Introduction
The circadian clock is a fundamental biological
phenomenon observed across the natural world (1). As an endogenous regulatory system, it
is present in nearly all organisms and has evolved to enable
adaptation to time-dependent environmental changes (2,3).
This system comprises a network of oscillators in which a central
clock synchronizes multiple peripheral clocks in alignment with
light and dietary cycles, thereby coordinating internal rhythms,
regulating metabolism, and influencing the onset and progression of
various diseases (4).
Epidemiological studies have demonstrated that
individuals with circadian rhythm disruptions in the central master
clock, such as those caused by shift work or jet lag, have an
elevated risk of developing tumors, digestive disorders,
neurological diseases, immune dysfunction, cardiovascular
conditions and endocrine disorders (5–9).
This suggests that disturbances of the master clock contribute to
the development of diverse pathologies. Similarly, disruptions in
the peripheral circadian clock have been associated with damaging
gene mutations, inflammation and fibrosis in organs such as the
heart, kidneys, lungs and pancreas (10–12).
These findings highlight that both master and peripheral clock
disruptions can drive disease. Importantly, the master and
peripheral clocks interact to regulate physiological and
pathological processes. Light signals are transmitted to the
suprachiasmatic nucleus (SCN) via the hypothalamic tract, resetting
the central clock, while the peripheral clock is synchronized with
the master clock through neural and hormonal pathways (13–15).
However, external environmental factors may disrupt this
synchronization; for example, in nocturnal animals under food
restriction, the peripheral clock is altered while the central
clock remains unaffected (6,16).
Disruptions in the circadian clock of the liver
contribute to the onset and progression of acute and chronic liver
disorders, including non-alcoholic fatty liver disease (NAFLD),
alcoholic liver disease (ALD), liver fibrosis (LF) and
hepatocellular carcinoma (HCC) (17–20).
Therapeutic approaches, such as pharmacological interventions,
time-restricted feeding, chronotherapy, phototherapy and
time-dependent pharmacology, have shown potential in the treatment
of circadian rhythm-related disorders (21–24).
The present review summarizes recent advances in the exploration of
the mechanisms underlying the relationship between circadian
rhythms, liver homeostasis and disease. It also discusses
time-based treatment strategies with potential in the management of
liver diseases.
Basic structure and function of the
biological clock
Core molecular oscillation
mechanism
The mammalian circadian clock operates on an ~24-h
cycle, regulating various rhythmic behaviors such as sleep, feeding
and hormone secretion. The SCN, located in the hypothalamus, serves
as the central oscillator of the circadian system, coordinating
peripheral clocks by modulating the nervous system, glucocorticoid
signaling and feeding behavior (14). The circadian system is synchronized
daily by external cues, including light and food. Light is the most
critical factor, conveying information via the retina and the
retinohypothalamic tract to the SCN (15). Peripheral circadian clocks are
present in various tissues and organs, including the liver,
kidneys, adipose tissue, pancreas and heart (25–28)
(Fig. 1). The SCN maintains the
synchronized oscillation of peripheral clocks through neuronal
connections and rhythmic humoral factors. At the molecular level,
several core proteins form basic feedback loops for the circadian
clock. These include circadian locomotor output cycles kaput
(Clock), period circadian regulator 1–3 (Per1-3), cryptochrome 1
and 2 (Cry1 and 2), brain and muscle ARNT-like protein 1 (Bmal1),
retinoic acid receptor-related orphan receptor α (RORα) and nuclear
receptor subfamily 1, group D member 1, also known as reverse
erythroblastosis virus α (Rev-erbα) (29–34)
(Table I).
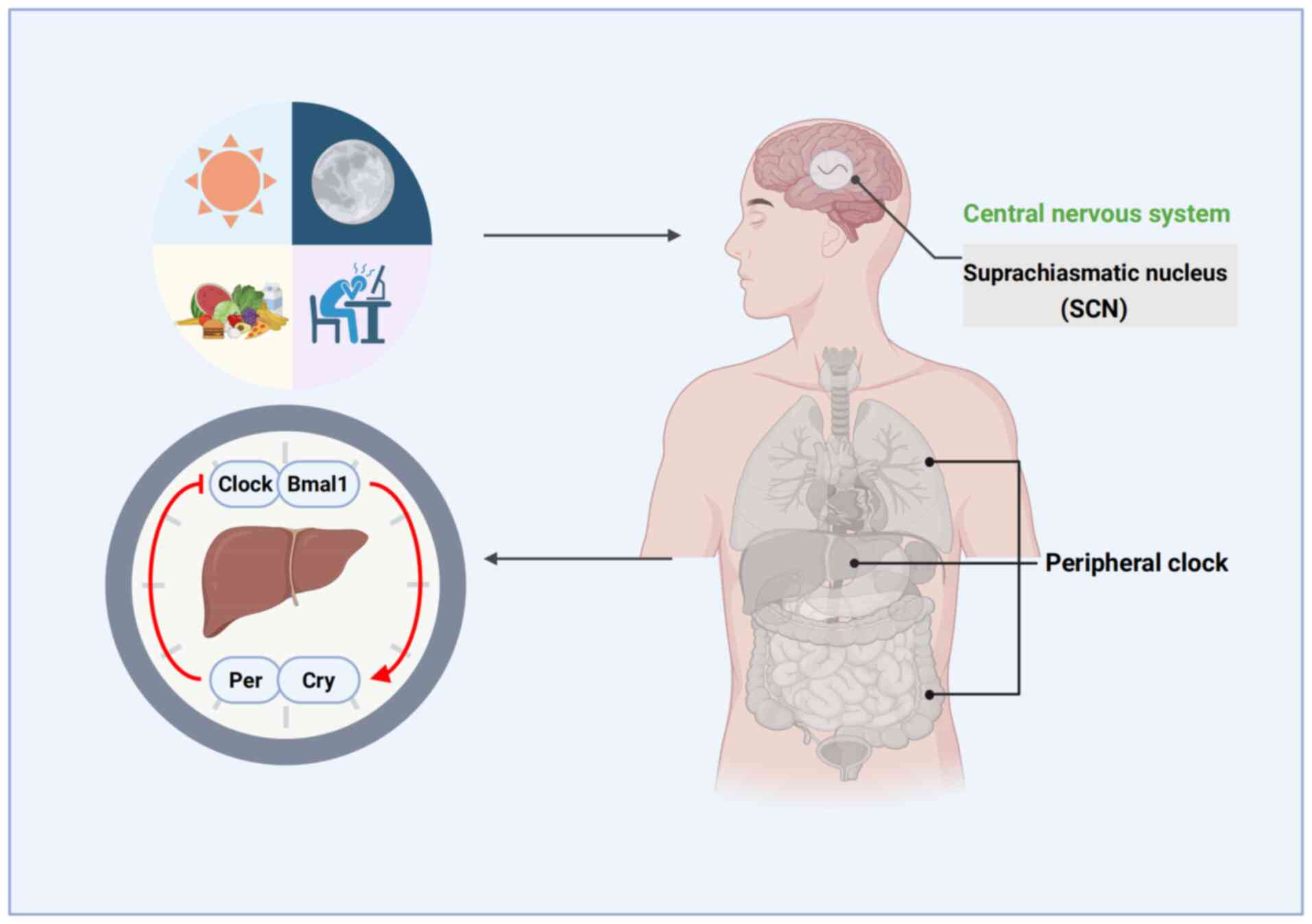 | Figure 1.Regulation process of the central and
peripheral biological clocks. The circadian system undergoes daily
regulation by natural light, food intake and other external cues.
Light serves as the principal zeitgeber, transmitting signals via
the retina and retinohypothalamic tract to the suprachiasmatic
nucleus, thereby resetting the central pacemaker. While the master
clock resides within the hypothalamic center, peripheral
oscillators exist in the liver, kidneys, adipose tissue, pancreas
and heart, all synchronized by the master clock. Bmal1, brain and
muscle ARNT-like protein 1; Clock, circadian locomotor output
cycles kaput; Cry, cryptochrome; Per, period circadian
regulator. |
 | Table I.Circadian clock proteins and their
functions. |
Table I.
Circadian clock proteins and their
functions.
| Circadian gene | Circadian
function |
|---|
| Bmal1 | bHLH-PAS
domain-containing transcription factor; positive regulator |
| Clock | bHLH-PAS
domain-containing transcription factor with histone
acetyltransferase activity; co-activator of Per-Cry
transcription; positive regulator |
| Cry1/2 | Negative
regulator/co-repressor of the Clock-Bmal1 complex |
| Per1/2 |
PAS-domain-containing protein;
co-repressor of the Clock-Bmal1 complex; negative regulator |
| PGC-1α | Transcriptional
coactivator |
| PPARg | Regulator of
metabolism and adipocyte differentiation |
|
Rev-erbα | Nuclear receptor;
repressor of Bmal1 transcription and regulator of
clock-controlled genes; negative regulator |
| RORα | Activator of
Bmal1 transcription; regulator of clock-controlled
genes |
The core feedback loop of the circadian clock begins
with the heterodimerization of Bmal1 and Clock, which activates
Per and Cry genes, along with other circadian
regulators, by binding to E-box elements in their promoters
(13,32,33).
As Cry protein accumulates in the cytosol, it binds with Per
protein to form a stable complex that re-enters the nucleus. This
complex binds to the Bmal1-Clock dimer, inhibiting its
transcriptional activity and reducing the synthesis of Per and Cry
proteins. This negative feedback cycle generates the oscillatory
expression of circadian clock molecules (13,14,34).
A second stabilizing loop involves Bmal1 expression. The
Bmal1-Clock dimer binds to E-box elements in the promoters of the
Rev-erbα and RORα genes. The Rev-erbα and RORα
proteins compete for the RRE site on the Bmal1 promoter,
with RORα activating Bmal1 transcription and Rev-erbα
inhibiting it (14) (Fig. 2).
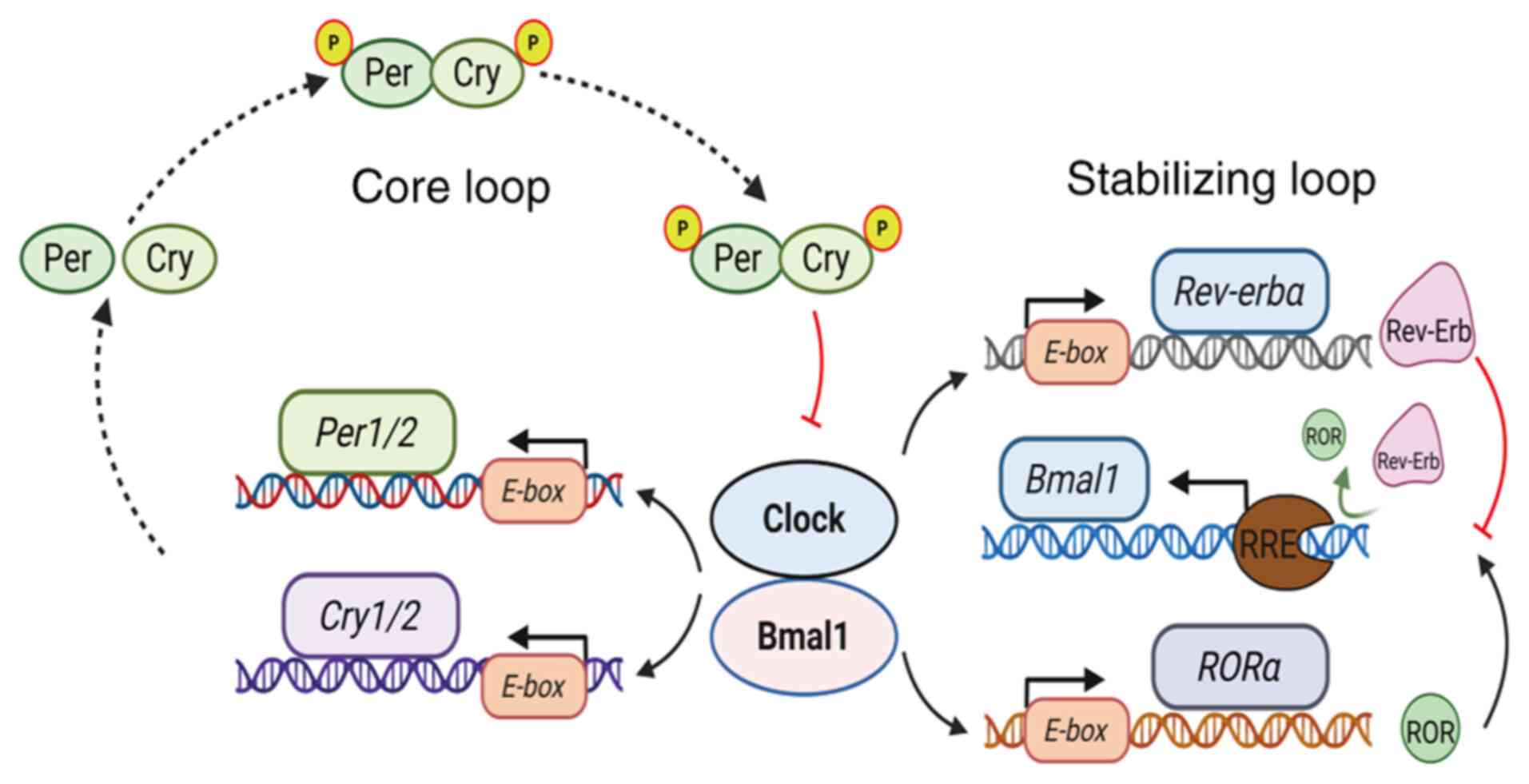 | Figure 2.Schematic diagram of the liver
circadian clock. The liver clock is composed of three parts: The
input pathway, central oscillator and output pathway. The central
oscillator comprises two regulatory loops: The core loop and the
stabilizing loop. In the core loop, Bmal1 and Clock form
heterodimers in the cytoplasm and translocate to the nucleus, where
they bind to E-box sequences in the promoters of target
genes Per and Cry, initiating their transcription. As
Cry protein accumulates in the cytosol, it combines with Per
protein to form a stable complex that re-enters the nucleus and
binds to the Bmal1-Clock heterodimer, inhibiting its
transcriptional activity and reducing Per and Cry protein
synthesis. This cycle forms the oscillatory expression of circadian
clock molecules. The stabilizing loop regulates Bmal1 expression.
Bmal1-Clock dimers bind to E-box elements in the promoters
of the nuclear receptor genes Rev-erbα and RORα,
activating the transcription of Rev-erbα and RORα,
which compete for the RRE site of the Bmal1 promoter: RORα
activates Bmal1 expression, while Rev-erbα inhibits it, thereby
maintaining the stability of the circadian rhythm (32–34).
