Introduction
Estrogen deficiency-induced osteoporosis is a bone
metabolic disease characterized by changes in bone density and
microstructure, resulting in increased bone fragility and a higher
risk of fracture. After menopause, patients lose ~50% of trabecular
bone mass and 30% of cortical bone mass compared with those at
20–30 years of age (1).
Postmenopausal osteoporosis (PMOP) is directly related to an
increased risk of bone fracture. A European Union study showed that
a 50-year-old female patient has a 46% chance of developing an
osteoporotic fracture in their lifetime (2). Currently, the commonly used
therapeutic drugs in clinics for PMOP include hormone replacement
therapy, bisphosphonate, parathyroid hormone and selective estrogen
receptor modulators. Hormone replacement therapy is the most
effective treatment, but is associated with risks such as breast
cancer, uterine bleeding and cardiovascular disease (3).
YC is the dry aboveground part of Artemisia
scoparia Waldst. et Kit. or Artemisia capillaris Thunb.
YC extract alleviates bone loss through a dual mechanism: Enhancing
osteoblast-mediated mineralization and suppressing osteoclast
differentiation (4). Using mass
spectrometric analysis and activity screening, subsequent studies
have further identified specific bioactive constituents responsible
for inhibiting osteoclast formation and bone resorption, primarily
via attenuation of acidification processes. These findings indicate
the potential of YC as a natural candidate for the treatment of
osteoporosis (4,5). The main components of YC include
coumarin, flavonoids, organic acids, volatile oil and terpenoids,
which have anti-inflammatory, antioxidative, antibacterial,
cytoprotective and anti-osteoporotic effects. Chlorogenic acid,
artemisinolide, hyperoside and scopoletin have been reported to be
the most active ingredients in the extract (6–11).
Chlorogenic acid can promote bone marrow stromal
cell (BMSC) proliferation and osteogenic differentiation through
the Shp2 pathway (12). In
addition, chlorogenic acid upregulates the neuronatin gene in
ovariectomized (OVX) mice, activates the MAPK signaling pathway and
promotes the osteogenic differentiation of BMSCs (13). It can also activate the nuclear
factor erythroid 2-related factor 2 (Nrf2)/heme oxygenase 1 (HO-1)
pathway, inhibit the excessive production of reactive oxygen
species (ROS) and reduce oxidative stress levels (14). Chlorogenic acid has also been shown
to downregulate the receptor activator of NF-κB ligand
(RANKL)-induced phosphorylation of p38 and ERK in bone marrow
macrophages, and to inhibit the expression of nuclear factor of
activated T-cells, cytoplasmic 1, thereby inhibiting osteoclast
differentiation and bone resorption (15,16).
Hyperoside can promote the proliferation and osteogenic
differentiation of BMSCs and improve the bone microstructure of OVX
mice by enhancing microRNA-19a-5p and downregulating IL-17A
(16–18). Furthermore, YC can downregulate the
expression of NADPH oxidase 1 and inhibit RANKL-induced osteoclast
differentiation, thereby inhibiting bone loss (17). Scopoletin can inhibit osteoclast
differentiation by scavenging ROS (19).
Cell death consists of programmed cell death and
non-programmed cell death. In 2012, Dixon et al (20) proposed ferroptosis as a new mode of
cell death that is different from other methods of programmed cell
death. When cells are subjected to excess stimulation or the
antioxidant system is defective, lipid peroxides will gradually
accumulate and eventually trigger ferroptosis. Glutathione
peroxidase 4 (Gpx4) was the first core inhibitor of ferroptosis
identified, and other Gpx4-independent pathways or factors that
inhibit ferroptosis have since been found, including the system
xc−/Gpx4, ferroptosis suppressor protein
1/NAD(P)H/coenzyme Q10 and GTP cyclohydrolase
1/Tetrahydrobiopterin/dihydrofolate reductase axes (21–23).
System xc-is a cystine/glutamate antiporter composed of the solute
carrier family 7 member 11 (Slc7a11) and Slc3a2 subunits, which is
crucial for cellular glutathione synthesis and antioxidant defense.
Dysfunction of Slc7a11 can impair cystine uptake, leading to
glutathione depletion and heightened susceptibility to ferroptosis
(24). Lipid peroxidation is an
important factor for ROS-induced oxidative stress in bone tissue.
Gpx4-knockout mice show a notable reduction in bone mineral density
(BMD) and ovariectomy can aggravate osteoporosis in Gpx4-knockout
mice (25). Nrf2 is also important
for regulating ferroptosis in osteocytes. Knockdown of Nrf2 in
osteocytes can induce ferroptosis and activation of Nrf2 can
inhibit RANKL expression (26).
YC is a potential drug for preventing osteoporosis;
ferroptosis may be a potential target for the treatment of
osteoporosis; and the Nrf2/Slc7a11/Gpx4 pathway is an important
pathway in regulating ferroptosis. However, the regulatory effects
of YC on ferroptosis in osteoporosis remain ambiguous. Therefore,
the present study aimed to investigate the regulatory effect of YC
on PMOP from the perspective of ferroptosis and to further explore
the effect of YC on the Nrf2/Slc7a11/Gpx4 ferroptosis regulatory
pathway.
Materials and methods
Experimental drugs
YC (TongRenTang Chinese Medicine) was mixed with
distilled water in a magnetic stirrer for 2 h at room temperature
and then crushed using a juicer. The juice was then filtered and
concentrated in a rotary evaporator to obtain 1 and 0.5 g/ml of
solution, which was stored at −20°C for later use. Estradiol
valerate acid tablets (EV) (cat. no. J20130009; Bayer) were ground
into powder, dissolved in distilled water a suspension of estradiol
was obtained in an ultrasound-assisted manner with a concentration
of 0.013 mg/ml.
Experimental animals
The study was conducted in strict accordance with
the animal experiment ethics regulations of the Institute of Basic
Theory for Chinese Medicine, China Academy of Chinese Medical
Sciences and were approved by the Institutional Animal Ethics
Committee (approval no. IBTCMCACMS21-2303-04; Beijing, China). A
total of 40 specific pathogen-free female C57BL/6 mice (age, 8
weeks; weight, 20±2 g) were obtained from SPF Biotechnology Co.,
Ltd. [license no. SCXK(Beijing)2021-0010]. The mice were housed
under conventional conditions (room temperature 22±1°C, humidity
50±5%, 12/12-h light/dark cycle) with free access to food and
water. They were randomly divided into five groups: i) Sham
operation (SHAM) group treated with 10 ml/kg distilled water via
oral gavage; ii) OVX model group treated with 10 ml/kg distilled
water via oral gavage; iii) EV group treated with 0.13 mg/kg EV for
12 weeks via oral gavage; iv) YC low dose (YCL), treated with 5
g/kg YC water extract via oral gavage for 12 weeks; and v) YC high
dose (YCH) group treated with 10 g/kg YC water extract via oral
gavage for 12 weeks. Bilateral ovariectomy was performed on mice in
the OVX and medication groups. Briefly, the mice were anesthetized
by intraperitoneal injection of 1% pentobarbital sodium (50 mg/kg).
An incision was made on the dorsal side, cauliflower-like ovarian
tissue was ligated, the ovaries were removed and the wound was
sutured. In the Sham group, the operation was the same as for the
experimental groups but only a small amount of adipose tissue
surrounding the ovary was ligated and excised. Drugs were given
starting 1 week after surgery, continuing for 12 weeks, with all
treatments administered once daily at the same time each day.
After the last administration, the mice were
euthanized by an intraperitoneal overdose of pentobarbital sodium
(100 mg/kg) and death was confirmed by a cessation of heartbeat and
respiration. Blood was collected via cardiac puncture (0.8–1.2 ml
per mouse) and allowed to stand at room temperature for 2 h. The
uterus was dissected and weighed, and the uterine coefficient was
calculated as uterine wet weight (mg)/body weight (g). The liver
was removed and fixed in 4% paraformaldehyde (PFA) fixation
solution at room temperature for 24 h, whereas the femur was fixed
in 4% PFA fixation solution at room temperature for 48 h after
removing the muscle from the bone. Subsequently, the fixation
solution was replaced with fresh 4% PFA for long-term storage at
room temperature. The tibia was preserved at −80°C. Humane
endpoints were defined as follows: Severe weight loss (>20%),
inability to access food or water, signs of severe pain or
distress, such as hunched posture, lethargy or vocalization, or any
other condition that would compromise animal welfare. No animals
reached these endpoints during the study.
Ultra-performance liquid
chromatography-tandem mass spectrometry (UPLC-MS/MS)
The aforementioned prepared water solution of YC was
added to an equal amount of internal standard extract (70%
methanol), centrifuged at ~13,800 × g for 3 min at 4°C and the
supernatant was filtered through a 0.22-µm filter membrane to
obtain the sample for later use. Analysis was performed on Agilent
SB-C18 columns (.8 µm, 2.1 mm × 100.0 mm; Agilent Technologies,
Inc.) using an ExionLC AD UPLC system (Sciex). The liquid phase
conditions consisted of a mobile phase, in which phase A was
ultrapure water with 0.1% formic acid and phase B was acetonitrile
with 0.1% formic acid. The elution gradient was as follows: i) 0
min, the proportion of phase B was 5%; ii) 9 min, the proportion of
phase B linearly increased to 95% and was maintained for 1 min; and
iii) subsequently the proportion of phase B decreased to 5% and was
equilibrated at 5% for 14 min. The flow rate was 0.35 ml/min, the
column temperature was 40°C and the injection volume was 2 µl. MS
conditions were as follows: Electrospray ionization source
temperature, 550°C; ion spray voltage, 5,500 V (positive ion
mode)/-4,500 V (negative ion mode); ion source gas I, gas II and
curtain gas were set to 50, 60 and 25 psi, respectively; and the
collision-induced ionization parameter was set to high. Scans were
performed in multiple reaction monitoring (MRM) mode with the
collision gas, nitrogen, set to medium. The de-clustering potential
and collision energy of each MRM ion pair were further optimized. A
specific set of MRM ion pairs was monitored at each period based on
the metabolites eluted within each period. A specific set of MRM
transitions was monitored for each period according to the
metabolites eluted within this period, based on the optimized
precursor ion (Q1) and product ion (Q3) m/z values. Finally,
components that have been proven to have anti-osteoporotic activity
in existing literature were selected from the 2,072 compounds for
further investigation in the present study (5,18,19).
