studies indicating
a clearer understanding of its potential adverse drug reactions
(35). Notably, while both agents
carry a risk of bleeding, the specific safety data of rTPA provide
clinicians with more reliable information for risk assessment.
Additionally, rTPA has a shorter half-life, which is beneficial for
managing acute ischemic events, as it allows for rapid clinical
adjustments based on the patient's response (36). These distinctions not only
elucidate the pharmacological advantages of rTPA but also
underscore its role in improving therapeutic outcomes in patients
requiring acute thrombolytic therapy (37).
Potential benefits of thrombolysis in
restoring coronary and pulmonary blood flow
Thrombolytic therapy, particularly with agents such
as rtPA, holds significant potential benefits during CPR.
Thrombotic occlusions during cardiac arrest can impair coronary and
pulmonary circulation, worsening myocardial and cerebral ischemia
and lowering resuscitation success rates. By dissolving these
clots, thrombolytic therapy can restore blood flow and improve
oxygen delivery to vital organs (38). In acute myocardial infarction or
massive pulmonary embolism (PE)-induced arrest, rtPA can rapidly
dissolve the causative thrombi, reestablish coronary perfusion and
potentially convert non-perfusing rhythms, such as ventricular
fibrillation or pulseless electrical activity, into perfusing ones
(39,40). Similarly, in PE, thrombolysis can
alleviate the obstruction in the pulmonary arteries, reduce right
ventricular strain and enhance cardiac output. Restoring coronary
and pulmonary circulation during CPR can improve resuscitation
efficacy, stabilize hemodynamics and increase return of spontaneous
circulation (ROSC) rates (41).
Reducing ischemic time may also lessen post-resuscitation
myocardial dysfunction and limit ischemic damage to the heart and
other organs (42,43). Consequently, integrating
thrombolytic therapy into CPR protocols, especially in cases where
thrombotic occlusions are suspected, can be a crucial strategy to
improve survival outcomes and neurological recovery in cardiac
arrest patients (44).
Diagnosing PE and ST-elevation myocardial infarction
(STEMI) during CPR poses significant challenges for clinicians.
Rapid decision-making is crucial, often relying on clinical
history, symptoms and immediate ECG findings. In STEMI, chest pain
and ST-segment elevation guide diagnosis, while PE may present with
sudden dyspnea and hemodynamic instability (45). To confirm thrombotic causes
quickly, point-of-care ultrasound and imaging techniques such as
computed tomography pulmonary angiography are helpful, though not
always available during CPR. Clinicians may also use risk
stratification tools, such as the Wells score for PE and the TIMI
score for STEMI (38), along with
biomarkers such as cardiac troponins and D-dimer levels, to inform
their decisions regarding rtPA administration. Ultimately, timely
identification requires a high level of clinical awareness and
effective teamwork (46).
Application of thrombolytic therapy in
CPR
Clinical studies and trials evaluating
thrombolytic therapy in CPR
Thrombolytic therapy, particularly rtPA, has been
explored as an adjunct to standard CPR protocols in several
clinical studies and trials (Fig.
2). For instance, the TICA trial indicated that rtPA could
improve ROSC rates, with reported rates of ~35% in the rtPA group
compared with ~20% in standard CPR cases (47). However, it also raised concerns
about safety, necessitating further research on optimal dosing and
outcomes. In a study by Bozeman et al (48), the use of tenecteplase (TNK) after
failed Advanced Cardiac Life Support (ACLS) measures resulted in a
ROSC rate of 26% compared with 12.4% in the control group (P=0.04).
Additionally, 12% of TNK patients survived to admission compared
with none in the control group (P=0.0007). This study emphasized
the potential benefit of timely intervention, as TNK was given
after an average of 30 min of cardiac arrest.
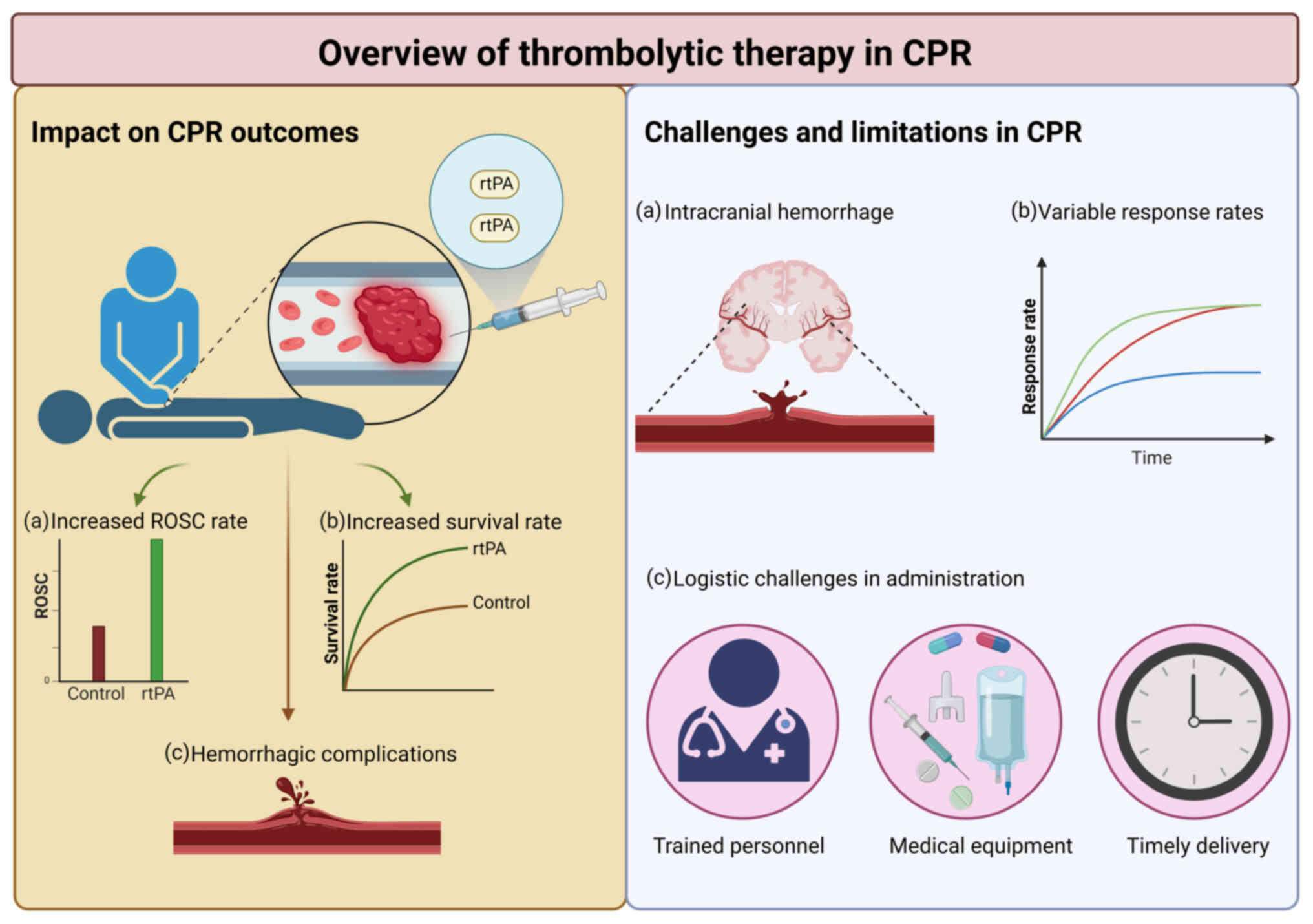 | Figure 2.The potential benefits of
thrombolytic therapy, particularly rtPA, in improving rates of ROSC
during CPR are highlighted. It emphasizes observed outcomes, such
as increased ROSC rates and early survival, while also addressing
significant challenges and limitations, including the risk of
bleeding complications, variable patient responses and logistical
hurdles in therapy administration. Together, these elements
underscore the complex considerations that healthcare providers
must navigate when integrating thrombolytic therapy into CPR
protocols. Created with BioRender.com. rtPA, recombinant tissue
plasminogen activator; ROSC, return of spontaneous circulation;
CPR, cardiopulmonary resuscitation. |
In a prospective study, 108 patients received rtPA
during CPR, while 216 matched controls received standard CPR
without thrombolysis. The rtPA group demonstrated markedly improved
outcomes, with ROSC achieved in 70.4% compared with 51.0% in the
control group. Additionally, 24-h survival was 48.1% in the rtPA
group compared with 32.9% in controls. Survival to hospital
discharge was also higher in the rtPA group at 25.9%, although
exact comparative data for the control group was not specified.
Among patients with presumed PE, outcomes were even more favorable:
24-h survival was 57.9% and discharge survival reached 31.6%.