Bmal1, brain and muscle ARNT-like protein 1; Clock, circadian
locomotor output cycles kaput; Cry, cryptochrome; P, phosphor; Per,
period circadian regulator; Rev-erbα, reverse erythroblastosis
virus α; RORα, retinoic acid receptor-related orphan receptor
α. |
Physiological function of the
biological clock
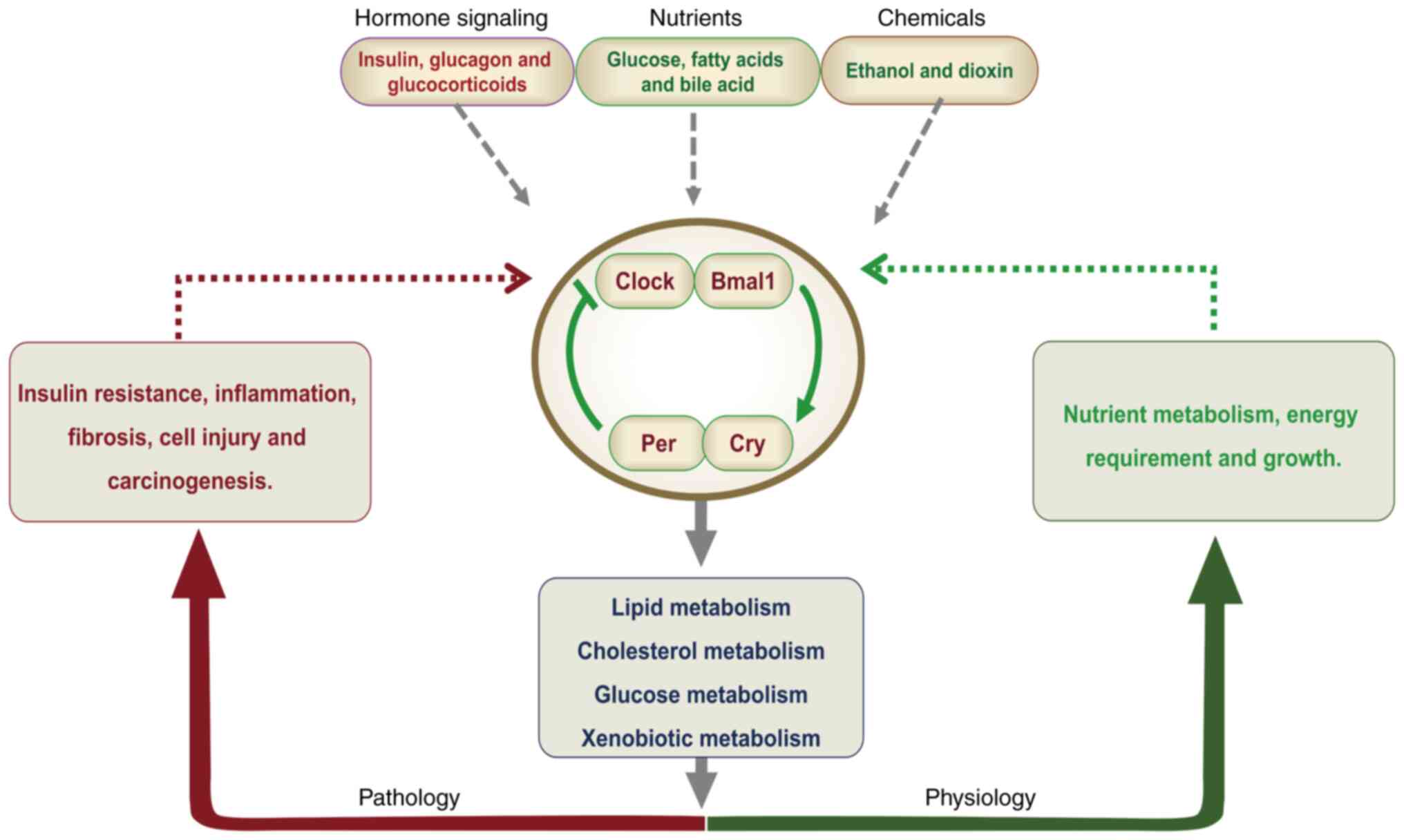
Participating in metabolic balance
The biological clock regulates circadian rhythms as
well as various physiological and metabolic processes within the
body. Disruptions in core circadian clock genes can lead to
metabolic disturbances, affecting the metabolism of sugars, lipids
and bile acids (BAs) (Fig. 3)
(35–37). For example, mice with
liver-specific knockout (KO) of the Bmal1 gene exhibit
dysregulated expression of glucose-regulated genes, leading to
hypoglycemia and increased glucose uptake following fasting
(38). In addition, Bmal1
KO mice exhibit elevated levels of circulating triglycerides (TGs)
and free fatty acids (FFAs), along with increased hepatic fat
accumulation (39). Furthermore,
when these mice are fed a high-cholesterol diet, they exhibit
excessive cholesterol accumulation in the liver compared with that
in wild-type (WT) mice. This may occur due to the circadian
dysregulation of genes involved in cholesterol metabolism,
including 3-hydroxy-3-methylglutaryl-CoA reductase, low-density
lipoprotein receptor and cytochrome P450 7A1 (CYP7A1), the
gene encoding cholesterol 7α-hydroxylase (40). In addition, Clock regulates the
circadian rhythm of hepatic glycogen synthesis by transcriptionally
activating glycogen synthase 2 (41). In a diabetic mouse model, the
overexpression of Cry1 or Cry 2 protein interferes with cyclic
adenosine monophosphate-mediated gluconeogenesis, which reduces
fasting blood glucose levels in these mice (42). Furthermore, Rev-erbα KO mice
exhibit abnormal BA metabolism and altered expression of CYP7A1. In
mice, Rev-erbα regulates BA and CHOL biosynthesis by modulating the
expression of sterol regulatory element binding protein-1c
(SREBP-1c) and CYP7A1. The activity of Rev-erbα is regulated by the
oxysterol-mediated activation of liver X receptor signaling
(43). These findings highlight
the critical role of clock genes and proteins in metabolic
regulation. In addition, clock genes interact with other signaling
pathways to maintain metabolic homeostasis, ensuring efficient
energy utilization and the timely synthesis of essential
biomolecules.
Regulating endocrine rhythm
The expression patterns of circadian clock genes not
only govern the sleep-wake cycle but also play a pivotal role in
the regulation of human metabolism, growth, development and
emotional well-being. For example, melatonin secretion promotes
sleep, modulates immune function and exerts antioxidant effects. It
indirectly protects against oxidative stress by upregulating the
expression of glutathione and antioxidant enzymes such as
superoxide dismutase and catalase (44). Melatonin, which is secreted by the
pineal gland and regulated by the hypothalamic SCN, follows a
distinct circadian rhythm, peaking at night and decreasing during
the day (45). In turn, melatonin
provides feedback that influences the SCN, thereby modulating the
oscillation of core clock genes (42).
Xiang et al (46) investigated the differential
expression of core circadian clock genes, including Clock,
Bmal1, Per2, as well as clock-controlled genes (CCGs) in
MCF-10A human mammary epithelial cells and MCF-7 breast cancer
cells, focusing on the regulatory role of melatonin. The study
revealed that circadian gene oscillations are impaired in breast
cancer cells, and melatonin partially restores rhythmicity by
activating melatonin receptor 1, thereby inhibiting RORα
transcriptional activity and leading to reduced Bmal1
promoter activity (46).
The morning surge in cortisol not only facilitates
wakefulness but also contributes to stress responses by enhancing
alertness and the adaptive capacity of the body (47–49).
Cortisol suppresses Bmal1 gene transcription by promoting
Cry expression, thereby regulating its own circadian peak (50). Glioblastoma cells can synchronize
their biological clock with the host circadian rhythm in response
to cortisol signaling via Bmal1 and Cry genes, and
the inhibition of cortisol signaling or Bmal1 function
significantly suppresses tumor growth (50).
Quagliarini et al (51) explored how the glucocorticoid
receptor (GR) regulates hepatic metabolism through circadian
chromatin binding. The study identified synergistic interactions
between the GR and core clock genes. Specifically, the GR
cooperates with Clock and Bmal1 to co-regulate genes involved in
carbohydrate, lipid and amino acid metabolism, including peroxisome
proliferator-activated receptor γ (PPARγ), CD36 and
4-aminobutyrate aminotransferase. In addition, GR directly
influences core clock components: GR binding sites are present in
the promoters or enhancers of all core clock genes, including
Per1, Per2 and Rev-erbα. The expression of Per1 and
Per2 is induced by GR and Clock/Bmal1, with GR binding preceding
the accumulation of Per1 and Per2. By contrast, GR suppresses
Rev-erbα transcription, while Cry1 and Cry2 inhibit the
GR-mediated activation of gluconeogenic genes, such as
phosphoenolpyruvate carboxykinase 1 (Pck1), through direct
interaction (51). These findings
enhance our understanding of the roles of GR and circadian clock
genes in hepatic metabolic regulation and offer new insights and
strategies for the treatment of metabolic disorders.
The secretion of growth hormone (GH) during deep
sleep is essential for the growth and repair of bones, muscles and
visceral organs (52,53). A study performed by Schoeller et
al (54) demonstrated that
male Bmal1 KO mice exhibit feminized GH secretion patterns,
characterized by increased pulse frequency, reduced regularity and
shortened trough periods. In addition, liver-specific Bmal1
KO mice display intermediate GH pulsatility patterns, lying between
those of global Bmal1 KO and WT mice, suggesting that
Bmal1 regulates GH secretion in tissues other than the
liver. Notably, serum insulin-like growth factor-1 (IGF-1) levels
are significantly lower in Bmal1 KO mice compared with WT
controls, while hepatic IGF-1 mRNA levels are elevated,
indicating disruption of the IGF-1/GH feedback loop. By contrast,
liver-specific Bmal1 KO mice show no significant difference
in serum IGF-1 levels compared with WT controls, suggesting the
involvement of extrahepatic mechanisms in IGF-1 regulation
(54). This research highlights
the critical role of Bmal1 in the GH axis and hepatic metabolism,
offering new insights into the interaction between the circadian
clock and endocrine regulation.
The oscillatory secretion of sex hormones and
thyroid hormones plays a vital role in the regulation of
reproductive function and basal metabolic rate, thereby maintaining
reproductive health and energy balance (55,56).
Sex steroids such as estrogen and testosterone also exhibit
circadian rhythmicity. By binding to their respective receptors,
these hormones modulate the expression of core circadian clock
genes, influencing overall circadian rhythms (15,57).
Research has revealed sex-specific differences in the distribution
and effects of estrogen and testosterone within the SCN. Estrogen
primarily stabilizes the central circadian clock by modulating
astrocytes in the SCN shell subregion, while testosterone
influences the responsiveness of the clock to light signals by
affecting neuronal activity in the SCN core (58,59).
Circadian rhythms often undergo significant changes
with aging, and thyroid function is also subject to circadian
regulation (60). The expression
of core circadian regulators Bmal1 and Clock is downregulated in
aging thyroid follicular epithelial cells, suggesting a decline in
circadian regulatory capacity, contributing to age-related
circadian disruptions (60).
Furthermore, the downregulation of Bmal1 expression suppresses the
expression of NF-κB inhibitor a, leading to persistent activation
of the NF-κB signaling pathway. This chronic activation promotes
thyroid cell senescence (60).
These findings indicate that circadian rhythm disruption
accelerates thyroid functional decline by inducing thyroid cell
senescence, providing a theoretical framework for understanding
thyroid aging and potential strategies to maintain thyroid
function.
Regulating immunity and cell
repair
Circadian clock genes regulate immune system
functions through intricate molecular mechanisms (61). Liu et al (62) demonstrated that the administration
of concanavalin A to mice at the start of the light phase leads to
more severe liver injury and inflammation, evidenced by elevated
alanine aminotransferase (ALT) and aspartate aminotransferase (AST)
levels and increased necrotic areas, compared with those induced by
administration at the beginning of the rest phase. This effect is
mediated by rhythmic regulation of the Bmal1-jun B proto-oncogene
(Junb) axis in macrophages. Specifically, Bmal1 protein directly
binds to the Junb promoter, enhancing its transcription.
Junb then activates the AKT/ERK signaling pathway, promoting the
secretion of pro-inflammatory cytokines, including TNF-α and IL-6,
in M1 macrophages (62). These
findings deepen our understanding of how circadian regulation
influences immune responses in liver diseases. In addition, Bmal1
modulates the transcriptional activity of nuclear factor erythroid
2-related factor 2 (NRF2) in macrophages by binding to E-box
sequences within the NRF2 gene promoter. This regulation
influences antioxidant responses and IL-1β production (63). Bmal1 deficiency reduces NRF2
activity, leading to the accumulation of reactive oxygen species
and excessive IL-1β secretion, thereby inducing a pro-inflammatory
phenotype (63). These findings
provide mechanistic insights into the circadian regulation of
immune responses in liver diseases and suggest that targeting the
Bmal1-NRF2-IL-1β axis may offer therapeutic benefits in
inflammatory conditions such as rheumatoid arthritis and
sepsis.
Circadian clock genes play a critical role in
cellular repair by ensuring that damaged cells are restored at
optimal times to maintain homeostasis (64). Peng et al (64) demonstrated that Bmal1 collaborates
with hypoxia-inducible factor-1α to bind the E'-box motif in the
glucose-6-phosphate dehydrogenase X promoter, driving rhythmic
transcriptional activity and pentose phosphate pathway (PPP)
metabolic flux, which is essential for supplying the nucleotide
precursors, such as ribose-5-phosphate, and NADPH required for DNA
replication and repair. Chronodisruption, due to Bmal1 KO or
mistimed feeding, impairs PPP function, leading to nucleotide
deficiency, oxidative stress and delayed hepatocyte S-phase
progression. This compromises post-hepatectomy regeneration,
exacerbates DNA damage-induced cellular senescence, and promotes
inflammation through the stimulator of interferon genes pathway.
These findings highlight how the circadian clock coordinates tissue
regeneration through metabolic reprogramming, emphasizing the
critical role of nucleotide availability in repair processes
(64). They also provide new
perspectives on circadian-immune interactions and suggest potential
therapeutic targets for disorders associated with immune
dysfunction and cellular damage.
Neurological and behavioral
regulation
Circadian clock genes precisely regulate behavioral
rhythms through a tripartite pathway involving molecular
oscillatory networks, SCN-based synchronization mediated by cilia
and glial cells, and coordination with peripheral organs (65). Genetic variations or aberrant
expression of these genes are associated with neurobehavioral
disorders, including sleep disorders, major depressive disorder and
anxiety. For example, Bmal1-KO rhesus monkeys exhibit
disrupted locomotor rhythms, fragmented sleep and psychiatric
abnormalities (66).
Mechanistically, SCN astrocytes rhythmically release γ-aminobutyric
acid (GABA) to synchronize neuronal oscillations. Inhibition of
GABA synthesis leads to circadian desynchronization, which has been
suggested to increase the risk of Alzheimer's disease (67). These findings underscore the
essential role of circadian clock genes in neural and behavioral
regulation.
Disruption of circadian rhythm
The biological clock uses environmental signals to
optimize energy use, enabling the body to adapt to cycles of rest
and activity, as well as eating and fasting within the circadian
rhythm (68,69). Factors such as sleep deprivation
(70), sleep quality (71,72),
exposure to nighttime light (73),
shift work (74) and altered
feeding patterns (75) can disrupt
circadian timing. Such disruptions may impair metabolic homeostasis
and contribute to the development of various diseases.