Micro-CT
To evaluate the changes in bone morphology in mice,
micro-CT was performed on the femurs of mice using Skyscan1276
(Bruker) with the following scan parameters: 6.5 µm, 70 kV and 200
mA. The lowest point of the lateral growth plate of the femoral
knee joint was taken as the baseline and the area with a thickness
of 1 mm was set as the 3D reconstruction area of interest. The
NRecon software (version 1.7.1.0, Bruker) was used for 3D image
reconstruction and the CTAn software (version 1.17.7.2, Bruker) was
used to evaluate the BMD (mg/cm3), bone volume/total
volume (BV/TV, %), trabecular number (Tb.N, 1/mm), trabecular
separation (Tb.Sp, mm) and structure model index (SMI).
ELISA
Blood was collected following euthanasia by an
overdose of pentobarbital sodium (as aforementioned) via cardiac
puncture, with 0.8–1.2 ml collected per mouse. Serum was collected
by centrifugation at 1,900 × g for 15 min at 4°C after 2 h at room
temperature. in addition, mouse tibias were ground into a powdery
form under liquid nitrogen using a grinding mill. PBS was added to
the powdered tibia samples, and homogenized using a low-temperature
grinder. Subsequently, the homogenate was centrifuged at 1,900 × g
for 15 min at 4°C and the supernatant was collected for analysis.
The levels of malondialdehyde (MDA), 4-hydroxynonenal (4-HNE), and
glutathione (GSH) in the mouse tibia and serum, as well as
estradiol (E2), tartrate-resistant acid phosphatase (TRAP), and
osteocalcin (OCN) in serum alone, were detected using commercial
ELISA kits strictly according to the manufacturers' protocols. The
specific kits employed were as follows: Mouse MDA ELISA Kit (cat.
no. F9264-A; Shanghai Kexing Trading Co., Ltd.), Mouse 4-HNE ELISA
Kit (cat. no. F9213-A; Shanghai Kexing Trading Co., Ltd.), Mouse
GSH ELISA Kit (cat. no. F2658-A; Shanghai Kexing Trading Co.,
Ltd.), and Mouse E2 ELISA Kit (cat. no. MB-3302A; Jiangsu Meibiao
Biotechnology Co., Ltd.). The absorbance was recorded at 450 nm. A
standard curve was generated from the absorbance values of the
standard wells, which allowed for the calculation of sample
concentration.
Hematoxylin and eosin (H&E)
staining
H&E staining was performed on 5-µm
paraffin-embedded mouse femur sections to distinguish bone
trabeculae from bone marrow. The femurs were dehydrated through a
graded ethanol series, cleared in xylene, and embedded in paraffin
prior to sectioning. The sections were baked at 67°C for 2 h,
deparaffinized in xylene I and II (10 min each) and rehydrated
through a graded ethanol series (100, 95 and 75%; 3 min each).
Subsequently, the sections were stained with hematoxylin at room
temperature for 3 min, rinsed with tap water, differentiated, blued
and washed again. After dehydration in 75 and 95% ethanol (5 min
each), eosin staining was carried out at room temperature for 5
min, followed by rinsing with distilled water. Sections were
further dehydrated in absolute ethanol (three changes, 5 min each),
cleared in xylene and mounted with neutral resin. Finally, images
were acquired using a slide scanner.
Masson's trichrome
Masson's staining can stain collagen fibers blue,
reflecting the maturity of bone tissue. Femoral specimens of mice
were fixed and decalcified, before being embedded as
aforementioned. The slices were baked at 67°C for 2 h,
deparaffinized, and rehydrated. The slices were stained
sequentially at room temperature using Weigert's iron hematoxylin
for 5 min, ponceau magenta for 5 min and aniline blue for 1 min.
Following dehydration, the slices were sealed with neutral resin.
Finally, the images were acquired by scanning the slices using a
slide scanner.
Prussian blue dyeing
Under acidic conditions, potassium ferrocyanide can
react with iron trivalent to form a blue compound, Prussian blue,
to detect liver iron ion levels. The liver tissue was dehydrated
with a gradient alcohol series and permeabilized by xylene after
fixation but before embedding. The paraffin-embedded liver was then
cut into 5-µm sections and placed on a slide to dry. Following
dewaxing as aforementioned, the Prussian blue stain was prepared
according to the manufacturer's instructions (Solarbio, G1422) and
added at room temperature for 20 min, before sections were washed
with distilled water for 5 min. Sections were stained with nuclear
solid red at room temperature for 8 min and rinsed for 3 sec. After
dehydration, the sections were sealed with neutral resin and the
images were scanned by a slide scanner.
Immunohistochemistry staining
Mouse femur specimens were fixed, decalcified,
embedded and sectioned as aforementioned. Sections were
deparaffinized, antigen retrieval was performed using EDTA antigen
retrieval solution A and B (cat. no. SBT10013; Shunbai
Biotechnology Co., Ltd.; both incubated at 37°C for 30 min), and
the sections blocked with 3% hydrogen peroxide at room temperature
for 10 min for 30 min. Triton X-100 (0.04%) was used to
permeabilize the sections for 20 min at room temperature and goat
serum (10%; cat. no. ZLI-9022; Beijing Zhongshan Jinqiao
Biotechnology Co., Ltd.) was used to block the sections for 60 min
at room temperature. Sections were incubated with the following
primary antibodies: Anti-osteoprotegerin (OPG; 1:50; cat. no.
ab73400; Abcam), anti-runt-related transcription factor 2 (RUNX2;
1:50; cat. no. ab236639; Abcam), Nrf2 polyclonal antibody (1:200;
cat. no. 16396-1-AP; Proteintech Group, Inc.), Slc7a11 polyclonal
antibody (1:50; cat. no. 26864-1-AP; Proteintech Group, Inc.) and
Gpx4 monoclonal antibody (1:50; cat. no. 67763-1-Ig; Proteintech
Group, Inc.) at 37°C overnight. Sections were incubated with an
HRP-conjugated goat anti-rabbit IgG secondary antibody (1:2000;
cat. no. GB23204; Wuhan Servicebio Technology Co., Ltd.) at 37°C
for 120 min and biotinylated secondary antibodies (1:2,000; cat.
no. PV-9001; Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.) for
120 min at room temperature. For the comparative analysis of marker
expression, additional sections were processed using optimized
antibody concentrations and incubation conditions: primary antibody
incubation at 37°C for 1 h, followed by secondary antibody
incubation at 37°C for 20 min. The sections were treated with color
developing solution (DAB Kit; cat. no. ZLI-9017; Beijing Zhongshan
Jinqiao Biotechnology Co., Ltd.). The staining results were
observed under a light microscope.
Dual immunofluorescence staining
Immunofluorescence staining was performed by binding
fluorescently labeled secondary antibodies to primary antibodies to
label corresponding proteins. Paraffin-embedded sections prepared
as aforementioned were deparaffinized and rehydrated in absolute,
95 and 75% ethanol, then antigen retrieval was performed using
antigen retrieval reagents A and B (both incubated at 37°C for 30
min), endogenous peroxidase blocker was added and the sections were
incubated in the dark at room temperature for 10 min. Subsequently,
the sections were blocked with 10% rabbit serum (cat. no. ZLI-9026;
Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.) at room
temperature for 30 min, and incubated with Gpx4 monoclonal antibody
(1:50; cat. no. 67763-1-Ig; Proteintech Group, Inc.) and OCN
antibody (1:100; cat. no. ab93876; Abcam) at 4°C overnight. The
corresponding fluorescent secondary antibodies (Alexa Fluor
488-conjugated goat anti-mouse IgG, 1:200, cat. no. ab150113,
Abcam; Alexa Fluor 594-conjugated goat anti-rabbit IgG, 1:200, cat.
no. ab150080, both Abcam) were added at room temperature for 50 min
in the dark, followed by tyramide signal amplification reaction at
room temperature for 10 min in the dark. Finally, DAPI staining was
performed, the slices were dehydrated and blocked with anti-fade
mounting medium and fluorescence was excited according to the
corresponding wavelength of the dye using a fluorescence
microscope.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The expression levels of genes related to
ferroptosis were detected by RT-qPCR. Tibia samples were ground
into powder in liquid nitrogen using a grinder, collected into
centrifuge tubes and placed on ice. RNA was extracted from the
tissue using the TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.) method according to standard operating
procedures. RNA was reverse transcribed into cDNA using the
PrimeScript RT reagent kit (Takara Bio, Inc.) according to the
manufacturer's protocol. The primer sequences utilized for
quantifying the expression levels of nuclear receptor coactivator 4
(Ncoa4), ferritin heavy chain (Fth), Nrf2, Slc7a11 and Gpx4 genes
are provided in Table I.
Amplification was performed using Hieff qPCR SYBR Green Master Mix
(High Rox Plus; cat. no. 11203ES08; Shanghai Yeasen Biotechnology
Co., Ltd.) under the following conditions: Initial denaturation at
95°C for 30 sec, followed by 40 cycles of denaturation at 95°C for
5 sec and annealing/extension at 60°C for 20 sec. Gene expression
levels were quantified using the 2−ΔΔCq method (27), with Gapdh as the reference gene for
data normalization.
 | Table I.Primer sequence. |
Table I.
Primer sequence.
| Gene | Forward, 5′-3′ | Reverse, 5′-3′ |
|---|
| Ncoa4 |
CCCTTCCAGAAATGAGCTAACA |
GCCACTCTGACAAGGAACTATT |
| Fth |
TGACCACGTGACCAACTTAC |
CGTCAGCTTAGCTCTCATCAC |
| Nrf2 |
GGCTCAGCACCTTGTATCTT |
CACATTGCCATCTCTGGTTTG |
| Slc7a11 |
CTGGTCAGCCAGCTTATGAA |
AGGAGGATGCTGCCAATAAC |
| Gpx4 |
TGTGCATCCCGCGATGATT |
CCCTGTACTTATCCAGGCAGA |
| Gapdh |
GAATGGGAAGCTGGTCATCAA |
CCAGTAGACTCCACGACATACT |
Statistical analysis
SPSS v.26.0 (IBM Corp.) was used for data analysis.
