Introduction
Interstitial lung disease (ILD), a heterogeneous
pulmonary parenchymal disease, is a major cause of pulmonary
fibrosis characterized by inflammation and fibrosis of the lung
parenchyma (1). The etiology of
most ILDs remains unknown, with idiopathic pulmonary fibrosis (IPF)
being the most common and characterized by progressive lung tissue
hardening, breathing difficulties, and eventual respiratory failure
(2). Known ILDs include connective
tissue disease (CTD)-ILD, which is more likely to develop into
progressive fibrosing ILD (PF-ILD), particularly in patients with
conditions such as rheumatoid arthritis (RA), systemic lupus
erythematosus, polymyositis and dermatomyositis, with PF-ILD
progression rates as high as 20% (3,4).
Fibrosis is primarily caused by the activation of fibroblasts,
which is triggered by chronic injury or inflammation, resulting in
cell destruction and abnormal tissue repair. Fibroblasts migrate to
injury sites, release growth factors and profibrotic mediators, and
transform into myofibroblasts that secrete excessive amounts of
extracellular matrix, causing tissue stiffness and progressive
interstitial pneumonia (5,6). All PF-ILDs are triggered by chronic
epithelial or vascular injury or granulomatous inflammation,
leading to abnormal repair processes and ultimately causing
fibrosis (5,6).
Human parvovirus B19 (B19V), a member of the
Erythrovirus genus within the Parvoviridae family, is a
small, non-enveloped virus with a linear, single-stranded DNA
genome (7). The capsid of B19V is
a stable icosahedral structure composed mainly of viral protein
(VP) 1 and VP2 proteins. VP1 and VP2 are identical except for a
unique 227-amino-acid region at the N-terminus of VP1 (VP1u)
(8). The unique VP1 region (VP1u)
of B19V plays roles in viral tropism, uptake, and nuclear entry. It
contains a nuclear localization signal, targets erythroid
progenitor cells, and is targeted by neutralizing antibodies
(8). The nonstructural protein
(NS)-1 of B19V is essential for viral DNA replication and gene
regulation. B19V NS1 contains nuclear localization signals, a
DNA-binding/nuclease domain, an ATPase and NTP-binding motif, and a
transactivation domain (7,8). B19V NS1 can transactivate host genes,
induce apoptosis, cause cell cycle arrest, and elicit inflammatory
cytokines and DNA damage responses (7,8).
Although most individuals infected with B19V are
asymptomatic or present with mild, nonspecific symptoms resembling
those of the common cold (7–9),
accumulating evidence has linked B19V to the pathogenesis of ILDs
(10–12). B19V has been associated with
chronic vasculopathy syndromes, including Wegener's granulomatosis,
dermatomyositis, and scleroderma (13–15)
and has been implicated in the progression of IPF through the
induction of endothelial immunogenicity (16). Case reports and clinical studies
have identified B19V DNA in lung biopsies and BALF from ILD
patients (11,17) and morphological evidence of septal
capillary injury has been observed in ILD case series with chronic
B19V infection (18).
Despite this, the mechanisms underlying the role of
B19V in ILD remain unclear. Prior studies have shown that B19V NS1
can transactivate expression of the IL-6 gene (19,20),
a cytokine recognized as a key mediator and biomarker of lung
fibrosis (21,22). An in vitro study
demonstrated that B19V can also activate human dermal fibroblasts,
indicating its role in fibrosis and systemic sclerosis (23). Given the antifibrotic effects of
nintedanib in patients with CTD-ILD, the present study was
conducted to elucidate the effect of B19V NS1 on pulmonary fibrosis
using a bleomycin (BLM)-treated mouse model and to evaluate the
therapeutic effect of nintedanib, thereby clarifying the
mechanistic association between B19V infection and ILD in CTD
patients.
Materials and methods
Human samples of CTD patients
A total of 86 patients diagnosed with CTD (19 males
and 67 females, aged 34–84 years) with or without ILD according to
the clinical criteria (24) were
recruited between November 1, 2021 and October 31, 2024, at the
Rheumatology and Immunology Center of the China Medical University
Hospital, Taichung, Taiwan. The China Medical University Hospital's
Institutional Review Board approved the current study (IRB approval
no. CMUH110-REC2-178) and all participants provided written
informed consent following the Declaration of Helsinki's ethical
guidelines for medical research involving human subjects.
Peripheral blood samples (10 ml) were collected into glass tubes
containing no additive or anticoagulant. The clotted blood samples
were centrifuged at 2,000 × g for 10 min at 4°C, and the sera were
collected and stored at −80°C until use. Human parvovirus B19
infection was determined using parvovirus B19V IgM and IgG ELISA
kits (cat. nos. IB79807 and IB79806; IBL-America), and
Cytomegalovirus (CMV) infection was determined using CMV IgM and
IgG test system (ZEUS Scientific, Inc.) from serum samples
according to the manufacturer's instructions. COVID-19 infection
was determined using the cobas SARS-CoV-2 RT-PCR test (Roche
Molecular Diagnostics) from nasopharyngeal swab samples. Herpes
zoster was determined to be caused by a varicella-zoster virus
infection, as indicated by the presence of the virus in blood or
urine samples.
Animals and treatments
A total of 25 male C57BL/6 mice (age, 6 weeks;
weight, 17–19 g) were obtained from the National Laboratory Animal
Center and housed at Chung Shan Medical University under controlled
lighting (12-h light/dark cycle), temperature (22–24°C) and
humidity (50–60%) conditions, with free access to water and
standard laboratory chow (Lab Diet 5001; PMI Nutrition
International Inc.). The experimental protocols were approved by
the Institutional Animal Care and Use Committee, Chung Shan Medical
University, Taiwan (IACUC approval nos. 2803 and 112065). Based on
a previous publication, a BLM-induced lung fibrosis mouse model was
performed (25). Bleomycin,
commonly administered intratracheally in mice, is widely used for
pulmonary fibrosis models due to its ability to induce cellular
damage and fibrosis (2,26). The present study employed the
intratracheal administration route. At eight weeks of age, the mice
were randomly divided into five groups (n=5 per group): i) Control
(PBS) group; ii) Bleomycin (BLM) group; iii) BLM + nintedanib
group; iv) BLM + NS1 group; and v) BLM + NS1 + nintedanib group.
The Control and BLM groups received intratracheal PBS and
intratracheal BLM (3 mg/kg) on day 0, respectively. The BLM +
nintedanib group received intratracheal BLM on day 0 and nintedanib
(50 mg/kg) intraperitoneally every 2 days, starting from day 10,
for a total of 5 injections (27).
The BLM + NS1 group received intratracheal BLM (3 mg/kg) and
B19V-NS1 recombinant protein (0.8 mg/kg) on day 0. The BLM + NS1 +
nintedanib group received intratracheal BLM (3 mg/kg) and B19V-NS1
recombinant protein (0.8 mg/kg) on day 0. nintedanib (50 mg/kg) was
administered intraperitoneally every 2 days, starting from day 10,
for a total of 5 injections. On day 19, the mice were sacrificed
using a carbon dioxide (CO2) euthanasia chamber. The
CO2 flow rate was 50% of the chamber volume/min. After
visually confirming that the mice had stopped breathing, the
CO2 flow was maintained for an additional minute to
ensure mortality. Then, bronchoalveolar lavage fluid (BALF) and
lung tissues were collected.
Hydroxyproline analysis
Lung tissues were processed and hydrolyzed according
to the manufacturer's instructions using a Hydroxyproline Assay Kit
(ab222941, Abcam). After homogenization, hydrolysis, and
neutralization, samples were centrifuged at 10,000 × g for 5 min at
4°C and dried. The hydroxyproline standard curve was generated, and
absorbance was measured at 560 nm using a SpectraMax M5 Microplate
Reader (Molecular Devices LLC).
BALF
The collection of BALF was performed as described
previously (28). Briefly, mice
were sacrificed and 1 ml of PBS was injected into the trachea and
aspirated three times. The collected lavage fluid was centrifuged
at 200 × g for 10 min at 4°C, and the supernatant was reserved for
ELISA analysis. Additionally, the cell pellet was re-suspended in
150 µl of PBS, and the cell populations were detected using a
cytospin to prepare the slides, which were then stained with Liu's
stain (Liu's A for 30 sec and Liu's B for 90 sec at room
temperature) for microscopic observation and enumeration of
macrophages, neutrophils, and lymphocytes.
