Respiratory tract diseases are a major health
problem, associated with high incidence and mortality rates
worldwide (1). Respiratory tract
infections are caused by the invasion and reproduction of
pathogenic microorganisms in the respiratory tract, and can be
broadly divided into upper and lower respiratory tract infections.
Upper respiratory tract infections mainly affect areas at and above
the throat, and include acute and chronic rhinitis, laryngitis and
pharyngitis, while lower respiratory tract infections affect areas
below the throat, and include acute and chronic bronchitis and
pneumonia. The World Health Organization estimates that respiratory
tract diseases were the fourth leading cause of death worldwide in
2016, accounting for nearly 3 million deaths, which corresponds to
~40 deaths per 100,000 population (2).
Children are particularly vulnerable to respiratory
tract diseases, with each child reported to experience up to 12
cases of respiratory tract infection per year (3). Due to their underdeveloped immune
systems, children are also prone to complications from respiratory
tract diseases, including bronchitis, pneumonia, sinusitis and
otitis media (4). Therefore,
respiratory tract infections in children pose a great threat to
health. Mild cases present with local symptoms such as sneezing,
nasal congestion and dry cough, while some children may also
experience symptoms such as a sore throat or discomfort in the
throat (5). However, severe
illness can occur, particularly in infants and young children, with
rapid onset, mild local symptoms and severe systemic symptoms,
manifesting as irritability, high fever, anorexia and fatigue, with
shock and death occurring in the most severe cases (6).
The human microbiota and its surrounding
microenvironment are closely linked to metabolism and health, and
dysbiosis of the microbiota is strongly associated with respiratory
tract diseases. As one of the organs with the most complex
microbiota, the intestines have a marked impact on human health.
The gut microbiota has various effects on human physiology, which
are mediated via a number of different axes, including the gut-lung
(7), gut-brain (8), gut-skeletal muscle (9), gut-organ (10) and gut-cardiac axes (11). Research into the gut microbiota has
explored its influence on a wide range of conditions, including
cancer (12), hypertension
(13), obesity (14) and respiratory diseases (15).
With the advancement of microbial research
techniques, the field of human microbial ecosystems has been
increasingly studied (16).
Although most studies on the microbiome have focused on diseases in
adults, researchers have now started to investigate the role of
microbial ecosystems in pediatric respiratory diseases. Studies of
adults suggest that the gut microbiota can indirectly regulate
pulmonary immune function (17).
The bacterial population in the gut is large and diverse compared
with that in other parts of the body, making it a particularly
valuable topic of research. The development of the gut microbiota
begins at birth, undergoes highly dynamic changes during in the
first years of life and typically stabilizes after 1–3 years
(18).
In the present review, the development of the gut
microbiota during early life and its roles in human health are
described. In addition, the associations of the gut microbiota with
pediatric respiratory tract diseases are reviewed, with a focus on
syncytial viral infections, childhood asthma and cystic fibrosis.
Finally, probiotic-related therapeutic approaches are discussed.
The review aims to provide new insights into the relationship
between gut flora and pediatric respiratory diseases.
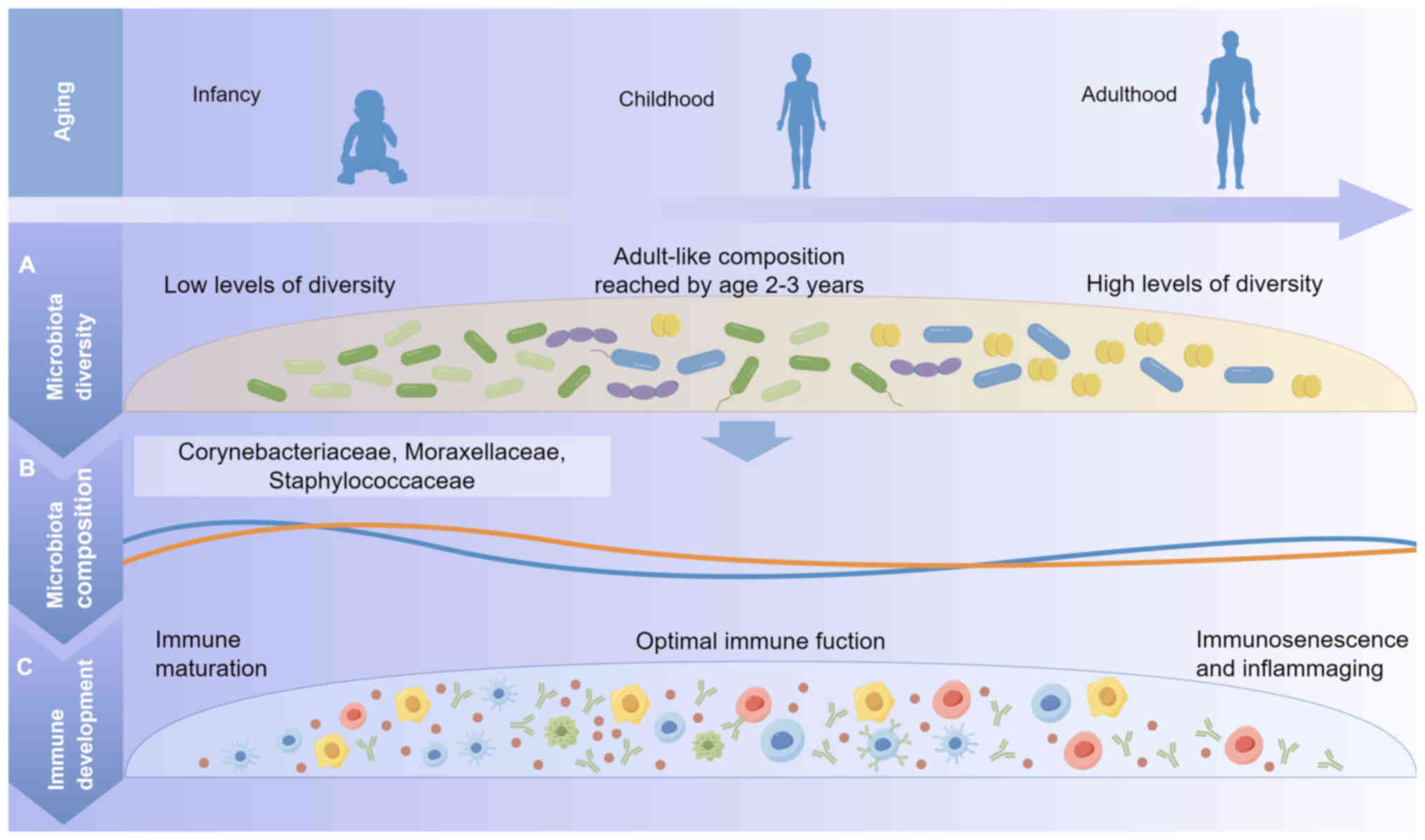
The innate and adaptive immune systems are known to
be influenced by the composition of the gut microbiota during the
first year of life (19). After
birth, the immune system is not yet mature, and the first few years
of life are a critical period for both microbiota establishment and
maturation of the immune system (20). The microbial community participates
in various processes within the body, including metabolism and
regulation of the immune system, thereby playing a vital role in
the overall health of infants (Fig.
1) (21).
The composition of the gut microbiota varies from
birth, and differs in infants born vaginally from those born by
cesarean section (22). Vaginally
delivered infants are exposed to maternal vaginal and fecal
microbiota, resulting in the presence of Lactobacillus,
Prevotella and/or Sneathia in the gut microbiota
(21,23). By contrast, infants delivered by
cesarean section acquire microbiota from maternal skin, hospital
staff or the hospital environment, including Staphylococcus,
Corynebacterium and Propionibacterium (24,25).
Infants born by cesarean section generally exhibit decreased
microbial diversity compared with that of vaginally delivered
infants (26). However, the gut
microbiology of infants delivered by emergency cesarean section
more closely resembles that of vaginal deliveries, likely due to
partial exposure to the birth canal during early labor (27,28).
Although the intrauterine environment was originally assumed to be
sterile, the presence of a unique microbial environment has been
identified in the placenta, comprising non-pathogenic commensal
microbiota. This indicates that even during embryonic development,
certain microorganisms may be in contact with and influence the
fetus (29).
The earliest microbiota colonizing the infant gut
are aerobic or parthenogenetic anaerobic bacteria, such as
Enterobacteriaceae, Enterococcus and Staphylococcus.
As anerobic bacteria increase and oxygen is consumed, the first
strict anaerobes begin to proliferate, including
Bifidobacterium, Clostridium and Lactobacillus. Among
these, Bifidobacterium is one of the most predominant
bacterial genera in the gut microbiota of human infants (30). In addition, preterm infants born at
less than 37 weeks' gestation typically exhibit a reduced gut
microbial diversity and lower levels of Bifidobacterium and
Lactobacillus acidophilus (L. acidophilus) (31). Their gut microbiota may be altered
due to factors such as exposure to medication or artificial feeding
(32). Preterm infants also
exhibit delayed gut colonization by commensal anerobic
microorganisms, such as Bifidobacterium or
Lactobacillus, and significantly elevated fecal levels of
pathogenic microorganisms, including Enterobacteriaceae and
Enterococcus (33,34).
Research has consistently demonstrated that
breastfeeding provides a wide range of health benefits for infants
(35). Breast milk contains
various bioactive components essential for infant growth and
development, including immunomodulatory factors, growth hormones,
antimicrobials and prebiotics (36). It has been hypothesized that the
inability of human milk oligosaccharides, which are abundant in
breast milk, to be digested by infants plays a crucial role in
shaping of the infant gut microbiota and protecting against
infection (37,38).
The gut microbiota of formula-fed infants consists
primarily of parthenogenetic anaerobes, such as
Lactobacillus and Clostridium. Differences between
the microbial communities of breastfed and formula-fed infants have
been associated with long-term health outcomes, including an
increased risk of developing allergic disease in formula-fed
infants, whereas breastfeeding is protective (40). To address this, prebiotics such as
short-chain galacto-oligosaccharides and long-chain
fructo-oligosaccharides are often added to formula milk. They
selectively promote the growth of Bifidobacterium and reduce
the abundance of Enterococcus and Escherichia coli,
thereby improving the composition of the gut microbiota (41). Although current infant formulas are
unable to fully replicate the beneficial effects of breast milk on
the gut microbiota, advances in prebiotic formulations have been
shown to have a positive effect on infants (such as establish the
gut microbiota and promote the colonization of bifidobacteria)
(42).
