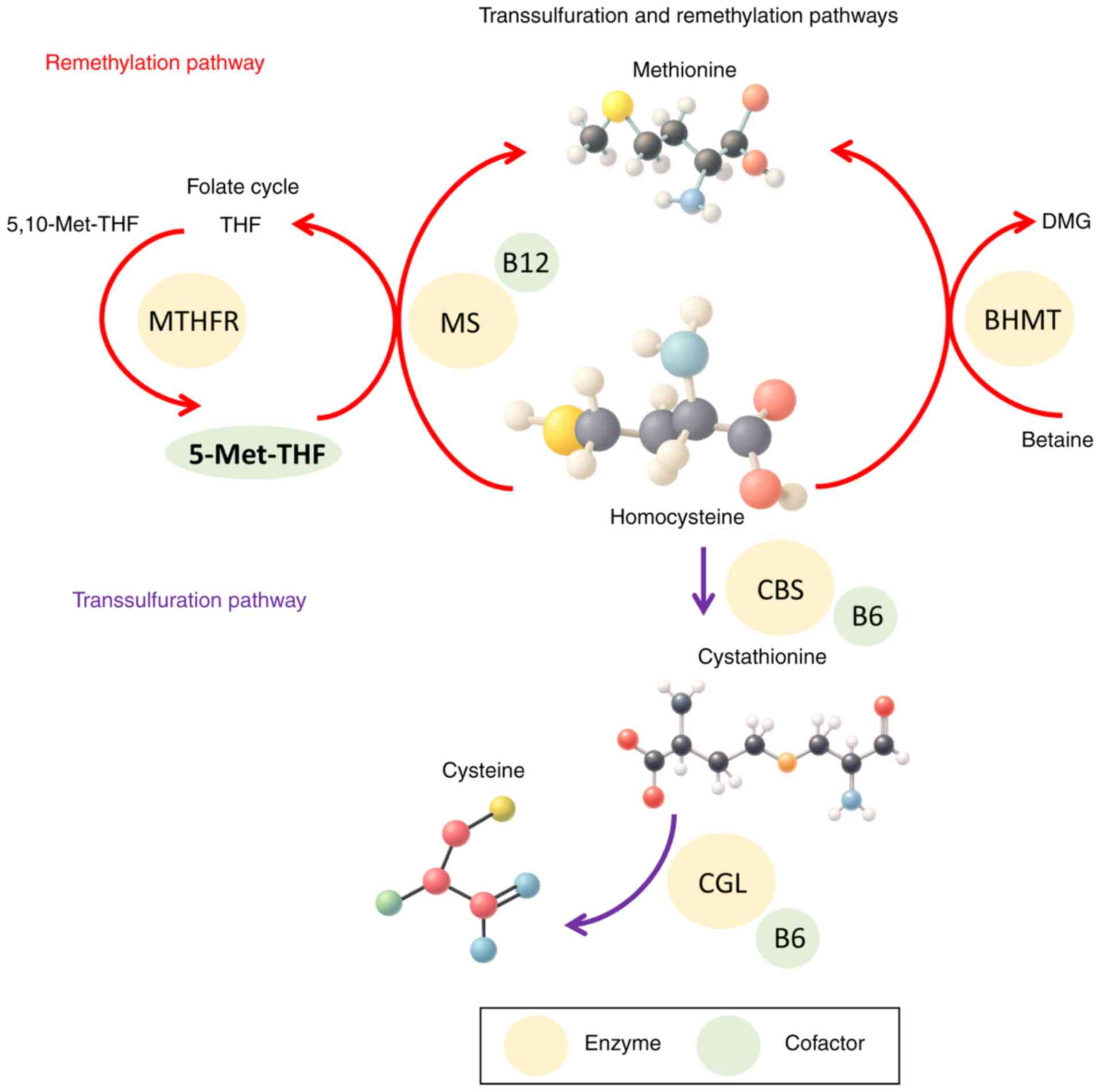
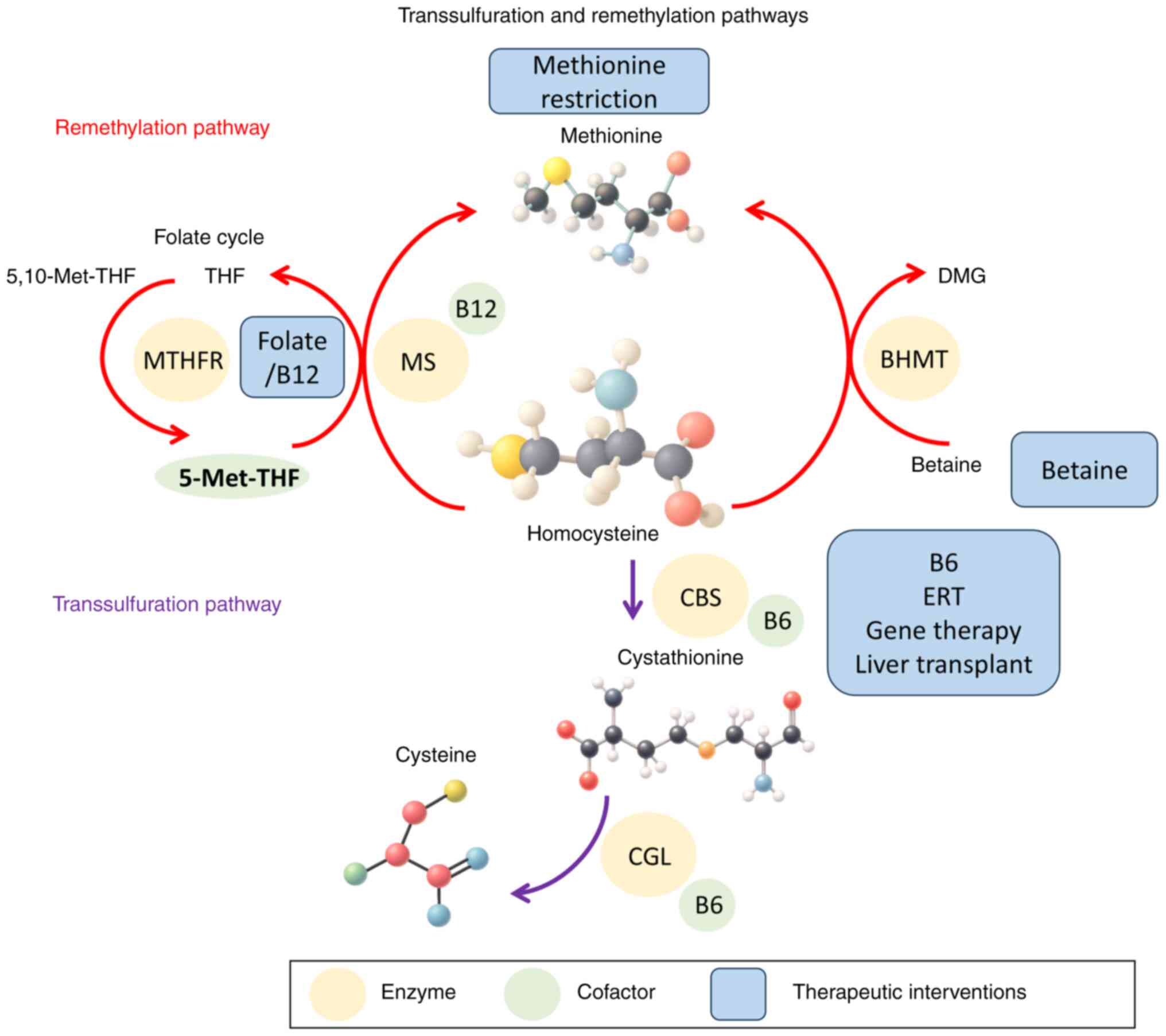
|
1
|
Carson NA, Dent C, Field CM and Gaull GE:
Homocystinuria: Clinical and pathological review of ten cases. J
Pediatr. 66:565–583. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jain M, Shah M, Thakker KM, Rava A,
Pelts-Block A, Ndiba-Markey C and Pinto L: High clinical burden of
classical homocystinuria in the United States: A retrospective
analysis. Orphanet J Rare Dis. 20:372025. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schneede J, Refsum H and Ueland PM:
Biological and environmental determinants of plasma homocysteine.