ELISA
A total of 100 µl BALF samples was added to the
ELISA plate wells and incubated at 37°C for 90 min. After washing,
a biotinylated antibody (100 µl) was added and incubated for 60 min
at the same temperature. The wells were then washed, and 100 µl
enzyme conjugate was added for a 30-min incubation. Subsequently,
substrate (100 µl) was added and incubated in the dark at 37°C
after washing thoroughly. The quantification of essential ILD
biomarkers, including interleukin (IL)-6, tumor necrosis factor
(TNF)-α, and Krebs von den Lungen (KL)-6 (29,30),
was performed by duplicating ELISA kits for mouse IL-6, TNF-α
(Invitrogen; Thermo Fisher Scientific, Inc.), and KL-6
(MyBioSource, Inc.), following the manufacturer's protocol.
Absorbance was measured at 450 nm.
Histological analysis
Histological evaluation of lung tissues was
performed with paraffin sections undergoing hematoxylin and eosin
(H&E) staining and Masson's trichrome staining. For H&E
staining, the tissues were immersed in 10% formalin at 25°C for 24
h and then embedded in paraffin. The tissue blocks were sliced into
5-µm sections, deparaffinized using xylene, and dehydrated by
passing through decreasing concentrations of ethanol (100, 90, 80
and 70%) and water. Subsequently, the slides were stained with
hematoxylin for 3 min at 25°C, rinsed with water and stained with
eosin for 5 min at 25°C. Each slide was subsequently immersed in 95
and 100% ethanol twice (1 min each immersion). Finally, the slides
were immersed in xylene twice for 2 min and then air-dried. For
Masson's trichrome staining, sections were deparaffinized,
rehydrated, and incubated in Bouin's fluid at 60°C for 60 min. They
were stained with Weigert's hematoxylin for 2 min at 25°C, Biebrich
scarlet/acid fuchsin for 10 min and aniline blue for 10 min at
25°C. Finally, sections were treated with 1% acetic acid,
dehydrated, cleared, and mounted, with collagen fibers staining
blue, muscle fibers red, cytoplasm pink and nuclei dark brown or
black. Photomicrographs were observed using TissueFAX Plus
(TissueGnostics GmbH) to analyze stained sections. A total of five
100-µm fields of view were examined in each section. The fibrosis
score was evaluated and graded using the modified Ashcroft score
(31). Grade 0 signified normal
lung tissue without fibrosis, and grades 1–8 represent varying
levels of fibrosis from minor to serious.
Immunohistochemistry (IHC)
The aforementioned paraffin-embedded sections (5 µm)
were fixed in acetone for 5 min at 25°C, air-dried for 10 min, and
then immersed in 0.3% H2O2/PBS for 5 min at
25°C to block peroxidase activity and blocked with 3% BSA
(Sigma-Aldrich; Merck KGaA) in PBS + 0.05% Tween 20 (PBS-Tween) for
1 h at 25°C. After washing twice with PBS-Tween, each for 10 min,
antibodies against transforming growth factor (TGF)-β (1:100; cat.
no. A16640; ABclonal Biotech Co., Ltd.), collagen I (1:100; cat.
no. A5786; ABclonal Biotech Co., Ltd.), IL-18 (1:200; cat. no.
A23076; ABclonal Biotech Co., Ltd.), IL-17A (1:200; cat. no.
A12454; ABclonal Biotech Co., Ltd.), citrullinated histone H3
(Cit-H3; (1:100; cat no. ab5103; Abcam) and myeloperoxidase (MPO;
1:500; cat. no. ab208670; Abcam) were applied and incubated for 1
h, followed by incubation with horseradish peroxidase (HRP)-labeled
secondary antibodies (1:5,000; cat. no. sc-2004; Santa Cruz
Biotechnology, Inc.) for 1 h. Sections were developed with DAB for
3 min, then washed with distilled water for an additional 5 min.
Subsequently, hematoxylin was used as a counterstain for 3 min at
25°C and the sections were washed with distilled water for 10 min.
The sections were then dehydrated using a graded ethanol series
(80, 95 and 100%; 5 min each), followed by immersion in xylene
twice for 5 min each and air-drying. A tissue quantification
analyzer (TissueFAX Plus; TissueGnostics GmbH) was used to analyze
stained sections, counting positive cells in randomly selected
fields and comparing the results with those of a negative control.
A total of five 100 µm fields of view were examined in each
section, across five fields of view counted and averaged.
Statistical analysis
Data were recorded and analyzed using Excel
(Microsoft Corporation) and GraphPad Prism 8.0 (Dotmatics)
software. Experimental results are presented as mean ± SD, and
statistical significance was determined using one-way ANOVA with
Tukey's multiple comparisons post hoc test. Fibrosis score results
were presented as medians and ranges and were analyzed using
one-way ANOVA with the Kruskal-Wallis test and Dunn's post hoc
test. BALF cell counts were analyzed using two-way ANOVA with
Tukey's multiple comparisons post hoc test, and the χ2
test was used to determine the significant differences in clinical
characteristics between the data sets. P<0.05 was considered
statistically significant.
Results
Prevalence of B19V infection in CTD
patients
As illustrated in Table
I, B19V infection (24/33 vs. 12/53; P<0.001) and
hypertension (14/33 vs. 11/53; P=0.03) were significantly more
prevalent in CTD patients with ILD than in those without ILD. By
contrast, no significant differences were detected in age at study
entry, sex distribution, smoking status, disease duration, CMV,
COVID-19, herpes zoster infection, or diabetes mellitus or
cardiovascular disease status between the two groups. In terms of
CTD type, among the 53 CTD patients without ILD, 50 had RA, one had
idiopathic inflammatory myopathy (IIM), and two had systemic
sclerosis (SSc). Among the 33 CTD patients with ILD, 25 had RA,
five had IIM, and three had SSc. Additionally, the ILD subtypes
included 26 cases of nonspecific interstitial pneumonia, five cases
of usual interstitial pneumonia (UIP), and two cases of organizing
pneumonia (OP).
 | Table I.Clinical characteristics of 86 CTD
patients with or without ILD. |
Table I.
Clinical characteristics of 86 CTD
patients with or without ILD.
| Clinical
characteristic | CTH without ILD
(n=53) | CTH with ILD
(n=33) | P-value |
|---|
| Age, years (mean ±
SD) [range] | 56.2±8.6
[34-75] | 61.8±9.9
[36-84] |
|
| Female (n=67) | 43 | 24 | 0.36 |
| Smoking (n=12) | 7 | 5 | 0.80 |
| B19V IgM or IgG (+)
(n=36) | 12 | 24 |
<0.001a |
| CMV IgM or IgG (+)
(n=67) | 41 | 26 | 0.84 |
| COVID-19 (+)
(n=33) | 19 | 14 | 0.54 |
| Herpes zoster
(n=19) | 15 | 4 | 0.08 |
| CTD duration,
years | 9.6±2.7 | 10.7±2.6 |
|
| CTD types |
|
|
|
| RA | 50 | 25 |
|
|
IIM | 1 | 5 |
|
| SSc | 2 | 3 |
|
| ILD patterns by
HRCT |
|
|
|
|
NSIP | NA | 26 | NA |
|
UIP | NA | 5 | NA |
| OP | NA | 2 | NA |
| Hypertension
(n=25) | 11 | 14 | 0.03a |
| DM (n=8) | 3 | 5 | 0.14 |
| CVD (n=9) | 4 | 5 | 0.26 |
Influence of B19V NS1 on body weights,
lung weights, and survival rate in mice with BLM-induced pulmonary
fibrosis
To assess the effects of B19V NS1 on BLM-induced
pulmonary fibrosis, the body weights of the mice were recorded
daily until day 19. By contrast, lung weights were measured on day
19 (Fig. 1A). Compared with those
of the control group, the body weights of the mice in the BLM, BLM
+ nintedanib, BLM + NS1, and BLM + NS1 + nintedanib groups
significantly decreased (Fig. 1B;
P=0.008, 0.002, <0.001 and <0.001, respectively). Treatment
with nintedanib improved body weight loss in mice from both the BLM
and the BLM + NS1 groups but was not sufficient to restore normal
weight (Fig. 1B). Compared with
the mice in the PBS group, the mice in the BLM and BLM + NS1 groups
had significantly greater lung weights (P=0.025 and 0.037,
respectively), whereas normal lung weight was restored in the
nintedanib treatment groups (Fig.
1C; BLM + nintedanib vs. BLM, P=0.035; BLM + NS1 + nintedanib
vs. BLM + NS1, P=0.044).