Shortened sleep duration leads to marked metabolic
disturbances (70,76). In animal models, restricted sleep
results in increased leptin secretion, heightened lipogenesis and
elevated appetite, all of which contribute to obesity (77). A meta-analysis of 11 longitudinal
studies confirmed that inadequate sleep increases the risk of
becoming overweight or obese (78). Furthermore, sleep deprivation can
interfere with the expression of core circadian clock genes,
including Per1 (77),
contributing to sleep-wake cycle disorders by altering the
rhythmicity of physiological processes such as body temperature and
hormone secretion.
Sleep quality is influenced by multiple factors,
including potential sleep disorders, eating times and environmental
factors such as alcohol and caffeine consumption (79,80).
In young, healthy adults without total sleep deprivation,
interruptions in light or slow-wave sleep for three consecutive
nights lead to reduced insulin sensitivity and impaired glucose
tolerance (81).
The industrialization of society has led to a
substantial increase in nighttime exposure to artificial light,
which alters the circadian rhythm and disrupts the biological clock
(82). Individuals engaged in
night shift work are particularly vulnerable to the adverse effects
of excessive nighttime light exposure (83). A meta-analysis of 13 studies
revealed that such workers face a significantly higher risk of
developing metabolic syndrome. Furthermore, a dose-response
relationship between the duration of night shift work and the risk
of metabolic syndrome was identified, further supporting the
association between circadian disruption and metabolic disease
(73).
Eating time has been identified as an independent
time cue for the peripheral circadian oscillator (84). Shifts in eating patterns can lead
to elevated plasma glucose and insulin levels, as well as reduced
nighttime plasma peaks of melatonin and leptin, all of which are
associated with the onset of metabolic syndrome (75,85).
The interplay between eating time, peripheral circadian rhythms and
hormonal fluctuations may represent a fundamental mechanism linking
eating habits to metabolic diseases. Further human studies are
necessary to determine the optimal timing of food intake and its
association with circadian rhythms.
The regulatory mechanisms of the biological clock
are both complex and precise, allowing it to respond to
environmental cues and anticipate future changes. Disruption of
this system impairs homeostasis, thereby increasing the risk of
metabolic disorders.
Circadian clock genes and liver
diseases
The liver plays a pivotal role in the maintenance of
metabolic homeostasis, performing vital functions such as
regulating blood glucose and ammonia levels, detoxifying drugs,
synthesizing bile, and storing and transforming nutrients (86,87).
Studies have demonstrated that cell-autonomous rhythm oscillators
exist in peripheral organs, including the liver, where they exert
significant effects on the metabolism of nutrients and exogenous
substances. Furthermore, key genes involved in hepatic metabolic
functions also exhibit circadian rhythms and reciprocally regulate
the hepatic circadian clock system (17,88,89).
Disruptions in the circadian clock of the liver can accelerate the
progression of liver diseases, including NAFLD, ALD, LF and HCC
(90–93) (Fig.
4 and Table II); conversely,
these diseases can also impair circadian clock function.
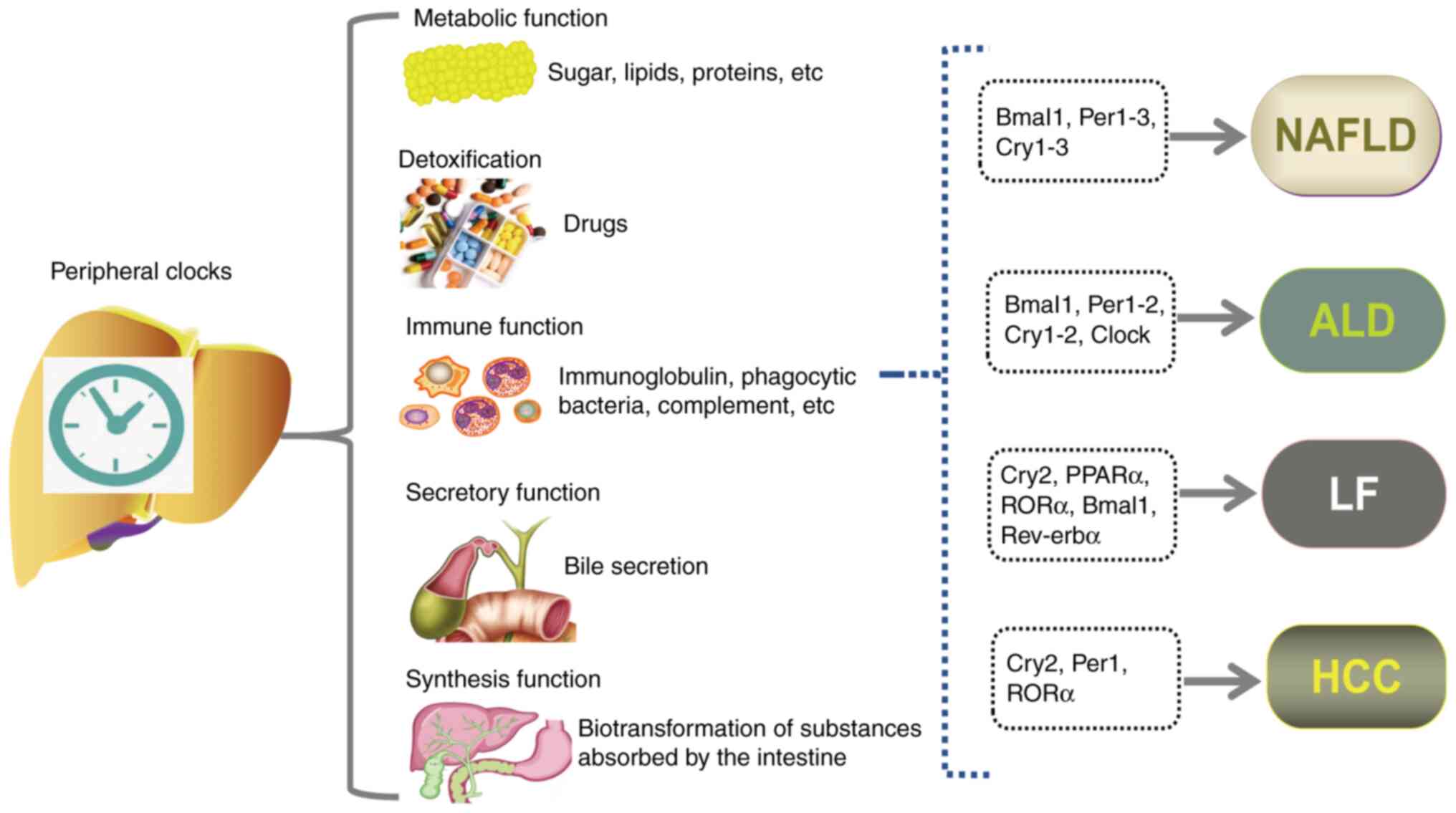 | Figure 4.Impact of circadian clock disturbance
on liver diseases. The liver performs multiple critical functions,
including metabolism, detoxification, immune defense, secretion and
synthesis. Disruption in the expression of circadian clock genes
within the liver can impair its normal physiological functions,
contributing to the pathogenesis of liver diseases, including
NAFLD, ALD, LF and HCC. ALD, alcohol-related liver disease; Bmal1,
brain and muscle ARNT-like protein 1; Clock, circadian locomotor
output cycles kaput; Cry, cryptochrome; HCC, hepatocellular
carcinoma; LF, liver fibrosis; NAFLD, non-alcoholic fatty liver
disease; Per, period circadian regulator; PPAR, peroxisome
proliferator-activated receptor; Rev-erbα, reverse erythroblastosis
virus α; RORa, retinoic acid receptor-related orphan receptor
α. |
 | Table II.Molecular mechanisms associated with
circadian clock dysfunction and liver disease. |
Table II.
Molecular mechanisms associated with
circadian clock dysfunction and liver disease.
| Disease | Mechanism | Impact | (Refs.) |
|---|
| NAFLD | Bmal1↓ → PPARγ↓ →
CD36↓ → fatty acid intake↓; Bmal1↓ → SIRT2↓ → inflammation↑; PPARα↓
→Bmal1↓ → steatosis↑ | Aggravation | (99,101,103) |
| ALD | Alcohol → NAD/NADH
ratio↓ → Clock/Bmal1↓ → bile acid synthesis↓, inflammation↑; Clock
mutation → serum LPS↑ → inflammation↑; SHP↓ → Rev-erbα↓ → CHOP↓ →
inflammation↑; | Aggravation | (112,114,115,116) |
|
| Bmal1↓ → AKT↓ →
ChREBP↓ → lipogenesis, fatty acid oxidation↓ |
|
|
| LF | Per2↓ → CHOP↓ →
TRAIL-R2↓ → HSC apoptosis↓; Per2↓ → CYP7A1, NTCP↓ → accumulation of
bile acids↑; Per2↓ → α-SMA↑ → collagen deposition↑; Bmal1↓,
Rev-erbα↓ → TGF-β/Smad↓ → Col1α, α-SMA↑ → HSC activation↑ | Aggravation | (92,127,128,130) |
|
| Bmal1↑ → IDH1↑,
α-KG↑, HK2↓, PKM2↓ → glycolysis↓ → HSC activation↓ | Alleviation | (131) |
| HCC | Per1/2↓, Cry1/2↓,
Rev-erbα↓ → c-Myc↑, c-Fos↑, FAH↓, Nr1h4↓ → inflammation and
EMT↑ | Aggravation | (142) |
NAFLD
The pathogenesis of NAFLD remains incompletely
understood, and likely involves a multifactorial interplay of
genetic and environmental factors (94). Given the role of circadian rhythms
in metabolic homeostasis, disruptions to these rhythms may
contribute to the development of metabolic syndrome and NAFLD
(95,96). For example, Clock mutant
mice fed a high-fat diet (HFD) exhibit significantly higher hepatic
TG levels than those observed in control mice. Similarly,
Bmal1 KO mice develop hepatic steatosis even when fed a
standard chow diet (97),
suggesting that circadian disruption is a key predisposing factor
for liver pathology. In addition, a study of WT mice fed an HFD for
11 months identified upregulated expression levels of genes
associated with gluconeogenesis, such as Pck1, and genes
associated with lipid metabolism, such as pyruvate dehydrogenase
kinase 4. The hepatic expression levels of the core clock genes
Bmal1, Per1-3, Cry1 and Cry2 were also significantly
elevated in these mice (98),
indicating that chronic HFD intake disrupts metabolic rhythms by
altering the expression of circadian genes, thereby exacerbating
obesity and metabolic syndrome. The interaction between circadian
clock genes and energy metabolism pathways, encompassing glucose
and lipid metabolism as well as fluid-electrolyte balance, may
underpin these disruptions.
A recent study found that Bmal1 KO mice fed
an HFD for 20 weeks exhibited significantly lower fasting blood
glucose, insulin levels and homeostatic model assessment of insulin
resistance values compared with those of HFD-fed WT controls.
Additionally, glucose tolerance and insulin sensitivity were
markedly improved in the KO mice. Liver weight and hepatic TG
content in the KO group were reduced by ~50% compared with those in
the controls, accompanied by significantly fewer lipid vacuoles and
a reduction in lipid droplet deposition (99). Mechanistic analysis revealed that
Bmal1 deficiency mitigated HFD-induced obesity and NAFLD by
suppressing the PPARγ-CD36 pathway, thereby reducing hepatic fatty
acid uptake (99). This was the
first study to implicate Bmal1 in the regulation of hepatic lipid
uptake via the PPARγ-CD36 axis, suggesting a novel therapeutic
target for NAFLD. However, it did not establish a direct regulatory
relationship between PPARγ and Bmal1, highlighting that further
experimental validation is necessary.
Saturated fatty acids can disrupt circadian rhythms
(100). Palmitic acid, the most
abundant circulating saturated fatty acid, has been shown to induce
liver cell toxicity and inflammation. Using the PH5CH8
non-cancerous immortalized hepatocyte cell line and HepG2 liver
cancer cells, Aggarwal et al (101) revealed that palmitic acid induces
hepatocyte lipotoxicity and lipoinflammation by disrupting the
Bmal1-sirtuin 2 (SIRT2) axis. Specifically, it suppresses the
chromatin-binding capacity of Bmal1 and impairs its interaction
with E-box elements in the SIRT2 promoter, thereby
downregulating SIRT2 transcription. The study also found that in
the liver tissues of patients with NAFLD, Bmal1 expression levels
positively correlate with SIRT2 levels, and Bmal1 nuclear
localization is significantly decreased during cirrhosis (101). This study was the first to
identify Bmal1 as a direct transcriptional regulator of SIRT2 and
implicate palmitic acid-induced circadian disruption in hepatic
inflammation. Activation of the Bmal1-NAD+-SIRT2 axis is
thus suggested to be a novel therapeutic strategy for
NAFLD/nonalcoholic steatohepatitis (NASH), while SIRT2 and Bmal1
levels may serve as biomarkers for NAFLD progression. However,
given the small sample size, with only 10 healthy controls and 15
NAFLD cases, further clinical validation is necessary before these
findings can be translated into clinical practice.
PPARα is a target gene of the Clock/Bmal1
heterodimer and contributes to the upregulation of Bmal1 expression
in peripheral tissues. In PPARα KO mouse models,
Bmal1 mRNA levels remain stable in most peripheral tissues
but are significantly decreased in the liver compared with those in
WT mice, indicating the critical role of PPARα in the circadian
regulation of the liver (102).
One study demonstrated that hepatic PPARα mRNA exhibits
diurnal oscillations in WT mice, peaking during the light phase and
declining in the dark phase (103), suggesting rhythmic regulation of
PPARα expression. In addition, fatty acid synthase (FASN)
and acetyl-CoA carboxylase (ACC) mRNA levels markedly
increase during the dark (feeding) phase of WT mice, but not in
PPARα KO mice, which instead exhibit persistently low
expression levels (103). This
finding underscores the role of PPARα as a regulator of the
circadian rhythm of hepatic lipid metabolism genes. Following
prolonged fasting, both systemic PPARα−/− and
liver-specific PPARαhep-/− mice develop hepatic
steatosis. After 6 months of chronic HFD intervention, PPARα
KO mice exhibit exacerbated hepatic steatosis and steatohepatitis
compared with WT controls. However, PPARα agonists prevent
steatohepatitis and fibrosis and even ameliorate existing fibrosis
in mice with steatohepatitis (103). These results highlight
circadian-regulated PPARα as a critical factor in the pathogenesis
of NAFLD.
Sleep deprivation can alter the circadian rhythm of
GH and testosterone secretion; however, following liver
transplantation, improvements in testosterone and GH levels,
together with reduced sleep disturbances associated with NAFLD,
suggest that the liver plays a pivotal role in the regulation of
both endocrine and circadian rhythms (78,104). In humans, sleep-wake disorders
disrupt the secretion of insulin, leptin and norepinephrine, while
circadian rhythm disturbances caused by insufficient sleep increase
insulin resistance (78,105).
