Introduction
Klebsiella pneumoniae is a Gram-negative,
encapsulated, facultative anaerobic bacillus that has
conventionally been regarded as an opportunistic cause of
nosocomial infections, such as pneumonia, urinary tract infections
and bacteremia (1). However, the
clinical image of this organism has entirely changed over the past
30 years (2). A new clinical
syndrome, community-acquired pyogenic liver abscess (PLA), mainly
due to hypervirulent K. pneumoniae (hvKp), was first noted
in Taiwan in the 1980s (3). As a
result, hvKp has spread worldwide and is now considered a
significant public health threat (4,5).
hvKp strains can cause severe, invasive disease in otherwise
healthy hosts, unlike classical K. pneumoniae (cKp), which
is typically isolated from immunocompromised patients in healthcare
settings (6). Monomicrobial liver
abscess, traditionally associated with capsular serotypes K1 and K2
[such as sequence type (ST) 23 and ST65], and metastatic
complications such as endophthalmitis, meningitis, septic pulmonary
emboli and necrotizing fasciitis, are clinical hallmarks (7). This shift reflects how a previously
opportunistic organism has evolved to cause invasive disease even
in healthy hosts (8).
K. pneumoniae expresses a wide range of
capsular (K) types, currently described as K1 through K80, encoded
by distinct cps loci (9). Although
>80 K types are recognized, certain serotypes are repeatedly
linked to invasive disease. K1 and K2 remain most strongly
associated with PLA and metastatic complications; additional
serotypes reported from severe or invasive infections include K5,
K20, K54 and K57 (10). These
serotypes frequently co-associate with key virulence loci such as
rmpA/rmpA2 and the aerobactin locus and characteristic sequence
types such as ST23 with K1 and ST65 with K2, explaining their
propensity for bloodstream invasion and metastatic spread (11). As this issue escalates, the spread
of multidrug resistance (MDR) in K. pneumoniae continues to
intensify, driven by extended-spectrum β-lactamase (ESBL)
production and, even more concerning, resistance to carbapenems
mediated by enzymes such as K. pneumoniae carbapenemase
(KPC) (12), New Delhi
metallo-β-lactamase (NDM) (13)
and OXA-48 carbapenemase (14).
Although hvKp strains are generally susceptible to most
antibiotics, worrisome cases have been reported of strains carrying
both hypervirulence plasmids and MDR determinants (15). The convergence of hypervirulence
and antimicrobial resistance represents an emerging global health
threat, leading to a critical therapeutic gap with few effective
treatment options available (16).
PLA due to K. pneumoniae differs from other abscesses, such
as polymicrobial or enteric abscesses (17).
Historically, the majority of liver abscesses were
secondary infections (such as to biliary obstruction or
intra-abdominal sepsis) and are typically polymicrobial (18). However, a recent study also
highlighted gastrointestinal colonization and translocation as
essential portals of entry, even in cryptogenic cases (19). Another example is the pathogenesis
of K. pneumoniae where a variety of specialized virulence
factors cooperate (20). A
hypermucoviscous polysaccharide capsule protects bacteria from
phagocytosis, iron-uptake siderophore systems sequestering iron
from the environmentally restrictive conditions of the mammalian
host [such as aerobactin, salmochelin, yersiniabactin (ybt) and
enterobactin], colonization factors (such as fimbriae and pili) and
lipopolysaccharide (LPS), which enables protection from
complement-mediated killing (21).
These mechanisms together result in K. pneumoniae invasion
and traversal across the gut barrier, subsequent colonization of
liver parenchyma, evasion of host immunity and dissemination to
remote organs (22).
The management of PLA typically involves a
combination of antimicrobial therapy and guided percutaneous
drainage of the abscess, based on clinical grounds (19). However, the emerging problem of
antimicrobial resistance, in conjunction with the invasive capacity
of hvKp, challenges treatment success and leads to higher morbidity
and mortality (4). In addition,
there is currently no licensed vaccine or approved anti-virulence
therapy available for treating this pathogen, emphasizing the need
for new approaches (23,24). In the present review a
comprehensive synthesis of the current knowledge of K.
pneumoniae-associated PLA is provided. The present review
begins with an overview of the epidemiology and clinical importance
of these emerging syndromes and summarizes virulence mechanisms and
host-pathogen interactions. Then, trends in antimicrobial
resistance are discussed, a narrative review of current therapeutic
approaches is provided and new concepts such as anti-virulence
therapies, immunotherapies and bacteriophage therapy are
introduced. Lastly, translational research opportunities and future
hurdles related to combating the combined threat of hypervirulence
and resistance are highlighted.
Epidemiology and emerging clinical
threats
Global burden of K. pneumoniae
infections
K. pneumoniae is one of the most frequently
isolated Gram-negative pathogens worldwide and the World Health
Organization has designated this bacterial species as a ‘critical
priority pathogen’ due to the dual MDR and hypervirulence threat it
poses (25). cKp has been a
long-standing pathogen of importance in the healthcare setting,
having been implicated in ventilator-associated pneumonia,
catheter-associated urinary tract infections, intra-abdominal
infections and bloodstream infections (26). K. pneumoniae has become the
focus of numerous challenging nosocomial epidemics as it possesses
the unique ability to rapidly capture resistance determinants,
ESBLs and carbapenemases (14). At
the same time, hvKp is a relatively recently recognized pathotype
and its emergence has significantly broadened the clinical spectrum
of K. pneumoniae infections (6).
hvKp was first identified in the 1980s in Taiwan and
it has become an international concern as a source of both
community-acquired and virulent invasive syndromes in both
immunocompromised and healthy hosts (4). Of these, PLA is the most typical and
clinically meaningful manifestation. Recent surveillance data
derived from multinational hospital-based surveillance networks and
national reporting systems from routine culture and genomic
analyses collected between 2020 and 2024 documented sporadic
autochthonous hvKp-PLA cases outside Asia, including South America
and Africa, suggesting that the global spread may be underestimated
due to underdiagnosis (4).
PLA as a distinct clinical entity
hvKp-associated PLA differs significantly from
classical polymicrobial liver abscesses (27). Traditionally, the majority of liver
abscesses develop as polymicrobial infections secondary to biliary
tract disease, appendicitis or intra-abdominal sepsis (18). By contrast, hvKp-PLA is generally
monomicrobial and more often cryptogenic, without an evident
underlying hepatobiliary abnormality or secondary cause (3). Capsular serotypes K1 and K2 account
for the majority of cases and sequence types ST23, ST65 and related
clones are predominant (9).
Patients classically present with acute fever, localized epigastric
or right-upper-quadrant abdominal pain, along with evidence of
systemic inflammation (28). A
hallmark of hvKp is its propensity for bacteremia with metastatic
spread, most commonly presenting as endogenous endophthalmitis,
central nervous system infections such as meningitis or brain
abscess, or pulmonary complications such as septic emboli or
pneumonia (29). Overall, hvKp-PLA
has lower mortality rates than polymicrobial abscesses if detected
and treated early; however, complications, including severe
morbidity, are common when either diagnosis or drainage is delayed
(19).
Risk factors and host
predispositions
Although hvKp can cause disease in healthy hosts,
several risk factors significantly predispose to disease (4). The most common and well-recognized
predisposing factor is diabetes mellitus; nearly all cohorts report
greater than half of PLA patients with underlying diabetes
(30). Experimental data indicate
that hyperglycemia impairs neutrophil chemotaxis and phagocytic
function, leading to impaired host defence (31). Chronic liver disease, cirrhosis,
malignancy and other immunosuppressive therapies are some other
factors that can contribute to vulnerability (32). However, hvKp is characterized by
its capacity to cause severe disease in hosts without obvious
comorbidities, contrasting with cKp infections, and challenging
long-held assumptions about host susceptibility (6). While hvKp can produce invasive
disease in healthy individuals, a number of comorbidities confer a
marked increase in risk, including diabetes mellitus (Table I).
 | Table I.Host risk factors and the mechanistic
basis of susceptibility to K. pneumoniae-associated PLA. |
Table I.
Host risk factors and the mechanistic
basis of susceptibility to K. pneumoniae-associated PLA.
| First author,
year | Risk factor | Mechanistic
basis | Clinical
impact/prevalence | Remarks | (Refs.) |
|---|
| Zhang et al,
2025 | Diabetes
mellitus | Hyperglycemia
impairs neutrophil chemotaxis and phagocytosis | Strongest and most
consistent risk factor (50–70% of hvKp PLA cases in Asian
cohorts) | Independent risk
for metastatic complications | (119) |
| Li et al,
2021 | Chronic liver
disease/cirrhosis | Impaired hepatic
innate immunity (Kupffer cell dysfunction) | Increased incidence
and poorer prognosis | Often coexists with
alcohol use or hepatitis | (70) |
| Kaur et al,
2018 | Malignancy | Disease and
chemotherapy cause immunosuppression | Higher
susceptibility and poorer outcomes | Common in elderly
patients | (62) |
| Pope et al,
2019 | Immunosuppressive
therapy | Reduced
innate/adaptive immunity | Risk of severe and
relapsing disease | Steroids, biologics
and post-transplant | (120) |
|
Thirugnanasambantham et al,
2025 | No comorbidity | hvKp can overcome
intact defences | Up to 30–40% of
hvKp PLA cases occur in otherwise healthy individuals | Key feature
distinguishing hvKp from cKp | (11) |
| Al Ismail et
al, 2025 | Microbiome
imbalance/colonization | hvKp intestinal
carriage increases the risk of translocation | 5–10% carriage
rates in Asia; lower but rising in Europe/N. America | Emerging risk
factor | (4) |
Although hvKp can infect otherwise healthy
individuals, epidemiological studies consistently identify certain
patient populations as being at higher risk of developing PLA and
other severe complications (4).
