Introduction
Pancreatic cancer is a highly aggressive and
prognostically poor malignancy of the digestive system, which has
been referred to as the ‘king of cancers’ (1) and is steadily increasing in
prevalence worldwide (2,3). Due to its insidious onset and the
lack of reliable early diagnostic and therapeutic strategies, most
patients are diagnosed at an advanced stage, precluding curative
surgical intervention (4).
Consequently, chemotherapy remains the primary treatment modality
for pancreatic cancer. The current recommended adjuvant
chemotherapy regimen for patients with a good performance status is
a combination of fluorouracil, oxaliplatin, irinotecan and
leucovorin, known as modified FOLFIRINOX (5,6). For
patients who exhibit a suboptimal response to gemcitabine- or
capecitabine-based therapy, alternative options include
DNA-damaging agents that interfere with DNA synthesis and repair,
such as oxaliplatin and irinotecan, as well as antimetabolites,
including gemcitabine and fluorouracil (7,8).
However, the safety profiles of these agents and the development of
drug resistance pose major clinical challenges. Therefore, the
identification of effective therapeutic targets is critical for
achieving an early diagnosis and improved prognosis for patients
with pancreatic cancer.
Astragalus polysaccharides and astragalosides are
the primary active constituents of Astragalus membranaceus
(Radix Astragali, Huangqi), among which astragaloside IV (AST-IV;
C41H68O14; molecular weight,
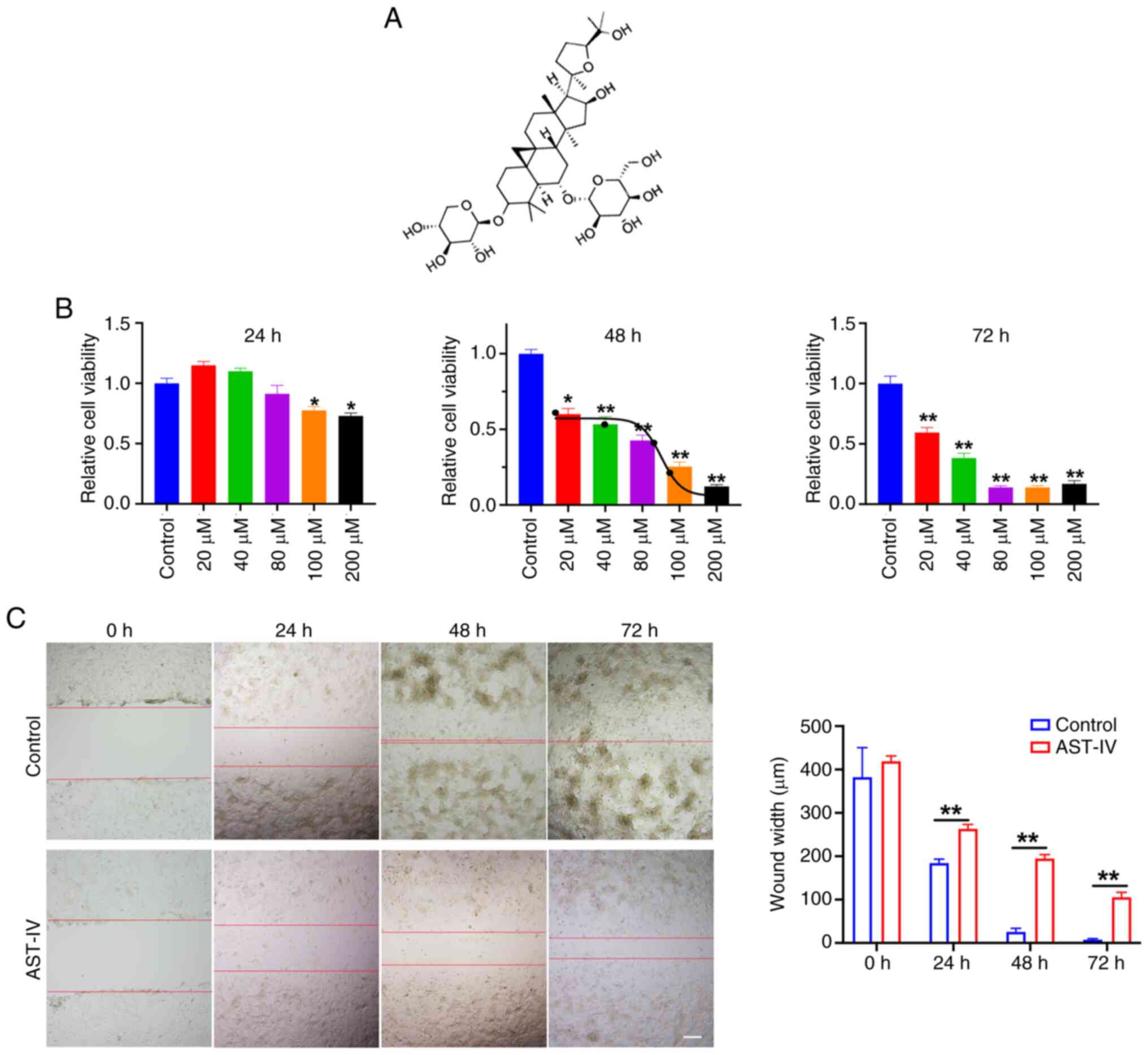
784.97 Da) is the most biologically potent small compound (9). AST-IV is a tetracyclic triterpenoid
saponin with a lanostane-type structure (Fig. 1A), and AST-IV exhibits a broad
spectrum of pharmacological activities. Notably, it displays
anti-inflammatory, antioxidant, antifibrotic, antidiabetic,
immunomodulatory and cardioprotective effects, mediated through the
modulation of diverse signaling pathways (10–12).
AST-IV has also been reported to exert therapeutic effects in
various pathological conditions, including cerebral
ischemia/reperfusion injury, pulmonary diseases, liver cirrhosis,
cardiovascular disorders and diabetic nephropathy (13,14).
Studies have demonstrated the efficacy of AST-IV as
an anticancer agent (13–16). Notably, Xu et al (15) reported that AST-IV effectively
suppresses the proliferation and migration of lung cancer cells,
and attenuates metastasis in vivo. In addition, a review by
Tang et al (16) reported
that AST-IV inhibits colorectal cancer cell proliferation, enhances
immune responses and reduces drug resistance. Although these
findings suggest that AST-IV has promising antitumor properties,
its precise mechanisms of action remain unclear. Therefore, further
investigation is required to elucidate the molecular pathways
underlying these anticancer effects.
In the present study, the ability of AST-IV to
inhibit the proliferation and migration of pancreatic cancer cells
was evaluated, and RNA-sequencing (RNA-seq) analysis was performed
to elucidate its mechanism of action. Furthermore, in vitro
analyses were performed to evaluate the effect of AST-IV on protein
kinase R-like endoplasmic reticulum kinase (PERK) signaling pathway
activation, and the expression of activating transcription factor 4
(ATF4) and apoptosis-related proteins, including
CCAAT/enhancer-binding protein homologous protein (CHOP). The
overall aim of the study was to explore the potential of AST-IV as
a candidate for pancreatic cancer treatment.
Materials and methods
Cell culture
The PANC-1 (cat. no. SCSP-535) human pancreatic
cancer cell line was obtained from The Cell Bank of Type Culture
Collection of The Chinese Academy of Sciences. Cells were cultured
in Dulbecco's Modified Eagle's Medium supplemented with 10% fetal
bovine serum and 1% penicillin/streptomycin (all Gibco; Thermo
Fisher Scientific, Inc.), and maintained under sterile conditions
at 37°C in a humidified incubator with a 5% CO2
atmosphere.
Drug
AST-IV (purity >99%; cat no. HY-N0431) was
obtained from MedChemExpress (MCE). The compound was dissolved in
dimethyl sulfoxide to prepare stock solutions of varying
concentrations, which were subsequently diluted in culture medium
to the desired working concentrations to treat the cells.
Cell viability assay
PANC-1 cells were detached using 0.25% trypsin
(Gibco; Thermo Fisher Scientific, Inc.) and resuspended at a
density of 1×104 cells/ml. The cell suspension (100
µl/well) was seeded into 96-well plates, and the cells were allowed
to adhere for 6 h under standard culture conditions. Following
attachment, the cells were treated with AST-IV at concentrations of
0, 20, 40, 80, 100 and 200 µM for 24, 48 or 72 h, at 37°C. Cell
viability was then assessed using a Cell Counting Kit-8 (CCK-8;
cat. no. HY-K0301; MCE). In brief, 10 µl diluted CCK-8 working
solution was added to each well, and after gentle agitation, the
plates were incubated at 37°C for 30 min. The absorbance at 450 nm
was measured using a microplate reader (Multiskan FC; Thermo Fisher
Scientific, Inc.), and the cell viability was calculated
accordingly.