All of the obtained data are presented as the mean ± standard
deviation. Groups were compared using one-way ANOVA followed by
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Main components of YC
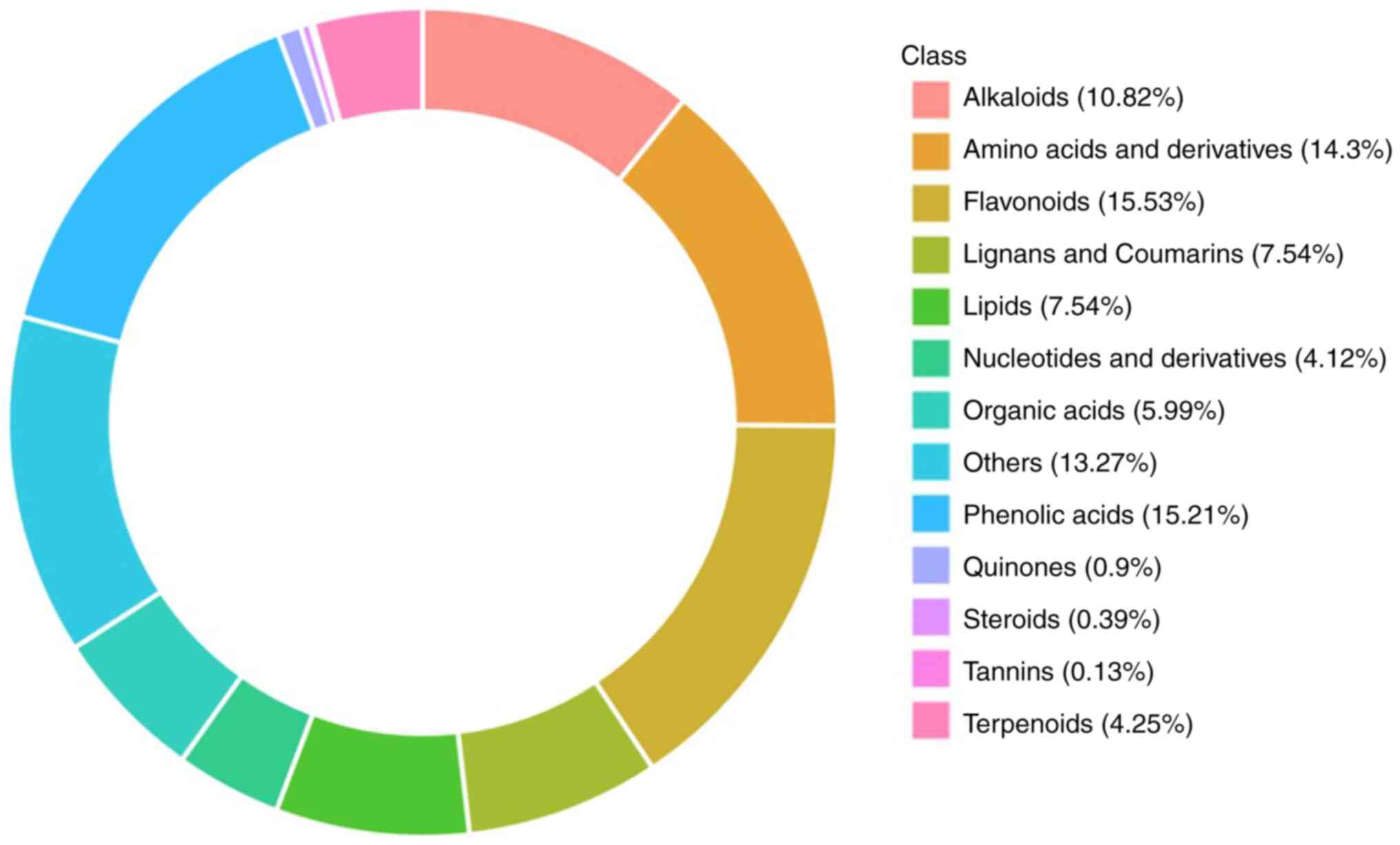
The main components of YC were determined using
UPLC-MS/MS and the results are shown in Fig. 1 according to the relative content
of various compounds. A total of 2,072 compounds were identified,
which were divided into 13 categories, including 333 phenolic
acids, 308 flavonoids, 259 amino acids and their derivatives, 233
alkaloids, 205 lipids, 134 lignans and coumarin, 127 organic acids,
113 terpenoids, 69 nucleotides and their derivatives, 23 quinones,
16 steroids, 5 tannins and 247 others. The main active ingredients
of YC included chlorogenic acid, ferulic acid, caffeic acid,
artemisininolide, artemisinin, scopoletin, quercetin, hyperoside,
isorhamnetin and isoquercitrin (data not shown). The 10 compounds
listed in Table II were selected
based on their documented anti-osteoporotic activities in previous
literature and their relative abundance in the extract (5,9,10,28).
 | Table II.Relative content of the active
components of Yinchen. |
Table II.
Relative content of the active
components of Yinchen.
| Chemical
compound | Molecular
formula | Category | Retention time,
min | Relative
content |
|---|
| Caffeic acid |
C9H8O4 | Phenolic acids | 3.4 | 2136.26 |
| Ferulic acid |
C10H10O4 | Phenolic acids | 4 | 1277.59 |
| Chlorogenic
acid |
C16H18O9 | Phenolic acids | 2.7 | 737.32 |
| Scopoletin |
C10H8O4 | Lignans and
coumarin | 4.1 | 659.48 |
| Isoquercitrin |
C21H20O12 | Flavone | 4 | 247.29 |
| Artemisinin |
C11H10O4 | Lignans and
coumarin | 5 | 97.44 |
| Quercetin |
C15H10O7 | Flavone | 5.1 | 74.37 |
| Chromenone |
C16H12O7 | Lignans and
coumarin | 5.8 | 57.39 |
| Isorhamnetin |
C16H12O7 | Flavone | 5.8 | 9.02 |
| Hyperoside |
C21H20O12 | Flavone | 3.6 | 1.00 |
Effect of YC on weight change, uterine
coefficient and serum estradiol in OVX mice
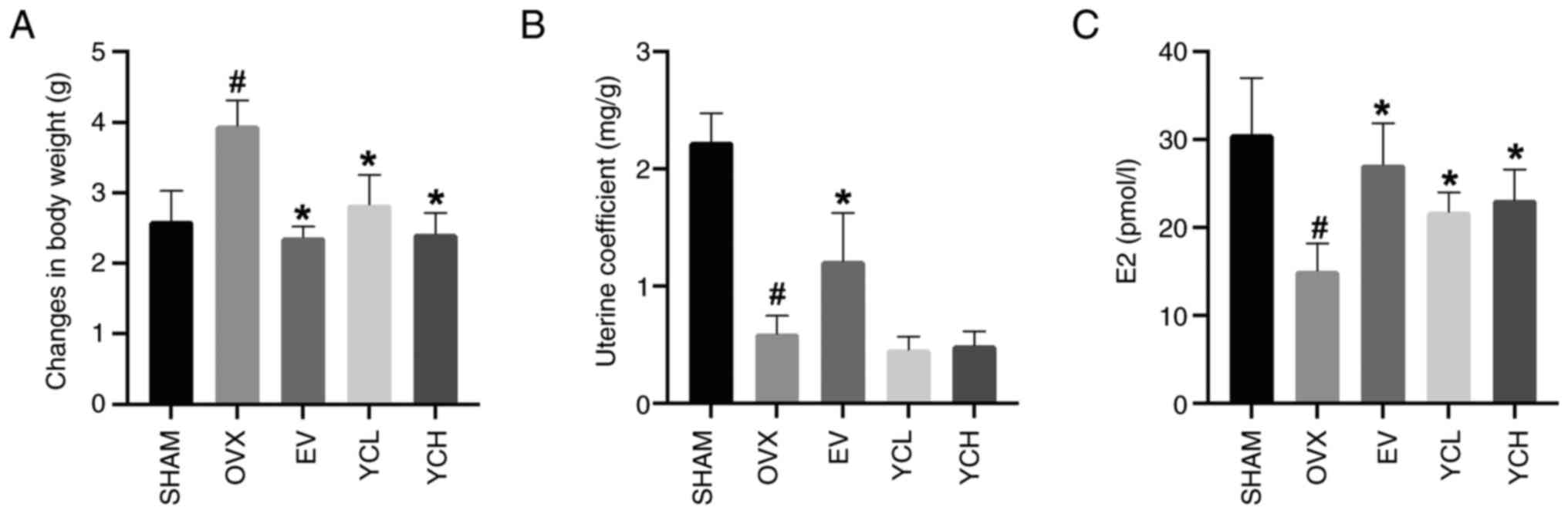
In the present study, as shown in Fig. 2, OVX mice had significantly lower
serum estradiol levels compared with those in SHAM mice. OVX mice
also showed a significant increase in body weight and a
significantly lower uterine coefficient compared with those in the
SHAM group. Notably, the administration of YC significantly
reversed these changes in body weight and estradiol levels,
although no significant difference in uterine coefficient was
observed between the OVX and YC groups. EV treatment also
significantly increased the uterine coefficient compared with the
OVX group. However, there were no significant differences observed
between the YCL and YCH groups.
Effect of YC on femoral bone
microstructure in OVX mice
Micro-CT analysis was used to investigate the effect
of YC on bone microstructure in OVX mice. As shown in Fig. 3A, the femoral trabeculae of mice in
the SHAM group were thick, closely arranged and continuous, whereas
the number of femoral trabeculae of mice in the OVX group was
markedly reduced, the bone microstructure was broken, and the
number and sizes of gaps increased. Both EV and YC treatment
markedly improved the deteriorated trabecular structure observed in
OVX mice. As shown in Fig. 3B-F
morphometric parameters further supported the observed changes in
trabecular microstructure in the different groups. Consistent with
the scanned images, OVX mice had significantly reduced BMD, BV/TV
and Tb.N compared with those in the SHAM group, whereas SMI and
Tb.Sp were significantly increased. However, the independent
application of YC and estradiol significantly ameliorated these
changes.
