Introduction
Primary lung cancer is the most common malignancy in
China, with non-small cell lung cancer (NSCLC) being the most
prevalent histological type, accounting for 80–85% of all cases
(1). The all-stage combined 5-year
relative survival for lung cancer in China is ~15% (2). The clinical treatment of NSCLC
primarily involves a combination of chemotherapy (platinum-based
doublets such as cisplatin-pemetrexed for non-squamous histology,
cisplatin-gemcitabine or carboplatin-paclitaxel for squamous
disease) and radiotherapy. In recent years, notable advances have
been made in the diagnosis and treatment of NSCLC, driven by
research into the molecular mechanisms (3,4).
Targeted therapy has led to more successful outcomes in the
treatment of NSCLC; however, despite the success of targeted
therapies in disease control, for example, EGFR-mutant disease is
treated with osimertinib, ALK-rearranged disease with
alectinib/lorlatinib, ROS1-positive disease with entrectinib, and
RET fusions with selpercatinib, the majority of patients with NSCLC
develop resistance and experience disease progression, highlighting
the persistent challenges in the treatment of NSCLC (5,6). To
more effectively address these issues, ongoing efforts to screen
for anticancer drugs and investigate novel therapeutic approaches
are important in identifying more effective treatment
strategies.
Ferroptosis is a recently discovered form of
iron-dependent non-apoptotic cell death characterized by the
accumulation of iron-dependent lipid peroxides and the loss of
glutathione (GSH) peroxidase 4 activity (7). The Hippo/yes-associated protein
(YAP)/transcriptional coactivator with PDZ-binding motif (TAZ)
signaling pathway is a highly conserved protein kinase signaling
pathway that regulates the phosphorylation status and nuclear
localization of the downstream transcriptional coactivators YAP and
TAZ (8,9). The Hippo/YAP/TAZ pathway controls
various cellular processes, including proliferation, apoptosis and
organ morphology. As a key regulator linking ferroptosis and the
tumor microenvironment (TME), the Hippo/YAP/TAZ signaling pathway
influences the biological behavior and susceptibility of tumor
cells to ferroptosis under the regulation of various non-genetic
factors (8,9). Reports have suggested that the human
gut absorbs 1–2 mg iron daily from dietary sources. Iron within
cells is either stored in ferritin or exported into the plasma by
iron transporters. In the presence of hepcidin, divalent iron is
oxidized to ferric iron and binds to transferrin (Tf), which then
associates with Tf receptors (TfRs) on the surface of target cell
membranes, facilitating intracellular iron release and initiating
iron cycling (9–11). When the iron concentration in
tissues increases excessively and exceeds the binding capacity of
Tf, non-Tf-bound iron (NTBI) is formed (9–11).
NTBI, along with some unstable ferrous ions, can easily promote
lipid peroxidation and H2O2 cleavage through
Fenton and Haber-Weiss reactions, accelerating the formation of
reactive oxygen species (ROS) and inducing ferroptosis.
Furthermore, acyl-CoA synthase long-chain family member 4 (ACSL4)
connects free polyunsaturated fatty acids (PUFAs) with acyl-CoA to
form PUFA-CoA, which is then incorporated into phospholipids (PLs)
by lysophosphatidylcholine acyltransferase 3 to generate PUFA-PL
(10,11). The H2O2 and
free iron in the cytoplasm undergo a Fenton reaction, producing
ROS, which, in turn, oxidizes PUFA-PL on the cell membrane, leading
to membrane rupture and ferroptosis (12,13).
Studies have confirmed that ACSL4 is an important regulatory site
in the Hippo/YAP/TAZ signaling pathway that mediates ferroptosis
(12,13). Hence, the Hippo/YAP/TAZ signaling
pathway serves an important role in regulating ferroptosis, and
targeting this pathway may be an important therapeutic strategy for
treating NSCLC.
Schisantherin A (Sch A) is a bioactive lignan
isolated from the traditional Chinese medicine (TCM) Schisandra
chinensis that has demonstrated notable pharmacological
effects, such as anti-inflammatory, antitumor, neuroprotective and
liver fibrosis-improving effects (14,15).
Despite its known bioactivity, to the best of our knowledge, the
specific role of Sch A in NSCLC has not yet been reported, making
it an unexplored area with potential for revealing new therapeutic
strategies. Given its diverse pharmacological effects,
investigating Sch A in the context of NSCLC may provide valuable
insights and offer a new option for targeted therapy in lung
cancer, which remains a major clinical challenge.
Materials and methods
Cell culture
Procell Life Science & Technology Co., Ltd.
provided the EGFR-mutant HCC827 cell line (human lung
adenocarcinoma origin, harboring the classic EGFR Exon19del
E746-A750 deletion mutation; cat. no. CL-0094) and the EGFR
wild-type A549 cell line (cat. no. CL-0016). These cell lines were
grown in an incubator at 37°C with 5% CO2. HCC827 cells
were cultured in RPMI 1640 medium (HyClone; Cytiva) supplemented
with 1% penicillin-streptomycin (Beijing Solarbio Science &
Technology Co., Ltd.) and 10% fetal bovine serum (HyClone; Cytiva).
A549 cells were grown in Ham's F-12K medium (Procell) supplemented
with 1% penicillin-streptomycin and 10% fetal bovine serum.
Cell Counting Kit-8 (CCK-8) assay
A total of 3,000 A549 and HCC827 cells/well were
plated into 96-well plates. After overnight incubation, various
concentrations of Sch A (0.5, 1, 2, 4, 8, 16, 32, 64, 128 and 256
µg/ml; MilliporeSigma) were added to each well, with three
replicates for each concentration. Drug-free medium served as a
blank control for 48 h at 37°C. Subsequently, 10 µl CCK-8 reagent
(Beijing Solarbio Science & Technology Co., Ltd.) was added for
2-h incubation. Next, the absorbance was measured at 450 nm and the
IC50 values were calculated using GraphPad Prism 10.0
(Dotmatics).
Inhibitor treatments
The following inhibitors were co-incubated with Sch
A: Z-VAD-FMK (pan-caspase inhibitor; HY-16658B; final
concentration: 20 µM); 3-Methyladenine (3-MA) (autophagy inhibitor,
HY-19312; final concentration: 5 mM); Necrostatin-1 (Nec-1)
(necroptosis inhibitor; MCE; HY-15760, 10 µM); Ferrostatin-1
(Fer-1) (ferroptosis inhibitor; MCE; HY-100579, 1 µM); Deferoxamine
(DFO) (iron chelator/ferroptosis inhibitor; HY-B1625; all MCE;
final concentration: 100 µM). Vehicle controls received matching
volumes of DMSO (≤0.1% v/v). Cells (A549 and HCC827) were
pretreated with inhibitors at 37°C for 2 h, and then co-incubated
with 6 µg/ml Sch A in A549 cells and 16 µg/ml Sch A in HCC827 cells
together with the same inhibitors for an additional 24 h at
37°C.
Detection of cell death by flow
cytometry
In 6-well plates, A549 and HCC827 cells were plated
at a density of 5×105 cells/well and were incubated
overnight. The cells were divided into Sch A-treated and control
groups, with A549 cells treated with 6 µg/ml Sch A and HCC827 cells
treated with 16 µg/ml Sch A for 48 h at 37°C. The cells were
collected using EDTA-free trypsin and then washed with 100 µl 1X
binding buffer (Annexin V-PE/7-AAD Apoptosis Kit; E-CK-A216, Wuhan
Elabscience Biotechnology Co., Ltd). A total of 5 µl each of
Annexin V-APC and 7-AAD were used to stain the cells. Following 15
min of staining at room temperature in the dark, the cells in
quadrant 3 were analyzed using a CytoFLEX S flow cytometer (Beckman
Coulter, Inc.) and FlowJo v10 software (BD Biosciences).
Gene-set retrieval and intersection
analysis
Ferroptosis- and NSCLC-associated genes were
retrieved from GeneCards (genecards.org;). Venn diagram were
generated using Venny 2.1 (https://bioinfogp.cnb.csic.es/tools/venny/), yielding
the overlapping gene set for downstream analyses.
PPI network construction and key node
identification
The overlapping genes were imported into the STRING
database (v11.x; string-db.org) to build the PPI) network. Network
topology (node degree) was used to prioritize key regulators; YAP1
emerged as a highly connected transcription factor within the
network.
Cell cycle detection by flow
cytometry
In 6-well plates, A549 and HCC827 cells were plated
at a density of 5×105 cells/well and incubated overnight
at 37°C. The cells were divided into Sch A-treated and control
groups, with A549 cells treated with 6 µg/ml Sch A and HCC827 cells
treated with 16 µg/ml Sch A for 48 h at 37°C. For 12 h, A549 and
HCC827 cells were harvested and washed once with PBS, adjusted to
1×106 cells/ml, and fixed with ice-cold 70% ethanol at
4°C for 12 h. Fixed cells were washed with PBS, then incubated with
100 µl RNase A solution (DNA Content Quantitation Assay; Beijing
Solarbio Science & Technology Co., Ltd.) at 37°C for 30 min. A
total of 400 µl PI staining solution was added and samples were
incubated for 30 min at 4°C before acquisition (Ex 488 nm). Next,
flow cytometry (CytoFLEX S) was used to assess cell cycle
progression, and FlowJo software was used to analyze the data.
