Introduction
The liver is the only human organ capable of
complete regeneration after partial resection (1). Even after resection of ≤70% of
hepatic mass, the remaining tissue can spontaneously restore liver
volume and function (2). This
notable regenerative capacity is important for favorable outcomes
in patients with severe liver injury, those undergoing living donor
liver transplantation, or patients undergoing surgical resection of
liver tumors and metastases (3).
Liver regeneration after hepatectomy can be broadly divided into
three phases: Initiation, growth and termination (4). Hepatocyte proliferation typically
peaks within the first 48 h post-surgery, after which the process
shifts towards functional restoration during the termination phase
(5). Failure to terminate
hepatocyte proliferation may impair subsequent differentiation,
hinder functional recovery of the liver, and may potentially lead
to poor outcomes or mortality (6).
Therefore, timely and effective termination of liver regeneration
is important for hepatocyte differentiation and restoration of
hepatic function.
At present, the initiation and growth phases of
liver regeneration have been extensively studied, but the molecular
mechanisms underlying the termination phase remain to be fully
elucidated (7,8). It has been reported that PP2Acα
regulates the termination of liver regeneration via the
AKT/GSK3β/cyclin D1 pathway (8).
Another study showed that hepatocyte nuclear factor 4α (HNF4α)
promotes hepatocyte cell cycle exit and the restoration of liver
function during the termination phase (6). In addition, hepatocyte
O-GlcNAcylation mediated by O-GlcNAc transferase represents an
important regulatory signal for termination of liver regeneration
(9).
Collagen, a major component of the extracellular
matrix (ECM), is important for functional remodeling during liver
regeneration (10). Furthermore,
it has been shown that endostatin, also known as collagen XVIII α1,
may regulate liver regeneration (11). A recent study has identified
characteristic clusters of collagen gene-expressing hepatic cells
following partial hepatectomy (PHx) (12). After PHx in rats, levels of
collagen I (col1) and collagen III (col3) have been reported to be
increased on postoperative days 3, 5 and 7, coinciding with the
timing of regeneration termination (13–15).
Previous studies have predominantly focused on total hepatic
collagen levels, with limited investigation into whether modulation
of collagen content affects liver regeneration (13–15).
However, collagen is highly expressed in areas surrounding
arterioles, venules and bile ducts, meaning that total hepatic
protein measurements may not accurately reflect the quantity of
collagen present in the hepatocyte-associated ECM (16). Therefore, the present study aimed
to explore how collagen in proximity to hepatocytes regulates the
termination of liver regeneration.
Materials and methods
Mouse PHx model
C57BL/6J mice (Hunan SJA Laboratory Animal Co.,
Ltd.; 69 female mice; age, 8 weeks; weight, 18–22 g) were housed in
the Animal Center of the Second Hospital Affiliated to Chongqing
Medical University (Chongqing, China). All animals were maintained
in temperature- and humidity-controlled facilities at 20–26°C and
60% humidity under a 12-h light/dark cycle, with food and water
supplied ad libitum. All animal experiments were approved by
the Ethics Committee of the Second Affiliated Hospital of Chongqing
Medical University (approval no. IACUC-SAHCQMU-2023-0027).
A 2/3 PHx model was established as previously
described (17). Briefly, under
isoflurane anesthesia (3% in the induction phase; 1.5–2% in the
maintenance phase), the left lateral and median lobes of the liver,
along with the gallbladder, were surgically removed. In the sham
group, mice underwent anesthesia and laparotomy for the same
average duration as the experimental groups but without hepatic
resection. Mice were subsequently sacrificed for tissue collection
at designated time points ranging from 0 h to 7 days
post-operation. Mice were euthanized by an overdose of 5%
isoflurane anesthesia, until complete cessation of the heartbeat
and respiration was observed. Subsequently, mouse liver tissues and
blood samples (from mouse fundus; ≥200 µl) were collected. Mouse
body weight was measured prior to euthanasia, and liver weight was
recorded after euthanasia. The serum liver function markers alanine
aminotransferase (ALT) and aspartate aminotransferase, as well as
the renal function marker blood urea nitrogen, were measured by
Wuhan Servicebio Technology Co., Ltd. using an automated
biochemical analyzer. ALT levels were evaluated in two different
experiments: One including the PHx, PHx + Coln0.2 and PHx + Coln0.2
+ MSAB groups, and the other including the Coln0.2 and Control
groups. For groups subjected to PHx alone, three mice were included
per group, whereas for experimental groups receiving PHx combined
with pharmacological interventions, including the vehicle control
group, six mice were included per group.
Administration of EdU, collagenase and
methyl-sulfonyl AB (MSAB)
To assess cell proliferation, EdU (50 mg/kg; cat.
no. ST067; Beyotime Biotechnology) fully dissolved in PBS was
administered to all mice via intraperitoneal injection 2 h prior to
the designated sampling time. For the low-dose collagenase group
(Coln0.1 group, 5 mg/kg/day), collagenase III, also known as matrix
metalloproteinase-13 (cat. no. BS238; Biosharp Life Sciences), was
injected intraperitoneally on postoperative days 4, 5 and 6
following PHx. The high-dose group (Coln0.2 group, 10 mg/kg/day)
received collagenase III injections at the same time points as the
low-dose group. For the high-dose collagenase + MSAB group (Coln0.2
+ MSAB group), 5 mg/kg/day MSAB (cat. no. HY-120697;
MedChemExpress) was dissolved in an ultrasonic ice bath and
administered intraperitoneally 2 h prior to each collagenase
injection. To evaluate the biosafety of collagenase III,
intraperitoneal injections of 10 mg/kg/day collagenase III for 3
days or an appropriate volume of vehicle were administered to
C57BL/6J mice (n=3, these mice did not undergo PHx), followed by
tissue collection on day 4. These mice only received the
administration of collagenase III; specifically, they did not
receive EdU injection, MSAB injection, PHx or sham operation. The
drug was administered in 0.2 ml PBS per injection, while the
control group received an equivalent volume of PBS.
Histology and immunofluorescence (IF)
staining
Tissues were fixed in 4% paraformaldehyde at room
temperature overnight, embedded in paraffin and sectioned at 5 µm.
Sections were deparaffinized in xylene for 30 min, followed by 100%
ethanol for 10 min and sequential rehydration in 95, 85 and 75%
ethanol for 5 min each, followed by ddH2O for 1 min.
Hematoxylin and eosin (H&E) staining was performed according to
the manufacturer's instructions (Hematoxylin-Eosin Stain Kit; cat.
no. G1120; Beijing Solarbio Science & Technology Co., Ltd.).
Sections were stained with hematoxylin for 3 min, differentiated in
1% hydrochloric acid for 5 sec, blued in 10% ammonium solution for
30 sec and stained with eosin for 1.5 min; all at room temperature.