Semin Thromb Hemost. 26:263–279. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Al-Sadeq DW and Nasrallah GK: The spectrum
of mutations of homocystinuria in the MENA region. Genes (Basel).
11:3302020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Naughten ER, Yap S and Mayne PD: Newborn
screening for homocystinuria: Irish and world experience. Eur J
Pediatr. 157:S84–S87. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alsharhan H, Ahmed AA, Ali NM, Alahmad A,
Albash B, Elshafie RM, Alkanderi S, Elkazzaz UM, Cyril PX,
Abdelrahman RM, et al: Early diagnosis of classic homocystinuria in
Kuwait through newborn screening: A 6-year experience. Int J
Neonatal Screen. 7:562021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moammar H, Cheriyan G, Mathew R and
Al-Sannaa N: Incidence and patterns of inborn errors of metabolism
in the Eastern Province of Saudi Arabia, 1983–2008. Ann Saudi Med.
30:271–277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sellos-Moura M, Glavin F, Lapidus D, Evans
K, Lew CR and Irwin DE: Prevalencecharacteristics, costs of
diagnosed homocystinuria, elevated homocysteine, phenylketonuria in
the United States: A retrospective claims-based comparison. BMC
Health Serv Res. 20:1832020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Refsum H, Fredriksen Å, Meyer K, Ueland PM
and Kase BF: Birth prevalence of homocystinuria. J Pediatr.
144:830–832. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schnabel E, Kölker S, Gleich F, Feyh P,
Hörster F, Haas D, Fang-Hoffmann J, Morath M, Gramer G, Röschinger
W, et al: Combined newborn screening allows comprehensive
identification also of attenuated phenotypes for methylmalonic
acidurias and homocystinuria. Nutrients. 15:33552023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yap S, Naughten E, Wilcken B, Wilcken D
and Boers GH: Vascular complications of severe hyperhomocysteinemia
in patients with homocystinuria due to cystathionine β-synthase
deficiency: Effects of homocysteine-lowering therapy. Semin Thromb
Hemost. 2000:335–340. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kalil MAB, Donis KC, de Oliveira Poswar F,
Dos Santos BB, Santos ÂBS and Schwartz IVD: Cardiovascular findings
in classic homocystinuria. Mol Genet Metab Rep.
25:1006932020.PubMed/NCBI
|
|
13
|
Rahman M, Sharma M, Aggarwal P, Singla S
and Jain N: Homocystinuria and ocular complications-A review.
Indian J Ophthalmol. 70:2272–2278. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weber DR, Coughlin C, Brodsky JL,
Lindstrom K, Ficicioglu C, Kaplan P, Freehauf CL and Levine MA: Low
bone mineral density is a common finding in patients with
homocystinuria. Mol Genet Metab. 117:351–354. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cordaro M, Siracusa R, Fusco R, Cuzzocrea
S, Di Paola R and Impellizzeri DJM: Involvements of
hyperhomocysteinemia in neurological disorders. Metabolites.
11:372021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yap S and Naughten E: Homocystinuria due
to cystathionine β-synthase deficiency in Ireland: 25 years'
experience of a newborn screened and treated population with
reference to clinical outcome and biochemical control. J Inherit
Metab Dis. 21:738–747. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gerrard A and Dawson C: Homocystinuria
diagnosis, management: It is not all classical. J Clin Pathol.