Western blot analysis
A549 and HCC827 cells were seeded in 6-well plates
at a density of 1×106 cells/well, and the cells were
grown for 24 h. After A549 and HCC827 cells were treated with 5,
10, 20, 40 µg/ml Sch A for 48 h at 37°C, they were lysed in RIPA
lysis buffer (Beijing Solarbio Science & Technology Co., Ltd.),
and the supernatant was centrifuged at 11,000 g for 15 min at 4°C
to extract all the cellular protein. With a BCA kit, protein
quantification was carried out (Beijing Solarbio Science &
Technology Co., Ltd.). After being separated by SDS-PAGE on 12%
gels, 20 µg proteins were transferred to a PVDF membrane (Thermo
Fisher Scientific, Inc.). After three washes with 0.1% PBS-Tween
(PBST), the membranes were blocked with 8% skimmed milk for 2 h at
room temperature, after which the primary antibodies were added at
4°C overnight. The primary antibodies used were against YAP (cat.
no. 14074; 1:1,000; Cell Signaling Technology, Inc.), ACSL4 (cat.
no. T510198, 1:1,000; Abmart Pharmaceutical Technology Co., Ltd.),
TfR (T56618; 1:1,000; Abmart Pharmaceutical Technology Co., Ltd.)
and GAPDH (2118, 1:5,000; Cell Signaling Technology, Inc.). The
membranes were then incubated with an ActivAb Goat Anti-Rabbit
IgG/HRP (1:4,000 dilution, Beijing Solarbio Science &
Technology Co., Ltd.) at room temperature for 2 h following three
PBST washes (5 min each). The blots were subsequently visualized
with ECL western blotting substrate (Beijing Solarbio Science &
Technology Co., Ltd.) for detection. GAPDH served as the internal
reference and ImageJ2 (National Institutes of Health) was used to
evaluate the density of the protein bands.
Detection of intracellular
malondialdehyde (MDA), Fe2+ and GSH levels
After A549 and HCC827 cells were centrifuged at
10,000 × g for 10 min at 4°C, the supernatant was collected.
According to the manufacturer's instructions, the total
intracellular levels of MDA, Fe2+ and GSH in 50 µl
supernatant were determined via the MDA Content Assay Kit (cat. no.
BC0025), Ferrous Ion Content Assay Kit (cat. no. BC5415) and
Reduced GSH Content Assay Kit (BC1175; all Beijing Solarbio Science
& Technology Co., Ltd.), respectively. The source of all the
assay kits was Beijing Solarbio Science & Technology Co.,
Ltd.
Transfection of small interfering RNA
(siRNA)
Shanghai GenePharma Co., Ltd. constructed both
negative control (NC) siRNA (forward: 5′-UUCUCCGAACGUGUCACGUTT-3′;
reverse: 5′-ACGUGACACGUUCGGAGAATT-3′) and YAP-targeting siRNA
(si-YAP; forward: 5′-AUGUGAUUUAAGAAGUAUCUC-3′; reverse:
5′-GAGAUACUUCUUAAAUCACAU-3′). In 6-well plates, A549 and HCC827
cells were plated at a density of 1×105 cells/well and
cultured overnight. Transfections were performed with the
siRNA-mate plus transfection kit (GenePharma; cat. no. G04026)
following the manufacturer's instructions. Briefly, 15 pmol siRNA
was mixed with Buffer (8.5 µl) to prepare the siRNA premix (final
~1.5 µM), combined with 3 µl siRNA-mate plus to form complexes, and
added directly to each 24-well (30 nM siRNA in 0.5 ml complete
medium). Cells were incubated with the transfection complexes at
37°C in a humidified incubator with 5% CO2 for 48 h
without changing the medium. Subsequent experiments were performed
immediately.
Reverse transcription-quantitative PCR
(RT-qPCR)
Using the Total RNA Extraction Kit (Beijing Solarbio
Science & Technology Co., Ltd.), total RNA was extracted from
each set of cells. A NanoDrop 2000 spectrophotometer (NanoDrop;
Thermo Fisher Scientific, Inc.) was used to measure the
concentration and purity of the RNA. For each sample, RT 1 µg total
RNA into cDNA was performed with the HiScript II One Step RT-PCR
Kit (Vazyme Biotech Co., Ltd.). The mRNA expression levels of
prostaglandin-endoperoxide synthase 2 (ptgs2) and GSH-specific
γ-glutamylcyclotransferase 1 (Chac1) were measured via AceQ qPCR
SYBR Green Master Mix (without ROX) (Vazyme Biotech Co., Ltd.),
with GAPDH serving as the internal reference gene. Thermocycling
conditions were as follows: initial denaturation at 95°C for 5 min;
followed by 40 cycles of denaturation at 95°C for 10 sec and
annealing/extension at 60°C for 30 sec. The 2−ΔΔCq
method was used to determine the relative expression levels of
target genes (16). The primers
are listed in Table I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Primer | Sequence
(5′-3′) |
|---|
| GAPDH-F |
GGGGAGCCAAAAGGGTCATC |
| GAPDH-R |
TGGTTCACACCCATGACGAA |
| YAP1-F |
ACATTGCAAAAGTGGGTGGC |
| YAP1-R |
TGCGAGGATAAAATCCACCTGA |
| FOXO1-F |
AACCCCAGCCCCAACTTAAA |
| FOXO1-R |
ACCTCAAGGAGAGGCCAACT |
| FOXP1-F |
GGCTTCCCTCTGTGTGTTG |
| FOXP1-R |
ATGGGGAAGGGTTAGGCTGA |
| FOXA2-F |
CAAACAGAGGGCCACACAGA |
| FOXA2-R |
CGAGACCTGGATTTCACCGT |
| FOXN4-F |
GTTCCGCAGATAGCTGGGTT |
| FOXN4-R |
AGCTTTGCTGGGAGAGCATT |
| HIF1A-F |
GTCACTTTGCCAGCTCAAAAGA |
| HIF1A-R |
ACCAACAGGGTAGGCAGAAC |
| RELA-F |
GGCATTGTCCCTGTGCCTAA |
| RELA-R |
GAAGTCCCAGACCAAACCCC |
| STAT3-F |
CAAAACCACCTTGCCTCAGC |
| STAT3-R |
GGCTCAGCTCCTCTCAGAAC |
| CEBPA-F |
GGACCCTCAGCCTTGTTTGT |
| CEBPA-R |
AGACGCGCACATTCACATTG |
| MYC-F |
TGGCTGCTTGTGAGTACAGG |
| MYC-R |
TGAACTGGCTTCTTCCCAGG |
| SP1-F |
ATTGTGGACAAGGGCAGGTC |
| SP1-R |
CAACAGTGGTGTGGACTGGT |
| JUN-F |
TCCTGCCCAGTGTTGTTTGT |
| JUN-R |
GACTTCTCAGTGGGCTGTCC |
| TP53-F |
GGAAATCTCACCCCATCCCA |
| TP53-R |
GCAGATGTGCTTGCAGAATGT |
| NFE2L2-F |
TCCCTGCAGCAAACAAGAGA |
| NFE2L2-R |
AACTAGCCCAAATGGTGTCCT |
| AP-1-F |
TCCTGCCCAGTGTTGTTTGT |
| AP-1-R |
GACTTCTCAGTGGGCTGTCC |
| PPARA-F |
TGACAGAAACACACGCGAGA |
| PPARA-R |
CGCTCTTCTCTGCACATCCT |
| NRF1-F |
TCTCCACGTCTTGCTCAACC |
| NRF1-R |
ATCCATGCTCTGCTACTGGG |
| GATA3 |
TTGCCGTTGAGGGTTTCAGA |
| GATA3 |
GCACGCTGGTAGCTCATACA |
| KLF2-F |
GTCTGGAAACCCACCTGGAG |
| KLF2-R |
TTGGTGGTCATGGTTACCCG |
| RUNX3-F |
CTGTAAGGCCCAAAGTGGGT |
| RUNX3-R |
CAGTTTCCACCCAGCTCCAT |
| BACH1-F |
ACAGGTTGCATGTGGACACT |
| BACH1-R |
TCACCTGAGAATTCACCGTAACA |
| ZEB1-F |
CAACAAGCCTGAACTGCTGTC |
| ZEB1-R |
GCAGGATGACAATGTACCCCA |
FerroOrange staining
After A549 and HCC827 cells were washed with
serum-free medium, 500 µl 1 µM FerroOrange working solution
(MedChemExpress) was added. The cells were then incubated at 37°C
in a 5% CO2 incubator for 30 min. A fluorescence
microscope (Olympus Corporation) was used to measure the
fluorescence intensity.
JC-1 staining
After A549 and HCC827 cells were treated with 6
µg/ml Sch A and HCC827 cells treated with 16 µg/ml Sch A for 48 h
at 37°C, 100 µl JC-1 fluorescence staining solution [mitochondrial
membrane potential (MMP) assay kit with JC-1; Beijing Solarbio
Science & Technology Co., Ltd.] was added to each well at 37°C
for 20 min, and the A549 and HCC827 cells were incubated in the
dark for 20 min. Subsequently, the cells were inspected under a
fluorescence microscope (Olympus Corporation). Green fluorescence
(excitation, 485 nm; emission, 535 nm; mono JC-1) was used to
identify apoptotic or necrotic cells, whereas red fluorescence
(excitation, 550 nm; emission, 600 nm; poly JC-1) was used to
identify healthy cells.
Xenograft nude mouse tumor model
Following centrifugation and trypsin digestion,
5×1010/l serum-free DMEM (HyClone; Cytiva) was used to
resuspend A549 cells at 300 × g for 5 min at 4°C. All BALB/c nude
mice were maintained at 22±2°C, 50±10% relative humidity, under a
12:12-h light/dark cycle, with free access to autoclaved chow and
water. Mice were acclimated for at least 7 days prior to
inoculation. The right axilla of male BALB/c nude mice (n=10; age,
6-weeks; weight, 16–18 g; Beijing Vital River Laboratory Animal
Technology Co., Ltd.; Charles River Laboratories, Inc.) was then
aseptically injected with a 0.1-ml aliquot of the cell mixture
(each mouse received an injection of 5×106 A549 cells).