Miyake et al (106) conducted a study of 2,429
participants, including 669 men and 1,760 women, without baseline
NAFLD to explore the association between sleep duration and the
onset of NAFLD. Sleep duration was self-reported and categorized as
≤4, 5–6 or 7–8 h, with a mean follow-up of 3.3 years. During the
follow-up period, 296 new cases of NAFLD were observed, affecting
145 men and 151 women. Among men, a sleep duration of ≤6 h was
significantly associated with a reduced risk of NAFLD [odds ratio
(OR), 0.551; 95% CI, 0.365–0.832; P=0.005], and NAFLD incidence
increased progressively with longer sleep (12.5% in the ≤4 h group
vs. 27.4% in the 7–8 h group; P=0.02) (106). However, no significant
association was observed between sleep duration and NAFLD incidence
in women. The authors suggested that prolonged waking hours in men
may increase basal energy expenditure, counteracting the negative
metabolic effects of sleep deprivation (106). This longitudinal cohort study was
the first to reveal sex-specific associations between sleep
duration and the development of NAFLD. The findings indicate that
short sleep duration may be a protective factor in men, suggesting
that differentiated intervention strategies may be appropriate for
this population. Another study, published the same year, explored
the same topic in a study of 2,172 Japanese individuals, including
731 men and 1,441 women. The study reported an overall NAFLD
prevalence of 29.6%, with rates of 38.0% in men and 25.3% in women
(107). In men, NAFLD prevalence
showed a non-significant decline with longer sleep duration, while
in women, a U-shaped relationship was observed, with prevalence
being highest in those having ≤6 or >8 h sleep and lowest in
those having 6–7 h sleep. Women with ≤6 h of sleep had a
significantly increased risk of NAFLD (age-adjusted OR, 1.44; 95%
CI, 1.06–1.96), which is potentially attributable to a higher body
fat percentage and unhealthy dietary habits such as skipping
breakfast (107). These two
studies emphasize sex-specific differences in sleep duration and
NAFLD within the Japanese population. The divergent findings may
reflect hormonal factors, body fat distribution or behavioral
factors such as dietary patterns. However, these studies have some
limitations, including a reliance on self-reported sleep duration
without objective monitoring of sleep quality or sleep apnea, and a
lack of adjustment for potential confounders such as psychological
health or occupational stress. Despite these limitations, the
evidence clearly implicates sleep deprivation in the development of
NAFLD.
Shift work can disrupt circadian rhythms, lead to
insufficient sleep and contribute to metabolic abnormalities.
Balakrishnan et al (108)
conducted a cross-sectional analysis of National Health and
Nutrition Examination Survey (NHANES) data from 8,159 employed
participants aged 20–79 years, to examine the potential link
between shift work and NAFLD risk. The overall prevalence of NAFDL
was 15.7%. Although a higher prevalence of NAFLD was observed among
shift workers, multivariate analysis did not find a significant
association between shift work and increased NAFLD risk. Despite
shift work being associated with conditions such as obesity and
diabetes, no significant relationship with NAFLD was observed in
the study, possibly due to the limitations of its cross-sectional
design, which cannot account for long-term exposure (108). Longitudinal studies are necessary
to clarify the effects of shift work duration and sleep quality on
NAFLD development.
ALD
Alcohol can directly influence the expression of
peripheral clock genes (109,110). Huang et al (111) examined circadian clock gene
expression in peripheral blood mononuclear cells (PBMCs) from 22
male alcohol-dependent (AD) patients and 12 healthy controls. The
results revealed that the mRNA expression levels of Clock1,
Bmal1, Per1, Per2, Cry1 and Cry2 in the PBMCs of
patients with AD were significantly lower compared with those in
the healthy controls. After 1 week of abstinence, gene expression
showed a limited recovery, but remained significantly lower than
that in the control group. This study was the first to demonstrate
an association between chronic alcohol consumption and circadian
gene dysregulation in humans, suggesting that alcohol may
exacerbate addictive behaviors by interfering with circadian
regulation. However, the small sample size, reliance on a single
time-point measurement (9:00 am), and lack of assessment of
circadian fluctuations limit the ability of the study to fully
capture the dynamics of circadian gene expression. The underlying
mechanisms require further investigation. In another study, Zhou
et al (112) explored how
chronic alcohol consumption disrupts hepatic circadian clock
function and promotes alcohol-induced hepatic steatosis in mice.
The results revealed that that short-term alcohol feeding for 1
week did not significantly alter the expression of core clock
genes, including Per2, Per3 and neuronal PAS domain protein
2 (Npas2), or hepatic lipid accumulation. However, after
long-term alcohol feeding for 4 weeks, Per2 and Per3 expression
significantly increased, while Npas2 expression decreased. These
changes were accompanied by hepatic steatosis and phase shifts in
the circadian rhythms of lipid metabolism genes, including
Cyp7a1 and D-box binding PAR bZIP transcription factor
(Dbp) (112). The mice
with long-term alcohol feeding also exhibited elevated hepatic TG
and CHOL levels, with TG rhythms phase-advanced by 5 h, a 12-h
phase shift in BA synthesis, and a significant reduction in the
NAD/NADH ratio, along with an inverted circadian rhythm. The
authors proposed that chronic alcohol intake disrupts the NAD/NADH
balance and impairs the DNA-binding activity of Clock/Bmal1, which
disturbs circadian gene expression and alters lipid and BA
synthesis pathways, thereby exacerbating alcohol-induced hepatic
inflammation and injury (112).
Although these findings underscore the critical role of the
circadian clock in metabolic homeostasis, as the study was
conducted in a mouse model, further clinical validation is
necessary to establish the relevance of these findings in
humans.
Alcohol directly disrupts the intestinal barrier and
induces gut microbiota dysbiosis, creating a harmful ‘gut-liver
axis’ cycle that accelerates the development of ALD (113). Therefore, targeting intestinal
permeability may represent a novel therapeutic approach for ALD.
Summa et al (114)
demonstrated that ClockΔ19/Δ19 mutant mice
exhibited significantly increased colonic permeability, with
further exacerbation of intestinal leakage following alcohol
feeding. Mice subjected to weekly 12-h light cycle phase shifts
showed increases in colonic permeability comparable to those
observed in alcohol-fed mice. Alcohol-fed
ClockΔ19/Δ19 mutant mice also displayed
significantly elevated serum lipopolysaccharide (LPS) levels and
aggravated hepatic steatosis. Mechanistic investigations revealed
that circadian disruption caused the translocation of occludin
protein from the cell membrane to the cytoplasm, impairing
intestinal barrier integrity and promoting endotoxin translocation.
The resultant endotoxemia activated inflammatory responses, further
aggravating alcoholic fatty liver and hepatocyte injury (114). These findings underscore the
critical role of circadian rhythms in the maintenance of intestinal
barrier function and metabolic health. Targeting the circadian
clock or components regulating the intestinal barrier, such as
occludin, may provide innovative therapeutic strategies for
alcohol-related liver disease.
In a clinical study, Swanson et al (115) explored the impact of circadian
disruption, such as night-work, on alcohol-induced increases in
intestinal permeability and its potential association with ALD. The
study enrolled 22 healthy adults, equally divided into day-shift
(07:00-19:00) and night-shift (19:00-07:00) workers, each with at
least 3 months of consistent work schedules. All participants
underwent a 7-day alcohol intervention, involving the intake of 0.5
g/kg/day red wine, with 24-h circadian assessments conducted before
and after the intervention. Following the intervention, night-shift
workers exhibited significantly increased colonic and whole-gut
permeability, as indicated by elevated 24-h urinary sucrose
excretion, whereas day-shift workers showed no significant changes.
In the night-shift group, melatonin secretion rhythms were delayed
by nearly 2 h and exhibited an inverse correlation with increased
colonic permeability. This group also had higher baseline levels of
the inflammatory markers LPS, LPS-binding protein and IL-6, which
lost circadian rhythmicity following alcohol exposure. Alcohol also
significantly altered the amplitude and phase of core clock genes,
including Clock, Bmal1 and Per1, in PBMCs; however,
differences between the groups were more pronounced in central
circadian markers than in peripheral gene expression (115). These findings provide the first
clinical evidence that circadian misalignment in shift workers
exacerbates alcohol-induced intestinal hyperpermeability, thereby
increasing susceptibility to ALD. They also highlight the vital
role of circadian rhythms in the maintenance of intestinal barrier
integrity and metabolic health, and suggest that targeting the
circadian clock or intestinal barrier components, such as occludin,
may help to mitigate alcohol-related hepatotoxicity. However, this
study is limited by a small sample size, reliance on indirect
inferences from PBMCs rather than intestinal epithelial tissue, and
the lack of circadian-targeted interventions, such as melatonin
supplementation or light therapy. Therefore, further investigation
is necessary to validate these observations.
Research has highlighted the critical roles of
circadian clock genes Rev-erbα and Bmal1 in the
pathogenesis of ALD (116,117).
Yang et al (116)
demonstrated that the nuclear receptor small heterodimer partner
(SHP) mitigates alcohol-induced hepatic steatosis by inhibiting the
Rev-erbα-mediated transcriptional activation of C/EBP homologous
protein (CHOP). In addition, dual deficiency of SHP and Rev-erbα
was found to prevent the development of ethanol-induced fatty
liver. The adenovirus-mediated knockdown of Rev-erbα in
SHP−/− mice alleviated ethanol-induced hepatic
steatosis, and reduced hepatic TG levels, serum ALT/AST levels and
the expression of lipogenic genes, including FASN and
ACC2. This establishes Rev-erbα as a key regulator in the
pathogenesis of alcoholic fatty liver (116). This study was the first to
provide evidence that SHP and Rev-erbα influence the development of
alcoholic steatosis by modulating diurnal oscillations of CHOP,
revealing novel chronotherapeutic targets for alcohol-related liver
disease.
In study by Zhang et al (117), liver-specific Bmal1 KO
mice developed aggravated steatosis, hepatic injury and
mitochondrial dysfunction following ethanol feeding. By contrast,
Bmal1 overexpression significantly alleviated ethanol-induced
hepatic lipid accumulation and injury. This protective effect
primarily resulted from enhanced carbohydrate response element
binding protein (ChREBP)-mediated de novo lipogenesis via
AKT signaling activation, along with increased PPARα-dependent
fatty acid β-oxidation (117).
Observations in patients with ALD also revealed significantly
reduced hepatic levels of Bmal1, AKT phosphorylation and ChREBP
protein. Animal experiments further demonstrated that treatment
with fenofibrate, a synthetic PPARα ligand, or the overexpression
of AKT or ChREBP partially reversed the metabolic defects and liver
injury in ethanol-fed mice with liver-specific Bmal1 KO
(117). These findings identify
Bmal1 in hepatocytes as a critical protective factor against ALD,
with the Bmal1-AKT-ChREBP axis representing a potential therapeutic
target (117). However, the exact
mechanism by which ethanol disrupts the Bmal1-Clock protein complex
remains unclear, warranting further investigation.
LF
LF is a precursor to cirrhosis, and patients with
cirrhosis often present with hepatic portal hypertension and
disturbances in circadian rhythms, including delayed sleep cycles,
altered melatonin and cortisol levels, and lethargy (118). However, the physiological
mechanisms underlying these phenomena remain unclear. Montagnese
et al (119) analyzed 87
patients with liver cirrhosis and found that 50–65% of them
experienced difficulty falling asleep or woke up during the night,
which was not directly associated with hepatic encephalopathy (HE).
The patients also exhibited a delayed sleep phase syndrome
(DSPS)-like pattern, characterized by staying up late and waking up
late, with a delay of 2–3 h in the peak secretion of melatonin.
Daytime melatonin levels were increased, nighttime clearance was
delayed, and the urinary excretion of 6-sulfamoxy melatonin was
reduced (120). Daytime
sleepiness correlated significantly with HE severity and blood
ammonia levels, with a negative predictive value of 92%. Abnormal
SCN function in the patients led to delayed synchronization of
melatonin and cortisol rhythms and a weakened response of melatonin
to light exposure, suggesting SCN dysfunction (120,121). The authors proposed that reduced
SCN photosensitivity involves disruption of the Clock/Bmal1 and
Per/Cry protein feedback loops, causing delayed melatonin rhythms
and a DSPS-like sleep phase. In addition, in a rat model of liver
cirrhosis with portal shunt, delayed activity rhythms, decreased
ammonia-induced deep sleep, and decreased liver SIRT1/PARP-1
activity were observed compared with those in controls (122–124). In the liver, an imbalance in
SIRT1 and PARP-1 activities leads to the asynchronous expression of
peripheral clock genes contributing to glucose and protein
metabolism disorders (124).
These findings indicate that both the peripheral and central
circadian clocks were disrupted, affecting the liver and SCN,
respectively. Notably, chronic light exposure accelerated liver
tumor growth in cirrhotic mice, indicating a self-reinforcing cycle
of circadian rhythm disruption and liver disease progression
(125). Subjecting cirrhotic mice
to chronic light exposure further accelerated liver tumor growth,
suggesting that circadian rhythm disruption may exacerbate disease
progression. These reports introduced a dual pathway model of
‘central-peripheral circadian clock disconnection’ and
‘metabolism-neurotransmitter imbalance’, supported by animal data
revealing a downstream molecular cascade effect. However, further
clinical translational research is required to validate these
findings.
Studies using animal models have demonstrated
disruptions in circadian clock gene regulation during LF (92,126). For example, in a carbon
tetrachloride (CCl4)-induced LF mouse model, a complete
loss of rhythmicity in the Cry2 gene was observed, along
with significantly reduced daytime expression. In addition, Clock
and Per1 expression exhibited an elevated mesor with reduced
amplitude, Bmal1 and Per1 expression levels were markedly weakened
in amplitude, and the peaks of Clock, Per1 and Cry1 expression were
delayed. Per2 rhythmicity remained largely unaffected, suggesting a
potential protective role against LF (126). Furthermore, the circadian
oscillations of CCGs involved in hepatic metabolism, such as
PPARα and cytochrome P450 oxidoreductase, were abolished,
with a marked reduction in expression levels. These findings imply
that LF disrupts the transcriptional-translational feedback loop of
Clock/Bmal1 and Per/Cry proteins (126). The loss of Cry2 rhythmicity may
exacerbate lipid metabolic disorders via downstream targets such as
PPARα. This study provided the first evidence that LF directly
causes circadian clock dysregulation in peripheral tissues,
particularly the liver, offering a molecular explanation for the
circadian rhythm disturbances observed in patients with cirrhosis.
However, as the data were validated only at the mRNA level, further
studies examining protein expression are warranted (126).
In a separate study, Chen et al (92) demonstrated that in a
CCl4-induced LF model, Per2 KO mice exhibited
more severe collagen deposition and hepatic stellate cell (HSC)
activation than WT mice. In addition, transfection of Per2
cDNA into the HSC-T6 cell line significantly increased HSC
apoptosis, accompanied by upregulation of the expression of
TNF-related apoptosis-inducing ligand-receptor 2 (TRAIL-R2), also
known as death receptor 5. Further analyses revealed that Per2
induces HSC apoptosis by upregulating CHOP transcription factors,
thereby promoting TRAIL-R2 expression (92). This study was the first to
demonstrate that Per2 protects the liver from chronic damage
through a dual mechanism, involving the inhibition of HSC
activation and promotion of apoptosis, highlighting Per2 and
TRAIL-R2 pathways as potential anti-fibrotic targets.
