A 2024 study in ‘The Lancet’ reported that, in 2021,
stroke accounted for almost 50% of cardiovascular disease-related
mortalities in the Western Pacific region, making it a leading
cause of mortality. As populations age, stroke-related mortalities
and disabilities are projected to rise, imposing an increasing
burden on healthcare systems (1,2).
Ischemic stroke (IS) represents 70–80% of all strokes. Mounting
evidence suggests that persistent systemic inflammation after IS
promotes infarct expansion, worsens neurological deficits and
aggravates brain injury (3–5).
Studies on post-stroke inflammation indicate that attenuating acute
inflammatory responses can limit cerebral damage, while
anti-inflammatory therapies may reduce recurrence in patients with
residual inflammation (6,7). Interleukin (IL)-6, a key mediator of
the inflammatory cascade following IS, serves a central role in its
pathophysiological progression (8). Advances in neuroimmunology in the
field of IS mean that elucidating the mechanism of action of IL-6,
investigating its association with IS and developing IL-6-targeted
interventions could lead to new methods for improving the outcomes
for patients with IS.
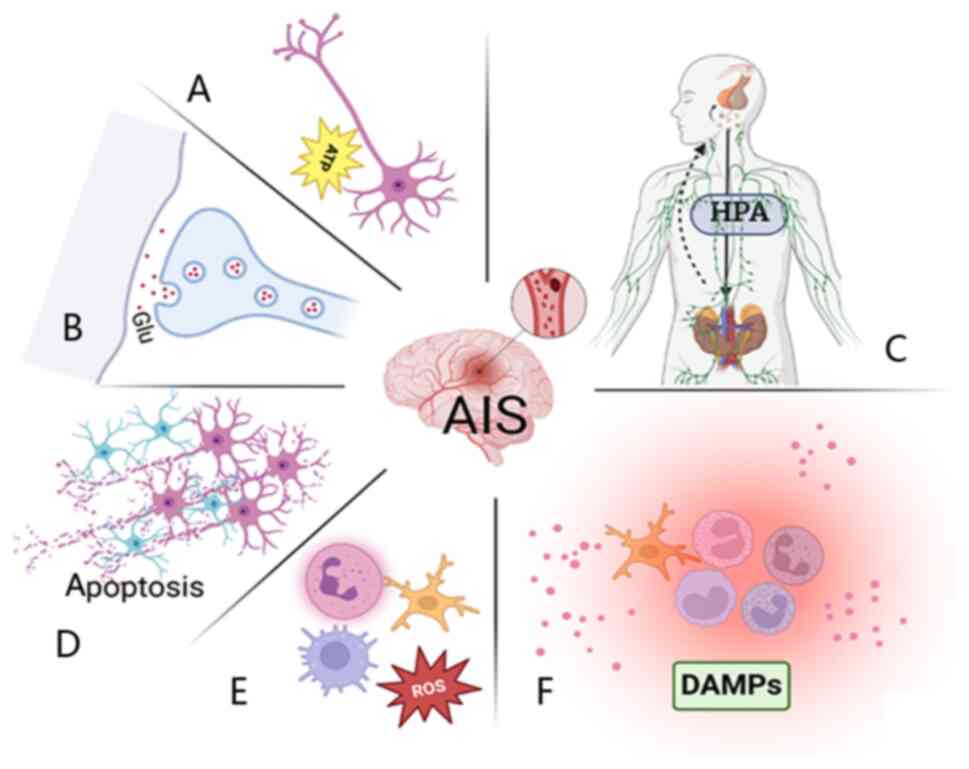
Following IS, the brain rapidly initiates the
ischemic cascade, in which disrupted energy metabolism triggers
excitotoxicity and oxidative stress, leading to neuronal injury and
the release of damage-associated molecular patterns (DAMPs)
(9,10). DAMPs activate downstream signaling
through pattern recognition receptors (PRRs), inducing microglial
M1 polarization and the production of pro-inflammatory cytokines,
including TNF-α and IL-6 (11,12).
Concurrently, activated neutrophils release reactive oxygen species
(ROS), amplifying inflammation and tissue damage (13,14).
These processes collectively disrupt the blood-brain barrier (BBB),
cause microcirculatory failure and promote vasogenic cerebral
edema. Neuroinflammation is not confined to the infarcted region
but propagates throughout the brain, persistently impairing
neurological recovery (15). In
the acute phase of IS, widespread pathological alterations, such as
excitotoxicity, oxidative stress and neuronal apoptosis, as well as
systemic responses, including autonomic dysfunction,
hypothalamic/pituitary/adrenal (HPA) axis overactivation and immune
dysregulation, further aggravate IS-induced injury (9,16)
(Fig. 1).
Notably, inflammation exerts a dual role in
regulating IS-induced neuronal injury: Excessive early responses
exacerbate ischemic damage, whereas moderate inflammation during
later phases facilitates debris clearance, glial scar formation and
neuroregeneration (17,18). Hence, timely modulation of acute
inflammation may slow infarct core expansion and alleviate ischemic
brain injury (19).
IL-6 is a globular glycoprotein composed of 184
amino acids, with a molecular weight of 21–30 kDa. IL-6 contains
two N-glycosylation sites and four conserved cysteine residues that
form a stable antiparallel α-helical structure, maintained by
hydrophobic interactions and disulfide bonds, which are important
for receptor binding and signal transduction. IL-6 has three
receptor-binding sites, one for IL-6 receptor (IL-6R) and two for
glycoprotein 130 (gp130), and is primarily secreted by macrophages
and activated T cells, although endothelial cells and hepatocytes
also contribute to its production (20,21).
IL-6 mediates three distinct signaling modes via two
types of receptors. The membrane-bound IL-6R (mIL-6R) activates the
classical pathway, which exerts regenerative and anti-inflammatory
effects, whereas the soluble IL-6R (sIL-6R) triggers
trans-signaling, which is predominantly pro-inflammatory (22,23).
Both pathways require gp130 for downstream activation (24,25).
Because gp130 is ubiquitously expressed, trans-signaling can occur
in nearly all cell types, amplifying inflammation (26). Additionally, in trans-presentation,
IL-6 bound to dendritic cells is presented to naïve CD4+
T cells, inducing their differentiation into pro-inflammatory T
helper 17 cells (27). Notably,
neurons respond to IL-6 only in the presence of sIL-6R (26).
Through the Janus kinase (JAK)/STAT pathway and
other related pathways, IL-6 regulates cell proliferation,
differentiation and immune homeostasis, serving as an important
bridge between innate and adaptive immunity (8). Under physiological conditions, IL-6
levels in the central nervous system are minimal but rise sharply
during AIS (28). Notable cellular
sources of IL-6 include neurons within the ischemic core,
endothelial cells, microglia and astrocytes (29). Endothelial cell-derived IL-6 and
chemokines recruit neutrophils, which cleave mIL-6R to generate
sIL-6R, thereby amplifying endothelial trans-signaling and
inflammatory responses (22).
Consequently, serum IL-6 levels are markedly elevated in patients
with AIS (30), and clinical
studies have demonstrated a strong association between IL-6
concentration and stroke risk (31). The present review provides a
detailed overview of the pathological mechanisms by which IL-6
contributes to AIS progression.