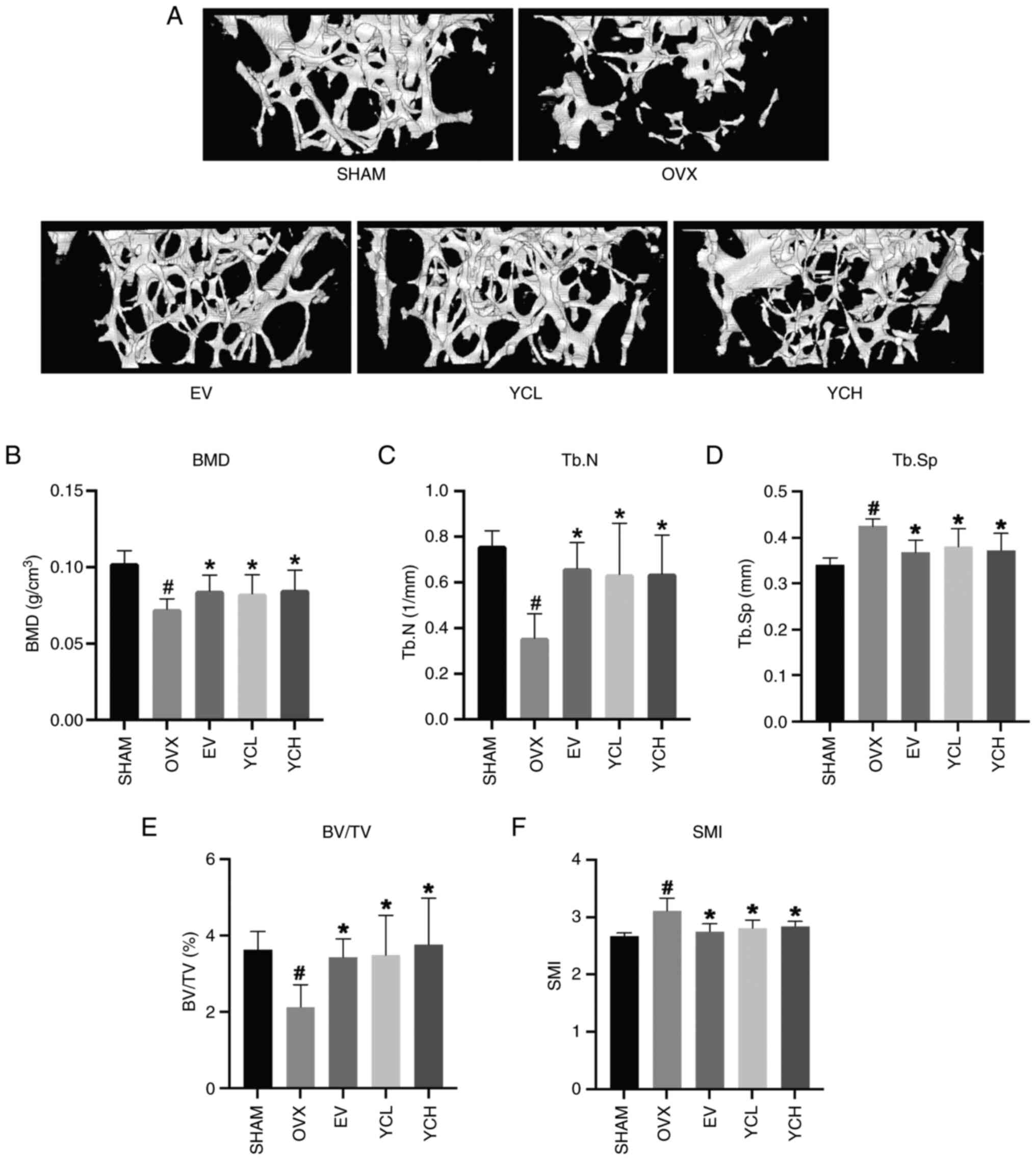 | Figure 3.Effect of YC on femoral bone
microstructure in OVX mice. (A) Micro-CT scanning images of bone
microstructure. Changes in (B) BMD, (C) Tb.N, (D) Tb.Sp, (E) BV/TV
and (F) SMI. Data are presented as the mean ± SD; n=8.
#P<0.05 vs. SHAM group; *P<0.05 vs. OVX group.
OVX, ovariectomized; EV, estradiol valerate; YC, Yinchen; YCL, YC
low dose; YCH, YC high dose; BMD, bone mineral density; Tb.N,
trabecular number; Tb.Sp, trabecular separation; BV/TV, bone
volume/total volume; SMI, structure model index. |
Effect of YC on the pathological
morphology of femurs in OVX mice
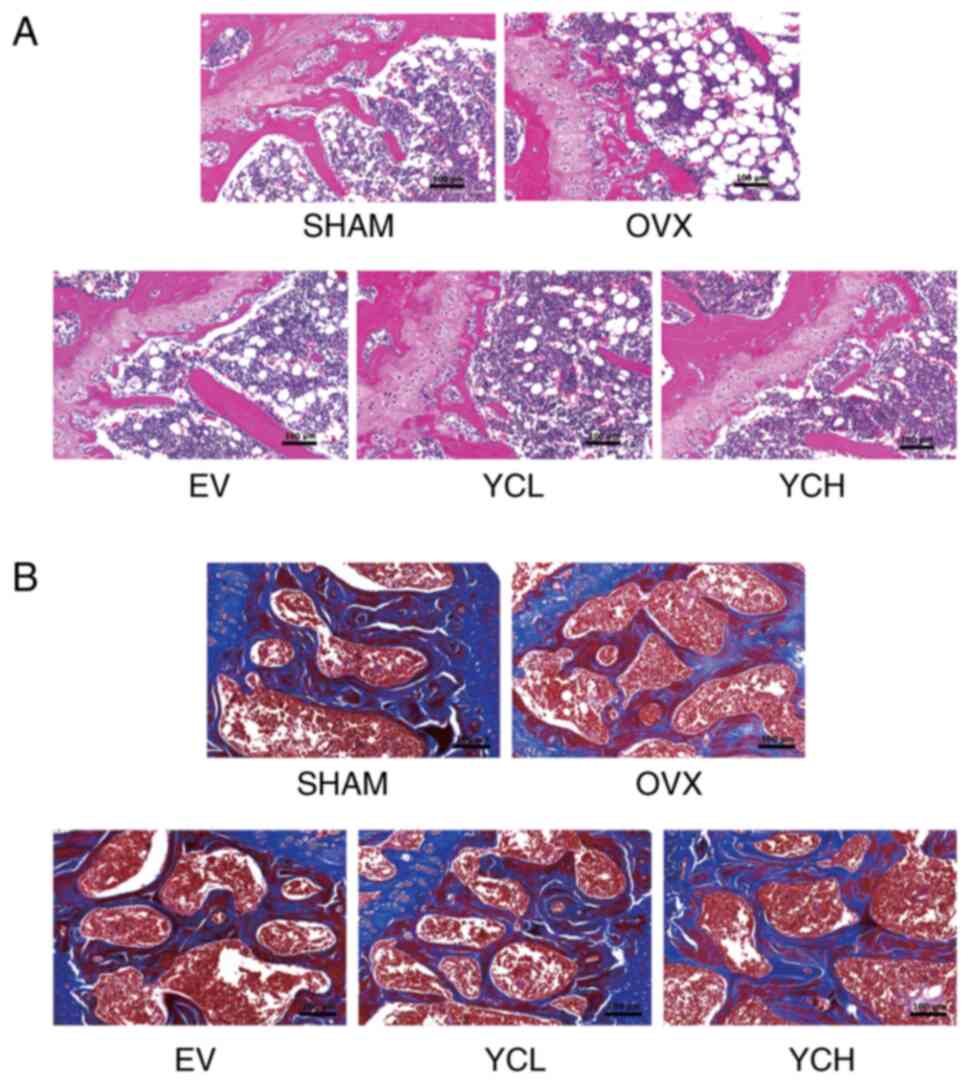
To further evaluate the effect of YC on trabecular
structure, H&E staining was performed on the femurs of mice. As
shown in Fig. 4A, a well-organized
trabecular meshwork and a small bone marrow cavity were observed in
the SHAM group. However, in the OVX group, it was found that the
bone marrow cavity was enlarged, the trabecular structure was
disordered and the lipid droplets in the bone marrow were markedly
increased. H&E staining also showed that EV and YC ameliorated
the histopathological changes in bone structure caused by
ovariectomy. Masson's staining in Fig.
4B showed that, compared with in the SHAM group, the femoral
bone trabeculae of the OVX group were thinner and more sparsely
arranged, with increased spacing and less collagen. These changes
were notably ameliorated in the EV and YC groups.
Effect of YC on bone metabolism in OVX
mice
The anti-osteoporotic effect of YC was monitored by
detecting the serum levels of TRAP and OCN through ELISA, the
results of which were shown in Fig. 5A
and B. Compared with those in the SHAM group, the serum levels
of TRAP and OCN in the OVX group were significantly increased (both
P<0.05). These results indicated that ovariectomy resulted in an
increased turnover rate of bone metabolism, whereas EV and YC
significantly reduced the concentrations of these serum markers
compared with those in the OVX group. However, there was no
significant difference observed between the YCL and YCH groups.
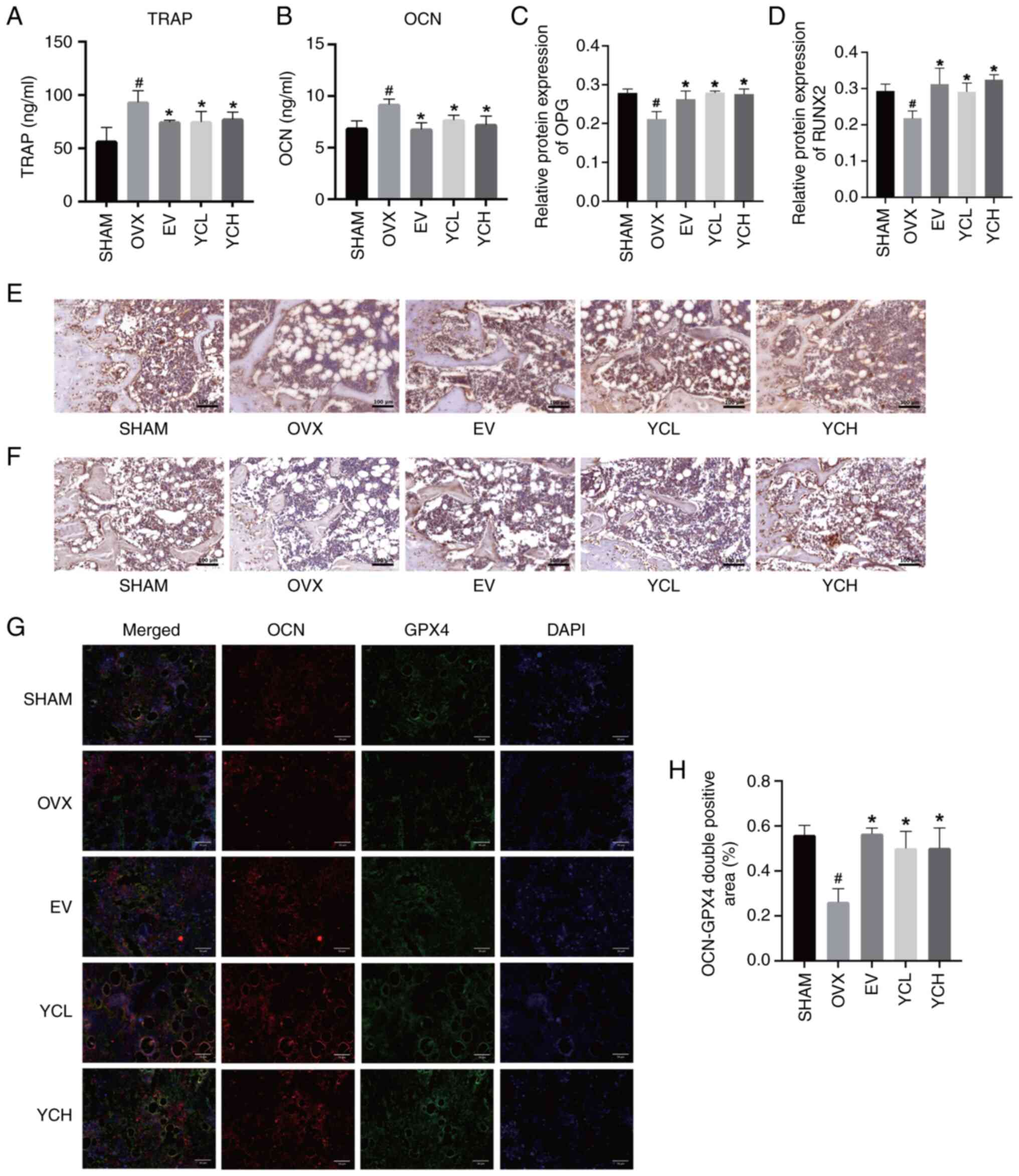 | Figure 5.Effect of YC on bone metabolism in
OVX mice. Results of (A) TRAP and (B) OCN ELISA. Relative protein
expression levels of (C) OPG and (D) RUNX2. Immunohistochemical
staining of (E) OPG and (F) RUNX2 protein expression (×200
magnification). (G) Double immunofluorescence staining images of
OCN and GPX4 (×400 magnification). (H) OCN-GPX4 double positive
area. Data are presented as the mean ± SD; n=8.