In a bile duct ligation (BDL)-induced mouse model,
Per2 KO (Per2−/−) mice exhibited more
severe cholestatic liver injury than WT mice, including larger bile
infarcts and increased hepatocyte necrosis (127). Serum and liver BA levels were
significantly elevated, accompanied by loss of circadian rhythm in
the expression of BA synthesis enzymes, such as CYP7A1, and
transporter proteins, including sodium taurocholate co-transporting
polypeptide (NTCP) and bile salt export pump. Furthermore, compared
with WT mice, the Per2−/− mice exhibited
aggravated LF, with increased collagen deposition, increased
activation of HSCs as indicated by a-smooth muscle actin (α-SMA),
and elevated mRNA levels of the pro-fibrotic factors transforming
growth factor-β1 (TGF-β1), TNF-α and collagen type I α1 (Col1α1) in
liver tissue (127).
Mechanistically, Per2 deficiency disrupts the circadian rhythm of
the rate-limiting BA synthesis enzyme CYP7A1, reducing its
efficiency and decreasing the expression of the BA uptake
transporter NTCP. This leads to hepatic BA accumulation, elevated
serum BA levels, and exacerbated BA toxicity. In parallel, Per2
deficiency promotes excessive HSC activation, further driving
collagen deposition and fibrosis (127). Similar findings were reported by
Chen et al (128),
confirming that Per2 plays a protective role in cholestatic liver
injury by maintaining the circadian rhythm of BA metabolism and HSC
function (92).
HSC activation plays a pivotal role in LF, with
evidence suggesting that circadian clock genes directly regulate
this process (92,128,129). Rev-erbα protein levels have been
observed to be significantly elevated in both BDL- and
CCl4-induced LF models, as well as in human cirrhotic
tissues, despite no significant changes in expression at the mRNA
level, indicating post-translational regulation (130). Notably, TGF-β induction increases
the cytoplasmic accumulation of Rev-erbα in HSCs, displaying a
distribution pattern similar to that of myosin, while the synthetic
Rev-erb ligand SR6452 reduces the cytoplasmic expression of
Rev-erbα. They developed a Rev-erbα truncation mutant in which the
N-terminus of the protein encompassing the DNA-binding domain
(which encodes a nuclear localization signal) was deleted.
Experiments using a Rev-erbα mutant demonstrated that
cytoplasmic Rev-erbα enhances cellular contractility in NIH3T3
mouse fibroblasts and promotes fibrotic progression (130). Notably, SR6452 potentiated the
ability of nuclear Rev-erbα to repress the transcription of its
target gene plasminogen activator inhibitor-1, thereby reducing
collagen deposition. Conversely, in the cytoplasm, SR6452
diminished Rev-erbα protein expression, thereby suppressing HSC
activation and collagen accumulation (130). These findings highlight a dual
role for Rev-erbα in HSCs, with both nuclear transcriptional
repression and cytoplasmic pro-fibrotic activity, modulated by
SR6452 (130). This study
provided the first evidence that Rev-erbα regulates HSC phenotypic
switching through differential nuclear-cytoplasmic distribution,
positioning Rev-erbα ligands such as SR6452 as potential targeted
therapies for LF and portal hypertension. A subsequent study
revealed that Bmal1 expression was significantly downregulated and
glycolytic activity increased in CCl4-induced mouse LF
models, and in primary HSCs and LX2 cells activated by TGF-β1
(131). The overexpression of
Bmal1 inhibited the glycolysis, proliferation and phenotypic
transformation of activated HSCs, including the downregulation of
α-SMA and COL1α1 expression. The underlying molecular mechanism was
shown to involve Bmal1-induced attenuation of HSC activation via
the promotion of isocitrate dehydrogenase 1 (IDH1) expression and
α-ketoglutarate (α-KG) generation, and the subsequent inhibition of
key glycolytic enzymes, including hexokinase 2 and pyruvate kinase
M2 (131). This study was the
first to demonstrate that Bmal1 regulates HSC glycolysis via the
IDH1/α-KG axis, and to propose a regulatory network linking the
biological clock, metabolism and fibrosis. These findings suggest
that Bmal1 or IDH1 agonists, such as α-KG supplementation, may be
promising therapeutic strategies for LF, although further research
is necessary to validate these findings.
In a recent study, Crouchet et al (20) found that in the livers of healthy
mice, the circadian rhythm plays a time-gating role by regulating
the expression of TGF-β target genes, including Smad3, Smad7
and TGF-βR1, and inhibiting persistent pro-fibrotic signals.
Analysis of a metabolic dysfunction-associated steatohepatitis
mouse model revealed that circadian clock disorders, characterized
by reduced expression of Rev-erbα, Per1, Per2 and Cry2, resulted in
a loss of control over TGF-β signaling, thereby promoting HSC
activation and collagen deposition. In addition, the treatment of
primary human hepatocytes (PHHs) with FFAs disrupted the expression
of circadian clock genes, such as Bmal1 and Rev-erbα,
and upregulated the expression of TGF-β target genes (20). Furthermore, reduced protein levels
of Rev-erbα, Per1 and Per2 were observed in the livers of patients
with cirrhosis, and these reductions were negatively correlated
with the degree of fibrosis (20).
These findings suggest that dysregulation of circadian clock genes
directly contributes to sustained activation of the TGF-β signaling
pathway and subsequent fibrosis. Subsequently, the roles of Bmal1
and Rev-erbα were examined using Bmal1 KO mice and
hepatocyte-specific Bmal1 KO (Bmal1hep-/−)
mice. In the livers of Bmal1 KO mice, the rhythmic
expression of TGF-β target genes was disrupted, confirming Bmal1 as
a key regulator of TGF-β signaling (20). In Bmal1hep-/−
mice, the circadian expression of TGF-β target genes was abolished,
and Bmal1/Clock complex binding to the promoter regions of TGF-β
target genes was significantly reduced. Rhythmic Bmal1/Clock
binding to the promoter regions of TGF-β target genes, such as
Smad7 and TGF-βR1, was detected in the livers of
healthy mice but not those of Bmal1hep-/− mice
(20). Furthermore, in normal
HSCs, Bmal1 and its downstream genes such as Per1/2 and
Rev-erbα, regulate the rhythmic expression of
fibrosis-related genes. However, in a choline-deficient amino
acid-defined high-fat diet (CDA-HFD) model of fibrosis in mice, the
rhythmic expression of Bmal1 in HSCs was lost, pro-fibrotic genes,
including actin a 2 (ACTA2) and TGF-βR1, were highly
expressed and the expression of anti-fibrotic genes, including
Smad7 and matrix metalloproteinase 9, was decreased.
Single-cell RNA sequencing confirmed that Bmal1 KO disrupts
the rhythmic dysregulation of fibrosis-related pathways, including
the TGF-β and extracellular matrix pathways in HSCs (20). These findings indicate that reduced
Bmal1 expression promotes HSC activation by modulating TGF-β
signaling.
The KO of Rev-erbα from LX2 HSCs upregulates the
expression of pro-fibrotic genes such as ACTA2 and
Col1α1 (20), while the
Rev-erbα agonist SR9009 reduces TGF-β-induced Smad2/3
phosphorylation and the expression of fibrosis marker proteins.
Furthermore, in PHHs, SR9009 reverses FFA-induced lipid
accumulation and the upregulation of TGF-β target genes (20). These findings suggest that Rev-erbα
maintains liver homeostasis by rhythmically inhibiting TGF-β
signaling, and its downregulation is a key driver of fibrosis
progression. The pharmacological targeting of Rev-erbα using SR9009
induced a significant reduction in collagen deposition,
hydroxyproline content and the expression of fibrosis-related genes
Col1α1 and TGF-β1 in the CDA-HFD mouse model. SR9009
also decreased α-SMA expression and collagen deposition in human
liver chimeric model mice, and significantly reduced the expression
of fibrosis markers in hepatic spheroids from fibrotic patients
(20). These results suggest that
targeting Rev-erbα can restore circadian clock function,
inhibit fibrosis, and provide a novel strategy for clinical
anti-fibrotic therapy.
HCC
The circadian mechanism strictly regulates the cell
cycle, cell proliferation, and the expression of tumor suppressor
genes and oncogenes (132,133).
The incidence of spontaneous tumors and radiation-induced HCC is
significantly increased in Per2−/−,
Bmal1±, Cry1−/− and
Cry2−/− mice (134,135). Diethylnitrosamine (DEN) disrupts
circadian rest-activity and body temperature rhythms (125,136,137), and reduces the oscillation
amplitude of Per1 in DEN-induced HCC mouse models (138). In such models, mice subjected to
conditions simulating chronic jet lag develop more tumors compared
with those under normal circadian conditions (125). DEN exposure upregulates the
expression of core clock genes, including Bmal1, Clock and
Npas2, while downregulating negative feedback genes,
including Per1-3 and Cry1, in mouse livers; melatonin
treatment reverses these effects (136). In vitro, a combination of
melatonin and SR9009 synergistically inhibits the proliferation of
Hep3B HCC cells, while Bmal1 knockdown attenuates the pro-apoptotic
and anti-proliferative effects of melatonin, indicating that Bmal1
is a key mediator of anticancer effects (136). These findings reveal a molecular
mechanism by which melatonin suppresses HCC progression through
circadian clock regulation. However, the study was limited to
DEN-induced mouse HCC models and Hep3B cells, without validation in
other models or clinical samples, and the downstream signaling
pathways remain to be clarified.
Disrupted circadian rhythms are associated with
hepatic metastasis (139).
Huisman et al (139)
compared the 24-h expression patterns of core clock genes in the
hepatic and renal tissues of mice with C26 colorectal cancer liver
metastases. In tumor tissues, the rhythmic expression of core clock
genes Bmal1, Rev-erbα and Per1/2, and CCGs including
Dbp, Wee1 and p21, was abolished, indicating severe
circadian dysfunction (139). In
peritumoral hepatic tissues, the core clock genes exhibited phase
advances relative to those in healthy controls, while renal tissues
displayed phase delays, suggesting that tumor-derived signals
disrupt clocks in distal organs (139). These findings highlight the phase
interference caused by colorectal cancer liver metastases at the
local and systemic levels, indicating that interactions occur
between the tumor microenvironment and host rhythms. However, the
molecular mechanisms mediating these shifts require further
exploration.
NAFLD can progress to NASH, which is linked to LF,
liver failure and HCC (140,141). Padilla et al (142) used humanized mouse models to
investigate the mechanisms by which disrupted circadian rhythms
contribute to NAFLD-associated HCC development. The study revealed
that core clock genes exhibit robust circadian rhythmicity in
healthy human and murine livers. However, during NAFLD and NASH
progression, this rhythmic regulation diminishes, with dampened
oscillation amplitudes and phase disturbances, and circadian gene
rhythmicity is nearly abolished in HCC. These changes coincide with
the upregulation of proto-oncogenes, including c-Myc and
c-Fos, and the suppression of tumor suppressor genes,
including fumarylacetoacetate hydrolase and nuclear receptor
subfamily 1 group H member 4 (142). In addition, a chronic jet lag
model in humanized mice accelerated the progression from NAFLD to
NASH to HCC (142).
Transcriptomic analysis showed that circadian disruption
dysregulated thousands of genes, leading to impaired BA and fatty
acid metabolism pathways, activation of NF-κB and TNF-α
inflammatory signaling, suppression of oxidative phosphorylation,
induction of epithelial-mesenchymal transition, and impaired DNA
repair (142). These findings
delineate how circadian dysregulation drives NAFLD-associated
hepatocarcinogenesis by a metabolism-inflammation-cancer axis,
emphasizing the importance of circadian homeostasis in HCC
prevention. However, the molecular mechanisms require further
investigation.
Human studies have established a strong association
between disrupted circadian clock gene expression rhythms and the
development and progression of HCC (93,143). A comparative analysis of 46
paired HCC and adjacent non-tumorous tissues found significantly
reduced mRNA and protein expression levels of Per1, Per2, Per3,
Cry2 and Timeless (TIM) in HCC tissues (93). This circadian gene dysregulation
was associated with the aberrant expression of cell cycle-related
genes, suggesting uncontrolled tumor cell proliferation (93). However, the study was limited by
its small sample size, incomplete coverage of core clock genes, and
reliance on correlative observations that require further
mechanistic validation. In another study, involving 337 patients
with HCC undergoing transarterial chemoembolization, a significant
association was observed between the rs2640908 Per3
single-nucleotide polymorphism (SNP) and overall survival; this
functional polymorphism correlated with reduced survival (143). A retrospective analysis using
data from The Cancer Genome Atlas and International Cancer Genome
Consortium demonstrated that high TIM expression or low Cry2, Per1
and RORα expression significantly predicted poor HCC prognosis
(144). Furthermore, Cry2, Per1,
and Rorα expression inversely correlated with B-cell and regulatory
T-cell infiltration, while TIM showed a positive correlation,
suggesting that these proteins contribute to the regulation of
tumor immune evasion (144). This
study highlights potential immune mechanisms that warrant
experimental validation. Overall, human clinical data on circadian
rhythms in HCC are limited, particularly regarding the practical
efficacy of chronotherapy in HCC management. However, these
findings support circadian clock genes as potential diagnostic
biomarkers and therapeutic targets for HCC.
Treatment of liver diseases based on the
biological clock
Drugs and active ingredients
A number of compounds have been shown to protect
against liver diseases by modulating circadian clock pathways. For
example, Melatonin treatment significantly ameliorates the
dysregulation of hepatic clock gene oscillations induced by
high-fat high-fructose diet and jet lag in mice, restoring the
rhythmic expression of Bmal1 and Clock while partially correcting
oscillations in the Nrf2-HO-1 pathway. This leads to reduced
hepatic lipid accumulation, decreased serum AST/ALT levels, and
improved expression of lipid metabolism-related genes (CPT-1, PPARα
and SREBP-1c) (145). In the LX-2
human HSC line, melatonin suppressed the expression of fibrosis
markers through the melatonin receptor 2-mediated upregulation of
Bmal1 and antioxidant enzymes (146), suggesting a molecular pathway for
the protective effects of melatonin against LF.
Ursolic acid (UA) has been shown to alleviate
hepatic steatosis, fibrosis and insulin resistance in HFD-induced
obese mice by regulating circadian gene expression (147,148). Treatment with UA reduced the
expression of Clock and Bmal1 in liver tissue, while
increasing that of Per1, Per2 and Per3 compared with
the respective levels in untreated HFD-fed mice. UA also promoted
the expression of genes involved in lipid and BA metabolism,
including nicotinamide phosphoribosyltransferase, forkhead box O3
and insulin-induced gene 1 (148).
A vitamin D-deficient diet has been shown to
disrupt circadian synchronization in the liver, while quercetin
supplementation alleviates this disorder. Specifically, a study
revealed that Bmal1 expression was upregulated while Clock and Cry1
expression was downregulated in mice fed a vitamin D-deficient
diet. However, following quercetin supplementation, Clock protein
expression increased significantly, while Bmal1 mRNA
expression decreased (149).
Nobiletin, a polymethoxyflavone abundant in citrus
fruits, enhances circadian rhythms and ameliorates diet-induced
hepatic steatosis (150–152). In models of ALD, nobiletin
activated AKT, increased the expression of ChREBP, ACC1 and FASN,
and decreased SREBP-1 levels and ACC1 phosphorylation in a
Bmal1-dependent manner, thereby alleviating liver injury (153).