Oxidative stress refers to a disruption of redox
homeostasis, characterized by excessive oxidant activity leading to
cellular injury. Cerebral ischemia and reperfusion provoke cellular
responses that generate excessive ROS (32). When ROS production surpasses
cellular antioxidant capacity, it triggers lipid peroxidation and
protein and DNA oxidation, also activating redox-sensitive kinases
such as IKKβ, thereby initiating inflammatory and cell death
signaling pathways (33).
Under ischemic conditions, ROS have been shown to
promote neuroinflammation and activate the NACHT, LRR and PYD
domains-containing protein 3 (NLRP3) inflammasome, while also
inducing IL-6 expression through NF-κB activation in vascular
smooth muscle cells (34). In
turn, IL-6 upregulates NADPH oxidase 4 via the JAK/STAT3 pathway,
sustaining ROS production (35).
This ROS/IL-6 positive feedback loop amplifies oxidative stress and
aggravates ischemic brain injury.
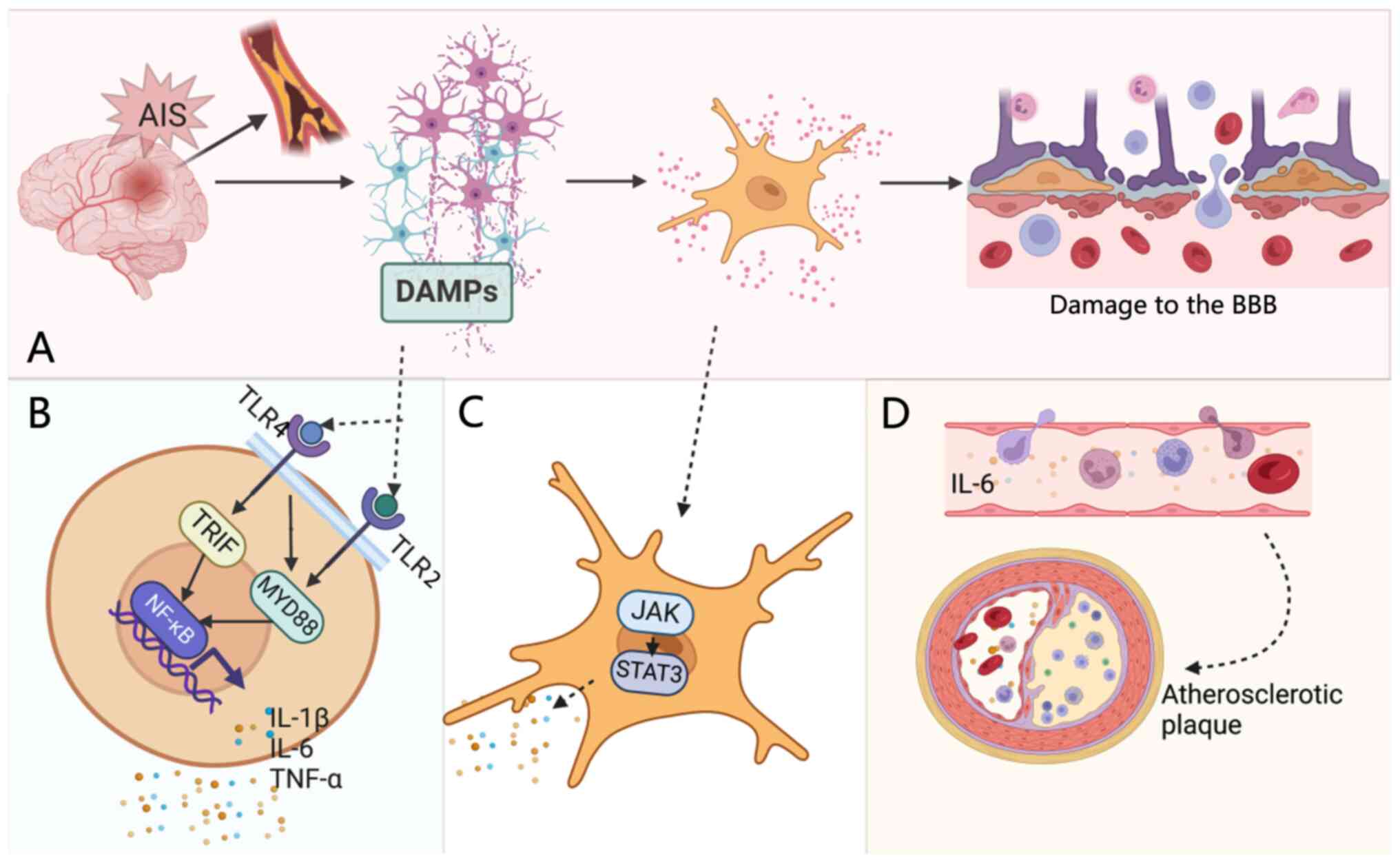
Following cerebral ischemia, DAMPs released from
injured neurons activate inflammatory cascades through PRRs
(6). PRR-mediated activation of
Toll-like receptor (TLR) 2 and TLR4 initiates myeloid
differentiation primary response protein MYD88 (MYD88)- and TIR
domain-containing adapter molecule 1 (TRIF)-dependent signaling
pathways, leading to NF-κB activation and the subsequent secretion
of pro-inflammatory cytokines, such as IL-1β and IL-6 (6,11,15).
Activated microglia and astrocytes further amplify the inflammatory
response by releasing IL-6, which stimulates the JAK/STAT3 pathway
(11). This results in STAT3
phosphorylation and nuclear translocation, driving the
transcription of pro-inflammatory genes and aggravating neuronal
injury and repair impairment (Fig.
2).
In AIS, endothelial injury and BBB disruption
promote sustained NF-κB activation, which enhances IL-6 production.
Elevated IL-6 in turn further amplifies NF-κB-mediated inflammatory
signaling, forming a positive feedback loop that exacerbates
neuroinflammation and tissue damage (36). Peripheral immune cells infiltrate
through the disrupted BBB, propagating local inflammation into a
widespread cerebral response (15). IL-6 promotes microglial M1
polarization via the JAK1/2/STAT1/3 pathway and upregulates
endothelial adhesion molecules, enhancing neutrophil recruitment
and amplifying local inflammation. Furthermore, IL-6 induces
endothelial cadherin disassembly and complement C5a receptor
expression, further compromising BBB integrity (37). It also activates platelets,
facilitating aggregation and contributing to thrombosis by
impairing endothelial function (38,39).
Activated M1-type microglia secrete pro-inflammatory cytokines,
including IL-1β, IL-6 and TNF-α, during the acute phase of IS.
These cytokines have a combined destructive effect, promoting
neuronal death, disrupting the BBB and causing overall brain tissue
damage (40). Clinically, serum
IL-6 levels are independently associated with infarct volume
(41).
Over the long term, as a key pro-inflammatory
mediator, IL-6 accelerates atherosclerotic plaque progression by
activating vascular endothelial cells, forming a notable
pathological basis for large-artery atherosclerotic stroke
(42,43). Through its classical signaling
pathway, IL-6 also stimulates hepatic synthesis of C-reactive
protein (CRP); both IL-6 and CRP serve as established biomarkers
for systemic inflammation and atherosclerotic cardiovascular
disease (ASCVD) risk assessments.