Images were captured using a light microscope. IF staining was
performed to evaluate the expression of col1, desmin, α-smooth
muscle actin (α-SMA), col3 and Ki-67. Antibody information can be
found in Table I. For col1,
desmin, α-SMA, col3 and Ki-67 IF staining, sections were subjected
to citrate buffer (cat. no. C1032; Beijing Solarbio Science &
Technology Co., Ltd.) at 90°C for 15 min. The sections were blocked
with 10% normal goat serum (cat. no. AR0009; Boster Biological
Technology) at room temperature for 30 min. Subsequently, sections
were incubated overnight at 4°C with primary antibodies. Sections
were subsequently incubated with fluorescently labeled secondary
antibodies at room temperature for 2 h, followed by DAPI staining
at room temperature for 20 min before mounting. For sections
requiring EdU double-labeling, EdU staining was performed using the
BeyoClick™ EdU-488 Cell Proliferation Assay Kit (cat.
no. C0071S; Beyotime Biotechnology) according to the manufacturer's
protocol prior to DAPI counterstaining. The fluorescence signals
were detected and visualized using a fluorescence microscope (Leica
Microsystems, Inc.). IF staining was independently scored and
reviewed by two blinded evaluators. For semi-quantification of col3
in the parenchymal area, regions lacking vessels and bile ducts
were preselected and the average fluorescence intensity was
measured. For EdU and Ki-67, the nuclear positivity rate was
calculated. Positive nuclei were identified and semi-quantified
using ImageJ version 2.1.0 (National Institutes of Health) by
applying the threshold, binary and analyze particles functions.
Each group contained three biological replicates (n=3).
 | Table I.Information on antibodies. |
Table I.
Information on antibodies.
| Target | Manufacturer | Cat. no. | Application | Dilution ratio |
|---|
| Collagen I | Abcam | ab270993 | IF | 1:500 |
| Desmin | Proteintech Group,
Inc. | 16520-1-AP | IF | 1:500 |
| α-SMA | Proteintech Group,
Inc. | 14395-1-AP | IF | 1:1,000 |
| Collagen III | Proteintech Group,
Inc. | 22734-1-AP | IF | 1:300 |
| Ki-67 | Proteintech Group,
Inc. | 28074-1-AP | IF | 1:500 |
| Cy3 conjugated
Goat | Wuhan
Servicebio | GB21303 | IF | 1:200 |
| Anti-Rabbit IgG
(H+L) | Technology Co.,
Ltd. |
|
|
|
| β-catenin | Abcam | ab305261 | WB | 1:1,000 |
| HNF4α | Santa Cruz | sc-101059 | WB | 1:100 |
|
| Biotechnology,
Inc. |
|
|
|
| Cyclin D1 | CST Biological | 2978S | WB | 1:1,000 |
|
| Reagents Co.,
Ltd. |
|
|
|
| GAPDH | HUABIO | HA721136 | WB | 1:5,000 |
| β-actin | HUABIO | HA722023 | WB | 1:20,000 |
| Goat anti-Rabbit
IgG-HRP | Absin Bioscience,
Inc. | abs20040 | WB | 1:5,000 |
| Goat anti-Mouse
IgG-HRP | Absin Bioscience,
Inc. | abs20039 | WB | 1:5,000 |
Western blotting
Total protein was extracted from liver tissue using
RIPA buffer (cat. no. P0013B; Beyotime Biotechnology) supplemented
with PMSF. The protein concentration was measured using the BCA
Protein Assay Kit (cat. no. P0010; Beyotime Biotechnology), and 20
µg of protein was loaded per lane. Proteins were separated by
SDS-PAGE on 10% gels and then transferred onto PVDF membranes. The
membranes were incubated at room temperature for 30 min in Protein
Free Rapid Blocking Buffer (cat. no. PS108P; Epizyme; Ipsen
Pharma). Subsequently, the membranes were incubated overnight at
4°C with primary antibodies against β-catenin, HNF4α, cyclin D1,
GAPDH and β-actin. GAPDH and β-actin served as loading controls.
The membranes were incubated with the secondary antibodies at room
temperature for 2 h. Antibody information can be found in Table I. Target protein expression was
detected using an ECL kit (cat. no. AR1191; Boster Biological
Technology). The intensity of each band was measured using ImageJ
version 2.1.0 (National Institutes of Health). Each group contained
three biological replicates (n=3).
Reverse transcription-quantitative PCR
(RT-qPCR)
RT-qPCR was performed as previously described
(18). Total mRNA was isolated
from liver tissues using RNAiso Plus (cat. no. 9108; Takara
Biotechnology Co., Ltd.). Subsequently, 500 ng mRNA was
reverse-transcribed into cDNA using a cDNA synthesis kit (cat. no.
HY-K0511A; MedChemExpress). The reverse transcription temperature
program was set according to the manufacturer's instructions. qPCR
was performed on the resulting cDNA using SYBR Green qPCR Master
Mix (cat. no. HY-K0501; MedChemExpress) on a CFX connect real-time
PCR detection system (Bio-Rad Laboratories, Inc.). qPCR was carried
out with an initial activation at 95°C for 5 min, followed by 40
cycles of 95°C for 15 sec and 60°C for 1 min, and a final melting
curve at 95°C for 15 sec, 60°C for 1 min and 95°C for 15 sec. GAPDH
served as the internal reference gene, and the relative expression
of the target gene was calculated using the 2−ΔΔCq
method (19). The primer sequences
used for RT-qPCR are provided in Table II. Each group contained three
biological replicates (n=3).
 | Table II.Reverse transcription-quantitative
PCR primers. |
Table II.
Reverse transcription-quantitative
PCR primers.
| Primer | Sequence,
5′-3′ |
|---|
| Col3a1 |
|
| Forward |
CTGTAACATGGAAACTGGGGAAA |
| Reverse |
CCATAGCTGAACTGAAAACCACC |
| Gapdh |
|
| Forward |
CAAGGAGTAAGAAACCCTGGAC |
| Reverse |
GGATGGAAATTGTGAGGGAGAT |
Statistical analysis
Statistical analysis was conducted using GraphPad
Prism version 9.0 (Dotmatics). Data are presented as the mean ±
standard deviation. An unpaired Student's t-test was used for
comparisons between two groups. One-way ANOVA was applied for
multiple group comparisons, and pairwise comparisons were performed
using Tukey's post hoc test. Two-tailed P<0.05 was considered to
indicate a statistically significant difference.