#P<0.05 vs. SHAM group; *P<0.05 vs. OVX group.
OVX, ovariectomized; EV, estradiol valerate; YC, Yinchen; YCL, YC
low dose; YCH, YC high dose; TRAP, tartrate-resistant acid
phosphatase; OCN, osteocalcin; OPG, osteoprotegerin; RUNX2,
runt-related transcription factor 2; GPX4, glutathione peroxidase
4. |
Subsequently, femurs were subject to
immunohistochemical staining, with RUNX2 and OPG being the key
factors regulating the dynamic balance of bone tissue (29,30).
As shown in Fig. 5C-F, the
expression of OPG and RUNX2 in the OVX group was significantly
lower than that in the SHAM group. Ovariectomy reduced bone
formation in mice, whereas the EV and YC groups significantly
increased the expression of OPG and RUNX2. The expression of RUNX2
in the femur of the YCH group was higher than that of the YCL
group, but the difference was not statistically significant.
The osteoblast-specific marker OCN and ferroptosis
inhibitor Gpx4 were co-stained by immunofluorescence staining to
observe the expression of Gpx4 in femoral osteoblasts (Fig. 5G and H). Compared with in the SHAM
group, the expression of Gpx4 in the femoral osteoblasts of OVX
mice was significantly decreased, whereas EV and YC treatment
significantly increased Gpx4 expression levels.
Effect of YC on iron metabolism in OVX
mice
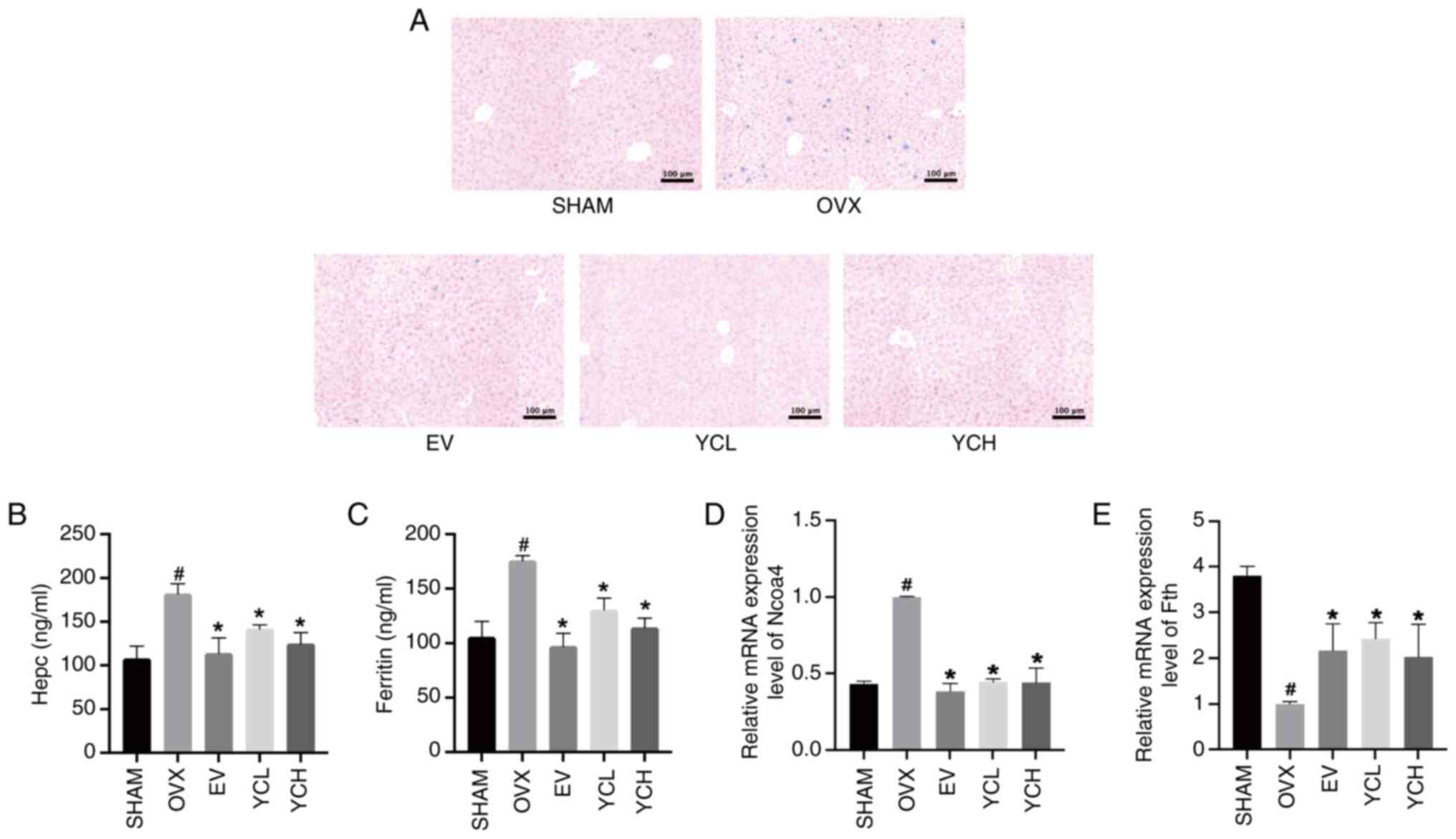
The liver is the main iron storage organ and when
iron accumulates, the liver exhibits increased iron deposition
(31). The iron ion content of
liver sections was determined by Prussian blue staining (Fig. 6A). Compared with that in the Sham
group, OVX mice demonstrated more punctate staining and heavier
iron deposition in the liver, which was effectively reduced in the
EV and YC groups.
Ferritin and hepcidin levels were subsequently
measured by ELISA, whereas Ncoa4 and Fth mRNA expression levels
were measured by RT-qPCR; the results of these assays were shown in
Fig. 6B-E. Compared with those in
the SHAM group, the levels of ferritin, hepcidin and Ncoa4 were
significantly increased in OVX mice whereas the expression of Fth
was significantly decreased. However, EV and YC administration
significantly reversed these changes (all P<0.05).
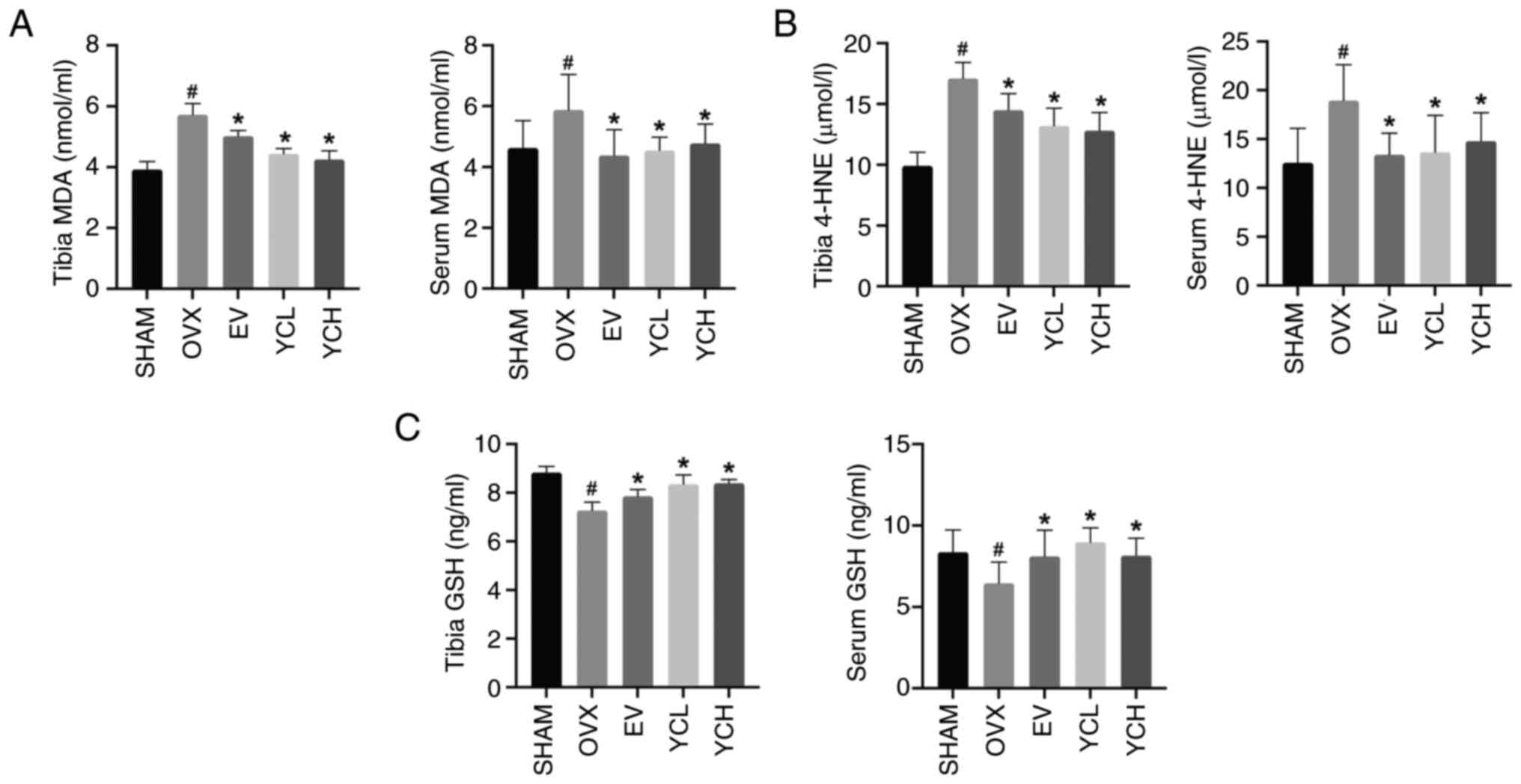
Effect of YC on lipid peroxidation in
OVX mice
Lipid peroxidation is an important link in
ferroptosis, and the accumulation of lipid peroxides in the local
membrane is the key reaction leading to cell death (32). MDA, 4-HNE and GSH contents in the
tibias and sera of mice were measured by ELISA (Fig. 7). Compared with those in the SHAM
mice, the levels of MDA and 4-HNE were significantly increased in
OVX mice, whereas the levels of GSH were significantly decreased.