According to relevant studies on lung cancer models (17,18),
sex has a minimal impact on experimental results; therefore, sex
was not considered a variable in the present study. After ~14 days,
the tumor model was considered successfully established once the
maximum tumor diameter was >5 mm or the tumor volume was >50
mm3.
Humane endpoints included maximum tumor diameter ≥15
mm, tumor volume ≥1,500 mm3,
ulceration/necrosis/infection, ≥20% body weight loss, or failure to
eat/drink. No mice reached humane endpoints before planned
termination. The mice with successfully established tumors were
randomly assigned to two groups: A control group and a Sch A
treatment group. During the 14-day treatment period, the Sch A
group of mice were given Sch A orally at a dose of 10 mg/kg/day,
whereas the control group was given an equivalent volume of saline.
Following treatment, the long and short diameters of the xenograft
tumors were measured, and their volumes were computed using the
following formula: Tumor volume=(long diameter × short
diameter2)/2. In the present study, the maximum tumor
diameter observed was 8.2 mm, and the maximum tumor volume was 107
mm3. Isoflurane anesthesia was administered at a
concentration of 4% for induction and 1.5% for maintenance, with an
oxygen flow rate of 1.5 l/min, during subcutaneous tumor cell
injection and tumor measurement procedures. For oral gavage,
anesthesia was not employed, as this procedure is generally
considered minimally invasive and does not typically require
anesthesia. The mice were anesthetized with 4% isoflurane 1 h after
the final administration and euthanized by decapitation. The tumors
were then removed and weighed for analysis.
The present study was conducted in accordance with
the Animal Research: Reporting of In Vivo Experiments
guidelines and the Guide for the Care and Use of Laboratory Animals
(US National Research Council) (19). All animal experiments were approved
by the Committee on the Ethics of Animal Experiments of Guangzhou
University of Chinese Medicine (Shenzhen, China; approval no.
2024055R).
Statistical analysis
SPSS 22.0 (IBM Corp.) was used to analyze the
experimental data, and the results are presented as the mean ± SD
of ≥3 independent experimental repeats. Pairwise comparisons were
performed using the unpaired Student's t-test, and group
comparisons were performed with one-way ANOVA followed by Tukey's
post hoc analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
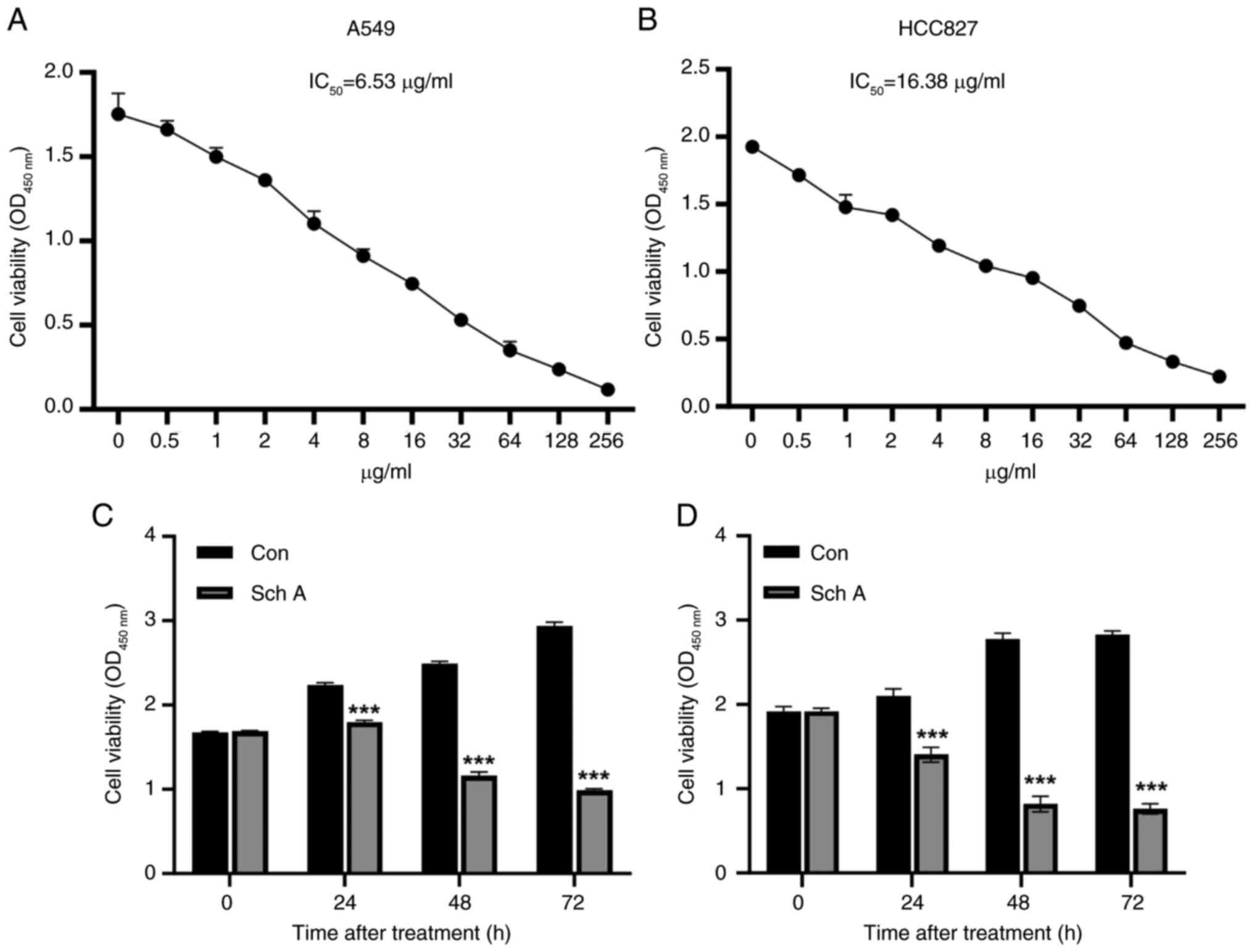
Concentration- and time-dependent
inhibition of A549 and HCC827 cell viability by Sch A
The viability of A549 and HCC827 cells was evaluated
following exposure to a range of Sch A concentrations. The CCK-8
assay revealed a concentration-dependent decrease in viability in
both cell lines (Fig. 1A and B).
The IC50 values of Sch A were determined to be 6.53
µg/ml for A549 cells and 16.38 µg/ml for HCC827 cells. Based on
these results, concentrations of 6 and 16 µg/ml were selected for
subsequent experiments in A549 and HCC827 cells, respectively.
Furthermore, the inhibitory effect of Sch A on the viability of
both cell lines increased significantly with prolonged treatment
(Fig. 1C and D).
Fer-1 and deferoxamine (DFO) abolish
Sch A-induced lung cancer cell death
Compared with the control, treatment with Sch A
significantly increased late apoptosis in both A549 and HCC827
cells (Fig. 2A and B).
Additionally, cell cycle analysis revealed a substantial
accumulation of A549 and HCC827 cells in the G1 phase,
accompanied by a notable reduction in the percentage of cells in
the S and G2 phases (Fig.
2C and D). Upon preincubation for 1 h with a range of
inhibitors, including the apoptosis inhibitor Z-VAD-FMK, the
autophagy inhibitor 3-methyladenine, the necrosis inhibitor
necrostatin-1 and the ferroptosis inhibitors Fer-1 and DFO, only
the latter two inhibitors partially reversed the Sch A-induced
reduction in cell viability. By contrast, apoptosis, autophagy and
necrosis inhibitors had no significant effect on Sch A-induced cell
death, highlighting the specific involvement of ferroptosis in this
process (Fig. 2E and F).