These include individuals with diabetes mellitus, both type 1 and
type 2, where impaired neutrophil chemotaxis and phagocytic
function contribute to increased susceptibility; patients with
chronic liver disease or cirrhosis, in whom hepatic immune
dysfunction and portal hypertension facilitate bacterial
dissemination; and immunocompromised individuals receiving systemic
corticosteroids, cytotoxic chemotherapy or biological immune
modulators (31). Increased risk
is also observed among patients with malignancies, those with
indwelling devices or a history of abdominal instrumentation that
disrupts mucosal barriers, as well as in the elderly and neonates,
where host defence mechanisms are often diminished (32). Notably, hvKp can occasionally cause
severe infections even in young, previously healthy individuals,
emphasizing that the absence of comorbidities does not preclude the
risk of invasive disease.
Geographical trends and strain
distribution
Epidemiology reveals a precise geographical
distribution associated with hvKp PLA (4). In East Asia, especially Taiwan,
China, South Korea and Singapore, hvKp has become the predominant
agent of community-acquired liver abscess (33). Initial cases described outside Asia
were predominantly associated with immigrants or travellers
(34). However, more recently,
autochthonous infections have been documented in Europe and North
America, indicating that hvKp is now establishing endemic
transmission in some non-Asian regions (35). As for South Asia, the Middle East
and Africa, fewer reports from these regions exist; however, this
is most likely due to a lack of recognition rather than an absence
(36). Surveillance from
developing nations, notably parts of South Asia, the Middle East
and selected African countries, indicates a complex image: A number
of reports show both classical MDR K. pneumoniae and hvKp
lineages, sometimes in mixed circulation (35). In South Asia, for example, hvKp
lineages (such as ST23) have been reported alongside ST11/ST15
clones carrying NDM or other carbapenemases, creating regional
hotspots for convergence events (36). Resource and capacity limitations
for routine molecular surveillance likely understate true incidence
in many low- and middle-income countries (LMICs).
Accordingly, terms such as ‘global trends’ should be
interpreted as geographically heterogeneous, with high burdens of
hvKp-PLA reported in East Asia, rising autochthonous cases in
high-income regions, and concerning reports of
resistance-associated hvKp from LMICs where surveillance is
improving (37). The lineage types
involved in PLA are also well-characterized using molecular
epidemiology, with ST23 (typically K1), ST65, ST86 and ST375 being
the most prominent lineages associated with PLA (10). The emergence of hvKp with its
virulence plasmids harbouring the rmpA gene and siderophore
biosynthesis clusters on different genetic backgrounds indicates
the genomic flexibility of hvKp for horizontal gene transfer and
genetic adaptation (11). Recent
genomic epidemiology studies also highlight convergence events
involving ST11 and ST15 lineages, which carry both carbapenem
resistance and virulence plasmids (36,37).
Notably, different geographical patterns have been documented, with
hvKp first described in East Asia but now found worldwide (Table II).
 | Table II.Geographical distribution,
predominant strains and clinical features of K.
pneumoniae-associated PLA. |
Table II.
Geographical distribution,
predominant strains and clinical features of K.
pneumoniae-associated PLA.
| Region/country | Predominant
strains/serotypes | Major risk
factors | Distinctive
clinical features | Remarks/trends | (Refs.) |
|---|
| Taiwan, China,
South Korea and Singapore | K1 (ST23) and K2
(ST65 and ST86) | Diabetes, no
hepatobiliary disease | Monomicrobial PLA
and high metastatic rates (endophthalmitis and meningitis) | Endemic focus;
earliest recognition | (3,121) |
| North America and
Europe | K1, K2 and emerging
ST375 | Often in healthy
individuals and still common in diabetes | Increasing
autochthonous cases | Expanding
incidence; community-onset clusters | (118) |
| South Asia (India
and Pakistan) | Mixed strains;
including hvKp ST23 and ST11 (CR-hvKp) | Diabetes, cirrhosis
and malignancy | Resistant hvKp
reported | Rising
ESBL/NDM-positive hvKp | (36) |
| Middle East | Mixed strains (K1,
K2 and ST11) | Diabetes and
chronic liver disease | Increasing
carbapenemase producers (NDM and OXA-48) | Likely
underreported | (122) |
| Africa | Sparse data and
sporadic case reports | Unknown | Sporadic reports
only | Likely
underrecognized; limited data due to lack of diagnostic capacity
and surveillance infrastructure | (123) |
Convergence of hypervirulence and
antimicrobial resistance
Between the two critical epidemiological
developments, the global rise of hypervirulent hvKp strains and the
rapid spread of MDR among classical K. pneumoniae, the most
worrying is the convergence of hypervirulence and MDR. Despite
their invasive behaviour, hvKp strains were susceptible to most
antibiotics for a number of years, which allowed for high treatment
effectiveness (4). In comparison,
classical strains rapidly acquired resistance genes, such as ESBLs
and carbapenemases, including KPC, NDM and OXA-48 (38). However, researchers recently
reported hvKp isolates with those resistance genes in China, India,
Europe and North America (4,39).
These strains represent a worst-case scenario, as they combine
extensive drug resistance with high metastatic potential (40). Molecular studies have shown that
homologous mobile plasmids constitute a significant part of this
phenomenon, transferring resistance genes to hvKp lineages or
virulence genes to MDR classical strains (41,42).
However, the emergence of carbapenem-resistant hypervirulent K.
pneumoniae (CR-hvKp) has raised an urgent need for surveillance
and containment (42).
Clinical and public health
implications
The changing epidemiology of hvKp-associated PLA has
important implications for clinicians and public health. The
heterogeneous virulence of hvKp complicates diagnosis for
clinicians, as distinguishing hvKp from cKp is not straightforward
in routine clinical settings. While differential testing by the
hypermucoviscosity ‘string test’ is widely used, it is not
sensitive or specific and advanced molecular assays are still
limited in clinical routine (11).
Rapid molecular assays targeting aerobactin (iuc locus) or rmpA
genes have shown higher accuracy but remain largely confined to
research settings (43). The
emergence of hvKp with reduced susceptibility to the standard
antibiotic regimen (including image-guided drainage) is
progressively leading to therapeutic dilemmas (44). However, outside East Asia,
surveillance is often opportunistic, and the actual global burden
is likely underestimated due to underdiagnosis and limited
surveillance infrastructure outside Asia. Given its ability to
produce life-threatening, invasive disease in patients who were
previously healthy, coupled with its rapid acquisition of
antimicrobial resistance, hvKp has the potential to become a
pandemic organism. The global emergence of hvKp exemplifies how a
classical opportunistic pathogen has evolved from being community
and healthcare-associated to becoming a significant threat with
consequences far beyond liver abscess.
Pathogenesis and virulence mechanisms
The liver abscesses caused by hvKp result from
multiple virulence mechanisms (33). These virulence determinants, which
include polysaccharide capsule, siderophore systems, adhesins, LPS
modifications and plasmid-encoded factors, exhibit 2- to 1,000-fold
upregulated expression in vivo (45). This section will evaluate these
mechanisms in-depth.
Capsule and hypermucoviscosity
phenotype
The polysaccharide capsule may be the most unique
virulence factor of K. pneumoniae (46). Hypermucoviscous capsule production
(commonly evaluated with the ‘string test’) is one of the markers
that is highly associated with hvKp (47). Capsule regulators rmpA and rmpA2,
encoded on large virulence plasmids, primarily control this
phenotype (48). K1 and K2
serotypes typically form very mucoid capsules that are resistant to
phagocytosis and the complement system. The hypermucoviscous
phenotype is lost in rmpA/rmpA2 mutants and the virulence of these
mutants is impaired in animal models (49). Additionally, the capsule prevents
neutrophil phagocytosis and protects the organism from recognition
by antibodies (50). A recent
narrative review showed that sequencing studies have identified
structural differences in capsular operons between hvKp and cKp,
which may contribute to the enhanced virulence of hvKp (11).
Siderophore-mediated iron
acquisition
Although iron is a mineral that bacteria need to
grow, it is locked away by host proteins, including transferrin and
lactoferrin (51). The high
affinity siderophores produced by K. pneumoniae enable it to
bypass this nutritional immunity. Hypervirulent strains encode
multiple iron acquisition systems (such as enterobactin, ybt,
salmochelin and aerobactin), with aerobactin being nearly universal
and the most critical for hvKp virulence in PLA (52). Siderophores mediate bacterial
expansion in liver parenchyma and blood by rapidly scavenging iron
from the host environment (53).
Besides iron acquisition, certain siderophores serve as
immunomodulators, leading to oxidative stress and a diminished
responses from neutrophils (54).
The redundancy affords hvKp with a significant evolutionary
advantage in a host environment where nutrients are often limited.
Recent studies also suggest that aerobactin expression correlates
with increased metastatic potential and poorer clinical outcomes
(33,55).
Fimbrial adhesins and
colonization
Fimbriae play a crucial role in the initial
attachment to host tissues (22).
At present, type 1 and type 3 fimbriae in K. pneumoniae have
been best characterized. Type 1 fimbriae allow binding to
mannose-containing receptors on epithelial cells, whereas type 3
fimbriae promote biofilm formation individually or on biotic and
abiotic surfaces, including medical devices. Fimbrial adhesins have
been shown to promote gastrointestinal colonization of bacteria and
increase the likelihood of translocating microbes through the
intestinal mucosa into the portal circulation, potentially
facilitating the formation of liver abscesses (22). Once in the liver, fimbriae
facilitate adhesion to hepatic tissue, allowing for persistence and
the formation of abscesses. Together with capsule and siderophores,
these adhesive structures enable hvKp to establish a robust
infection niche. K. pneumoniae employs a wide array of
virulence factors, from capsule, siderophores, fimbrial adhesins
and LPSs (Table III).
 | Table III.Major virulence determinants of K.
pneumoniae in PLA. |
Table III.