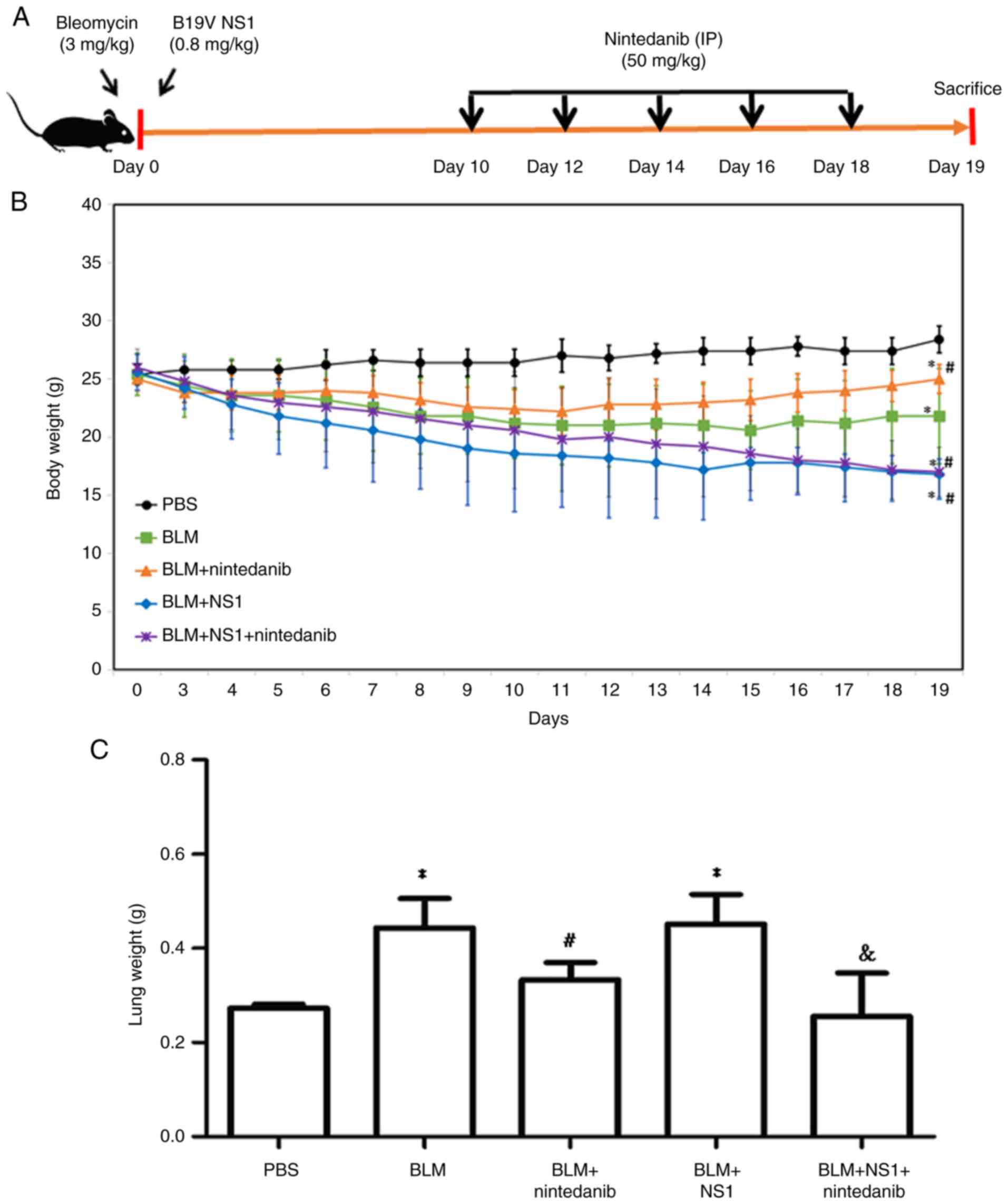 | Figure 1.Effects of nintedanib and B19V NS1 on
bleomycin-induced pulmonary fibrosis in mice. (A) Schematic diagram
of the experimental animal model. (B) Daily body weight
measurements across the PBS, BLM, BLM + nintedanib, BLM + NS1, and
BLM + NS1 + nintedanib groups (BLM vs. PBS, P=0.008; BLM +
nintedanib vs. PBS, P=0.002; BLM + NS1 vs. PBS, P<0.001; BLM +
nintedanib + NS1 vs. PBS, P<0.001; BLM + NS1 vs. BLM, P=0.031;
BLM + nintedanib + NS1 vs. BLM, P=0.048; BLM + nintedanib + NS1 vs.
BLM + nintedanib, P<0.001). (C) Lung weight of mice on day 19
(BLM vs. PBS, P=0.025; BLM + NS1 vs. PBS, P=0.037; BLM + nintedanib
vs BLM, P=0.035; BLM + NS1 + nintedanib vs BLM + NS1, P=0.044).
*P<0.05 vs. the PBS (Control) group, #P<0.05 vs.
the BLM group, and &P<0.05 vs. the BLM + NS1
group. B19V, human parvovirus B19; NS1, nonstructural protein 1;
BLM, bleomycin. |
B19V NS1 administration changes immune
cell compositions and increases levels of fibrotic markers in the
BALF of mice with BLM-induced pulmonary fibrosis
To evaluate the influence of B19V NS1 on immune cell
distribution in the BALF of mice treated with BLM, the proportions
of macrophages, neutrophils and lymphocytes were analyzed. No
significant difference in lymphocyte proportion was observed among
the groups (Fig. 2A). Treatment
with BLM resulted in a significant increase in the numbers of
macrophages and neutrophils. Treatment with nintedanib partially
reversed this trend by increasing the number of macrophages and
reducing the number of neutrophils. Notably, the proportions of
neutrophils remained elevated in the BLM + NS1 + nintedanib group,
indicating that the NS1-induced effects were not fully mitigated by
nintedanib (Fig. 2A; macrophages:
BLM vs. PBS, P<0.001; BLM + nintedanib vs. PBS, P<0.001; BLM
+ NS1 vs. PBS, P=0.02; BLM + nintedanib + NS1 vs. PBS, P=0.005; BLM
+ NS1 vs. BLM, P<0.001; neutrophils: BLM vs. PBS, P<0.001;
BLM + nintedanib vs. PBS, P<0.001; BLM + NS1 vs. PBS,
P<0.001; BLM + nintedanib + NS1 vs. PBS, P<0.001; and BLM +
NS1 vs. BLM, P=0.006). Additionally, an ELISA was performed to
measure the levels of lung fibrosis-related markers, including
TNF-α, KL-6, and IL-6, in the BALF. Compared with those in the
control group (PBS), TNF-α (Fig.
2B; P=0.014 and 0.003, respectively), KL-6 (Fig. 2C; P<0.001 and P<0.001,
respectively) and IL-6 (Fig. 2D;
P=0.039 and 0.009, respectively) levels in the BALF of mice from
the BLM and BLM + NS1 groups were markedly elevated. nintedanib
treatment significantly reduced the expression of these markers in
the BALF of mice from both the BLM and BLM + NS1 groups (Fig. 2B-D; TNF-α, BLM + nintedanib vs.
BLM, P=0.039; BLM + NS1 + nintedanib vs. BLM + NS1, P=0.009; KL-6,
BLM + nintedanib vs. BLM, P<0.001; BLM + NS1 + nintedanib vs.
BLM + NS1, P<0.001; IL-6, BLM + nintedanib vs. BLM, P=0.013; BLM
+ NS1 + nintedanib vs. BLM + NS1, P=0.014).