Wound healing assay
The migratory capacity of PANC-1 cells was assessed
using a wound healing assay. The cells were seeded in 12-well
plates at a density of 1×105 cells/well and cultured to
90–100% confluency. Following aspiration of the culture medium,
wounds were created by scraping the cell monolayer with a sterile
pipette tip in a perpendicular direction, after which dislodged
cells were removed by gentle washing with PBS. The cells were then
treated with serum-free medium containing 80 µM AST-IV. The width
of the wound was documented at 0 h (baseline), and wound closure
was evaluated at 24, 48 and 72 h post-treatment under a light
microscope (Eclipse Ci-S; Nikon Corporation) using Image J software
(v1.8.0, National Institutes of Health),
RNA-seq analysis
PANC-1 cells were seeded at a density of
1×106 cells and cultured as aforementioned, followed by
treatment with 80 µM AST-IV or an equivalent volume of DMSO for 48
h at 37°C. Total RNA was extracted using TRIzol® Reagent
(cat. no. 15596026CN, Invitrogen; Thermo Fisher Scientific. Inc.),
and the concentration, quality and integrity of the RNA were
quantified using a NanoDrop spectrophotometer (Thermo Fisher
Scientific, Inc.). A total of 3 µg RNA per sample was used as an
input for library preparation. Illumina Paired End Sample Prep kits
(cat. no. 67700, Illumina, Inc.) were used to prepare libraries.
Remaining overhangs were converted into blunt ends via
exonuclease/polymerase activities and the enzymes were removed.
After adenylation of the 3′ ends of the DNA fragments, Illumina PE
adapter oligonucleotides were ligated to prepare for hybridization.
To select cDNA fragments of the preferred 400–500 bp in length, the
library fragments were purified using the AMPure XP system (Beckman
Coulter). Each cDNA library was sequenced using an Illumina Hiseq
4000 (cat. no. PE150; Illumina, Inc.). Sequencing analysis was
performed by Shanghai Personal Biotechnology Co. Ltd. The raw data
were processed and analyzed using the Personalbio GenesCloud online
platform (genescloud.cn).
Read counts for each gene were quantified using
HTSeq (v0.9.1, htseq.readthedocs.io/en/release_0.9.1/) to obtain
the raw expression values. Gene expression levels were then
normalized to fragments per kilobase of transcript per million
fragments. Differential gene expression analysis was performed
using DESeq2 (v1.38.3, bioconductor.org) with screening thresholds
set at |log2FoldChange| >1 and P<0.05.
Bi-directional clustering analysis of all differentially expressed
genes (DEGs) was conducted using the ComplexHeatmap (v2.16.0,
http://www.bioconductor.org) software
package. Clustering was based on the Euclidean distance and the
complete linkage method, and heatmaps were generated to visualize
gene expression patterns across samples.
Gene Ontology (GO) enrichment analysis was performed
to identify the biological functions associated with the DEGs. The
number of DEGs enriched in each term was calculated, and GO
enrichment analysis was performed on the upregulated and
downregulated DEGs using ClusterProfiler (v4.6.0). P-values were
calculated using the hypergeometric distribution method, with a
threshold of P<0.05. Significantly enriched GO terms were used
to determine the main biological functions, molecular functions and
cellular components associated with the DEGs. Similarly, Kyoto
Encyclopedia of Genes and Genomes (KEGG) pathway enrichment
analysis of the DEGs was performed using ClusterProfiler (v4.6.0)
software, with a threshold of P<0.05. Detailed results of these
analyses are presented in Table
SI. In addition, the RNA-seq data generated in the present
study are all publicly available at the Sequence Read Archive (SRA)
under accession number PRJNA1312337
(ncbi.nlm.nih.gov/sra/PRJNA1312337).
Western blot analysis
Western blotting was performed as previously
described (17), with brief
modifications. Cells were lysed in whole-cell lysis buffer (cat.
no. P0013; Beyotime Institute of Biotechnology) and collected by
scraping. The lysates were pelleted by centrifugation at 10,000 × g
for 10 min at room temperature, and protein concentrations were
determined using a BCA protein assay kit (cat. no. P0012; Beyotime
Institute of Biotechnology). Equal amounts of protein (25 µg/lane)
were separated by 10% SDS-polyacrylamide gel electrophoresis and
transferred onto polyvinylidene difluoride membranes
(MilliporeSigma). Membranes were blocked with 10% skimmed milk for
1 h at room temperature, then incubated overnight at 4°C with the
following primary antibodies (1:1,000 dilution): 78-kDa
glucose-regulated protein (GRP78; cat. no. 80849-1-RR), PERK (cat.
no. 20582-1-AP), phosphorylated (p-)PERK (cat. no. 82534-1-RR),
ATF4 (cat. no. 81798-1-RR), CHOP (cat. no. 81462-1-RR) and Bax
(cat. no. 50599-2-Ig) from Proteintech Group, Inc., and eukaryotic
translation initiation factor 2 a (eIF2α; cat. no. 9722), p-eIF2α
(cat. no. 3398) and Bcl-2 (cat. no. 15071) from CST Biological
Reagents Co., Ltd. After washing, the membranes were incubated with
horseradish peroxidase-conjugated secondary antibodies
(anti-rabbit; cat. no. SA00001-2) or anti-mouse (cat. no.
SA00001-1) IgG; 1:5,000 dilution) for 30–60 min at room
temperature. Protein bands were visualized using an enhanced
chemiluminescence detection system (Amersham; Cytiva) and
quantitatively analyzed using ImageJ software (v1.8.0, National
Institutes of Health).
Statistical analysis
All data are presented as the mean ± standard error
of the mean. Statistical comparisons were performed using Student's
t-test or one-way analysis of variance followed by post-hoc
analysis with Tukey's test. P<0.05 was considered to indicate a
statistically significant result. All statistical analyses were
performed using GraphPad Prism 8 (Dotmatics).
Results
AST-IV inhibits the proliferation and
migration ability of PANC-1 pancreatic cancer cells
The potential effect of AST-IV on PANC-1 pancreatic
cell viability was evaluated through comprehensive concentration-
and time-dependent CCK-8 analyses. Only high concentrations of
AST-IV (100 and 200 µM) significantly reduced PANC-1 cell viability
after a 24-h treatment (Fig. 1B).
With extended exposure, all tested concentrations (20–200 µM)
significantly reduced cell viability at 48 and 72 h, with an
inhibitory trend apparent over time (Fig. 1B). Growth curve analysis determined
that the half-maximal inhibitory concentration of AST-IV at 48 h
was ~80 µM, which was selected as the standard treatment
concentration for 48-h exposure in subsequent experiments. Wound
healing assays demonstrated that 80 µM AST-IV significantly
suppressed PANC-1 cell migration at all assessed timepoints (24, 48
and 72 h; Fig. 1C). Collectively,
these findings indicate the anticancer potential of AST-IV,
revealing its dual ability to suppress the proliferative and
migratory capacities of pancreatic cancer cells.