Antibiotic treatment and malnutrition are two major
factors that affect the gut microbiota, leading to gut dysbiosis
(43). Disturbances during early
life can impair the composition, maturation and function of the gut
microbiota, leading to adverse health consequences later in life.
While antibiotics are commonly used to treat bacterial infections,
they destroy commensal bacteria in addition to harmful bacteria,
thereby triggering intestinal dysbiosis (44). Early use of antibiotics has been
shown to reduce the number of bifidobacteria in the neonatal gut,
and broad-spectrum antibiotics can significantly alter the
composition and structure of the gut microbiota, reducing its
diversity by >25% (45,46). In addition, maternal antibiotic use
during pregnancy and breastfeeding has been linked with neonatal
microflora dysbiosis (47,48). Antibiotics reduce the diversity of
the breast milk microbiota, thereby decreasing the abundance of
Bifidobacterium in the neonatal gut (49). Malnourished children also exhibit
disturbed intestinal flora, characterized by a significant
reduction in Bifidobacterium and an altered ratio of aerobic
to anaerobic bacteria in fecal samples, resembling immature
intestinal flora (50).
Malnutrition may also promote inflammation, impair nutrient
absorption and exacerbate gut microflora dysbiosis (51,52).
Some of the factors influencing the gut microbiota in early life
are summarized in Table I
(23,53–58).
The close relationship between the gut and lungs can
be partly attributed to their shared embryonic origin and the fact
that both are exposed to the external environment via the oral
cavity and pharynx, which share physiological and structural
features (59,60). Although the mechanisms underlying
the gut-lung axis are not fully understood, the gut microbiota and
its metabolites play an important role in host defense. Metabolites
produced by the commensal gut microbiota activate and regulate
certain cellular responses required to maintain inflammatory tone,
thereby promoting microbiota-host homeostasis (61). The anaerobic fermentation of
dietary fiber by the gut microbiota produces short-chain fatty
acids with immunomodulatory functions, including the inhibition of
immune cell chemotaxis and adhesion, induction of anti-inflammatory
cytokine expression, and stimulation of apoptosis in immune cells
(62). These fatty acid
metabolites also increase the number and function of T regulatory
(Treg), T helper (Th) 1 and Th17 effector cells through the
inhibition of histone deacetylases, thereby reducing excessive
inflammation and immune responses in respiratory tract diseases
(63).
There are significant differences in the overall gut
microbiome composition between patients with chronic obstructive
pulmonary disease (COPD) and healthy individuals (64). The fecal microbiome of patients
with COPD shows increased abundances of Streptococcus, Rothia,
Romboutsia, Streptococcus spp., and Escherichia, with
Streptococcus considered to be a key factor differentiating
samples from patients with COPD from those from healthy individuals
(65). The gut microbiota is
particularly relevant to respiratory health. For example, exposure
to gut commensal bacteria immediately after birth has been shown to
promote the migration of group 3 innate lymphoid cells into the
lungs of neonatal mice to help defend against pneumonia, whereas
the same treatment in adult mice has limited effects on pneumonia
susceptibility (66). Another
study in mice demonstrated that colonization with gut microbes
during the neonatal period reduces the likelihood of allergic
asthma by preventing ovalbumin-induced aggregation of invariant
natural killer T cells to the lungs and by reducing
hypermethylation of the CC motif chemokine ligand 16 gene (67).
The administration of microbial metabolites,
microbial components or probiotics to mice improves their immune
response and survival when exposed to lung pathogens, with
detectable changes in the microbiota of the gut and lungs (68). A study by Luoto et al
(69) found that prebiotic
treatment with a mixture of galacto-oligosaccharides and
polydextrose, or probiotic supplementation with Lactobacillus
rhamnosus GG (LGG), reduced the incidence of upper respiratory
tract infections in preterm neonates. Similarly, Maldonado et
al (70) observed a reduced
incidence of respiratory and gastrointestinal infections in
neonates following the administration of Lactobacillus
fermentum and galacto-oligosaccharides. In addition, a
meta-analysis of 23 trials and 6,269 children performed by Wang
et al (71) demonstrated
that probiotic supplementation reduced the incidence of upper
respiratory tract infections, with the evidence rated as moderate
quality.
Gastrointestinal symptoms such as loss of appetite,
nausea and vomiting occur in 20–60% of patients with coronavirus
disease 19 (COVID-19). These symptoms may appear earlier than
respiratory symptoms exhibit an association with severe disease
progression (72). Differences in
the composition of the gut microbiota between individuals infected
with COVID-19 and controls have been detected, with reductions of
Faecalibacterium prausnitzii and Bifidobacterium bifidum
(B. bifidum) populations in COVID-19-infected patients, which
are inversely correlated with disease severity (73,74).
The remainder of this section examines the
relationship between the gut microbiota and respiratory tract
diseases, focusing on respiratory syncytial virus (RSV) infections,
childhood asthma and cystic fibrosis.
Early in life, RSV infection can bias immune
responses toward Th2 or Th17 pathways. This is due to the presence
of thymic stromal lymphopoietin released by airway epithelial
cells, which activates the Th2 response via CD4+ T cells
and type 2 innate lymphocytes (87). Another study found that the RSV
infection of dendritic cells alters histone methylation in the
promoter region of pro-inflammatory cytokines, thereby reducing
innate Th1-associated inflammatory responses and shifting immunity
towards Th2 inflammation (88).
Both clinical and animal studies indicate that this RSV-induced
immune profile persists even after recovery from infection
(89,90). Notably, the gut microbiota can
suppress inflammation and modulate the immune response. A
systematic review of the literature suggests that the gut
microbiome of RSV-infected individuals differs from that of healthy
controls (91), a finding
supported by a study of animals (92). Furthermore, a summary review
reports that the administration of bacterial lysates designed to
act on the gut microbiota modulates immunity and reduces the
frequency and severity of respiratory infections in children
(93).
The prevalence of asthma in children aged 5–14 years
is ~10%, making it the most common chronic disease worldwide.
Validated tools or methods to confirm the diagnosis of asthma in
children <5 years of age are lacking, despite asthma-like
symptoms appearing before the age of 2 years in some cases
(94,95). Asthma is a complex disease
characterized by clinical symptoms such as wheezing, cough, chest
tightness and dyspnoea, along with bronchial obstruction,
hyper-responsiveness to triggers such as infections, allergies,
pollution, climate or physical activity, and airway inflammation.
Symptoms vary with age, exposure to triggers or treatment (96). Asthma can also be classified into
subtypes, with the most common childhood form being allergy-related
and mediated by Th2 cells (97).
Th2 cell-mediated asthma is characterized by airway eosinophilic
inflammation, activated by innate epithelial mediators, including
IL-25 and IL-33, and thymic stromal lymphopoietin. These mediators
are secreted by airway epithelial cells in response to allergens,
smoke, pollutants, microbes and other irritants. Genetic and
epigenetic factors have been shown to influence the development of
asthma (98).
The gut microbiota has been demonstrated to play a
role in childhood asthma. In a cohort study, children born to
mothers with asthma had immature gut microbiota at 1 year of age,
which was associated with an increased risk of developing asthma by
age 5 years, whereas this association was not observed in children
without maternal asthma (99). The
study observed that the abundance of Veillonella in the
intestines of children born to asthmatic mothers at 1 year was
positively associated with asthma at 5 years, while the abundance
of Enterococcus faecalis (E. faecalis), Bifidobacterium,
Roseburia, Alistipes, Dialister, Lachnospiraceae incertae
sedis and Ruminococcus was negatively associated.
Another study found that newborns with a higher abundance of E.
faecalis, Bifidobacterium, Lactobacillus and Akkermansia
in the gut microbiota had a lower 4-year risk of developing asthma
(100). Factors such as mode of
delivery, feeding practices and antibiotic use have been shown to
influence the gut microbiota in infants, and these same factors are
epidemiologically associated with the development of asthma in
children. It has been suggested that the adverse effects of
antibiotic use on childhood asthma may be mediated by interference
with the gut microbiota in infancy, for example, by altering the
relative abundance of various microbes in the gut microbiota and
inducing dysbiosis (101). A
study on breastfeeding and childhood asthma confirmed that part of
the protective effect of breast milk on childhood asthma is
mediated by the composition of the early gut microbiota (102). In addition, a higher abundance of
beneficial bacteria, such as Bifidobacterium longum (B.
longum), in the gut microbiota early in life has been
associated with a reduced risk of asthma (103). A recent population-based and
prospective cohort study conducted in British Columbia, Canada
further suggested that the judicious use of antibiotics in infancy
and early childhood may protect the gut microbiota and help to
reduce the incidence of asthma in children (104). Collectively, these findings
support a link between childhood asthma and the gut microbiota.
Studies have shown that the neonatal microbiota in
children with allergic diseases, such as allergic asthma, is
associated with increased Th2 and decreased Treg cell numbers
(105). In addition, the colon,
skin and lungs of germ-free mice have been found to exhibit
increased Th2 and reduced Treg cell numbers, which can be altered
by exposure to commensal microbes early in life (106). Furthermore, an increased
abundance of commensal bacteria, including Lactobacillus,
Clostridium, Lactobacillus and Veillonella, is
associated with improved Treg cell function and may provide
protection against diseases such as childhood asthma (107).
Cystic fibrosis is an autosomal recessive disorder
affecting mucus- and sweat-producing cells that affects multiple
organs, primarily the lungs and digestive system. It leads to
impaired mucus clearance and bacterial infections of the airways
(108). This disease is
predominantly prevalent in Caucasian populations, with estimated
prevalence rates of 1/3,000 in Europe and 1/6,000 worldwide
(109). Cystic fibrosis lung
disease is characterized by thickened airway secretions, bacterial
infection and inflammation, which progressively cause airway
destruction, leading to bronchiectasis and ultimately respiratory
failure (110). This condition is
caused by mutations in the cystic fibrosis transmembrane
conductance regulator (CFTR) gene, which leads to the defective
function or absence of CFTR protein, affects the cells that produce
mucus and sweat, causing the mucus to thicken and block the airways
in the lungs. This protein is a chloride channel in the epithelial
cell membrane that also regulates the activity of sodium channels
(111).