In a hypoxia-ischemia (HI) rat model, small
interfering RNA-mediated silencing of the IL-6 gene markedly
inhibited the activation of the pro-apoptotic protein Bax and the
apoptotic executor protein caspase-3, thereby reducing brain injury
and neuronal apoptosis (44). In
both middle cerebral artery occlusion (MCAO) and oxygen-glucose
deprivation/reoxygenation models, targeting IL-6R was found to
block IL-6 signaling, resulting in reduced expression of the
pro-apoptotic proteins Bax and caspase-3 and upregulation of the
anti-apoptotic protein Bcl-2. This was shown to ultimately lead to
the inhibition of neuronal apoptosis (45). Following IS, inflammatory factors
induce neuronal and glial cell apoptosis by regulating
apoptosis-related genes and signaling pathways, and this apoptosis
is one of the key mechanisms underlying brain injury (44,45).
Following AIS, activation of the HPA axis promotes
the release of corticotropin-releasing hormone (CRH) and
adrenocorticotropic hormone, markedly increasing cortisol (CORT)
levels, which disrupt BBB integrity, facilitate inflammatory
cytokine entry into brain tissue and exacerbate neuroinflammation
(46). Stroke also induces
sympathetic dominance and systemic immune dysregulation,
characterized by increased gut permeability, translocation of
bacterial components into the bloodstream and dysfunction of
peripheral immune organs, such as the bone marrow and thymus
(16).
Under physiological conditions, CORT exerts
anti-inflammatory effects; however, chronic HPA axis
hyperactivation leads to excessive CORT release, resulting in
sustained activation of microglia and astrocytes. Elevated IL-6
suppresses HPA axis negative feedback, maintaining its hyperactive
state (46). IL-6 also promotes
CORT secretion either by directly stimulating hypothalamic CRH
release or by enhancing pituitary sensitivity to CRH (47). Prolonged CORT upregulation may also
induce glucocorticoid receptor resistance, leading to increased
secretion of pro-inflammatory cytokines such as IL-6 (48).
Patients with diabetes frequently exhibit HPA axis
dysregulation and elevated CORT levels (49). A study performed by Kim et
al (50) reported that in
diabetic mice subjected to MCAO, the HPA axis was markedly
overactivated, with mice displaying elevated IL-6, IL-1β and TNF-α
expression. Treatment with metyrapone, a glucocorticoid synthesis
inhibitor, subsequently reduced IL-6 expression and infarct volume.
These findings suggest that under diabetic conditions, IL-6 may
exacerbate stroke-related inflammation by modulating the HPA axis,
providing an extracerebral mechanism linking metabolic and
neuroinflammatory pathways and offering theoretical support for
IL-6-targeted strategies to improve stroke outcomes in diabetic
patients.
IL-6 serves a notable role in atherosclerotic
development and progression, as well as in the pathogenesis of
associated complications. Persistent elevation of IL-6 is a notable
risk factor for atherosclerotic and thrombotic vascular events
(42). Clinical and imaging
studies have demonstrated the potential of IL-6 in predicting
carotid plaque severity, instability and progression (51,52).
As a key pro-inflammatory cytokine, circulating IL-6 reflects
chronic low-grade inflammation that promotes atherothrombosis.
Elevated IL-6 concentrations are consistently associated with
increased risks of coronary artery disease, stroke and myocardial
infarction (52–54). However, the standardization of IL-6
measurement remains a core bottleneck in clinical practice,
constraining its application (8).
Unified testing standards must be established; otherwise, the
adoption of IL-6 measurements in routine clinical practice will
remain limited.
In IS, IL-6 demonstrates notable prognostic
potential. Large-scale prospective studies, including the Reasons
for Geographic and Racial Differences in Stroke cohort, have shown
a dose-dependent relationship between baseline IL-6 levels and
future IS risk, independent of conventional vascular risk factors
(55,56). In AIS, higher IL-6 levels are
associated with multiple acute infarctions (43), greater recurrence risk (57) and worse functional outcomes
(58,59). Kaplan-Meier analyses further reveal
that IL-6 levels above specific thresholds at AIS onset predict
higher rates of stroke recurrence (30). Individual-participant data
meta-analyses have corroborated these findings, showing that IL-6
independently predicts post-stroke vascular events regardless of
underlying atherosclerosis (60).
Genetic evidence indicates that reduced IL-6 pathway
activity markedly lowers the risk of IS, particularly large artery
atherosclerosis (LAA) and small artery occlusion (SAO) subtypes, as
well as coronary and peripheral artery disease, and this protective
association, consistently observed in both European and East Asian
populations, is also linked to a lower incidence of type 2 diabetes
(61). Furthermore, clinical
findings of IL-6 inhibition with ziltivekimab align with these
genetic observations, providing mechanistic support for ongoing
phase III trials and highlighting IL-6 as a promising therapeutic
target for cardiovascular-metabolic disease prevention and
treatment (62). Table I (43,51,54–61)
lists the relevant studies.
The intensity of the inflammatory response in AIS is
closely associated with disease severity. Numerous studies have
provided evidence that higher serum or cerebrospinal fluid levels
of pro-inflammatory cytokines, particularly IL-6, associate with
greater neurological deficits, larger infarct volumes and worse
functional outcomes (63–77). Compared with healthy individuals,
patients with AIS show markedly elevated serum IL-6 levels, and
IL-6-based prediction models for AIS demonstrate strong diagnostic
and prognostic performance (63).
Experimental evidence also indicates that IL-6 measurements can
help estimate stroke onset time (64). Ischemia, hypoxia, oxidative stress,
vascular occlusion and inflammation itself can all induce IL-6
upregulation, which in turn promotes hepatic acute-phase response
protein synthesis, leukocyte recruitment and thrombogenesis,
thereby exacerbating ischemic injury (65). Prospective studies have further
validated the clinical relevance of IL-6. Elevated IL-6 levels
reflect acute disease severity and independently predict short-term
functional outcomes (66–70). In patients undergoing endovascular
therapy (EVT) for large-vessel occlusion, both circulating and
intrathrombus IL-6 concentrations are notably associated with poor
prognosis (71). Longitudinal
studies have demonstrated that dynamic IL-6 changes mirror the
intensity and extent of post-stroke inflammation: Patients with
lower IL-6 levels before thrombolysis achieve improved 90-day
outcomes compared with patients with high IL-6 levels, suggesting
that IL-6 may serve as a potential marker of neurorecovery
(72). After reperfusion, a
decline in patient IL-6 levels indicates successful recanalization
and neurological stabilization, whereas persistent elevation of
IL-6 reflects failed or incomplete reperfusion (73).
IL-6 also exhibits early warning value for early
neurological deterioration (END). Serial monitoring shows that
increased IL-6 levels associate with worsening National Institutes
of Health Stroke Scale scores and adverse neurological evolution
(74–77). In EVT-treated patients, elevated
postoperative IL-6 independently predicts END, underscoring its
role as a quantitative biomarker for early IS risk stratification
and treatment optimization (77).