RNA-seq and DEG analysis
To elucidate the molecular mechanisms underlying the
effects of AST-IV on pancreatic cancer cells, comprehensive RNA-seq
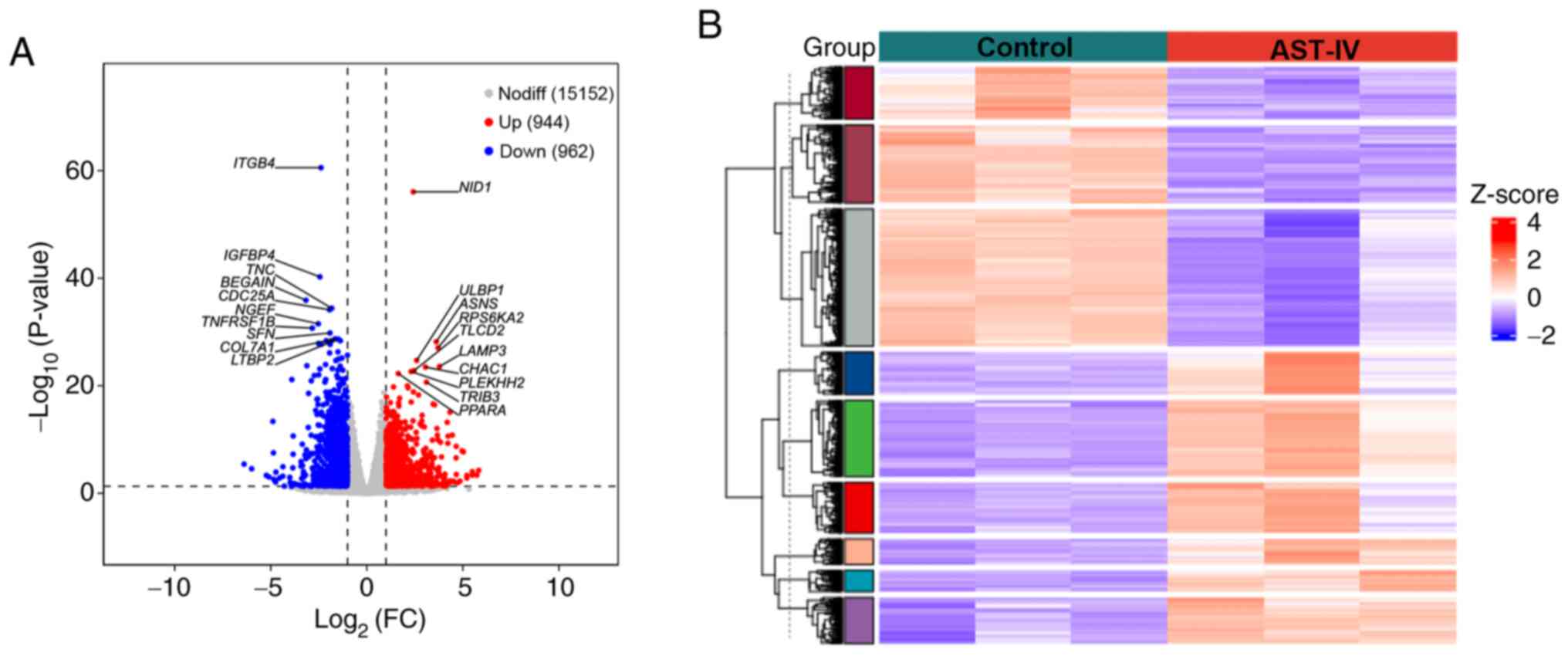
analysis was performed and DEGs were identified. As shown in
Fig. 2A, AST-IV treatment induced
substantial alterations in the transcriptome of the pancreatic
cancer cells, with 1,906 DEGs identified relative to the control
group, of which 944 were significantly upregulated and 962 were
significantly downregulated. Hierarchical clustering analysis
(Fig. 2B) revealed clear
separation between the treatment groups and consistent gene
expression patterns within replicates, confirming the
reproducibility of the data. The expression profiles of all
identified DEGs are provided in Table
SI. This transcriptomic analysis establishes a solid foundation
for subsequent functional investigations of the mechanism of action
of AST-IV in pancreatic cancer cells.
Enrichment analysis of upregulated
DEGs in the AST-IV group
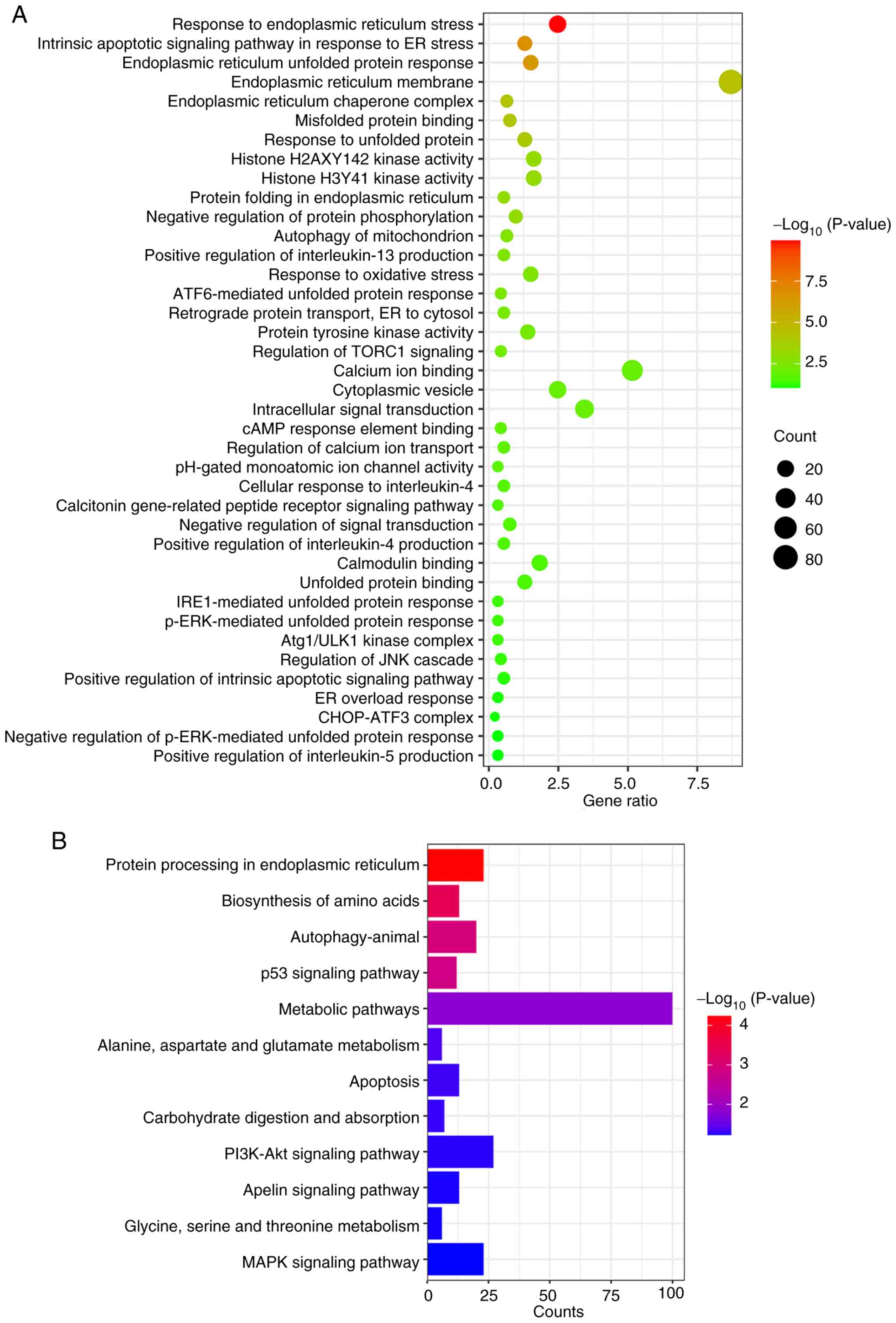
To investigate the mechanistic basis of the effects
of AST-IV on pancreatic cancer cells, a comprehensive functional
enrichment analysis of significantly upregulated DEGs was performed
using GO and KEGG pathway analyses. GO enrichment revealed three
primary functional categories affected by AST-IV treatment: i)
Endoplasmic reticulum (ER) stress response, encompassing processes
including ‘endoplasmic reticulum unfolded protein response’,
‘misfolded protein binding’, ‘response to unfolded protein’,
‘protein folding in endoplasmic reticulum’, ‘ATF6-mediated unfolded
protein response’, ‘retrograde protein transport, ER to cytosol’
and ‘p-ERK-mediated unfolded protein response’; ii) protein
activity regulation, including ‘histone H2AXY142 kinase activity’,
‘protein tyrosine kinase activity’ and ‘Atg1/ULK1 kinase complex’;
and iii) signal transduction processes, including ‘intracellular
signal transduction’, ‘regulation of TORC1 signaling’ and
‘calcitonin gene-related peptide receptor signaling pathway’.
Additional enriched processes included ‘response to oxidative
stress’, ‘positive regulation of interleukin-4 production’ and
‘positive regulation of interleukin-5 production’ (Fig. 3A).
KEGG pathway analysis demonstrated significant
enrichment of upregulated genes in ‘protein processing in
endoplasmic reticulum’, ‘metabolic pathways’ and ‘apoptosis’, with
notable involvement of key signaling pathways including the ‘p53
signaling pathway’, ‘PI3K-Akt signaling pathway’ and ‘MAPK
signaling pathway’ (Fig. 3B).