Bleeding complications occurred in 5.6% of rtPA patients, including
one case of intracranial hemorrhage (0.9%), which was equal to the
ICH rate of the control group (0.9%) (30). In a retrospective study of 66
patients with CA, rtPA administered during or shortly after CPR was
associated with improved early survival outcomes compared with
standard CPR protocols. ROSC was achieved in 67% of patients in the
rtPA group compared with 43% in the standard CPR protocol group and
24-h survival was markedly higher at 53% compared with 23% in
controls. Importantly, prolonged CPR (≥10 min) did not correlate
with increased bleeding risk. The data suggest that administration
of rtPA during CPR for CA could improve ROSC and short-term
survival compared with the standard CPR protocols (49).
In another prospective study involving 90 patients
with out-of-hospital cardiac arrest, 40 received rt-PA during CPR,
while 50 received standard CPR alone. Thrombolytic therapy markedly
improved clinical outcomes. ROSC occurred in 68% of rt-PA patients
compared with 44% of controls and 58% were admitted to a cardiac
intensive care unit compared with 30% of controls. Although 24-h
survival (35 vs. 22%) and hospital discharge rates (15 vs. 8%)
favored the rtPA group, these differences were not statistically
significant. Notably, no bleeding complications related to CPR were
observed. However, two rtPA patients experienced gastrointestinal
bleeding requiring transfusion, occurring 2- and 12-days
post-arrest (19). In a
randomized, double-blind, placebo-controlled trial involving 233
patients with cardiac arrest and presumed cardiovascular etiology,
the efficacy of tPA during CPR was evaluated against standard CPR
alone. Patients were randomized to receive either tPA or placebo in
addition to standard CPR. ROSC occurred in 21.4% of the tPA group
and 23.3% of the placebo group, showing no significant benefit.
Only one patient (0.9%) in the tPA group survived to hospital
discharge, compared with none in the placebo group. Four tPA
patients survived more than 24 h post-admission, but this did not
translate into improved overall outcomes (50).
The Thrombolysis in Cardiac Arrest (TROICA) study
(51), an international
multicenter trial, evaluated the efficacy and safety of
tenecteplase during CPR for out-of-hospital cardiac arrest. Despite
its larger scale, TROICA found no significant improvement in
long-term survival or neurological outcomes compared with placebo.
The study reported ROSC rates of ~30% in the rtPA group compared
with 20% in the control group. Timing was critical; rtPA was
administered as soon as feasible after ROSC, typically within 30
min. However, the quality of post-resuscitation care varied, which
may have obscured the potential benefits of thrombolysis.
Furthermore, Bakkum et al (36) demonstrated that an accelerated
regimen of rtPA (0.6 mg/kg in 15 min) resulted in a trend toward
higher ROSC rates (67 compared with 43%; P=0.06) compared with no
thrombolysis in patients with pulmonary embolism-induced
circulatory arrest. Amini et al (35) investigated the efficacy and safety
of reduced doses of rtPA in patients with acute pulmonary embolism,
comparing it to standard doses and anticoagulation therapy.
Analyzing data from 13 studies, including four observational
studies with 4,223 patients and nine randomized controlled trials
involving 780 patients, the authors found that the reduced dose was
associated with a markedly lower incidence of total bleeding events
(RR 5.08; 95%CI 1.39–18.6) compared with anticoagulants.
Furthermore, when comparing standard and reduced doses of rtPA, the
standard dose showed a greater incidence of bleeding (RR 1.48;
95%CI 1.00–2.19). Importantly, there were no significant
differences in all-cause mortality or recurrence of PE between the
treatment groups, suggesting that a reduced dose of rtPA offers a
safer alternative without compromising efficacy. Doelare et
al (52) investigated the
relationship between fibrinogen levels and major bleeding risk
during catheter-directed thrombolysis for acute limb ischemia. Of
443 patients treated with rtPA, the overall incidence of major
bleeding was 7%. Patients with fibrinogen levels below 1.0 g/l had
a markedly higher bleeding rate (15 vs. 6%; P=0.041), highlighting
low fibrinogen as a critical risk factor. Additionally, high-dose
thrombolytic regimens and older age were independent predictors of
major bleeding. The TROICA study reported a lower incidence of
major bleeding complications at ~3%, attributed to careful patient
selection and monitoring (51).
The contrasting results across these studies may be attributed to
several factors, including variability in patient characteristics
(such as age or comorbidities) that may influence outcomes, as seen
in the TROICA study where patient selection criteria were stringent
(53). The timing of rtPA
administration markedly affected outcomes; studies that
administered rtPA more quickly after cardiac arrest generally
reported improved results. Additionally, differences in dosing
strategies (standard compared with accelerated or reduced doses)
could explain variations in efficacy and safety profiles observed
in different trials (54).
Finally, variability in post-resuscitation care protocols across
studies can markedly affect observed outcomes. Factors such as the
availability of advanced monitoring, therapeutic hypothermia and
comprehensive cardiac care can differ widely between institutions
and trials. Inconsistent care may obscure the potential benefits of
thrombolytic therapy, as optimal post-resuscitation management is
crucial for patient recovery and overall survival (55) (Table
I). It is important to note that neurological outcomes have not
been evaluated in most of these studies.
 | Table I.Comparison of rtPA and standard CPR
protocols. |
Table I.
Comparison of rtPA and standard CPR
protocols.
| Trial or first
author/s, year | Intervention | ROSC rates | Survival rates | Key findings | (Refs.) |
|---|
| Böttiger et
al, 2001 | rtPA | 68 (rtPA) vs. 44%
(control) | 24 h: 35 vs. 22%;
discharge: 15 vs. 8% | rtPA improved
outcomes, but survival differences were not statistically
significant. No CPR-related bleeding; 2 GI bleeds in rtPA
group | (19) |
| Lederer et
al, 2001 | rtPA | 70.4% (rtPA) vs.
51.0% (control) | 24 h survival: 48.1
vs. 32.9%; discharge: 25.9% (rtPA); control not specified | rtPA improved ROSC
and survival. PE subgroup had even higher 24 h (57.9%) and
discharge (31.6%) survival. Bleeding: 5.6, ICH: 0.9% (same as
control) | (30) |
| Amini et al,
2021 | Reduced dose
rtPA | Not specified | No significant
differences | Reduced doses led
to fewer bleeding events without compromising efficacy | (35) |
| Bakkum et
al, 2021 | Accelerated
rtPA | 67 (accelerated)
vs. 43% (no thrombolysis) | Not specified | Higher ROSC rates
with accelerated dosing for pulmonary embolism | (36) |
| TICA trial,
2004 | rtPA | 35 (rtPA) vs. 20%
(standard CPR) | Not specified | Improved ROSC with
rtPA; raised safety concerns | (47) |
| Bozeman et
al, 2006 | Tenecteplase
(rtPA) | 26 (rtPA) vs. 12.4%
(control) | 12 (rtPA) vs. 0%
(control) | Emphasized timely
intervention; rtPA given after 30 min | (48) |
| Janata et
al, 2003 | rtPA | 67 (rtPA) vs. 43%
(control) | 24 h survival: 53
vs. 23% | rtPA improved ROSC
and 24h survival. No increased bleeding with prolonged CPR. | (49) |
| Abu-Laban et
al, 2002 | tPA | 21.4 (tPA) vs.
23.3% (placebo) | Discharge: 0.9
(tPA) vs. 0% (placebo); 4 tPA patients survived >24h | No significant
benefit of tPA. Only one discharge survivor in tPA group | (50) |
| TROICA study,
2005 | Tenecteplase
(rtPA) | 30 (rtPA) vs. 20%
(control) | No significant
improvement | No long-term
survival benefit; variability in post-resuscitation care | (51) |
| Doelare et
al, 2023 | rtPA | Not specified | Major bleeding
incidence: 7% | High fibrinogen
levels linked to increased bleeding risk | (52) |
Efficacy outcomes, rates of ROSC and
survival to hospital admission and discharge
The efficacy of thrombolytic therapy in the context
of CPR is primarily measured through rates of ROSC, survival to
hospital admission and survival to hospital discharge with
favorable neurological outcomes (56). In studies where thrombolytics were
administered, an increase in ROSC rates was often observed
(57). For instance, a
meta-analysis of several small trials indicated a statistically
significant improvement in ROSC rates when rtPA was used. However,
the effect on survival to hospital discharge remains less
definitive (58). This highlights
the need to distinguish between short-term efficacy (such as ROSC
and hospital admission) and long-term outcomes (such as survival to
discharge and neurological recovery). While some studies have
reported higher survival rates for hospital admission, these
improvements do not always translate to long-term survival or
favorable neurological outcomes (59). The TROICA trial, for example, found
no significant difference in survival to hospital discharge between
the thrombolytic and placebo groups, highlighting the complexity
and potential limitations of thrombolytic therapy in CPR. Moreover,
even when ROSC is achieved, the potential for hypoxic brain injury
and subsequent neurological impairment remains high, underlining
the importance of evaluating therapies not only for their ability
to restore circulation but also for their influence on neurological
prognosis and functional recovery (60,61).