It is now widely accepted that the relationship
between the microbiota and cystic fibrosis is bidirectional. CFTR
dysfunction leads to aberrant colonization of the gut and
respiratory microbiota, which in turn alters the intestinal and
airway microenvironment (112). A
study showed that cystic fibrosis-induced changes in the
lungmicrobiota cause changes in the gut microbiota (113). Patients with cystic fibrosis have
been found to exhibit reduced abundances of Ruminococcaceae,
Bifidobacterium, Bacteroides and Roseburia in the
gut, which are normally considered to be healthy commensal gut
microbiota (114–116), and are associated with
anti-inflammatory activity, fermentation, and immune regulation
in vivo (117,118). Unlike the gut microbiota of
healthy children, an increased abundance of Enterococcus,
Enterobacter and Escherichia has been observed in the
intestines of patients with cystic fibrosis, however, there is no
evidence indicating that this variation is definitively associated
with pathogenicity (119–121). The functional characteristics of
the gut microbiota in pediatric patients with cystic fibrosis
differ significantly from those without cystic fibrosis (122). Manor et al (123) found that the gut micribiota of
pediatric patients with cystic fibrosis have an increased
propensity to metabolize short-chain fatty acids, nutrients and
antioxidants, with enriched short-chain fatty acid metabolic
pathways and reduced fatty acid biosynthetic pathways, microbiota
dysbiosis and functional imbalance are highly evident, with
functional disparities linked to malabsorption and inflammation. In
addition, another study showed that, despite severe dysbiosis, the
gut microbiota in patients with cystic fibrosis retains the ability
to produce short-chain fatty acids by the fermentation of starch
(124).
The dynamic relationship between the gut microbiota
and respiratory tract diseases in children is increasingly being
recognized. Further in-depth research is required to provide
researchers and clinicians with a more comprehensive and innovative
perspective on the management of childhood respiratory
diseases.
Currently, traditional treatments for respiratory
diseases rely heavily on antibiotics and antiviral drugs, which
must be used with caution in infants and young children to avoid
short- or long-term adverse effects. Widespread use of antibiotics
can lead to serious issues, such as drug resistance, reduced
therapeutic efficacy and a substantial societal burden (125). Probiotics have emerged as
effective agents for the regulation of intestinal microecology and
have gained attention in clinical studies for the prevention and
treatment of respiratory diseases in children. Probiotics are
generally defined as live microorganisms that can positively
influence host health (126).
They have been widely used as medicines, food additives or
nutritional supplements for the prevention and treatment of
pediatric diseases due to their recognized beneficial effects
(127). Evidence-based medical
findings have shown that oral probiotics are effective in
preventing respiratory diseases and reducing recurrence rates in
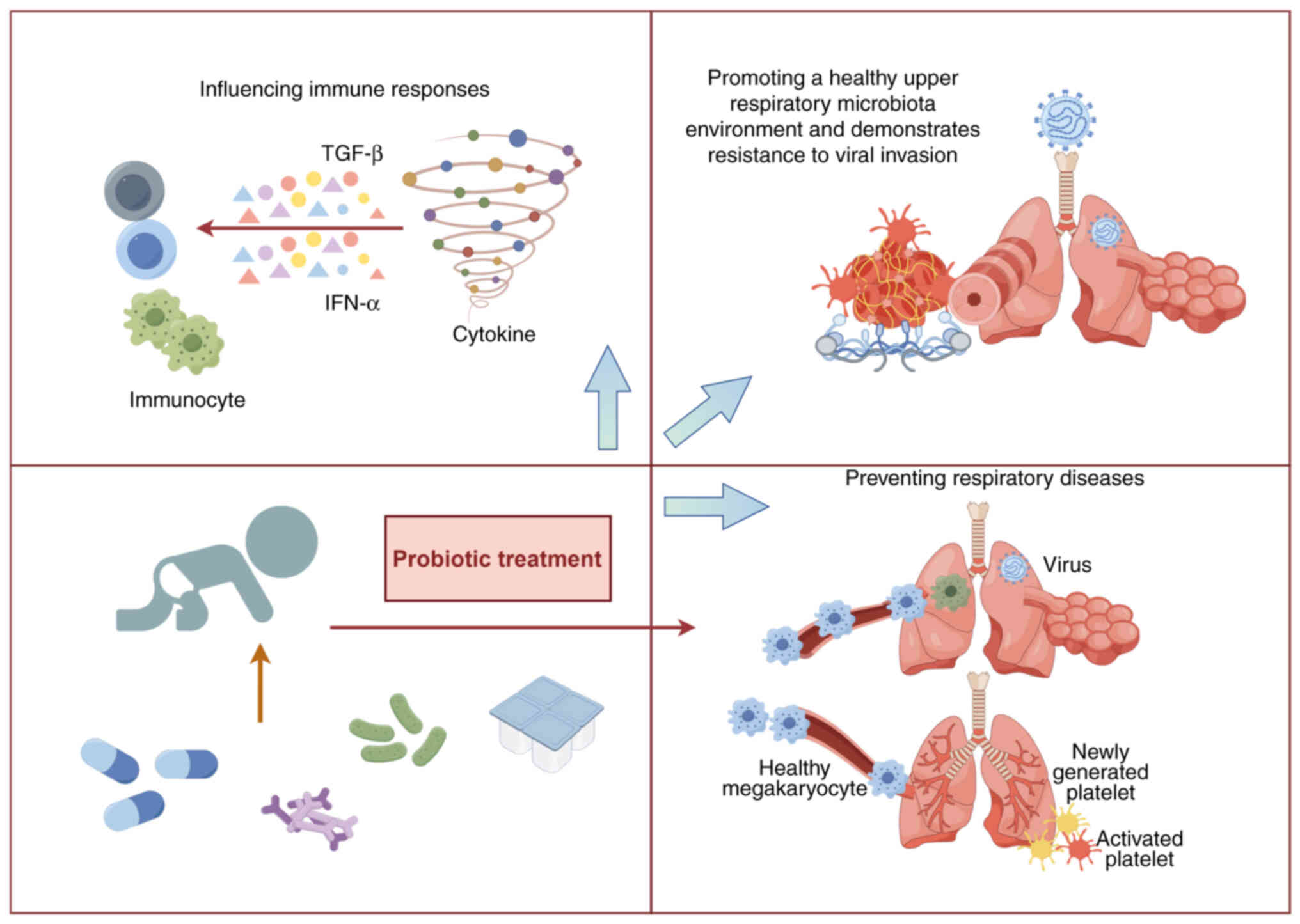
healthy children without any adverse side effects (128). Probiotic intake can promote a
healthy upper respiratory microbiota and enhance resistance to
viral invasion (129,130). The gut microbiota influences
immune responses in multiple mucosal systems, playing a crucial
role in the maintenance of physiological homeostasis and human
health, while dysbiosis of the gut microbiota increases
susceptibility to respiratory diseases (131). Although the etiology of
respiratory diseases is multifaceted, the ability of probiotics to
modulate microecological balance and immune responses has attracted
considerable attention in treatment strategies for respiratory
diseases (Fig. 2) (132).
Probiotics protect the integrity of the epithelial
barrier by activating pattern recognition receptors on epithelial
cells through microbe-associated molecular patterns that regulate
tight and adhesion junctions (136). They also disrupt the
microenvironments of pathogens through competition for epithelial
cell adhesion sites, nutrient depletion and the secretions of
antimicrobial compounds (137).
In addition, probiotic metabolites play an important role in
respiratory diseases by modulating the differentiation of immune
cells and controlling the immune response via G protein-coupled
receptors and histone deacetylases (138).
Gut-associated lymphoid tissue is an important
component of the peripheral immune system, and probiotics have been
shown to enhance systemic immunity and indirectly strengthen
respiratory defense by the modulation of this tissue (139). They also mediate innate immune
responses through pattern recognition receptors, particularly
toll-like receptors (TLRs), which recognize and bind to
pathogen-associated molecular patterns on the surface of pathogenic
microorganisms and activate signaling pathways, such as the nuclear
factor-κB and mitogen-activated protein kinase pathways, to
regulate the secretion of pro-inflammatory cytokines (140). For example, Lactococcus
lactis (L. lactis) enhances Th1 cell differentiation and
upregulates the expression of cytokines IL-12, IFN-γ and TNF-α via
TLR2, TLR3 and TLR9 pathways (141).
LGG is currently one of the most widely used
probiotics due to its tolerance of stomach acid and bile. Studies
have shown that LGG can regulate the balance of microbiota in the
gut, reducing harmful bacteria such as Bacteroides and
Proteus, and increasing beneficial bacteria such as
Lactobacillus, Bifidobacterium and butyric acid-producing
bacteria (142,143). In addition, LGG modulates the
host immune system, helping to prevent and treat infections by
triggering an inflammatory response and activating macrophages,
which protect intestinal epithelial cells (144). Several studies have demonstrated
that LGG has favorable preventive, therapeutic and curative effects
on respiratory diseases in children. In a randomized trial
involving 281 children, LGG significantly reduced the risk of upper
respiratory tract infections and shortened the duration of
respiratory symptoms (145),
while a meta-analysis of four randomized controlled trials with
1,805 participants showed that LGG reduces the incidence of acute
otitis media while also reducing antibiotic use (146). Other studies have demonstrated
that LGG significantly reduces the overall risk of respiratory
infection and shortens the duration of respiratory symptoms in
children (147). However,
although LGG significantly improves respiratory symptoms, it does
not appear to inhibit viral activity in respiratory tract
infections in children (148).
Interestingly, in another randomized study involving 619
participants aged 2–6 years, LGG effectively relieved symptoms of
upper respiratory tract infection, but was not effective in
reducing the incidence of these infections (149). Despite the multifaceted health
benefits of LGG, the results of the studies show some
inconsistency, suggesting that the efficacy of probiotics may
differ among individuals.