Major virulence determinants of K.
pneumoniae in PLA.
| First author,
year | Virulence
factor | Genetic basis | Role in
pathogenesis | Clinical
relevance | (Refs.) |
|---|
| Zhu et al,
2021 | Capsule (K1 and
K2;) hypermucoviscosity | cps locus;
rmpA/rmpA2 | Resistance to
phagocytosis, inhibition of complement activation leading to serum
resistance and enhanced bloodstream survival | Strongly associated
with invasive PLA and metastatic spread; vaccine/antibody
target | (59) |
| Monteiro et
al, 2024 | Siderophores
(aerobactin, salmochelin, yersiniabactin and enterobactin) | iuc, iro, ybt and
ent loci | Iron acquisition;
immune modulation; oxidative stress induction | Aerobactin nearly
universal in hvKp PLA strains; candidate for anti-virulence
therapy | (124) |
| Clegg and Murphy,
2017 | Fimbrial adhesins
(Type 1 and 3 fimbriae) | fim and mrk
operons | Colonization of
gut; adherence in liver; biofilm formation | Facilitate
gut-to-liver translocation | (20) |
| Miller et
al, 2024 |
Lipopolysaccharide | waa gene
cluster | Resistance to serum
killing; immune modulation | Contributes to
serum survival and sepsis severity | (105) |
| Liao et al,
2024 | Virulence
plasmids | pLVPK-like plasmids
(~200 kb) | Encode capsule
regulators (rmpA/rmpA2) and siderophores | Horizontal transfer
spreads hypervirulence traits | (125) |
| Lam et al,
2018 | Pathogenicity
islands | ICEKp elements (ybt
and clb) | Iron scavenging
(ybt) and genotoxicity (colibactin) | Colibactin linked
to DNA damage and possible cancer risk | (126) |
| Bossuet-Greif et
al, 2018 | Colibactin | clb genes on
ICEKp | Genotoxic effects
and DNA damage in host cells | Recently linked to
colorectal cancer; under study | (127) |
LPS and immune evasion
The LPS of K. pneumoniae also protects
bacteria from complement-mediated lysis, thereby contributing to
its pathogenesis (56). Various
O-antigen structures of LPS directly impact serum resistance and
binding by host pattern recognition receptors (PRRs) (57). HvKp strains are proposed to have
adapted LPS that confer serum resistance, with the capsule playing
a protective role simultaneously. Beyond structural defence, LPS is
a potent inducer of inflammation, driving hepatocellular injury and
systemic inflammatory responses that exacerbate PLA severity
(58).
Virulence plasmids and mobile genetic
elements
Most hvKp virulence factors are encoded on large
virulence plasmids (200–220 kb) (59). Such plasmids encode genes for
rmpA/rmpA2 and the aerobactin (iuc locus), salmochelin (iro locus)
and other accessory factors (60).
This is especially concerning since their horizontal mobility
enables transfer of hypervirulence traits to classical MDR strains,
producing the so-called convergence phenotype. In addition, hvKp
virulence is further expanded by chromosomal pathogenicity islands,
including those coding for ybt and colibactin (clb), which provide
unique mechanisms for iron acquisition and genotoxicity,
respectively (6). A study has
linked clb production to colorectal carcinogenesis, highlighting
potential long-term sequelae of hvKp colonization (61). The adaptability and clinical
success of hvKp stems from the complex interplay among plasmids,
pathogenicity islands and host genomic backgrounds.
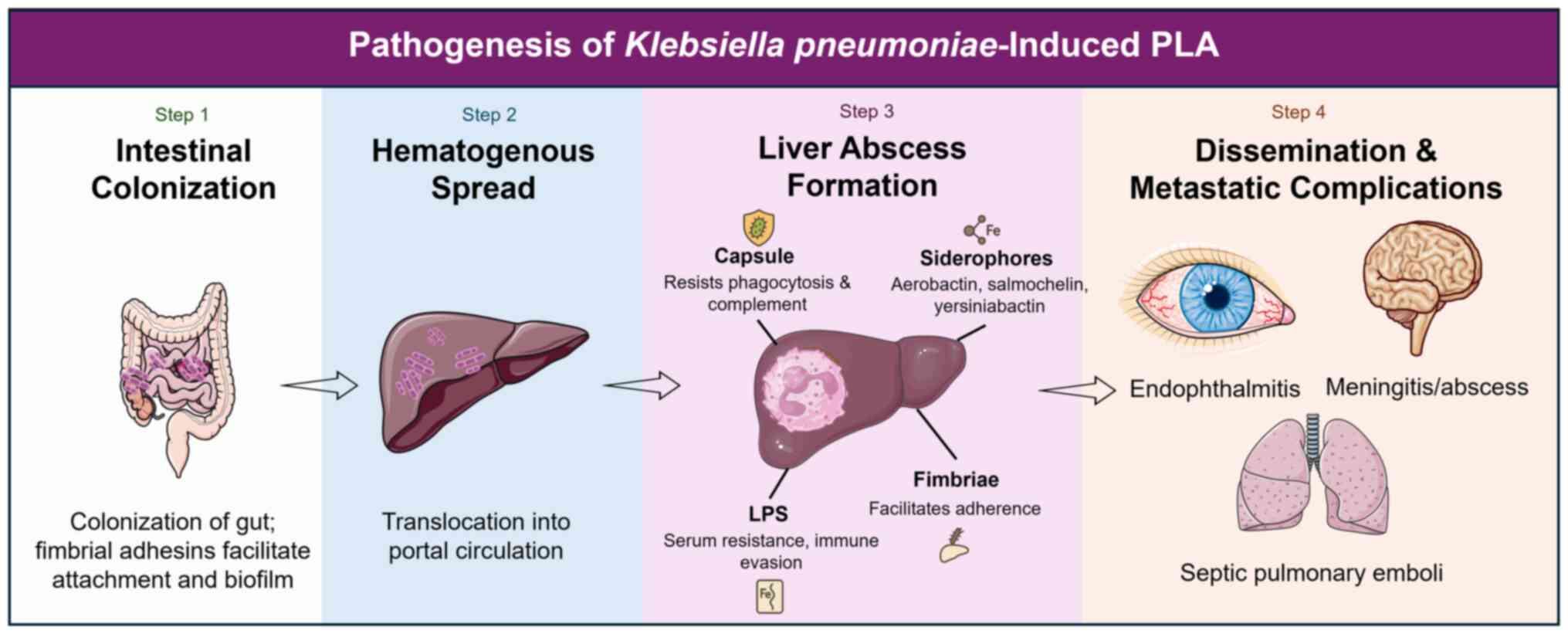
Mechanistic basis of liver abscess
formation
PLA is the result of a series of microbial-host
interactions that lead to disease pathogenesis (17). The initial reservoir is intestinal
colonization, followed by bacterial translocation into the portal
venous circulation (3). The hvKp
capsule and LPS facilitate the avoidance of phagocytic clearance
once the bacterium reaches the liver (50). The production of siderophores
ensures survival in the iron-limited hepatic microenvironment and
fimbrial adhesins facilitate tissue adherence (51). Neutrophil and macrophage
recruitment lead to regional inflammation, necrosis and the
formation of an abscess cavity (31). By contrast, hvKp liver abscesses
are almost always monomicrobial rather than polymicrobial, as is
the case for most abscesses caused by anaerobes and enteric flora,
emphasizing the intrinsic capacity of the pathogen to establish
infection independently (33). The
rapidity of metastasis in these patients is therefore closely
related to the ability of hvKp to penetrate the bloodstream and
disseminate systemically via its capsule and plasmid-borne
virulence arsenal. Virulence factors, including capsule,
siderophores, fimbrial adhesins and LPS, work together to
facilitate colonization of the liver, evade the immune response and
promote systemic spread (Fig.
1).
Host-pathogen interactions and disease
manifestation
Colonization of the host by K. pneumoniae and
subsequent progression to PLA are multifaceted processes involving
the interplay of virulence factors and immune responses of the host
(22). Such hypervirulent strains
appear to have developed mechanisms that enable them to both evade
immune clearance and exploit host physiological weaknesses, thereby
facilitating invasive disease with metastatic sequelae. This
section elaborates on the mechanisms of maintaining virulence while
evading both innate and adaptive immunity, ultimately culminating
in hepatic invasion, abscess formation and systemic
complications.
Hepatic invasion and abscess
formation
K. pneumoniae translocates from the
gastrointestinal tract and reaches the liver via portal circulation
(62). The gastrointestinal
mucosa, especially during dysbiosis or increased permeability,
serves as a significant reservoir. After entering the portal venous
system, hypervirulent strains are not phagocytosed or killed by
complement as they possess a thick polysaccharide capsule and a
hypermucoid phenotype (63). This
allows the bacterium to survive in the bloodstream and seed hepatic
tissues. In the liver parenchyma, bacteria attach to hepatocytes
and endothelial cells through fimbrial adhesins, and iron
acquisition through siderophores promotes bacterial replication in
the iron-limited hepatic microenvironment (64). With advancing infection, this leads
to local necrosis and tissue liquefaction, promoted by bacterial
toxins and host mediators of inflammation. The result is the
development of defined abscess cavities filled with pus material,
often multiloculated, which may increase in size or number.
Innate immune responses
Innate immune cells are abundant in the liver,
including Kupffer cells, neutrophils and natural killer cells,
which form the first line of defence against invading pathogens
(65). PRRs recognize bacterial
components, such as LPS and capsular polysaccharides, initiating an
inflammatory cascade that is associated with cytokine release and
the recruitment of neutrophils (66). Nevertheless, hypervirulent K.
pneumoniae strains evade neutrophil extracellular traps,
inhibit complement activation and resist phagocytosis, thereby
allowing them to persist in hepatic tissue. Neutrophils are a
hallmark of PLA pathophysiology; however, excessive neutrophil
accumulation contributes to collateral tissue injury, hepatocyte
necrosis and the progression of abscesses (67). Similarly, with the activation of
Kupffer cells, pro-inflammatory cytokines are produced, which
enhance local inflammation but typically do not clear the pathogen
from the liver (68). This
inappropriate immune reaction leads to an environment that favours
bacterial persistence and abscess maturation.
Adaptive immune responses
The adaptive immune system plays a crucial role in
containing and clearing liver abscesses (33). Antibody-mediated responses against
capsular polysaccharides and siderophores enhance
opsonophagocytosis, while T cell-mediated immunity contributes to
bacterial clearance. However, in individuals with diabetes, chronic
liver disease or immunosuppression, adaptive immune responses are
impaired, resulting in dysregulated bacterial growth and severe
disease (66). The diversity of
capsule and antigenic variability makes hypervirulent strains
unusually adept at evading antibody-mediated recognition.