Similarly, when the effects of sulforaphane were
examined in HFD-fed mice with a disrupted circadian rhythm,
significant enhancements in the abundance of beneficial gut flora,
including Lachnospiraceae, Lactobacillus and
Alistipes were observed. This was accompanied by a reduction
in the expression of Rev-erbα, Per1 and Bmal1 proteins in the
liver, and increased Clock expression in the hypothalamus (154).
Time-restricted feeding
Time-restricted feeding, which limits nutrient
intake to a defined time window, significantly impacts biological,
physiological, biochemical and behavioral processes. This regimen
not only resets the liver clock but also dissociates it from the
central circadian oscillator (16,155,156). In mice, restricted feeding
prevented HFD-induced weight gain, hyperglycemia, hyperinsulinemia
and insulin resistance. Long-term food restriction also reduced the
expression of inflammatory cytokines in the liver, small intestine
and white adipose tissue (156–158). By aligning the timing of nutrient
intake with circadian timing, time-restricted feeding improves
weight loss and metabolic homeostasis, supporting its integration
with standard treatment strategies for liver diseases.
Phototherapy
Morning light therapy effectively advances
circadian rhythms and sleep phases, with short blue-light pulses in
the morning improving sleep integrity and quality. Intermittent
light exposure appears to be more effective in altering circadian
rhythms than continuous light (43). Therefore, phototherapy may offer
potential benefits for liver diseases associated with circadian
rhythm disruptions, although further studies are necessary to
evaluate this.
Chronopharmacology
Temporal pharmacology highlights that numerous
pharmacodynamic and pharmacokinetic parameters follow circadian
rhythms, meaning that the efficacy and safety of drugs fluctuate
with the time of administration. The circadian clock regulates
every stage of hepatic drug metabolism, including absorption,
distribution, transformation and elimination (159). During absorption, the liver takes
up lipophilic drugs more rapidly in the morning than at night. In
the distribution phase, the expression of several transporters,
including organic cationic transporter 1 and organic anion
transporting polypeptides 1 and 1A4, exhibits circadian variation.
In metabolism, xenobiotic metabolism is divided into three phases,
with the expression of relevant genes following a biological rhythm
in the mouse liver (160).
Furthermore, circadian-regulated transcription factors, including
RORα/γ, DBP, hepatic leukemic factor and thyrotroph embryonic
factor, control the expression of liver metabolic genes that encode
enzymes involved in drug metabolism and elimination (159). Notably, the timing of
administration is a crucial factor influencing drug efficacy. For
example, circadian disruption induced by HFD feeding can be
corrected with appropriately timed drug interventions (160). Due to strict timing requirements,
circadian-based drug administration is often impractical, reducing
patient compliance. As a result, most drugs are not administered
according to circadian rhythms. However, given accumulating
evidence linking circadian rhythm disorders with metabolic
diseases, the role of time-based therapy in disease prevention and
treatment merits more attention.
Conclusions and future prospects
Circadian clock research remains a prominent
frontier in the life sciences, highlighted by the 2017 Nobel Prize
in Physiology or Medicine being awarded for the discovery of its
molecular mechanisms. As a key peripheral clock, the liver clock
plays a pivotal role in the regulation of energy balance and
metabolic homeostasis, coordinating the daily rhythms of processes
such as glucose and lipid metabolism. Disruptions in circadian
clock genes have been observed in several chronic liver diseases,
and circadian rhythm disorders promote the onset and progression of
these conditions. For liver diseases associated with circadian
disturbances, promising therapeutic approaches include natural
drugs, time-restricted feeding, chronotherapy, phototherapy and the
development of clock-targeting drugs.
Despite progress, research on the relationship
between circadian rhythms and liver diseases remains limited.
Mechanistic understanding is insufficient, with most studies
relying on animal models, such as Bmal1 KO mice, and lacking
a comprehensive dissection of the spatiotemporal regulatory
networks governing human circadian genes, including the
Bmal1-PPARγ-CD36 axis. Critical molecular events, such as the
nucleocytoplasmic shuttling of Rev-erbα, are largely described
without dynamic single-cell tracking. Multi-organ interactions,
particularly the cooperation between hepatic rhythms, gut
microbiota and the central clock, remain underexplored.
Methodological shortcomings also persist: Human studies such as the
NHANES, predominantly use a cross-sectional design, which cannot
establish causality, while animal models fail to replicate the
long-term effects of human societal behaviors, such as shift work.
Circadian monitoring technologies remain underdeveloped; static
sampling methods, such as PBMC analysis, cannot capture dynamic
oscillations of liver clock genes in real time. Clinical
translation faces major barriers, as current interventions,
including Rev-erbα targeting with SR9009 and time-restricted
feeding. Time-restricted feeding has only been validated in small
cohorts and lacks multi-center randomized controlled trial (RCT)
evidence (21). In addition,
inter-individual variations in circadian disruption have not been
adequately addressed.
To address current limitations, several strategies
are suggested. First, mechanistic investigations should be
deepened: Single-cell sequencing technologies could be used to map
circadian-specific regulatory networks within hepatocytes and HSCs.
In addition, humanized mouse models could be established to
simulate circadian disruption-related diseases in humans, with the
synchronized acquisition of cross-organ parameters being achieved
by the integration of functional magnetic resonance imaging for SCN
neural activity, nanopore real-time sequencing for the 16S rRNA
profiling of gut microbiota, and hepatic microdialysis for
single-cell metabolic flux analysis. Second, methodological
improvements are essential. Since cross-sectional studies, such as
the NHANES, cannot establish causality, humanized mouse models
mimicking human shift-work behaviors may be constructed. In
addition, technological innovation could focus on the development
of liver-specific biosensors paired with wearable devices for
continuous circadian parameter monitoring. These wearables can be
integrated with artificial intelligence predictive algorithms, such
as long short-term memory time-series models, to generate
personalized circadian profiles. Finally, clinical translation
should be accelerated: Multi-center RCTs are needed to validate the
efficacy of chronotherapeutic interventions, such as timed
melatonin administration, in patients with cirrhosis. In addition,
circadian gene variants, such as the Per3 SNP rs2640908,
along with broader expression signatures, should be developed as
early diagnostic biomarkers for liver diseases.
To enhance clinical translation, circadian
assessments may be incorporated into routine liver disease
screening protocols, including genotyping for Per3 variants
alongside salivary melatonin rhythm profiling. Existing evidence
should be integrated into liver disease management guidelines,
advising patients with NAFLD to avoid nocturnal feeding and
optimizing light-exposure schedules for shift workers. Long-term
goals involve the development of small-molecule therapeutics
targeting circadian proteins, such as the Rev-erbα agonist SR9009,
and the refining of metabolic syndrome treatment regimens through
combination with time-restricted feeding strategies. In conclusion,
circadian research introduces a critical temporal dimension to
hepatology, necessitating the integration of basic and clinical
resources to overcome translational barriers and facilitate the
transition from molecular mechanisms to precision medicine.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
JW was responsible for conceptualization,
validation and writing the original draft of the manuscript. MC and
SL revised and edited the manuscript. WP contributed to contributed
to acquisition and analysis of data. JL and ZW ontributed to the
conception and design, and critically revised the manuscript. All
authors read and approved the final version of the manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviation
Abbreviations:
|
ACC
|
acetyl CoA carboxylase
|
|
ACTA2
|
actin α2
|
|
α-SMA
|
α smooth muscle actin
|
|
α-KG
|
α-ketoglutarate
|
|
ALD
|
alcoholic liver disease
|
|
Bmal1
|
brain and muscle ARNT-like protein
1
|
|
CYP7A1
|
cholesterol 7α-hydroxylase
|
|
CCl4
|
carbon tetrachloride
|
|
CHOP
|
C/EBP homologous protein
|
|
ChREBP
|
carbohydrate response element binding
protein
|
|
COL1A1
|
collagen type I α1
|
|
Cry1/2
|
cryptochrome 1/2
|
|
DEN
|
diethylnitrosamine
|
|
FASN
|
fatty acid synthase
|
|
HCC
|
hepatocellular carcinoma
|
|
HFD
|
high-fat diet
|
|
HSC
|
hepatic stellate cell
|
|
IDH1
|
isocitrate dehydrogenase 1
|
|
LF
|
liver fibrosis
|
|
NAFLD
|
non-alcoholic fatty liver disease
|
|
LPS
|
lipopolysaccharide
|
|
NTCP
|
sodium taurocholate co-transporting
polypeptide
|
|
Per1-3
|
period circadian regulator 1–3
|
|
PPARα/γ
|
peroxisome proliferator-activated
receptor α/γ
|
|
Rev-erbα
|
reverse erythroblastosis virus α
|
|
SCN
|
suprachiasmatic nucleus
|
|
SHP
|
small heterodimer partner
|
|
SIRT2
|
sirtuin 2
|
|
SREBP-1c
|
sterol regulatory element binding
protein-1c
|
|
TGF-β1
|
transforming growth factor-β1
|
References
|
1
|
Sanchez REA, Kalume F and de la Iglesia
HO: Sleep timing and the circadian clock in mammals: Past, present
and the road ahead. Semin Cell Dev Biol. 126:3–14. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim P, Oster H, Lehnert H, Schmid SM,
Salamat N, Barclay JL, Maronde E, Inder W and Rawashdeh O: Coupling
the circadian clock to homeostasis: The role of period in timing
physiology. Endocr Rev. 40:66–95. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Masri S, Cervantes M and Sassone-Corsi P:
The circadian clock and cell cycle: Interconnected biological
circuits. Curr Opin Cell Biol. 25:730–734. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yagita K: Emergence of the circadian clock
oscillation during the developmental process in mammals. Curr Opin
Genet Dev. 84:1021522024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oosterman JE, Wopereis S and Kalsbeek A:
The circadian clock, shift work, and tissue-specific insulin
resistance. Endocrinology. 161:bqaa1802020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Santhi N, Duffy JF, Horowitz TS and
Czeisler CA: Scheduling of sleep/darkness affects the circadian
phase of night shift workers. Neurosci Lett. 384:316–320. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rizza S, Luzi A, Mavilio M, Ballanti M,
Massimi A, Porzio O, Magrini A, Hannemann J, Menghini R, Cridland
J, et al: Impact of light therapy on rotating night shift workers:
The EuRhythDia study. Acta Diabetol. 59:1589–1596. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hasenmajer V, Sbardella E, Sciarra F,
Simeoli C, Pivonello C, Ceccato F, Pofi R, Minnetti M, Rizzo F,
Ferrari D, et al: Circadian clock disruption impairs immune
oscillation in chronic endogenous hypercortisolism: A multi-level
analysis from a multicentre clinical trial. EBioMedicine.
110:1054622024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Škrlec I, Milić J, Cilenšek I, Petrovič D,
Wagner J and Peterlin B: Circadian clock genes and myocardial
infarction in patients with type 2 diabetes mellitus. Gene.
701:98–103. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fletcher EK, Morgan J, Kennaway DR,
Bienvenu LA, Rickard AJ, Delbridge LMD, Fuller PJ, Clyne CD and
Young MJ: Deoxycorticosterone/Salt-mediated cardiac inflammation
and fibrosis are dependent on functional CLOCK signaling in male
mice. Endocrinology. 158:2906–2917. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cunningham PS, Meijer P, Nazgiewicz A,
Anderson SG, Borthwick LA, Bagnall J, Kitchen GB, Lodyga M, Begley
N, Venkateswaran RV, et al: The circadian clock protein REVERBα
inhibits pulmonary fibrosis development. Proc Natl Acad Sci USA.
117:1139–1147. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen WD, Yeh JK, Peng MT, Shie SS, Lin SL,
Yang CH, Chen TH, Hung KC, Wang CC, Hsieh IC, et al: Circadian
CLOCK mediates activation of transforming growth factor-β signaling
and renal fibrosis through cyclooxygenase 2. Am J Pathol.
185:3152–3163. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cox KH and Takahashi JS: Circadian clock
genes and the transcriptional architecture of the clock mechanism.
J Mol Endocrinol. 63:R93–R102. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Partch CL, Green CB and Takahashi JS:
Molecular architecture of the mammalian circadian clock. Trends
Cell Biol. 24:90–99. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Patke A, Young MW and Axelrod S: Molecular
mechanisms and physiological importance of circadian rhythms. Nat
Rev Mol Cell Biol. 21:67–84. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chaix A, Lin T, Le HD, Chang MW and Panda
S: Time-restricted feeding prevents obesity and metabolic syndrome
in mice lacking a circadian clock. Cell Metab. 29:303–319.e4. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mukherji A, Bailey SM, Staels B and
Baumert TF: The circadian clock and liver function in health and
disease. J Hepatol. 71:200–211. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Udoh US, Valcin JA, Gamble KL and Bailey
SM: The molecular circadian clock and alcohol-induced liver injury.
Biomolecules. 5:2504–2537. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
de Assis LVM, Demir M and Oster H: The
role of the circadian clock in the development, progression, and
treatment of non-alcoholic fatty liver disease. Acta Physiol (Oxf).
237:e139152023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Crouchet E, Dachraoui M, Jühling F,
Roehlen N, Oudot MA, Durand SC, Ponsolles C, Gadenne C,
Meiss-Heydmann L, Moehlin J, et al: Targeting the liver clock
improves fibrosis by restoring TGF-β signaling. J Hepatol.
82:120–133. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jamshed H, Beyl RA, Della Manna DL, Yang
ES, Ravussin E and Peterson CM: Early Time-restricted feeding
improves 24-hour glucose levels and affects markers of the
circadian clock, aging, and autophagy in humans. Nutrients.
11:12342019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zeitzer JM, Fisicaro RA, Ruby NF and
Heller HC: Millisecond flashes of light phase delay the human
circadian clock during sleep. J Biol Rhythms. 29:370–376. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Burke TM, Markwald RR, Chinoy ED, Snider
JA, Bessman SC, Jung CM and Wright KP Jr: Combination of light and
melatonin time cues for phase advancing the human circadian clock.
Sleep. 36:1617–1624. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lundell LS, Parr EB, Devlin BL, Ingerslev
LR, Altıntaş A, Sato S, Sassone-Corsi P, Barrès R, Zierath JR and
Hawley JA: Time-restricted feeding alters lipid and amino acid
metabolite rhythmicity without perturbing clock gene expression.
Nat Commun. 11:46432020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Heyde I and Oster H: Differentiating
external zeitgeber impact on peripheral circadian clock resetting.
Sci Rep. 9:201142019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lekkas D and Paschos GK: The circadian
clock control of adipose tissue physiology and metabolism. Auton
Neurosci. 219:66–70. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Solocinski K and Gumz ML: The circadian
clock in the regulation of renal rhythms. J Biol Rhythms.
30:470–486. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yoo SH: Circadian regulation of cardiac
muscle function and protein degradation. Chronobiol Int. 40:4–12.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xiong W, Li J, Zhang E and Huang H: BMAL1
regulates transcription initiation and activates circadian clock
gene expression in mammals. Biochem Biophys Res Commun.