Results
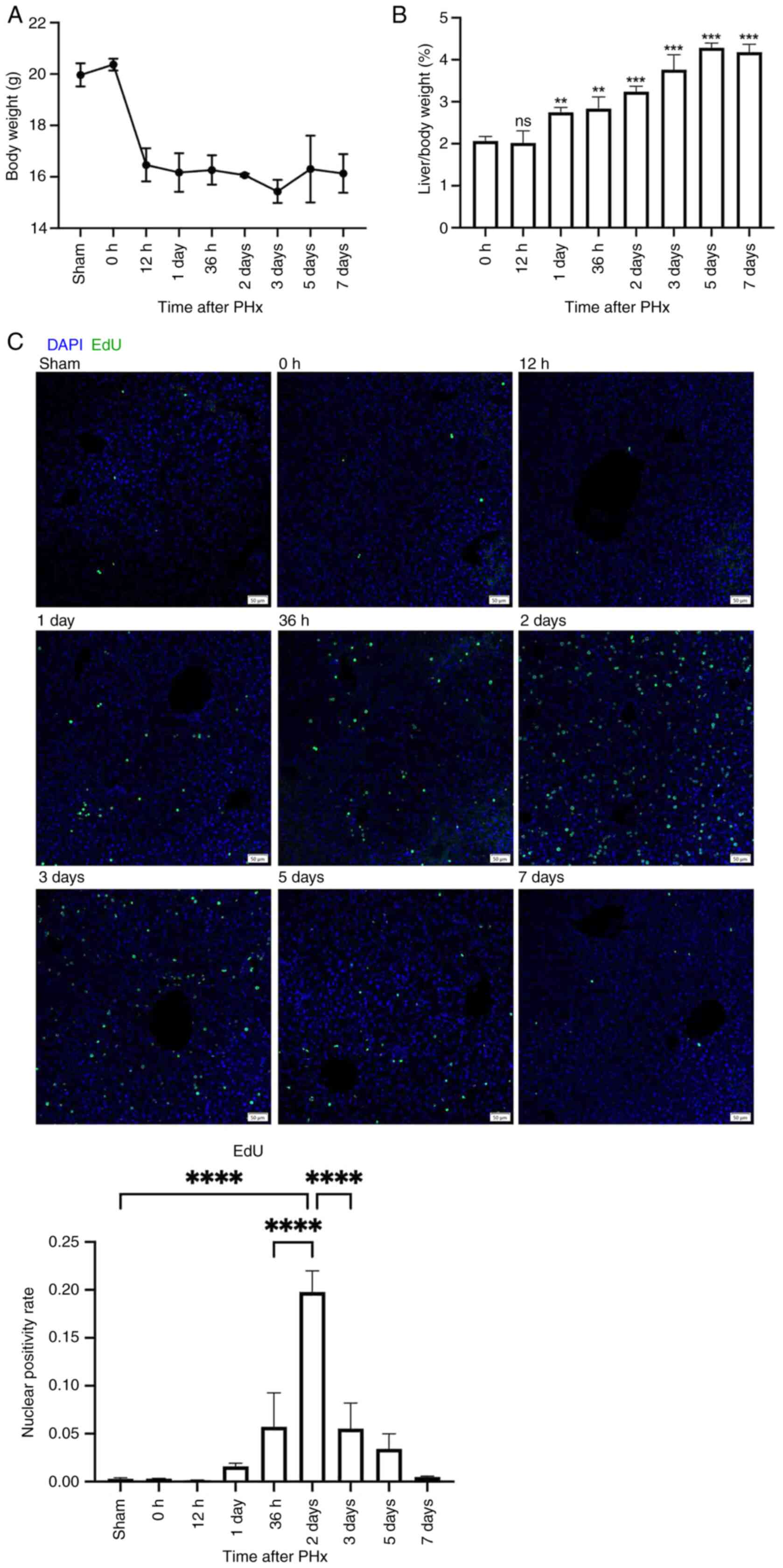
Spontaneous liver regeneration after
2/3 PHx in mice
The present study established a mouse model of 2/3
PHx and collected liver tissues at specific time points
post-surgery. EdU was intraperitoneally injected 2 h before tissue
harvest. The present study observed a partial reduction in body
weight after PHx (Fig. 1A), while
the liver-to-body weight ratio began to significantly recover from
day 1 post-PHx (Fig. 1B). The
nuclear positivity rate of EdU increased starting from day 1,
peaked significantly at day 2 and gradually decreased from days 2
to 7 (Fig. 1C). These findings
indicated that spontaneous liver regeneration occurred after 2/3
PHx in mice. The growth phase occurred between days 1 and 2, with
the highest hepatocyte proliferation rate observed on day 2,
followed by a termination phase of liver regeneration from days 2
to 7.
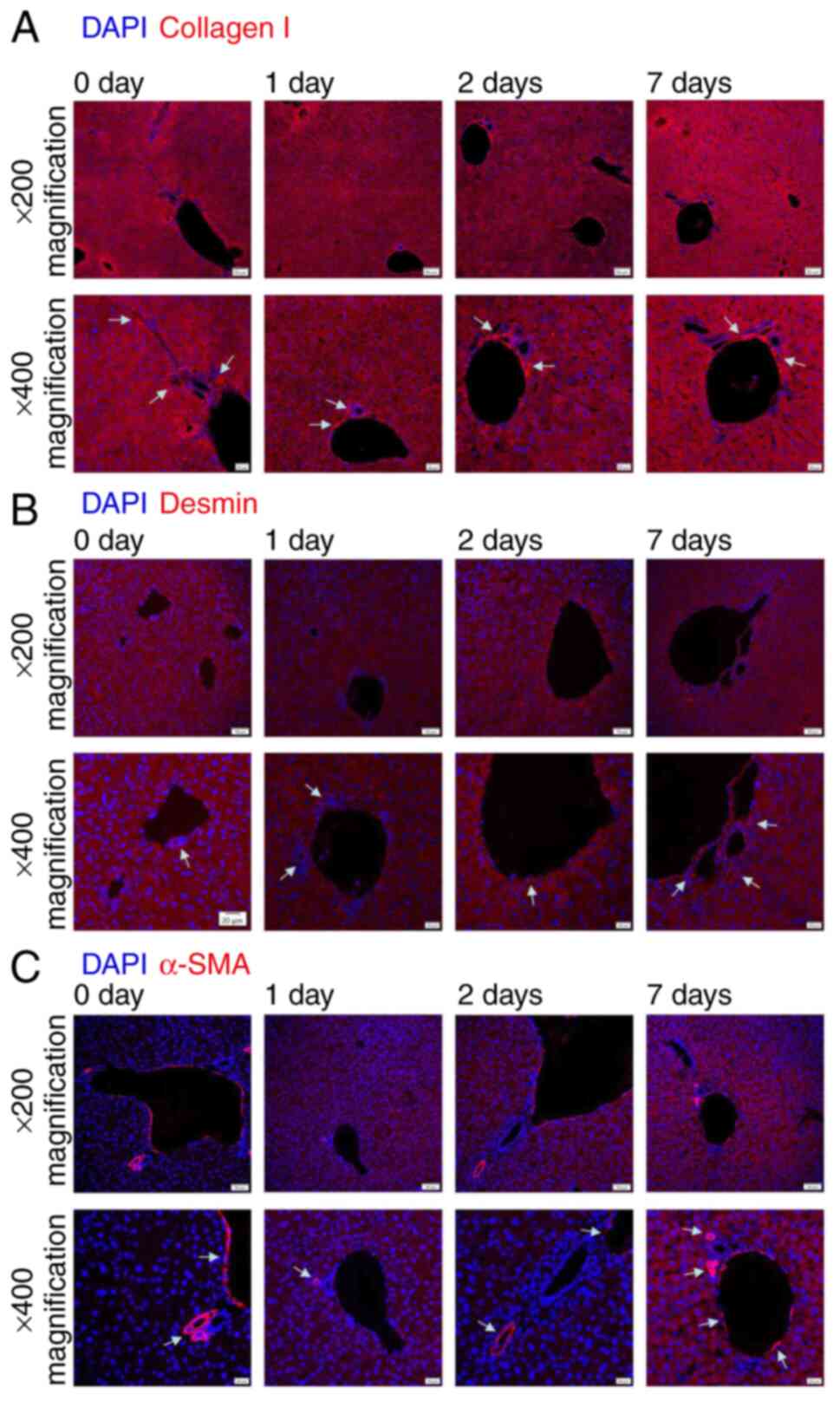
Expression of collagen after PHx
The present study hypothesized that specific
components within the hepatocyte-associated ECM may regulate liver
regeneration. These components were hypothesized to be distributed
in proximity to hepatocytes and exhibit dynamic changes across
different stages of regeneration. Therefore, the present study
evaluated the expression of major collagens or proteins related to
collagens during liver regeneration via IF staining, including
col1, col3, desmin and α-SMA. Col1, desmin and α-SMA were
predominantly expressed surrounding hepatic blood vessels and bile
ducts across all experimental time points, with comparatively low