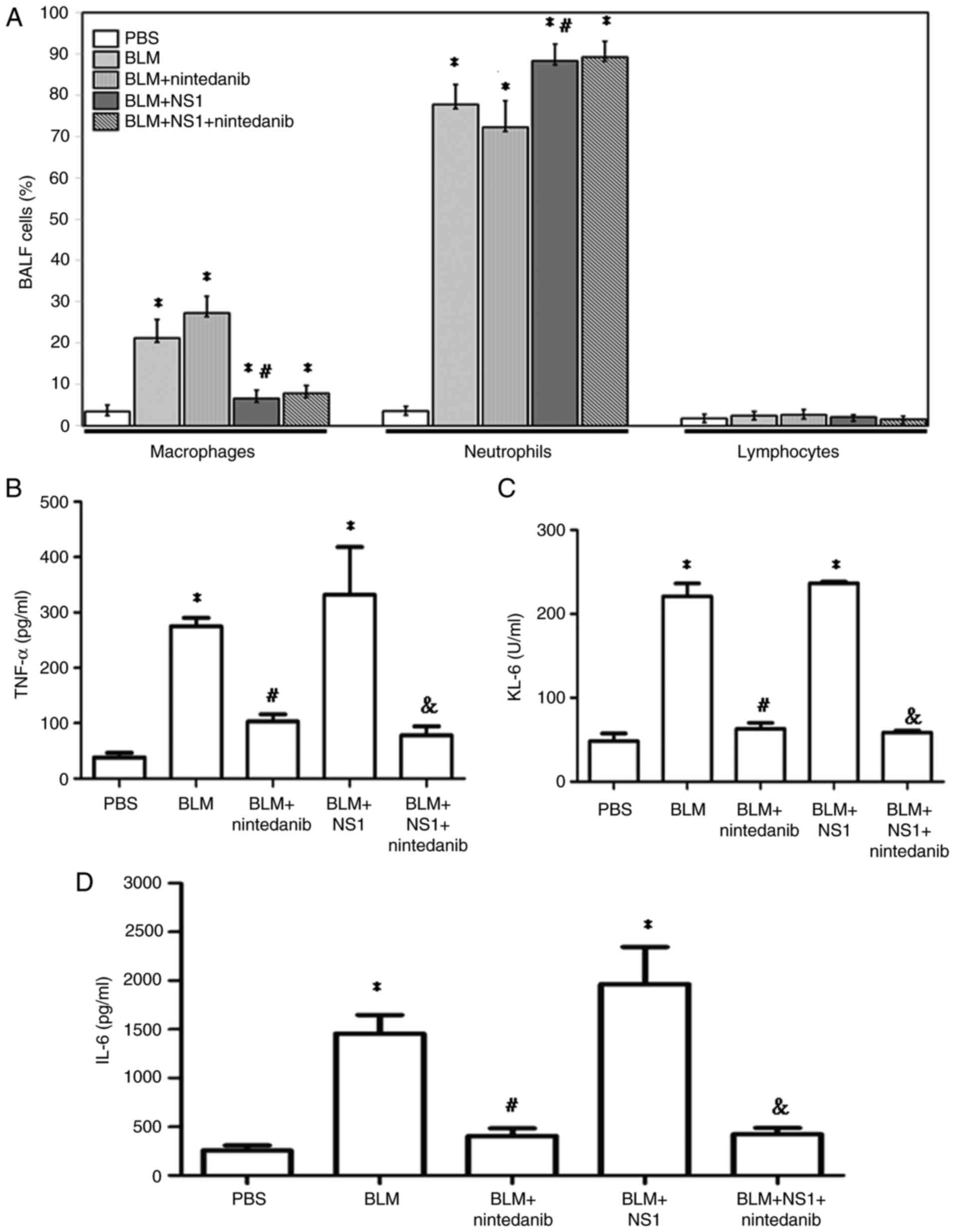 | Figure 2.Proportions of immune cells in BALF
of mice. (A) Percentages of macrophage, neutrophil, and lymphocyte
in BALF of mice from different groups (Macrophages: BLM vs. PBS,
P<0.001; BLM + nintedanib vs. PBS, P<0.001; BLM + NS1 vs.
PBS, P=0.02; BLM + nintedanib + NS1 vs. PBS, P=0.005; BLM + NS1 vs.
BLM, P<0.001; Neutrophils: BLM vs. PBS, P<0.001; BLM +
nintedanib vs. PBS, P<0.001; BLM + NS1 vs. PBS, P<0.001; BLM
+ nintedanib + NS1 vs. PBS, P<0.001; BLM + NS1 vs. BLM, P=0.01).
The concentrations of (B) TNF-α (BLM vs. PBS, P=0.014; BLM + NS1
vs. PBS, P=0.003; BLM + nintedanib vs. BLM, P=0.039; BLM +
nintedanib + NS1 vs. BLM + NS1 P=0.009), (C) KL-6 (BLM vs. PBS,
P<0.001; BLM + NS1 vs. PBS, P<0.001; BLM + nintedanib vs.
BLM, P<0.001; BLM + nintedanib + NS1 vs. BLM + NS1 P<0.001)
and (D) IL-6 (BLM vs. PBS, P= 0.039; BLM + NS1 vs. PBS, P=0.009;
BLM + nintedanib vs. BLM, P=0.013; BLM + nintedanib + NS1 vs. BLM +
NS1, P=0.014) in BALF of mice from different groups. *P<0.05 vs.
the PBS (Control) group, #P<0.05 vs. the BLM group,
and &P<0.05 vs. the BLM + NS1 group. BALF,
bronchoalveolar lavage fluid; BLM, bleomycin; NS1, nonstructural
protein 1; IL, interleukin. |
B19V NS1 administration increases the
lung fibrosis score and hydroxyproline content in mice with
BLM-induced pulmonary fibrosis
Histological analyses were also performed to assess
the effect of B19V NS1 on BLM-induced pulmonary fibrosis in mice.
Significantly higher fibrosis scores were observed in the lungs of
mice from the BLM and BLM + NS1 groups than in those from the PBS
group (P<0.05 and P<0.001, respectively). Nintedanib reduced
fibrosis levels in both the BLM + nintedanib group [3 (3–4)] and
the BLM + NS1 + nintedanib group [4 (4–5)]
(Fig. 3A-B). However, the lung
fibrosis score remained more severe in the mice from the BLM + NS1
group [7 (7–8)] than in those from the BLM group [6
(5–7)] (Fig.
3B). Similarly, lung hydroxyproline content, an indicator of
collagen deposition, was significantly greater in the lungs of mice
from the BLM and BLM + NS1 groups than in those from the PBS group
(P<0.001 and P<0.001, respectively). Hydroxyproline levels
remained higher in the mice in the BLM + NS1 group than in the mice
in the BLM group (P=0.016). Nintedanib treatment markedly reduced
hydroxyproline levels, although the reduction was more pronounced
in the BLM group and the BLM + NS1 group (P=0.004 and P=0.036,
respectively) (Fig. 3C). Notably,
significantly higher hydroxyproline levels were detected in the BLM
+ NS1 + nintedanib group than in the BLM + nintedanib group
(P=0.006) (Fig. 3C). Moreover,
quantification of key fibrotic marker levels, including those of
TGF-β and collagen I, revealed that compared with BLM treatment
alone, B19V NS1 administration significantly exacerbated fibrosis
(P=0.015 and P<0.001, respectively). Conversely, the
administration of nintedanib reduced the levels of these markers in
mice from both the BLM and the BLM + NS1 groups (Fig. 4A-C; TGF-β: BLM vs. PBS, P<0.001;
BLM + NS1 vs. PBS, P<0.001; BLM + nintedanib vs. BLM, P=0.001;
BLM + nintedanib + NS1 vs. BLM + NS1, P<0.001; collagen I: BLM
vs. PBS, P=0.019; BLM + NS1 vs. PBS, P<0.001; BLM + nintedanib
vs. BLM, P=0.024; and BLM + nintedanib + NS1 vs. BLM + NS1,
P<0.001).
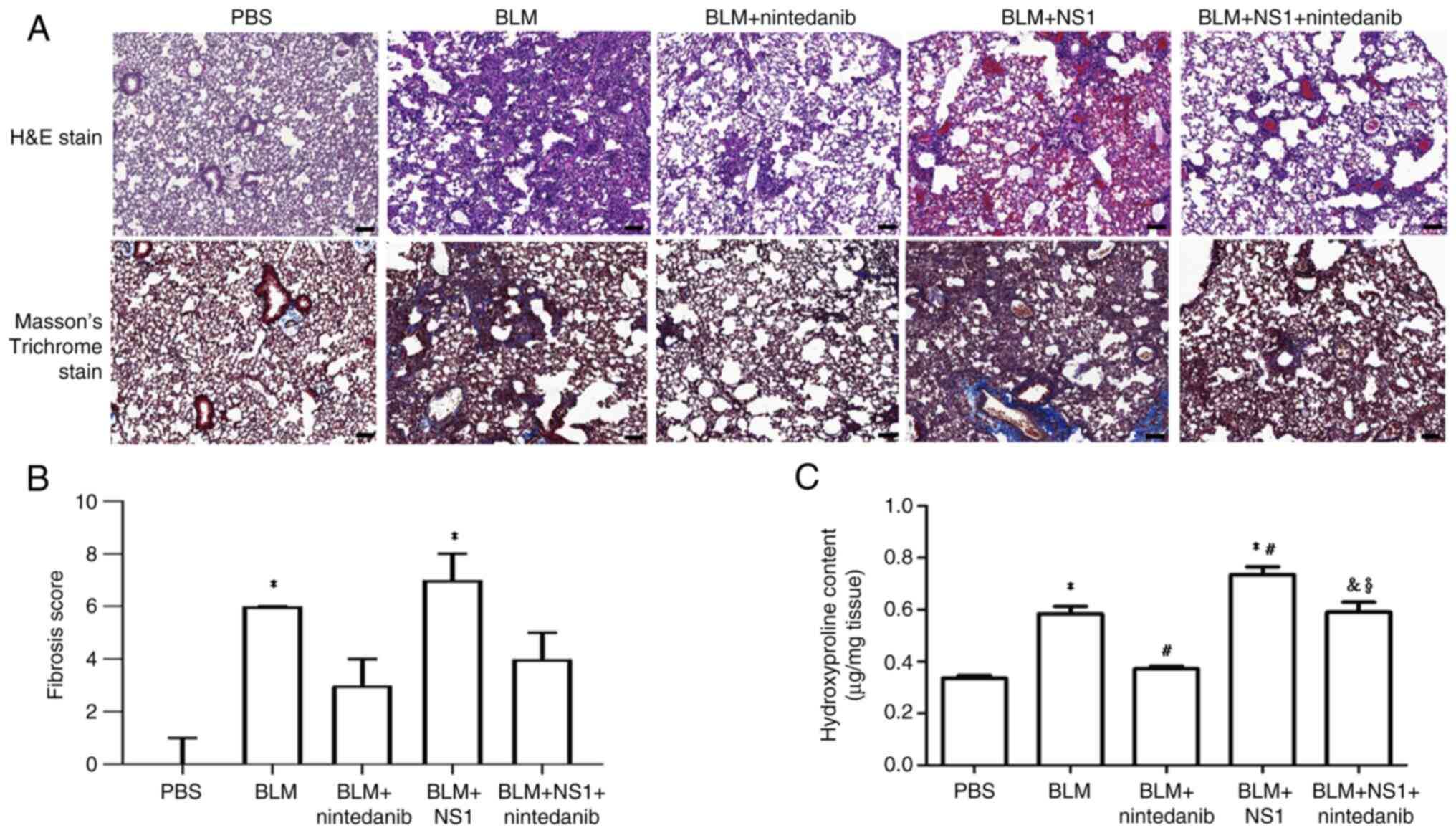 | Figure 3.Histological staining of the lung
tissues of mice. (A) Representative images of H&E and Masson's
trichrome-stained mouse lung sections. Scale bar, 100 µm.