These systematic analyses collectively suggest that AST-IV may
accelerate pancreatic cancer cell apoptosis via the modulation of
ER stress-related processes and associated signaling cascades.
Enrichment analysis of downregulated
DEGs in the AST-IV group
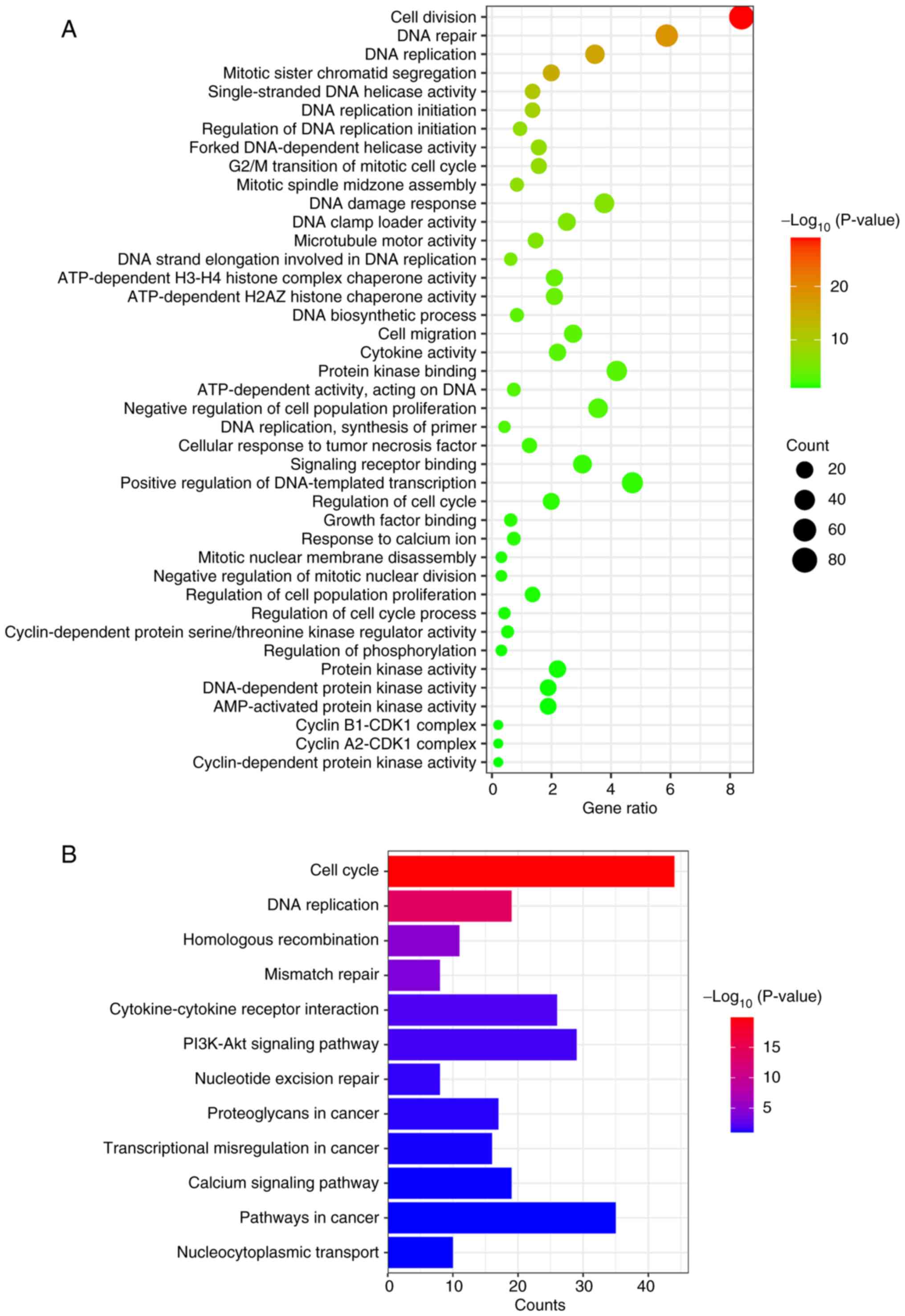
Similarly, comprehensive GO and KEGG enrichment
analyses were conducted for the significantly downregulated genes
following AST-IV treatment. GO analysis revealed two primary
functional categories: i) Cell cycle-related processes, including
‘G2/M transition of mitotic cell cycle’, ‘growth factor binding’,
‘cyclin-dependent protein serine/threonine kinase regulator
activity’, ‘cyclin B1-CDK1 complex formation’, ‘cyclin-dependent
protein kinase activity’ and ‘microtubule motor activity’; and ii)
DNA damage processes, encompassing ‘DNA repair’, ‘DNA replication’,
‘single-stranded DNA helicase activity’, ‘forked DNA-dependent
helicase activity’, ‘DNA clamp loader activity’, ‘DNA strand
elongation involved in DNA replication’ and ‘DNA-dependent protein
kinase activity’. Additional enriched processes included ‘signaling
receptor binding’, ‘AMP-activated protein kinase activity’,
‘cytokine activity’ and ‘protein kinase binding’ (Fig. 4A).
KEGG pathway analysis demonstrated significant
enrichment in cell cycle regulation and DNA damage repair pathways,
along with ‘homologous recombination’, ‘cytokine-cytokine receptor
interaction’, ‘PI3K-Akt signaling pathway’, ‘nucleotide excision
repair’ and ‘nucleocytoplasmic transport’. These systematic
findings collectively indicate that AST-IV may exert its
therapeutic effects against pancreatic cancer through the
coordinated modulation of cell cycle progression and DNA damage
response mechanisms.
Analysis of the mechanism of AST-IV in
the treatment of pancreatic cancer
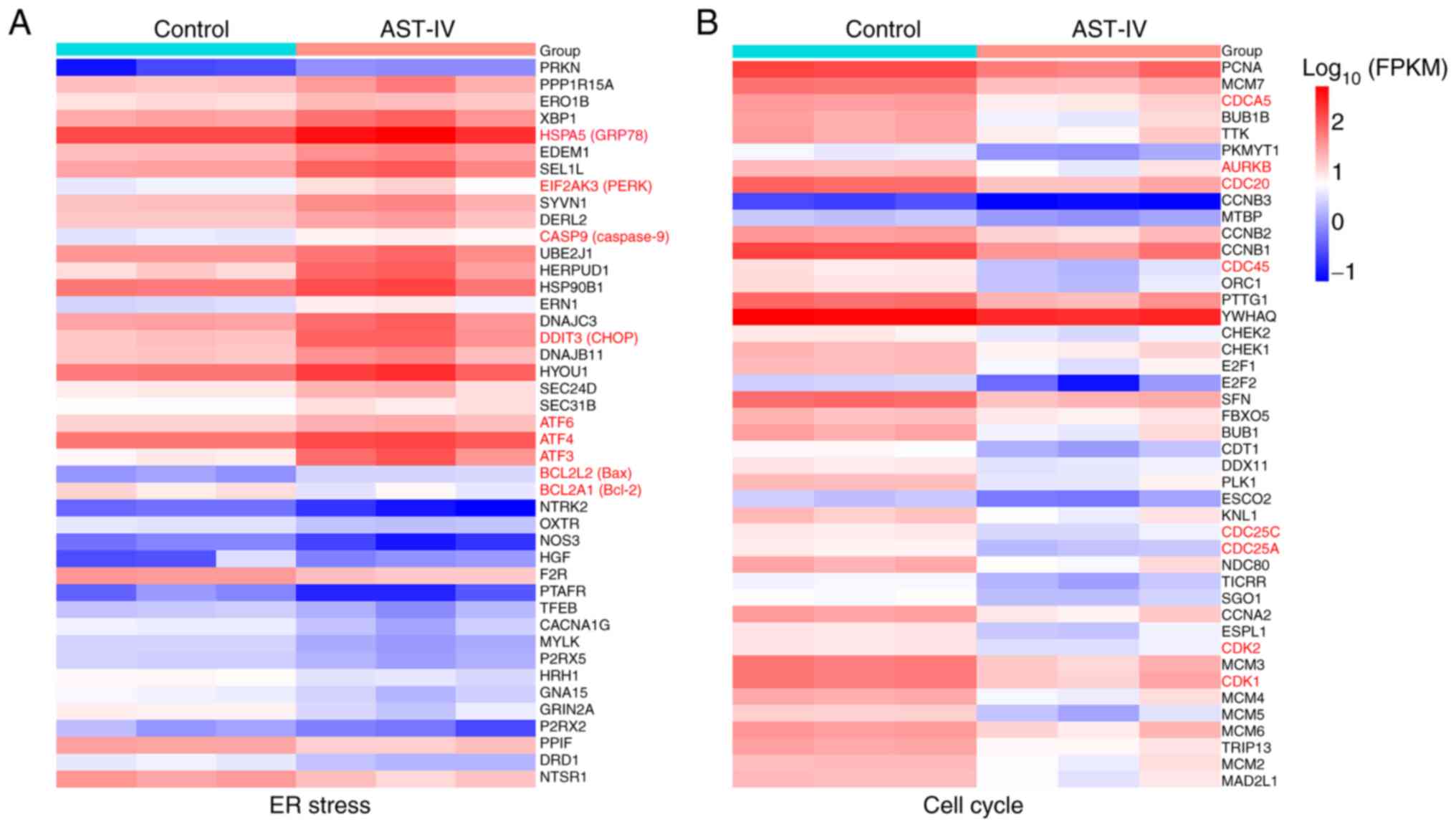
The comprehensive RNA-seq analysis revealed that
AST-IV exerts its antitumor effects in pancreatic cancer through
the coordinated regulation of ER stress and cell cycle pathways
(Fig. 5A). The systematic
evaluation of DEGs identified through GO and KEGG enrichment
analyses demonstrated distinct molecular signatures associated with
these processes. Key ER stress markers were significantly
upregulated (Fig. 5A), including
the molecular chaperone GRP78 [encoded by heat shock protein family
A (Hsp70) member 5; HSPA5], the ER stress sensor PERK
(eukaryotic translation initiation factor 2 a kinase 3;
EIF2AK3), activating transcription factor 4 (ATF4)
and the pro-apoptotic transcription factor CHOP (DNA
damage-inducible transcript 3; DDIT3). This ER stress
response was accompanied by the activation of downstream apoptotic
effectors, including caspase 9 (encoded by CASP9) and Bax,
indicating robust induction of programmed cell death (Fig. 5A).