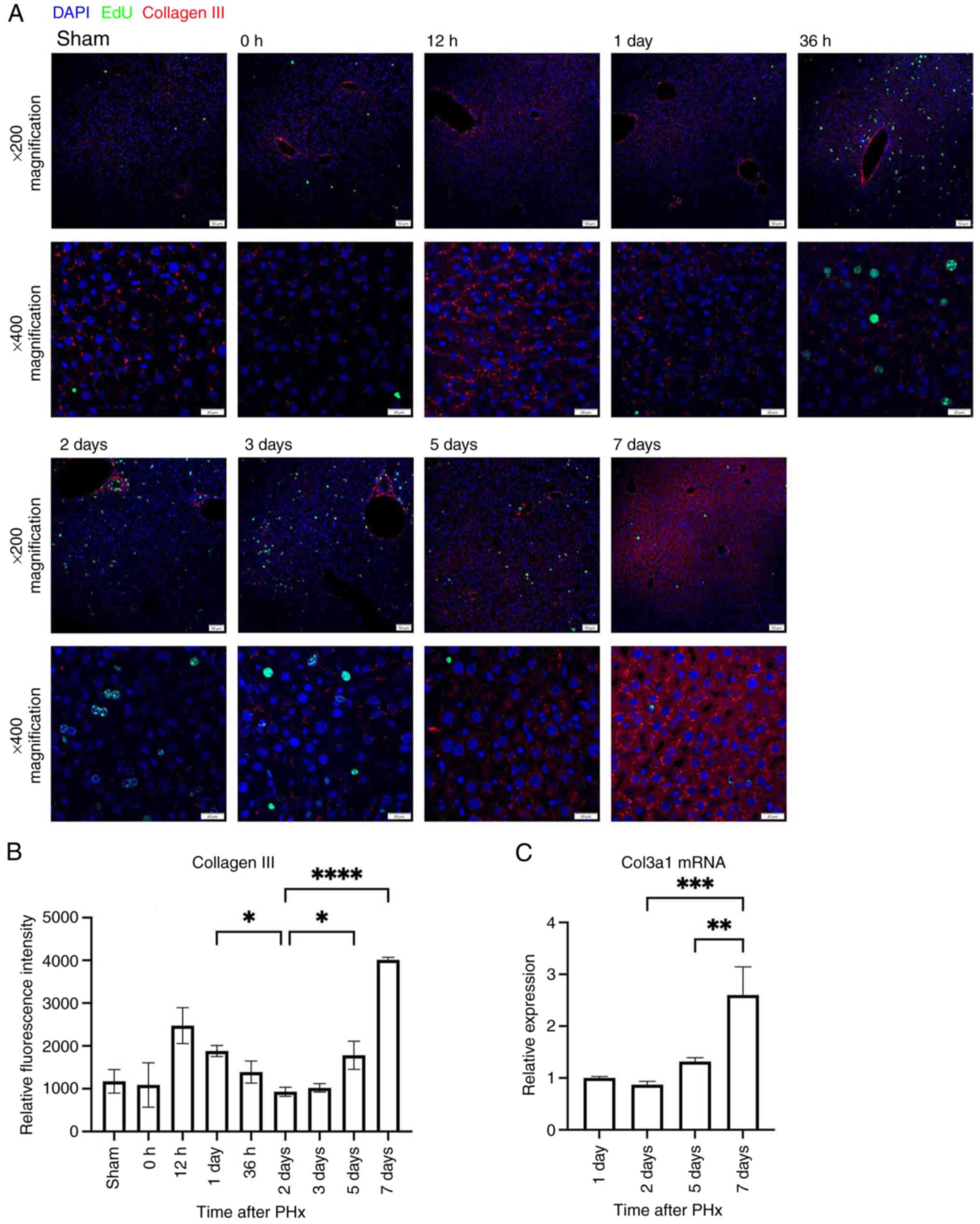
expression observed in parenchymal areas (Fig. 2). By contrast, col3 was abundantly
expressed not only in proximity to blood vessels and bile ducts but
also surrounding hepatocytes in parenchymal areas (Fig. 3A). Semi-quantitative analysis of
average fluorescence intensity in parenchymal areas showed that
col3 expression was significantly decreased during the
proliferative phase from days 1–2 and significantly increased again
during the termination phase across days 2–7 (Fig. 3B). RT-qPCR supported that Col3α1
mRNA expression increased significantly from days 2 to 7 (Fig. 3C). These findings suggested that
col3 levels in parenchymal areas may have been inversely associated
with hepatocyte proliferation and that col3 may have acted as a
negative regulator of regeneration.
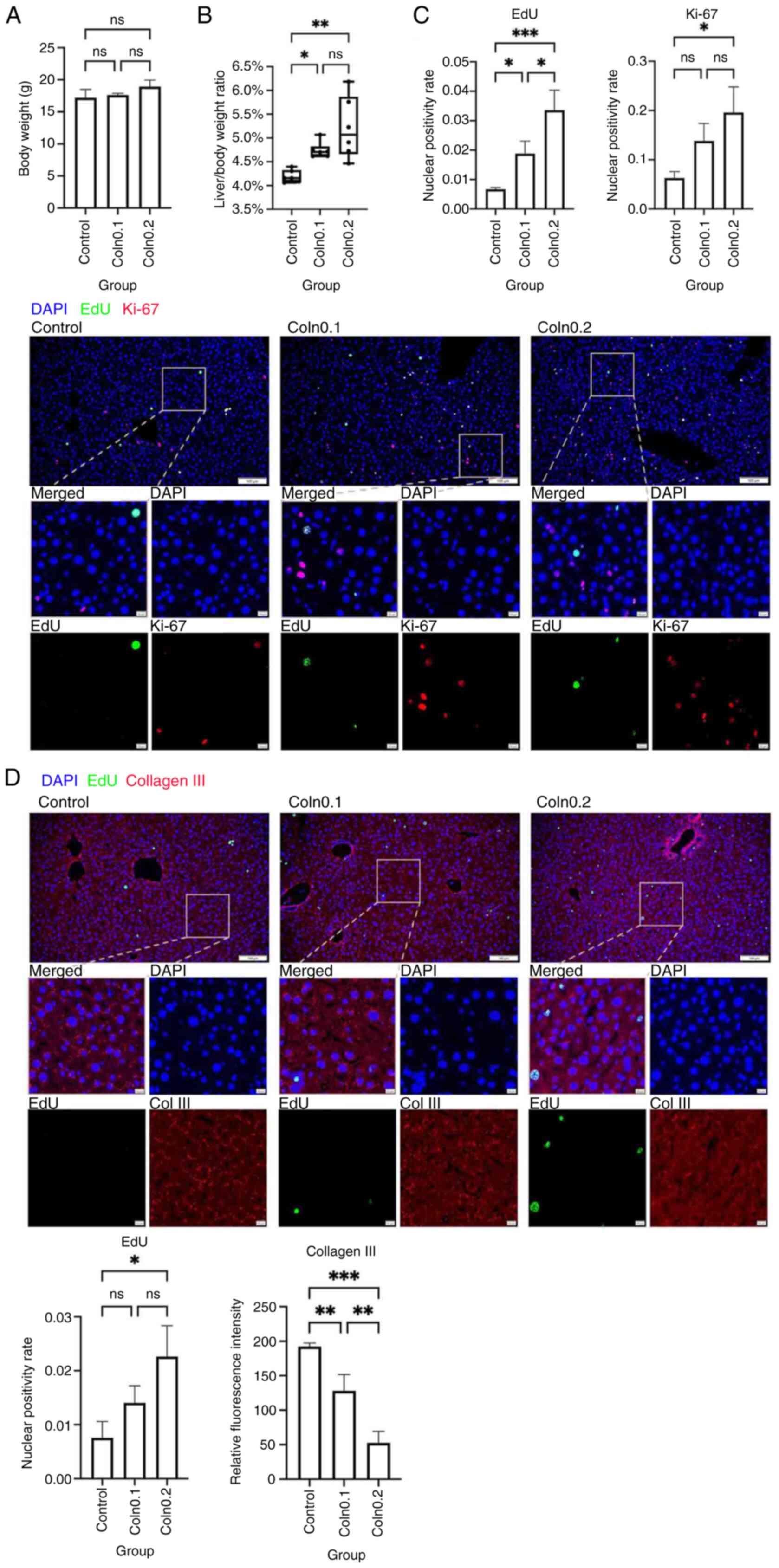
Degradation of col3 promotes
proliferation and delays regenerative termination
To assess whether col3 suppressed hepatocyte
proliferation, mice were administered either low or high doses of
collagenase III on days 4, 5 and 6 post-hepatectomy; samples of
liver tissue were subsequently collected on day 7. Compared with in
the control group, body weight was unchanged in collagenase-treated
mice, but the liver-to-body weight ratio was significantly higher
in both collagenase groups (Fig. 4A
and B). Dual IF staining for Ki-67 and EdU provided evidence of
a significantly higher proportion of proliferating cells in the
collagenase groups, with the highest proliferation rates observed
in the high-dose group (Fig. 4C).