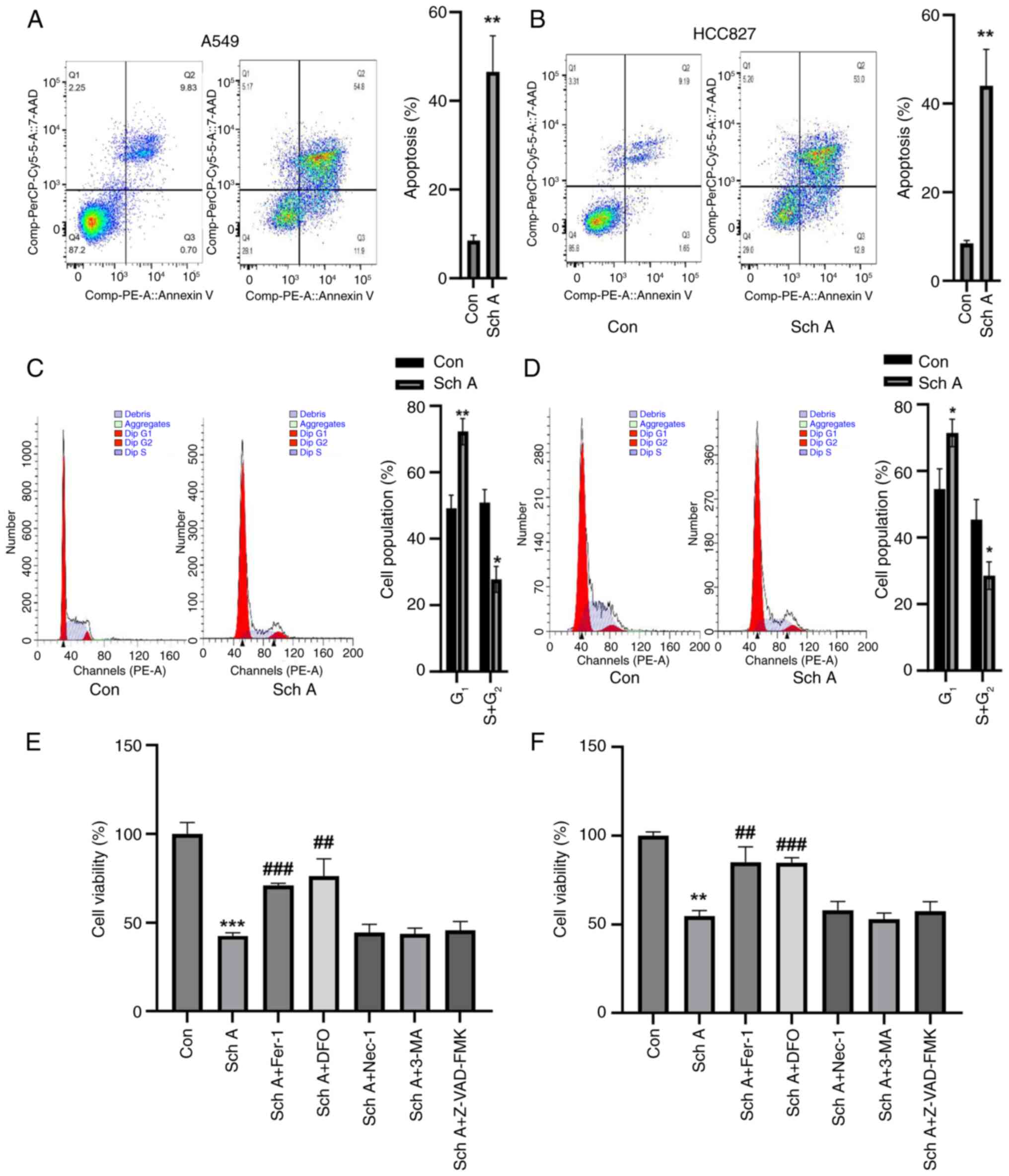 | Figure 2.Sch A induces cell death and cell
cycle arrest in A549 and HCC827 cells. Sch A considerably increased
the death of (A) A549 and (B) HCC827 cells in comparison with the
control group, according to flow cytometric analysis. Compared with
Con, (C) A549 and (D) HCC827 cells presented a significant decrease
in the number of G2 + S-phase cells and a significant
increase in the number of G1-phase cells. Fer-1 and DFO
were able to significantly reverse the Sch A-induced decrease in
(E) A549 and (F) HCC827 cell viability, according to the results of
the Cell Counting Kit-8 analysis. *P<0.05, **P<0.01 and
***P<0.001 vs. Con; ##P<0.01 and
###P<0.001 vs. Sch A. DFO, deferoxamine; Con,
control; Sch A, Schisantherin A; Fer-1, ferrostatin-1; Nec-1,
necrostatin-1; 3-MA, 3-methyladenine. |
Sch A induces ferroptosis in A549 and
HCC827 cells by modulating MMP and lipid peroxidation
Changes were also observed in the MMP, where Sch A
treatment led to a decrease in JC-1 aggregation and an increase in
monomeric JC-1 levels, indicating a reduction in the MMP compared
with that in the control group (Fig.
3A and B). Preincubation with the ferroptosis inhibitors Fer-1
and DFO resulted in a reduction in monomeric JC-1 levels and an
increase in polymeric JC-1 levels, suggesting the restoration of
the MMP. Furthermore, Sch A treatment led to elevated MDA levels in
both A549 and HCC827 cells, an effect that was reversed by
pre-treatment with Fer-1 and DFO (Fig.
3C and D). Sch A also caused a decrease in GSH levels in both
cell lines, which was reversed by pre-treatment with Fer-1 and DFO
(Fig. 3E and F). Additionally, an
increase in the expression of the ferroptosis markers Chac1 and
ptgs2 was observed following Sch A treatment, with Fer-1 and DFO
effectively reversing the Sch A-induced upregulation of these
markers (Fig. 3G and H).
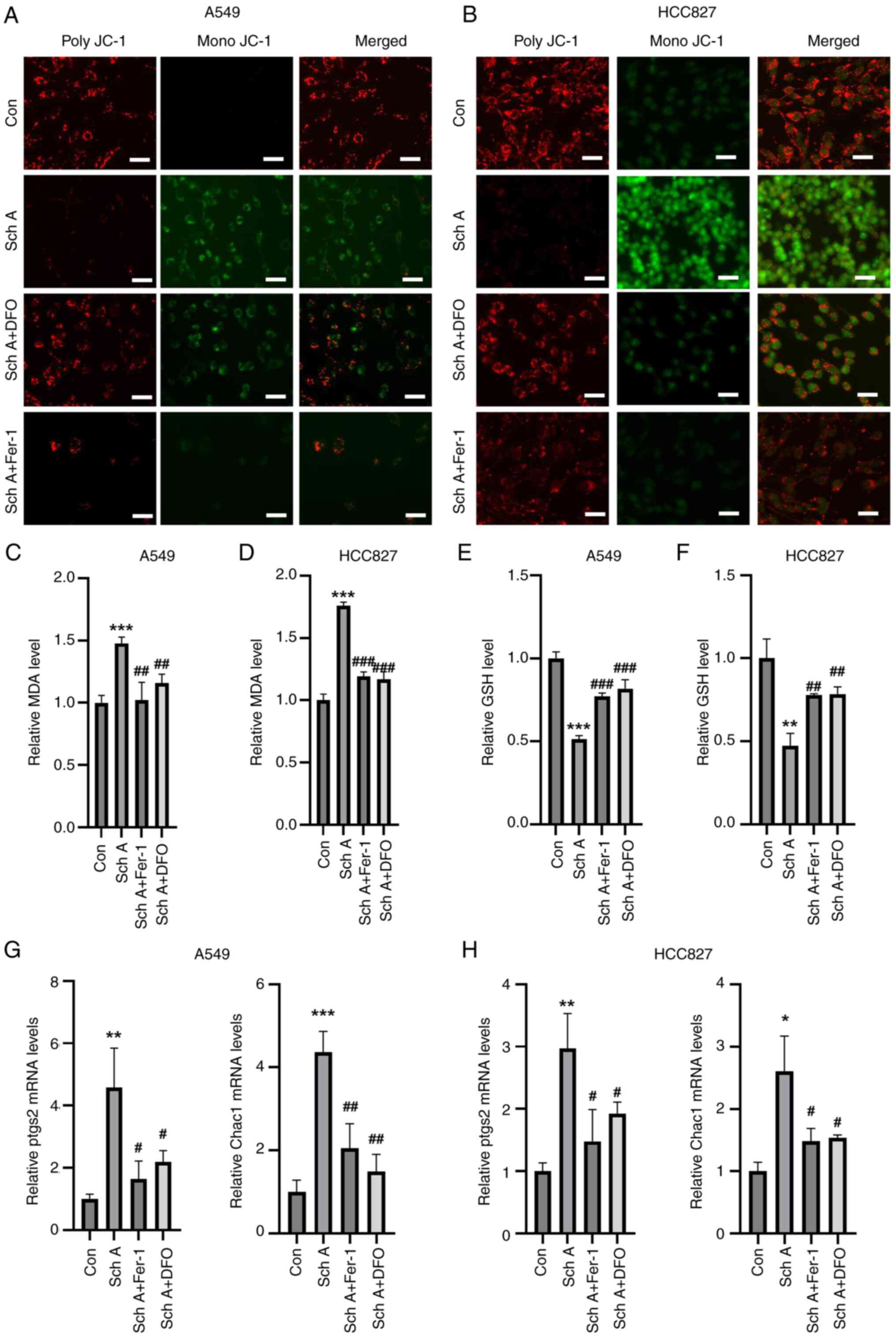 | Figure 3.In A549 and HCC827 cells, ferroptosis
inhibitors prevent Sch A-induced apoptosis. Fer-1 and DFO were
shown by JC-1 staining to reverse the Sch A-induced MMP reduction
in (A) A549 and (B) HCC827 cells (scale bar, 30 µm). Fer-1 and DFO
inhibited the Sch A-induced increase in MDA in (C) A549 and (D)
HCC827 cells. Fer-1 and DFO counteracted the Sch A-induced
reduction in GSH in (E) A549 and (F) HCC827 cells. Reverse
transcription-quantitative PCR analysis revealed that Fer-1 and DFO
reversed the Sch A-induced increase in Chac1 and ptgs2 levels in
(G) A549 and (H) HCC827 cells. *P<0.05, **P<0.01 and
***P<0.001 vs. Con; #P<0.05,
##P<0.01 and ###P<0.001 vs. Sch A. Con,
control; Chac1, glutathione-specific γ-glutamylcyclotransferase 1;
ptgs2, prostaglandin-endoperoxide synthase 2; Fer-1, ferrostatin-1;
DFO, deferoxamine; Sch A, Schisantherin A; MDA, malondialdehyde;
GSH, glutathione. |
Sch A upregulates YAP1 and modulates
ferroptosis in NSCLC through transcriptional activation of
ferroptosis-related proteins
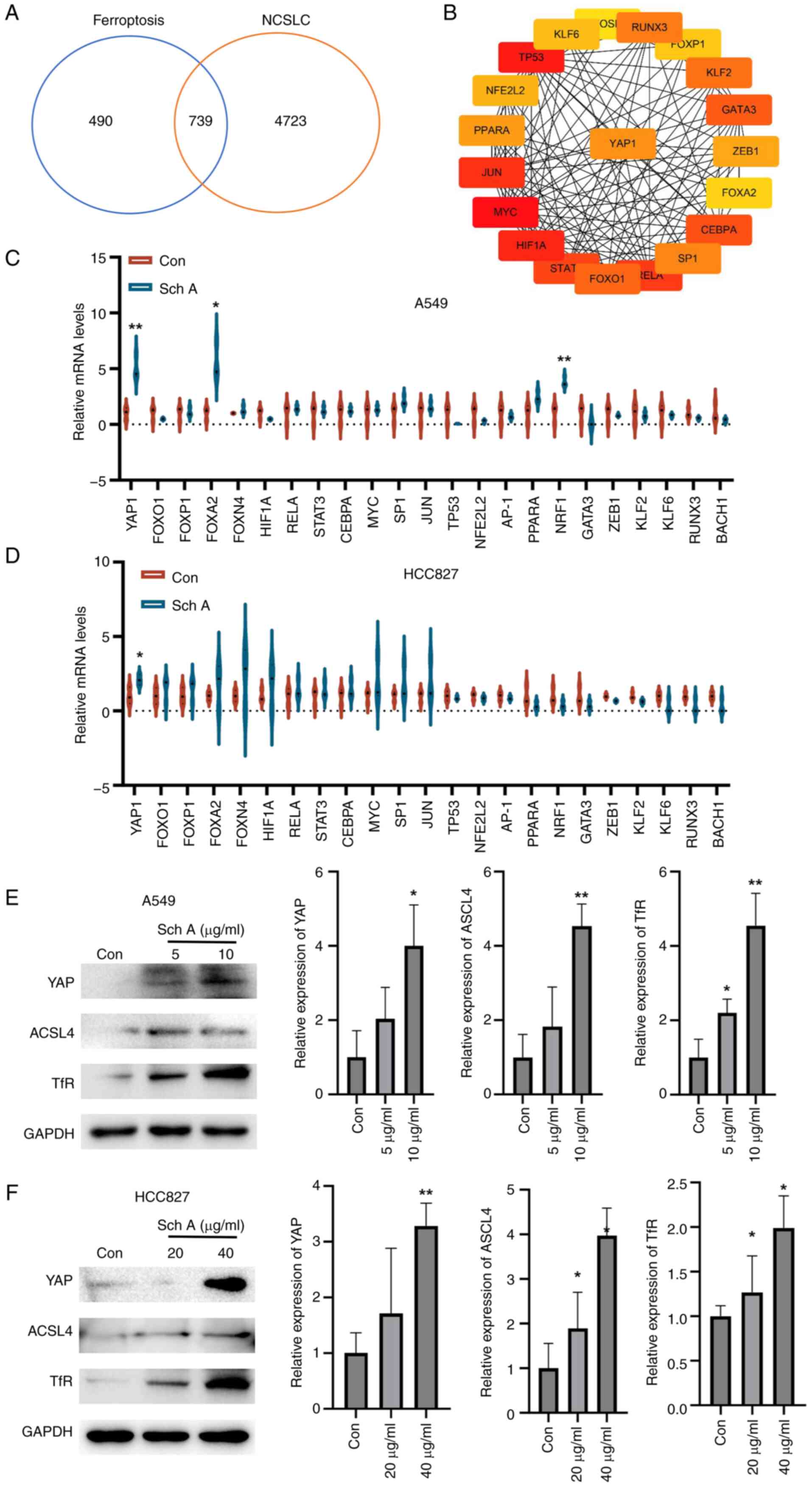
Using GeneCards, an intersection analysis of
ferroptosis-related and NSCLC-associated target genes revealed 739