Concurrently, marked downregulation of critical cell
cycle regulator genes, including cell division cycle associated 5
(CDCA5), CDC20, CDC45, cyclin-dependent kinase 1
(CDK1) and CDK2, was observed, reflecting suppression
of cell cycle progression (Fig.
5B). Collectively, these molecular patterns demonstrate that
AST-IV has a dual mechanism of action, involving the simultaneous
induction of ER stress-mediated apoptosis and suppression of cell
cycle progression in pancreatic cancer cells.
AST-IV activates ER stress via the
PERK signaling pathway to induce apoptosis in pancreatic cancer
cells
Growing evidence indicates that ER stress is a
critical driver of apoptotic cell death (18,19).
Therefore, the ability of AST-IV to activate ER stress and the
apoptotic pathway in pancreatic cancer cells was investigated.
Treatment with AST-IV significantly upregulated the classical ER
stress marker GRP78 (Fig. 6A and
B), and the activation of PERK signaling, by significantly
increasing p-PERK levels, while total PERK expression appeared to
remain stable (Fig. 6A and B).
This activation was propagated through downstream effectors, as
evidenced by the significant elevation of p-eIF2α levels (Fig. 6A and B) and ATF4 expression
(Fig. 6C and D). In addition,
CHOP, recognized as a pivotal pro-apoptotic transcription factor in
ER stress-mediated apoptosis (20), exhibited significantly upregulated
protein expression following AST-IV exposure (Fig. 6C and D). By contrast, the
expression of the anti-apoptotic protein Bcl-2, a key regulator of
cell survival in multiple malignancies (21), was significantly suppressed
(Fig. 6C and D), while that of its
pro-apoptotic counterpart Bax was significantly upregulated
(Fig. 6C and D), indicating
activation of the apoptotic pathway. Collectively, these findings
demonstrate that AST-IV initiates ER stress, activates the PERK
pathway, and promotes apoptosis through the coordinated regulation
of both pro-death and pro-survival factors in pancreatic cancer
cells. The sequential activation from early ER stress to downstream
apoptotic effectors is suggested as a comprehensive mechanism
underlying the cytotoxic activity of AST-IV.
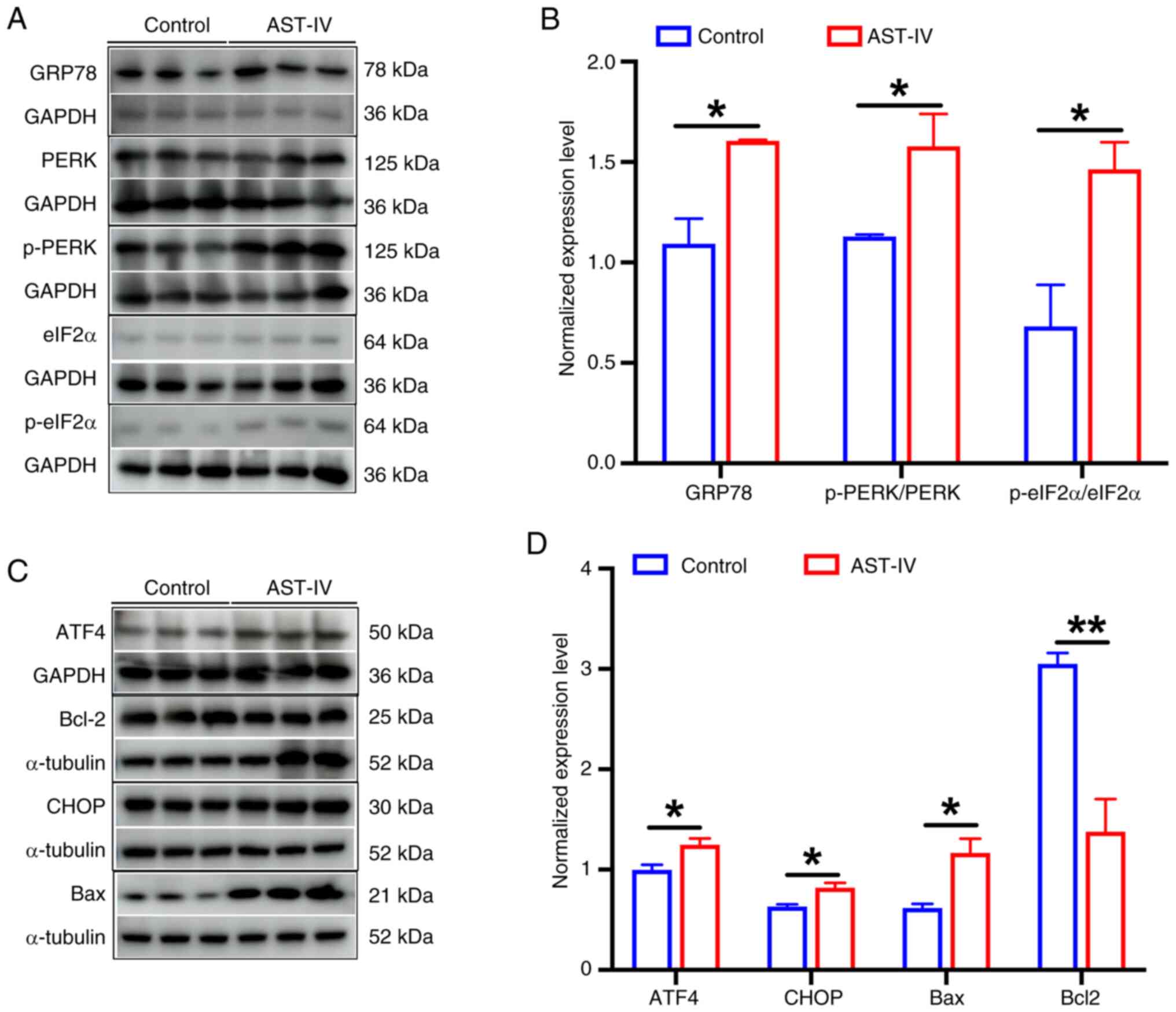 | Figure 6.AST-IV activates the PERK signaling
pathway and induces the apoptosis of pancreatic cancer cells. (A)
Representative western blots of GRP78, PERK, p-PERK, eIF2α and
p-eIF2α and (B) semi-quantitative analysis of the blots. (C)
Representative western blots of ATF4, CHOP, Bcl-2 and Bax and (D)
semi-quantitative analysis of the blots. Data are presented as the
mean ± SEM (n=3). *P<0.05 and **P<0.01 as indicated. AST-IV,
astragaloside IV; eIF2a, eukaryotic translation initiation factor 2
a; ATF4, activating transcription factor 4; CHOP,
CCAAT/enhancer-binding protein homologous protein; GRP78, 78-kDa
glucose-regulated protein; p-, phosphorylated; PERK, protein kinase
R-like endoplasmic reticulum kinase. |
Discussion
Recent epidemiological data from the United States
identify pancreatic cancer as the fourth leading cause of
cancer-related mortality, with projections indicating it may become
the second most lethal malignancy by 2030 (22). The current therapeutic landscape
remains largely centered on gemcitabine as the first-line
chemotherapeutic standard; however, it has limited efficacy and the
development of resistance inevitably occurs, which compromises
long-term treatment outcomes (23,24).