Dual IF staining of col3 and EdU showed that, compared with that in
the control group, col3 expression in parenchymal areas was
significantly reduced in collagenase groups, while EdU nuclear
positivity increased significantly after high-dose collagenase
treatment (Fig. 4D). These results
indicated that collagenase III effectively degraded col3 in
parenchymal areas and promoted hepatocyte proliferation, delaying
the termination of regeneration in a dose-dependent manner.
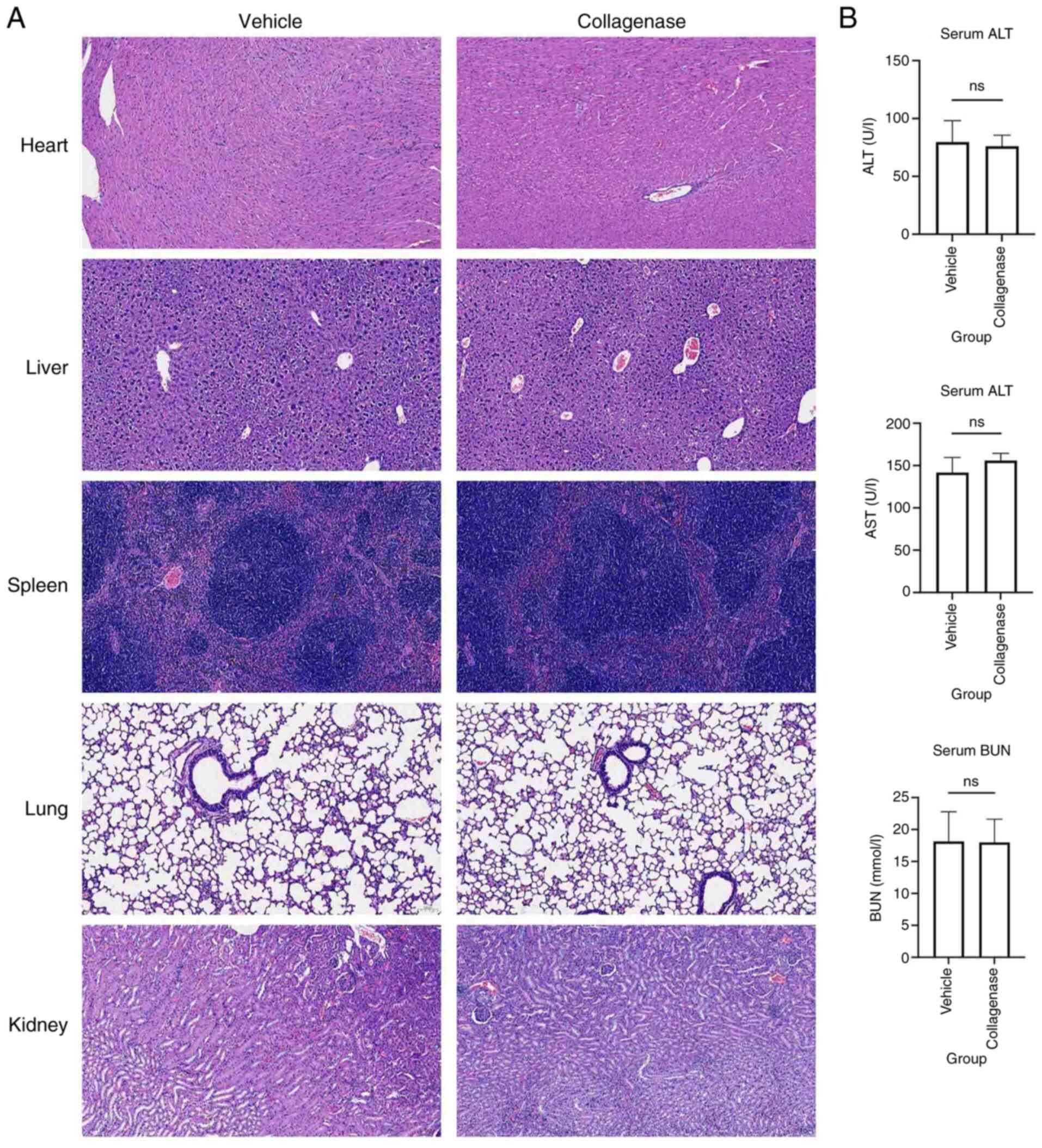
To evaluate the biosafety of collagenase III,
intraperitoneal injections of 10 mg/kg/day collagenase III for 3
days or an appropriate volume of vehicle were administered to
C57BL/6J mice, followed by tissue collection on day 4. H&E
staining showed no notable tissue damage or abnormalities in the
heart, liver, spleen, lungs or kidneys of collagenase III-treated
mice compared with vehicle-treated control mice (Fig. 5A). Additionally, levels of serum
markers of liver and renal function revealed no significant
differences between the two groups (Fig. 5B). These results suggested that
intraperitoneal injections of collagenase III at the dosage used in
the present study were well tolerated and exhibited sufficient
biosafety.
Col3 degradation triggers
β-catenin-mediated abnormal proliferation, which is rescued by
MSAB
Given the well-established role of the β-catenin
pathway in liver regeneration (20), the present study subsequently
investigated whether the proliferative effect of col3 degradation
involved this pathway. Western blot analysis showed that
administration of high-dose collagenase significantly increased
β-catenin expression compared with both the control and low-dose
groups, indicating pathway activation (Fig. 6B).