overlapping genes, suggesting that these genes may serve an
important role in regulating ferroptosis within NSCLC (Fig. 4A). Among these 739 overlapping
genes, transcription factors were further analyzed to identify key
node genes, with YAP emerging as a critical regulator within this
intersecting gene network, highlighting its potential role in
modulating ferroptosis in NSCLC (Fig.
4B). RT-qPCR analysis revealed that Sch A treatment of A549 and
HCC827 cells led to a significant increase in YAP1 mRNA expression,
indicating that Sch A can upregulate YAP1 in NSCLC cell lines
(Fig. 4C and D). Given the
multifaceted role of the Hippo pathway in regulating tumor cell
behavior, the phosphorylation status of YAP, a key downstream
effector of this pathway, was further examined. Although no changes
in YAP phosphorylation levels were observed (data not shown), a
marked increase in YAP protein levels was detected (Fig. 4E and F), suggesting that YAP may
participate in ferroptosis through mechanisms independent of
phosphorylation. To further investigate this finding, it was
hypothesized that YAP might regulate ferroptosis by
transcriptionally activating ferroptosis-related proteins,
specifically TfR and ACSL4. Western blot analysis confirmed the
involvement of YAP in regulating ferroptosis, as Sch A treatment
increased the expression of the ferroptosis-related proteins TfR
and ACSL4.
YAP knockdown abolishes Sch A-induced
ferroptosis in lung cancer cells
To determine whether YAP activation has a role in
Sch A-induced ferroptosis, the present study employed siRNAs
targeting YAP. Western blot analysis demonstrated that transfection
with si-YAP resulted in a marked reduction in the protein levels of
YAP in both A549 and HCC827 cell lines when compared with cells
transfected with the NC siRNA (Fig. 5A
and B). The results, presented in Fig. 5A and B, demonstrated a significant
reduction in YAP expression in both A549 and HCC827 cells following
siRNA transfection, indicating efficient knockdown of YAP. Along
with the knockdown of YAP, the downstream targets ACSL4 and TfR
were also significantly suppressed by si-YAP. Following Sch A
treatment conditions, si-YAP was still able to efficiently knock
down YAP expression as well as the downstream targets ACSL4 and TfR
in both cell lines. FerroOrange staining revealed that Sch A
treatment led to notable Fe2+ accumulation in both A549
and HCC827 cells. However, upon YAP silencing, this increase in
Fe2+ was effectively reversed, further indicating that
YAP serves an important role in regulating iron metabolism during
Sch A-induced ferroptosis (Fig. 5C and
D). Additionally, quantification of Fe2+ and MDA
levels in A549 and HCC827 cells revealed that Sch A treatment
significantly elevated both Fe2+ and MDA levels compared
with those in the control group, whereas YAP silencing effectively
alleviated these Sch A-induced increases (Fig. 5E and F).
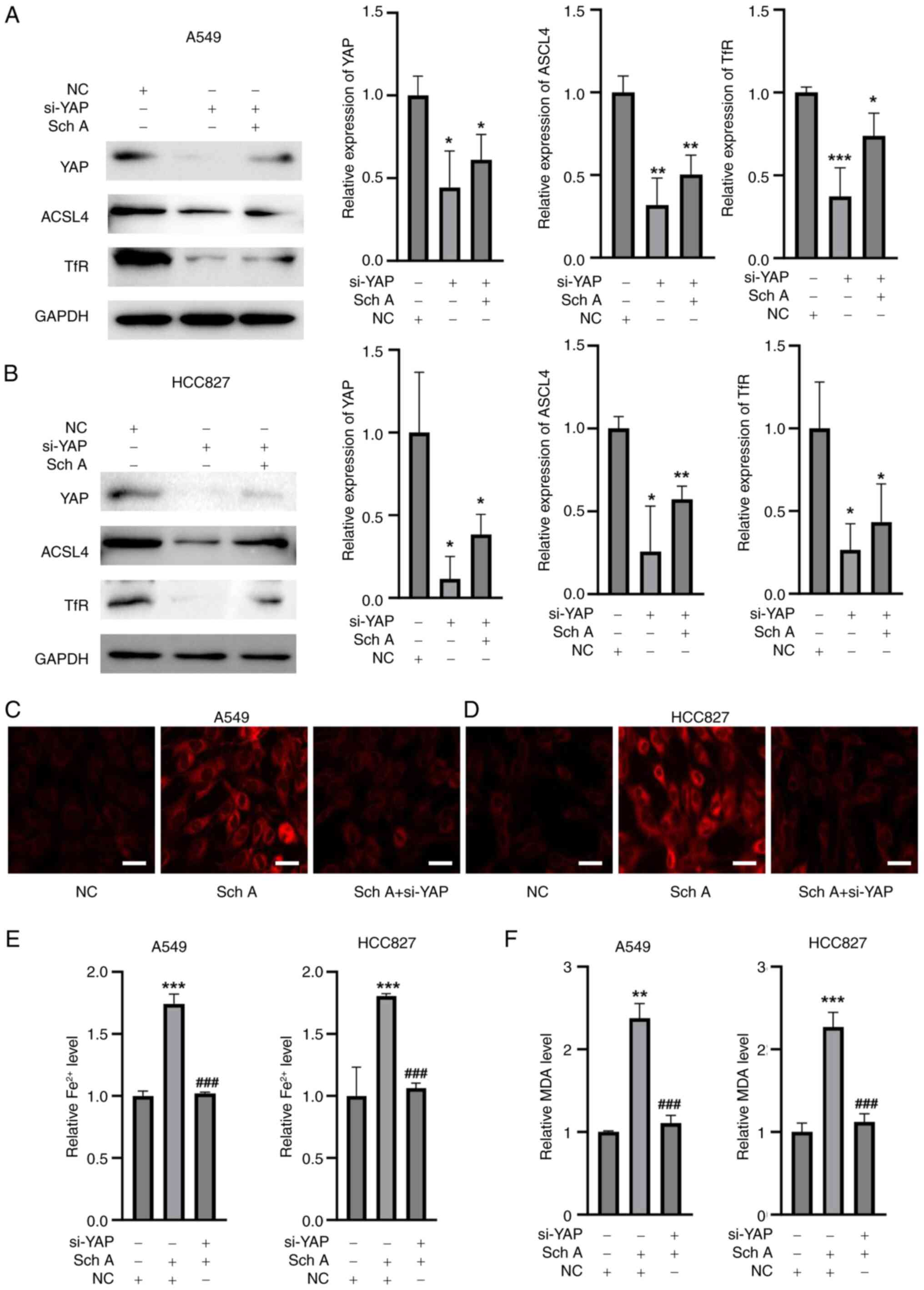 | Figure 5.Silencing of YAP reverses Sch
A-induced ferroptosis. Western blot analysis showed that the
expression of YAP was decreased in (A) A549 and (B) HCC827 cells.