Quantified result of (B) fibrosis score (BLM vs. PBS, P<0.05;
BLM + NS1 vs. PBS, P<0.001) and (C) hydroxyproline content (BLM
vs. PBS, P<0.001; BLM + NS1 vs. PBS, P<0.001; BLM +
nintedanib vs. BLM, P<0.001; BLM + NS1 vs. BLM, P= P=0.016; BLM
+ nintedanib + NS1 vs. BLM + NS1, P=0.036; BLM + NS1 + nintedanib
vs. BLM + nintedanib, P=0.006). *P<0.05 vs. the PBS (Control)
group, #P<0.05 vs. the BLM group,
&P<0.05 vs. the BLM + NS1 group, and
§P<0.05 vs. the BLM + nintedanib group. H&E,
hematoxylin and eosin; BLM, bleomycin; NS1, nonstructural protein
1. |
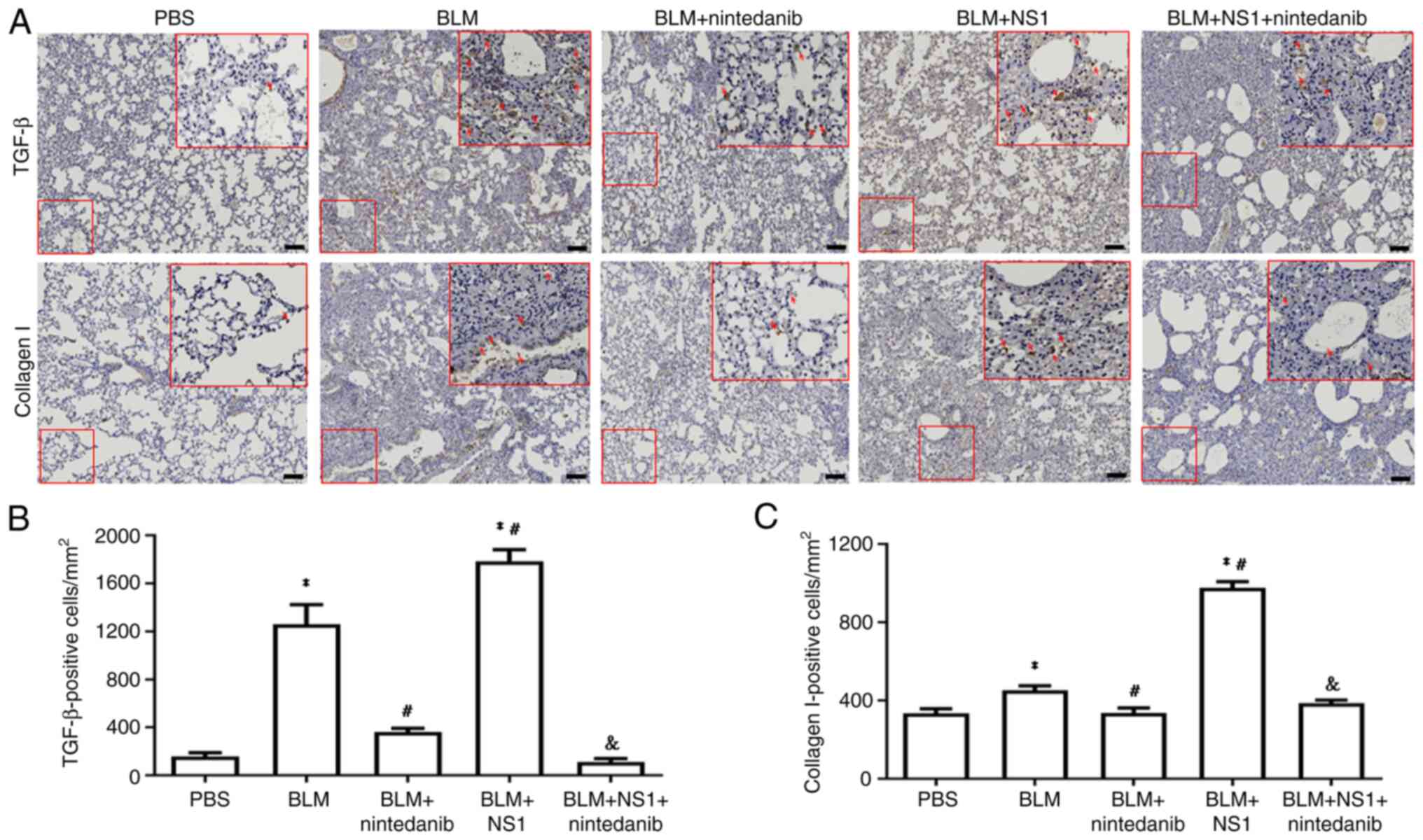 | Figure 4.IHC staining of lung tissue of mice.
(A) Representative images of lung sections of mice stained with
TGF-β and collagen I. Scale bar, 100 µm. Quantified results of (B)
TGF-β (BLM vs. PBS, P<0.001; BLM + NS1 vs. PBS, P<0.001; BLM
+ nintedanib vs. BLM, P=0.001; BLM + NS1 vs. BLM, P=0.015; BLM +
nintedanib + NS1 vs. BLM + NS1, P<0.001) and (C) collagen I
positive cells (BLM vs. PBS, P=0.019; BLM + NS1 vs. PBS,
P<0.001; BLM + nintedanib vs. BLM, P=0.024; BLM + NS1 vs. BLM,
P<0.001; BLM + nintedanib + NS1 vs. BLM + NS1, P<0.001) in
lung sections of mice. *P<0.05 vs. the PBS (Control) group,
#P<0.05 vs. the BLM group, and
&P<0.05 vs. the BLM + NS1 group. IHC,
immunohistochemistry; TGF transforming growth factor; BLM,
bleomycin; NS1, nonstructural protein 1. |
B19V NS1 administration increases
neutrophil and inflammasome involvement in the lungs of mice with
BLM-induced pulmonary fibrosis
To confirm the involvement of neutrophils in the
lungs of mice with BLM-induced pulmonary fibrosis treated with B19V
NS1, levels of key neutrophil-related markers, including Cit-H3 and
MPO, were determined. Significantly elevated Cit-H3 (P<0.001 and
P<0.001, respectively) and MPO (P=0.001 and P<0.001,
respectively) expression was detected in lung sections from mice in
the BLM and BLM + NS1 groups compared with those from the PBS group
(Fig. 5A-C), reflecting heightened
neutrophil responses. Conversely, nintedanib treatment
significantly reduced the levels of Cit-H3 (P=0.013 and P<0.001,
respectively) and MPO (P=0.001 and P<0.001, respectively)
compared with those in the BLM and BLM + NS1 groups. However,
significantly more MPO-positive signals were detected in the BLM +
NS1 + nintedanib group than in the BLM + nintedanib group (P=0.015;
Fig. 5C). Additionally, the
expression of inflammasome signaling factors, including IL-18 and
IL-17A, significantly increased in the lungs of mice in the BLM and
BLM + NS1 groups compared with those in the control group (IL-18:
P<0.001 and P<0.001; IL-17A: P<0.001 and P<0.001,
respectively; Fig. 5D and E).