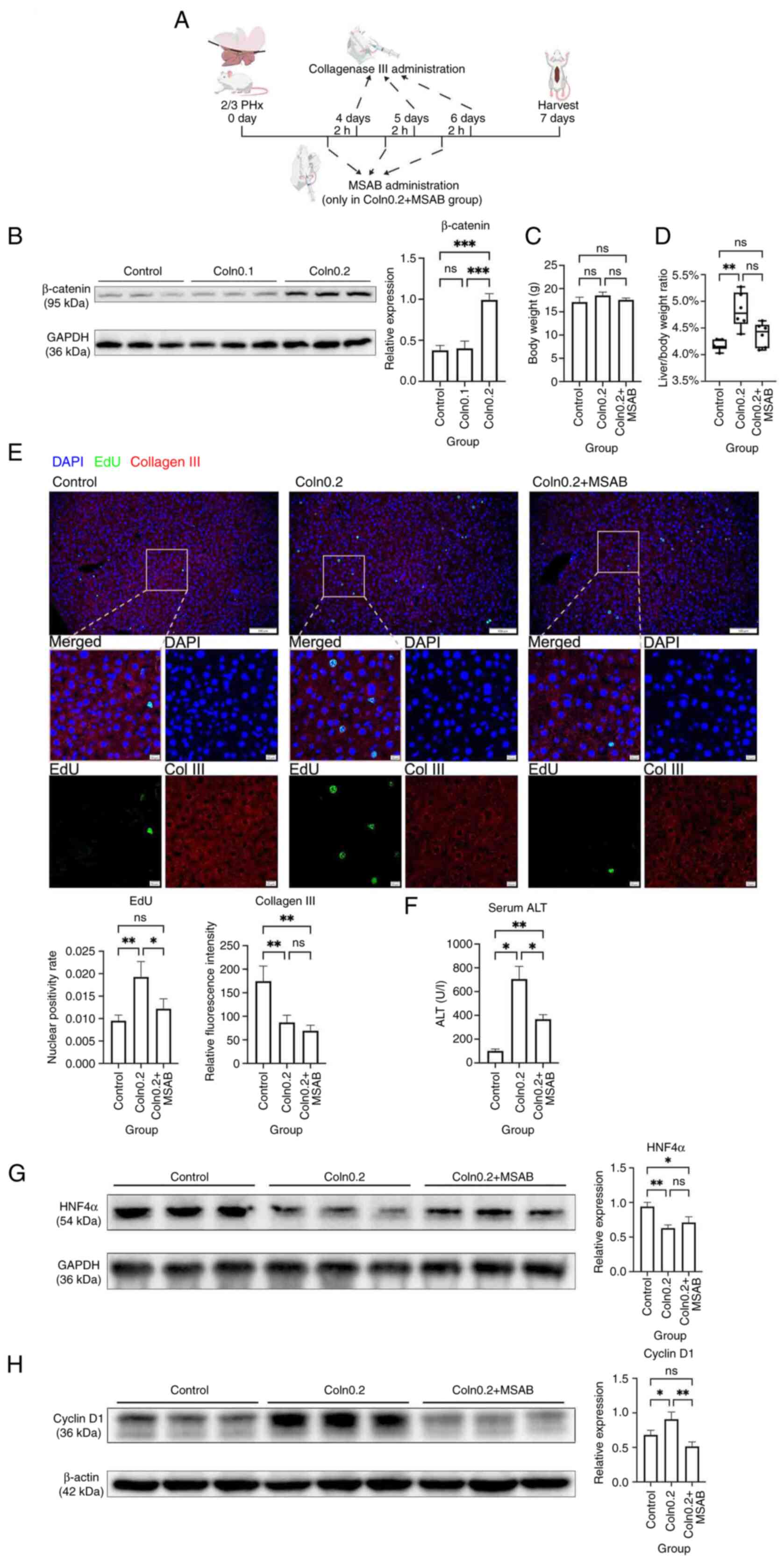 | Figure 6.Inhibition of β-catenin partially
rescues impaired regeneration termination and liver dysfunction
induced by collagen III degradation. (A) Schematic diagram of the
animal experimental protocol; created with BioGDP.com (agreement
no. GDP2025X2AT2F) (35). (B) WB
analysis of β-catenin after collagen III degradation. (C) Body
weight and (D) liver-to-body weight ratio of mice following
collagen III degradation and β-catenin inhibition. (E) Dual
immunofluorescence staining of EdU and collagen III following
collagen III degradation and β-catenin inhibition. Scale bars, 100
and 10 µm. (F) Serum liver function marker levels following
collagen III degradation and β-catenin inhibition. (G) WB analysis
of HNF4α following collagen III degradation and β-catenin
inhibition. (H) WB analysis of cyclin D1 following collagen III
degradation and β-catenin inhibition. *P<0.05, **P<0.01 and
***P<0.001. ns, not significant; WB, western blot; PHx, partial
hepatectomy; MSAB, methyl-sulfonyl AB; Coln0.1, low-dose
collagenase III; Coln0.2, high-dose collagenase III; Col III,
collagen III; ALT, alanine aminotransferase; HNF4α, hepatocyte
nuclear factor 4α. |
To further assess causality, the β-catenin inhibitor
MSAB was administered to mice intraperitoneally 2 h before each
collagenase injection on days 4, 5 and 6 (Fig. 6A). MSAB did not significantly
affect body weight but partially reversed the collagenase-induced
increase in the liver-to-body weight ratio, returning it close to
control levels, although this difference was not statistically
significant (Fig. 6C and D). Dual
IF staining for col3 and EdU showed that administration of MSAB
alongside high-dose collagenase treatment did not alter col3
expression but significantly reduced EdU positivity (Fig. 6E), suggesting that β-catenin
inhibition partially reversed the proliferative effect induced by
col3 degradation.
To evaluate the effect of abnormal hepatocyte
proliferation on liver function, serum ALT levels were measured.
Mice treated with collagenase exhibited significantly elevated ALT
compared with control mice, which was indicative of liver injury,
while co-treatment with MSAB significantly reduced ALT levels
(Fig. 6F). Western blotting showed
that the hepatocyte differentiation marker HNF4α was significantly
decreased after collagenase treatment (Fig. 6G). Additionally, cyclin D1, a
downstream target of β-catenin, was significantly upregulated
following collagenase administration compared with in the control
group, and was subsequently significantly downregulated upon MSAB
co-treatment (Fig. 6H).
Taken together, these results demonstrated that col3
degradation during the termination phase activated the β-catenin
pathway, disrupted the termination of liver regeneration, promoted
abnormal hepatocyte proliferation and impaired liver function.
Inhibition of β-catenin by MSAB partially rescued these
effects.
Discussion
Liver regeneration is regulated by multiple
mechanisms and signaling pathways; however, the processes governing
its termination remain to be fully elucidated. The present study
investigated the role of col3 in the termination of liver
regeneration following 2/3 PHx. The findings of the present study
suggested that col3 may regulate the termination of liver
regeneration via the β-catenin pathway, while promoting functional
recovery of the liver.
ECM is a complex and well-organized
three-dimensional network that provides structural support to cells
and tissues (21). Beyond its
mechanical role, the ECM coordinates intercellular signaling, and
mediates communication between tissues and organs (22). Through the regulation of cell
proliferation, differentiation and adhesion, the ECM serves an
important role in guiding tissue morphogenesis, development and
homeostatic maintenance (23). ECM
remodeling under pathological conditions serves as a key driver of
disease progression (24,25). Collagen is a major structural
component of the ECM, accounting for >30% of total ECM protein,
with col1, col2 and col3 comprising 80–90% of all collagens in the
body (23). In the context of
liver disease, col1 and col3 are primarily known for forming the
structural scaffold of the liver and providing tensile strength to
the hepatic lobule (23). Their
excessive accumulation is a hallmark of liver fibrosis (23). Previous studies have examined the
temporal changes of hepatic col1 and col3 in a rat PHx model
(13–15). Consistent with the results of the
present study performed in mice, the expression of both col1 and
col3 gradually increased during the termination phase of liver
regeneration. However, the present study found that only col3 was
expressed surrounding hepatocytes in parenchymal areas, suggesting
that col3 may have represented the major collagen component acting
within the hepatocyte-associated ECM. Recent studies have reported
an elevated expression of col3 in poorly regenerating skin tissue,
and have indicated that this could suppress scar formation during
wound healing (26,27). The findings of these studies
suggest that col3 may serve as a negative regulator of
regeneration, possibly by limiting proliferation or tissue
remodeling. This raises the possibility that col3 may serve a
similar regulatory role in the termination of liver
regeneration.