473:1019–1025. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li B, Chen Q, Feng Y, Wei T, Zhong Y,
Zhang Y and Feng Q: Glucose restriction induces AMPK-SIRT1-mediated
circadian clock gene Per expression and delays NSCLC progression.
Cancer Lett. 576:2164242023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kotwica-Rolinska J, Chodáková L, Smýkal V,
Damulewicz M, Provazník J, Wu BC, Hejníková M, Chvalová D and
Doležel D: Loss of timeless underlies an evolutionary transition
within the circadian clock. Mol Biol Evol. 39:msab3462022.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Farshadi E, Yan J, Leclere P, Goldbeter A,
Chaves I and van der Horst GTJ: The positive circadian regulators
CLOCK and BMAL1 control G2/M cell cycle transition through Cyclin
B1. Cell Cycle. 18:16–33. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rosensweig C and Green CB: Periodicity,
repression, and the molecular architecture of the mammalian
circadian clock. Eur J Neurosci. 51:139–165. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Takahashi JS: Transcriptional architecture
of the mammalian circadian clock. Nat Rev Genet. 18:164–179. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sato T and Sassone-Corsi P: Nutrition,
metabolism, and epigenetics: Pathways of circadian reprogramming.
EMBO Rep. 23:e524122022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stenvers DJ, Scheer F, Schrauwen P, la
Fleur SE and Kalsbeek A: Circadian clocks and insulin resistance.
Nat Rev Endocrinol. 15:75–89. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang Y and Zhang J: Bile acid metabolism
and circadian rhythms. Am J Physiol Gastrointest Liver Physiol.
319:G549–G563. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lamia KA, Storch KF and Weitz CJ:
Physiological significance of a peripheral tissue circadian clock.
Proc Natl Acad Sci USA. 105:15172–15177. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rudic RD, McNamara P, Curtis AM, Boston
RC, Panda S, Hogenesch JB and Fitzgerald GA: BMAL1 and CLOCK, two
essential components of the circadian clock, are involved in
glucose homeostasis. PLoS Biol. 2:e3772004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kudo T, Kawashima M, Tamagawa T and
Shibata S: Clock mutation facilitates accumulation of cholesterol
in the liver of mice fed a cholesterol and/or cholic acid diet. Am
J Physiol Endocrinol Metab. 294:120–130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Doi R, Oishi K and Ishida N: CLOCK
regulates circadian rhythms of hepatic glycogen synthesis through
transcriptional activation of Gys2. J Biol Chem. 285:22114–22121.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang EE, Liu Y, Dentin R, Pongsawakul PY,
Liu AC, Hirota T, Nusinow DA, Sun X, Landais S, Kodama Y, et al:
Cryptochrome mediates circadian regulation of cAMP signaling and
hepatic gluconeogenesis. Nat Med. 16:1152–1156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gnocchi D, Custodero C, Sabbà C and
Mazzocca A: Circadian rhythms: A possible new player in
non-alcoholic fatty liver disease pathophysiology. J Mol Med
(Berl). 97:741–759. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lebda MA, Sadek KM, Abouzed TK, Tohamy HG
and El-Sayed YS: Melatonin mitigates thioacetamide-induced hepatic
fibrosis via antioxidant activity and modulation of proinflammatory
cytokines and fibrogenic genes. Life Sci. 192:136–143. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Brzezinski A, Rai S, Purohit A and
Pandi-Perumal SR: Melatonin, clock genes, and mammalian
reproduction: What is the link? Int J Mol Sci. 22:132402021.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xiang S, Mao L, Duplessis T, Yuan L,
Dauchy R, Dauchy E, Blask DE, Frasch T and Hill SM: Oscillation of
clock and clock controlled genes induced by serum shock in human
breast epithelial and breast cancer cells: Regulation by melatonin.
Breast Cancer (Auckl). 6:137–150. 2012.PubMed/NCBI
|
|
47
|
Engel S, Laufer S, Klusmann H, Schulze L,
Schumacher S and Knaevelsrud C: Cortisol response to traumatic
stress to predict PTSD symptom development-a systematic review and
meta-analysis of experimental studies. Eur J Psychotraumatol.
14:22251532023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lightman SL, Birnie MT and Conway-Campbell
BL: Dynamics of ACTH and cortisol secretion and implications for
disease. Endocr Rev. 41:bnaa0022020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pulopulos MM, Baeken C and De Raedt R:
Cortisol response to stress: The role of expectancy and
anticipatory stress regulation. Horm Behav. 117:1045872020.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gonzalez-Aponte MF, Damato AR, Simon T,
Aripova N, Darby F, Jeon MS, Luo J, Rubin JB and Herzog ED: Daily
glucocorticoids promote glioblastoma growth and circadian synchrony
to the host. Cancer Cell. 43:144–160.e7. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Quagliarini F, Mir AA, Balazs K, Wierer M,
Dyar KA, Jouffe C, Makris K, Hawe J, Heinig M, Filipp FV, et al:
Cistromic reprogramming of the diurnal glucocorticoid hormone
response by High-Fat diet. Mol Cell. 76:531–545.e5. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Aguiar-Oliveira MH and Bartke A: Growth
hormone deficiency: Health and longevity. Endocr Rev. 40:575–601.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ranke MB and Wit JM: Growth hormone-past,
present and future. Nat Rev Endocrinol. 14:285–300. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Schoeller EL, Tonsfeldt KJ, Sinkovich M,
Shi R and Mellon PL: Growth hormone pulses and liver gene
expression are differentially regulated by the circadian clock gene
Bmal1. Endocrinology. 162:bqab0232021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Decaroli MC and Rochira V: Aging and sex
hormones in males. Virulence. 8:545–570. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Accorroni A, Chiellini G and Origlia N:
Effects of thyroid hormones and their metabolites on learning and
memory in normal and pathological conditions. Curr Drug Metab.
18:225–236. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tsang AH, Astiz M, Friedrichs M and Oster
H: Endocrine regulation of circadian physiology. J Endocrinol.
230:R1–R11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Koop S and Oster H: Eat, sleep,
repeat-endocrine regulation of behavioural circadian rhythms. FEBS
J. 289:6543–6558. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Neumann AM, Schmidt CX, Brockmann RM and
Oster H: Circadian regulation of endocrine systems. Auton Neurosci.
216:1–8. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zong D, Sun B, Ye Q, Cao H and Guan H:
Circadian Gene BMAL1 regulation of cellular senescence in thyroid
aging. Aging Cell. 24:e701192025. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Cermakian N, Lange T, Golombek D, Sarkar
D, Nakao A, Shibata S and Mazzoccoli G: Crosstalk between the
circadian clock circuitry and the immune system. Chronobiol Int.
30:870–888. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Liu Z, Zhang J, Li S, Wang H, Ren B, Li J,
Bao Z, Liu J, Guo M, Yang G, et al: Circadian control of
ConA-induced acute liver injury and inflammatory response via Bmal1
regulation of Junb. JHEP Rep. 5:1008562023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Early JO, Menon D, Wyse CA,
Cervantes-Silva MP, Zaslona Z, Carroll RG, Palsson-McDermott EM,
Angiari S, Ryan DG, Corcoran SE, et al: Circadian clock protein
BMAL1 regulates IL-1β in macrophages via NRF2. Proc Natl Acad Sci
USA. 115:E8460–E8468. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Peng L, Xiang S, Wang T, Yang M, Duan Y,
Ma X, Li S, Yu C, Zhang X, Hu H, et al: The hepatic clock
synergizes with HIF-1α to regulate nucleotide availability during
liver damage repair. Nat Metab. 7:148–165. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Tu HQ, Li S, Xu YL, Zhang YC, Li PY, Liang
LY, Song GP, Jian XX, Wu M, Song ZQ, et al: Rhythmic cilia changes
support SCN neuron coherence in circadian clock. Science.
380:972–979. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Qiu P, Jiang J, Liu Z, Cai Y, Huang T,
Wang Y, Liu Q, Nie Y, Liu F, Cheng J, et al: BMAL1 knockout macaque
monkeys display reduced sleep and psychiatric disorders. Natl Sci
Rev. 6:87–100. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ness N, Díaz-Clavero S, Hoekstra MMB and
Brancaccio M: Rhythmic astrocytic GABA production synchronizes
neuronal circadian timekeeping in the suprachiasmatic nucleus. EMBO
J. 44:356–381. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Dibner C, Schibler U and Albrecht U: The
mammalian circadian timing system: Organization and coordination of
central and peripheral clocks. Annu Rev Physiol. 72:517–549. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Leger D, Metlaine A and Gronfier C; et le
Consensus Chronobiologie et sommeil de la Société française de
recherche et médecine du sommeil (SFRMS), : Physiology of the
biological clock. Presse Med. 47:964–968. 2018.(in French).
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Archer SN and Oster H: How sleep and
wakefulness influence circadian rhythmicity: Effects of
insufficient and mistimed sleep on the animal and human
transcriptome. J Sleep Res. 24:476–493. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Meyer N, Harvey AG, Lockley SW and Dijk
DJ: Circadian rhythms and disorders of the timing of sleep. Lancet.
400:1061–1078. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Shen Y, Lv QK, Xie WY, Gong SY, Zhuang S,
Liu JY, Mao CJ and Liu CF: Circadian disruption and sleep disorders
in neurodegeneration. Transl Neurodegener. 12:82023. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wang F, Zhang L, Zhang Y, Zhang B, He Y,
Xie S, Li M, Miao X, Chan EY, Tang JL, et al: Meta-analysis on
night shift work and risk of metabolic syndrome. Obes Rev.
15:709–720. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Sooriyaarachchi P, Jayawardena R, Pavey T
and King NA: Shift work and the risk for metabolic syndrome among
healthcare workers: A systematic review and meta-analysis. Obes
Rev. 23:e134892022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Birketvedt GS, Sundsfjord J and Florholmen
JR: Hypothalamic-pituitary-adrenal axis in the night eating
syndrome. Am J Physiol Endocrinol Metab. 282:E366–E369. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Horne J: Short sleep is a questionable
risk factor for obesity and related disorders: Statistical versus
clinical significance. Biol Psychol. 77:266–276. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Husse J, Hintze SC, Eichele G, Lehnert H
and Oster H: Circadian clock genes Per1 and Per2 regulate the
response of metabolism-associated transcripts to sleep disruption.
PLoS One. 7:e529832012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Fatima Y, Doi SA and Mamun AA:
Longitudinal impact of sleep on overweight and obesity in children
and adolescents: A systematic review and bias-adjusted
meta-analysis. Obes Rev. 16:137–149. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Chaput JP, Dutil C, Featherstone R, Ross
R, Giangregorio L, Saunders TJ, Janssen I, Poitras VJ, Kho ME,
Ross-White A, et al: Sleep timing, sleep consistency, and health in
adults: A systematic review. Appl Physiol Nutr Metab. 45 (10 Suppl
2):S232–S247. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhong L, Han X, Li M and Gao S: Modifiable
dietary factors in adolescent sleep: A systematic review and
meta-analysis. Sleep Med. 115:100–108. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Tasali E, Leproult R, Ehrmann DA and Van
Cauter E: Slow-wave sleep and the risk of type 2 diabetes in
humans. Proc Natl Acad Sci USA. 105:1044–1049. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Stevens RG: Circadian disruption and
health: Shift work as a harbinger of the toll taken by electric
lighting. Chronobiol Int. 33:589–594. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Haus EL and Smolensky MH: Shift work and
cancer risk: Potential mechanistic roles of circadian disruption,
light at night, and sleep deprivation. Sleep Med Rev. 17:273–284.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Damiola F, Le Minh N, Preitner N, Kornmann
B, Fleury-Olela F and Schibler U: Restricted feeding uncouples
circadian oscillators in peripheral tissues from the central
pacemaker in the suprachiasmatic nucleus. Genes Dev. 14:2950–2961.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Scheer FA, Hilton MF, Mantzoros CS and
Shea SA: Adverse metabolic and cardiovascular consequences of
circadian misalignment. Proc Natl Acad Sci USA. 106:4453–4458.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Adeva-Andany MM, Pérez-Felpete N,
Fernández-Fernández C, Donapetry-García C and Pazos-García C: Liver
glucose metabolism in humans. Biosci Rep. 36:e004162016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Trefts E, Gannon M and Wasserman DH: The
liver. Curr Biol. 27:R1147–R1151. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Guan D, Xiong Y, Trinh TM, Xiao Y, Hu W,
Jiang C, Dierickx P, Jang C, Rabinowitz JD and Lazar MA: The
hepatocyte clock and feeding control chronophysiology of multiple
liver cell types. Science. 369:1388–1394. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Tahara Y and Shibata S: Circadian rhythms
of liver physiology and disease: Experimental and clinical
evidence. Nat Rev Gastroenterol Hepatol. 13:217–226. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Mukherji A, Dachraoui M and Baumert TF:
Perturbation of the circadian clock and pathogenesis of NAFLD.
Metabolism. 111S:1543372020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Gao J, Sun X, Zhou Q, Jiang S, Zhang Y, Ge
H and Qin X: Circadian clock disruption aggravates alcohol liver
disease in an acute mouse model. Chronobiol Int. 39:1554–1566.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Chen P, Han Z, Yang P, Zhu L, Hua Z and
Zhang J: Loss of clock gene mPer2 promotes liver fibrosis induced
by carbon tetrachloride. Hepatol Res. 40:1117–1127. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Lin YM, Chang JH, Yeh KT, Yang MY, Liu TC,
Lin SF, Su WW and Chang JG: Disturbance of circadian gene
expression in hepatocellular carcinoma. Mol Carcinog. 47:925–933.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Byrne CD and Targher G: NAFLD: A
multisystem disease. J Hepatol. 62 (Suppl 1):S47–S64. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Reinke H and Asher G: Circadian clock
control of liver metabolic functions. Gastroenterology.
150:574–580. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Saran AR, Dave S and Zarrinpar A:
Circadian rhythms in the pathogenesis and treatment of fatty liver
disease. Gastroenterology. 158:1948–1966.e1. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Tong X and Yin L: Circadian rhythms in
liver physiology and liver diseases. Compr Physiol. 3:917–940.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Hsieh MC, Yang SC, Tseng HL, Hwang LL,
Chen CT and Shieh KR: Abnormal expressions of circadian-clock and
circadian clock-controlled genes in the livers and kidneys of
long-term, high-fat-diet-treated mice. Int J Obes (Lond).
34:227–239. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zhan C, Chen H, Zhang Z, Shao Y, Xu B, Hua
R, Yao Q, Liu W and Shen Q: BMAL1 deletion protects against obesity
and non-alcoholic fatty liver disease induced by a high-fat diet.
Int J Obes (Lond). 48:469–476. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Li Z, Wu K, Zou Y, Gong W, Wang P and Wang
H: PREX1 depletion ameliorates high-fat diet-induced non-alcoholic
fatty liver disease in mice and mitigates palmitic acid-induced
hepatocellular injury via suppressing the NF-κB signaling pathway.