Bifidobacteria are a group of probiotics commonly
found in the human gut, particularly in infants. Their numbers and
species diversity tend to decline with age (150). Studies have shown that
bifidobacteria play an important preventive role in the maintenance
of a healthy gut microbiota by regulating gut microbial metabolism,
promoting intestinal motility, adhering to and degrading harmful
substances, and enhancing host immune function (151). B. longum has been shown to
regulate the Th1/Th2 immune system balance. In a study in Malaysian
preschool children, B. longum BB536 significantly increased
the abundance of anti-inflammatory and immunomodulatory bacteria in
the feces, thereby preventing upper respiratory tract diseases via
modulation of the gut microbiota (152). Another study showed that the
early administration of Bifidobacterium animalis subspecies
Lactobacillus bifidus BB-12 to infants reduced the risk of
early infections and respiratory tract infections (153).
Probiotic complexes are preparations containing
multiple probiotic strains that regulate the composition and
diversity of the intestinal flora, protect intestinal barrier
function, and reduce inflammation and intestinal damage. Probiotic
complexes have demonstrated superior efficacy than single strains
in maintaining gut health, modulating immune function and single
strain resistance issues (154).
Probiotic complexes have shown the potential to play a positive
role in the prevention and treatment of recurrent respiratory
infections. For example, oral quadruple probiotic tablets
containing B. bifidum, L. acidophilus, E. faecalis and
Bacillus cereus not only increased the abundance of the
beneficial gut bacteria B. bifidum and L. lactis, but
also significantly reduced the mean annual frequency of acute
respiratory infections and antibiotic use (155). Also, another probiotic
preparation containing Bifidobacterium animalis subspecies
Lactobacillus bifidus BB-12 and E. faecalis L3
significantly increased salivary immunoglobulin levels and reduced
the risk of upper respiratory tract infections in healthy children
(156). In addition, a nasal
spray comprising Streptococcus salivarius 24SMB and
Streptococcus oralis 89a was found to be effective in
relieving the symptoms of recurrent respiratory infections in
children (157). Another clinical
study also supports the benefits of probiotic complexes, with a
marked reduction in the incidence of respiratory infections in
children treated with oral probiotics compared with placebo-treated
controls, in addition to a lack of adverse effects (158).
Although numerous probiotic products have been shown
to be safe, their use can also cause adverse reactions (159). Probiotics have been reported to
cause gastrointestinal side effects, including diarrhea and
bloating (160). In addition,
probiotics may facilitate the lateral transfer of antibiotic
resistance genes to other microorganisms (161,162). There have been some reports that
probiotics can act as opportunistic pathogens in immunocompromised
individuals, potentially leading to life-threatening diseases such
as pneumonia, endocarditis and sepsis (163–165). Therefore, further research is
necessary to evaluate probiotic therapies for respiratory diseases,
particularly regarding individual differences and safety
issues.
Certain probiotics have demonstrated beneficial
effects. For example, a combination of Lactobacillus and
B. bifidum has exhibited an association with reduced
inflammation, and may have the ability to ameliorate inflammatory
conditions (166). In addition,
Lactobaccillus plantarum has been shown to regulate
oxidative stress, inflammation and gut microbiota dysbiosis in
vivo, while promoting intestinal motility and mucin production
(167). Some of the key effects
and underlying mechanisms of probiotic therapy are summarized in
Table II (168–172). However, the mechanisms by which
different probiotics exert their effects on the human body remain
unclear, highlighting the need for further comprehensive
research.
When considering any treatment, safety is a
priority for pediatric populations. The comprehensive reporting of
all adverse events, particularly long-term safety outcomes, is
critical to meaningfully advance the evidence base in this area
(173). A meta-analysis of COPD
performed by Su et al (174) suggested that probiotics can
improve lung function and structure, and reduce inflammation.
However, the experimental results were suggested to have some
limitations, particularly regarding the efficacy and safety of
long-term probiotic use. Li et al (175) conducted a 12-week randomized
controlled trial, which demonstrated that Bifidobacterium
infantis YLGB-1496 exhibited excellent safety and tolerability
in infants and effectively alleviated the gastrointestinal
discomfort associated with respiratory diseases.
Further research is necessary to validate the
long-term efficacy and safety of probiotics, even though a number
of studies have indicated that probiotic therapy is safe (176–178).
The present review first described the development
of gut microbiota in the early stages of life and their critical
role in human health. It then introduced the gut-lung axis as an
important bidirectional communication system, reviewed the
relationship between the gut microbiota and three pediatric
respiratory tract diseases, and concluded with a summary of
probiotic therapies.
The concept of the gut-lung axis is crucial for
understanding respiratory disease. The gut microbiota and human
health interact with each other, but the underlying mechanisms
remain unclear and require further study. The development of a
healthy gut microbiota is strongly associated with respiratory
health and the development of the immune system in children.
Therefore, in-depth investigation of the gut microbiota composition
and function during early childhood may help to predict future
health, prevent diseases and clarify the underlying mechanisms.
Probiotic therapy offers a novel approach for the
prevention and treatment of respiratory diseases. Increasing
evidence suggests that probiotics can be used to relieve symptoms,
reduce disease recurrence and reduce the use of antibiotics.
Reducing antibiotic exposure is important in children, as
antibiotics can lead to dysbiosis of the gut microbiota, leading to
lower host resistance and contributing to the global issue of
antibiotic resistance.
However, the limitations of probiotic treatments
must be acknowledged. Their exact mechanisms remain unclear, and
therapeutic effects may vary. In addition, the roles of probiotic
preparations in the microenvironments of different diseases require
further investigation. The development of more effective composite
probiotic preparations appears to be a promising area of research.
Although studies have shown that probiotic therapy is generally
safe, with no serious side effects or adverse symptoms reported,
safety assessments of probiotic treatments remain limited to
certain populations; therefore, more research on safety is
necessary. Finally, the lack of unified industry or clinical
guidelines for probiotic use remains a barrier. The standardization
of usage methods in daily life and clinical practice will be
essential to expand the prospects for probiotic treatments in
pediatric respiratory care.
Not applicable.
Funding: No funding was received.
Not applicable.
MZ and YH reviewed the literature and wrote the
manuscript. YJ and LC conceived and designed the study. All authors
have read and approved the final version of the manuscript. Data
authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
GBD 2015 Chronic Respiratory Disease
Collaborators, . Global, regional, and national deaths, prevalence,
disability-adjusted life years, and years lived with disability for
chronic obstructive pulmonary disease and asthma, 1990–2015: A
systematic analysis for the global burden of disease study 2015.
Lancet Respir Med. 5:691–706. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
World Health Organization (WHO), . Global
Health Estimates 2016: Deaths by cause, age, sex, by country and by
region, 2000–2016. WHO; Geneva: 2018
|
|
3
|
Ogal M, Johnston SL, Klein P and Schoop R:
Echinacea reduces antibiotic usage in children through respiratory
tract infection prevention: A randomized, blinded, controlled
clinical trial. Eur J Med Res. 26:332021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aitken M and Taylor JA: Prevalence of
clinical sinusitis in young children followed up by primary care
pediatricians. Arch Pediatr Adolesc Med. 152:244–248. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tian J, Wang XY, Zhang LL, Liu MJ, Ai JH,
Feng GS, Zeng YP, Wang R and Xie ZD: Clinical epidemiology and
disease burden of bronchiolitis in hospitalized children in China:
A national cross-sectional study. World J Pediatr. 19:851–863.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chatterjee A, Mavunda K and Krilov LR:
Current state of respiratory syncytial virus disease and
management. Infect Dis Ther. 10 (Suppl 1):S5–S16. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Keulers L, Dehghani A, Knippels L, Garssen
J, Papadopoulos N, Folkerts G, Braber S and van Bergenhenegouwen J:
Probiotics, prebiotics, and synbiotics to prevent or combat air
pollution consequences: The gut-lung axis. Environ Pollut.
302:1190662022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Asadi A, Shadab Mehr N, Mohamadi MH,
Shokri F, Heidary M, Sadeghifard N and Khoshnood S: Obesity and
gut-microbiota-brain axis: A narrative review. J Clin Lab Anal.
36:e244202022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mancin L, Wu GD and Paoli A: Gut
microbiota-bile acid-skeletal muscle axis. Trends Microbiol.
31:254–269. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ahlawat S and Asha Sharma KK: Gut-organ
axis: A microbial outreach and networking. Lett Appl Microbiol.
72:636–668. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Akshay A, Gasim R, Ali TE, Kumar YS and
Hassan A: Unlocking the gut-cardiac axis: A paradigm shift in
cardiovascular health. Cureus. 15:e510392023.PubMed/NCBI
|
|
12
|
Wong CC and Yu J: Gut microbiota in
colorectal cancer development and therapy. Nat Rev Clin Oncol.
20:429–452. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang Z, Wang Q, Liu Y, Wang L, Ge Z, Li Z,
Feng S and Wu C: Gut microbiota and hypertension: Association,
mechanisms and treatment. Clin Exp Hypertens. 45:21951352023.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wilkins AT and Reimer RA: Obesity, early
life gut microbiota, and antibiotics. Microorganisms. 9:4132021.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu W, Wu Y, Liu H, Jiang C and Huo L:
Gut-lung axis: Microbial crosstalk in pediatric respiratory tract
infections. Front Immunol. 12:7412332021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Walker AW and Hoyles L: Human microbiome
myths and misconceptions. Nat Microbiol. 8:1392–1396. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eribo OA, du Plessis N and Chegou NN: The
intestinal commensal, bacteroides fragilis, modulates host
responses to viral infection and therapy: Lessons for exploration
during mycobacterium tuberculosis infection. Infect Immun.
90:e00321212022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alcazar CG, Paes VM, Shao Y, Oesser C,
Miltz A, Lawley TD, Brocklehurst P, Rodger A and Field N: The
association between early-life gut microbiota and childhood
respiratory diseases: A systematic review. Lancet Microbe.
3:e867–e880. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zheng D, Liwinski T and Elinav E:
Interaction between microbiota and immunity in health and disease.