Future studies and clinical protocols should prioritize these
distinctions to improved assess the true benefit of thrombolytic
therapy in cardiac arrest scenarios.
Comparison of outcomes between
thrombolytic therapy and standard CPR protocols
The data reveals potential benefits and notable
risks when comparing outcomes between thrombolytic therapy and
standard CPR protocols. Standard CPR protocols, which do not
include thrombolytics, have established benchmarks for ROSC and
survival rates. Thrombolytic therapy, in theory, offers an
advantage by addressing underlying thrombotic causes of cardiac
arrest, potentially enhancing coronary and cerebral perfusion
during resuscitation efforts (19). However, while some studies suggest
improved ROSC rates with thrombolytics, the overall survival to
hospital discharge and neurological outcomes do not consistently
show significant improvements over standard protocols (62). Moreover, the risk of adverse
effects, particularly hemorrhagic complications, remains a critical
concern. Bleeding risks associated with thrombolytic therapy can
offset potential benefits, especially in the context of traumatic
cardiac arrests or in patients with underlying conditions
predisposing them to hemorrhage (63).
Indications and patient selection for
thrombolytic therapy in CPR
Identification of suitable candidates
for thrombolytic treatment in CPR
Identifying suitable candidates for thrombolytic
therapy during CPR involves assessing the underlying cause of
cardiac arrest, the patient's medical history and the likelihood of
a favorable outcome from thrombolysis. Thrombolytic therapy,
particularly with rtPA, is most beneficial in cases where cardiac
arrest is due to PE, MI or other thrombotic events (64). Clinicians should prioritize
patients with witnessed arrest, a short duration of arrest before
the initiation of CPR and those without severe comorbidities
(65). The presence of risk
factors such as recent surgery, active bleeding, or a history of
hemorrhagic stroke generally contraindicates the use of
thrombolytics due to the elevated risk of bleeding complications
(44,58). If available, advanced imaging
techniques such as echocardiography or computed tomography scans
can help confirm the presence of thrombi, guiding the decision to
administer rtPA (44).
Thrombolytic therapy in CPR involves careful consideration of
various factors, including indications such as pulmonary embolism
and myocardial infarction (44),
as well as exclusion criteria like active bleeding and recent
surgery (66) (Table II). Patient selection is critical,
taking into account age, sex, and pre-existing conditions such as
carotid artery stenosis (67,68).
Administering therapy promptly can improve survival and
neurological outcomes (69,70).
This therapy should be coupled with adjunctive treatments (71), and careful monitoring (72), while adhering to established
clinical guidelines for individualized care (73).
 | Table II.Overview of indications and patient
criteria for thrombolytic therapy in CPR. |
Table II.
Overview of indications and patient
criteria for thrombolytic therapy in CPR.
| First author/s,
year | Category | Details | (Refs.) |
|---|
| Javaudin et
al, 2019 | Indications | 1. Pulmonary
embolism: Suspected or confirmed massive pulmonary embolism causing
cardiac arrest. | (44) |
|
|
| 2. Myocardial
infarction: ST-elevation myocardial infarction leading to cardiac
arrest. |
|
| Page et al,
2021 | Exclusion
criteria | 1. Active internal
bleeding: contraindicated due to the risk of exacerbating
bleeding. | (66) |
|
|
| 2. Recent surgery
or trauma: Due to bleeding risk within the past 2–4 weeks. |
|
| Higgins, 2011/Blum
et al, 2019 | Patient
selection | 1. Age levels:
Males under 75 and females under 70 are often preferred for
thrombolytic therapy due to a more favorable risk-to-benefit
ratio. | (67,68) |
|
|
| 2. Sex factors: Sex
differences were observed, with more females excluded due to
clinical risk factors associated with age, such as carotid artery
stenosis and history of previous strokes. |
|
|
|
| 3. Health
conditions: Significant health conditions impacting eligibility for
thrombolytic therapy included: |
|
|
|
| • Carotid artery
stenosis: Higher exclusion rates in both males and females. |
|
|
|
| • Atrial
fibrillation: Particularly affected male patients. |
|
|
|
| • Previous stroke:
Increased likelihood of exclusion for both sexes. |
|
|
|
| 4. Time since
arrest: Best outcomes if administered within min to a few h of
arrest. |
|
| Stang, 2010/Qiao
et al, 2024 | Outcomes | 1. Survival rates:
Increased survival rates in selected patients, particularly those
with pulmonary embolism. | (69,70) |
|
|
| 2. Neurological
outcomes: Improves functional recovery and reduces post-stroke
complications, such as sleep disturbances, epilepsy, anxiety and
depression, thereby enhancing overall quality of life. |
|
| Broderick et
al, 2021 | Adjunctive
therapies | 1. Anticoagulation:
Heparin, in forms such as low molecular weight heparin or
unfractionated heparin, is used alongside Warfarin, which is
started after thrombolytic treatment to prevent re-thrombosis. | (71) |
|
|
| 2. Mechanical
support: Use devices such as extracorporeal membrane oxygenation
for hemodynamic support in select cases. |
|
| Kluft et al,
2017 | Monitoring and
follow-up | 1. Bleeding risks:
Continuous monitoring for bleeding complications involves clinical
assessments for signs such as bruising and unusual mucosal
bleeding, regular laboratory tests to evaluate coagulation
parameters such as activated partial thromboplastin time and
prothrombin time and event reporting to document bleeding
occurrences, categorizing them as major or minor based on
severity. | (72) |
|
|
| 2. Cardiac
function: Regular assessment of cardiac function post-therapy. |
|
| Renard et
al, 2011 | Clinical
guidelines | 1. The American
Heart Association and the American College of Cardiology
guidelines: Follow specific protocols for thrombolytic
administration in CPR. | (73) |
|
|
| 2. Individualized
care: Decisions tailored based on patient condition and
response. |
|
Considerations for timing, dosing and
administration route of rtPA
The timing of thrombolytic therapy during CPR is
crucial for its effectiveness. The administration of rtPA should
occur as early as possible after cardiac arrest, ideally within the
first few min of CPR, to maximize the chances of restoring
spontaneous circulation (74).
This approach is supported by findings from observational studies
and case series, which indicate that early administration of
thrombolytics, particularly within the first 10–15 min of arrest,
is associated with improved ROSC and neurological outcomes.
Additionally, recommendations from the European Resuscitation
Council and the American Heart Association suggest considering
thrombolytics during CPR in patients with suspected or confirmed
PE, provided that administration does not markedly delay other
resuscitative efforts (65,75–77).
The standard dosing regimen for rtPA in the context
of CPR often involves a bolus injection followed by a continuous
infusion. However, protocols may vary based on institutional
practices and patient-specific factors (54). The typical dose ranges from 50–100
mg of rtPA, administered over 15–60 min as observed in studies and
clinical guidelines addressing massive PE and cardiac arrest
management (75–77). In practice, a 50 mg bolus may be
used in out-of-hospital settings to allow rapid administration,
whereas a 100 mg infusion over 2 h is common in in-hospital PE
protocols, adapted for use during prolonged resuscitation. The
administration route is typically intravenous, but intraosseous
access can be considered if intravenous access is not readily
available. Continuous monitoring of the patient during and after
the administration is essential to manage potential complications,
such as bleeding or allergic reactions and to assess the
effectiveness of the therapy. These recommendations are
increasingly being refined through accumulating evidence from
registry data, observational cohorts and ongoing randomized
controlled trials (78–82) (Table
III).
 | Table III.Factors to consider regarding the
timing, dosing and administration route of rtPA. |
Table III.