Toxicol Appl Pharmacol. 448:1160742022. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Aggarwal S, Rastogi A, Maiwall R, Sevak
JK, Yadav V, Maras J, Thomas SS, Kale PR, Pamecha V, Perumal N, et
al: Palmitic acid causes hepatocyte inflammation by suppressing the
BMAL1-NAD+-SIRT2 axis. J Physiol Biochem. 80:845–864.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Canaple L, Rambaud J, Dkhissi-Benyahya O,
Rayet B, Tan NS, Michalik L, Delaunay F, Wahli W and Laudet V:
Reciprocal regulation of brain and muscle Arnt-like protein 1 and
peroxisome proliferator-activated receptor alpha defines a novel
positive feedback loop in the rodent liver circadian clock. Mol
Endocrinol. 20:1715–1727. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Patel DD, Knight BL, Wiggins D, Humphreys
SM and Gibbons GF: Disturbances in the normal regulation of
SREBP-sensitive genes in PPAR alpha-deficient mice. J Lipid Res.
42:328–337. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Jouffe C, Weger BD, Martin E, Atger F,
Weger M, Gobet C, Ramnath D, Charpagne A, Morin-Rivron D, Powell
EE, et al: Disruption of the circadian clock component BMAL1
elicits an endocrine adaption impacting on insulin sensitivity and
liver disease. Proc Natl Acad Sci USA. 119:e22000831192022.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Rao MN, Neylan TC, Grunfeld C, Mulligan K,
Schambelan M and Schwarz JM: Subchronic sleep restriction causes
tissue-specific insulin resistance. J Clin Endocrinol Metab.
100:1664–1671. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Miyake T, Kumagi T, Furukawa S, Hirooka M,
Kawasaki K, Koizumi M, Todo Y, Yamamoto S, Tokumoto Y, Ikeda Y, et
al: Short sleep duration reduces the risk of nonalcoholic fatty
liver disease onset in men: A community-based longitudinal cohort
study. J Gastroenterol. 50:583–589. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Imaizumi H, Takahashi A, Tanji N, Abe K,
Sato Y, Anzai Y, Watanabe H and Ohira H: The association between
sleep duration and non-alcoholic fatty liver disease among Japanese
men and women. Obes Facts. 8:234–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Balakrishnan M, El-Serag HB, Kanwal F and
Thrift AP: Shiftwork is not associated with increased risk of
NAFLD: Findings from the national health and nutrition examination
survey. Dig Dis Sci. 62:526–533. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Prosser RA and Glass JD: Assessing
Ethanol's actions in the suprachiasmatic circadian clock using in
vivo and in vitro approaches. Alcohol. 49:321–339. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Rosenwasser AM: Chronobiology of ethanol:
Animal models. Alcohol. 49:311–319. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Huang MC, Ho CW, Chen CH, Liu SC, Chen CC
and Leu SJ: Reduced expression of circadian clock genes in male
alcoholic patients. Alcohol Clin Exp Res. 34:1899–1904. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Zhou P, Ross RA, Pywell CM, Liangpunsakul
S and Duffield GE: Disturbances in the murine hepatic circadian
clock in alcohol-induced hepatic steatosis. Sci Rep. 4:37252014.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Shao T, Zhao C, Li F, Gu Z, Liu L, Zhang
L, Wang Y, He L, Liu Y, Liu Q, et al: Intestinal HIF-1α deletion
exacerbates alcoholic liver disease by inducing intestinal
dysbiosis and barrier dysfunction. J Hepatol. 69:886–895. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Summa KC, Voigt RM, Forsyth CB, Shaikh M,
Cavanaugh K, Tang Y, Vitaterna MH, Song S, Turek FW and
Keshavarzian A: Disruption of the circadian clock in mice increases
intestinal permeability and promotes alcohol-induced hepatic
pathology and inflammation. PLoS One. 8:e671022013. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Swanson GR, Gorenz A, Shaikh M, Desai V,
Kaminsky T, Van Den Berg J, Murphy T, Raeisi S, Fogg L, Vitaterna
MH, et al: Night workers with circadian misalignment are
susceptible to alcohol-induced intestinal hyperpermeability with
social drinking. Am J Physiol Gastrointest Liver Physiol.
311:G192–G201. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Yang Z, Tsuchiya H, Zhang Y, Lee S, Liu C,
Huang Y, Vargas GM and Wang L: REV-ERBα activates C/EBP homologous
protein to control small heterodimer partner-mediated oscillation
of alcoholic fatty liver. Am J Pathol. 186:2909–2920. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Zhang D, Tong X, Nelson BB, Jin E, Sit J,
Charney N, Yang M, Omary MB and Yin L: The hepatic
BMAL1/AKT/lipogenesis axis protects against alcoholic liver disease
in mice via promoting PPARα pathway. Hepatology. 68:883–896. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Montagnese S, De Pittà C, De Rui M,
Corrias M, Turco M, Merkel C, Amodio P, Costa R, Skene DJ and Gatta
A: Sleep-wake abnormalities in patients with cirrhosis. Hepatology.
59:705–712. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Montagnese S, Middleton B, Skene DJ and
Morgan MY: Night-time sleep disturbance does not correlate with
neuropsychiatric impairment in patients with cirrhosis. Liver Int.
29:1372–1382. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Montagnese S, Middleton B, Mani AR, Skene
DJ and Morgan MY: On the origin and the consequences of circadian
abnormalities in patients with cirrhosis. Am J Gastroenterol.
105:1773–1781. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Montagnese S, Middleton B, Mani AR, Skene
DJ and Morgan MY: Changes in the 24-h plasma cortisol rhythm in
patients with cirrhosis. J Hepatol. 54:588–591. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Coy DL, Mehta R, Zee P, Salchli F, Turek
FW and Blei AT: Portal-systemic shunting and the disruption of
circadian locomotor activity in the rat. Gastroenterology.
103:222–228. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Llansola M, Cantero JL, Hita-Yañez E,
Mirones-Maldonado MJ, Piedrafita B, Ahabrach H, Errami M, Agusti A
and Felipo V: Progressive reduction of sleep time and quality in
rats with hepatic encephalopathy caused by portacaval shunts.
Neuroscience. 201:199–208. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Nakahata Y, Sahar S, Astarita G, Kaluzova
M and Sassone-Corsi P: Circadian control of the NAD+ salvage
pathway by CLOCK-SIRT1. Science. 324:654–657. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Filipski E, Subramanian P, Carrière J,
Guettier C, Barbason H and Lévi F: Circadian disruption accelerates
liver carcinogenesis in mice. Mutat Res. 680:95–105. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Chen P, Kakan X and Zhang J: Altered
circadian rhythm of the clock genes in fibrotic livers induced by
carbon tetrachloride. FEBS Lett. 584:1597–1601. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Ma K, Xiao R, Tseng HT, Shan L, Fu L and
Moore DD: Circadian dysregulation disrupts bile acid homeostasis.
PLoS One. 4:e68432009. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Chen P, Kakan X, Wang S, Dong W, Jia A,
Cai C and Zhang J: Deletion of clock gene Per2 exacerbates
cholestatic liver injury and fibrosis in mice. Exp Toxicol Pathol.
65:427–432. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Chen L, Xia S, Wang F, Zhou Y, Wang S,
Yang T, Li Y, Xu M, Zhou Y, Kong D, et al: m6A
methylation-induced NR1D1 ablation disrupts the HSC circadian clock
and promotes hepatic fibrosis. Pharmacol Res. 189:1067042023.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Li T, Eheim AL, Klein S, Uschner FE, Smith
AC, Brandon-Warner E, Ghosh S, Bonkovsky HL, Trebicka J and Schrum
LW: Novel role of nuclear receptor Rev-erbα in hepatic stellate
cell activation: Potential therapeutic target for liver injury.
Hepatology. 59:2383–2396. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Xu L, Yang TY, Zhou YW, Wu MF, Shen J,
Cheng JL, Liu QX, Cao SY, Wang JQ and Zhang L: Bmal1 inhibits
phenotypic transformation of hepatic stellate cells in liver
fibrosis via IDH1/α-KG-mediated glycolysis. Acta Pharmacol Sin.
43:316–329. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Feillet C, van der Horst GT, Levi F, Rand
DA and Delaunay F: Coupling between the circadian clock and cell
cycle oscillators: Implication for healthy cells and malignant
growth. Front Neurol. 6:962015. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Kelleher FC, Rao A and Maguire A:
Circadian molecular clocks and cancer. Cancer Lett. 342:9–18. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Fu L, Pelicano H, Liu J, Huang P and Lee
C: The circadian gene Period2 plays an important role in tumor
suppression and DNA damage response in vivo. Cell. 111:41–50. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Lee S, Donehower LA, Herron AJ, Moore DD
and Fu L: Disrupting circadian homeostasis of sympathetic signaling
promotes tumor development in mice. PLoS One. 5:e109952010.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Sánchez DI, González-Fernández B, Crespo
I, San-Miguel B, Álvarez M, González-Gallego J and Tuñón MJ:
Melatonin modulates dysregulated circadian clocks in mice with
diethylnitrosamine-induced hepatocellular carcinoma. J Pineal Res.
65:e125062018. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Verma D, Hashim OH, Jayapalan JJ and
Subramanian P: Effect of melatonin on antioxidant status and
circadian activity rhythm during hepatocarcinogenesis in mice. J
Cancer Res Ther. 10:1040–1044. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Davidson AJ, Straume M, Block GD and
Menaker M: Daily timed meals dissociate circadian rhythms in
hepatoma and healthy host liver. Int J Cancer. 118:1623–1627. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Huisman SA, Oklejewicz M, Ahmadi AR,
Tamanini F, Ijzermans JN, van der Horst GT and de Bruin RW:
Colorectal liver metastases with a disrupted circadian rhythm phase
shift the peripheral clock in liver and kidney. Int J Cancer.
136:1024–1032. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Fernández-Tussy P, Sun J, Cardelo MP,
Price NL, Goedeke L, Xirouchaki CE, Yang X, Pastor-Rojo O, Bennett
AM, Tiganis T, et al: Hepatocyte-specific miR-33 deletion
attenuates NAFLD-NASH-HCC progression. bioRxiv. Jan 20–2023.doi:
10.1101/2023.01.18.523503. PubMed/NCBI
|
|
141
|
Compagnoni C, Capelli R, Zelli V, Corrente
A, Vecchiotti D, Flati I, Di Vito Nolfi M, Angelucci A, Alesse E,
Zazzeroni F, et al: MiR-182-5p is upregulated in hepatic tissues
from a diet-induced NAFLD/NASH/HCC C57BL/6J mouse model and
modulates cyld and foxo1 expression. Int J Mol Sci. 24:92392023.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Padilla J, Osman NM, Bissig-Choisat B,
Grimm SL, Qin X, Major AM, Yang L, Lopez-Terrada D, Coarfa C, Li F,
et al: Circadian dysfunction induces NAFLD-related human liver
cancer in a mouse model. J Hepatol. 80:282–292. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Zhao B, Lu J, Yin J, Liu H, Guo X, Yang Y,
Ge N, Zhu Y, Zhang H and Xing J: A functional polymorphism in PER3
gene is associated with prognosis in hepatocellular carcinoma.
Liver Int. 32:1451–1459. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Liang Y, Wang S, Huang X, Chai R, Tang Q,
Yang R, Huang X, Wang X and Zheng K: Dysregulation of circadian
clock genes as significant clinic factor in the tumorigenesis of
hepatocellular carcinoma. Comput Math Methods Med.
2021:82388332021. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Joshi A, Upadhyay KK, Vohra A, Shirsath K
and Devkar R: Melatonin induces Nrf2-HO-1 reprogramming and
corrections in hepatic core clock oscillations in Non-alcoholic
fatty liver disease. FASEB J. 35:e218032021. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Kim JI and Cheon HG: Melatonin ameliorates
hepatic fibrosis via the melatonin receptor 2-mediated upregulation
of BMAL1 and anti-oxidative enzymes. Eur J Pharmacol.
966:1763372024. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Woźniak Ł, Skąpska S and Marszałek K:
Ursolic Acid-A pentacyclic triterpenoid with a wide spectrum of
pharmacological activities. Molecules. 20:20614–20641. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Kwon EY, Shin SK and Choi MS: Ursolic acid
attenuates hepatic steatosis, fibrosis, and insulin resistance by
modulating the circadian rhythm pathway in diet-induced obese mice.
Nutrients. 10:17192018. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Li R, Wang G, Liu R, Luo L, Zhang Y and
Wan Z: Quercetin improved hepatic circadian rhythm dysfunction in
middle-aged mice fed with vitamin D-deficient diet. J Physiol
Biochem. 80:137–147. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Kim E, Mawatari K, Yoo SH and Chen Z: The
Circadian nobiletin-ROR axis suppresses adipogenic differentiation
and IκBα/NF-κB signaling in adipocytes. Nutrients. 15:39192023.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Mileykovskaya E, Yoo SH, Dowhan W and Chen
Z: Nobiletin: Targeting the circadian network to promote
bioenergetics and healthy aging. Biochemistry (Mosc). 85:1554–1559.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Neba Ambe GNN, Breda C, Bhambra AS and
Arroo RRJ: Effect of the citrus flavone nobiletin on circadian
rhythms and metabolic syndrome. Molecules. 27:77272022. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Li X, Zhuang R, Zhang K, Zhang Y, Lu Z, Wu
F, Wu X, Li W, Zhang Z, Zhang H, et al: Nobiletin protects against
alcoholic liver disease in mice via the BMAL1-AKT-lipogenesis
pathway. Mol Nutr Food Res. 68:e23008332024. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
He C, Chen M, Jiang X, Ren J,
Ganapathiraju SV, Lei P, Yang H, Pannu PR, Zhao Y and Zhang X:
Sulforaphane improves liver metabolism and gut microbiota in
circadian rhythm disorder mice models fed with High-Fat diets. Mol
Nutr Food Res. 68:e24005352024. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Hatori M, Vollmers C, Zarrinpar A,
DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M,
Fitzpatrick JA, et al: Time-restricted feeding without reducing
caloric intake prevents metabolic diseases in mice fed a High-Fat
diet. Cell Metab. 15:848–860. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Ramanathan C, Johnson H, Sharma S, Son W,
Puppa M, Rohani SN, Tipirneni-Sajja A, Bloomer RJ and van der Merwe
M: Early time-restricted feeding amends circadian clock function
and improves metabolic health in male and female nile grass rats.
Medicines (Basel). 9:152022.PubMed/NCBI
|
|
157
|
Shiba A, de Goede P, Tandari R, Foppen E,
Korpel NL, Coopmans TV, Hellings TP, Jansen MW, Ruitenberg A,
Ritsema W, et al: Synergy between time-restricted feeding and
time-restricted running is necessary to shift the muscle clock in
male wistar rats. Neurobiol Sleep Circadian Rhythms. 17:1001062024.
View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Sun S, Hanzawa F, Umeki M, Ikeda S,
Mochizuki S and Oda H: Time-restricted feeding suppresses excess
sucrose-induced plasma and liver lipid accumulation in rats. PLoS
One. 13:e02012612018. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Dallmann R, Brown SA and Gachon F:
Chronopharmacology: New insights and therapeutic implications. Annu
Rev Pharmacol Toxicol. 54:339–361. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Woller A, Duez H, Staels B and Lefranc M:
A Mathematical model of the liver circadian clock linking feeding
and fasting cycles to clock function. Cell Rep. 17:1087–1097. 2016.
View Article : Google Scholar : PubMed/NCBI
|