Cell Res. 30:492–506. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van den Elsen LWJ, Garssen J, Burcelin R
and Verhasselt V: Shaping the gut microbiota by breastfeeding: The
gateway to allergy prevention? Front Pediatr. 7:472019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shao Y, Forster SC, Tsaliki E, Vervier K,
Strang A, Simpson N, Kumar N, Stares MD, Rodger A, Brocklehurst P,
et al: Stunted microbiota and opportunistic pathogen colonization
in caesarean-section birth. Nature. 574:117–121. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Munyaka PM, Khafipour E and Ghia JE:
External influence of early childhood establishment of gut
microbiota and subsequent health implications. Front Pediatr.
2:1092014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dominguez-Bello MG, Costello EK, Contreras
M, Magris M, Hidalgo G, Fierer N and Knight R: Delivery mode shapes
the acquisition and structure of the initial microbiota across
multiple body habitats in newborns. Proc Natl Acad Sci USA.
107:11971–11975. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bokulich NA, Chung J, Battaglia T,
Henderson N, Jay M, Li H, D Lieber A, Wu F, Perez-Perez GI, Chen Y,
et al: Antibiotics, birth mode, and diet shape microbiome
maturation during early life. Sci Transl Med. 8:343ra3822016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rodríguez JM, Murphy K, Stanton C, Ross
RP, Kober OI, Juge N, Avershina E, Rudi K, Narbad A, Jenmalm MC, et
al: The composition of the gut microbiota throughout life, with an
emphasis on early life. Microb Ecol Health Dis.
26:260502015.PubMed/NCBI
|
|
26
|
Jakobsson HE, Abrahamsson TR, Jenmalm MC,
Harris K, Quince C, Jernberg C, Björkstén B, Engstrand L and
Andersson AF: Decreased gut microbiota diversity, delayed
Bacteroidetes colonisation and reduced Th1 responses in infants
delivered by caesarean section. Gut. 63:559–566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chu DM, Ma J, Prince AL, Antony KM,
Seferovic MD and Aagaard KM: Maturation of the infant microbiome
community structure and function across multiple body sites and in
relation to mode of delivery. Nat Med. 23:314–326. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fouhy F, Watkins C, Hill CJ, O'Shea CA,
Nagle B, Dempsey EM, O'Toole PW, Ross RP, Ryan CA and Stanton C:
Perinatal factors affect the gut microbiota up to four years after
birth. Nat Commun. 10:15172019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stupak A and Kwaśniewski W: Evaluating
current molecular techniques and evidence in assessing microbiome
in placenta-related health and disorders in pregnancy.
Biomolecules. 13:9112023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Le Huërou-Luron I, Blat S and Boudry G:
Breast- v. formula-feeding: Impacts on the digestive tract and
immediate and long-term health effects. Nutr Res Rev. 23:23–36.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Korpela K, Blakstad EW, Moltu SJ, Strømmen
K, Nakstad B, Rønnestad AE, Brække K, Iversen PO, Drevon CA and de
Vos W: Intestinal microbiota development and gestational age in
preterm neonates. Sci Rep. 8:24532018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Milani C, Duranti S, Bottacini F, Casey E,
Turroni F, Mahony J, Belzer C, Delgado Palacio S, Arboleya Montes
S, Mancabelli L, et al: The first microbial colonizers of the human
gut: Composition, activities, and health implications of the infant
gut microbiota. Microbiol Mol Biol Rev. 81:e00036–17. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Arboleya S, Sánchez B, Milani C, Duranti
S, Solís G, Fernández N, de los Reyes-Gavilán CG, Ventura M,
Margolles A and Gueimonde M: Intestinal microbiota development in
preterm neonates and effect of perinatal antibiotics. J Pediatr.
166:538–544. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cong X, Xu W, Janton S, Henderson WA,
Matson A, McGrath JM, Maas K and Graf J: Gut microbiome
developmental patterns in early life of preterm infants: Impacts of
feeding and gender. PLoS One. 11:e01527512016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Binns C, Lee M and Low WY: The long-term
public health benefits of breastfeeding. Asia Pac J Public Health.
28:7–14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Perrella S, Gridneva Z, Lai CT, Stinson L,
George A, Bilston-John S and Geddes D: Human milk composition
promotes optimal infant growth, development and health. Semin
Perinatol. 45:1513802021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Berger B, Porta N, Foata F, Grathwohl D,
Delley M, Moine D, Charpagne A, Siegwald L, Descombes P, Alliet P,
et al: Linking human milk oligosaccharides, infant fecal community
types, and later risk to require antibiotics. mBio. 11:e03196–19.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zuurveld M, van Witzenburg NP, Garssen J,
Folkerts G, Stahl B, Van't Land B and Willemsen LEM:
Immunomodulation by human milk oligosaccharides: The potential role
in prevention of allergic diseases. Front Immunol. 11:8012020.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bogaert D, van Beveren GJ, de Koff EM,
Lusarreta Parga P, Balcazar Lopez CE, Koppensteiner L, Clerc M,
Hasrat R, Arp K, Chu MLJN, et al: Mother-to-infant microbiota
transmission and infant microbiota development across multiple body
sites. Cell Host Microbe. 31:447–460.e6. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yagi K, Asai N, Huffnagle GB, Lukacs NW
and Fonseca W: Early-life lung and gut microbiota development and
respiratory syncytial virus infection. Front Immunol.
13:8777712022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Borewicz K, Suarez-Diez M, Hechler C,
Beijers R, de Weerth C, Arts I, Penders J, Thijs C, Nauta A,
Lindner C, et al: The effect of prebiotic fortified infant formulas
on microbiota composition and dynamics in early life. Sci Rep.
9:24342019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhu B, Zheng S, Lin K, Xu X, Lv L, Zhao Z
and Shao J: Effects of infant formula supplemented with prebiotics
and OPO on infancy fecal microbiota: A pilot Randomized clinical
trial. Front Cell Infect Microbiol. 11:6504072021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ratsika A, Codagnone MC, O'Mahony S,
Stanton C and Cryan JF: Priming for Life: Early life nutrition and
the microbiota-gut-brain axis. Nutrients. 13:4232021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ianiro G, Tilg H and Gasbarrini A:
Antibiotics as deep modulators of gut microbiota: Between good and
evil. Gut. 65:1906–1915. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dierikx TH, Visser DH, Benninga MA, van
Kaam AHLC, de Boer NKH, de Vries R, van Limbergen J and de Meij
TGJ: The influence of prenatal and intrapartum antibiotics on
intestinal microbiota colonisation in infants: A systematic review.
J Infect. 81:190–204. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Panda S, El khader I, Casellas F, López
Vivancos J, García Cors M, Santiago A, Cuenca S, Guarner F and
Manichanh C: Short-term effect of antibiotics on human gut
microbiota. PLoS One. 9:e954762014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Blaser MJ and Dominguez-Bello MG: The
human microbiome before birth. Cell Host Microbe. 20:558–560. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Azad MB, Konya T, Persaud RR, Guttman DS,
Chari RS, Field CJ, Sears MR, Mandhane PJ, Turvey SE, Subbarao P,
et al: Impact of maternal intrapartum antibiotics, method of birth
and breastfeeding on gut microbiota during the first year of life:
A prospective cohort study. BJOG. 123:983–993. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hermansson H, Kumar H, Collado MC,
Salminen S, Isolauri E and Rautava S: Breast milk microbiota is
shaped by mode of delivery and intrapartum antibiotic exposure.
Front Nutr. 6:42019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Subramanian S, Huq S, Yatsunenko T, Haque
R, Mahfuz M, Alam MA, Benezra A, DeStefano J, Meier MF, Muegge BD,
et al: Persistent gut microbiota immaturity in malnourished
Bangladeshi children. Nature. 510:417–421. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Blanton LV, Barratt MJ, Charbonneau MR,
Ahmed T and Gordon JI: Childhood undernutrition, the gut
microbiota, and microbiota-directed therapeutics. Science.
352:15332016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kane AV, Dinh DM and Ward HD: Childhood
malnutrition and the intestinal microbiome. Pediatr Res.
77:256–262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Moles L, Gómez M, Heilig H, Bustos G,
Fuentes S, de Vos W, Fernández L, Rodríguez JM and Jiménez E:
Bacterial diversity in meconium of preterm neonates and evolution
of their fecal microbiota during the first month of life. PLoS One.
8:e669862013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Fernández L, Langa S, Martín V, Maldonado
A, Jiménez E, Martín R and Rodríguez JM: The human milk microbiota:
Origin and potential roles in health and disease. Pharmacol Res.
69:1–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mountzouris KC, McCartney AL and Gibson
GR: Intestinal microflora of human infants and current trends for
its nutritional modulation. Br J Nutr. 87:405–420. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Barrett E, Kerr C, Murphy K, O'Sullivan O,
Ryan CA, Dempsey EM, Murphy BP, O'Toole PW, Cotter PD, Fitzgerald
GF, et al: The individual-specific and diverse nature of the
preterm infant microbiota. Arch Dis Child Fetal Neonatal Ed.
98:F334–F340. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Fouhy F, Guinane CM, Hussey S, Wall R,
Ryan CA, Dempsey EM, Murphy B, Ross RP, Fitzgerald GF, Stanton C
and Cotter PD: High-throughput sequencing reveals the incomplete,
short-term recovery of infant gut microbiota following parenteral
antibiotic treatment with ampicillin and gentamicin. Antimicrob
Agents Chemother. 56:5811–5820. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Fallani M, Young D, Scott J, Norin E,
Amarri S, Adam R, Aguilera M, Khanna S, Gil A, Edwards CA, et al:
Intestinal microbiota of 6-week-old infants across Europe:
Geographic influence beyond delivery mode, breast-feeding, and
antibiotics. J Pediatr Gastroenterol Nutr. 51:77–84. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Budden KF, Gellatly SL, Wood DL, Cooper
MA, Morrison M, Hugenholtz P and Hansbro PM: Emerging pathogenic
links between microbiota and the gut-lung axis. Nat Rev Microbiol.