Factors to consider regarding the
timing, dosing and administration route of rtPA.
| First author/s,
year | Factor | Key
considerations | (Refs.) |
|---|
| Nadkarni et
al, 2006 | Timing | Early
administration: Best outcomes observed when administered within
3–4.5 h of symptom onset. | (80) |
|
|
| Delayed treatment:
Reduced efficacy and increased risk of complications beyond 4.5
h. |
|
|
|
| Extended window:
Some guidelines suggest up to 6 h in specific cases with advanced
imaging. |
|
|
|
| Monitoring:
Continuous assessment of symptoms and contraindications. |
|
| Nolan et al,
2007 | Dosing | Standard dose:
Typically, 0.9 mg/kg body weight, with a maximum dose of 90
mg. | (81) |
|
|
| Initial bolus: 10%
of the total dose given as an initial intravenous bolus over 1
min. |
|
|
|
| Infusion: Remaining
90% infused over 60 min. |
|
|
|
| Adjustments: Dose
adjustments may be necessary for patients with renal or hepatic
impairment or extreme body weight. |
|
|
|
| Pediatric
considerations: Different dosing strategies are required, not well
established for children. |
|
| Miyazaki et
al, 2019 | Administration
route | IV: Standard and
most commonly used route for administration. IA: Used in cases
where IV administration is contraindicated or unsuc cessful, often
combined with mechanical thrombectomy. | (82) |
|
|
| Combination
therapy: IV rtPA followed by IA intervention in selected cases for
improved outcomes. |
|
|
|
| Site of
administration: Ensure proper venous access and infusion monitoring
to prevent extravasation and complications. |
|
|
|
| Alternative routes:
Investigational studies on novel administration routes (such as
intraosseous) for specific clinical scenarios. |
|
Contraindications and potential risks
associated with thrombolytic therapy in CPR
Thrombolytic therapy during CPR carries significant
risks, which necessitate careful consideration of contraindications
(51). Absolute contraindications
include active internal bleeding, a history of intracranial
hemorrhage, recent major surgery or trauma, known bleeding
disorders and severe uncontrolled hypertension (83–85).
Relative contraindications, which require a risk-benefit analysis,
include recent gastrointestinal bleeding, peptic ulcer disease and
pregnancy (86). The most
significant potential risk of thrombolytic therapy is hemorrhage,
including intracranial hemorrhage, which can lead to devastating
outcomes (87). Other risks
include reperfusion arrhythmias, allergic reactions and an
increased risk of further thrombotic events in certain conditions
(88).
The decision to use rtPA during CPR must balance
these risks against the potential for improved survival and
neurological outcomes, particularly in patients with a high
likelihood of thrombotic cardiac arrest etiology. This
decision-making process often depends not only on patient-specific
factors but also on the availability of institutional protocols,
access to rapid diagnostic imaging and multidisciplinary team input
(89,90). In emergency settings, bedside tools
such as focused cardiac ultrasound can assist in identifying
possible thrombotic causes (such as massive PE), providing a
rationale for thrombolytic administration even when formal imaging
is not feasible (91).
Furthermore, institutions with predefined algorithms for cardiac
arrest management that include thrombolytic therapy in select cases
help streamline decision-making and reduce hesitation during
critical moments (92,93). Comprehensive patient assessment and
adherence to established guidelines are essential to optimizing the
benefits of thrombolytic therapy while minimizing its risks
(29). Ultimately, a tailored
approach that integrates clinical judgment, real-time diagnostics
and institutional capabilities is crucial in determining the
appropriateness of rtPA during CPR.
Challenges and limitations of thrombolytic
therapy in CPR
While promising in specific scenarios, thrombolytic
therapy poses medical and logistical challenges when used during
CPR. These issues encompass medical and logistical aspects, which
must be carefully addressed to utilize thrombolytic agents such as
rtPA effectively. This section outlines key limitations, including
bleeding risks, inconsistent efficacy and difficulties with timely
administration in diverse settings (94,95).
Bleeding complications
A primary concern with thrombolytic therapy in CPR
is the increased risk of bleeding, particularly intracranial
hemorrhage (96). While the goal
is to dissolve obstructive coronary clots and restore myocardial
blood flow, this benefit comes with a higher risk of bleeding,
especially in critical areas such as the brain (49,97).
The delicate balance between thrombolysis and hemorrhage must be
meticulously managed, as any imbalance can lead to catastrophic
outcomes (49). Moreover, CPR
patients often have impaired clotting due to pre-existing
conditions or prior treatments, further heightening bleeding risks
(98).
Variable response rates and
effectiveness
Response to thrombolytic therapy during CPR varies
markedly among patients (99).
While some patients may exhibit a robust response to thrombolytic
agents, experiencing rapid clot dissolution and successful
resuscitation, others may demonstrate limited or no response
despite timely administration (69,100). This variability stems from
multiple factors, including the cause of cardiac arrest, the degree
of vessel occlusion and individual patient characteristics such as
age, comorbidities and coagulation status. As a result, identifying
patients most likely to benefit remains a challenge and calls for
more personalized and evidence-based approaches (67).
Logistic challenges in administering
thrombolytic therapy
Besides clinical concerns, logistical barriers
hinder effective thrombolytic administration during CPR,
particularly in pre-hospital and in-hospital environments (101,102). Unlike standard CPR interventions,
thrombolytic therapy requires trained personnel, specialized
equipment and infrastructure (103,104). In pre-hospital care, EMS teams
must be trained in thrombolysis and the required drugs and
monitoring tools must be readily available en route. In hospitals,
issues such as drug storage, preparation and protocol adherence can
delay timely administration (64,73).
Addressing these challenges demands standardized protocols,
cross-disciplinary teamwork and ongoing system improvements.
Future directions and research
perspectives
Optimization of thrombolytic therapy
protocols in CPR
As CPR methods evolve, research is focusing on
refining thrombolytic therapy, especially regarding timing, dosage
and delivery of agents such as rtPA. Ongoing studies explore how
patient-specific factors, including comorbidities and arrest
etiology, can inform protocol adjustments (78,105). There is also growing interest in
personalized medicine approaches that tailor thrombolytic regimens
to individual biomarker profiles. This could maximize therapeutic
efficacy while minimizing adverse outcomes such as bleeding.
Collaborative research across clinical and industry sectors aims to
integrate thrombolysis more effectively into ACLS frameworks.
Exploration of novel thrombolytic
agents and delivery methods
In parallel with efforts to optimize existing
thrombolytic agents, researchers are exploring novel agents and
delivery methods to enhance the efficacy and safety of thrombolytic
therapy in CPR. Novel thrombolytic agents, such as modified
variants of rtPA or entirely new classes of thrombolytic drugs, are
being investigated for their potential to improve clot dissolution
kinetics and reduce the risk of reperfusion injury (106). Furthermore, advancements in drug
delivery technologies enable targeted and controlled delivery of
thrombolytic agents to the site of vascular occlusion, thereby
minimizing systemic exposure and off-target effects.
Nanoparticle-based drug delivery systems, microneedle patches and
catheter-based delivery platforms are among the innovative
approaches being explored to optimize the delivery of thrombolytic
therapy during CPR (107).
Integration of thrombolytic therapy
with other resuscitative interventions
As the complexity of resuscitative interventions
continues to expand, there is growing interest in integrating
thrombolytic therapy with other advanced techniques, such as
extracorporeal membrane oxygenation (ECMO), to improve outcomes in
refractory cardiac arrest cases. ECMO provides temporary
cardiopulmonary support by oxygenating blood outside the body,
allowing for prolonged resuscitative efforts and stabilization of
patients with profound hemodynamic instability (108). Research endeavors are underway to
elucidate the optimal strategies for combining thrombolytic therapy
with ECMO in the management of cardiac arrest patients with
suspected or confirmed pulmonary embolism or massive myocardial
infarction (109). By
synergistically addressing both the underlying cause of cardiac
arrest (such as acute coronary syndrome and pulmonary embolism) and
the resultant hemodynamic collapse, the integration of thrombolytic
therapy with ECMO holds promise for improving survival and
neurological outcomes in select patient populations (110). Moreover, ongoing studies are
investigating the potential synergistic effects of combining
thrombolytic therapy with other advanced resuscitative
interventions, such as targeted temperature management and
mechanical circulatory support devices, to optimize outcomes in
refractory cardiac arrest cases further (111). Through interdisciplinary
collaboration and rigorous clinical research, these integrated
approaches aim to redefine the standard of care for patients in
cardiac arrest, ultimately improving survival rates and
neurological recovery.
Ethical and legal considerations
Ethical implications of administering
thrombolytic therapy
Using thrombolytics during CPR involves complex
ethical issues related to autonomy, benefit, harm and resource
fairness. In emergencies, informed consent is often not feasible,
raising concerns about respecting patient autonomy while aiming to
save lives (112). The risk of
adverse effects, especially bleeding, must be weighed against the
potential benefit of restoring circulation. Additionally, the
uneven availability of thrombolytic therapy across healthcare
settings raises questions of equity and access. Clinicians must
carefully navigate these ethical concerns within
resource-constrained environments (113).
Legal issues surrounding informed
consent, liability and documentation
Legally, thrombolytic use during CPR touches on
informed consent, liability and documentation. Given the urgency of
CPR, formal consent may not always be possible, but providers
should communicate risks and alternatives whenever feasible
(114). Failing to inform or
document appropriately could result in legal consequences,
including malpractice claims. Comprehensive documentation of
treatment rationale, consent discussions (if possible) and patient
responses is vital to protect both patients and clinicians
(115).