The findings of the present study suggested that the
accumulation of col3 in close proximity to hepatocytes may create a
microenvironment which suppresses excessive hepatocyte
proliferation, while promoting hepatocyte differentiation,
maturation and functional recovery. In the present study, col3 was
associated with reduced expression of proliferative markers,
supporting the notion that col3 may function as a negative
regulator of proliferative cues. Furthermore, col3 appeared to
facilitate the transition from proliferation to functional
recovery. By upregulating HNF4α, col3 may promote hepatocyte
differentiation and maturation, which is important for restoring
hepatic function once regenerative growth subsides (6). Such dual actions, including the
restriction of excessive hepatocyte proliferation and the promotion
of functional reconstruction, highlight col3 as a key ECM component
that may ensure the sufficient termination of liver regeneration.
These mechanistic insights provide a foundation for future studies
aimed at manipulating ECM remodeling to improve postoperative liver
recovery.
β-catenin, a central component of the Wnt/β-catenin
signaling pathway, is activated during the early phase of liver
regeneration and promotes hepatocyte proliferation (20). In the present study, the
degradation of col3 during the termination phase of liver
regeneration was followed by the upregulation of β-catenin and
abnormal proliferation. Notably, col3 is generally considered a
downstream target of β-catenin during hepatic fibrosis, being
upregulated following β-catenin activation (28). The results of the present study
suggested that the degradation of col3 upregulated β-catenin
expression in return, indicating a potential bidirectional feedback
loop between col3 and β-catenin. However, the mechanisms underlying
this interaction require further investigation.
Following PHx, once liver mass and volume are
largely restored, proliferation is gradually terminated and
hepatocytes enter a redifferentiation process to recover normal
liver function (6). A previous
study has reported that HNF4α serves an important role during the
termination phase of liver regeneration by promoting hepatocyte
maturation and maintaining hepatic function (6). HNF4α is a key regulator of hepatocyte
differentiation and controls as much as 60% of all hepatic genes
(29,30). In the present study, degradation of
col3 during the termination phase led to the downregulation of
HNF4α, suggesting that hepatocytes failed to properly differentiate
and regain normal function. A previous study has shown that mice
with impaired termination of liver regeneration may develop small
nodules at 28 days after PHx, indicating that defects in
regeneration termination may further progress to tumorigenesis
(9). The results of the present
study also showed that the body weight of mice at 12 h after PHx
was lower than that of mice in the sham and PHx 0 h groups. This
may have been due to a number of factors. On the one hand,
postoperative pain may have led to reduced food and water intake.
On the other hand, abdominal surgery may have been accompanied by
fluid loss. Furthermore, transient liver injury could have caused
digestive dysfunction.
There are several limitations to the present study.
Primarily, the present study used collagenase III to degrade
collagen. Although collagenase III is known to preferentially
digest col3 located in proximity to cells, it can also cleave
various other ECM proteins (31).
Therefore, the present study cannot exclude the possibility that
other ECM proteins may have exerted similar or opposite effects on
hepatic regeneration to those observed. Furthermore, although IF
staining did not reveal positive col1 staining surrounding
hepatocytes in parenchymal areas, the present study cannot rule out
potential contributions of col1 or other collagens in the
hepatocyte-associated ECM to the observed results. Additionally,
considering that liver regeneration is regulated by multiple
signaling pathways (7), disruption
of one pathway may be compensated by the activation of others. The
present study only empirically evaluated the involvement of the
β-catenin pathway and did not explore the contribution of other
potential pathways. Nevertheless, inhibition of β-catenin partially
rescued the impaired termination of liver regeneration, providing
support for β-catenin serving at least a partial role in this
process. Finally, the present study focused exclusively on
hepatocytes, with limited investigation of other cell types. A
growing body of evidence has demonstrated that non-epithelial cells
have important roles in various liver diseases (32–34).
Although hepatocytes ultimately execute hepatic functions, they are
also regulated by other cell types. Further studies are warranted
to elucidate the complex interactions among different cell
populations in the liver.
In summary, the present study provided preliminary
evidence that col3 may regulate the termination of liver
regeneration by suppressing hepatocyte proliferation and promoting
functional recovery. Further studies are needed to elucidate the
underlying mechanisms in greater detail.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Nature Science
Foundation of China (grant no. 82102337), the Natural Science
Foundation of Chongqing (grant no. CSTB2022NSCQ-MSX0899) and the
Special Funding for Postdoctoral Research Project of Chongqing
(grant no. 2021XM3071).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
AZ and JG were responsible for the conception and
design of the experiments. HP, ZC, QZ, YZ and PY contributed
towards the acquisition and analysis of data. HP, ZC and AZ
interpreted the data. HP, YZ and PY were responsible for drafting
the manuscript. JG and AZ reviewed and edited the manuscript. HP,
ZC, QZ, YZ, PY, JG and AZ confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Ethics
Committee of the Second Affiliated Hospital of Chongqing Medical
University (approval no. IACUC-SAHCQMU-2023-0027).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PHx
|
partial hepatectomy
|
|
col3
|
collagen III
|
|
col1
|
collagen I
|
|
ALT
|
alanine aminotransferase
|
|
ECM
|
extracellular matrix
|
References
|
1
|
Wu Y, Min J, Ge C, Shu J, Tian D, Yuan Y
and Zhou D: Interleukin 22 in liver injury, inflammation and
cancer. Int J Biol Sci. 16:2405–2413. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shao C, Jing Y, Zhao S, Yang X, Hu Y, Meng
Y, Huang Y, Ye F, Gao L, Liu W, et al: LPS/Bcl3/YAP1 signaling
promotes Sox9+HNF4α+ hepatocyte-mediated
liver regeneration after hepatectomy. Cell Death Dis. 13:2772022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Satilmis B, Akbulut S, Sahin TT, Dalda Y,
Tuncer A, Kucukakcali Z, Ogut Z and Yilmaz S: Assessment of liver
regeneration in patients who have undergone living donor
hepatectomy for living donor liver transplantation. Vaccines
(Basel). 11:2442023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Haan LR, van Golen RF and Heger M:
Molecular pathways governing the termination of liver regeneration.
Pharmacol Rev. 76:500–558. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang L, Zhou Y, Huang Z, Li W, Lin J,
Huang W, Sang Y, Wang F, Sun X, Song J, et al: Electroacupuncture
promotes liver regeneration by activating DMV acetylcholinergic
neurons-vagus-macrophage axis in 70% partial hepatectomy of mice.