Collectively, serum IL-6 represents a reliable indicator of AIS
severity and prognosis, and early dynamic monitoring of IL-6 levels
may facilitate the identification of high-risk patients and guide
individualized therapeutic strategies. Table II (41,66–77)
lists the relevant studies.
Biologic agents targeting the IL-6/IL-6R axis are
generally divided into three classes: Anti-IL-6R antibodies,
anti-IL-6 antibodies and soluble gp130 (sgp130)-Fc fusion proteins.
Representative IL-6R inhibitors include tocilizumab, sarilumab and
satralizumab, while siltuximab, ziltivekimab and pacibekitug
selectively target IL-6 itself (78).
Animal studies have shown that targeting the
IL-6/IL-6R axis is therapeutically beneficial. In MCAO mouse
models, sgp130-Fc administration improved long-term survival
outcomes by blocking IL-6 trans-signaling (79). Tocilizumab administered during the
acute phase of IS was shown to reduce infarct volume, with female
mice requiring higher doses compared with males due to elevated
sIL-6R levels (80). Inhibition of
IL-6 trans-signaling also slows or reverses atherosclerotic plaque
formation (81). Table III (79–82)
lists the relevant studies.
Phase II clinical evidence further supports the
therapeutic potential of IL-6 pathway inhibition. The IL-6R
Inhibition in Stroke (IRIS) trial demonstrated that in patients
with anterior circulation large-vessel occlusion-induced AIS, EVT
combined with tocilizumab within 24 h of onset markedly reduced
infarct core volume and may have improved functional outcomes
(83). The Assessing the Effect of
Anti-IL-6 Treatment in Myocardial Infarction trial showed that a
single dose of tocilizumab attenuated myocardial injury in patients
with acute ST-segment elevation myocardial infarction without
serious adverse events (84). In
the RESCUE trial, treatment with the fully human monoclonal
antibody ziltivekimab markedly reduced the levels of
atherosclerosis-related inflammatory and thrombotic biomarkers in
patients with moderate-to-severe chronic kidney disease, likely
through inhibition of collagen-induced platelet activation
(82,85). Furthermore, the TRANQUILITY trial
demonstrated that quarterly administration of the IL-6 inhibitor
pacibekitug effectively lowered high-sensitivity (hs)-CRP levels,
prompting a phase III trial for ASCVD (86). The ongoing ZEUS trial, with an
expected completion date during 2026, aims to determine whether
ziltivekimab can reduce the incidence of cardiovascular events in
the high-risk population that is patients with ASCVD (87,88).
Table IV (83–86)
lists the relevant studies.
IL-6 exerts a prototypical dual effect in
neuroinflammation, with its biological outcomes determined by the
dynamic balance between the anti-inflammatory classical signaling
pathway, mediated by mIL-6R/gp130 signaling and the
pro-inflammatory trans-signaling pathway comprising sIL-6R/gp130
signaling. Notably, sgp130-Fc selectively binds the IL-6/-sIL-6R
binary complex, resulting in blockage of trans-signaling without
interfering with classical IL-6 signaling (89,90).
This property makes sgp130-Fc a promising precision therapeutic
target. IL-6 antagonists have been shown to protect BBB integrity,
reduce cerebral edema and inflammatory injury and attenuate
systemic inflammation by downregulating CRP expression and
inhibiting the JAK/STAT3 pathway (91). Clinically, serum IL-6 levels rise
sharply within the first week after AIS, whereas sgp130 levels
decrease markedly, with sIL-6R levels showing no notable change
(92). This imbalance increases
the ratio of pro-inflammatory IL-6/sIL-6R binary (B) complexes to
inactive IL-6/sIL-6R/sgp130-Fc ternary (T) complexes (B/T ratio). A
biomarker study has shown that elevated B/T ratios are
independently associated with higher future stroke risks in
non-atrial fibrillation populations, suggesting the potential of
this ratio as a biomarker for individualized anti-IL-6 therapy
(93).
Numerous clinical trials have demonstrated that
reducing IL-6 levels can slow the progression of atherosclerosis,
prevent recurrent strokes and reduce the severity of
ischemia-reperfusion injury following stroke recanalization
(51,58,61,85,94).
The CANTOS trial further demonstrated that IL-1β inhibition
indirectly suppresses IL-6 signaling, reducing cardiovascular event
incidence independent of lipid lowering (94). Similarly, colchicine, by inhibiting
NLRP3 inflammasome activation and subsequently reducing IL-6
levels, markedly decreases IS incidence and the occurrence of major
adverse cardiovascular events in secondary prevention without
increasing bleeding risk (95,96).
Reperfusion therapies themselves can exacerbate inflammation.
Tissue plasminogen activator, while restoring perfusion, may
disrupt the BBB and increase hemorrhagic transformation risk
through IL-6-driven inflammatory cascades (97). In mechanical thrombectomy (MT),
postoperative systemic inflammation has been identified as a
notable determinant of poor patient outcomes (98). Patients that develop systemic
inflammatory response syndrome after MT exhibit higher mortality
and worse neurological recovery compared with patients that do not
(99). The IRIS trial demonstrated
that adjunctive IL-6R blockade with tocilizumab during EVT markedly
reduced infarct core size and improved neurological outcomes
(83).
Genetic evidence also supports the notion that
decreased IL-6 levels are associated with improved patient outcomes
for a number of diseases: Attenuation of IL-6 signaling reduces the
risk of poor IS outcomes among patients with rheumatoid arthritis
(100). Genetic variability
further influences IL-6 bioactivity. The 3′ untranslated region
polymorphism rs2228145 on the C allele of IL-6R markedly increases
sIL-6R levels, enhancing the buffering capacity of patients against
IL-6-mediated inflammation (61).
As serum sgp130 concentrations generally exceed sIL-6R levels, the
latter becomes the rate-limiting component of this neutralization
system (101). Carriers of the
rs2228145-C allele exhibit protective effects against
cardiovascular and autoimmune diseases akin to treatment with
tocilizumab (102). Mendelian
randomization studies confirm that genetically-reduced IL-6
signaling lowers atherosclerotic and IS risk, particularly in LAA
and SAO subtypes, although this potentially occurs at the cost of
increased infection susceptibility (61,103).
Due to the low serum concentration, short half-life
and multifactorial regulation of IL-6, including factors such as
circadian rhythm, glucose level, age and comorbidities (104,105), precise monitoring and
individualized assessment of IL-6 levels are necessary. Future
studies should integrate differences in model sex, genetic
polymorphisms, dynamic B/T ratio monitoring and hs-CRP trajectories
to develop adaptive dosing algorithms, facilitating the transition
of IL-6-related indicators from biomarkers to therapeutic targets.
The present review also had certain limitations regarding the
previous studies discussed, including small cohort sizes across
individual studies, inconsistent timing of IL-6 testing and assay
variability.
In summary, accumulating mechanistic and clinical
evidence positions the IL-6/IL-6R axis as a notable link between
neuroinflammation, atherosclerosis and ischemic injury. Targeted
IL-6/IL-6R inhibition holds promise not only for neuroprotection in
AIS but also for secondary cardiovascular prevention (83–86).