Clear guidelines and protocols for the
use of thrombolytic therapy in CPR
Clear guidelines and protocols are paramount for the
safe and effective use of thrombolytic therapy in CPR. These
guidelines offer healthcare providers with standardized procedures
for patient assessment, medication administration and monitoring
during resuscitative efforts (65). Guidelines help ensure practice
consistency, reduce care variability and promote patient safety.
They outline criteria for patient selection, dosing regimens and
monitoring parameters to minimize the risk of adverse events
associated with thrombolytic therapy. Additionally, clear protocols
aid in informed decision-making and facilitate communication among
healthcare team members (116).
They provide a framework for addressing ethical dilemmas, such as
determining futility or prioritizing resources in resource-limited
settings. Regular review and updates of guidelines and protocols
are essential to incorporate new evidence and best practices. This
iterative process helps healthcare institutions adapt to
advancements in medical knowledge and technology, ultimately
improving patient outcomes in the challenging context of CPR.
Conclusion
The present review provided compelling evidence
supporting the efficacy of thrombolytic therapy, specifically rtPA,
in improving outcomes for patients undergoing CPR. The
administration of rtPA has been shown to increase the likelihood of
ROSC and favorable neurological outcomes. However, it is imperative
to carefully consider the potential risks, including hemorrhagic
complications. Future research should focus on refining patient
selection criteria, optimizing dosing regimens and identifying
ideal timing for rtPA administration to maximize benefit and
minimize adverse events. Additionally, large-scale randomized
controlled trials are needed to further elucidate the role of
thrombolytic therapy in specific patient populations, such as those
with acute myocardial infarction or stroke. By integrating
evidence-based guidelines and protocols, healthcare providers can
harness the potential of thrombolytic therapy to markedly improve
patient survival and quality of life following cardiac arrest.
Acknowledgements
Not applicable.
Funding
The present study was supported by Jilin Provincial Health and
Health Commission (grant no. 2022LC038).
Availability of data and materials
Not applicable.
Authors' contributions
All authors contributed to the study conception and
design. Original draft preparation was conducted by WZ, YP and JD.
Writing, reviewing and editing of the draft manuscript was
completed by WZ, YP, DX and WW. In addition, WW supervised the
writing, analyses and revision of the manuscript. All authors
commented on previous versions of the manuscript. Data
authentication is not applicable. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Professor Wenhai Wang
ORCID: 0009-0002-3879-4259
References
|
1
|
Riggs M, Franklin R and Saylany L:
Associations between cardiopulmonary resuscitation (CPR) knowledge,
self-efficacy, training history and willingness to perform CPR and
CPR psychomotor skills: A systematic review. Resuscitation.
138:259–272. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Z, Zhai ZG, Liang LR, Liu FF, Yang
YH and Wang C: Lower dosage of recombinant tissue-type plasminogen
activator (rt-PA) in the treatment of acute pulmonary embolism: A
systematic review and meta-analysis. Thromb Res. 133:357–363. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Monsieurs KG, Nolan JP, Bossaert LL, Greif
R, Maconochie IK, Nikolaou NI, Perkins GD, Soar J, Truhlář A,
Wyllie J, et al: European resuscitation council guidelines for
resuscitation 2015: Section 1. Executive summary. Resuscitation.
95:1–80. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Taffet GE, Teasdale TA and Luchi RJ:
In-hospital cardiopulmonary resuscitation. JAMA. 260:2069–2072.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kamali MJ, Salehi M and Fath MK: Advancing
personalized immunotherapy for melanoma: Integrating
immunoinformatics in multi-epitope vaccine development, neoantigen
identification via NGS, and immune simulation evaluation. Comput
Biol Med. 188:1098852025. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Konstantinides SV, Meyer G, Becattini C,
Bueno H, Geersing GJ, Harjola VP, Huisman MV, Humbert M, Jennings
CS, Jiménez D, et al: 2019 ESC guidelines for the diagnosis and
management of acute pulmonary embolism developed in collaboration
with the European respiratory society (ERS) the task force for the
diagnosis and management of acute pulmonary embolism of the
European Society of Cardiology (ESC). Eur Heart J. 41:543–603.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gilbert CR, Wilshire CL, Chang SC and
Gorden JA: The use of intrapleural thrombolytic or fibrinolytic
therapy, or both, via indwelling tunneled pleural catheters with or
without concurrent anticoagulation use. Chest. 160:776–783. 2021.
View Article : Google Scholar
|
|
8
|
Ortel TL, Neumann I, Ageno W, Beyth R,
Clark NP, Cuker A, Hutten BA, Jaff MR, Manja V, Schulman S, et al:
American Society of Hematology 2020 guidelines for management of
venous thromboembolism: Treatment of deep vein thrombosis and
pulmonary embolism. Blood Adv. 4:4693–4738. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Altaf F, Wu S and Kasim V: Role of
fibrinolytic enzymes in anti-thrombosis therapy. Front Mol Biosci.
8:6803972021. View Article : Google Scholar
|
|
10
|
Grines CL, Serruys P and O'Neill WW:
Fibrinolytic therapy: Is it a treatment of the past? Circulation.
107:2538–2542. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang S, Wang D and Li L: Recombinant
tissue-type plasminogen activator (rt-PA) effectively restores
neurological function and improves prognosis in acute ischemic
stroke. Am J Transl Res. 15:34602023.PubMed/NCBI
|
|
12
|
Leal Rato M, Diógenes MJ and Sebastião A:
Pharmacodynamics and pharmacokinetics of stroke therapy. Precision
Medicine in Stroke. Springer Nature; pp. 41–69. 2021, View Article : Google Scholar
|
|
13
|
Marko M, Posekany A, Szabo S, Scharer S,
Kiechl S, Knoflach M, Serles W, Ferrari J, Lang W, Sommer P, et al:
Trends of r-tPA (recombinant tissue-type plasminogen activator)
treatment and treatment-influencing factors in acute ischemic
stroke. Stroke. 51:1240–1247. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Llucià-Carol L, Muiño E, Gallego-Fabrega
C, Cárcel-Márquez J, Martín-Campos J, Lledós M, Cullell N and
Fernández-Cadenas I: Pharmacogenetics studies in stroke patients
treated with rtPA: A review of the most interesting findings.
Pharmacogenomics. 22:1091–1097. 2021. View Article : Google Scholar
|
|
15
|
Peppard SR, Parks AM and Zimmerman J:
Characterization of alteplase therapy for presumed or confirmed
pulmonary embolism during cardiac arrest. Am J Health Syst Pharm.
75:870–875. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kotb E: The biotechnological potential of
fibrinolytic enzymes in the dissolution of endogenous blood
thrombi. Biotechnol Prog. 30:656–672. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sharifi M, Berger J, Beeston P, Bay C,
Vajo Z and Javadpoor S; ‘PEAPETT’ investigators, : Pulseless
electrical activity in pulmonary embolism treated with thrombolysis
(from the ‘PEAPETT’ study). Am J Emerg Med. 34:1963–1967. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dejouvencel T, Doeuvre L, Lacroix R,
Plawinski L, Dignat-George F, Lijnen HR and Anglés-Cano E:
Fibrinolytic cross-talk: A new mechanism for plasmin formation.
Blood. 115:2048–2056. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Böttiger BW, Bode C, Kern S, Gries A, Gust
R, Glätzer R, Bauer H, Motsch J and Martin E: Efficacy and safety
of thrombolytic therapy after initially unsuccessful
cardiopulmonary resuscitation: A prospective clinical trial.
Lancet. 357:1583–1585. 2001. View Article : Google Scholar
|
|
20
|
Iyer A, Zurolo E, Boer K, Baayen J,
Giangaspero F, Arcella A, Di Gennaro GC, Esposito V, Spliet WG, van
Rijen PC, et al: Tissue plasminogen activator and urokinase
plasminogen activator in human epileptogenic pathologies.
Neuroscience. 167:929–945. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Badimon L, Padró T and Vilahur G:
Atherosclerosis, platelets and thrombosis in acute ischaemic heart
disease. Eur Heart J Acute Cardiovasc Care. 1:60–74.