Although the combination of gemcitabine with nab-paclitaxel has
emerged as a clinically meaningful advancement for advanced
disease, it provides only marginal survival benefits, typically
measured in weeks rather than months or years (25,26).
This stark therapeutic gap highlights the critical unmet
requirement for novel treatment strategies that can meaningfully
improve patient outcomes.
In this context, natural product-derived compounds
present a compelling alternative to conventional cytotoxic agents,
offering comparable antitumor efficacy with potentially improved
safety profiles. Among these, AST-IV has emerged as a particularly
promising candidate due to its diverse biological activities and
documented anticancer properties (27,28).
The present aimed to characterize the pharmacological effects of
AST-IV and to deepen the understanding of pancreatic cancer
biology, with the ultimate goal of establishing an innovative
therapeutic approach grounded in rigorous scientific evidence.
The results of the present study demonstrate that
AST-IV effectively inhibits the proliferation and migration of
pancreatic cancer cells, confirming its therapeutic potential
against this malignancy. These findings are consistent with
previous reports showing that AST-IV induces apoptosis via the
mitochondrial-dependent intrinsic pathway and death
receptor-dependent extrinsic pathway in various cancers, including
colorectal cancer, breast cancer, lung cancer, vulvar squamous cell
carcinoma and hepatocellular carcinoma (29–31),
primarily by increasing the Bax/Bcl-2 ratio. Furthermore, Xia et
al (32) reported that AST-IV
exerts anticancer effects on SiHa cervical cancer cells by
modulating autophagy-related proteins, including
microtubule-associated protein 1 light chain 3, ubiquitin-like
modifier-activating enzyme ATG7 and ubiquitin-like protein ATG12.
In addition, AST-IV has been shown to suppress angiogenesis and
reduce migration in HepG2 lung cancer cells by inhibiting the
cyclooxygenase-2/prostaglandin E2/vascular endothelial growth
factor axis (33,34). Distinct from these established
mechanisms, the RNA-seq analysis performed in the current study
revealed that AST-IV activates ER stress and induces apoptosis in
PANC-1 pancreatic cancer cells, a previously unidentified mechanism
underlying the anticancer activity of AST-IV. This novel insight
expands our understanding of the multifaceted therapeutic potential
of AST-IV beyond the established pathways observed in other
malignancies.
The ER represents is a vital organelle that
regulates cellular stress responses and serves as the primary site
for protein synthesis, folding, transport and intracellular calcium
storage (35,36). Both physiological and pathological
stimuli can disrupt calcium homeostasis and alter protein folding,
leading to the accumulation of misfolded proteins within the ER
lumen and subsequent ER stress (37). Upon activation, ER stress signals
are transduced across the ER membrane to the nucleus, initiating a
cascade of molecular events collectively known as the unfolded
protein response (UPR), a complex signaling network that exerts
dual effects by either restoring cellular homeostasis or triggering
apoptosis (37). ER
stress-mediated apoptosis constitutes a distinct mechanism of cell
death that differs from mitochondrial and death receptor-mediated
pathways. This pathway is activated via three principal signaling
branches, namely the PERK, inositol-requiring enzyme 1 (IRE1) and
ATF6 signaling pathways (38).
Under physiological conditions, these transmembrane sensors remain
inactive through their association with the chaperone protein
GRP78. During ER stress, GRP78 dissociates from these sensors to
bind misfolded proteins, thereby allowing the activation of PERK
and IRE1 by autophosphorylation (39). This initiates the UPR cascade and
subsequent regulation of downstream transcription factors,
ultimately leading to ER stress-induced apoptosis.
When cells accumulate excessive unfolded or
misfolded proteins, GRP78 dissociates from PERK to preferentially
bind these aberrant polypeptides (40). This dissociation triggers PERK
activation, which subsequently phosphorylates eIF2α, resulting in a
global attenuation of protein synthesis, serving as a crucial
adaptive mechanism that alleviates ER protein loading and maintains
metabolic homeostasis (41). The
protein growth arrest and DNA damage-inducible protein 34
facilitates the dephosphorylation of eIF2α during ER-stress in
cells (42). Paradoxically,
p-eIF2α enhances the translation of ATF4, which upregulates the
pro-apoptotic factor CHOP (43,44).
Under conditions of persistent or unresolved ER stress, this
PERK/ATF4/CHOP signaling axis becomes fully activated, ultimately
committing the cells to apoptosis (45).
The RNA-seq analysis performed in the present study
revealed the significant upregulation of key ER stress markers,
including the molecular chaperone GRP78 (HSPA5), ER stress
sensor PERK (EIF2AK3), ATF4 and the pro-apoptotic
transcription factor CHOP (DDIT3). These findings were
confirmed at the protein level by western blotting. The AST-IV
treatment significantly upregulated the expression of GRP78 and
activation of PERK in the pancreatic cancer cells, accompanied by a
marked elevation of downstream ATF4 expression, collectively
indicating the induction of sustained ER stress.
CHOP is a pivotal pro-apoptotic transcription factor
in ER stress-mediated apoptosis, which integrates signals from all
three ER stress sensors. While CHOP expression is tightly regulated
at the transcriptional level in normal cells, accumulation of CHOP
protein was observed in PANC-1 cells following AST-IV treatment,
indicating the activation of irreversible apoptotic signaling via
downstream effector regulation.
AST-IV was also observed to trigger the Bax/Bcl-2
pathway. Bax, a water-soluble protein homologous to Bcl-2 and a
pro-apoptotic member of the Bcl-2 gene family, functions as a
promoter of apoptosis (46). By
contrast, Bcl-2 is embedded in the mitochondrial membrane where it
sequesters Bax, thereby inhibiting the initiation of apoptosis. The
overexpression of Bax can counteract the effect of Bcl-2,
predisposing cells to apoptosis (47). The ratio of Bax to Bcl-2 is a
well-established determinant of the balance between pro- and
anti-apoptotic signaling (46,47).
In the present study, AST-IV effectively promoted Bax expression
and concurrently suppressed Bcl-2 expression, providing substantial
evidence that AST-IV induces apoptosis in pancreatic cancer
cells.
Due to technical limitations, although the present
study identified the PERK signaling pathway as a mediator of
AST-IV-induced ER stress, the direct molecular target(s) of AST-IV
remain unclear and require further investigation. Furthermore, as
the development and progression of pancreatic cancer are influenced
by multiple factors within the in vivo microenvironment,
future studies should include animal experiments to facilitate the
drug development progress of AST-IV. Collectively, the data support
the proposed mechanism (Fig. 7),
in which AST-IV initiates persistent ER stress in pancreatic cancer
cells, leading to activation of the PERK/ATF4/CHOP axis and
culminating in caspase-mediated apoptosis. This represents a
comprehensive ER stress-driven pathway distinct from the previously
reported mechanisms of AST-IV in other malignancies.