In (C) A549 and (D) HCC827 cells, si-YAP decreased the expression
of YAP and downstream signaling molecules. Fe2+
accumulation was induced by Sch A in (E) A549 and (F) HCC827 cells,
and the increase in Fe2+ was reversed by YAP silencing,
as demonstrated by FerroOrange staining (scale bar, 10 µm). After
silencing YAP, the Sch A-induced increase in Fe2+ and
MDA in (G) A549 and (H) HCC827 cells was reduced. *P<0.05,
**P<0.01 and ***P<0.001 vs. NC; ###P<0.001 vs.
Sch A. NC, negative control; YAP, yes-associated protein; Sch A,
Schisantherin A; si, small interfering RNA; MDA, malondialdehyde;
ACSL4, acyl-CoA synthase long-chain family member 4; TfR,
transferrin receptor. |
Sch A inhibits tumor growth in nude
mice
To assess the effect of Sch A on tumor growth in
vivo, a nude mouse xenograft model was used. Compared with the
control treatment, Sch A treatment resulted in a significant
reduction in both tumor mass and volume, demonstrating its potent
inhibitory effect on tumor growth in this animal model (Fig. 6A and B). Furthermore, Sch A
treatment led to a marked increase in MDA levels and
Fe2+ accumulation within the tumors, indicating the
promotion of ferroptosis-related oxidative stress and iron overload
in the TME (Fig. 6C and D). This
increase was accompanied by a significant decrease in GSH levels,
suggesting a depletion of antioxidant defenses in the tumor tissues
(Fig. 6E). Additionally, Sch A
treatment upregulated the protein expression levels of YAP, ACSL4
and TfR in tumor tissues, which was consistent with the in
vitro findings, further supporting the involvement of these
proteins in Sch A-induced ferroptosis and tumor suppression
(Fig. 6F).
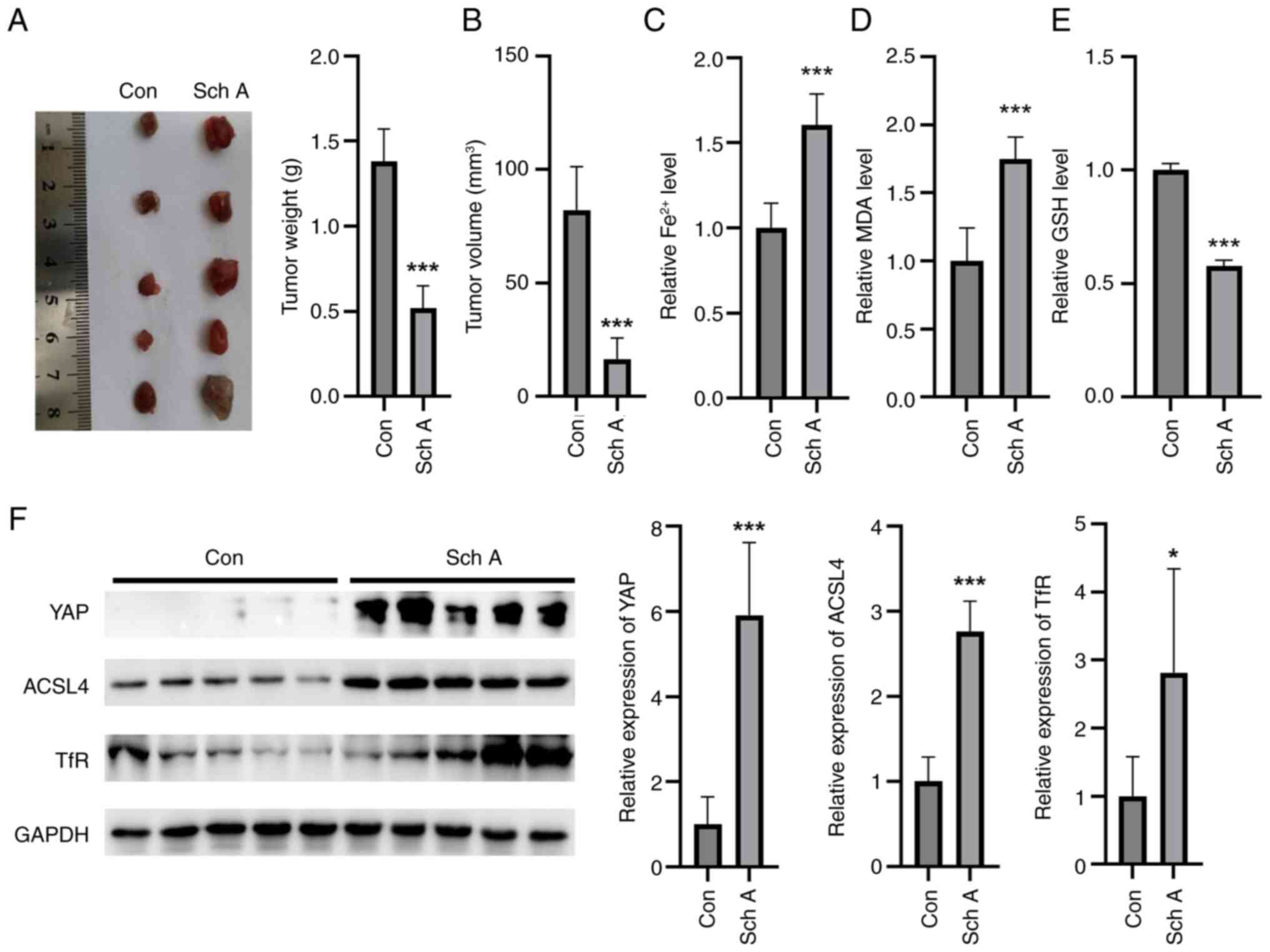 | Figure 6.In vivo, Sch A prevents the
formation of tumors in nude mice. Compared with Con, Sch A
inhibited (A) tumor mass and (B) volume in vivo in nude
mice. Sch A increased the (C) Fe2+ and (D) MDA contents
in nude mouse tumors. (E) Sch A diminished the GSH levels in the
tumors compared with those in the Con group. (F) Compared with
those in the Con group, Sch A induced the expression levels of YAP,
ACSL4 and TfR in the tumors of nude mice. *P<0.05 and
***P<0.001 vs. Con. Con, control; Sch A, Schisantherin A; MDA,
malondialdehyde; GSH, glutathione; YAP, yes-associated protein;
ACLS4, acyl-CoA synthase long-chain family member 4; TfR,
transferrin receptor. |
Discussion
Lung cancer poses a notable threat to global human
health and life, with >50% of patients being diagnosed at
advanced stages, leading to a low chance of curative surgery
(20). Currently, chemotherapy and
radiotherapy are the primary treatment methods for lung cancer, but
owing to their pronounced toxic side effects and the tendency for
drug resistance to develop, patients often find it difficult to
adhere to treatment, resulting in low cure rates and high
recurrence rates (20,21). In recent years, TCM has attracted
notable attention from researchers due to its potent therapeutic
effects, low toxicity and multitargeted approach for tumor
treatment (14). Sch A, a major
bioactive lignan isolated from the TCM plant Schisandra
chinensis, has been shown to inhibit hepatocellular carcinoma
cell proliferation by suppressing glucose metabolism, thereby
exerting anti-liver cancer effects (22). Additionally, Sch A induces ROS
generation, activates the JNK signaling pathway and inhibits
nuclear factor erythroid 2-related factor 2, effectively
suppressing gastric cancer cell proliferation and migration, and
promoting apoptosis (23).
However, to the best of our knowledge, no studies to date have
reported the potential anticancer effects of Sch A on NSCLC and its
underlying mechanisms.
To the best of our knowledge, the present study
explored the molecular mechanisms underlying the anticancer effects
of Sch A in NSCLC for the first time. The present results
demonstrated that Sch A effectively inhibited the viability of
NSCLC cells in a dose- and time-dependent manner. Treatment with
Sch A significantly led to an increase in NSCLC cell apoptosis and
induced cell cycle arrest, highlighting its notable anticancer
activity. Further mechanistic investigations revealed that Sch A
triggered ferroptosis in NSCLC cells, as supported by the reversal
of the Sch A-induced reduction in cell viability and MMP loss upon
treatment with the ferroptosis inhibitors Fer-1 and DFO.
Additionally, Sch A treatment resulted in elevated intracellular
levels of Fe2+ and MDA, alongside a significant decrease
in the content of GSH, a key indicator of ferroptosis activation
(24). Collectively, these
findings provide evidence that Sch A induces ferroptosis in lung
cancer cells, suggesting its potential as a novel therapeutic
strategy for NSCLC.