2012.PubMed/NCBI
|
|
22
|
Yepes M: The uPA/uPAR system in astrocytic
wound healing. Neural Regen Res. 17:2404–2406. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang B, Leung L, Su EJ and Lawrence DA:
PA system in the pathogenesis of ischemic stroke. Arterioscler
Thromb Vasc Biol. 45:600–608. 2025. View Article : Google Scholar
|
|
24
|
Kadir RRA and Bayraktutan U: Urokinase
plasminogen activator: A potential thrombolytic agent for ischaemic
stroke. Cell Mol Neurobiol. 40:347–355. 2020. View Article : Google Scholar
|
|
25
|
Emberson J, Lees KR, Lyden P, Blackwell L,
Albers G, Bluhmki E, Brott T, Cohen G, Davis S, Donnan G, et al:
Effect of treatment delay, age, and stroke severity on the effects
of intravenous thrombolysis with alteplase for acute ischaemic
stroke: A meta-analysis of individual patient data from randomised
trials. Lancet. 384:1929–1935. 2014. View Article : Google Scholar
|
|
26
|
Katsanos AH, Psychogios K, Turc G, Sacco
S, de Sousa DA, De Marchis GM, Palaiodimou L, Filippou DK, Ahmed N,
Sarraj A, et al: Off-label use of tenecteplase for the treatment of
acute ischemic stroke: A systematic review and meta-analysis. JAMA
Netw Open. 5:e2245062022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fredriksson L, Lawrence DA and Medcalf RL:
tPA modulation of the blood-brain barrier: A unifying explanation
for the pleiotropic effects of tPA in the CNS. Semin Thromb Hemost.
43:154–168. 2017.
|
|
28
|
Katz JM and Tadi P: Physiology,
plasminogen activation. StatPearls Publishing; Treasure Island:
2019
|
|
29
|
Gurman P, Miranda O, Nathan A, Washington
C, Rosen Y and Elman N: Recombinant tissue plasminogen activators
(rtPA): A review. Clin Pharmacol Ther. 97:274–285. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lederer W, Lichtenberger C, Pechlaner C,
Kroesen G and Baubin M: Recombinant tissue plasminogen activator
during cardiopulmonary resuscitation in 108 patients with
out-of-hospital cardiac arrest. Resuscitation. 50:71–76. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sabermoghadam R, Shahmahmood TM, Sarvghadi
P and Toosi MB: The gross, fine, and oral motor functions in a
patient with megalencephalic leukoencephalopathy with subcortical
cyst: A case report. Iran J Child Neurol. 17:149–161. 2023.
|
|
32
|
Hasanoğlu H, Hezer H, Karalezli A, Argüder
E, Kiliç H, Şentürk A, Er M and Soytürk AN: Half-dose recombinant
tissue plasminogen activator treatment in venous thromboembolism. J
Investig Med. 62:71–77. 2014. View Article : Google Scholar
|
|
33
|
Wang X, Li X, Xu Y, Li R, Yang Q, Zhao Y,
Wang F, Sheng B, Wang R, Chen S, et al: Effectiveness of
intravenous r-tPA versus UK for acute ischaemic stroke: A
nationwide prospective Chinese registry study. Stroke Vasc Neurol.
6:603–609. 2021. View Article : Google Scholar
|
|
34
|
Li BH, Wang JH, Wang H, Wang DZ, Yang S,
Guo FQ and Yu NW: Different doses of intravenous tissue-type
plasminogen activator for acute ischemic stroke: A network
meta-analysis. Front Neurol. 13:8842672022. View Article : Google Scholar
|
|
35
|
Amini S, Bakhshandeh H, Mosaed R, Abtahi
H, Sadeghi K and Mojtahedzadeh M: Efficacy and safety of different
dosage of recombinant tissue-type plasminogen activator (rt-PA) in
the treatment of acute pulmonary embolism: A systematic review and
meta-analysis. Iran J Pharm Res. 20:441–454. 2021.PubMed/NCBI
|
|
36
|
Bakkum MJ, Schouten VL, Smulders YM,
Nossent EJ, van Agtmael MA and Tuinman PR: Accelerated treatment
with rtPA for pulmonary embolism induced circulatory arrest. Thromb
Res. 203:74–80. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhong K, An X, Kong Y and Chen Z:
Predictive model for the risk of hemorrhagic transformation after
rt-PA intravenous thrombolysis in patients with acute ischemic
stroke: A systematic review and meta-analysis. Clin Neurol
Neurosurg. 239:1082252024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hezer H, Kiliç H, Abuzaina O, Hasanoğlu HC
and Karalezli A: Long-term results of low-dose tissue plasminogen
activator therapy in acute pulmonary embolism. J Investig Med.
67:1142–1147. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ucar EY, Araz O, Kerget B, Yilmaz N, Akgun
M and Saglam L: Comparison of long-term outcomes of 50 and 100 mg
rt-PA in the management of acute pulmonary thromboembolism. Clin
Respir J. 12:1628–1634. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Salehi M, Kamali MJ, Ashuori Z, Ghadimi F,
Shafiee M, Babaei S and Moghadam AA: Functional significance of
tRNA-derived fragments in sustained proliferation of tumor cells.
Gene Rep. 35:1019012024. View Article : Google Scholar
|
|
41
|
Le Conte P, Huchet L, Trewick D, Longo C,
Vial I, Batard E, Yatim D, Touzé MD and Baron D: Efficacy of
alteplase thrombolysis for ED treatment of pulmonary embolism with
shock. Ame J Emerg Med. 21:438–440. 2003. View Article : Google Scholar
|
|
42
|
Cokkinos P: Post-resuscitation care:
Current therapeutic concepts. Acute Card Care. 11:131–137. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Herlitz J, Castren M, Friberg H, Nolan J,
Skrifvars M, Sunde K and Steen PA: Post resuscitation care: What
are the therapeutic alternatives and what do we know?
Resuscitation. 69:15–22. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Javaudin F, Lascarrou JB, Le Bastard Q,
Bourry Q, Latour C, De Carvalho H, Le Conte P, Escutnaire J, Hubert
H, Montassier E, et al: Thrombolysis during resuscitation for
out-of-hospital cardiac arrest caused by pulmonary embolism
increases 30-day survival: Findings from the French National
Cardiac Arrest Registry. Chest. 156:1167–1175. 2019. View Article : Google Scholar
|
|
45
|
Dasa O, Ruzieh M, Ammari Z, Syed MA,
Brickman KR and Gupta R: It's a ST-elevation myocardial infarction
(STEMI), or is it? Massive pulmonary embolism presenting as STEMI.
J Emerg Med. 55:125–127. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Villablanca PA, Vlismas PP, Aleksandrovich
T, Omondi A, Gupta T, Briceno DF, Garcia MJ and Wiley J: Case
report and systematic review of pulmonary embolism mimicking
ST-elevation myocardial infarction. Vascular. 27:90–97. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fatovich DM, Dobb GJ and Clugston RA: A
pilot randomised trial of thrombolysis in cardiac arrest (The TICA
trial). Resuscitation. 61:309–313. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bozeman WP, Kleiner DM and Ferguson KL:
Empiric tenecteplase is associated with increased return of
spontaneous circulation and short term survival in cardiac arrest
patients unresponsive to standard interventions. Resuscitation.
69:399–406. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Janata K, Holzer M, Kürkciyan I, Losert H,
Riedmüller E, Pikula B, Laggner AN and Laczika K: Major bleeding
complications in cardiopulmonary resuscitation: The place of
thrombolytic therapy in cardiac arrest due to massive pulmonary
embolism. Resuscitation. 57:49–55. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Abu-Laban RB, Christenson JM, Innes GD,
van Beek CA, Wanger KP, McKnight RD, MacPhail IA, Puskaric J,
Sadowski RP, Singer J, et al: Tissue plasminogen activator in
cardiac arrest with pulseless electrical activity. N Engl J Med.
346:1522–1528. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Spöhr F, Arntz HR, Bluhmki E, Bode C,
Carli P, Chamberlain D, Danays T, Poth J, Skamira C, Wenzel V and
Böttiger BW: International multicentre trial protocol to assess the
efficacy and safety of tenecteplase during cardiopulmonary
resuscitation in patients with out-of-hospital cardiac arrest: The
thrombolysis in cardiac arrest (TROICA) study. Eur J Clin Invest.
35:315–323. 2005. View Article : Google Scholar
|
|
52
|
Doelare SA, Oukrich S, Ergin K, Jongkind
V, Wiersema AM, Lely RJ, Ebben HP, Yeung KK and Hoksbergen AWJ:
Collaborators: Major bleeding during thrombolytic therapy for acute
lower limb ischaemia: Value of laboratory tests for clinical
decision making, 17 years of experience. Eur J Vasc Endovasc Surg.
65:398–404. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Pereira R, Bowen M, Rapezzano G, Redpath
A, Pratt S and Hallowell G: Use of recombinant tissue plasminogen
activator (rTPA) for treatment of fibrin in the anterior chamber of
the horse. Vet Med Sci. 10:e14482024. View Article : Google Scholar
|
|
54
|
Vandebroek AC, Rickmann A, Boden KT, Seitz
B, Szurman P and Schulz A: Thrombolytic activity of recombinant
tissue-type plasminogen activator (rtPA) in in-vitro model for
subretinal hemorrhages. Int Ophthalmol. 45:1642025. View Article : Google Scholar
|
|
55
|
Li X, Chen Y, Dong Z, Zhang F, Chen P and
Zhang P: A study of the safety and efficacy of multi-mode
NMR-guided double-antiplatelet pretreatment combined with low-dose
rtPA in the treatment of acute mild ischemic stroke. Front Neurol.