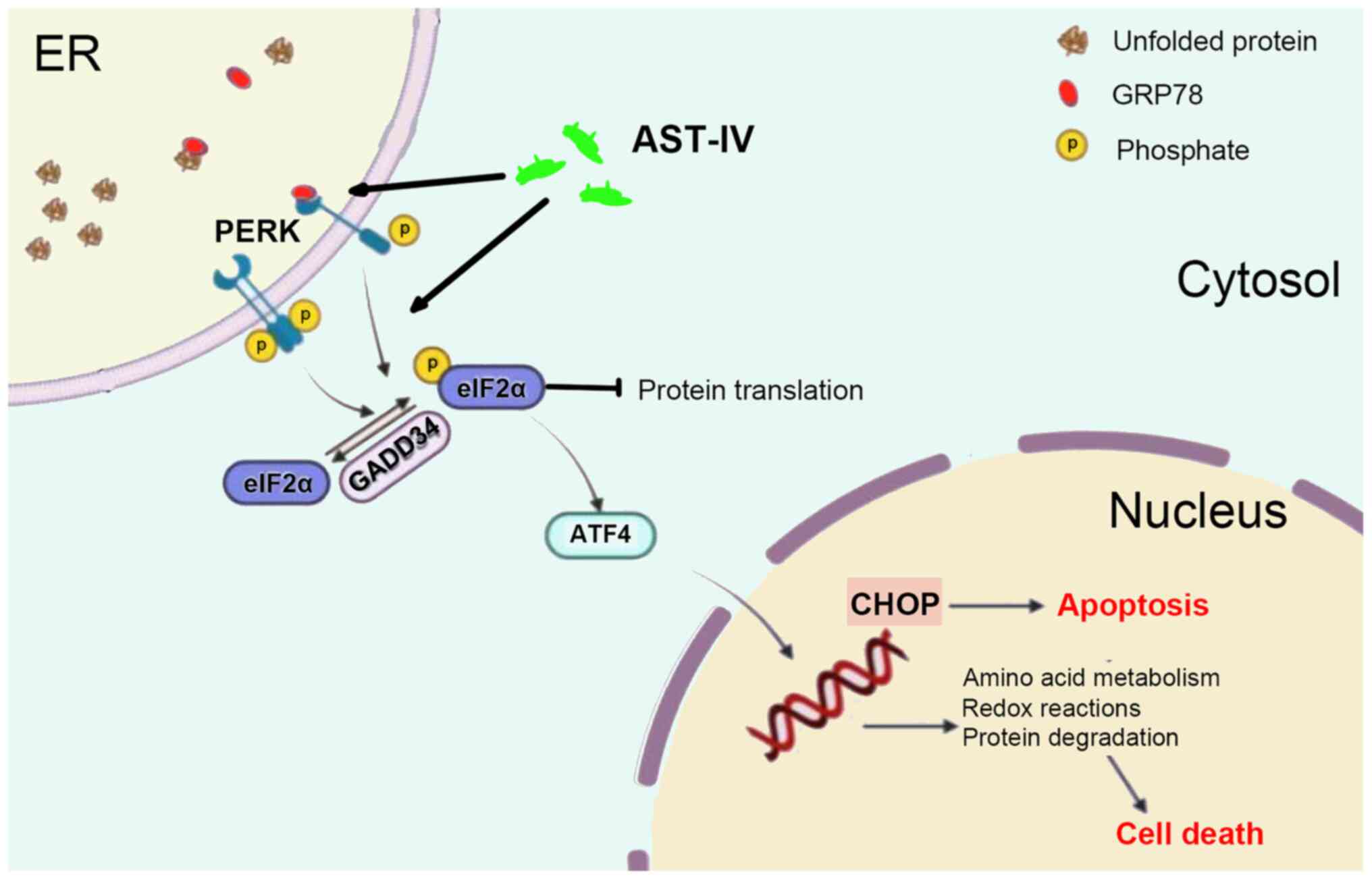 | Figure 7.Schematic model illustrating the
proposed mechanism by which AST-IV promotes apoptosis in pancreatic
cancer cells. AST-IV induces ER stress via activation of the PERK
signaling pathway, leading to upregulation of the downstream
signaling factor ATF4. This subsequently upregulates the expression
of pro-apoptotic proteins CHOP and Bax, while downregulating the
anti-apoptotic protein Bcl-2, thereby promoting the apoptosis of
pancreatic cancer cells. AST-IV, astragaloside IV; ATF, activating
transcription factor; CHOP, CCAAT/enhancer-binding protein
homologous protein; eIF2a, eukaryotic translation initiation factor
2 a; ER, endoplasmic reticulum; GADD34, growth arrest and DNA
damage-inducible protein 34; GRP78, 78-kDa glucose-regulated
protein; PERK, protein kinase R-like endoplasmic reticulum kinase;
SP1/2, site-1/2 protease; UPR, unfolded protein response. |
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Yujie Zhang
(Peking University, Beijing, China) for discussions and critical
reading of the manuscript.
Funding
The present study was supported by the Science and Technology
Research and Development Program Project of Henan Province (grant
no. 192102310382).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author. The RNA-seq data generated
in the present study may be found in the Sequence Read Archive
under accession number PRJNA1312337 or at the following URL:
(ncbi.nlm.nih.gov/sra/PRJNA1312337).
Authors' contributions
YW, MZ, SL, RZ and TG contributed to study design.
YW, MZ and SL and TG analyzed data. MZ, SL and RZ performed
experiments. The first draft of the manuscript was written by YW
and RZ, and TG revise the manuscript. YW, MZ, SL, RZ and TG confirm
the authenticity of all the raw data. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Klein AP: Pancreatic cancer epidemiology:
Understanding the role of lifestyle and inherited risk factors. Nat
Rev Gastroenterol Hepatol. 18:493–502. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang RQ, Zhou Y, Zheng HX, Wang D, Zheng
XY, Li ZS and Hu LH: Transparency of clinical trials in pancreatic
cancer: An analysis of availability of trial results from the
ClinicalTrials.gov database. Front Oncol. 12:10262682022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stoffel EM, Brand RE and Goggins M:
Pancreatic cancer: Changing epidemiology and new approaches to risk
assessment, early detection, and prevention. Gastroenterology.
164:752–765. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gong J, Li X, Feng Z, Lou J, Pu K, Sun Y,
Hu S, Zhou Y, Song T, Shangguan M, et al: Sorcin can trigger
pancreatic cancer-associated new-onset diabetes through the
secretion of inflammatory cytokines such as serpin E1 and CCL5. Exp
Mol Med. 56:2535–2547. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang B, Zhou F, Hong J, Ng DM, Yang T,
Zhou X, Jin J, Zhou F, Chen P and Xu Y: The role of FOLFIRINOX in
metastatic pancreatic cancer: A meta-analysis. World J Surg Oncol.
19:1822021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eshmuminov D, Aminjonov B, Palm RF, Malleo
G, Schmocker RK, Abdallah R, Yoo C, Shaib WL, Schneider MA,
Rangelova E, et al: FOLFIRINOX or gemcitabine-based chemotherapy
for borderline resectable and locally advanced pancreatic cancer: A
multi-institutional, patient-level, meta-analysis and systematic
review. Ann Surg Oncol. 30:4417–4428. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao J, Logan KA, Nesbitt H, Callan B,
McKaig T, Taylor M, Love M, McHale AP, Griffith DM and Callan JF: A
single microbubble formulation carrying 5-fluorouridine, Irinotecan
and oxaliplatin to enable FOLFIRINOX treatment of pancreatic and
colon cancer using ultrasound targeted microbubble destruction. J
Control Release. 338:358–366. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nichetti F, Rota S, Ambrosini P, Pircher
C, Gusmaroli E, Droz Dit Busset M, Pusceddu S, Sposito C, Coppa J,
Morano F, et al: NALIRIFOX, FOLFIRINOX, and gemcitabine with
nab-paclitaxel as first-line chemotherapy for metastatic pancreatic
cancer: A systematic review and meta-analysis. JAMA Netw Open.
7:e23507562024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liang Y, Chen B, Liang D, Quan X, Gu R,
Meng Z, Gan H, Wu Z, Sun Y, Liu S and Dou G: Pharmacological
effects of astragaloside IV: A review. Molecules. 28:61182023.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang L, Liu C, Wang L and Tang B:
Astragaloside IV mitigates cerebral ischaemia-reperfusion injury
via inhibition of P62/Keap1/Nrf2 pathway-mediated ferroptosis. Eur
J Pharmacol. 944:1755162023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yao M, Zhang L and Wang L: Astragaloside
IV: A promising natural neuroprotective agent for neurological
disorders. Biomed Pharmacother. 159:1142292023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li XX, Li D, Cui XY, Zhou K, Liu J, Lu JJ,
Wu Y, Lin Q and Li Y: Astragaloside IV for heart failure:
Preclinical evidence and possible mechanisms, A systematic review
and meta-analysis. Chin J Integr Med. 29:626–633. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang M, Wang W, Liu K, Jia C, Hou Y and
Bai G: Astragaloside IV protects against lung injury and pulmonary
fibrosis in COPD by targeting GTP-GDP domain of RAS and
downregulating the RAS/RAF/FoxO signaling pathway. Phytomedicine.