15:55–63. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Espírito Santo C, Caseiro C, Martins MJ,
Monteiro R and Brandão I: Gut microbiota, in the halfway between
nutrition and lung function. Nutrients. 13:17162021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chistiakov DA, Bobryshev YV, Kozarov E,
Sobenin IA and Orekhov AN: Intestinal mucosal tolerance and impact
of gut microbiota to mucosal tolerance. Front Microbiol. 5:7812015.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ratajczak W, Rył A, Mizerski A,
Walczakiewicz K, Sipak O and Laszczyńska M: Immunomodulatory
potential of gut microbiome-derived short-chain fatty acids
(SCFAs). Acta Biochim Pol. 66:1–12. 2019.PubMed/NCBI
|
|
63
|
Li M, van Esch BCAM, Wagenaar GTM, Garssen
J, Folkerts G and Henricks PAJ: Pro- and anti-inflammatory effects
of short chain fatty acids on immune and endothelial cells. Eur J
Pharmacol. 831:52–59. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lee SH, Yun Y, Kim SJ, Lee EJ, Chang Y,
Ryu S, Shin H, Kim HL, Kim HN and Lee JH: Association between
cigarette smoking status and composition of gut microbiota:
Population-based cross-sectional study. J Clin Med. 7:2822018.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Bowerman KL, Rehman SF, Vaughan A, Lachner
N, Budden KF, Kim RY, Wood DLA, Gellatly SL, Shukla SD, Wood LG, et
al: Disease-associated gut microbiome and metabolome changes in
patients with chronic obstructive pulmonary disease. Nat Commun.
11:58862020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Gray J, Oehrle K, Worthen G, Alenghat T,
Whitsett J and Deshmukh H: Intestinal commensal bacteria mediate
lung mucosal immunity and promote resistance of newborn mice to
infection. Sci Transl Med. 9:eaaf94122017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Olszak T, An D, Zeissig S, Vera MP,
Richter J, Franke A, Glickman JN, Siebert R, Baron RM, Kasper DL
and Blumberg RS: Microbial exposure during early life has
persistent effects on natural killer T cell function. Science.
336:489–493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Brown RL, Sequeira RP and Clarke TB: The
microbiota protects against respiratory infection via GM-CSF
signaling. Nat Commun. 8:15122017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Luoto R, Ruuskanen O, Waris M, Kalliomäki
M, Salminen S and Isolauri E: Prebiotic and probiotic
supplementation prevents rhinovirus infections in preterm infants:
a randomized, placebo-controlled trial. J Allergy Clin Immunol.
133:405–413. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Maldonado J, Cañabate F, Sempere L, Vela
F, Sánchez AR, Narbona E, López-Huertas E, Geerlings A, Valero AD,
Olivares M and Lara-Villoslada F: Human milk probiotic
Lactobacillus fermentum CECT5716 reduces the incidence of
gastrointestinal and upper respiratory tract infections in infants.
J Pediatr Gastroenterol Nutr. 54:55–61. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wang Y, Li X, Ge T, Xiao Y, Liao Y, Cui Y,
Zhang Y, Ho W, Yu G and Zhang T: Probiotics for prevention and
treatment of respiratory tract infections in children: A systematic
review and meta-analysis of randomized controlled trials. Medicine
(Baltimore). 95:e45092016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Redd WD, Zhou JC, Hathorn KE, McCarty TR,
Bazarbashi AN, Thompson CC, Shen L and Chan WW: Prevalence and
characteristics of gastrointestinal symptoms in patients with
severe acute respiratory syndrome coronavirus 2 infection in the
United States: A multicenter cohort study. Gastroenterology.
159:765–767.e2. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zuo T, Zhang F, Lui GCY, Yeoh YK, Li AYL,
Zhan H, Wan Y, Chung ACK, Cheung CP, Chen N, et al: Alterations in
gut microbiota of patients with COVID-19 during time of
hospitalization. Gastroenterology. 159:944–955.e8. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Yeoh YK, Zuo T, Lui GC, Zhang F, Liu Q, Li
AY, Chung AC, Cheung CP, Tso EY, Fung KS, et al: Gut microbiota
composition reflects disease severity and dysfunctional immune
responses in patients with COVID-19. Gut. 70:698–706. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Liu Y, Ni F, Huang J, Hu Y, Wang J, Wang
X, Du X and Jiang H: PPAR-α inhibits DHEA-induced ferroptosis in
granulosa cells through upregulation of FADS2. Biochem Biophys Res
Commun. 715:1500052024. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Mazur NI, Higgins D, Nunes MC, Melero JA,
Langedijk AC, Horsley N, Buchholz UJ, Openshaw PJ, McLellan JS,
Englund JA, et al: The respiratory syncytial virus vaccine
landscape: Lessons from the graveyard and promising candidates.
Lancet Infect Dis. 18:e295–e311. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Janet S, Broad J and Snape MD: Respiratory
syncytial virus seasonality and its implications on prevention
strategies. Hum Vaccin Immunother. 14:234–244. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Russell CD, Unger SA, Walton M and
Schwarze J: The human immune response to respiratory syncytial
virus infection. Clin Microbiol Rev. 30:481–502. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Bénet T, Sánchez Picot V, Messaoudi M,
Chou M, Eap T, Wang J, Shen K, Pape JW, Rouzier V, Awasthi S, et
al: Microorganisms associated with pneumonia in children <5
years of age in developing and emerging countries: The GABRIEL
pneumonia multicenter, prospective, case-control study. Clin Infect
Dis. 65:604–612. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Pneumonia Etiology Research for Child
Health (PERCH) Study Group, : Causes of severe pneumonia requiring
hospital admission in children without HIV infection from Africa
and Asia: the PERCH multi-country case-control study. Lancet.
394:757–779. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Lozano R, Naghavi M, Foreman K, Lim S,
Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et
al: Global and regional mortality from 235 causes of death for 20
age groups in 1990 and 2010: A systematic analysis for the Global
Burden of Disease Study 2010. Lancet. 380:2095–2128. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Harding JN, Siefker D, Vu L, You D,
DeVincenzo J, Pierre JF and Cormier SA: Altered gut microbiota in
infants is associated with respiratory syncytial virus disease
severity. BMC Microbiol. 20:1402020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Jang MJ, Kim YJ, Hong S, Na J, Hwang JH,
Shin SM and Ahn YM: Positive association of breastfeeding on
respiratory syncytial virus infection in hospitalized infants: A
multicenter retrospective study. Clin Exp Pediatr. 63:135–140.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Hasegawa K, Linnemann RW, Mansbach JM,
Ajami NJ, Espinola JA, Petrosino JF, Piedra PA, Stevenson MD,
Sullivan AF, Thompson AD and Camargo CA Jr: The fecal microbiota
profile and bronchiolitis in infants. Pediatrics.
138:e201602182016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Nishimura T, Suzue J and Kaji H:
Breastfeeding reduces the severity of respiratory syncytial virus
infection among young infants: A multi-center prospective study.
Pediatr Int. 51:812–816. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Kristensen K, Fisker N, Haerskjold A, Ravn
H, Simões EA and Stensballe L: Caesarean section and
hospitalization for respiratory syncytial virus infection: A
population-based study. Pediatr Infect Dis J. 34:145–148. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Lee HC, Headley MB, Loo YM, Berlin A, Gale
M Jr, Debley JS, Lukacs NW and Ziegler SF: Thymic stromal
lymphopoietin is induced by respiratory syncytial virus-infected
airway epithelial cells and promotes a type 2 response to
infection. J Allergy Clin Immunol. 130:1187–1196.e1185. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Ptaschinski C, Mukherjee S, Moore ML,
Albert M, Helin K, Kunkel SL and Lukacs NW: RSV–Induced H3K4
demethylase KDM5B leads to regulation of dendritic cell-derived
innate cytokines and exacerbates pathogenesis in vivo. PLoS Pathog.
11:e10049782015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Lu S, Hartert TV, Everard ML, Giezek H,
Nelsen L, Mehta A, Patel H, Knorr B and Reiss TF: Predictors of
asthma following severe respiratory syncytial virus (RSV)
bronchiolitis in early childhood. Pediatr Pulmonol. 51:1382–1392.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Malinczak CA, Fonseca W, Rasky AJ,
Ptaschinski C, Morris S, Ziegler SF and Lukacs NW: Sex-associated
TSLP-induced immune alterations following early-life RSV infection
leads to enhanced allergic disease. Mucosal Immunol. 12:969–979.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Yagi K, Lukacs NW, Huffnagle GB, Kato H
and Asai N: Respiratory and gut microbiome modification during
respiratory syncytial virus infection: A systematic review.
Viruses. 16:2202024. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Fonseca W, Malinczak CA, Fujimura K, Li D,
McCauley K, Li J, Best SKK, Zhu D, Rasky AJ, Johnson CC, et al:
Maternal gut microbiome regulates immunity to RSV infection in
offspring. J Exp Med. 218:e202102352021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Ballarini S, Ardusso L, Ortega Martell JA,
Sacco O, Feleszko W and Rossi GA: Can bacterial lysates be useful
in prevention of viral respiratory infections in childhood? The
results of experimental OM-85 studies. Front Pediatr.
10:10510792022. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
National Institute for Health and Care
Excellence (NICE), . Asthma: Diagnosis, monitoring and chronic
asthma management. NICE Guideline, No. 80. NICE; London: 2021
|
|
95
|
Asher MI, Rutter CE, Bissell K, Chiang CY,
El Sony A, Ellwood E, Ellwood P, García-Marcos L, Marks GB, Morales
E, et al: Worldwide trends in the burden of asthma symptoms in
school-aged children: Global asthma network phase I cross-sectional
study. Lancet. 398:1569–1580. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Fainardi V, Esposito S, Chetta A and Pisi
G: Asthma phenotypes and endotypes in childhood. Minerva Med.
113:94–105. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Martinez FD and Vercelli D: Asthma.
Lancet. 382:1360–1372. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ntontsi P, Photiades A, Zervas E, Xanthou
G and Samitas K: Genetics and epigenetics in asthma. Int J Mol Sci.
22:24122021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Stokholm J, Blaser MJ, Thorsen J,
Rasmussen MA, Waage J, Vinding RK, Schoos AM, Kunøe A, Fink NR,
Chawes BL, et al: Maturation of the gut microbiome and risk of
asthma in childhood. Nat Commun. 9:1412018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Fujimura KE, Sitarik AR, Havstad S, Lin
DL, Levan S, Fadrosh D, Panzer AR, LaMere B, Rackaityte E, Lukacs
NW, et al: Neonatal gut microbiota associates with childhood
multisensitized atopy and T cell differentiation. Nat Med.