16:14820782025. View Article : Google Scholar
|
|
56
|
Oh TK, Park YM, Do SH, Hwang JW and Song
IA: ROSC rates and live discharge rates after cardiopulmonary
resuscitation by different CPR teams-a retrospective cohort study.
BMC Anesthesiol. 17:1–9. 2017. View Article : Google Scholar
|
|
57
|
Gräsner JT, Wnent J, Herlitz J, Perkins
GD, Lefering R, Tjelmeland I, Koster RW, Masterson S, Rossell-Ortiz
F, Maurer H, et al: Survival after out-of-hospital cardiac arrest
in Europe-Results of the EuReCa TWO study. Resuscitation.
148:218–226. 2020. View Article : Google Scholar
|
|
58
|
Wang Y, Wang M, Ni Y, Liang B and Liang Z:
Can systemic thrombolysis improve prognosis of cardiac arrest
patients during cardiopulmonary resuscitation? A systematic review
and meta-analysis. J Emerg Med. 57:478–487. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Nolan JP, Soar J, Smith GB, Gwinnutt C,
Parrott F, Power S, Harrison DA, Nixon E and Rowan K; National
Cardiac Arrest Audit, : Incidence and outcome of in-hospital
cardiac arrest in the United Kingdom National Cardiac Arrest audit.
Resuscitation. 85:987–992. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hoiland RL, Ainslie PN, Wellington CL,
Cooper J, Stukas S, Thiara S, Foster D, Fergusson NA, Conway EM,
Menon DK, et al: Brain hypoxia is associated with neuroglial injury
in humans post-cardiac arrest. Circ Res. 129:583–597. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hoiland RL, Robba C, Menon DK, Citerio G,
Sandroni C and Sekhon MS: Clinical targeting of the cerebral oxygen
cascade to improve brain oxygenation in patients with
hypoxic-ischaemic brain injury after cardiac arrest. Intensive Care
Med. 49:1062–1078. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Panchal AR, Bartos JA, Cabañas JG, Donnino
MW, Drennan IR, Hirsch KG, Kudenchuk PJ, Kurz MC, Lavonas EJ,
Morley PT, et al: Part 3: Adult basic and advanced life support:
2020 American Heart Association guidelines for cardiopulmonary
resuscitation and emergency cardiovascular care. Circulation. 142
(16_Suppl_2):S366–S468. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Soar J, Böttiger BW, Carli P, Couper K,
Deakin CD, Djärv T, Lott C, Olasveengen T, Paal P, Pellis T, et al:
European resuscitation council guidelines 2021: Adult advanced life
support. Resuscitation. 161:115–151. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Stadlbauer KH, Krismer AC, Arntz HR, Mayr
VD, Lienhart HG, Böttiger BW, Jahn B, Lindner KH and Wenzel V:
Effects of thrombolysis during out-of-hospital cardiopulmonary
resuscitation. Am J Cardiol. 97:305–308. 2006. View Article : Google Scholar
|
|
65
|
Lott C, Truhlář A, Alfonzo A, Barelli A,
González-Salvado V, Hinkelbein J, Nolan JP, Paal P, Perkins GD,
Thies KC, et al: European resuscitation council guidelines 2021:
Cardiac arrest in special circumstances. Resuscitation.
161:152–219. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372:n712021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Higgins J, Altman DG, Gøtzsche PC, Jüni P,
Moher D, Oxman AD, Savović J, Schulz KF, Weeks L and SterneJA C;
Cochrane Bias Methods GroupCochrane Statistical Methods Group, :
The cochrane collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343:d59282011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Blum B, Wormack L, Holtel M, Penwell A,
Lari S, Walker B and Nathaniel TI: Gender and thrombolysis therapy
in stroke patients with incidence of dyslipidemia. BMC Womens
Health. 19:112019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010. View Article : Google Scholar
|
|
70
|
Qiao Y, Wang J, Nguyen T, Liu L, Ji X and
Zhao W: Intravenous thrombolysis with urokinase for acute ischemic
stroke. Brain Sci. 14:9892024. View Article : Google Scholar
|
|
71
|
Broderick C, Watson L and Armon MP:
Thrombolytic strategies versus standard anticoagulation for acute
deep vein thrombosis of the lower limb. Cochrane Database Syst Rev.
1:CD0027832021.PubMed/NCBI
|
|
72
|
Kluft C, Sidelmann JJ and Gram JB:
Assessing safety of thrombolytic therapy. Semin Thromb Hemost.
43:300–310. 2017.
|
|
73
|
Renard A, Verret C, Jost D, Meynard JB,
Tricehreau J, Hersan O, Fontaine D, Briche F, Benner P, de
Stabenrath O, et al: Impact of fibrinolysis on immediate prognosis
of patients with out-of-hospital cardiac arrest. J Thromb
Thrombolysis. 32:405–409. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wardlaw JM, Koumellis P and Liu M:
Thrombolysis (different doses, routes of administration and agents)
for acute ischaemic stroke. Cochrane Database Syst Rev.
31:CD0005142013.
|
|
75
|
Ramaswamy VV, Abiramalatha T, Weiner GM
and Trevisanuto D: A comparative evaluation and appraisal of 2020
American Heart Association and 2021 European Resuscitation Council
neonatal resuscitation guidelines. Resuscitation. 167:151–159.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Semeraro F, Greif R, Böttiger BW, Burkart
R, Cimpoesu D, Georgiou M, Yeung J, Lippert F, Lockey AS,
Olasveengen TM, et al: European resuscitation council guidelines
2021: Systems saving lives. Resuscitation. 161:80–97. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Nolan JP, Sandroni C, Böttiger BW, Cariou
A, Cronberg T, Friberg H, Genbrugge C, Haywood K, Lilja G, Moulaert
VRM, et al: European resuscitation council and European society of
intensive care medicine guidelines 2021: Post-resuscitation care.
Resuscitation. 161:220–269. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Yousuf T, Brinton T, Ahmed K, Iskander J,
Woznicka D, Kramer J, Kopiec A, Chadaga AR and Ortiz K: Tissue
plasminogen activator use in cardiac arrest secondary to fulminant
pulmonary embolism. J Clin Med Res. 8:190–195. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Sobala R, Kibbler J, Yip K, Thompson R,
Rafique C, Steward M, Samanta R; INSPIRE ERUPT collaborators, ; Jha
A, Rahman N and De Soyza A: Evaluation of Real-world use of
pulmonary embolism thrombolysis (ERUPT). ERJ Open Res.
11:00935–2024. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Nadkarni VM, Larkin GL, Peberdy MA, Carey
SM, Kaye W, Mancini ME, Nichol G, Lane-Truitt T, Potts J, Ornato
JP, et al: First documented rhythm and clinical outcome from
in-hospital cardiac arrest among children and adults. JAMA.
295:50–57. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Nolan J, Laver S, Welch C, Harrison D,
Gupta V and Rowan K: Outcome following admission to UK intensive
care units after cardiac arrest: A secondary analysis of the ICNARC
case mix programme database. Anaesthesia. 62:1207–1216. 2007.
View Article : Google Scholar
|
|
82
|
Miyazaki K, Hikone M, Kuwahara Y, Ishida
T, Sugiyama K and Hamabe Y: Extracorporeal CPR for massive
pulmonary embolism in a ‘hybrid 2136 emergency department’. Am J
Emerg Med. 37:2132–2135. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wallentin L, Goldstein P, Armstrong PW,
Granger CB, Adgey AA, Arntz HR, Bogaerts K, Danays T, Lindahl B,
Makijarvi M, et al: Efficacy and safety of tenecteplase in
combination with the low-molecular-weight heparin enoxaparin or
unfractionated heparin in the prehospital setting: the assessment
of the safety and efficacy of a new thrombolytic regimen (ASSENT)-3
PLUS randomized trial in acute myocardial infarction. Circulation.
108:135–142. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ali MR, Hossain MS, Islam MA, Arman MS,
Raju GS, Dasgupta P and Noshin TF: Aspect of thrombolytic therapy:
A review. ScientificWorldJournal. 2014:5865102014. View Article : Google Scholar
|
|
85
|
Saber-Moghadam R: Investigating the
efficacy of noninvasive brain stimulation on poststroke dysphagia:
A systematic review. JNFH. 10:P2862022.
|
|
86
|
Beales I: Recent advances in the
management of peptic ulcer bleeding. F1000Res. 6:17632017.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Scott JH, Gordon M, Vender R, Pettigrew S,
Desai P, Marchetti N, Mamary AJ, Panaro J, Cohen G, Bashir R, et
al: Venoarterial extracorporeal membrane oxygenation in massive
pulmonary embolism-related cardiac arrest: A systematic review.