EV and YC significantly reversed the changes in MDA, 4-HNE and GSH
in the serum and tibias after ovariectomy.
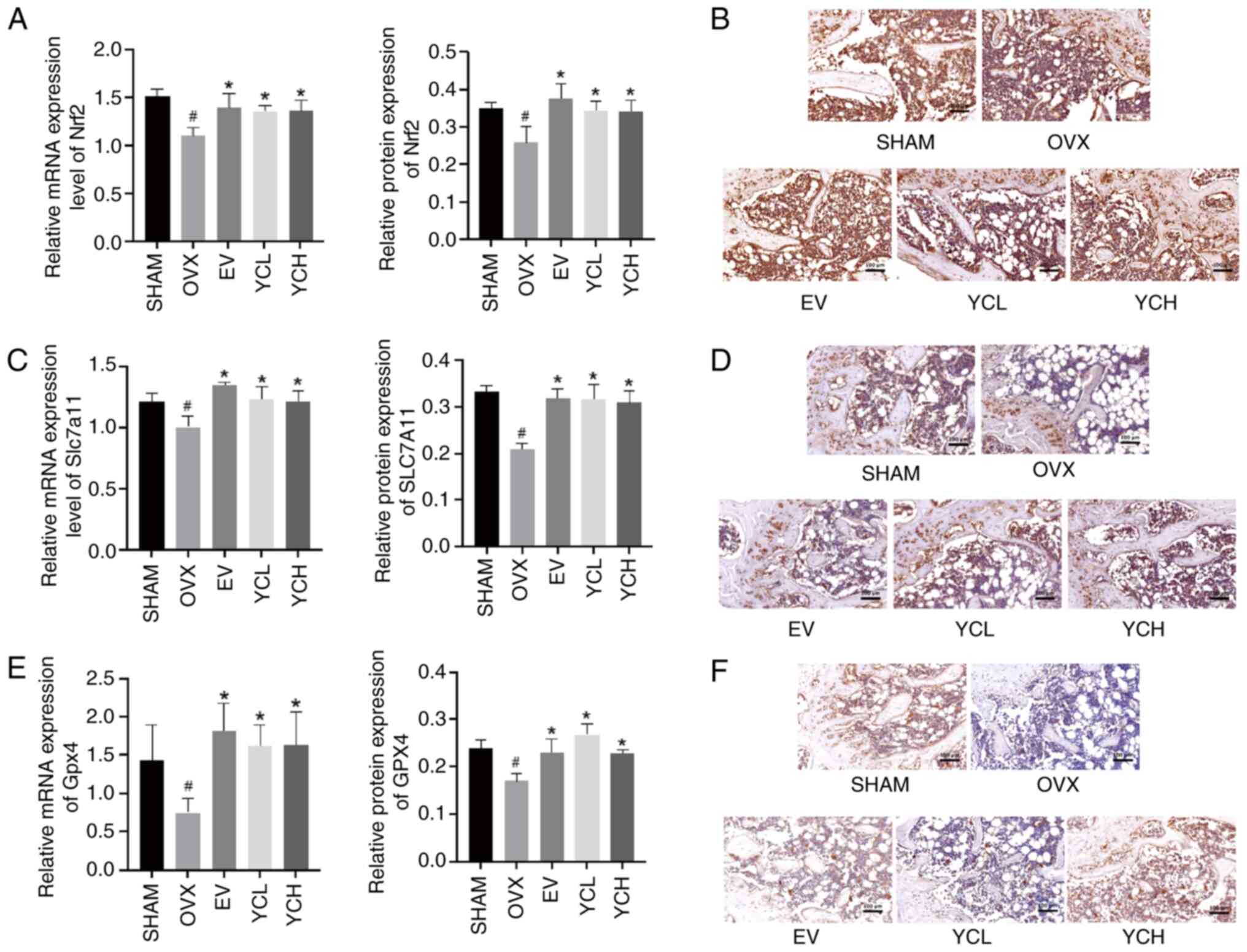
Effect of YC on the Nrf2/Slc7a11/Gpx4
pathway
The expression levels of Nrf2, Slc7a11 and Gpx4 in
the bone tissue of mice were detected by RT-qPCR and
immunohistochemistry (Fig. 8).
Compared with those in the SHAM group, the mRNA and protein
expression levels of Nrf2, Slc7a11 and Gpx4 in the bone tissue of
OVX mice were significantly decreased. By contrast, compared with
those in the OVX mice, EV and YC treatment significantly increased
their expression levels.
Discussion
In the present study, UPLC-MS/MS was used to detect
the active components of YC. The OVX osteoporotic mouse model was
used to simulate PMOP, and the effects and mechanism of YC in the
treatment of osteoporosis were explored. Reduced estrogen levels
lead to a gradual decrease in osteoblast number and activity,
resulting in a reduced rate of bone formation (26). When osteoporosis occurs, BMSCs are
more likely to transform into adipocytes than in healthy
individuals (33). In the present
study, estradiol levels in the serum of YC-treated mice were
increased and mouse body weight was decreased, indicating that YC
improved the reduction of estrogen levels and body weight gain
caused by ovariectomy, but could not restore the uterine atrophy
caused by ovariectomy, as indicated by the lack of significant
difference in uterine coefficient. In addition, in OVX mice, the
trabecular bone destruction was notable, the formation of lipid
droplets in the bone marrow cavity increased and bone formation
decreased.
TRAP and OCN are serum markers of bone turnover
(34). Increased levels of bone
turnover markers may precede changes in BMD. In OVX mice, the
present study detected elevated levels of TRAP and OCN in mouse
serum. RUNX2 is a key osteogenic transcription factor. In immature
osteoblasts, RUNX2 can regulate the expression of bone matrix
proteins, such as secreted phosphoprotein 1, collagen type I α1,
collagen type I α2 and OCN, and can induce the maturation of
osteoblasts (35,36). OPG is secreted by osteoblasts and
can competitively bind to RANKL. It also inhibits the binding of
RANKL to RANK. OPG-null mice exhibit reduced whole-body BMD,
whereas the use of RANKL inhibitors can enhance Wnt/β-catenin
signaling and induce bone formation in OPG null mice (37,38).
Immunohistochemistry showed that the expression levels of OPG and
RUNX2 were decreased in the femurs of OVX mice compared with those
in the Sham group, indicating that bone formation was inhibited in
the bone tissue of mice with ovariectomy-induced osteoporosis,
whereas the administration of YC was shown to promote the
expression of osteogenesis-related factors in bone tissue.
Ferroptosis is a novel mode of cell death
characterized by iron accumulation and lipid peroxidation.
Ferroptosis is involved in high-fat-induced bone loss, and
ferroptosis in high-fat-fed osteoporotic mice can be inhibited by
intraperitoneal injection of ferrostatin-1 (39). In a mouse model of diabetic
osteoporosis, the levels of lipid peroxides in the body are
increased and the levels of osteogenic markers are decreased
(40). Another study found that
type 2 diabetes can reduce the expression of mitochondrial ferritin
in bone tissue and cause ferroptosis (41). Serum levels of the ferroptosis
markers Gsh, Gpx4 and MDA can more effectively predict the
occurrence of PMOP compared to conventional bone turnover markers
such as OCN and C-terminal telopeptide of type I collagen. Iron
overload occurs in postmenopausal women, and the increase in iron
levels after menopause may be related to the loss of the pathway of
menstrual blood loss to expel ferritin (42,43).
This implies that ferroptosis may be an important mechanism in the
pathogenesis of osteoporosis.
Hepcidin is the core factor regulating the balance
of iron metabolism, and the level of hepcidin is regulated by the
feedback of iron level in the body. Hepcidin-knockout mice display
increased serum iron and ferritin content, increased liver and bone
iron content and inhibited osteogenic differentiation (44,45).
Ferritin is the predominant iron storage protein in cells, which is
composed of Fth and ferritin light chain. Fth is the principal
iron-storing subunit of ferritin. In a state of iron deficiency,
Ncoa4 can recognize and bind to Fth, degrade Fth, release iron ions
into cells, promote lipid peroxidation and induce ferroptosis
(46). The etiology of
osteoporosis is notably associated with the overproduction of lipid
peroxides, which represents the terminal stage of ferroptosis
(47). In the present study, serum
ferritin levels were elevated in OVX mice, indicating that iron
accumulation has occurred. At the same time, the liver iron ion
level of OVX mice was notably increased.
Ncoa4 can promote the degradation of Fth and release
iron ions into the cells, which is a process involved in
ferroptosis (32). Therefore, the
present study examined the expression levels of Ncoa4 and Fth in
the tibia of OVX mice; RT-qPCR analysis revealed that Ncoa4 was
found to be significantly increased, whereas Fth levels were
decreased in OVX mice compared with the Sham group. On the other
hand, lipid peroxidation is an important feature of ferroptosis.