22:1187–1191. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Abreo A, Gebretsadik T, Stone CA and
Hartert TV: The impact of modifiable risk factor reduction on
childhood asthma development. Clin Transl Med. 7:152018. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Rosas-Salazar C, Shilts MH, Tang ZZ, Hong
Q, Turi KN, Snyder BM, Wiggins DA, Lynch CE, Gebretsadik T, Peebles
RS Jr, et al: Exclusive breast-feeding, the early-life microbiome
and immune response, and common childhood respiratory illnesses. J
Allergy Clin Immunol. 150:612–621. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Saeed NK, Al-Beltagi M, Bediwy AS,
El-Sawaf Y and Toema O: Gut microbiota in various childhood
disorders: Implication and indications. World J Gastroenterol.
28:1875–1901. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Patrick DM, Sbihi H, Dai DLY, Al Mamun A,
Rasali D, Rose C, Marra F, Boutin RCT, Petersen C, Stiemsma LT, et
al: Decreasing antibiotic use, the gut microbiota, and asthma
incidence in children: Evidence from population-based and
prospective cohort studies. Lancet Respir Med. 8:1094–1105. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Kloepfer KM and Kennedy JL: Childhood
respiratory viral infections and the microbiome. J Allergy Clin
Immunol. 152:827–834. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Gensollen T, Iyer SS, Kasper DL and
Blumberg RS: How colonization by microbiota in early life shapes
the immune system. Science. 352:539–544. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Renz H and Skevaki C: Early life microbial
exposures and allergy risks: Opportunities for prevention. Nat Rev
Immunol. 21:177–191. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Fahy JV and Dickey BF: Airway mucus
function and dysfunction. N Engl J Med. 363:2233–2247. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Scotet V, L'Hostis C and Férec C: The
changing epidemiology of cystic fibrosis: Incidence, Survival and
impact of the CFTR gene discovery. Genes (Basel). 11:5892020.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Elborn JS: Cystic fibrosis. Lancet.
388:2519–2531. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Rafeeq MM and Murad HAS: Cystic fibrosis:
Current therapeutic targets and future approaches. J Transl Med.
15:842017. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Bassis CM, Erb-Downward JR, Dickson RP,
Freeman CM, Schmidt TM, Young VB, Beck JM, Curtis JL and Huffnagle
GB: Analysis of the upper respiratory tract microbiotas as the
source of the lung and gastric microbiotas in healthy individuals.
mBio. 6:e000372015. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Rogers GB, Carroll MP, Hoffman LR, Walker
AW, Fine DA and Bruce KD: Comparing the microbiota of the cystic
fibrosis lung and human gut. Gut Microbes. 1:85–93. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Dayama G, Priya S, Niccum DE, Khoruts A
and Blekhman R: Interactions between the gut microbiome and host
gene regulation in cystic fibrosis. Genome Med. 12:122020.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Kristensen M, Prevaes SMPJ, Kalkman G,
Tramper-Stranders GA, Hasrat R, de Winter-de Groot KM, Janssens HM,
Tiddens HA, van Westreenen M, Sanders EAM, et al: Development of
the gut microbiota in early life: The impact of cystic fibrosis and
antibiotic treatment. J Cyst Fibros. 19:553–561. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Vernocchi P, Del Chierico F, Russo A, Majo
F, Rossitto M, Valerio M, Casadei L, La Storia A, De Filippis F,
Rizzo C, et al: Gut microbiota signatures in cystic fibrosis: Loss
of host CFTR function drives the microbiota enterophenotype. PLoS
One. 13:e02081712018. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Burke DG, Fouhy F, Harrison MJ, Rea MC,
Cotter PD, O'Sullivan O, Stanton C, Hill C, Shanahan F, Plant BJ
and Ross RP: The altered gut microbiota in adults with cystic
fibrosis. BMC Microbiol. 17:582017. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Miragoli F, Federici S, Ferrari S, Minuti
A, Rebecchi A, Bruzzese E, Buccigrossi V, Guarino A and Callegari
ML: Impact of cystic fibrosis disease on archaea and bacteria
composition of gut microbiota. FEMS Microbiol Ecol. 93:fiw2302017.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
de Freitas MB, Moreira EAM, Tomio C,
Moreno YMF, Daltoe FP, Barbosa E, Ludwig Neto N, Buccigrossi V and
Guarino A: Altered intestinal microbiota composition, antibiotic
therapy and intestinal inflammation in children and adolescents
with cystic fibrosis. PLoS One. 13:e01984572018. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Matamouros S, Hayden HS, Hager KR,
Brittnacher MJ, Lachance K, Weiss EJ, Pope CE, Imhaus AF, McNally
CP, Borenstein E, et al: Adaptation of commensal proliferating
Escherichia coli to the intestinal tract of young children with
cystic fibrosis. Proc Natl Acad Sci USA. 115:1605–1610. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Nielsen S, Needham B, Leach ST, Day AS,
Jaffe A, Thomas T and Ooi CY: Disrupted progression of the
intestinal microbiota with age in children with cystic fibrosis.
Sci Rep. 6:248572016. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Coffey MJ, Nielsen S, Wemheuer B, Kaakoush
NO, Garg M, Needham B, Pickford R, Jaffe A, Thomas T and Ooi CY:
Gut microbiota in children with cystic fibrosis: A taxonomic and
functional dysbiosis. Sci Rep. 9:185932019. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Manor O, Levy R, Pope CE, Hayden HS,
Brittnacher MJ, Carr R, Radey MC, Hager KR, Heltshe SL, Ramsey BW,
et al: Metagenomic evidence for taxonomic dysbiosis and functional
imbalance in the gastrointestinal tracts of children with cystic
fibrosis. Sci Rep. 6:224932016. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Wang Y, Leong LEX, Keating RL, Kanno T,
Abell GCJ, Mobegi FM, Choo JM, Wesselingh SL, Mason AJ, Burr LD and
Rogers GB: Opportunistic bacteria confer the ability to ferment
prebiotic starch in the adult cystic fibrosis gut. Gut Microbes.
10:367–381. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Vaezi A, Healy T, Ebrahimi G, Rezvankhah
S, Hashemi Shahraki A and Mirsaeidi M: Phage therapy: breathing new
tactics into lower respiratory tract infection treatments. Eur
Respir Rev. 33:2400292024. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Hong Y and Luo T: The potential protective
effects of probiotics, prebiotics, or yogurt on chronic obstructive
pulmonary disease: Results from NHANES 2007–2012. Food Sci Nutr.
12:7233–7241. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Depoorter L and Vandenplas Y: Probiotics
in pediatrics. A review and practical guide. Nutrients.
13:21762021. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Zhang Y, Xu Y, Hu L and Wang X:
Advancements related to probiotics for preventing and treating
recurrent respiratory tract infections in children. Front Pediatr.
13:15086132025. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
O'Donnell A, Murray A, Nguyen A, Salmon T,
Taylor S, Morton JP and Close GL: Nutrition and golf performance: A
systematic scoping review. Sports Med. 54:3081–3095. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Wang Q, Lin X, Xiang X, Liu W, Fang Y,
Chen H, Tang F, Guo H, Chen D, Hu X, et al: Oropharyngeal probiotic
ENT-K12 prevents respiratory tract infections among frontline
medical staff fighting against COVID-19: A pilot study. Front
Bioeng Biotechnol. 9:6461842021. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Samuelson DR, Charles TP, de la Rua NM,
Taylor CM, Blanchard EE, Luo M, Shellito JE and Welsh DA: Analysis
of the intestinal microbial community and inferred functional
capacities during the host response to Pneumocystis pneumonia. Exp
Lung Res. 42:425–439. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Dong Y, Li M and Yue X: Current research
on probiotics and fermented products. Foods. 13:14062024.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Mazziotta C, Tognon M, Martini F,
Torreggiani E and Rotondo JC: Probiotics mechanism of action on
immune cells and beneficial effects on human health. Cells.
12:1842023. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Suissa R, Oved R, Jankelowitz G, Turjeman
S, Koren O and Kolodkin-Gal I: Molecular genetics for probiotic
engineering: Dissecting lactic acid bacteria. Trends Microbiol.
30:293–306. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Chiappini E, Santamaria F, Marseglia GL,
Marchisio P, Galli L, Cutrera R, de Martino M, Antonini S,
Becherucci P, Biasci P, et al: Prevention of recurrent respiratory
infections : Inter-society Consensus. Ital J Pediatr. 47:2112021.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Yamanishi S and Pawankar R: Current
advances on the microbiome and role of probiotics in upper airways
disease. Curr Opin Allergy Clin Immunol. 20:30–35. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Lopes SA, Roque-Borda CA, Duarte JL, Di
Filippo LD, Borges Cardoso VM, Pavan FR, Chorilli M and Meneguin
AB: delivery strategies of probiotics from nano- and
microparticles: Trends in the treatment of inflammatory bowel
disease-an overview. Pharmaceutics. 15:26002023. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Sun W, Zhou T, Ding P, Guo L, Zhou X and
Long K: Bibliometric analysis of intestinal microbiota and lung
diseases. Front Cell Infect Microbiol. 14:13471102024. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Chakradhar S: A curious connection:
Teasing apart the link between gut microbes and lung disease. Nat
Med. 23:402–404. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Han X, Hu X, Jin W and Liu G: Dietary
nutrition, intestinal microbiota dysbiosis and post-weaning
diarrhea in piglets. Anim Nutr. 17:188–207. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Zhang T, Wei X, Li Y, Huang S, Wu Y, Cai
S, Aipire A and Li J: Dendritic cell-based vaccine prepared with
recombinant Lactococcus lactis enhances antigen cross-presentation
and antitumor efficacy through ROS production. Front Immunol.
14:12083492023. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Ni C, Li X, Wang L, Li X, Zhao J, Zhang H,
Wang G and Chen W: Lactic acid bacteria strains relieve
hyperuricaemia by suppressing xanthine oxidase activity via a
short-chain fatty acid-dependent mechanism. Food Funct.