75:744–750. 2022. View Article : Google Scholar
|
|
18
|
Kožich V, Ditrói T, Sokolová J, Křížková
M, Krijt J, Ješina P and Nagy P: Metabolism of sulfur compounds in
homocystinurias. Br J Pharmacol. 176:594–606. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Horowitz JH, Rypins EB, Henderson JM,
Heymsfield SB, Moffitt SD, Bain RP, Chawla RK, Bleier JC and Rudman
D: Evidence for impairment of transsulfuration pathway in
cirrhosis. Gastroenterology. 81:668–675. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sacharow SJ, Picker JD and Levy HL:
Homocystinuria caused by cystathionine beta-synthase deficiency.
2017.
|
|
21
|
Richard E, Desviat LR, Ugarte M and Pérez
B: Oxidative stress and apoptosis in homocystinuria patients with
genetic remethylation defects. J Cell Biochem. 114:183–191. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Obeid R: The metabolic burden of methyl
donor deficiency with focus on the betaine homocysteine
methyltransferase pathway. Nutrients. 5:3481–3495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Münke M, Kraus JP, Ohura T and Francke U:
The gene for cystathionine beta-synthase (CBS) maps to the
subtelomeric region on human chromosome 21q and to proximal mouse
chromosome 17. Am J Hum Genet. 42:5501988.PubMed/NCBI
|
|
24
|
Liu X, Liu X, Liu J, Guo J, Nie D and Wang
J: Identification and functional analysis of cystathionine
beta-synthase gene mutations in chinese families with classical
homocystinuria. Biomedicines. 13:9192025. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Poloni S, Sperb-Ludwig F, Borsatto T, Hoss
GW, Doriqui MJR, Embiruçu EK, Boa-Sorte N, Marques C, Kim CA, de
Souza CFM, et al: CBS mutations are good predictors for
B6-responsiveness: A study based on the analysis of 35 Brazilian
Classical Homocystinuria patients. Mol Genet Genomic Med.
6:160–170. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li DX, Li XY, Dong H, Liu YP, Ding Y, Song
JQ, Jin Y, Zhang Y, Wang Q and Yang YL: Eight novel mutations of
CBS gene in nine Chinese patients with classical homocystinuria.
World J Pediatr. 14:197–203. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sibani S, Christensen B, O'Ferrall E,
Saadi I, Hiou-Tim F, Rosenblatt DS and Rozen R: Characterization of
six novel mutations in the methylenetetrahydrofolate reductase
(MTHFR) gene in patients with homocystinuria. Hum Mutat.
15:280–287. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li WX, Dai SX, Zheng JJ, Liu JQ and Huang
JFJN: Homocysteine metabolism gene polymorphisms (MTHFR C677T,
MTHFR A1298C, MTR A2756G and MTRR A66G) jointly elevate the risk of
folate deficiency. Nutrients. 7:6670–6687. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li WX, Cheng F, Zhang AJ, Dai SX, Li GH,
Lv WW, Zhou T, Zhang Q, Zhang H, Zhang T, et al: Folate deficiency
and gene polymorphisms of MTHFR, MTR and MTRR elevate the
hyperhomocysteinemia risk. Clin Lab. 63:523–533. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zaric BL, Obradovic M, Bajic V, Haidara
MA, Jovanovic M and Isenovic ER: Homocysteine and
hyperhomocysteinaemia. Curr Med Chem. 26:2948–2961. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sellos-Moura M, Glavin F, Lapidus D, Evans
KA, Palmer L and Irwin DE: Estimated prevalence of moderate to
severely elevated total homocysteine levels in the United States: A
missed opportunity for diagnosis of homocystinuria? Mol Genet
Metab. 130:36–40. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Levy HL, Mudd SH, Schulman JD, Dreyfus PM
and Abeles RH: A derangement in B12 metabolism associated with
homocystinemia, cystathioninemia, hypomethioninemia and
methylmalonic aciduria. Am J Med. 48:390–397. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Carey MC, Fennelly JJ and FitzGerald O:
Homocystinuria: II. Subnormal serum folate levels, increased folate
clearance and effects of folic acid therapy. Am J Med. 45:26–31.