Xian et al (25) and Zhu et al (26) reported that Sch A can induce
apoptosis in NSCLC by inhibiting EGFR phosphorylation or
synergizing with gefitinib. However, the present study was, to the
best of our knowledge, the first to demonstrate that Sch A
triggered ferroptosis through activation of the YAP/ACSL4/TfR axis,
a cell death mechanism unexplored in prior research. While previous
work, such as the study by Zhu et al (26), focused on apoptosis or, in the case
of the study by Xian et al (25), apoptosis and cell cycle arrest, the
systematic inhibitor screening experiments performed in the present
study established ferroptosis as the predominant cell death
modality under Sch A monotherapy, with the apoptosis inhibitor
Z-VAD-FMK showing no significant effect on Sch A-induced
cytotoxicity. To ensure translational relevance across NSCLC
subtypes, two genetically distinct cell lines were employed, A549
(EGFR-wild type) and HCC827 (EGFR Exon19del E746-A750 mutant),
which are tyrosine kinase inhibitor-sensitive lines frequently used
in targeted-therapy studies (27,28).
The results of the present study demonstrated that Sch A induced
ferroptotic features in both cell lines, suggesting that its
mechanism was not dependent on the EGFR mutation status and may
therefore offer therapeutic potential across a range of NSCLC
genetic backgrounds. Notably, comparable increases in
Fe2+ accumulation, and ACSL4 and TfR expression were
observed in both A549 and HCC827 cells following Sch A treatment,
indicating genotype-independent vulnerability to ferroptosis. The
apparent discrepancy with the apoptotic phenotype described in the
study by Zhu et al (26)
may arise from key methodological differences: i) The
aforementioned study used 50 µM Sch A for 24 h to induce apoptosis,
whereas the present study employed lower concentrations that may
activate distinct cell death pathways; and ii) the study by Zhu
et al (26) quantified
total apoptosis (quadrants 2 + 3) via a FITC-PE kit, whereas the
present study specifically analyzed late apoptosis (quadrant 3) via
an Annexin V-7-AAD kit alongside ferroptosis markers, such as
Fe2+ accumulation. The Annexin V-FITC-PE approach cannot
exclude interference from necrotic or ferroptotic cells in
PI+ populations, which represent membrane-disrupted
cells. By contrast, the Annexin V-7-AAD kit precisely distinguishes
early apoptosis (quadrant 3, Annexin
V+/7-AAD−), late apoptosis (quadrant 2,
Annexin V+/7-AAD+) and primary necrosis
(quadrant 1, Annexin V−/7-AAD+). The present
study observed a significant Sch A-induced increase in
7-AAD+ cells in quadrant 2 (late apoptosis/secondary
necrosis), which co-occurred with ferroptosis biomarkers. Combined
with specific rescue by the ferroptosis inhibitors Fer-1 and DFO,
the present study concluded that ferroptosis, not classical
apoptosis, was the primary death mechanism. The present study
explored the anticancer mechanism of Sch A in NSCLC, which shifted
the paradigm from apoptosis to YAP-dependent ferroptosis. Hence,
the present study investigated a novel signaling axis with broad
therapeutic implications, advancing the potential of Sch A as a
precision oncology agent.
Furthermore, emerging evidence links EGFR signaling
to ferroptosis regulation (29,30).
In colorectal cancer, glucose deprivation inhibits Hippo signaling
and activates EGFR, which enhances cell anti-ferroptotic defenses
through PPAR-ACSL1/4 lipid-metabolic remodeling, thereby limiting
lipid peroxidation (29). In
triple-negative breast cancer, EGFR inhibition promotes ferroptosis
via YAP/mTOR-mediated autophagy (30). In the NSCLC models of the present
study, Sch A-induced YAP activation led to the upregulation of
ACSL4 and TfR, which are hallmark mediators of ferroptosis, in both
EGFR-mutant (HCC827) and -wild-type (A549) cells, indicating
YAP-driven ferroptosis across distinct EGFR backgrounds. This
cascade may have been modulated by the tissue context and EGFR
mutational status but ultimately converged on a shared ferroptotic
axis. In summary, the present study provided new mechanistic
insight into the anti-NSCLC potential of Sch A. By demonstrating
consistent ferroptosis induction across both EGFR-wild-type and
mutant backgrounds, the present study highlighted the promise of
Sch A as a mutation-agnostic therapeutic.
While traditional molecular research has revealed
that Sch A induced ferroptosis in NSCLC cells via the YAP/ACSL4/TfR
axis, this perspective alone may not have fully accounted for
complex clinical phenomena such as drug resistance and tumor
recurrence. By adopting the cancer ecology paradigm (31), the role of Sch A within the TME was
interpreted as part of a dynamic ecological system. In this
context, YAP may function not only as a molecular signaling node
but also as an ecological sensor that enables cancer cells to
perceive and adapt to microenvironmental stresses. For example,
under glucose-deprived conditions, which are common in advanced
NSCLC, YAP activation typically confers survival advantages to
cancer cells by promoting metabolic reprogramming (32). However, the present findings
suggested that Sch A disrupted this adaptive mechanism by
potentially activating YAP, leading to the upregulation of ACSL4
and TfR expression. This shift triggered mitochondrial lipid
peroxidation and induced ferroptosis, effectively converting the
adaptive evolution of cancer cells into a self-destructive process.
This mechanism was analogous to embedding a self-destructive
program within an invasive species, in this case cancer cells,
thereby undermining their population stability. The present study,
therefore, transitioned from a purely molecular focus to a novel
paradigm of cancer ecological regulation. Sch A exploited the
evolutionary adaptability of cancer cells, specifically YAP
activation, and transformed them into ecologically vulnerable cells
through the induction of ferroptosis. This ecological perspective
underscores the potential of Sch A to modulate the dynamic
interactions between cancer cells and the TME in NSCLC, offering
novel avenues for predicting cancer progression and developing more
effective therapeutic strategies.
Furthermore, cancer stem cells (CSCs) constitute a
small subpopulation within tumors characterized by self-renewal,
differentiation and tumor-initiating capacity (33). Numerous studies have demonstrated
that lung CSCs are closely associated with recurrence, metastasis
and therapeutic resistance in NSCLC, making them both major
challenges and important targets in current anticancer strategies
(33,34). Although the present study focused
primarily on A549 and HCC827 cells, Sch A significantly activated
the YAP/ACSL4/TfR signaling axis, leading to intracellular
Fe2+ accumulation and lipid peroxidation, which are
hallmarks of ferroptosis. Notably, previous studies have reported a
close relationship between the malignant proliferation of lung CSCs
and ferroptosis (35,36). Therefore, the upregulation of TfR
induced by Sch A may further sensitize lung CSCs to ferroptotic
stress, suggesting that CSCs may serve as potential cellular
targets of Sch A. In future studies, it should be investigated
whether Sch A can effectively suppress the survival and
functionality of lung CSCs through ferroptosis induction, thereby
providing a novel approach to overcoming therapeutic resistance in
NSCLC.
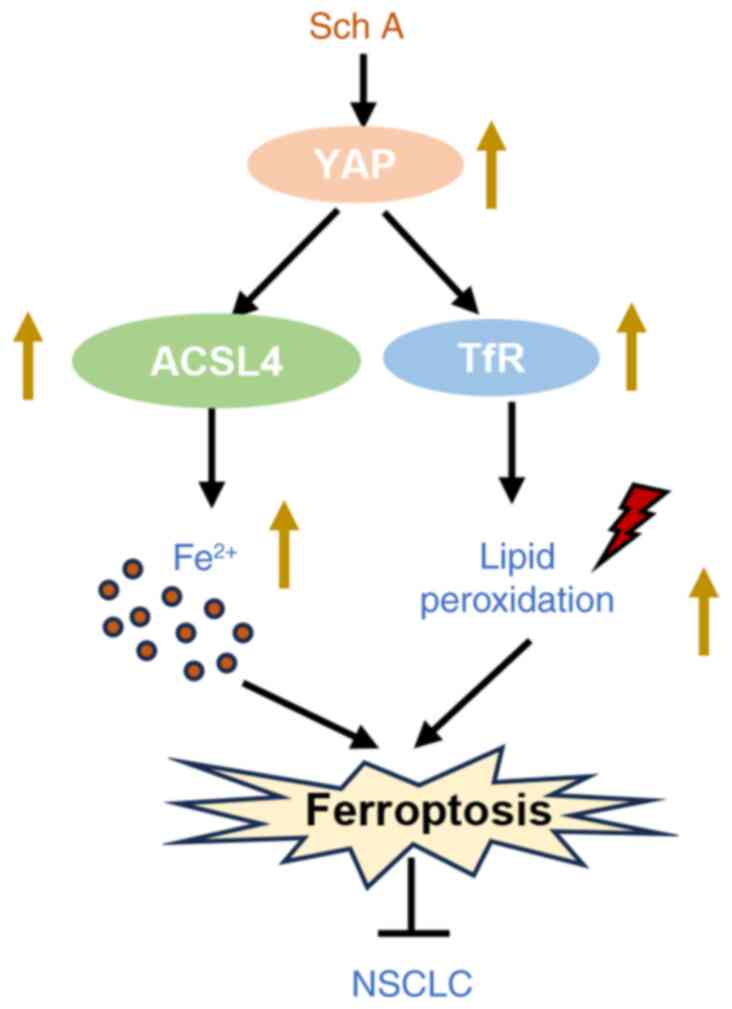
In conclusion, the results obtained in the present
study demonstrated that Sch A induced ferroptosis in lung cancer
cells through the YAP/ACSL4/TfR signaling pathway, leading to the
disruption of iron homeostasis and the generation of oxidative
stress (Fig. 7). This novel
mechanism highlights the potential of Sch A as a therapeutic agent
that promotes ferroptotic cell death, particularly in the treatment
of NSCLC.