Excess intracellular iron participates in the production of
peroxide and then participates in the formation of lipid peroxide
with polyunsaturated fatty acids (48). The significant increase in MDA and
4-HNE levels, and decrease in GSH levels in the serum and bone
tissues of OVX mice compared with in the SHAM mice in the present
study indicated that lipid peroxidation was elevated in the OVX
mice. The results of immunofluorescence double staining of OCN and
Gpx4 showed that the co-staining area of OCN and Gpx4 in the femurs
of OVX mice was significantly reduced compared with that in the
SHAM mice, indicating that Gpx4 expression was decreased, and the
level of iron accumulation and lipid peroxidation in the OVX mice
was increased, which suggested that the bone tissue of OVX mice
exhibited ferroptosis.
Upon treatment with YC, mice displayed the following
changes compared with OVX mice: i) Serum ferritin and hepcidin
levels, and liver iron ion content were decreased; ii) Ncoa4
expression in bone tissue was decreased; iii) the expression levels
of Fth in bone tissue was increased; iv) MDA and 4-HNE levels in
serum and bone tissue were decreased; v) GSH levels were increased;
and Gpx4 expression was increased. These results suggested that YC
improved osteoporosis in OVX mice by inhibiting ferroptosis.
Nrf2 is a key regulator of cellular antioxidant
responses, which drive transcriptional responses that inhibit
ferroptosis (26,49). Under physiological conditions, Nrf2
is maintained at low levels in the cytoplasm by interacting with
Kelch-like ECH-associated protein 1 (Keap1) to form a complex.
Under oxidative stress, Keap1 is degraded by autophagy and Nrf2 is
released into the cytoplasm. Subsequently, free Nrf2 is transported
to the nucleus where it binds to antioxidant response elements and
initiates downstream reactions (26). Nrf2 can regulate factors related to
GSH synthesis and metabolism, including the catalytic and
regulatory subunits of glutamate-cysteine ligase, GSH synthetase
and the system xc− subunits Slc7a11 and Gpx4 (24). Thus, Nrf2 is a key repressor of the
ferroptosis response. In pancreatic cancer cells, Nrf2 can activate
microsomal GSH S-transferase 1, inhibiting lipoxygenase 5 (Alox5)
and ferroptosis (50). Studies
have confirmed that increased Nrf2 expression can inhibit
ferroptosis and that downregulation of Nrf2 expression can increase
the sensitivity of cells to ferroptosis (26,51).
The results of the present study showed that this pathway was
inhibited in OVX mice. By contrast, YC increased the expression of
Gpx4 and OCN in OVX mouse osteoblasts and activated the
Nrf2/Slc7a11/Gpx4 pathway. Previous studies have confirmed that
kakaferol can enhance cellular antioxidant capacity, inhibit the
accumulation of lipid peroxides in neurons and inhibit ferroptosis
by activating the Nrf2/Slc7a11/Gpx4 pathway; pachynic acid has also
been shown to inhibit oxygen-glucose
deprivation/re-oxygenation-induced lipid peroxidation and
ferroptosis in cardiomyocytes by activating this pathway (26,52).
Taken together, it can be inferred that YC may inhibit osteoblast
ferroptosis by activating the Nrf2/Slc7a11/Gpx4 pathway, thereby
promoting bone formation and improving osteoporosis in OVX mice.
While YC may exert its effects through multiple pathways, the
present study focused on the Nrf2/Slc7a11/Gpx4 axis due to its
established role in ferroptosis and bone metabolism. Future studies
should investigate other potential pathways, such as HO-1 and
RANKL-mediated osteoclast differentiation, to provide an improved
comprehensive mechanistic understanding of the effects of YC on
osteoporosis.
In addition, Nrf2 is a key transcription factor that
regulates cellular antioxidant responses and iron metabolism. It
modulates susceptibility to ferroptosis by regulating the
expression of a series of downstream genes, including several
directly involved in ferroptosis. For example, Fth1 facilitates
iron storage, reducing the intracellular labile iron pool and
thereby attenuating ferroptotic cell death. Similarly, Gpx4 is a
notable enzyme that suppresses lipid peroxidation and Nrf2 can
upregulate Gpx4 expression to inhibit ferroptosis (53,54).
Based on the association between the Nrf2 pathway and ferroptosis,
the present study investigated the regulatory role of YC in this
pathway (55).
The present study had a number of limitations to be
acknowledged. Primarily, the causal relationship between YC
treatment and the activation of the Nrf2 pathway was not directly
validated through genetic or pharmacological interventions in the
present study, with the inference primarily relying on changes in
biomarker expression and existing literature. Furthermore, although
UPLC-MS/MS identified numerous compounds, the synergistic or
antagonistic interactions among the constituents within the YC
extract remain unexplored and the exact bioactive components
responsible for the observed effects require further isolation and
functional verification. Additionally, biomechanical assessments,
such as the three-point bending test, were not performed to
evaluate bone strength improvement. Finally, while the dosage used
in the present study (5–10 g/kg) is within the range of clinical
applications of YC in traditional Chinese medicine, which can reach
up to 100 g/day in human patients (56,57),
chronic toxicity and comprehensive safety profiles of YC at these
doses were not systematically investigated, which are important for
evaluating its clinical translational potential. Future studies
employing Nrf2-knockout models and ferroptosis inducers are
warranted to conclusively establish the causal role of the
Nrf2/Slc7a11/Gpx4 axis in mediating the anti-osteoclastogenic
effects of YC while the incorporation of functional biomechanical
tests and component-specific approaches will be important to fully
elucidate the therapeutic potential of YC for bone disorders.
In conclusion, the present study suggested the
effectiveness of YC in potentially mitigating ovariectomy-induced
osteoporosis in mice. YC promoted bone formation and improved bone
microstructure by potentially inhibiting ferroptosis via activation
of the Nrf2/Slc7a11/Gpx4 pathway in OVX mice.
Acknowledgements
Not applicable.
Funding
The present work was supported by the National Natural Science
Foundation of China (grant no. 82074297) and the Fundamental
Research Funds for the Central Public Welfare Research Institutes
(grant nos. YZ-202244, YZX202335 and YZX202235).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author. The UPLC-MS/MS data
generated in the present study may be found in the MetaboLights
public database under accession number MTBLS13042 or at the
following URL: http://www.ebi.ac.uk/metabolights/editor/MTBLS13042).
Authors' contributions
PL and ZZ conceived the study. PL, YC and ZZ
contributed to the methodology development. WL performed the
experiments. XW, YY and DC analyzed data. PL wrote the manuscript.
RZ made substantial contributions to the interpretation of data and
critically revised the manuscript for important intellectual
content. ZZ and HL also participated in critical revision of the
manuscript. YW contributed to project administration, participated
in data interpretation and revised the manuscript. HL supervised
the overall research design, interpreted the key results and
acquired the funding. All authors have read and approved the final
manuscript. PL and XW confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
All animal experiments were performed in strict
accordance with the ethical guidelines of the Institute of Basic
Theory for Chinese Medicine, China Academy of Chinese Medical
Sciences, and were approved by the Institutional Animal Ethics
Committee (approval no. IBTCMCACMS21-2303-04). Efforts were made to
minimize animal suffering and reduce the number of animals
used.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Garnero P, Sornay-Rendu E, Duboeuf F and
Delmas PD: Markers of bone turnover predict postmenopausal forearm
bone loss over 4 years: The OFELY study. J Bone Miner Res.
14:1614–1621. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hernlund E, Svedbom A, Ivergård M,
Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B and Kanis
JA: Osteoporosis in the European union: Medical management,
epidemiology and economic burden. A report prepared in
collaboration with the International Osteoporosis Foundation (IOF)
and the European Federation of Pharmaceutical Industry Associations
(EFPIA). Arch Osteoporos. 8:1362013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
An J, Yang H, Zhang Q, Liu C, Zhao J,
Zhang L and Chen B: Natural products for treatment of osteoporosis:
The effects and mechanisms on promoting Osteoblast-mediated bone
formation. Life Sci. 147:46–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee CJ, Shim KS and Ma JY: Artemisia
capillaris alleviates bone loss by stimulating osteoblast
mineralization and suppressing osteoclast differentiation and bone
resorption. Am J Chin Med. 44:1675–1691. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee SH, Lee JY, Kwon YI and Jang HD:
Anti-Osteoclastic activity of Artemisia capillaris thunb.
Extract depends upon attenuation of osteoclast differentiation and
bone Resorption-associated acidification due to chlorogenic acid,
hyperoside, and scoparone. Int J Mol Sci. 18:3222017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu YP, Qiu XY, Liu Y and Ma G: Research
progress on the pharmacological effects of Artemisiae Scopariae
Herba. Chinese Traditional and Herbal Drugs. 50:2235–2241.
2019.(In Chinese).
|
|
7
|
Witaicenis A, Seito LN, Da Silveira Chagas
A, de Almeida LD Jr, Luchini AC, Rodrigues-Orsi P, Cestari SH and
Di Stasi LC: Antioxidant and intestinal Anti-inflammatory effects
of Plant-derived coumarin derivatives. Phytomedicine. 21:240–246.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zeinali M, Rezaee SA and Hosseinzadeh H:
An overview on immunoregulatory and Anti-inflammatory properties of
chrysin and flavonoids Substances. Biomed Pharmacother.
92:998–1009. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gupta A, Atanasov AG, Li Y, Kumar N and
Bishayee A: Chlorogenic acid for cancer prevention and therapy:
Current status on efficacy and mechanisms of Action. Pharmacol Res.
186:1065052022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ding J, Wang L, He C, Zhao J, Si L and
Huang H: Artemisia scoparia: Traditional uses, active
constituents and pharmacological effects. J Ethnopharmacol.
273:1139602021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gan DL, Yao Y, Su HW, Huang YY, Shi JF,
Liu XB and Xiang MX: Volatile oil of Platycladus orientalis
(L.) Franco leaves exerts strong Anti-inflammatory effects via
inhibiting the IκB/NF-κB pathway. Curr Med Sci. 41:180–186. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou RP, Deng MT, Chen LY, Fang N, Du C,
Chen LP, Zou YQ, Dai JH, Zhu ML, Wang W, et al: Shp2 regulates
chlorogenic acid-induced proliferation and adipogenic
differentiation of bone Marrow-derived mesenchymal stem cells in
adipogenesis. Mol Med Rep. 11:4489–4495. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu T, Wu X, Zhou Z, Ye Y, Yan C, Zhuge N
and Yu J: Hyperoside ameliorates periodontitis in rats by promoting
osteogenic differentiation of BMSCs via activation of the NF-κB
pathway. FEBS Open Bio. 10:1843–1855. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han D, Chen W, Gu X, Shan R, Zou J, Liu G,
Shahid M, Gao J and Han B: Cytoprotective effect of chlorogenic
acid against hydrogen Peroxide-induced oxidative stress in MC3T3-E1
cells through PI3K/Akt-mediated Nrf2/HO-1 signaling pathway.