Significantly reduced levels of IL-18 and IL-17A were observed in
the lung tissues of mice from both the BLM + nintedanib and the BLM
+ NS1 + nintedanib groups (Fig. 5D and
E; IL-18: BLM + nintedanib vs. BLM, P=0.043; BLM + nintedanib +
NS1 vs. BLM + NS1, P<0.001; IL-17A: BLM + nintedanib vs. BLM,
P<0.03; BLM + nintedanib + NS1 vs. BLM + NS1; P<0.001).
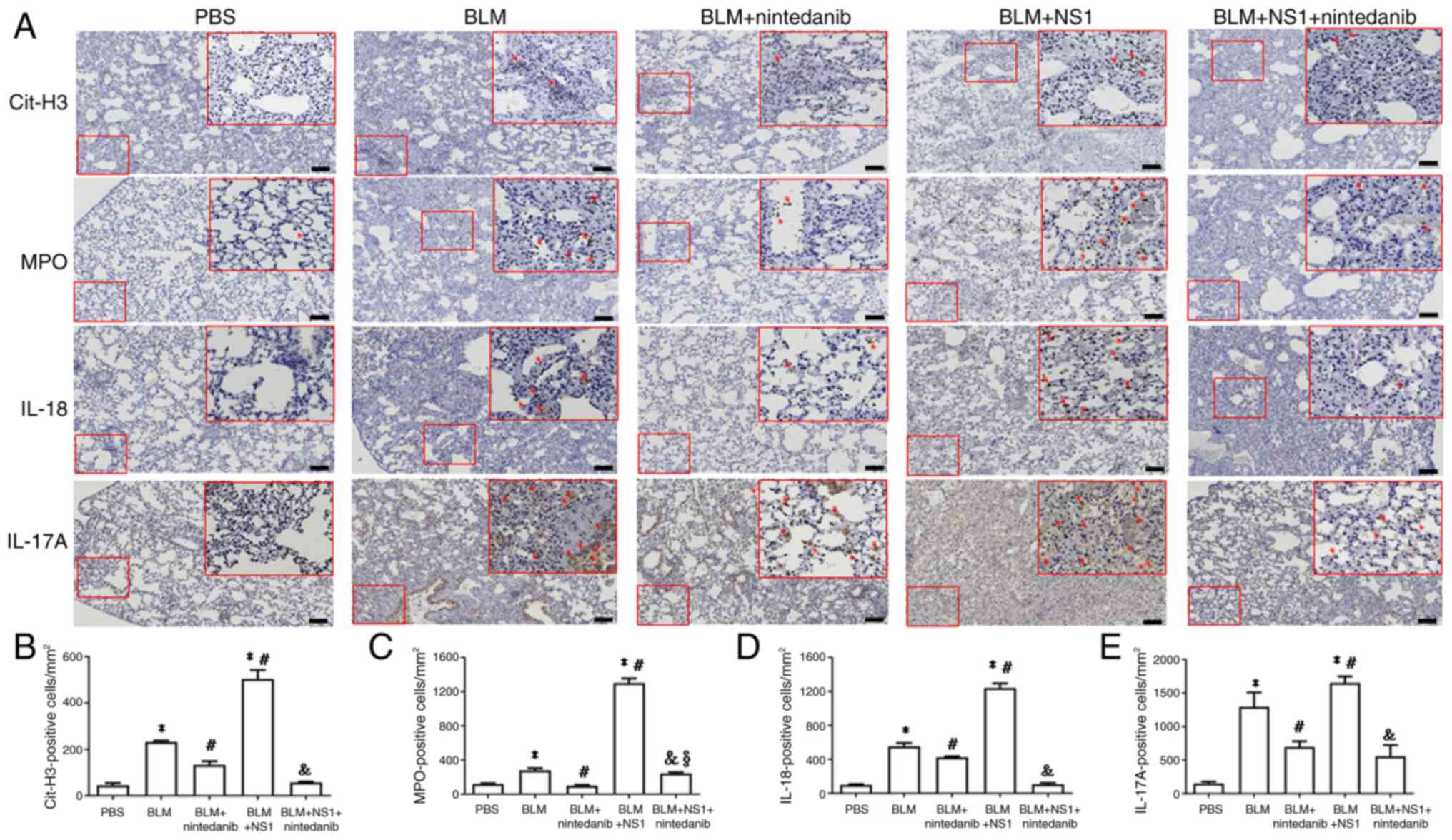 | Figure 5.Expressions of neutrophil-related and
inflammasome markers. (A) Representative immunohistochemistry
images of the lung section of mice stained with (A) Cit-H3, MPO,
IL-18 and IL-17. Scale bar, 100 µm. Quantification of (B) Cit-H3
(BLM vs. PBS, P<0.001; BLM + NS1 vs. PBS, P<0.001; BLM +
nintedanib vs. BLM, P=0.013; BLM + NS1 vs. BLM, P<0.001; BLM +
nintedanib + NS1 vs. BLM + NS1, P<0.001), (C) MPO (BLM vs. PBS,
P=0.001; BLM + NS1 vs. PBS, P<0.001; BLM + nintedanib vs. BLM,
P<0.001; BLM + NS1 vs. BLM, P= P<0.001; BLM + nintedanib +
NS1 vs. BLM + NS1, P<0.001; BLM + nintedanib + NS1 vs. BLM +
nintedanib, P=0.015), (D) IL-18 (BLM vs. PBS, P<0.001; BLM + NS1
vs. PBS, P<0.001; BLM + nintedanib vs. BLM, P=0.043; BLM + NS1
vs. BLM, P<0.001; BLM + nintedanib + NS1 vs. BLM + NS1,
P<0.001), and (E) IL-17 (BLM vs. PBS, P<0.001; BLM + NS1 vs.
PBS, P<0.001; BLM + nintedanib vs. BLM, P<0.03; BLM + NS1 vs.
BLM, P<0.001; BLM + nintedanib + NS1 vs. BLM + NS1, P<0.001)
positive cells in lung sections of mice. *P<0.05 vs. the PBS
(Control) group, #P<0.05 vs. the BLM group,
&P<0.05 vs. the BLM + NS1 group, and
§P<0.05 vs. the BLM + nintedanib group. Cit-H3,
citrullinated histone H3; MPO, myeloperoxidase; IL, interleukin;
BLM, bleomycin; NS1, nonstructural protein 1. |
Discussion
The present study revealed for the first time that
B19V NS1 significantly exacerbates BLM-induced pulmonary fibrosis
in mice by increasing inflammation and fibrosis, mainly through
inflammasome activation and neutrophil-driven responses.
Conversely, the administration of nintedanib reduced the expression
of key fibrotic markers, such as IL-18, IL-17A, Cit-H3, and MPO,
suggesting the therapeutic potential of nintedanib in exacerbating
lung fibrosis associated with B19V NS1. In addition, there were
significant differences in the proportions of B19V infection and
hypertension between CTD patients with and without ILD. These
findings revealed a mechanistic link between B19V infection and
ILD, indicating that B19V, through NS1, acts as a potent
exacerbator of ILD, further aggravating fibrosis in patients with
already compromised pulmonary conditions.
Bleomycin induces fibrosis primarily through
reactive oxygen species (ROS)-mediated oxidative stress, which
triggers epithelial apoptosis, activates fibroblasts and promotes
collagen deposition (32). ROS
also activate the NLRP3 inflammasome, promoting fibrosis via the
IL-1β/IL-1R/MyD88/NF-κB pathway and caspase-1-mediated activation
of IL-1β and IL-18 (33–37). These findings are consistent with
previous reports showing that B19V NS1 induces IL-6 and TGF-β
expression and activates fibroblasts (21,23,38–40).
In the present study, compared with BLM alone, combined treatment
with BLM and B19V NS1 resulted in higher levels of
inflammasome-related cytokines and collagen deposition. These
findings provided a possible explanation for why B19V NS1
exacerbates BLM-induced pulmonary fibrosis. However, further
studies are needed to clarify the precise role of B19V NS1 in ILD
aggravation.
In a mouse model of BLM-induced fibrosis, neutrophil
recruitment driven by chemokines such as CXCR2 contributes to
tissue damage and fibrosis through the release of NETs,
proinflammatory cytokines, and neutrophil elastase (41–43).
Although evidence for B19V NS1-induced NET formation is limited,
studies on dengue and Zika virus NS1 proteins have demonstrated
their ability to promote NET release through platelet activation
and neutrophil infiltration (44,45).
In the present study, B19V NS1 exacerbated lung fibrosis through
mechanisms that parallel those of BLM, including neutrophil-driven
responses. The elevated levels of neutrophil markers, such as
Cit-H3 and MPO, in the lung tissues of mice treated with both BLM
and B19V NS1 indicated a strong correlation with increased
neutrophil invasion, contributing to the chronic tissue damage
characteristic of BLM-induced fibrosis.