Acknowledgements
Not applicable.
Funding
The present work was funded by the Science and Technology
Planning Project of Shenzen Municipality (grant no.
JSGG20220226090203006).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
WZ, YC and ZZ designed the research. WZ, YC and XW
performed the cell line experiments. XF analyzed the data. YH, YM,
QZ and MT performed the animal experiments. WZ, YC, XW and ZZ wrote
the manuscript and were responsible for making revisions. ZZ
secured funding. All authors confirm the authenticity of all the
raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Animal Research: Reporting of In Vivo Experiments
guidelines and the Guide for the Care and Use of Laboratory Animals
(US National Research Council). All animal experiments were
approved by the Committee on the Ethics of Animal Experiments of
Guangzhou University of Chinese Medicine (approval no.
2024055R).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cao M and Chen W: Epidemiology of lung
cancer in China. Thorac Cancer. 10:3–7. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li C, Lei S, Ding L, Xu Y, Wu X, Wang H,
Zhang Z, Gao T, Zhang Y and Li L: Global burden and trends of lung
cancer incidence and mortality. Chin Med J (Engl). 136:1583–1590.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li D, Shi J, Liang D, Ren M and He Y: Lung
cancer risk and exposure to air pollution: A multicenter North
China case-control study involving 14604 subjects. BMC Pulm Med.
23:1822023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fang Y, Li Z, Chen H, Zhang T, Yin X, Man
J, Yang X and Lu M: Burden of lung cancer along with attributable
risk factors in China from 1990 to 2019, and projections until
2030. J Cancer Res Clin Oncol. 149:3209–3218. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Imyanitov EN, Iyevleva AG and Levchenko
EV: Molecular testing and targeted therapy for non-small cell lung
cancer: Current status and perspectives. Crit Rev Oncol Hematol.
157:1031942021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xiang Y, Liu X, Wang Y, Zheng D, Meng Q,
Jiang L, Yang S, Zhang S, Zhang X, Liu Y and Wang B: Mechanisms of
resistance to targeted therapy and immunotherapy in non-small cell
lung cancer: Promising strategies to overcoming challenges. Front
Immunol. 15:13662602024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lei G and Zhuang Gan B: Targeting
ferroptosis as a vulnerability in cancer. Nat Rev Cancer.
22:381–396. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fang K, Du S, Shen D, Xiong Z, Jiang K,
Liang D, Wang J, Xu H, Hu L, Zhai X, et al: SUFU suppresses
ferroptosis sensitivity in breast cancer cells via Hippo/YAP
pathway. iScience. 25:1046182022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Niu X, Han P, Liu J, Chen Z and Ma X,
Zhang T, Li B and Ma X: Regulation of Hippo/YAP signaling pathway
ameliorates cochlear hair cell injury by regulating ferroptosis.
Tissue Cell. 82:1020512023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cui J, Wang Y, Tian X, Miao Y, Ma L, Zhang
C, Xu X, Wang J, Fang W and Zhang X: LPCAT3 is transcriptionally
regulated by YAP/ZEB/EP300 and collaborates with ACSL4 and YAP to
determine ferroptosis sensitivity. Antioxid Redox Signal.
39:491–511. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li L, Ye Z, Xia Y, Li B, Chen L, Yan X,
Yuan T, Song B, Yu W, Rao T, et al: YAP/ACSL4 pathway-mediated
ferroptosis promotes renal fibrosis in the presence of kidney
stones. Biomedicines. 11:26922023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu Z, Sun J, Liao Z, Sun T, Huang L, Qiao
J, Ling C, Chen C, Zhang B and Wang H: Activation of PAR1
contributes to ferroptosis of Schwann cells and inhibits
regeneration of myelin sheath after sciatic nerve crush injury in
rats via Hippo-YAP/ACSL4 pathway. Exp Neurol. 384:1150532025.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gu Y, Wu S, Fan J, Meng Z, Gao G, Liu T,
Wang Q, Xia H, Wang X and Wu K: CYLD regulates cell ferroptosis
through Hippo/YAP signaling in prostate cancer progression. Cell
Death Dis. 15:792024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiao Z, Xiao W and Li G: Research progress
on the pharmacological action of Schisantherin A. Evid Based
Complement Alternat Med. 2022:64208652022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mi X, Zhang Z, Cheng J, Xu Z, Zhu K and
Ren Y: Cardioprotective effects of Schisantherin A against
isoproterenol-induced acute myocardial infarction through
amelioration of oxidative stress and inflammation via modulation of
PI3K-AKT/Nrf2/ARE and TLR4/MAPK/NF-κB pathways in rats. BMC
Complement Med Ther. 23:2772023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shen Y, Yang Y, Zhao Y, Nuerlan S, Zhan Y
and Liu C: YY1/circCTNNB1/miR-186-5p/YY1 positive loop aggravates
lung cancer progression through the Wnt pathway. Epigenetics.
19:23690062024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xie C, Zhou X, Wu J, Chen W, Ren D, Zhong
C, Meng Z, Shi Y and Zhu J: ZNF652 exerts a tumor suppressor role
in lung cancer by transcriptionally downregulating cyclin D3. Cell
Death Dis. 15:7922024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lussier G, Evans AJ, Houston I, Wilsnack
A, Russo CM, Vietor R and Bedocs P: Compact arterial monitoring
device use in resuscitative endovascular balloon occlusion of the
aorta (REBOA): A simple validation study in swine. Cureus.
16:e707892024.PubMed/NCBI
|
|
20
|
Jonna S and Subramaniam DS: Molecular
diagnostics and targeted therapies in non-small cell lung cancer
(NSCLC): An update. Discov Med. 27:167–170. 2019.PubMed/NCBI
|
|
21
|
Wang M, Herbst RS and Boshoff C: Toward
personalized treatment approaches for non-small-cell lung cancer.
Nat Med. 27:1345–1356. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng F, Pan L, Wu J, Liu M, He L, Yang L
and Zhou W: Schisantherin A inhibits cell proliferation by
regulating glucose metabolism pathway in hepatocellular carcinoma.
Front Pharmacol. 13:10194862022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Z, Yu K, Hu Y, Su F, Gao Z, Hu T,
Yang Y, Cao X and Qian F: Schisantherin A induces cell apoptosis
through ROS/JNK signaling pathway in human gastric cancer cells.
Biochem Pharmacol. 173:1136732020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang X, Stockwell BR and Conrad M:
Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol
Cell Biol. 22:266–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xian H, Feng W and Zhang J: Schizandrin A
enhances the efficacy of gefitinib by suppressing IKKβ/NF-κB
signaling in non-small cell lung cancer. Eur J Pharmacol.
855:10–19. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu L, Wang Y, Huang X, Liu X, Ye B, He Y,
Yu H, Lv W, Wang L and Hu J: Schizandrin A induces non-small cell
lung cancer apoptosis by suppressing the epidermal growth factor
receptor activation. Cancer Med. 13:e69422024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nilsson MB, Yang Y, Heeke S, Patel SA,
Poteete A, Udagawa H, Elamin YY, Moran CA, Kashima Y, Arumugam T,
et al: CD70 is a therapeutic target upregulated in EMT-associated
EGFR tyrosine kinase inhibitor resistance. Cancer Cell. 41:340–355.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shyam Sunder S, Sharma UC and Pokharel S:
Adverse effects of tyrosine kinase inhibitors in cancer therapy:
Pathophysiology, mechanisms and clinical management. Signal
Transduct Target Ther. 8:2622023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qian LH, Wen KL, Guo Y, Liao YN, Li MY, Li
ZQ, Li SX and Nie HZ: Nutrient deficiency-induced downregulation of
SNX1 inhibits ferroptosis through PPARs-ACSL1/4 axis in colorectal
cancer. Apoptosis. 30:1391–1409. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu X, Sheng H, Zhao L, Jiang M, Lou H,
Miao Y, Cheng N, Zhang W, Ding D and Li W: Co-loaded lapatinib/PAB
by ferritin nanoparticles eliminated ECM-detached cluster cells via
modulating EGFR in triple-negative breast cancer. Cell Death Dis.
13:5572022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Luo W: Nasopharyngeal carcinoma ecology
theory: Cancer as multidimensional spatiotemporal ‘unity of ecology
and evolution’ pathological ecosystem. Theranostics. 13:1607–1631.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hsu PC, Yang CT, Jablons DM and You L: The
crosstalk between Src and Hippo/YAP signaling pathways in non-small
cell lung cancer (NSCLC). Cancers (Basel). 12:13612020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu M, Wu H and Xu C: Targeting cancer
stem cell pathways for lung cancer therapy. Curr Opin Oncol.
35:78–85. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zakaria N, Satar NA, Abu Halim NH, Ngalim
SH, Yusoff NM, Lin J and Yahaya BH: Targeting lung cancer stem
cells: Research and clinical impacts. Front Oncol. 7:802017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zheng Y, Yang W, Wu W, Jin F, Lu D, Gao J
and Wang S: Diagnostic and predictive significance of the
ferroptosis-related gene TXNIP in lung adenocarcinoma stem cells
based on multi-omics. Transl Oncol. 45:1019262024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou J, Zhang L, Yan J, Hou A, Sui W and
Sun M: Curcumin induces ferroptosis in A549 CD133+ cells
through the GSH-GPX4 and FSP1-CoQ10-NAPH Pathways. Discov Med.
35:251–263. 2023. View Article : Google Scholar : PubMed/NCBI
|