120:1550662023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tan YQ, Chen HW and Li J: Astragaloside
IV: An effective drug for the treatment of cardiovascular diseases.
Drug Des Devel Ther. 14:3731–3746. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu F, Cui WQ, Wei Y, Cui J, Qiu J, Hu LL,
Gong WY, Dong JC and Liu BJ: Astragaloside IV inhibits lung cancer
progression and metastasis by modulating macrophage polarization
through AMPK signaling. J Exp Clin Cancer Res. 37:2072018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang Z, Hu X, An C and Li T: The potential
molecular pathways of Astragaloside-IV in colorectal cancer: A
systematic review. Biomed Pharmacother. 167:1156252023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng L, Fang S, Hui J, Rajamanickam V,
Chen M, Weng Q, Wu X, Zhao Z and Ji J: Triptonide modulates MAPK
signaling pathways and exerts anticancer effects via ER
stress-mediated apoptosis induction in human osteosarcoma cells.
Cancer Manag Res. 12:5919–5929. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee YS, Lee DH, Choudry HA, Bartlett DL
and Lee YJ: Ferroptosis-induced endoplasmic reticulum stress:
Cross-talk between ferroptosis and apoptosis. Mol Cancer Res.
16:1073–1076. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim C and Kim B: Anti-cancer natural
products and their bioactive compounds inducing ER stress-mediated
apoptosis: A review. Nutrients. 10:10212018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Y, Guo Y, Tang J, Jiang J and Chen Z:
New insights into the roles of CHOP-induced apoptosis in ER stress.
Acta Biochim Biophys Sin (Shanghai). 46:629–640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ashkenazi A, Fairbrother WJ, Leverson JD
and Souers AJ: From basic apoptosis discoveries to advanced
selective BCL-2 family inhibitors. Nat Rev Drug Discov. 16:273–284.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
He R, Jiang W, Wang C, Li X and Zhou W:
Global burden of pancreatic cancer attributable to metabolic risks
from 1990 to 2019, with projections of mortality to 2030. BMC
Public Health. 24:4562024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu H, Li T, Du Y and Li M: Pancreatic
cancer: Challenges and opportunities. BMC Med. 16:2142018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Carpenter ES, Vendramini-Costa DB,
Hasselluhn MC, Maitra A, Olive KP, Cukierman E, Pasca di Magliano M
and Sherman MH: Pancreatic cancer-associated fibroblasts: Where do
we go from here? Cancer Res. 84:3505–3508. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen H, Zhuo Q, Ye Z, Xu X and Ji S:
Organoid model: A new hope for pancreatic cancer treatment? Biochim
Biophys Acta Rev Cancer. 1875:1884662021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brozos-Vázquez E, Toledano-Fonseca M,
Costa-Fraga N, García-Ortiz MV, Díaz-Lagares Á, Rodríguez-Ariza A,
Aranda E and López-López R: Pancreatic cancer biomarkers: A pathway
to advance in personalized treatment selection. Cancer Treat Rev.
125:1027192024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xia D, Li W, Tang C and Jiang J:
Astragaloside IV, as a potential anticancer agent. Front Pharmacol.
14:10655052023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou L, Li M, Chai Z, Zhang J, Cao K, Deng
L, Liu Y, Jiao C, Zou GM, Wu J and Han F: Anticancer effects and
mechanisms of astragaloside-IV (Review). Oncol Rep. 49:52023.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zheng Y, Dai Y, Liu W, Wang N, Cai Y, Wang
S, Zhang F, Liu P, Chen Q and Wang Z: Astragaloside IV enhances
taxol chemosensitivity of breast cancer via caveolin-1-targeting
oxidant damage. J Cell Physiol. 234:4277–4290. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jia L, Lv D, Zhang S, Wang Z and Zhou B:
Astragaloside IV inhibits the progression of non-small cell lung
cancer through the Akt/GSK-3β/β-catenin pathway. Oncol Res.
27:503–508. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao Y, Wang L, Wang Y, Dong S, Yang S,
Guan Y and Wu X: Astragaloside IV inhibits cell proliferation in
vulvar squamous cell carcinoma through the TGF-β/Smad signaling
pathway. Dermatol Ther. 32:e128022019.PubMed/NCBI
|
|
32
|
Xia C, He Z and Cai Y: Quantitative
proteomics analysis of differentially expressed proteins induced by
astragaloside IV in cervical cancer cell invasion. Cell Mol Biol
Lett. 25:252020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qi H, Wei L, Han Y, Zhang Q, Lau AS and
Rong J: Proteomic characterization of the cellular response to
chemopreventive triterpenoid astragaloside IV in human
hepatocellular carcinoma cell line HepG2. Int J Oncol. 36:725–735.
2010.PubMed/NCBI
|
|
34
|
Hashemi Goradel N, Najafi M, Salehi E,
Farhoo B and Mortezaee K: Cyclooxygenase-2 in cancer: A review. J
Cell Physiol. 234:5683–5699. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Merighi A and Lossi L: Endoplasmic
reticulum stress signaling and neuronal cell death. Int J Mol Sci.
23:151862022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Reggiori F and Molinari M: ER-phagy:
mechanisms, regulation, and diseases connected to the lysosomal
clearance of the endoplasmic reticulum. Physiol Rev. 102:1393–1448.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen Z and Zhang SL: Endoplasmic reticulum
stress: A key regulator of cardiovascular disease. DNA Cell Biol.
42:322–335. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang Z, Sun B, Lu J, Bai P, Su Y and Li
Y: Norcantharidin inhibits the malignant progression of cervical
cancer by inducing endoplasmic reticulum stress. Mol Med Rep.
29:712024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen X and Cubillos-Ruiz JR: Endoplasmic
reticulum stress signals in the tumour and its microenvironment.
Nat Rev Cancer. 21:71–88. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen Y, Shen H, Wang Z, Huang C, Zhang H,
Shao Y, Tong Y, Xu L, Lu Y and Fu Z: Recruitment of USP10 by GCS1
to deubiquitinate GRP78 promotes the progression of colorectal
cancer via alleviating endoplasmic reticulum stress. J Exp Clin
Cancer Res. 43:2612024. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ji X, Chen Z, Lin W, Wu Q, Wu Y, Hong Y,
Tong H, Wang C and Zhang Y: Esculin induces endoplasmic reticulum
stress and drives apoptosis and ferroptosis in colorectal cancer
via PERK regulating eIF2α/CHOP and Nrf2/HO-1 cascades. J
Ethnopharmacol. 328:1181392024. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hicks D, Giresh K, Wrischnik LA and Weiser
DC: The PPP1R15 family of eIF2-alpha phosphatase targeting subunits
(GADD34 and CReP). Int J Mol Sci. 24:173212023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Krzyzosiak A, Pitera AP and Bertolotti A:
An overview of methods for detecting eIF2α phosphorylation and the
integrated stress response. Methods Mol Biol. 2428:3–18. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rozpedek W, Pytel D, Mucha B, Leszczynska
H, Diehl JA and Majsterek I: The role of the PERK/eIF2α/ATF4/CHOP
signaling pathway in tumor progression during endoplasmic reticulum
stress. Curr Mol Med. 16:533–544. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pan X, Liu Y, Liu L, Pang B, Sun Z, Guan
S, Yan Q, Mo T, Chen R, Xu M, et al: Bushen Jieyu Tiaochong Formula
reduces apoptosis of granulosa cells via the PERK-ATF4-CHOP
signaling pathway in a rat model of polycystic ovary syndrome with
chronic stress. J Ethnopharmacol. 292:1149232022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Alam M, Alam S, Shamsi A, Adnan M,
Elasbali AM, Al-Soud WA, Alreshidi M, Hawsawi YM, Tippana A,
Pasupuleti VR and Hassan MI: Bax/Bcl-2 cascade is regulated by the
EGFR pathway: Therapeutic targeting of non-small cell lung cancer.
Front Oncol. 12:8696722022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Azimian H, Dayyani M, Toossi MTB and
Mahmoudi M: Bax/Bcl-2 expression ratio in prediction of response to
breast cancer radiotherapy. Iran J Basic Med Sci. 21:325–332.
2018.PubMed/NCBI
|