Progressive fibrosing ILD poses a significant
clinical challenge, as conventional treatments for pulmonary
fibrosis often yield poor outcomes and only manage symptoms
(12). nintedanib, a tyrosine
kinase inhibitor targeting platelet-derived growth factor receptor,
fibroblast growth factor receptor and vascular endothelial growth
factor receptor, exerts antifibrotic and antiangiogenic effects and
is approved by the United States Food and Drug Administration for
treating IPF and SSc-associated ILD (46–48).
In the current study, MPO and hydroxyproline levels remained
increased in the BLM + NS1 + nintedanib group. Although nintedanib
treatment mitigated several inflammatory and fibrotic responses
exacerbated by NS1 in the BLM model, it did not fully reverse all
pathological changes. These findings suggested that nintedanib only
partly alleviates NS1-exacerbated lung injury and that its efficacy
may be limited in the presence of NS1-mediated fibrosis. Further
studies are needed to explore combination strategies or alternative
treatments that more effectively counteract NS1-driven fibrotic
pathways.
Notably, the present study has several limitations
that should be acknowledged. First, the sample size was relatively
small, particularly in the subgroup of CTD patients with ILD
(n=33), which may limit the statistical power and generalizability
of the findings. These factors may influence disease severity and
outcomes, and their absence could have introduced residual
confounding. Therefore, the results should be interpreted with
caution and further studies with larger, well-characterized cohorts
are needed to confirm these findings in the future. Second, a group
receiving NS1 treatment alone was not included in the present
study. While the primary objective was to investigate the effect of
NS1 in the context of BLM-induced lung injury, the lack of an
NS1-only control group made it difficult to fully determine whether
NS1 itself can independently induce fibrotic changes in the lungs.
Including such a group would help clarify whether the observed
effects are specific to the interaction between NS1 and BLM-induced
injury or partially attributable to NS1 alone. Therefore, future
studies should incorporate an NS1-only group to delineate the
individual more precisely and combined effects of NS1 and BLM on
the progression of pulmonary fibrosis. Third, direct mechanistic
evidence to confirm the causal role of neutrophil activation and
inflammasome signaling in the NS1-induced exacerbation of fibrosis
was lacking. While the findings suggested that NS1 may promote
pulmonary fibrosis through enhanced neutrophil activity and
activation of the NLRP3 inflammasome pathway, these conclusions are
based on correlative data. The absence of experiments employing
specific inhibitors, such as NLRP3 or NETosis inhibitors, limits
the ability of the present study to definitively establish
causality. Therefore, future studies incorporating targeted
inhibition or loss-of-function approaches are needed to validate
these mechanistic links and strengthen the causal interpretation of
our findings. Finally, a key limitation is that the experiments
were conducted in mouse models, which may not fully replicate the
complex pathophysiology of human disease. Consequently, the
findings and therapeutic effects observed here may not directly
translate to humans due to species-specific differences in immune
responses, disease progression, and drug metabolism (49). Therefore, further validation in
clinical settings or human-derived models is necessary to confirm
the relevance of these results to human patients.
Overall, the results of the current study provided a
clinical and mechanistic connection between B19V infection and the
progression of pulmonary fibrosis. The clinical data indicated a
higher incidence of B19V infection among CTD patients with ILD,
suggesting a potential association with the development of ILD.
Experimental findings further demonstrated that B19V, through its
NS1 protein, may exacerbate ILD and worsen fibrosis in patients
already affected by lung conditions, particularly by activating the
inflammasome and driving neutrophil responses. These findings build
on previous evidence of B19V in ILD patients, reinforcing the
possible role of B19V NS1 in exacerbating fibrogenesis.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the National
Science and Technology Council (grant no. NSTC 112-2314-B040-015)
and Chung Shan Medical University (grant no. CSMU-INT-113-04). The
funders had no role in the study design, data collection and
analysis, the decision to publish, or the preparation of the
manuscript.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Author's contribution
TCH, CCT, DYC and BST were involved in the study
conception and design, drafting and revising of the manuscript and
data analysis. CWK and ZHW performed the experiments and analyzed
the data. TCH, DYC and BST confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The China Medical University Hospital's
Institutional Review Board approved the study (IRB approval number
CMUH110-REC2-178), and all participants provided written informed
consent following the Declaration of Helsinki's ethical guidelines
for medical research involving human subjects. The animal study
protocol was approved by the Institutional Animal Care and Use
Committee of Chung Shan Medical University, Taiwan (approval
numbers 2803 and 112065).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cottin V, Wollin L, Fischer A, Quaresma M,
Stowasser S and Harari S: Fibrosing interstitial lung diseases:
knowns and unknowns. Eur Respir Rev. 28:1801002019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Podolanczuk AJ, Thomson CC, Remy-Jardin M,
Richeldi L, Martinez FJ, Kolb M and Raghu G: Idiopathic pulmonary
fibrosis: State of the art for 2023. Eur Respir J. 61:22009572023.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matson SM and Demoruelle MK: Connective
tissue disease-associated interstitial lung disease. Rheum Dis Clin
North Am. 50:423–438. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wan Q, Zhang X, Zhou D, Xie R, Cai Y,
Zhang K and Sun X: Inhaled nano-based therapeutics for pulmonary
fibrosis: Recent advances and future prospects. J
Nanobiotechnology. 21:2152023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Andersson-Sjöland A, de Alba CG, Nihlberg
K, Becerril C, Ramírez R, Pardo A, Westergren-Thorsson G and Selman
M: Fibrocytes are a potential source of lung fibroblasts in
idiopathic pulmonary fibrosis. Int J Biochem Cell Biol.
40:2129–2140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Al Oweidat KS, Abdulelah AA, Toubasi AA,
Abdulelah M, Alatteili NZ and Abdulelah ZA: The clinical efficacy
and safety of nintedanib in the treatment of interstitial lung
disease among patients with systemic sclerosis: Systematic review.
Can Respir J. 2025:16825462025.PubMed/NCBI
|
|
7
|
Qiu J, Söderlund-Venermo M and Young NS:
Human parvoviruses. Clin Microbiol Rev. 30:43–113. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arvia R, Stincarelli MA, Manaresi E,
Gallinella G and Zakrzewska K: Parvovirus B19 in rheumatic
diseases. Microorganisms. 12:17082024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tzang CC, Chi LY, Lee CY, Chang ZY, Luo
CA, Chen YH, Lin TA, Yu LC, Chen YR, Tzang BS and Hsu TC: Clinical
implications of human Parvovirus B19 infection on autoimmunity and
autoimmune diseases. Int Immunopharmacol. 147:1139602025.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Janner D, Bork J, Baum M and Chinnock R:
Severe pneumonia after heart transplantation as a result of human
parvovirus B19. J Heart Lung Transplant. 13:336–338.
1994.PubMed/NCBI
|
|
11
|
Bousvaros A, Sundel R, Thorne GM, McIntosh
K, Cohen M, Erdman DD, Perez-Atayde A, Finkel TH and Colin AA:
Parvovirus B19-associated interstitial lung disease, hepatitis, and
myositis. Pediatr Pulmonol. 26:365–369. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Atzeni F, Alciati A, Gozza F, Masala IF,
Siragusano C and Pipitone N: Interstitial lung disease in rheumatic
diseases: an update of the 2018 review. Expert Rev Clin Immunol.
21:209–226. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nikkari S, Mertsola J, Korvenranta H,
Vainionpää R and Toivanen P: Wegener's granulomatosis and
parvovirus B19 infection. Arthritis Rheum. 37:1707–1710. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Magro CM, Crowson AN, Dawood M and Nuovo
GJ: Parvoviral infection of endothelial cells and its possible role
in vasculitis and autoimmune diseases. J Rheumatol. 29:1227–1235.
2002.PubMed/NCBI
|
|
15
|
Magro CM, Nuovo G, Ferri C, Crowson AN,
Giuggioli D and Sebastiani M: Parvoviral infection of endothelial
cells and stromal fibroblasts: A possible pathogenetic role in
scleroderma. J Cutan Pathol. 31:43–50. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Magro CM, Allen J, Pope-Harman A, Waldman
WJ, Moh P, Rothrauff S and Ross P Jr: The role of microvascular
injury in the evolution of idiopathic pulmonary fibrosis. Am J Clin
Pathol. 119:556–567. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li X, Chen X and Zhang Y: Interstitial
pneumonia with pulmonary parvovirus B19 infection. Med Clin (Barc).
159:e17–e18. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Magro CM, Wusirika R, Frambach GE, Nuovo
GJ, Ferri C and Ross P Jr: Autoimmune-like pulmonary disease in
association with parvovirus B19: A clinical, morphologic, and
molecular study of 12 cases. Appl Immunohistochem Mol Morphol.