Future clinical translation should establish unified standards for
IL-6 detection, and focus on identifying appropriate patient
subgroups for testing, optimizing treatment timing and dosage and
balancing the anti-inflammatory efficacy of IL-6/IL-6R inhibition
with immune safety to realize the full therapeutic potential of
IL-6-targeted interventions in stroke management.
Not applicable.
The present review was funded by the Dalian Medical Science
Research Program (grant no. 2211046).
Not applicable.
XW was responsible for the conceptualization of the
research project and drafting of the initial manuscript. XZ
contributed to study conceptualization and reviewed the manuscript.
JL contributed to the conclusion and provided critical insights. PL
contributed towards conceptualization of the research project, as
well as reviewing and revising the manuscript. Data authentication
is not applicable. All authors read and approved the final version
of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Wang H, Yu X, Guo J, Ma S, Liu Y, Hu Y, Li
J, Song Y and Zou Z: Burden of cardiovascular disease among the
Western Pacific region and its association with human resources for
health, 1990–2021: A systematic analysis of the global burden of
disease study 2021. Lancet Reg Health West Pac.
51:1011952024.PubMed/NCBI
|
|
2
|
Ji C, Ge X, Zhang J and Tong H: The stroke
burden in china and its long-term trends: Insights from the global
burden of disease (GBD) study 1990–2021. Nutr Metab Cardiovasc Dis.
35:1038482025. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fu Y, Liu Q, Anrather J and Shi FD: Immune
interventions in stroke. Nat Rev Neurol. 11:524–535. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li M, Li Z, Yao Y, Jin WN, Wood K, Liu Q,
Shi FD and Hao J: Astrocyte-derived interleukin-15 exacerbates
ischemic brain injury via propagation of cellular immunity. Proc
Natl Acad Sci USA. 114:E396–E405. 2017.PubMed/NCBI
|
|
5
|
Zhang F, Yan C, Wei C, Yao Y, Ma X, Gong
Z, Liu S, Zang D, Chen J, Shi FD and Hao J: Vinpocetine inhibits
NF-κB-dependent inflammation in acute ischemic stroke patients.
Transl Stroke Res. 9:174–184. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Endres M, Moro MA, Nolte CH, Dames C,
Buckwalter MS and Meisel A: Immune pathways in etiology, acute
phase, and chronic sequelae of ischemic stroke. Circ Res.
130:1167–1186. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zietz A, Gorey S, Kelly PJ, Katan M and
McCabe JJ: Targeting inflammation to reduce recurrent stroke. Int J
Stroke. 19:379–387. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pawluk H, Woźniak A, Tafelska-Kaczmarek A,
Kosinska A, Pawluk M, Sergot K, Grochowalska R and Kołodziejska R:
The role of IL-6 in ischemic stroke. Biomolecules. 15:4702025.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cui P, McCullough LD and Hao J: Brain to
periphery in acute ischemic stroke: Mechanisms and clinical
significance. Front Neuroendocrinol. 63:1009322021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stanzione R, Forte M, Cotugno M, Bianchi
F, Marchitti S and Rubattu S: Role of DAMPs and of leukocytes
infiltration in ischemic stroke: Insights from animal models and
translation to the human disease. Cell Mol Neurobiol. 42:545–556.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kumari S, Dhapola R, Sharma P, Nagar P,
Medhi B and HariKrishnaReddy D: The impact of cytokines in
neuroinflammation-mediated stroke. Cytokine Growth Factor Rev.
78:105–119. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lai TW, Zhang S and Wang YT:
Excitotoxicity and stroke: Identifying novel targets for
neuroprotection. Prog Neurobiol. 115:157–188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tang C, Wang C, Zhang Y, Xue L, Li Y, Ju C
and Zhang C: Recognition, intervention, and monitoring of
neutrophils in acute ischemic stroke. Nano Lett. 19:4470–4477.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dhanesha N, Patel RB, Doddapattar P,
Ghatge M, Flora GD, Jain M, Thedens D, Olalde H, Kumskova M, Leira
EC and Chauhan AK: PKM2 promotes neutrophil activation and cerebral
thromboinflammation: Therapeutic implications for ischemic stroke.
Blood. 139:1234–1245. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi K, Tian DC, Li ZG, Ducruet AF, Lawton
MT and Shi FD: Global brain inflammation in stroke. Lancet Neurol.
18:1058–1066. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iadecola C, Buckwalter MS and Anrather J:
Immune responses to stroke: Mechanisms, modulation, and therapeutic
potential. J Clin Invest. 130:2777–2788. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Q, Tang XN and Yenari MA: The
inflammatory response in stroke. J Neuroimmunol. 184:53–68. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jayaraj RL, Azimullah S, Beiram R, Jalal
FY and Rosenberg GA: Neuroinflammation: Friend and foe for ischemic
stroke. J Neuroinflammation. 16:1422019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu D, Chen WQ, Wang YP, Duan WY, Guo L,
Wang L, Liu L, Xu AD and Wang YJ: Scientific Statements on Brain
Cytoprotection in Ischemic Stroke—A Scientific Statement from the
Chinese Stroke Association. Chin J Stroke. 19:938–55. 2024.(In
Chinese).
|
|
20
|
Tanaka T and Kishimoto T: The biology and
medical implications of interleukin-6. Cancer Immunol Res.
2:288–294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Taher MY, Davies DM and Maher J: The role
of the interleukin (IL)-6/IL-6 receptor axis in cancer. Biochem Soc
Trans. 46:1449–1462. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Scheller J, Chalaris A, Schmidt-Arras D
and Rose-John S: The pro- and anti-inflammatory properties of the
cytokine interleukin-6. Biochim Biophys Acta. 1813:878–888. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Montgomery A, Tam F, Gursche C, Cheneval
C, Besler K, Enns W, Manku S, Rey K, Hanson PJ, Rose-John S, et al:
Overlapping and distinct biological effects of IL-6 classic and
trans-signaling in vascular endothelial cells. Am J Physiol Cell
Physiol. 320:C554–C565. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mihara M, Hashizume M, Yoshida H, Suzuki M
and Shiina M: IL-6/IL-6 receptor system and its role in
physiological and pathological conditions. Clin Sci (Lond).
122:143–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Heo TH, Wahler J and Suh N: Potential
therapeutic implications of IL-6/IL-6R/gp130-targeting agents in
breast cancer. Oncotarget. 7:15460–15473. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
März P, Cheng JG, Gadient RA, Patterson
PH, Stoyan T, Otten U and Rose-John S: Sympathetic neurons can
produce and respond to interleukin 6. Proc Natl Acad Sci USA.
95:3251–3256. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Heink S, Yogev N, Garbers C, Herwerth M,
Aly L, Gasperi C, Husterer V, Croxford AL, Möller-Hackbarth K,
Bartsch HS, et al: Trans-presentation of IL-6 by dendritic cells is
required for the priming of pathogenic T(H)17 cells. Nat Immunol.
18:74–85. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gu Y, He M, Zhou X, Liu J, Hou N, Bin T,
Zhang Y, Li T and Chen J: Endogenous IL-6 of mesenchymal stem cell
improves behavioral outcome of hypoxic-ischemic brain damage
neonatal rats by supressing apoptosis in astrocyte. Sci Rep.