Adv Sci (Weinh). 11:e24028562024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huck I, Gunewardena S, Espanol-Suner R,
Willenbring H and Apte U: Hepatocyte Nuclear factor 4 alpha
activation is essential for termination of liver regeneration in
mice. Hepatology. 70:666–681. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsomaia K, Patarashvili L, Karumidze N,
Bebiashvili I, Azmaipharashvili E, Modebadze I, Dzidziguri D,
Sareli M, Gusev S and Kordzaia D: Liver structural transformation
after partial hepatectomy and repeated partial hepatectomy in rats:
A renewed view on liver regeneration. World J Gastroenterol.
26:3899–3916. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lai SS, Zhao DD, Cao P, Lu K, Luo OY, Chen
WB, Liu J, Jiang EZ, Yu ZH, Lee G, et al: PP2Acα positively
regulates the termination of liver regeneration in mice through the
AKT/GSK3β/cyclin D1 pathway. J Hepatol. 64:352–360. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Robarts DR, McGreal SR, Umbaugh DS, Parkes
WS, Kotulkar M, Abernathy S, Lee N, Jaeschke H, Gunewardena S,
Whelan SA, et al: Regulation of liver regeneration by hepatocyte
O-GlcNAcylation in mice. Cell Mol Gastroenterol Hepatol.
13:1510–1529. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Akhtam R, Nuraliyevna SN, Kadham MJ,
Mirzakhamitovna KS, Tursunaliyevna RM, Shakhnoz K, Shakhzod T,
Otabek B, Baxtiyorovich MI, Shakhboskhanovna AF, et al: Biomarkers
in liver regeneration. Clin Chim Acta. 576:1204132025. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yazici SE, Gedik ME, Leblebici CB,
Kosemehmetoglu K, Gunaydin G and Dogrul AB: Can endocan serve as a
molecular ‘hepatostat’ in liver regeneration? Mol Med. 29:292023.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kimura Y, Koyama Y, Taura K, Kudoh A,
Echizen K, Nakamura D, Li X, Nam NH, Uemoto Y, Nishio T, et al:
Characterization and role of collagen gene expressing hepatic cells
following partial hepatectomy in mice. Hepatology. 77:443–455.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Klaas M, Kangur T, Viil J, Mäemets-Allas
K, Minajeva A, Vadi K, Antsov M, Lapidus N, Järvekülg M and Jaks V:
The alterations in the extracellular matrix composition guide the
repair of damaged liver tissue. Sci Rep. 6:273982016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rudolph KL, Trautwein C, Kubicka S,
Rakemann T, Bahr MJ, Sedlaczek N, Schuppan D and Manns MP:
Differential regulation of extracellular matrix synthesis during
liver regeneration after partial hepatectomy in rats. Hepatology.
30:1159–1166. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamamoto H, Murawaki Y and Kawasaki H:
Hepatic collagen synthesis and degradation during liver
regeneration after partial hepatectomy. Hepatology. 21:155–161.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rho H, Terry AR, Chronis C and Hay N:
Hexokinase 2-mediated gene expression via histone lactylation is
required for hepatic stellate cell activation and liver fibrosis.
Cell Metab. 35:1406–1423.e8. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wei S, Guan G, Luan X, Yu C, Miao L, Yuan
X, Chen P and Di G: NLRP3 inflammasome constrains liver
regeneration through impairing MerTK-mediated macrophage
efferocytosis. Sci Adv. 11:eadq57862025. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Zhang Z, Dong J and Liu C: HNRNPC
as a pan-cancer biomarker and therapeutic target involved in tumor
progression and immune regulation. Oncol Res. 33:83–102.
2024.PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−∆∆C(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu S, Cao C, Poddar M, Delgado E, Singh S,
Singh-Varma A, Stolz DB, Bell A and Monga SP: Hepatocyte β-catenin
loss is compensated by insulin-mTORC1 activation to promote liver
regeneration. Hepatology. 77:1593–1611. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saraswathibhatla A, Indana D and Chaudhuri
O: Cell-extracellular matrix mechanotransduction in 3D. Nat Rev Mol
Cell Biol. 24:495–516. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sack KD, Teran M and Nugent MA:
Extracellular matrix stiffness controls VEGF signaling and
processing in endothelial cells. J Cell Physiol. 231:2026–2039.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Karamanos NK, Theocharis AD, Piperigkou Z,
Manou D, Passi A, Skandalis SS, Vynios DH, Orian-Rousseau V,
Ricard-Blum S, Schmelzer CEH, et al: A guide to the composition and
functions of the extracellular matrix. FEBS J. 288:6850–6912. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mavrogonatou E, Pratsinis H, Papadopoulou
A, Karamanos NK and Kletsas D: Extracellular matrix alterations in
senescent cells and their significance in tissue homeostasis.
Matrix Biol. 75–76. 27–42. 2019.PubMed/NCBI
|
|
25
|
Karamanos NK: Extracellular matrix: Key
structural and functional meshwork in health and disease. FEBS J.
286:2826–2829. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stewart DC, Brisson BK, Yen WK, Liu Y,
Wang C, Ruthel G, Gullberg D, Mauck RL, Maden M, Han L and Volk SW:
Type III collagen regulates matrix architecture and mechanosensing
during wound healing. J Invest Dermatol. 145:919–938.e14. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tan PC, Zhou SB, Ou MY, He JZ, Zhang PQ,
Zhang XJ, Xie Y, Gao YM, Zhang TY and Li QF: Mechanical stretching
can modify the papillary dermis pattern and papillary fibroblast
characteristics during skin regeneration. J Invest Dermatol.
142:2384–2394.e8. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamaji K, Iwabuchi S, Tokunaga Y,
Hashimoto S, Yamane D, Toyama S, Kono R, Kitab B, Tsukiyama-Kohara
K, Osawa Y, et al: Molecular insights of a CBP/β-catenin-signaling
inhibitor on nonalcoholic steatohepatitis-induced liver fibrosis
and disorder. Biomed Pharmacother. 166:1153792023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kotulkar M, Robarts DR and Apte U: HNF4α
in hepatocyte health and disease. Semin Liver Dis. 43:234–244.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ko HL, Zhuo Z and Ren EC: HNF4α
combinatorial isoform heterodimers activate distinct gene targets
that differ from their corresponding homodimers. Cell Rep.
26:2549–2557.e3. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li S, Pritchard DM and Yu LG: Regulation
and function of matrix metalloproteinase-13 in cancer progression
and metastasis. Cancers (Basel). 14:32632022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang Z, Mou T, Luo Y, Pu X, Pu J, Wan L,
Gong J, Yang H, Liu Y, Li Z, et al: Inhibition of miR-450b-5p
ameliorates hepatic ischemia/reperfusion injury via targeting
CRYAB. Cell Death Dis. 11:4552020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li TT, Luo YH, Yang H, Chai H, Lei ZL,
Peng DD, Wu ZJ and Huang ZT: FBXW5 aggravates hepatic
ischemia/reperfusion injury via promoting phosphorylation of ASK1
in a TRAF6-dependent manner. Int Immunopharmacol. 99:1079282021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang Z, Zheng D, Pu J, Dai J, Zhang Y,
Zhang W and Wu Z: MicroRNA-125b protects liver from
ischemia/reperfusion injury via inhibiting TRAF6 and NF-κB pathway.
Biosci Biotechnol Biochem. 83:829–835. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jiang S, Li H, Zhang L, Mu W, Zhang Y,
Chen T, Wu J, Tang H, Zheng S, Liu Y, et al: Generic diagramming
platform (GDP): A comprehensive database of high-quality biomedical
graphics. Nucleic Acids Res. 53(D1): D1670–D1676. 2025. View Article : Google Scholar : PubMed/NCBI
|