12:7054–7067. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Zhao H, Chen X, Zhang L, Meng F, Zhou L,
Pang X, Lu Z and Lu Y: Lacticaseibacillus rhamnosus Fmb14 prevents
purine induced hyperuricemia and alleviate renal fibrosis through
gut-kidney axis. Pharmacol Res. 182:1063502022. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Ren D, Ding M, Su J, Ye J, He X, Zhang Y
and Shang X: Stachyose in combination with L. rhamnosus GG
ameliorates acute hypobaric hypoxia-induced intestinal barrier
dysfunction through alleviating inflammatory response and oxidative
stress. Free Radic Biol Med. 212:505–519. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Hojsak I, Snovak N, Abdović S, Szajewska
H, Misak Z and Kolacek S: Lactobacillus GG in the prevention of
gastrointestinal and respiratory tract infections in children who
attend day care centers: A randomized, double-blind,
placebo-controlled trial. Clin Nutr. 29:312–316. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Liu S, Hu P, Du X, Zhou T and Pei X:
Lactobacillus rhamnosus GG supplementation for preventing
respiratory infections in children: A meta-analysis of randomized,
placebo-controlled trials. Indian Pediatr. 50:377–381. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Laursen RP and Hojsak I: Probiotics for
respiratory tract infections in children attending day care
centers-a systematic review. Eur J Pediatr. 177:979–994. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Kumpu M, Lehtoranta L, Roivainen M, Rönkkö
E, Ziegler T, Söderlund-Venermo M, Kautiainen H, Järvenpää S,
Kekkonen R, Hatakka K, et al: The use of the probiotic
Lactobacillus rhamnosus GG and viral findings in the nasopharynx of
children attending day care. J Med Virol. 85:1632–1638. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Damholt A, Keller MK, Baranowski K, Brown
B, Wichmann A, Melsaether C, Eskesen D, Westphal V, Arltoft D,
Habicht A, et al: Lacticaseibacillus rhamnosus GG DSM 33156 effects
on pathogen defence in the upper respiratory tract: A randomised,
double-blind, placebo-controlled paediatric trial. Benef Microbes.
13:13–23. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Sibanda T, Marole TA, Thomashoff UL,
Thantsha MS and Buys EM: Bifidobacterium species viability in
dairy-based probiotic foods: Challenges and innovative approaches
for accurate viability determination and monitoring of probiotic
functionality. Front Microbiol. 15:13270102024. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Li M, Ding J, Stanton C, Ross RP, Zhao J,
Yang B and Chen W: Bifidobacterium longum subsp. infantis FJSYZ1M3
ameliorates DSS-induced colitis by maintaining the intestinal
barrier, regulating inflammatory cytokines, and modifying gut
microbiota. Food Funct. 14:354–368. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Lau AS, Yanagisawa N, Hor YY, Lew LC, Ong
JS, Chuah LO, Lee YY, Choi SB, Rashid F, Wahid N, et al:
Bifidobacterium longum BB536 alleviated upper respiratory illnesses
and modulated gut microbiota profiles in Malaysian pre-school
children. Benef Microbes. 9:61–70. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Taipale TJ, Pienihäkkinen K, Isolauri E,
Jokela JT and Söderling EM: Bifidobacterium animalis subsp. lactis
BB-12 in reducing the risk of infections in early childhood.
Pediatr Res. 79:65–69. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Li W, Zhang S, Wang Y, Bian H, Yu S, Huang
L and Ma W: Complex probiotics alleviate ampicillin-induced
antibiotic-associated diarrhea in mice. Front Microbiol.
14:11560582023. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Li KL, Wang BZ, Li ZP, Li YL and Liang JJ:
Alterations of intestinal flora and the effects of probiotics in
children with recurrent respiratory tract infection. World J
Pediatr. 15:255–261. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
DI Pierro F, Lo Russo P, Danza ML, Basile
I, Soardo S, Capocasale G, Paparone SB, Paletta V, Lanza C,
Schiavone E, et al: Use of a probiotic mixture containing
Bifidobacterium animalis subsp. lactis BB-12 and Enterococcus
faecium L3 as prophylaxis to reduce the incidence of acute
gastroenteritis and upper respiratory tract infections in children.
Minerva Pediatr (Torino). 73:222–229. 2021.PubMed/NCBI
|
|
157
|
Manti S, Parisi GF, Papale M, Licari A,
Salpietro C, Miraglia Del Giudice M, Marseglia GL and Leonardi S:
Bacteriotherapy with Streptococcus salivarius 24SMB and
Streptococcus oralis 89a nasal spray for treatment of upper
respiratory tract infections in children: A pilot study on
short-term efficacy. Ital J Pediatr. 46:422020. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Campanella V, Syed J, Santacroce L, Saini
R, Ballini A and Inchingolo F: Oral probiotics influence oral and
respiratory tract infections in pediatric population: A randomized
double-blinded placebo-controlled pilot study. Eur Rev Med
Pharmacol Sci. 22:8034–8041. 2018.PubMed/NCBI
|
|
159
|
Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M
and Gil A: Mechanisms of action of probiotics. Adv Nutr. 10
(Suppl_1):S49–s66. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Liu R and Sun B: Lactic acid bacteria and
aging: Unraveling the interplay for healthy longevity. Aging Dis.
15:1487–1498. 2023.PubMed/NCBI
|
|
161
|
Crits-Christoph A, Hallowell HA,
Koutouvalis K and Suez J: Good microbes, bad genes? The
dissemination of antimicrobial resistance in the human microbiome.
Gut Microbes. 14:20559442022. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Merenstein D, Pot B, Leyer G, Ouwehand AC,
Preidis GA, Elkins CA, Hill C, Lewis ZT, Shane AL, Zmora N, et al:
Emerging issues in probiotic safety: 2023 perspectives. Gut
Microbes. 15:21850342023. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Boumis E, Capone A, Galati V, Venditti C
and Petrosillo N: Probiotics and infective endocarditis in patients
with hereditary hemorrhagic telangiectasia: A clinical case and a
review of the literature. BMC Infect Dis. 18:652018. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Doern CD, Nguyen ST, Afolabi F and Burnham
CA: Probiotic-associated aspiration pneumonia due to Lactobacillus
rhamnosus. J Clin Microbiol. 52:3124–3126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Zbinden A, Zbinden R, Berger C and
Arlettaz R: Case series of Bifidobacterium longum bacteremia in
three preterm infants on probiotic therapy. Neonatology. 107:56–59.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Rizzatti G, Lopetuso LR, Gibiino G, Binda
C and Gasbarrini A: Proteobacteria: A common factor in human
diseases. Biomed Res Int. 2017:93515072017. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Zhu M, Liu X, Ye Y, Yan X, Cheng Y, Zhao
L, Chen F and Ling Z: Gut microbiota: A novel therapeutic target
for Parkinson's disease. Front Immunol. 13:9375552022. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Gentle SJ and Lal CV: Predicting BPD:
Lessons learned from the airway microbiome of preterm infants.
Front Pediatr. 7:5642020. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Carvalho JL, Miranda M, Fialho AK,
Castro-Faria-Neto H, Anatriello E, Keller AC and Aimbire F: Oral
feeding with probiotic Lactobacillus rhamnosus attenuates cigarette
smoke-induced COPD in C57Bl/6 mice: Relevance to inflammatory
markers in human bronchial epithelial cells. PLoS One.
15:e02255602020. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Fangous MS, Alexandre Y, Hymery N, Gouriou
S, Arzur D, Blay GL and Berre RL: Lactobacilli intra-tracheal
administration protects from Pseudomonas aeruginosa pulmonary
infection in mice - a proof of concept. Benef Microbes. 10:893–900.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Zelaya H, Villena J, Lopez AG, Alvarez S
and Agüero G: Modulation of the inflammation-coagulation
interaction during pneumococcal pneumonia by immunobiotic
Lactobacillus rhamnosus CRL1505: Role of Toll-like receptor 2.
Microbiol Immunol. 58:416–426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Fangous MS, Gosset P, Galakhoff N, Gouriou
S, Guilloux CA, Payan C, Vallet S, Héry-Arnaud G and Le Berre R:
Priming with intranasal lactobacilli prevents Pseudomonas
aeruginosa acute pneumonia in mice. BMC Microbiol. 21:1952021.
View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Wallace C, Gordon M, Sinopoulou V and
Akobeng AK: Probiotics for management of functional abdominal pain
disorders in children. Cochrane Database Syst Rev.
2:Cd0128492023.PubMed/NCBI
|
|
174
|
Su Z, Ma C, Ru X, Zhang S, Wu C, Huang Y,
Cen H, Yin Z and Zhang J: Effects of probiotic treatment on
patients and animals with chronic obstructive pulmonary disease: A
systematic review and meta-analysis of randomized control trials.
Front Cell Infect Microbiol. 14:14112222024. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Li P, Uma Mageswary M, Taib F, Koo TH,
Yusof A, Hamid IJA, Jiang H, Liong MT, Ali A and Zhang Y: Safety
and tolerance of bifidobacterium longum subsp. Infantis YLGB-1496
in toddlers with respiratory symptoms. Nutrients. 17:21272025.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Zeng L, Yang K, He Q, Zhu X, Long Z, Wu Y,
Chen J, Li Y, Zeng J, Cui G, et al: Efficacy and safety of gut
microbiota-based therapies in autoimmune and rheumatic diseases: A
systematic review and meta-analysis of 80 randomized controlled
trials. BMC Med. 22:1102024. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Bettocchi S, Comotti A, Elli M, De Cosmi
V, Berti C, Alberti I, Mazzocchi A, Rosazza C, Agostoni C and
Milani GP: Probiotics and fever duration in children with upper
respiratory tract infections: A Randomized clinical trial. JAMA
Netw Open. 8:e2506692025. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Hiraku A, Nakata S, Murata M, Xu C, Mutoh
N, Arai S, Odamaki T, Iwabuchi N, Tanaka M, Tsuno T and Nakamura M:
Early probiotic supplementation of healthy term infants with
bifidobacterium longum subsp. infantis M-63 is safe and leads to
the development of bifidobacterium-predominant gut microbiota: A
double-blind, placebo-controlled trial. Nutrients. 15:14022023.
View Article : Google Scholar : PubMed/NCBI
|