6:185872016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu H, Hu S, Li Y, Sun Y, Xiong X, Hu X,
Chen J and Qiu S: Interleukins and ischemic stroke. Front Immunol.
13:8284472022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pacinella G, Ciaccio AM, Casuccio A,
Daidone M, Pecoraro R, Di Bona D, Del Cuore A, Puleo MG, Di
Raimondo D, Di Chiara T, et al: Genetic polymorphisms and cytokine
levels in ischemic stroke: Associations with subtypes and
prognosis. Expert Rev Clin Immunol. 21:961–976. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang X, Zhang M, Wang J and Hu F:
Association between interleukin-6 levels and stroke: A systematic
review and meta-analysis. J Int Med Res. 52:30006052412746262024.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sanderson TH, Reynolds CA, Kumar R,
Przyklenk K and Hüttemann M: Molecular mechanisms of
ischemia-reperfusion injury in brain: Pivotal role of the
mitochondrial membrane potential in reactive oxygen species
generation. Mol Neurobiol. 47:9–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shirley R, Ord EN and Work LM: Oxidative
stress and the use of antioxidants in stroke. Antioxidants (Basel).
3:472–501. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dong X, Wu D, Zhang Y, Jia L, Pan X, Sun J
and Pan LL: Cathelicidin modulates vascular smooth muscle cell
phenotypic switching through ROS/IL-6 pathway. Antioxidants
(Basel). 9:4912020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yan M, Sun Z, Zhang S, Yang G, Jiang X,
Wang G, Li R, Wang Q and Tian X: SOCS modulates JAK-STAT pathway as
a novel target to mediate the occurrence of neuroinflammation:
Molecular details and treatment options. Brain Res Bull.
213:1109882024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang SR, Li CT, Yang Y and Zhang LH:
Oxidative stress, inflammation and autophagy in cerebral ischemic
injury: mechanisms and therapeutic strategies. Chin J Pharmacol
Toxicol. 32:651–660. 2018.(In Chinese).
|
|
37
|
Kang S and Kishimoto T: Interplay between
interleukin-6 signaling and the vascular endothelium in cytokine
storms. Exp Mol Med. 53:1116–1123. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Henein MY, Vancheri S, Longo G and
Vancheri F: The role of inflammation in cardiovascular disease. Int
J Mol Sci. 23:129062022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Webb CE, Vautrinot J and Hers I: IL-6 as a
mediator of platelet hyper-responsiveness. Cells. 14:7662025.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang W, Tian T, Gong SX, Huang WQ, Zhou
QY, Wang AP and Tian Y: Microglia-associated neuroinflammation is a
potential therapeutic target for ischemic stroke. Neural Regen Res.
16:6–11. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Purroy F, Farré-Rodriguez J,
Mauri-Capdevila G, Vicente-Pascual M and Farré J: Basal IL-6 and
S100b levels are associated with infarct volume. Acta Neurol Scand.
144:517–523. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ohta M, Kihara T, Toriuchi K, Aoki H,
Iwaki S, Kakita H, Yamada Y and Aoyama M: IL-6 promotes cell
adhesion in human endothelial cells via microRNA-126-3p
suppression. Exp Cell Res. 393:1120942020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mo J, Chen Z, Wang M, Cheng A, Li J, Pan
Y, Jiang Y, Jing J, Wang Y, Pu Y and Li Z: Association between
interleukin-6 and multiple acute infarctions in symptomatic
intracranial atherosclerotic disease. Curr Neurovasc Res.
21:292–299. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang X, Liao KH, Deng IB and Zhang LC:
Knockdown of interleukin-6 plays a neuroprotective role against
hypoxia-ischemia in neonatal rats via inhibition of caspase 3 and
Bcl-2-associated X protein signaling pathway. Ibrain. 8:413–428.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhan L, Mu Z, Jiang H, Zhang S, Pang Y,
Jin H, Chen J, Jia C and Guo H: MiR-21-5p protects against ischemic
stroke by targeting IL-6R. Ann Transl Med. 11:1012023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Radak D, Resanovic I and Isenovic ER:
Changes in hypothalamus-pituitary-adrenal axis following transient
ischemic attack. Angiology. 65:723–732. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Crofford LJ, Kalogeras KT, Mastorakos G,
Magiakou MA, Wells J, Kanik KS, Gold PW, Chrousos GP and Wilder RL:
Circadian relationships between interleukin (IL)-6 and
hypothalamic-pituitary-adrenal axis hormones: Failure of IL-6 to
cause sustained hypercortisolism in patients with early untreated
rheumatoid arthritis. J Clin Endocrinol Metab. 82:1279–1283. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hassamal S: Chronic stress,
neuroinflammation, and depression: An overview of
pathophysiological mechanisms and emerging anti-inflammatories.
Front Psychiatry. 14:11309892023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Roy M, Collier B and Roy A:
Hypothalamic-pituitary-adrenal axis dysregulation among diabetic
outpatients. Psychiatry Res. 31:31–37. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kim S, Park ES, Chen PR and Kim E:
Dysregulated hypothalamic-pituitary-adrenal axis is associated with
increased inflammation and worse outcomes after ischemic stroke in
diabetic mice. Front Immunol. 13:8648582022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kamtchum-Tatuene J, Saba L, Heldner MR,
Poorthuis MHF, de Borst GJ, Rundek T, Kakkos SK, Chaturvedi S,
Topakian R, Polak JF, et al: Interleukin-6 predicts carotid plaque
severity, vulnerability, and progression. Circ Res. 131:e22–e33.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mehta NN, deGoma E and Shapiro MD: IL-6
and cardiovascular risk: A narrative review. Curr Atheroscler Rep.
27:122024. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lundin JI, Peters U, Hu Y, Ammous F,
Benjamin EJ, Bis JC, Brody JA, Cushman M, Fuller H, Gignoux C, et
al: Epigenetic mechanisms underlying variation of IL-6, a
well-established inflammation biomarker and risk factor for
cardiovascular disease. Atherosclerosis. 407:1202192025. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kitagawa K, Toi S, Yoshizawa H, Sato Y and
Todo K: Association between serum levels of interleukin-6 and
stroke, cardiovascular events, and Alzheimer's disease dementia. J
Atheroscler Thromb. 32:1390–1399. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Jenny NS, Callas PW, Judd SE, McClure LA,
Kissela B, Zakai NA and Cushman M: Inflammatory cytokines and
ischemic stroke risk: The REGARDS cohort. Neurology.
92:e2375–e2384. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Papadopoulos A, Palaiopanos K, Björkbacka
H, Peters A, de Lemos JA, Seshadri S, Dichgans M and Georgakis MK:
Circulating interleukin-6 levels and incident ischemic stroke: A
systematic review and meta-analysis of prospective studies.