14:208–216. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moffatt S, Tanaka N, Tada K, Nose M,
Nakamura M, Muraoka O, Hirano T and Sugamura K: A cytotoxic
nonstructural protein, NS1, of human parvovirus B19 induces
activation of interleukin-6 gene expression. J Virol. 70:8485–8491.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hsu TC, Tzang BS, Huang CN, Lee YJ, Liu
GY, Chen MC and Tsay GJ: Increased expression and secretion of
interleukin-6 in human parvovirus B19 non-structural protein (NS1)
transfected COS-7 epithelial cells. Clin Exp Immunol. 144:152–157.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martinović Kaliterna D and Petrić M:
Biomarkers of skin and lung fibrosis in systemic sclerosis. Expert
Rev Clin Immunol. 15:1215–1223. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dsouza NN, Alampady V, Baby K, Maity S,
Byregowda BH and Nayak Y: Thalidomide interaction with inflammation
in idiopathic pulmonary fibrosis. Inflammopharmacology.
31:1167–1182. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Arvia R, Margheri F, Stincarelli MA,
Laurenzana A, Fibbi G, Gallinella G, Ferri C, Del Rosso M and
Zakrzewska K: Parvovirus B19 activates in vitro normal human dermal
fibroblasts: A possible implication in skin fibrosis and systemic
sclerosis. Rheumatology (Oxford). 59:3526–3532. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jeganathan N and Sathananthan M:
Connective tissue disease-related interstitial lung disease:
Prevalence, patterns, predictors, prognosis, and treatment. Lung.
198:735–759. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Smith RE, Strieter RM, Phan SH, Lukacs NW,
Huffnagle GB, Wilke CA, Burdick MD, Lincoln P, Evanoff H and Kunkel
SL: Production and function of murine macrophage inflammatory
protein-1 alpha in bleomycin-induced lung injury. J Immunol.
153:4704–4712. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Maher TM, Wells AU and Laurent GJ:
Idiopathic pulmonary fibrosis: Multiple causes and multiple
mechanisms? Eur Respir J. 30:835–839. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mor A, Segal Salto M, Katav A, Barashi N,
Edelshtein V, Manetti M, Levi Y, George J and Matucci-Cerinic M:
Blockade of CCL24 with a monoclonal antibody ameliorates
experimental dermal and pulmonary fibrosis. Ann Rheum Dis.
78:1260–1268. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chiang SR, Lin CY, Chen DY, Tsai HF, Lin
XC, Hsu TC and Tzang BS: The effects of human parvovirus VP1 unique
region in a mouse model of allergic asthma. PLoS One.
14:e02167992019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ishikawa Y, Iwata S, Hanami K, Nawata A,
Zhang M, Yamagata K, Hirata S, Sakata K, Todoroki Y, Nakano K, et
al: Relevance of interferon-gamma in pathogenesis of
life-threatening rapidly progressive interstitial lung disease in
patients with dermatomyositis. Arthritis Res Ther. 20:2402018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tzang CC, Lin WC, Huang ES, Kang YF, Cheng
YH, Tzang BS and Hsu TC: Interstitial lung disease biomarkers: A
systematic review and meta-analysis. Clin Chim Acta.
577:1204732025. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hübner RH, Gitter W, El Mokhtari NE,
Mathiak M, Both M, Bolte H, Freitag-Wolf S and Bewig B:
Standardized quantification of pulmonary fibrosis in histological
samples. Biotechniques. 44:507–511. 514–517. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mohammed SM, Al-Saedi HFS, Mohammed AQ,
Amir AA, Radi UK, Sattar R, Ahmad I, Ramadan MF, Alshahrani MY,
Balasim HM and Alawadi A: Mechanisms of bleomycin-induced lung
fibrosis: A review of therapeutic targets and approaches. Cell
Biochem Biophys. 82:1845–1870. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Song C, He L, Zhang J, Ma H, Yuan X, Hu G,
Tao L, Zhang J and Meng J: Fluorofenidone attenuates pulmonary
inflammation and fibrosis via inhibiting the activation of NALP3
inflammasome and IL-1β/IL-1R1/MyD88/NF-κB pathway. J Cell Mol Med.
20:2064–2077. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jegal Y, Kim DS, Shim TS, Lim CM, Do Lee
S, Koh Y, Kim WS, Kim WD, Lee JS, Travis WD, et al: Physiology is a
stronger predictor of survival than pathology in fibrotic
interstitial pneumonia. Am J Respir Crit Care Med. 171:639–644.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Artlett CM, Sassi-Gaha S, Hope JL,
Feghali-Bostwick CA and Katsikis PD: Mir-155 is overexpressed in
systemic sclerosis fibroblasts and is required for NLRP3
inflammasome-mediated collagen synthesis during fibrosis. Arthritis
Res Ther. 19:1442017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang WJ, Chen SJ, Zhou SC, Wu SZ and Wang
H: Inflammasomes and fibrosis. Front Immunol. 12:6431492021.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Colunga Biancatelli RML, Solopov PA and
Catravas JD: The Inflammasome NLR family pyrin domain-containing
protein 3 (NLRP3) as a novel therapeutic target for idiopathic
pulmonary fibrosis. Am J Pathol. 192:837–846. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hsu TC, Tsai CC, Chiu CC, Hsu JD and Tzang
BS: Exacerbating effects of human parvovirus B19 NS1 on liver
fibrosis in NZB/W F1 mice. PLoS One. 8:e683932013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Arvia R, Zakrzewska K, Giovannelli L,
Ristori S, Frediani E, Del Rosso M, Mocali A, Stincarelli MA,
Laurenzana A, Fibbi G and Margheri F: Parvovirus B19 induces
cellular senescence in human dermal fibroblasts: Putative role in
systemic sclerosis-associated fibrosis. Rheumatology (Oxford).
61:3864–3874. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang CL, Chen DY, Tzang CC, Lin JW, Tzang
BS and Hsu TC: Celastrol attenuates human parvovirus B19
NS1-induced NLRP3 inflammasome activation in macrophages. Mol Med
Rep. 28:1932023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Suzuki M, Ikari J, Anazawa R, Tanaka N,
Katsumata Y, Shimada A, Suzuki E and Tatsumi K: PAD4 deficiency
improves bleomycin-induced neutrophil extracellular traps and
fibrosis in mouse lung. Am J Respir Cell Mol Biol. 63:806–818.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen WC, Chen NJ, Chen HP, Yu WK, Su VY,
Chen H, Wu HH and Yang KY: Nintedanib reduces neutrophil chemotaxis
via activating GRK2 in bleomycin-induced pulmonary fibrosis. Int J
Mol Sci. 21:47352020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cheng IY, Liu CC, Lin JH, Hsu TW, Hsu JW,
Li AF, Ho WC, Hung SC and Hsu HS: Particulate matter increases the
severity of bleomycin-induced pulmonary fibrosis through
KC-mediated neutrophil chemotaxis. Int J Mol Sci. 21:2272019.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Garishah FM, Rother N, Riswari SF,
Alisjahbana B, Overheul GJ, van Rij RP, van der Ven A, van der Vlag
J and de Mast Q: Neutrophil extracellular traps in dengue are
mainly generated NOX-independently. Front Immunol. 12:6291672021.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
de Siqueira Santos R, Rochael NC, Mattos
TRF, Fallett E, Silva MF, Linhares-Lacerda L, de Oliveira LT, Cunha
MS, Mohana-Borges R, Gomes TA, et al: Peripheral nervous system is
injured by neutrophil extracellular traps (NETs) elicited by
nonstructural (NS) protein-1 from Zika virus. FASEB J.
37:e231262023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ghazipura M, Mammen MJ, Herman DD, Hon SM,
Bissell BD, Macrea M, Kheir F, Khor YH, Knight SL, Raghu G, et al:
Nintedanib in progressive pulmonary fibrosis: A systematic review
and meta-analysis. Ann Am Thorac Soc. 19:1040–1049. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Singh P, Thampi G, Gupta K, Gangte N,
Pattnaik B, Agrawal A and Kukreti R: Clinical efficacy and safety
evaluation of drug therapies for the treatment of progressive
fibrotic-interstitial lung diseases (PF-ILDs): A network
meta-analysis of randomized controlled trials. Expert Rev Clin
Immunol. 21:1135–1170. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Srivali N, De Giacomi F, Moua T and Ryu
JH: Perioperative antifibrotic therapy for patients with idiopathic
pulmonary fibrosis undergoing lung cancer surgery: A systematic
review and meta-analysis. Heart Lung. 74:266–275. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tang Z, Yang H, Liang X, Chen J, He Q, Zhu
D and Liu Y: Animal models for connective tissue disease-associated
interstitial lung disease: Current status and future directions.
Autoimmun Rev. 24:1039192025. View Article : Google Scholar : PubMed/NCBI
|



