Additionally, bacterial metabolites and endotoxins may suppress
adaptive immunity, thereby prolonging the infection and
facilitating its dissemination (66).
Metastatic infections and extrahepatic
complications
A hallmark of hvKp-mediated PLA is its ability to
induce metastatic infections at non-contiguous sites (66). Through bacteremia, it can spread to
the eyes, central nervous system, lungs and other organs.
Endophthalmitis (often leading to irreversible vision loss),
meningitis and brain abscesses are among the most devastating
complications, associated with high morbidity and mortality
(69). These processes are
critical mechanisms driving metastatic spread, including complement
resistance, neutrophil resistance and vascular invasion. The
metabolic flexibility enabled by siderophore systems supports
survival in these nutrient-limited niches, such as the vitreous
humour and cerebrospinal fluid. The ability to disseminate despite
host immune defences underscores the enhanced virulence potential
of hvKp compared with cKp.
Clinical outcomes and prognosis
K. pneumoniae PLA can present from simple
localized hepatic abscesses with fever and abdominal pain to
fulminant sepsis with multi-organ involvement (18). Therapy and prognosis are influenced
by three key factors: The immune status of the host, bacterial
virulence and the timing of intervention. Older patients, patients
with diabetes and patients with underlying liver disease are
predisposed towards severe disease and poor outcomes. Patients with
resistant infections, metastatic complications or delayed diagnosis
experience substantially higher mortality (70). The host-pathogen interactions
underlying hvKp-mediated PLA are ultimately defined by a
combination of the evasion of the pathogen from the innate and
adaptive immunity, the immunological background of the host and the
fine line between protective inflammation and harmful immune
pathologies (29). Understanding
these interactions is essential for designing targeted therapies
that bolster host defences while neutralizing bacterial
virulence.
Antimicrobial resistance in K. pneumoniae
PLA
K. pneumoniae PLA is becoming increasingly
challenging to treat due to the widespread emergence of
antimicrobial resistance (19).
The division between hypervirulent strains, which were initially
mostly susceptible to most antibiotics, and classical strains,
which were primarily associated with MDR in a nosocomial
background, is also no longer absolute. Hypervirulent and resistant
strains are increasingly appearing, posing a significant
therapeutic challenge (4). This
section summarizes current resistance patterns, mechanisms and
clinical consequences specific to PLA.
Global resistance trends
Antimicrobial resistance in K. pneumoniae has
been reported worldwide, with the highest prevalence observed in
regions where PLA is endemic (71). The increase in ESBL production of
K. pneumoniae is particularly high in East and Southeast
Asia, where multidrug class co-resistance is common (72). Carbapenem-resistant K.
pneumoniae (CRKP) has also emerged globally, driven by
carbapenemases such as KPC (prevalent in the Americas and Europe),
NDM (dominant in the Indian subcontinent) and OXA-48-like enzymes
(common in the Middle East and Europe) (73,74).
Resistance genes have begun to emerge in a significant proportion
of hvKp isolates associated with liver abscesses (27). The rate surpassed 20% in the
networks for ESBL-hvKp-PLA strains in both China and Taiwan between
2020 and 2024 (75). Although
fundamentally uncommon, carbapenem resistance is increasingly
reported in hvKp-PLA, particularly among healthcare-associated
cases or in endemic countries with CRKP (76). A surveillance study has indicated
that up to 10–15% of hvKp isolates in China and India now harbour
carbapenemase genes, reflecting a rising convergence of resistance
and hypervirulence (73).
Mechanisms of resistance
Resistant mechanisms in K. pneumoniae are
heterogeneous and often act cooperatively to produce strong MDR
profiles (77). The most common
mechanism is the production of β-lactamases, including ESBLs and
carbapenemases, which inactivate extended-spectrum cephalosporins
and carbapenems (78). Resistance
to β-lactam class antibiotics typically develops from ESBLs, such
as Cefotaximase-Munich (CTX-M) and sulfhydryl variable, and
carbapenemases, such as KPC, NDM, OXA-48 and Verona
integron-encoded metallo-β-lactamase (79). These enzymes are often accompanied
by porin loss (OmpK35/36) and upregulation of efflux pumps, thereby
enhancing resistance. Plasmids containing blaCTX-M and
blaKPC are frequently associated with fluoroquinolone and
aminoglycoside resistance genes (80). A broader concern regarding salvage
therapy is the development of colistin and tigecycline resistance,
which can occur via mcr genes or chromosomal alterations in the
case of colistin, and through ramR mutations or efflux pumps in the
case of tigecycline, in K. pneumoniae in general (81). The development of mutations or loss
of outer membrane porins, particularly OmpK35 and OmpK36, results
in a decrease in drug permeability, thereby increasing the potency
of β-lactamase activity (82).
Efflux pumps, such as the AcrAB-TolC system, when upregulated,
render lower intracellular concentrations of numerous antibiotic
classes, which also include fluoroquinolones and tigecycline
(83). Aminoglycoside resistance
typically arises from enzymatic modification or ribosomal
methylation. By contrast, colistin resistance develops through
plasmid-borne mcr genes or chromosomal regulatory mutations
resulting from chromosomal alterations in the regulatory pathways
or the acquisition of plasmid-borne mcr genes (84). This can occur in a single isolate,
resulting in organisms that are resistant to nearly all clinically
available antibiotics. Resistance mechanisms, including β-lactamase
production, porin loss and efflux pumps, act synergistically
(Table IV).
 | Table IV.Major antimicrobial resistance
mechanisms in K. pneumoniae and their clinical
implications. |
Table IV.
Major antimicrobial resistance
mechanisms in K. pneumoniae and their clinical
implications.
| Resistance
mechanism | Genetic basis | Antibiotic classes
affected | Clinical
relevance |
|---|
| Extended-spectrum
β-lactamases | blaCTX-M,
blaSHV and blaTEM | Third generation
cephalosporins | Undermines
cephalosporin therapy; common in Asia (30–50%) |
| Carbapenemases | KPC, NDM, OXA-48
and VIM | Carbapenems | Critical driver of
CR-hvKp; global spread |
| Porin loss
(OmpK35/36) | Chromosomal
mutations | β-lactams and
carbapenems | Enhances
β-lactamase resistance |
| Efflux pumps
(AcrAB-TolC) | Regulatory
mutations | Fluoroquinolones
and tigecycline | Contributes to MDR
profiles |
| Aminoglycoside
resistance | AMEs and armA
methyltransferase |
Aminoglycosides | Limits the use of
amikacin/gentamicin |
| Colistin
resistance | mcr plasmid genes
and pmrA/B mutations | Colistin (last
line) | Increasingly
reported, especially in Asia and the Middle East |
| Fosfomycin
resistance | fosA gene
(plasmid-borne) | Fosfomycin | Emerging, limits
use in urinary/liver infections |
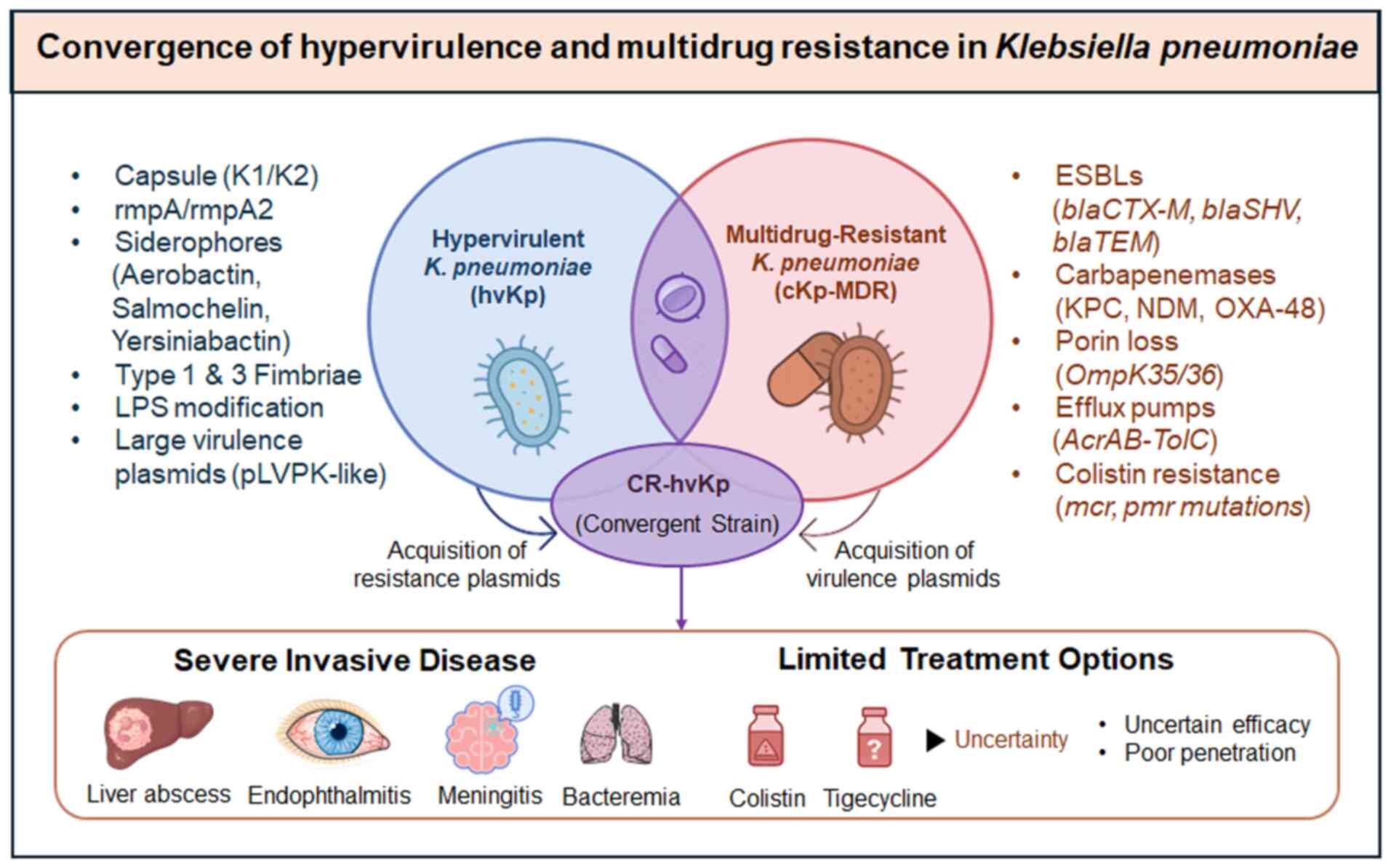
Convergence of hypervirulence and
MDR
A growing and serious threat is the convergence of
hypervirulence with MDR (6,25).