Crit Care Med. 49:760–769. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Vanden Hoek TL, Morrison LJ, Shuster M,
Donnino M, Sinz E, Lavonas EJ, Jeejeebhoy FM and Gabrielli A: Part
12: Cardiac arrest in special situations: 2010 American Heart
Association guidelines for cardiopulmonary resuscitation and
emergency cardiovascular care. Circulation. 122
(18_suppl_3):S829–S861. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Amacher SA, Bohren C, Blatter R, Becker C,
Beck K, Mueller J, Loretz N, Gross S, Tisljar K, Sutter R, et al:
Long-term survival after out-of-hospital cardiac arrest: A
systematic review and meta-analysis. JAMA Cardiol. 7:633–643. 2022.
View Article : Google Scholar
|
|
90
|
Cho Y, Oh J, Shin JH, Kim BS, Park JK, Lee
JH, Kim JH and Park M: Long-term prognosis and causes of death
among survivors after out-of-hospital cardiac arrest: A
population-based longitudinal study. Resuscitation. 173:31–38.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Chocron R, Fahrenbruch C, Yin L, Guan S,
Drucker C, Shin J, Eisenberg M, Chatterjee NA, Kudenchuk PJ and Rea
T: Association between functional status at hospital discharge and
long-term survival after out-of-hospital-cardiac-arrest.
Resuscitation. 164:30–37. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Berg KM, Soar J, Andersen LW, Böttiger BW,
Cacciola S, Callaway CW, Couper K, Cronberg T, D'Arrigo S, Deakin
CD, et al: Adult advanced life support: 2020 international
consensus on cardiopulmonary resuscitation and emergency
cardiovascular care science with treatment recommendations.
Circulation. 142 (16_suppl_1):S92–S139. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Maconochie IK, Aickin R, Hazinski MF,
Atkins DL, Bingham R, Couto TB, Guerguerian AM, Nadkarni VM, Ng KC,
Nuthall GA, et al: Pediatric life support 2020 international
consensus on cardiopulmonary resuscitation and emergency
cardiovascular care science with treatment recommendations.
Pediatrics. 147 (Suppl 1):e2020038505B2021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Kireyev D, Tan HC and Poh KK: Management
of acute ST-elevation myocardial infarction: Reperfusion options.
Ann Acad Med Singap. 39:927–933. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Pinto DS, Frederick PD, Chakrabarti AK,
Kirtane AJ, Ullman E, Dejam A, Miller DP, Henry TD and Gibson CM;
National Registry of Myocardial Infarction Investigators, : Benefit
of transferring ST-segment-elevation myocardial infarction patients
for percutaneous coronary intervention compared with administration
of onsite fibrinolytic declines as delays increase. Circulation.
124:2512–2521. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Böttiger BW and Martin E: Thrombolytic
therapy during cardiopulmonary resuscitation and the role of
coagulation activation after cardiac arrest. Curr Opin Crit Care.
7:176–183. 2001.
|
|
97
|
Böttiger BW, Arntz HR, Chamberlain DA,
Bluhmki E, Belmans A, Danays T, Carli PA, Adgey JA, Bode C, Wenzel
V, et al: Thrombolysis during resuscitation for out-of-hospital
cardiac arrest. N Engl J Med. 359:2651–2662. 2008. View Article : Google Scholar
|
|
98
|
Higgins JP and Altman DG: Assessing risk
of bias in included studies. Cochrane handbook for systematic
reviews of interventions: Cochrane Book Series. 2008:187–241. 2008.
View Article : Google Scholar
|
|
99
|
Vedantham S, Piazza G, Sista AK and
Goldenberg NA: Guidance for the use of thrombolytic therapy for the
treatment of venous thromboembolism. J Thromb Thrombolysis.
41:68–80. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Luchini C, Veronese N, Nottegar A, Shin
JI, Gentile G, Granziol U, Soysal P, Alexinschi O, Smith L and
Solmi M: Assessing the quality of studies in meta-research:
Review/guidelines on the most important quality assessment tools.
Pharm Stat. 20:185–195. 2021. View Article : Google Scholar
|
|
101
|
Zhou Y, Yan S, Song X, Gong Y, Li W, Wang
M, Yin X, Hu B and Lu Z: Intravenous thrombolytic therapy for acute
ischemic stroke in Hubei, China: A survey of thrombolysis rate and
barriers. BMC Neurol. 19:1–9. 2019. View Article : Google Scholar
|
|
102
|
Berger AK, Radford MJ, Wang Y and Krumholz
HM: Thrombolytic therapy in older patients. J Am Coll Cardiol.
36:366–374. 2000. View Article : Google Scholar
|
|
103
|
Sølling C, Ashkanian M, Hjort N,
Gyldensted C, Andersen G and Østergaard L: Feasibility and
logistics of MRI before thrombolytic treatment. Acta Neurol Scand.
120:143–149. 2009. View Article : Google Scholar
|
|
104
|
Thortveit ET, Bøe MG, Ljøstad U, Mygland Å
and Tveiten A: Organizational changes aiming to reduce iv tPA
door-to-needle time. Acta Neurol Scand. 130:248–252. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Salehi M, Kamali MJ, Rajabzadeh A, Minoo
S, Mosharafi H, Saeedi F and Daraei A: tRNA-derived fragments: Key
determinants of cancer metastasis with emerging therapeutic and
diagnostic potentials. Arch Biochem Biophys. 753:1099302024.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Ma H, Jiang Z, Xu J, Liu J and Guo ZN:
Targeted nano-delivery strategies for facilitating thrombolysis
treatment in ischemic stroke. Drug Deliv. 28:357–371. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zamanlu M, Farhoudi M, Eskandani M,
Mahmoudi J, Barar J, Rafi M and Omidi Y: Recent advances in
targeted delivery of tissue plasminogen activator for enhanced
thrombolysis in ischaemic stroke. J Drug Target. 26:95–109. 2018.
View Article : Google Scholar
|
|
108
|
Yang Y, Chang Q, Chen J, Zou X, Xue Q and
Song A: Application of integrated emergency care model based on
failure modes and effects analysis in patients with ischemic
stroke. Front Surg. 9:8745772022. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Taccone FS, Nobile L and Annoni F:
Thrombolysis for ECMO oxygenator thrombosis. Crit Care. 27:1422023.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Reisinger AC, Fandler-Höfler S, Kreuzer P,
Toth-Gayor G, Schmidt A, Gary T, Rief P, Eller P and Brodmann M:
VA-ECMO and thrombus aspiration in a pulmonary embolism patient
with cardiac arrest and contraindications to thrombolytic therapy.
Vasa. 51:315–319. 2022. View Article : Google Scholar
|
|
111
|
Lin TW, Tsai MT, Hu YN, Wang YC, Wen JS,
Wu HY, Luo CY and Roan JN: Simultaneous thrombolysis and
extracorporeal membrane oxygenation for acute massive pulmonary
emboli. Ann Thorac Surg. 111:923–929. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Han TS, Gulli G, Fry CH, Affley B, Robin
J, Fluck D, Kakar P and Sharma P: Adverse consequences of immediate
thrombolysis-related complications: A multi-centre registry-based
cohort study of acute stroke. J Thromb Thrombolysis. 53:1–10. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Zonjee VJ, Slenders JP, de Beer F, Visser
MC, Ter Meulen BC, Van den Berg-Vos RM and van Schaik SM: Practice
variation in the informed consent procedure for thrombolysis in
acute ischemic stroke: A survey among neurologists and neurology
residents. BMC Med Ethics. 22:1–9. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Szalados JE: The ethics and laws governing
informed decision-making in healthcare: Informed consent, refusal,
and discussions regarding resuscitation and life-sustaining
treatment. The medical-legal aspects of acute care medicine: A
resource for clinicians, administrators, and risk managers.
Springer; 2021, pp. 43–73. View Article : Google Scholar
|
|
115
|
Milling L, Binderup LG, de Muckadell CS,
Christensen EF, Lassen A, Christensen HC, Nielsen DC and Mikkelsen
S; The Danish Cardiac Arrest Registry Group, : Documentation of
ethically relevant information in out-of-hospital resuscitation is
rare: A Danish nationwide observational study of 16,495
out-of-hospital cardiac arrests. BMC Med Ethics. 22:822021.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zenych A, Fournier L and Chauvierre C:
Nanomedicine progress in thrombolytic therapy. Biomaterials.
258:1202972020. View Article : Google Scholar : PubMed/NCBI
|