Neurology. 98:e1002–e1012. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Jiang Y and Fan T: IL-6 and stroke
recurrence in ischemic stroke. Biomark Med. 18:739–747. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Xu J, Mo J, Zhang X, Chen Z, Pan Y, Yan H,
Meng X and Wang Y: Nontraditional risk factors for residual
recurrence risk in patients with ischemic stroke of different
etiologies. Cerebrovasc Dis. 51:630–638. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li J, Lin J, Pan Y, Wang M, Meng X, Li H,
Wang Y, Zhao X, Qin H, Liu L, et al: Interleukin-6 and YKL-40
predicted recurrent stroke after ischemic stroke or TIA: analysis
of 6 inflammation biomarkers in a prospective cohort study. J
Neuroinflammation. 19:1312022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
McCabe JJ, Harris K, Walsh C, Gorey S,
Arnold M, De Marchis GM, Hervella P, Iglesias-Rey R, Jern C, Katan
M, et al: Interleukin-6, C-reactive protein, and vascular
recurrence after stroke with and without atherosclerosis. Stroke.
56:2588–2596. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Georgakis MK, Malik R, Gill D,
Franceschini N, Sudlow CLM and Dichgans M; INVENT Consortium,
CHARGE Inflammation Working Group, : Interleukin-6 signaling
effects on ischemic stroke and other cardiovascular outcomes: A
mendelian randomization study. Circ Genom Precis Med.
13:e0028722020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang L, Omarov M, Xu L, deGoma E,
Natarajan P and Georgakis MK: IL6 genetic perturbation mimicking
IL-6 inhibition is associated with lower cardiometabolic risk. Nat
Cardiovasc Res. 4:1172–1186. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Cui JJ, Zhang CZ, Zhu B, Xu ZP and Liu YY:
Serum miR-21-5p, IL-6, and IL-8 Expression Levels and Predictive
Model Construction in Patients with Cerebral Infarction. Chin J
Gerontology. 43:4103–4106. 2023.(In Chinese).
|
|
64
|
Kowalski RG, Ledreux A, Violette JE,
Paustian W, Sillau S, Thompson JA, Neumann RT, Ornelas D, Monte AA,
Dylla L, et al: Circulating interleukin-6 levels and timing of
acute ischemic stroke onset. Ann Clin Transl Neurol. 12:1926–1931.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Su JH, Luo MY, Liang N, Gong SX, Chen W,
Huang WQ, Tian Y and Wang AP: Interleukin-6: A novel target for
cardio-cerebrovascular diseases. Front Pharmacol. 12:7450612021.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Mosarrezaii A, Amiri-Nikpour MR, Mehryar
HR, Choobi Anzali B, Nourooz-Zadeh S, Babaei S and Farrokhi H:
Investigating the relationship between interleukin-6 serum levels
and outcome in acute ischemic CVA. Brain Behav. 10:e016682020.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Alfieri DF, Lehmann MF, Flauzino T, de
Araújo MCM, Pivoto N, Tirolla RM, Simão ANC, Maes M and Reiche EMV:
Immune-inflammatory, metabolic, oxidative, and nitrosative stress
biomarkers predict acute ischemic stroke and short-term outcome.
Neurotox Res. 38:330–343. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Li X, Lin S, Chen X, Huang W, Li Q, Zhang
H, Chen X, Yang S, Jin K and Shao B: The prognostic value of serum
cytokines in patients with acute ischemic stroke. Aging Dis.
10:544–556. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Jia WL, Jiang YY, Jiang Y, Meng X, Li H,
Zhao XQ, Wang YL, Wang YJ, Gu HQ and Li ZX: Associations between
admission levels of multiple biomarkers and subsequent worse
outcomes in acute ischemic stroke patients. J Cereb Blood Flow
Metab. 44:742–756. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Gu HQ, Yang KX, Li JJ, Lin JX, Jing J,
Xiong YY, Zhao XQ, Wang YL, Liu LP, Meng X, et al: Mediation effect
of stroke recurrence in the association between post-stroke
interleukin-6 and functional disability. CNS Neurosci Ther.
29:3579–3587. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Dargazanli C, Blaquière M, Moynier M, de
Bock F, Labreuche J, Ter Schiphorst A, Derraz I, Radu RA, Gascou G,
Lefevre PH, et al: Inflammation biomarkers in the intracranial
blood are associated with outcome in patients with ischemic stroke.
J Neurointerv Surg. 17:159–166. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Pawluk H, Kołodziejska R, Grześk G,
Kozakiewicz M, Woźniak A, Pawluk M, Kosinska A, Grześk M, Wojtasik
J and Kozera G: Selected mediators of inflammation in patients with
acute ischemic stroke. Int J Mol Sci. 23:106142022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Hervella P, Rodríguez-Castro E,
Rodríguez-Yáñez M, Arias S, Santamaría-Cadavid M, López-Dequidt I,
Estany-Gestal A, Maqueda E, López-Loureiro I, Sobrino T, et al:
Intra- and extra-hospital improvement in ischemic stroke patients:
Influence of reperfusion therapy and molecular mechanisms. Sci Rep.
10:35132020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Băcilă CI, Vlădoiu MG, Văleanu M, Moga DF
and Pumnea PM: The role of IL-6 and TNF-alpha biomarkers in
predicting disability outcomes in acute ischemic stroke patients.
Life Basel). 15:472025. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yi L, Li ZX, Jiang YY, Jiang Y, Meng X, Li
H, Zhao XQ, Wang YL, Liu LP, Wang YJ and Gu HQ: Inflammatory marker
profiles and in-hospital neurological deterioration in patients
with acute minor ischemic stroke. CNS Neurosci Ther. 30:e146482024.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Sabir Rashid A, Huang-Link Y, Johnsson M,
Wetterhäll S and Gauffin H: Predictors of early neurological
deterioration and functional outcome in acute ischemic stroke: The
importance of large artery disease, hyperglycemia and inflammatory
blood biomarkers. Neuropsychiatr Dis Treat. 18:1993–2002. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Deng QW, Huang S, Li S, Zhai Q, Zhang Q,
Wang ZJ, Chen WX, Sun H, Lu M and Zhou J: Inflammatory factors as
potential markers of early neurological deterioration in acute
ischemic stroke patients receiving endovascular therapy-the AISRNA
study. J Inflamm Res. 14:4399–4407. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Grebenciucova E and VanHaerents S:
Interleukin 6: At the interface of human health and disease. Front
Immunol. 14:12555332023. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Hall C, Nguyen DT, Mendoza K, Tan C and
Chauhan A: Inhibition of IL-6 trans-signaling promotes post-stroke
functional recovery in a sex and dose-dependent manner. J
Neuroinflammation. 22:522025. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Hudobenko J, Chauhan A and McCullough L:
Abstract 128: Amelioration of ischemic stroke damage through
inhibition of interleukin-6 signaling with tocilizumab requires sex
specific dosing. Stroke. 50 (Suppl 1):A1282019. View Article : Google Scholar
|
|
81
|
Schuett H, Oestreich R, Waetzig GH, Annema
W, Luchtefeld M, Hillmer A, Bavendiek U, von Felden J, Divchev D,
Kempf T, et al: Transsignaling of interleukin-6 crucially
contributes to atherosclerosis in mice. Arterioscler Thromb Vasc
Biol. 32:281–290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Ministrini S, Liberale L, Puspitasari YM,
Han J, Kirmes K, Unkelbach LP, Tirandi A, Niederberger R, Bengs S,
Beer JH, et al: Direct interleukin-6 inhibition blunts arterial
thrombosis by reducing collagen-mediated platelet activation.