Hypervirulent strains, known for causing severe disease with
metastatic complications, were long considered manageable due to
their susceptibility to antibiotics, providing clinicians with an
essential therapeutic window. However, this advantage is rapidly
diminishing as hypervirulent strains acquire plasmids carrying
ESBLs and carbapenemases (35).
This convergence is not an isolated event, but a global problem, as
evidenced by reports of outbreaks caused by carbapenem-resistant
hypervirulent strains. hvKp and MDR-cKp lineages have historically
circulated separately, but recent plasmid exchanges are blurring
this distinction (85).
Nonetheless, recent surveillance data have described hvKp strains,
often ST23 or ST11 clones, that have acquired ESBL or carbapenemase
genes on plasmids (86,87). The transfer of plasmids and the
integration of resistance loci into the chromosomal genome drive
this convergence. Some examples include hvKp isolates associated
with blaNDM or blaKPC, which have been reported from
China and India, with some found in the absence of PLA in Europe.
The recent rise of strains with simultaneous hypervirulent (K1/K2
capsule, aerobactin) and carbapenem-resistant traits has been
coined as ‘hypervirulent carbapenem-resistant K. pneumoniae
(hv-CRKP)’ with global alerts issued (10). Such convergence is illustrated in
Fig. 2, which contrasts the
historical separation of cKp and hvKp, as well as the recent
emergence of CR-hvKp due to the plasmid-mediated exchange of
resistance or virulence determinants (88). These hybrid strains exhibit the
worst aspects of both phenotypes and pose a significant clinical
and public health challenge.
Clinical implications of resistance in
liver abscess
Antimicrobial resistance notably affects the
clinical presentation and treatment of liver abscesses (33). Empirical therapy with
cephalosporins often fails in areas with high ESBL prevalence,
resulting in delayed bacterial clearance and adverse outcomes
(33). This leaves few options,
especially with carbapenem resistance, frequently restricting
clinicians to last-line drugs such as colistin and tigecycline.
However, both agents have limitations, such as poor penetration
into abscess spaces, toxicity and variable efficacy (33). Consequently, patients with
resistant infections may require more extended hospital stays,
combination regimens with complex and challenging combinations and
invasive procedures. Outcomes are particularly poor in hvKp strains
that are also multidrug resistant, as they are strongly associated
with bacteremia, septic shock, metastatic complications and high
mortality.
Limitations of current antibiotic
therapies
The treatment of K. pneumoniae PLA with
conventional antibiotic therapy has become less reliable in recent
years (33). The widespread
resistance against once highly effective third-generation
cephalosporins and carbapenems is undermining this progress
(89). Target-site mutations and
efflux contribute to fluoroquinolone resistance, and
aminoglycosides are destroyed by enzymatic modification. Colistin,
otherwise a last-line antibiotic, faces increasing resistance, as
well as nephrotoxicity and uncertain effects in liver abscesses
(45). In addition to emerging
resistance, several commonly used agents have clinically important
adverse effects and pharmacological limitations that influence
therapeutic decision-making in PLA. Colistin is nephrotoxic and its
pharmacodynamics in poorly vascularized abscess cavities are
uncertain; renal adverse effects often limit dose escalation
(90). Tigecycline has poor serum
concentrations, can cause significant gastrointestinal side effects
and has been associated with increased mortality signals in a
meta-analysis; its penetration into abscess cavities is variable
and often suboptimal (91).
Aminoglycosides have nephro- and ototoxicity risks
that restrict prolonged use, and β-lactams, including many
third-generation cephalosporins and even carbapenems, may
inadequately penetrate the necrotic centre of large, poorly
vascularized abscesses (92).
These adverse events and pharmacokinetic limitations, such as
reduced vascular delivery, high protein binding and sequestration
in necrotic material, help explain treatment failures and motivate
alternative local or adjunctive delivery strategies, such as
intra-cavitary instillation and drug-eluting devices (93). A number of antibiotics have
pharmacokinetics that limit their penetration into abscess
cavities, thereby complicating complete eradication (93). This bottleneck highlights the need
for alternative strategies that target both antimicrobial
resistance mechanisms and the pathogenic potential of hypervirulent
strains, beyond classical antibiotics.
Current therapeutic approaches
Management of K. pneumoniae PLA involves a
combination of empirical antimicrobial therapy and interventional
procedures that target abscess drainage. Although the early
initiation of antibiotics is crucial, the rising prevalence of
antimicrobial resistance complicates the choice of empirical
regimens. Source control, which is often required for definitive
cure, is also obtained with the aid of interventional radiology and
surgical procedures.
Antimicrobial therapy
Empirical antibiotic therapy for PLA is initiated as
soon as the diagnosis is confirmed, with adjustments made based on
culture and susceptibility results (94). Third-generation cephalosporins,
such as ceftriaxone or ceftazidime, have historically been the
preferred choice due to their effectiveness against susceptible
strains and adequate biliary penetration (95). However, in areas of high ESBL
prevalence, carbapenems have frequently been used as first-line
therapy. Carbapenem resistance has further complicated management,
leaving tigecycline, colistin and, in some instances,
aminoglycosides as the only alternative agents (73). Combination therapy is often
employed in severe or resistant infections, although supporting
evidence remains limited. Carbapenems may also be used in
combination with aminoglycosides or fluoroquinolones to facilitate
bacterial clearance, although the evidence for these combination
regimens is mixed.
For MDR or extensively drug-resistant strains,
salvage therapies such as colistin-tigecycline combinations are
occasionally used, although concerns are present regarding efficacy
and toxicity (95). The length of
antimicrobial treatment ranges from weeks to months, depending upon
clinical condition, radiological resolution of the abscess and
clearance of microbiological measures. The pharmacokinetics of
antimicrobials represent a crucial aspect of managing PLA (96). Therapeutic concentrations of
antibiotics are not only in the blood but also in hepatic tissue
and the abscess cavity. The encapsulated nature of liver abscesses
and necrotic material can limit the penetration of drugs requiring
long-course treatment, and in most cases, also drainage procedures
(97).
The efficacy of antibiotic therapy in PLA depends
not only on systemic susceptibility profiles but also on achieving
effective drug concentrations within the abscess cavity (19). Several pathophysiological factors
can markedly reduce intra-cavitary antibiotic exposure, including
poor vascularity and the presence of necrotic debris that impede
drug delivery, the high protein and lipid content of abscess
material can bind and inactivate antimicrobials and acidic or
hypoxic microenvironments that alter drug activity (96). Although β-lactams generally attain
favourable biliary and hepatic tissue concentrations, their
penetration into poorly perfused abscess cores may remain
suboptimal (97). Lipophilic
agents such as, tigecycline may distribute to hepatic tissue but
often display low serum concentrations; conversely, aminoglycosides
have limited penetration into abscess fluid (91,92).
Local pharmacokinetic studies are limited, but measured
abscess/plasma ratios have shown substantial variability across
agents and individual patients (92). These limitations provide a
rationale for local delivery approaches such as intra-cavitary
instillation, catheter-directed infusion or drug-eluting beads and
for tailoring systemic therapy guided by
pharmacodynamic/pharmacokinetic principles when possible.
Interventional management
Besides antibiotics, drainage of the abscess is an
essential form of source control (98). Ultrasonography or computed
tomography-guided percutaneous catheter drainage is the standard of
care for the majority of cases and has replaced open surgical
approaches in most cases (99).
Drainage reduces bacterial load and relieves pressure from
expanding cavities, thereby enhancing antibiotic penetration and
alleviating mass effect. The drainage will be based on the
size/number and location as well as the clinical condition of the
patient. However, small abscesses of <3 cm may be managed with
antibiotics alone and close follow-up (19). Percutaneous drainage is typically
needed for larger abscesses, multiloculated cavities or abscesses
that show a poor response to medical therapy. Surgical drainage is
reserved for complicated cases, including multiloculated abscesses,
failed percutaneous drainage, rupture or perforation with
peritonitis (100). Both
diagnosis and management are imaging centred. Ultrasonography is
frequently used for initial diagnosis and to guide drainage
procedures, while computed tomography has higher sensitivity for
diagnosing abscess formation, especially in cases of multiple or
small lesions. Imaging follow-up is also necessary to assess both
response to treatment and to identify recurrence.
Integrated management
considerations
Prompt intervention combined with appropriate
antibiotics is the optimal treatment for K. pneumoniae PLA,
which requires a multidisciplinary approach (101). Glycaemic control and supportive
care are crucial for managing diabetes and other comorbidities, as
they significantly impact outcomes in such scenarios (102). Management also focuses on
preventing complications such as sepsis, metastatic spread and
organ failure. The identification of resistant organisms and the
prompt modification of antimicrobial therapy play crucial roles,
with delays in effective treatment being closely linked to patient
outcomes. Despite advances in diagnostic imaging and interventional
radiology, morbidity and mortality remain high, especially in MDR
infections and hvKp strains with metastatic spread. These
limitations underscore the critical need for innovative treatment
modalities that move beyond standard antibiotics and drainage
steps, setting the stage for the forthcoming innovative strategies
addressed in the next section.
Emerging and experimental therapeutic
strategies
The limitations of traditional antibiotic
treatments, combined with the notable increase in the incidence of
MDR strains of K. pneumoniae, have sparked renewed interest
in novel and complementary therapeutic approaches (101). These emerging approaches aim not
only to circumvent resistance but also to neutralize virulence and
strengthen host defences (103).