1968. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bartholomew DW, Batshaw ML, Allen RH, Roe
CR, Rosenblatt D, Valle DL and Francomano CA: Therapeutic
approaches to cobalamin-C methylmalonic acidemia and
homocystinuria. J Pediatr. 112:32–39. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kraus JP: Molecular basis of phenotype
expression in homocystinuria. J Inherit Metab Dis. 17:383–390.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tsai MY, Garg U, Key NS, Hanson NQ, Suh A
and Schwichtenberg K: Molecular and biochemical approaches in the
identification of heterozygotes for homocystinuria.
Atherosclerosis. 122:69–77. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mohamed AS, AlAnzi T, Alhashem A, Alrukban
H, Al Harbi F and Mohamed S: Clinicalbiochemical molecular
characteristics of classic homocystinuria in Saudi Arabia, the
impact of newborn screening on prevention of the complications: A
tertiary center experience. JIMD Rep. 66:e124542025.PubMed/NCBI
|
|
38
|
Alcaide P, Krijt J, Ruiz-Sala P, Ješina P,
Ugarte M, Kožich V and Merinero B: Enzymatic diagnosis of
homocystinuria by determination of cystathionine-ß-synthase
activity in plasma using LC-MS/MS. Clin Chim Acta. 438:261–265.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vyletal P, Sokolová J, Cooper DN, Kraus
JP, Krawczak M, Pepe G, Rickards O, Koch HG, Linnebank M,
Kluijtmans LA, et al: Diversity of cystathionine β-synthase
haplotypes bearing the most common homocystinuria mutation c.
833T> C: A possible role for gene conversion. Hum Mutat.
28:255–264. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kruger WD, Wang L, Jhee KH, Singh RH and
Elsas II LJ: Cystathionine β-synthase deficiency in Georgia (USA):
Correlation of clinical and biochemical phenotype with genotype.
Hum Mutat. 22:434–441. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Linnebank M, Janosik M, Kozich V, Pronicka
E, Kubalska J, Sokolova J, Linnebank A, Schmidt E, Leyendecker C,
Klockgether T, et al: The cystathionine β-synthase (CBS) mutation
c.1224-2A>C in Central Europe: Vitamin B6 nonresponsiveness and
a common ancestral haplotype. Hum Mutat. 24:352–353. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Al-Sadeq DW, Thanassoulas A, Islam Z,
Kolatkar P, Al-Dewik N, Safieh-Garabedian B, Nasrallah GK and
Nomikos M: Pyridoxine non-responsive p. R336C mutation alters the
molecular properties of cystathionine beta-synthase leading to
severe homocystinuria phenotype. Biochim Biophys Acta Gen Subj.
1866:1301482022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gdynia N, Gratzer A and Gdynia HJ:
Polyneuropathy due to vitamin B6 hypervitaminosis: A case series
and call for more education. J Neurosci Rural Pract. 16:99–103.
2025. View Article : Google Scholar
|
|
44
|
Liu Y, Guo J, Cheng H, Wang J, Tan Y,
Zhang J, Tao H, Liu H, Xiao J, Qu D, et al: Methionine restriction
diets: Unravelling biological mechanisms and enhancing brain
health. Trends Food Sci Technol. 149:1045322024. View Article : Google Scholar
|
|
45
|
Pokrzywinski R, Bartke D, Clucas C,
Machuzak K and Pinto L: ‘My dream is to not have to be on a diet’:
A qualitative study on burdens of classical homocystinuria (HCU)
from the patient perspective. Orphanet J Rare Dis. 20:1–11. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Skvorak K, Mitchell V, Teadt L, Franklin
KA, Lee HO, Kruse N, Huitt-Roehl C, Hang J, Du F, Galanie S, et al:
An orally administered enzyme therapeutic for homocystinuria that
suppresses homocysteine by metabolizing methionine in the
gastrointestinal tract. Mol Genet Metab. 139:1076532023. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Perreault M, Means J, Gerson E, James M,
Cotton S, Bergeron CG, Simon M, Carlin DA, Schmidt N, Moore TC, et
al: The live biotherapeutic SYNB1353 decreases plasma methionine
via directed degradation in animal models and healthy volunteers.