Arterioscler Thromb Vasc Biol. 45:1432–1439. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Chu X, Ma Z, Liu Y, Sun J, Wang N, Li C,
Feng X, Li J, Wang B, Zhou C, et al: IRIS, a randomised,
double-blind, placebo-controlled trial of interleukin-6 receptor
inhibition undergoing endovascular treatment in acute anterior
circulation ischaemic stroke: Study rationale and design. Stroke
Vasc Neurol. 10:514–519. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Huse C, Anstensrud AK, Michelsen AE,
Ueland T, Broch K, Woxholt S, Yang K, Sharma K, Tøllefsen IM, Bendz
B, et al: Interleukin-6 inhibition in ST-elevation myocardial
infarction: Immune cell profile in the randomised ASSAIL-MI trial.
EBioMedicine. 80:1040132022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Ridker PM, Devalaraja M, Baeres FMM,
Engelmann MDM, Hovingh GK, Ivkovic M, Lo L, Kling D, Pergola P, Raj
D, et al: IL-6 inhibition with ziltivekimab in patients at high
atherosclerotic risk (RESCUE): A double-blind, randomised,
placebo-controlled, phase 2 trial. Lancet. 397:2060–2069. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
GlobeNewswire, . Tourmaline Bio Announces
Positive Topline Results from the Ongoing Phase 2 TRANQUILITY Trial
Evaluating Pacibekitug in Patients with Elevated High-Sensitivity
C-reactive Protein and Chronic Kidney Disease. Tourmaline Inc.;
https://www.globenewswire.com/news-release/2025/05/20/3084742/0/en/Tourmaline-Bio-Announces-Positive-Topline-Results-from-the-Ongoing-Phase-2-TRANQUILITY-Trial-Evaluating-Pacibekitug-in-Patients-with-Elevated-High-Sensitivity-C-reactive-Protein-an.htmlAugust
7–2025
|
|
87
|
Ridker PM: From RESCUE to ZEUS: will
interleukin-6 inhibition with ziltivekimab prove effective for
cardiovascular event reduction? Cardiovasc Res. 117:e138–e140.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Ridker PM and Rane M: Interleukin-6
signaling and anti-interleukin-6 therapeutics in cardiovascular
disease. Circ Res. 128:1728–1746. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Rose-John S, Jenkins BJ, Garbers C, Moll
JM and Scheller J: Targeting IL-6 trans-signalling: Past, present
and future prospects. Nat Rev Immunol. 23:666–681. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Jostock T, Müllberg J, Ozbek S, Atreya R,
Blinn G, Voltz N, Fischer M, Neurath MF and Rose-John S: Soluble
gp130 is the natural inhibitor of soluble interleukin-6 receptor
transsignaling responses. Eur J Biochem. 268:160–167. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Weiss A and Ding Y: Beyond reperfusion:
Adjunctive therapies targeting inflammation, edema, and blood-brain
barrier dysfunction in ischemic stroke. Cerebrovasc Dis. 1–10.
2025.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Acalovschi D, Wiest T, Hartmann M, Farahmi
M, Mansmann U, Auffarth GU, Grau AJ, Green FR, Grond-Ginsbach C and
Schwaninger M: Multiple levels of regulation of the interleukin-6
system in stroke. Stroke. 34:1864–1869. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Ziegler L, Wallén H, Aspberg S, de Faire U
and Gigante B: IL6 trans-signaling associates with ischemic stroke
but not with atrial fibrillation. BMC Neurol. 21:3062021.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ridker PM, Libby P, MacFadyen JG, Thuren
T, Ballantyne C, Fonseca F, Koenig W, Shimokawa H, Everett BM and
Glynn RJ: Modulation of the interleukin-6 signalling pathway and
incidence rates of atherosclerotic events and all-cause mortality:
Analyses from the canakinumab anti-inflammatory thrombosis outcomes
study (CANTOS). Eur Heart J. 39:3499–3507. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhu S, Pan W, Yao Y and Shi K: The
efficacy of colchicine compared to placebo for preventing ischemic
stroke among individuals with established atherosclerotic
cardiovascular diseases: A systematic review and meta-analysis.
Scand Cardiovasc J. 59:24411122025. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Fiolet ATL, Poorthuis MHF, Opstal TSJ,
Amarenco P, Boczar KE, Buysschaert I, Budgeon C, Chan NC, Cornel
JH, Jolly SS, et al: Colchicine for secondary prevention of
ischaemic stroke and atherosclerotic events: A meta-analysis of
randomised trials. EClinicalMedicine. 76:1028352024. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Shi K, Zou M, Jia DM, Shi S, Yang X, Liu
Q, Dong JF, Sheth KN, Wang X and Shi FD: tPA mobilizes immune cells
that exacerbate hemorrhagic transformation in stroke. Circ Res.
128:62–75. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Wang H, Tian X, Liao Z, Yue X, Sun L, Li
X, Zou M and Ding J: Inflammatory biomarkers may be associated with
poor outcomes after mechanical thrombectomy. Thromb J. 22:582024.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Jafari M, Katlowitz K, De la Garza C,
Sellers A, Moore S, Hall H, Desai A, Singh V and Damani R: Impact
of systemic inflammatory response syndrome on acute ischemic stroke
patients treated with mechanical thrombectomy. J Neurol Sci.
430:1199882021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Jia Y, Zhang K, Shi M, Guo D, Yang P, Bu
X, Chen J, Wang A, Xu T, He J, et al: Associations of rheumatoid
factor, rheumatoid arthritis, and interleukin-6 inhibitor with the
prognosis of ischemic stroke: A prospective multicenter cohort
study and mendelian randomization analysis. Transl Stroke Res.
15:750–760. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Scheller J and Rose-John S: The
interleukin 6 pathway and atherosclerosis. Lancet. 380:3382012.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zhang M, Bai Y, Wang Y, Cui H, Tang M,
Wang L, Wang X and Gu D: Cumulative evidence for associations
between genetic variants in interleukin 6 receptor gene and human
diseases and phenotypes. Front Immunol. 13:8607032022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Georgakis MK, Malik R, Li X, Gill D, Levin
MG, Vy HMT, Judy R, Ritchie M, Verma SS; Regeneron Genetics Center,
; et al: Genetically downregulated interleukin-6 signaling is
associated with a favorable cardiometabolic profile: A phenome-wide
association study. Circulation. 143:1177–1780. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Said EA, Al-Reesi I, Al-Shizawi N, Jaju S,
Al-Balushi MS, Koh CY, Al-Jabri AA and Jeyaseelan L: Defining IL-6
levels in healthy individuals: A meta-analysis. J Med Virol.
93:3915–3924. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Docherty S, Harley R, McAuley JJ, Crowe
LAN, Pedret C, Kirwan PD, Siebert S and Millar NL: The effect of
exercise on cytokines: Implications for musculoskeletal health: A
narrative review. BMC Sports Sci Med Rehabil. 14:52022. View Article : Google Scholar : PubMed/NCBI
|