Other antimicrobial approaches, such as exploring new
antimicrobials and anti-virulence agents, immunotherapies and
biological modalities, including bacteriophage and microbiome
interventions, remain under consideration for the treatment of PLA
due to K. pneumoniae (14).
Novel antibiotics and combination
therapies
Novel β-lactam/β-lactamase inhibitor combinations
and non-β-lactam antibiotics have been developed in response to the
global spread of CRKP (104). For
KPC-producing strains, ceftazidime-avibactam and
meropenem-vaborbactam target KPC, and imipenem-relebactam extends
the coverage against some OXA-48 producers (38). Cefiderocol, a
siderophore-cephalosporin, demonstrates potent activity against
carbapenemase-producing bacteria via iron uptake pathways (103). Nonetheless, resistance is
reported to emerge to ceftazidime-avibactam in KPC-CRKP, warranting
stewardship. Other options include newer tetracycline derivatives
(such as eravacycline) and aminoglycoside formulations (such as
plazomicin), for which limited experience is available in PLA.
Anti-virulence approaches
Considering that hypervirulent strains do not
merely cause a more severe disease by being more resistant, but
have evolved a different set of pathogenic weapons, developing
therapies to neutralize these virulence factors provides an
appealing approach. Inhibition of capsule biosynthesis has been
explored to destabilize the protective layer that protects bacteria
from the host immune system (66).
Agents targeting siderophore systems prevent bacterial access to
iron, a key nutrient for survival and dissemination. Additionally,
the disruption of biofilm formation and quorum sensing, which
promote persistence and help avoid host responses, are also
promising targets. Although they may not eradicate bacteria
directly, anti-virulence therapies can render pathogens more
susceptible to immune clearance, thereby enhancing the efficacy of
companion antibiotics.
Immunotherapy and vaccine
development
Another promising direction involves immunological
strategies (50). An animal study
has demonstrated that monoclonal antibodies against capsular
polysaccharides, LPSs or siderophores enhance opsonophagocytosis
and provide passive protection (105). K1 and K2 serotypes, which are
most often implicated in hypervirulent liver abscess strains, are
primary targets of whole-cell and subunit vaccines being developed
as part of vaccination efforts (106). Preclinical and early clinical
studies suggest that immunization may provide long-lasting
protection in at-risk populations, such as individuals with
diabetes or chronic liver disease; however, no vaccine has yet been
licensed (23,106). Phase I/II trials of K1/K2
capsule-based vaccines and siderophore-conjugate vaccines are
ongoing, but clinical translation remains pending (107). Host-directed immunotherapies
target not only the pathogen but also aim to augment the innate
immune responses of the host and correct the disturbed immunity,
thereby tipping the host-pathogen balance in favour of bacterial
clearance.
Bacteriophage therapy and
phage-derived enzymes
Bacteriophage therapy has resurfaced as a potential
treatment for MDR K. pneumoniae infections, similar to the
scope of PLA (108). Natural
phages against hypervirulent strains have been effective in
preclinical studies, rapidly lysing bacteria resistant to
antibiotics (109,110). Phage cocktails are being explored
to mitigate resistance development, with early clinical trials now
underway (110). Moreover,
phage-derived lysins, enzymes that degrade bacterial cell walls,
also represent a promising strategy with broad lytic activity and
the ability to work synergistically with antibiotics. Although
phage-host specificity, regulatory considerations and in
vivo efficacy remain challenges, clinical case reports
demonstrate the potential of phage therapy to be lifesaving when
conventional treatment fails.
Microbiome-based and adjunctive
strategies
K. pneumoniae is a part of the gut
microbiota and its changes may initiate systemic invasion (22). This has sparked interest in
microbiome-targeted therapies, including probiotics and faecal
microbiota transplantation, to restore microbial balance and reduce
hvKp colonization. CRISPR, for example, is being explored not just
as a genomic editing tool that works at the gene-targeting level,
such as virulence determinants or resistance genes, but also as a
programmable antimicrobial system capable of selectively
eliminating resistant or hypervirulent bacteria while sparing
commensal flora (111). While
they are still experimental, these strategies represent new
approaches to reduce pathogen load and prevent reinfection.
CRISPR-based antimicrobials are also being investigated as
precision tools to target resistance or virulence genes in hvKp
selectively (103). The
management of K. pneumoniae PLA involves a combination of
antibiotics and drainage; however, new strategies are currently
under active investigation, including β-lactam/β-lactamase
inhibitors, cefiderocol, vaccines and phage therapy (Table V) (103). Fig.
3 highlights therapeutic strategies against K.
pneumoniae-induced PLA.
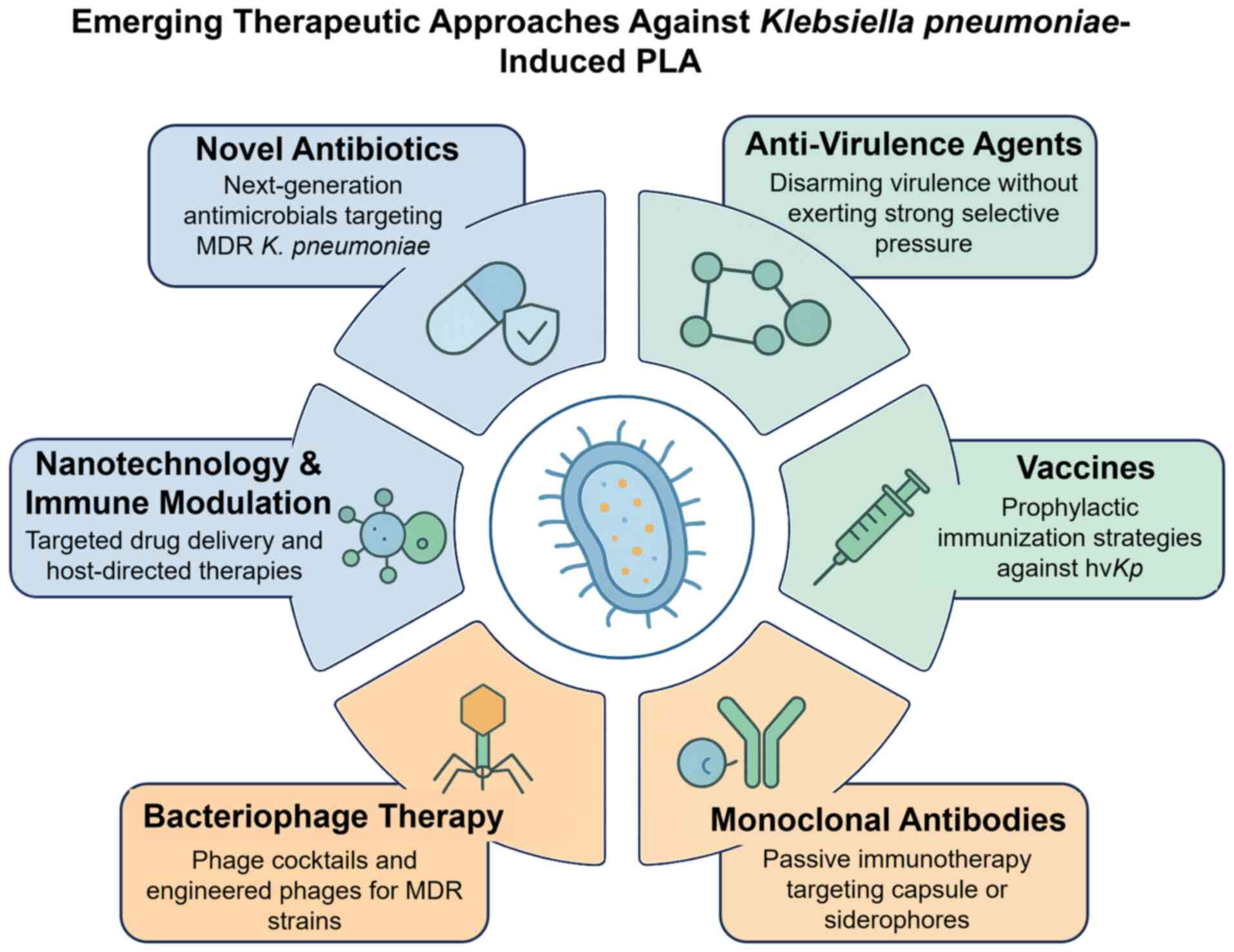 | Figure 3.Emerging therapeutic approaches
against Klebsiella pneumoniae-induced PLA. The central
illustration shows hypervirulent, drug-resistant K.
pneumoniae with capsule, pili and siderophores. Surrounding
panels highlight six strategies: Novel antibiotics, anti-virulence
agents, vaccines, monoclonal antibodies, bacteriophage therapy and
nanotechnology/immune modulation. Colour codes indicate
antibiotic-based (blue), immune/vaccine-based (green) and
innovative approaches (orange). PLA, pyogenic liver abscess; MDR,
multidrug resistance; hvKp, hypervirulent Klebsiella
pneumoniae. |
 | Table V.Current and emerging therapeutic
strategies for K. pneumoniae PLA. |
Table V.
Current and emerging therapeutic
strategies for K. pneumoniae PLA.
| Strategy | Mechanism of
action | Advantages | Limitations | Clinical
status |
|---|
| Cephalosporins
(Third generation) | Inhibit cell wall
synthesis | Effective if
susceptible | ESBL undermines
efficacy | Routine use;
declining efficacy in ESBL-prevalent regions |
| Carbapenems | Broad-spectrum
β-lactams | Active vs. ESBL
strains | Resistance
spreading | Standard therapy;
limited |
| Tigecycline and
Colistin | Ribosomal
inhibition/membrane disruption | Salvage for
MDR | Poor penetration,
toxicity | Last-line
therapy |
| Fosfomycin | Cell wall
inhibition (MurA) | Active vs. some
MDR | fosA resistance is
common | Off-label use |
| Novel
β-lactam/β-lactamase inhibitors (such as ceftazidime-avibactam,
meropenem-vaborbactam and imipenem-relebactam) | Inhibit
β-lactamases | Effective vs.