Cell Host Microbe. 32:382–395. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Truitt C, Hoff WD and Deole R: Health
functionalities of betaine in patients with homocystinuria. Front
Nutr. 8:6903592021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Singh RH, Kruger WD, Wang L, Pasquali M
and Elsas LJ II: Cystathionine β-synthase deficiency: Effects of
betaine supplementation after methionine restriction in
B6-nonresponsive homocystinuria. Genet Med. 6:90–95. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Schwab U, Törrönen A, Toppinen L, Alfthan
G, Saarinen M, Aro A and Uusitupa M: Betaine supplementation
decreases plasma homocysteine concentrations but does not affect
body weight, body composition, or resting energy expenditure in
human subjects. Am J Clin Nutr. 76:961–967. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lu XT, Wang YN, Mo QW, Huang BX, Wang YF,
Huang ZH, Luo Y, Maierhaba W, He TT, Li SY, et al: Effects of
low-dose B vitamins plus betaine supplementation on lowering
homocysteine concentrations among Chinese adults with
hyperhomocysteinemia: A randomized, double-blind, controlled
preliminary clinical trial. Eur J Nutr. 62:1599–1610. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Imbard A, Toumazi A, Magréault S,
Garcia-Segarra N, Schlemmer D, Kaguelidou F, Perronneau I, Haignere
J, de Baulny HO, Kuster A, et al: Efficacy pharmacokinetics of
betaine in CBS, cblC deficiencies: A cross-over randomized
controlled trial. Orphanet J Rare Dis. 17:4172022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zawieja EE, Bogna Z and Chmurzynska A:
Betaine supplementation moderately increases total cholesterol
levels: A systematic review and meta-analysis. J Diet Suppl.
18:105–117. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Marroncini G, Martinelli S, Menchetti S,
Bombardiere F and Martelli FS: Hyperhomocysteinemia and disease-is
10 µmol/l a suitable new threshold limit? Int J Mol Sci.
25:122952024. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
McCaddon A and Miller JW: Homocysteine-a
retrospective and prospective appraisal. Front Nutr.
10:11798072023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kok DEG, Dhonukshe-Rutten RAM, Lute C,
Heil SG, Uitterlinden AG, van der Velde N, van Meurs JB, van Schoor
NM, Hooiveld GJ, de Groot LC, et al: The effects of long-term daily
folic acid and vitamin B12 supplementation on genome-wide DNA
methylation in elderly subjects. Clin Epigenetics. 7:1212015.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Meier M, Janosik M, Kery V, Kraus JP and
Burkhard P: Structure of human cystathionine beta-synthase: A
unique pyridoxal 5′-phosphate-dependent heme protein. EMBO J.
20:3910–3916. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bublil EM and Majtan T: Classical
homocystinuria: From cystathionine beta-synthase deficiency to
novel enzyme therapies. Biochimie. 173:48–56. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bublil EM, Majtan T, Park I, Carrillo RS,
Hůlková H, Krijt J, Kožich V and Kraus JP: Enzyme replacement with
PEGylated cystathionine β-synthase ameliorates homocystinuria in
murine model. J Clin Invest. 126:2372–2384. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ficicioglu C, Ganesh J, Smith W, Lah M,
Kudrow D, Güner J, McDermott S, Vaidya S, Wilkening L, Thomas J and
Levy H: P012: Pegtibatinase, an investigational enzyme replacement
therapy for the treatment of classical homocystinuria: Latest
findings from the COMPOSE phase 1/2 trial. Genetics in Medicine
Open. 2:1008892024. View Article : Google Scholar
|
|
61
|
Majtan T, Jones W Jr, Krijt J, Park I,
Kruger WD, Kožich V, Bassnett S, Bublil EM and Kraus JP: Enzyme
replacement therapy ameliorates multiple symptoms of murine
homocystinuria. Mol Ther. 26:834–844. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Cramer SL, Saha A, Liu J, Tadi S, Tiziani
S, Yan W, Triplett K, Lamb C, Alters SE, Rowlinson S, et al:
Systemic depletion of L-cyst (e) ine with cyst (e) inase increases
reactive oxygen species and suppresses tumor growth. Nat Med.