Oncotarget. 8:14680–14692. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Folwarczna J, Pytlik M, Zych M, Cegieła U,
Nowinska B, Kaczmarczyk-Sedlak I, Sliwinski L, Trzeciak H and
Trzeciak HI: Effects of caffeic and chlorogenic acids on the rat
skeletal system. Eur Rev Med Pharmacol Sci. 19:682–693.
2015.PubMed/NCBI
|
|
16
|
Kwak SC, Lee C, Kim JY, Oh HM, So HS, Lee
MS, Rho MC and Oh J: Chlorogenic acid inhibits osteoclast
differentiation and bone resorption by down-regulation of receptor
activator of nuclear factor kappa-B ligand-induced nuclear factor
of activated T cells c1 expression. Biol Pharm Bull. 36:1779–1786.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee SH and Jang HD: Scoparone attenuates
RANKL-induced osteoclastic differentiation through controlling
reactive oxygen species production and scavenging. Exp Cell Res.
331:267–277. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
An H, Chu C, Zhang Z, Zhang Y, Wei R, Wang
B, Xu K, Li L, Liu Y, Li G and Li X: Hyperoside alleviates
postmenopausal osteoporosis via regulating miR-19a-5p/IL-17A axis.
Am J Reprod Immunol. 90:e137092023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee SH, Ding Y, Yan XT, Kim YH and Jang
HD: Scopoletin and scopolin isolated from Artemisia
iwayomogi suppress differentiation of osteoclastic macrophage
RAW 264.7 cells by scavenging reactive oxygen species. J Nat Prod.
76:615–620. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bersuker K, Hendricks JM, Li Z, Magtanong
L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, et al:
The CoQ oxidoreductase FSP1 acts parallel to Gpx4 to inhibit
ferroptosis. Nature. 575:688–692. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kraft VAN, Bezjian CT, Pfeiffer S,
Ringelstetter L, Müller C, Zandkarimi F, Merl-Pham J, Bao X,
Anastasov N, Kössl J, et al: GTP Cyclohydrolase
1/Tetrahydrobiopterin Counteract ferroptosis through lipid
remodeling. ACS Cent Sci. 6:41–53. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stockwell BR, Friedmann Angeli JP, Bayir
H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK,
Kagan VE, et al: Ferroptosis: A regulated cell death nexus linking
metabolism, redox biology, and disease. Cell. 171:273–285. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dodson M, Castro-Portuguez R and Zhang DD:
NRF2 plays a critical role in mitigating lipid peroxidation and
Ferroptosis. Redox Biol. 23:1011072019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang Z, Qi G, He X, Yu Y, Cao Y, Zhang C,
Zou W and Yuan H: Ferroptosis in osteocytes as a target for
protection against postmenopausal osteoporosis. Adv Sci (Weinh).
2024:e23073882024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R
and Tang D: Activation of the p62-Keap1-NRF2 pathway protects
against ferroptosis in hepatocellular carcinoma cells. Hepatology.
63:173–184. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim TE, Jo YH and Kim CT: Improvement of
quality characteristics of mulberry (Morus alba L.) fruit
extract using high-pressure enzymatic treatment. Food Bioprocess
Technol. 17:4106–4114. 2024. View Article : Google Scholar
|
|
29
|
Gnoumou E, Tran TA, Yang TT, Quach TT and
Wang CY: Antibacterial, anti-inflammatory and anti-osteoclastogenic
effects of synthetic mineralized lysozyme nanoparticles for
treating infectious osteoporosis. Int J Biol Macromol.
320:1457692025. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li P, Dai J, Li Y, Alexander D, Čapek J,
Geis-Gerstorfer J, Wan G, Han J, Yu Z and Li A: Zinc based
biodegradable metals for bone repair and regeneration: Bioactivity
and molecular mechanisms. Mater Today Bio. 25:1009322024.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pietrangelo A: Iron and the Liver. Liver
Int. 36 (Suppl 1):S116–S123. 2016. View Article : Google Scholar
|
|
32
|
Wu Z, Zhu Y, Liu W, Balasubramanian B, Xu
X, Yao J and Lei X: Ferroptosis in liver disease: Natural active
compounds and therapeutic implications. Antioxidants (Basel).
13:3522024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Karadeniz F, Oh JH, Lee JI, Seo Y and Kong
CS: 3,5-dicaffeoyl-epi-quinic acid from Atriplex gmelinii
enhances the osteoblast differentiation of bone marrow-derived
human mesenchymal stromal cells via WnT/BMP signaling and
suppresses adipocyte differentiation via AMPK activation.
Phytomedicine. 71:1532252020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bauer NB, Khassawna TE, Goldmann F, Stirn
M, Ledieu D, Schlewitz G, Govindarajan P, Zahner D, Weisweiler D,
Schliefke N, et al: Characterization of bone turnover and energy
metabolism in a rat model of primary and secondary osteoporosis.
Exp Toxicol Pathol. 67:287–296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Komori T: Whole aspect of Runx2 functions
in skeletal development. Int J Mol Sci. 23:57762022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fakhry M, Hamade E, Badran B, Buchet R and
Magne D: Molecular mechanisms of mesenchymal stem cell
differentiation towards osteoblasts. World J Stem Cells. 5:136–148.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee B, Thirunavukkarasu K, Zhou L, Pastore
L, Baldini A, Hecht J, Geoffroy V, Ducy P and Karsenty G: Missense
mutations abolishing DNA binding of the osteoblast-specific
transcription factor OSF2/CBFA1 in cleidocranial dysplasia. Nat
Genet. 16:307–310. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ozaki Y, Koide M, Furuya Y, Ninomiya T,
Yasuda H, Nakamura M, Kobayashi Y, Takahashi N, Yoshinari N and
Udagawa N: Treatment of OPG-deficient mice with WP9QY, a
Rankl-binding peptide, recovers alveolar bone loss by suppressing
osteoclastogenesis and enhancing osteoblastogenesis. PLoS One.
12:e01849042017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhu R, Wang Z, Xu Y, Wan H, Zhang X, Song
M, Yang H, Chai Y and Yu B: High-fat diet increases bone loss by
inducing ferroptosis in osteoblasts. Stem Cells Int.
2022:93594292022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang Y, Lin Y, Wang M, Yuan K, Wang Q, Mu
P, Du J, Yu Z, Yang S, Huang K, et al: Targeting ferroptosis
suppresses osteocyte glucolipotoxicity and alleviates diabetic
osteoporosis. Bone Res. 10:262022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang X, Ma H, Sun J, Zheng T, Zhao P, Li H
and Yang M: Mitochondrial ferritin deficiency promotes osteoblastic
ferroptosis via mitophagy in type 2 diabetic osteoporosis. Biol
Trace Elem Res. 200:298–307. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zimmermann MB and Hurrell RF: Nutritional
iron deficiency. Lancet. 370:511–520. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Clark SF: Iron deficiency anemia. Nutr
Clin Pract. 23:128–141. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shen GS, Yang Q, Jian JL, Zhao GY, Liu LL,
Wang X, Zhang W, Huang X and Xu YJ: Hepcidin1 knockout mice display
defects in bone microarchitecture and changes of bone formation
markers. Calcif Tissue Int. 94:632–639. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lu H, Lian L, Shi D, Zhao H and Dai Y:
Hepcidin promotes osteogenic differentiation through the bone
morphogenetic protein 2/small mothers against decapentaplegic and
mitogen-activated protein kinase/P38 signaling pathways in
mesenchymal stem cells. Mol Med Rep. 11:143–150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fang Y, Chen X, Tan Q, Zhou H, Xu J and Gu
Q: Inhibiting ferroptosis through disrupting the NCOA4-FTH1
interaction: A new mechanism of action. ACS Cent Sci. 7:980–989.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu P, Zhang Z, Cai Y, Li Z, Zhou Q and
Chen Q: Ferroptosis: Mechanisms and role in diabetes mellitus and
its complications. Ageing Res Rev. 94:1022012024. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Le Y, Liu Q, Yang Y, Stanford D, Freeman
WM and Ding XQ: The emerging role of nuclear receptor coactivator 4
in health and disease: A novel bridge between iron metabolism and
Immunity. Cell Death Discov. 10:3122024. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang Z, Ji C, Wang YN, Liu S, Wang M, Xu
X and Zhang D: Maresin1 Suppresses High-Glucose-induced ferroptosis
in osteoblasts via NRF2 Activation in type 2 diabetic osteoporosis.
Cells. 11:25602022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kuang F, Liu J, Xie Y, Tang D and Kang R:
MGST1 is a redox-sensitive repressor of ferroptosis in pancreatic
cancer cells. Cell Chem Biol. 28:765–775.e5. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fan Z, Wirth AK, Chen D, Wruck CJ, Rauh M,
Buchfelder M and Savaskan N: Nrf2-Keap1 pathway promotes cell
proliferation and diminishes ferroptosis. Oncogenesis. 6:e3712017.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Rojo de la Vega M, Chapman E and Zhang DD:
NRF2 and the hallmarks of cancer. Cancer Cell. 34:21–43. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chen Y, Jiang Z and Li X: New insights
into crosstalk between Nrf2 pathway and ferroptosis in lung
disease. Cell Death Dis. 15:8412024. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Živanović N, Lesjak M, Simin N and Srai
SKS: Beyond mortality: Exploring the influence of plant phenolics
on modulating Ferroptosis-A systematic review. Antioxidants
(Basel). 13:3342024. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhou HM, Liu Y, Shi F, Qiu HW, Li L, Shu
QP, Liu YY, Wang BQ, Gao M, Du RL, et al: CLK2 Regulates the
KEAP1/NRF2 and p53 pathways to suppress ferroptosis in colorectal
cancer. Cancer Res. 85:4734–4750. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Duan CH and Jiang LL: High-dose Yin Chen
combined with Western medicine for liver protection in treating
acute icteric hepatitis. Youjiang Med J. 3832001.
|
|
57
|
Chen GE and Huang XD: Large dose of Yin
Chen in the treatment of 84 cases of acute infectious icteric
hepatitis: A therapeutic effect observation. Jilin J Tradit Chin
Med. 191984.
|