KPC/OXA-48 | Limited vs.
NDM | Approved for
systemic infections; limited clinical data in PLA |
| Cefiderocol
(siderophore cephalosporin) | Exploits iron
uptake pathways | Potent vs.
MDR/CRKP | Resistance may
develop; limited liver data | Approved; clinical
use expanding |
| Eravacycline and
Plazomicin | Ribosomal
inhibition (tet)/next-generation aminoglycoside | Activity vs. MDR
strains | Limited
PLA-specific data | Approved, limited
use |
| Anti-virulence
agents | Block capsule or
siderophores | Low resistance
pressure | Preclinical
stage | Experimental |
| Vaccines (K1/K2 and
siderophore-based) | Prevent
capsule/siderophore infection | Prophylaxis
potential | Serotype
diversity | Preclinical/early
clinical |
| Monoclonal
antibodies | Neutralize capsule,
LPS and siderophores | Adjunctive
therapy | Expensive; narrow
targets |
Preclinical/early |
| Bacteriophage/phage
enzymes | Lytic activity vs.
MDR hvKp | Active vs.
CR-hvKp | Specificity,
regulatory hurdles | Case reports,
trials emerging |
| Microbiome
interventions (probiotics and FMT) | Restore gut
balance, reduce hvKp colonization | Prevent
recurrence | Experimental; early
preclinical data only | Preclinical |
Future perspectives and clinical
challenges
K. pneumoniae PLA remains a major clinical
challenge despite recent developments in diagnostic modalities and
treatment options (14). The
emerging epidemiology of this infection is characterised by the
convergence of hypervirulence and antimicrobial resistance, which
complicates both treatment and prevention measures. In the future,
breakthroughs in therapeutics, combined with enhanced clinical and
public health approaches, will be key to alleviating the disease
burden and improving outcomes (103).
Convergence of hypervirulence and
resistance
During recent years, some of the most concerning
findings have involved the emergence of hypervirulent phenotypes
that are also multidrug resistant (112). These strains challenge the
classic divide between more resistant but less virulent ‘classic’
isolates and more overtly virulent, although
antibiotic-susceptible, ‘hypervirulent’ strains. These strains can
be considered ‘superbug’ strains as they combine the invasiveness
of hvKp with the drug resistance of cKp, eliminating the
therapeutic advantage previously afforded by antibiotic
susceptibility. Future genomic surveillance and rapid diagnostics
will be necessary to ensure these strains are rapidly identified
and contained. The clinical manifestation of this convergence is
dire; infections due to these pathogens are associated with high
morbidity and mortality and are not amenable to treatment with
available antibiotics. Future research will focus on understanding
the genetic basis of this convergence and the ecological pressures
that drive it, as this may inform surveillance, containment and
drug development efforts.
Diagnostic and predictive
limitations
Diagnosis is one of the major limiting factors for
clinical management, which is crucial for timely and accurate
treatment (98). Culture-based
methods, which are standard for testing for antimicrobial
resistance, are slow, ultimately creating a vicious circle that
delays the start of targeted therapy (113). Molecular diagnostics and rapid
resistance detection platforms are not yet readily available in
numerous clinical settings. In addition, the severity of disease
and the risk of metastatic complications cannot be accurately
predicted since clinical features and routine laboratory parameters
lack specificity. Newer discoveries of biomarkers, genomic
profiling and machine learning-based prediction approaches should
enable the early identification of high-risk patients and
facilitate personalized therapeutic solutions. Recent advances in
metagenomic sequencing and rapid MALDI-TOF-based assays have the
potential to improve early pathogen identification; however, their
widespread implementation remains limited (114).
Recent diagnostic innovations hold considerable
promise for improving the management of hvKp-PLA (114). Metagenomic next-generation
sequencing of blood or pus can identify pathogens and resistance
determinants directly from clinical specimens without the need for
culture (115). However, its
routine use remains constrained by high cost, turnaround time and
bioinformatic complexity. MALDI-TOF mass spectrometry enables
species-level identification within hours from cultured isolates
and, in some centres, can be adapted for direct specimen testing
through rapid extraction workflows (114). In parallel, rapid PCR-based and
isothermal point-of-care assays targeting species markers,
virulence loci such as iuc and rmpA as well as common carbapenemase
genes, including blaNDM and blaKPC, provide
actionable results within a few hours (116). Although each of these modalities
has inherent trade-offs in sensitivity, cost and accessibility, an
integrated diagnostic approach, combining rapid molecular detection
for early guidance with culture and susceptibility testing for
definitive management, offers the greatest potential to reduce time
to effective therapy in real-world clinical settings. Successful
implementation will ultimately depend on local laboratory
infrastructure, cost-benefit considerations and integration with
antimicrobial stewardship programs.
Gaps in therapeutic options
The management options for K. pneumoniae PLA
are limited by poor penetration of drugs into abscess cavities, the
rapid emergence of resistance and a lack of targeted regimens for
hvKp (33). Antibiotics may not
reach adequate levels in the centre of abscesses as they are poorly
vascularized (69). Novel delivery
methods, such as drug-eluting beads or local catheter infusions,
may enhance intra-abscess drug concentrations and warrant further
investigation (117).
Additionally, there have been no clinical trials specifically for
hvKp-PLA, leaving no consensus on optimal empirical therapy. More
extensive registries and prospective studies are necessary to
determine the optimal antibiotic regimens and durations for
different resistance phenotypes.
Global and regional disparities
The regional distribution of K. pneumoniae
PLA is not homogenous (118).
These hypervirulent strains are most prevalent in East and
Southeast Asia, where the majority of the literature and expertise
reside (98). By contrast, MDR
strains have a longer epidemiological history in North America and
Europe, and hvKp-PLA clusters have only recently been described on
these continents. This has implications for both research
priorities and clinical approaches. For example, in Asia,
priorities may centre on vaccines and anti-virulence strategies to
curb hvKp and CRKP, whereas in Western countries, emphasis may lie
on antibiotic stewardship and infection control. This step requires
sharing surveillance data and best practices between countries.
Toward integrated and preventive
approaches
Integrated methods that apply existing and
potential novel strategies may represent a promising future for PLA
management (14). This encompasses
high-risk population prophylaxis (such as diabetes control and
potential vaccine usage), rapid diagnostic testing to target
therapy, judicious antimicrobial stewardship to limit resistance
selection and the deployment of novel therapeutic modalities. If
implemented, preventive measures, especially vaccines for the most
common capsule types or siderophores, could substantially reduce
incidence. Improved global surveillance systems and stronger health
infrastructure are crucial for detecting outbreaks and implementing
infection control measures promptly (50). A successful response to K.
pneumoniae PLA will eventually be coordinated across all
sectors of clinical medicine, microbiology and public health.
Conclusions
K. pneumoniae PLA has emerged as a
significant clinical and public health concern, driven by the dual
threats of hypervirulence and escalating antimicrobial resistance.
While advances in imaging, interventional drainage and antibiotic
therapy have improved outcomes, the increasing prevalence of
strains combining MDR with invasive virulence presents an urgent
global challenge. Therapeutic options are limited by poor
pharmacokinetics, inconsistent efficacy and the absence of
standardized regimens for resistant or hypervirulent infections.
The future of management will depend on a multipronged strategy.
Continued surveillance is necessary to track the evolving
epidemiology and the genetic determinants of virulence and
resistance. Innovative therapeutics, including novel antibiotics,
anti-virulence agents, immunotherapies and bacteriophage-based
approaches, offer promise for more effective, targeted
interventions. At the same time, advances in diagnostics and
predictive modelling may enable earlier recognition of high-risk
patients and more personalized care. Preventive strategies,
especially vaccines, together with global antimicrobial stewardship
and strengthened health systems, will be central to reducing
disease burden. Ultimately, addressing K. pneumoniae liver
abscess requires an integrated vision that bridges clinical
medicine, microbiology and public health. Only coordinated global
action and sustained translational research can counter this
formidable pathogen and its increasingly complex clinical
manifestations.
Acknowledgements
GMH would like to thank the Prince Sattam Bin
Abdulaziz University (Grant No. PSAU/2025/RV/7).
Funding
This work is sponsored by Prince Sattam Bin Abdulaziz University
(grant no. PSAU/2025/RV/7).
Availability of data and materials
Not applicable.
Authors' contribution
GMH contributed to conceptualization, funding
acquisition, and preparation of the original draft. TM contributed
to data analysis, graphics preparation, data validation and
critical revision of the manuscript. SZ contributed, graphics
design, data validation and manuscript review. AS contributed to
analysis, graphics, data curation (systematically collecting,
organizing, and verifying the literature, clinical reports, and
epidemiological datasets relevant to Klebsiella
pneumoniae–associated pyogenic liver abscess - this included
screening articles, extracting key findings, maintaining reference
databases, and ensuring accuracy and consistency of the compiled
information used for analysis), critical revisions and supervision.
SSS contributed to graphics, data validation, manuscript review and
supervision. MIH guided the conceptual framework, supervised the
study, critical revisions and managed project administration. SZ
and MIH contributed to the investigation, this involved conducting
an in-depth scientific inquiry into the clinical features,
virulence mechanisms, and therapeutic strategies associated with
Klebsiella pneumoniae infections - this covered critical
literature analysis, interpretation of current evidence,
identifying research gaps, and synthesizing mechanistic insights
necessary for the development of the review article. All authors
read and approved the final version of the manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, artificial
intelligence tools (ChatGPT) were used to improve the readability
and language of the manuscript or to generate images, and
subsequently, the authors revised and edited the content produced
by the artificial intelligence tools as necessary, taking full
responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Priyanka A, Akshatha K, Deekshit VK,
Prarthana J and Akhila DS: Klebsiella pneumoniae infections
and antimicrobial drug resistance. Model organisms for microbial
pathogenesis, biofilm formation and antimicrobial drug discovery.