23:120–127. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Majtan T, Kožich V and Kruger WD: Recent
therapeutic approaches to cystathionine beta-synthase-deficient
homocystinuria. Br J Pharmacol. 180:264–278. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
A Phase 1/2 Multiple Ascending-Dose Study
in Subjects With Homocystinuria Due to Cystathionine β-Synthase
(CBS) Deficiency to Investigate the Safety, Pharmacokinetics, and
Pharmacodynamics of ACN00177. https://clinicaltrials.gov/study/NCT05154890August
11–2025
|
|
65
|
Aeglea BioTherapeutics: Aeglea
BioTherapeutics Announces Interim Results from Ongoing Phase 1/2
Clinical Trial of Pegtarviliase for the Treatment of Classical
Homocystinuria and Begins Process to Explore Strategic
Alternatives. https://ir.aeglea.com/press-releases/news-details/2023/Aeglea-BioTherapeutics-Announces-Interim-Results-from-Ongoing-Phase-12-Clinical-Trial-of-Pegtarviliase-for-the-Treatment-of-Classical-Homocystinuria-and-Begins-Process-to-Explore-Strategic-Alternatives/default.aspxAugust
11–2025
|
|
66
|
Wang L, Chen X, Tang B, Hua X,
Klein-Szanto A and Kruger WD: Expression of mutant human
cystathionine β-synthase rescues neonatal lethality but not
homocystinuria in a mouse model. Hum Mol Genet. 14:2201–2208. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Syntis Bio, . Syntis Bio Launches to
Revolutionize Oral Therapy for Obesity, Diabetes and Rare Diseases
by Unlocking the Full Therapeutic Potential of the Small Intestine.
https://syntis.bio/syntis-bio-launches-to-revolutionize-oral-therapy-for-obesity-diabetes-and-rare-diseases-by-unlocking-the-full-therapeutic-potential-of-the-small-intestine/August
11–2025
|
|
68
|
Lee HO, Salami CO, Sondhi D, Kaminsky SM,
Crystal RG and Kruger WD: Long-term functional correction of
cystathionine β-synthase deficiency in mice by adeno-associated
viral gene therapy. J Inherit Metab Dis. 44:1382–1392. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Calcedo R, Vandenberghe LH, Gao G, Lin J
and Wilson JM: Worldwide epidemiology of neutralizing antibodies to
adeno-associated viruses. J Infect Dis. 199:381–390. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
George LA, Sullivan SK, Giermasz A, Rasko
JEJ, Samelson-Jones BJ, Ducore J, Cuker A, Sullivan LM, Majumdar S,
Teitel J, et al: Hemophilia B gene therapy with a
high-specific-activity factor IX variant. N Engl J Med.
377:2215–2227. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Chandler RJ, LaFave MC, Varshney GK,
Trivedi NS, Carrillo-Carrasco N, Senac JS, Wu W, Hoffmann V,
Elkahloun AG, Burgess SM and Venditti CP: Vector design influences
hepatic genotoxicity after adeno-associated virus gene therapy. J
Clin Invest. 125:870–880. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lee HO, Gallego-Villar L, Grisch-Chan HM,
Häberle J, Thöny B and Kruger WD: Treatment of cystathionine
β-synthase deficiency in mice using a minicircle-based naked DNA
vector. Hum Gene Ther. 30:1093–1100. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Collard R and Majtan T: Genetic and
pharmacological modulation of cellular proteostasis leads to
partial functional rescue of homocystinuria-causing
cystathionine-beta synthase variants. Mol Cell Biol. 43:664–674.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Majtan T, Pey AL, Gimenez-Mascarell P,
Martínez-Cruz LA, Szabo C, Kožich V and Kraus JP: Potential
pharmacological chaperones for cystathionine
beta-synthase-deficient homocystinuria. Handb Exp Pharmacol.
245:345–383. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kopecká J, Krijt J, Raková K and Kožich V:
Restoring assembly and activity of cystathionine β-synthase mutants
by ligands and chemical chaperones. J Inherit Metab Dis. 34:39–48.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kluijtmans LA, Boers GH, Stevens EM,
Renier WO, Kraus JP, Trijbels FJ, van den Heuvel LP and Blom HJ:
Defective cystathionine beta-synthase regulation by
S-adenosylmethionine in a partially pyridoxine responsive
homocystinuria patient. J Clin Invest. 98:285–289. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Mendes MI, Colaço HG, Smith DE, Ramos RJ,
Pop A, van Dooren SJ, de Almeida IT, Kluijtmans LA, Janssen MC,
Rivera I, et al: Reduced response of Cystathionine Beta-Synthase
(CBS) to S-Adenosylmethionine (SAM): Identification and functional
analysis of CBS gene mutations in Homocystinuria patients. J
Inherit Metab Dis. 37:245–254. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Melenovská P, Kopecká J, Krijt J, Hnízda
A, Raková K, Janošík M, Wilcken B and Kožich V: Chaperone therapy
for homocystinuria: The rescue of CBS mutations by heme arginate. J
Inherit Metab Dis. 38:287–294. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kim D and Li GC: Proteasome inhibitors
lactacystin and MG132 inhibit the dephosphorylation of HSF1 after
heat shock and suppress thermal induction of heat shock gene
expression. Biochem Biophys Res Commun. 264:352–358. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Gupta S, Wang L, Anderl J, Slifker MJ,
Kirk C and Kruger WD: Correction of cystathionine β-synthase
deficiency in mice by treatment with proteasome inhibitors. Hum
Mutat. 34:1085–1093. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Gupta S, Wang L and Kruger WD: The c.797
G>A (p.R266K) cystathionine β-synthase mutation causes
homocystinuria by affecting protein stability. Hum Mutat.
38:863–869. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Gupta S, Lee HO, Wang L and Kruger WD:
Examination of two different proteasome inhibitors in reactivating
mutant human cystathionine β-synthase in mice. PLoS One.
18:e02865502023. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Isabella VM, Ha BN, Castillo MJ, Lubkowicz
DJ, Rowe SE, Millet YA, Anderson CL, Li N, Fisher AB, West KA, et
al: Development of a synthetic live bacterial therapeutic for the
human metabolic disease phenylketonuria. Nat Biotechnol.
36:857–864. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Puurunen MK, Vockley J, Searle SL,
Sacharow SJ, Phillips JA III, Denney WS, Goodlett BD, Wagner DA,
Blankstein L, Castillo MJ, et al: Safety pharmacodynamics of an
engineered E. coli Nissle for the treatment of phenylketonuria: A
first-in-human phase 1/2a study. Nat Metab. 3:1125–1132. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Lin NC, Niu DM, Loong CC, Hsia CY, Tsai
HL, Yeh YC, Tsou MY and Liu CS: Liver transplantation for a patient
with homocystinuria. Pediatr Transplant. 16:E311–E314. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Kerkvliet SP, Rheault MN and Berry SA:
Liver transplant as a curative treatment in a pediatric patient
with classic homocystinuria: A case report. Am J Med Genet A.
185:1247–1250. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Li DX, Chen ZH, Jin Y, Song JQ, Li MQ, Liu
YP, Li XY, Chen YX, Zhang YN, Lyu GY, et al: Clinical
characteristics and CBS gene analysis of 13 cases with classic
homocystinuria. Zhonghua Er Ke Za Zhi. 60:533–538. 2022.(In
Chinese). PubMed/NCBI
|