Springer. (Singapore). 195–225. 2020. View Article : Google Scholar
|
|
2
|
Giacobbe DR, Di Pilato V, Karaiskos I,
Giani T, Marchese A, Rossolini GM and Bassetti M: Treatment and
diagnosis of severe KPC-producing Klebsiella pneumoniae
infections: A perspective on what has changed over last decades.
Ann Med. 55:101–113. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang S, Zhang X, Wu Q, Zheng X, Dong G,
Fang R, Zhang Y, Cao J and Zhou T: Clinical, microbiological, and
molecular epidemiological characteristics of Klebsiella
pneumoniae-induced pyogenic liver abscess in southeastern
China. Antimicrob Resist Infect Control. 8:1662019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Al Ismail D, Campos-Madueno EI, Donà V and
Endimiani A: Hypervirulent Klebsiella pneumoniae (hvKp):
Overview, epidemiology, and laboratory detection. Pathog Immun.
10:80–119. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marr CM and Russo TA: Hypervirulent
Klebsiella pneumoniae: A new public health threat. Expert
Rev Anti Infect Ther. 17:71–73. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Choby JE, Howard-Anderson J and Weiss DS:
Hypervirulent Klebsiella pneumoniae-clinical and molecular
perspectives. J Intern Med. 287:283–300. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sohrabi M, Alizade Naini M, Rasekhi A,
Oloomi M, Moradhaseli F, Ayoub A, Bazargani A, Hashemizadeh Z,
Shahcheraghi F and Badmasti F: Emergence of K1 ST23 and K2 ST65
hypervirulent Klebsiella pneumoniae as true pathogens with
specific virulence genes in cryptogenic pyogenic liver abscesses
Shiraz Iran. Front Cell Infect Microbiol. 12:9642902022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Berman JJ: Changing how we think about
infectious diseases. Taxonomic guide to infectious diseases.
(second edition). Elsevier; pp. 321–365. 2019, View Article : Google Scholar
|
|
9
|
Liao CH, Huang YT, Chang CY, Hsu HS and
Hsueh PR: Capsular serotypes and multilocus sequence types of
bacteremic Klebsiella pneumoniae isolates associated with
different types of infections. Eur J Clin Microbiol Infect Dis.
33:365–369. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mendes G, Santos ML, Ramalho JF, Duarte A
and Caneiras C: Virulence factors in carbapenem-resistant
hypervirulent Klebsiella pneumoniae. Front Microbiol.
14:13250772023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thirugnanasambantham MK, Thuthikkadu
Indhuprakash S and Thirumalai D: Phenotypic and genotypic
characteristics of hypervirulent Klebsiella pneumoniae
(hvKp): A narrative review. Curr Microbiol. 82:3412025. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ding L, Shen S, Chen J, Tian Z, Shi Q, Han
R, Guo Y and Hu F: Klebsiella pneumoniae carbapenemase
variants: The new threat to global public health. Clin Microbiol
Rev. 36:e00008232023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khan AU, Maryam L and Zarrilli R:
Structure, genetics and worldwide spread of New Delhi
metallo-β-lactamase (NDM): A threat to public health. BMC
Microbiol. 17:1012017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shah AA, Alwashmi AS, Abalkhail A and
Alkahtani AM: Emerging challenges in Klebsiella pneumoniae:
Antimicrobial resistance and novel approach. Microb. Pathog.
202:1073992025.PubMed/NCBI
|
|
15
|
Hetta HF, Alanazi FE, Ali MAS, Alatawi AD,
Aljohani HM, Ahmed R, Alansari NA, Alkhathami FM, Albogmi A,
Alharbi BM, et al: Hypervirulent Klebsiella pneumoniae:
Insights into virulence, antibiotic resistance, and fight
strategies against a superbug. Pharmaceuticals (Basel). 18:7242025.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pan Y, Chen H, Ma R, Wu Y, Lun H, Wang A,
He K, Yu J and He P: A novel depolymerase specifically degrades the
K62-type capsular polysaccharide of Klebsiella pneumoniae.
One Health Adv. 2:52024. View Article : Google Scholar
|
|
17
|
Xu Q, Liu C, Wu Z, Zhang S, Chen Z, Shi Y
and Gu S: Demographics and prognosis of patients with pyogenic
liver abscess due to Klebsiella pneumonia or other species.
Heliyon. 10:e294632024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pandita A, Javaid W and Fazili T: Liver
Abscess. In: Introduction to Clinical Infectious Diseases.
Domachowske J: Springer; Cham: pp. 147–155. 2019
|
|
19
|
Lam JC and Stokes W: Management of
pyogenic liver abscesses: Contemporary strategies and challenges. J
Clin Gastroenterol. 57:774–781. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Clegg S and Murphy CN: Epidemiology and
virulence of Klebsiella pneumoniae. Urinary tract
infections: Molecular pathogenesis and clinical management.
435–457. 2017.
|
|
21
|
Abbas R, Chakkour M, Zein El Dine H,
Obaseki EF, Obeid ST, Jezzini A, Ghssein G and Ezzeddine Z: General
overview of Klebsiella pneumonia: Epidemiology and the role
of siderophores in its pathogenicity. Biology (Basel).
13:782024.PubMed/NCBI
|
|
22
|
Chen Q, Wang M, Han M, Xu L and Zhang H:
Molecular basis of Klebsiella pneumoniae colonization in
host. Microb Pathog. 177:1060262023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wantuch PL and Rosen DA: Klebsiella
pneumoniae: Adaptive immune landscapes and vaccine horizons.
Trends Immunol. 44:826–844. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ke Y, Zeng Z, Liu J and Ye C: Capsular
polysaccharide as a potential target in hypervirulent and
drug-resistant Klebsiella pneumoniae treatment. Infect Drug
Resist. 18:1253–1262. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Igweonu CF: Molecular characterization of
antibiotic resistance genes in multidrug-resistant Klebsiella
pneumoniae clinical isolates. Int J Eng Technol Res Manag.
8:2412024.
|
|
26
|
Haque M, Sartelli M, McKimm J and Bakar
MA: Health care-associated infections-an overview. Infect Drug
Resist. 11:2321–2333. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ye M, Tu J, Jiang J, Bi Y, You W, Zhang Y,
Ren J, Zhu T, Cao Z, Yu Z, et al: Clinical and genomic analysis of
liver abscess-causing Klebsiella pneumoniae identifies new
liver abscess-associated virulence genes. Front Cell Infect
Microbiol. 6:1652016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wichmann D, Königsrainer A, Schweizer U,
Archid R, Nadalin S and Manncke S: Pyogenic liver abscesses caused
by acute appendicitis: Frequency and diagnostic and therapeutic
recommendations. Surg Infect (Larchmt). 22:253–257. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Serban D, Popa Cherecheanu A, Dascalu AM,
Socea B, Vancea G, Stana D, Smarandache GC, Sabau AD and Costea DO:
Hypervirulent Klebsiella pneumoniae endogenous
endophthalmitis-a global emerging disease. Life (Basel).
11:6762021.PubMed/NCBI
|
|
30
|
Neill L, Edwards F, Collin SM, Harrington
D, Wakerley D, Rao GG and McGregor AC: Clinical characteristics and
treatment outcomes in a cohort of patients with pyogenic and
amoebic liver abscess. BMC Infect Dis. 19:4902019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thimmappa PY, Vasishta S, Ganesh K, Nair
AS and Joshi MB: Neutrophil (dys)function due to altered
immuno-metabolic axis in type 2 diabetes: Implications in combating
infections. Hum Cell. 36:1265–1282. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gan C, Yuan Y, Shen H, Gao J, Kong X, Che
Z, Guo Y, Wang H, Dong E and Xiao J: Liver diseases: Epidemiology,
causes, trends and predictions. Signal Transduct Target Ther.
10:332025. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Angeles-Solano M, Tabashsum Z, Chen L and
Rowe SE: Klebsiella pneumoniae liver abscesses:
Pathogenesis, treatment, and ongoing challenges. Infect Immun.
93:e00508242025. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nakamura K, Nomoto H, Harada S, Suzuki M,
Yomono K, Yokochi R, Hagino N, Nakamoto T, Moriyama Y, Yamamoto K,
et al: Infection with capsular genotype K1-ST23 hypervirulent
Klebsiella pneumoniae isolates in Japan after a stay in East
Asia: Two cases and a literature review. J Infect Chemother.
27:1508–1512. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
García-Cobos S, Oteo-Iglesias J and
Pérez-Vázquez M: Hypervirulent Klebsiella pneumoniae:
Epidemiology outside Asian countries, antibiotic resistance
association, methods of detection and clinical management. Enferm
Infecc Microbiol Clin (Engl Ed). 43:102–109. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kain MJ, Reece NL, Parry CM, Rajahram GS,
Paterson DL and Woolley SD: The rapid emergence of hypervirulent
klebsiella species and burkholderia pseudomallei as major health
threats in southeast Asia: The urgent need for recognition as
neglected tropical diseases. Trop Med Infect Dis. 9:802024.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li X, Chen S, Lu Y, Shen W, Wang W, Gao J,
Gao J, Shao P and Zhou Z: Molecular epidemiology and genetic
dynamics of carbapenem-resistant hypervirulent Klebsiella
pneumoniae in China. Front Cell Infect Microbiol.
15:15299292025. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Isler B, Aslan AT, Akova M, Harris P and
Paterson DL: Treatment strategies for OXA-48-like and NDM producing
Klebsiella pneumoniae infections. Expert Rev Anti Infect
Ther. 20:1389–1400. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang Y, McNally A and Zong Z: Call for
prudent use of the term hypervirulence in carbapenem-resistant
Klebsiella pneumoniae. Lancet Microbe. 6:1010902025.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Russo TA, Alvarado CL, Davies CJ, Drayer
ZJ, Carlino-MacDonald U, Hutson A, Luo TL, Martin MJ, Corey BW,
Moser KA, et al: Differentiation of hypervirulent and classical
Klebsiella pneumoniae with acquired drug resistance. mBio.
15:e02867232024. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Han B, Feng C, Jiang Y, Ye C, Wei Y, Liu J
and Zeng Z: Mobile genetic elements encoding antibiotic resistance
genes and virulence genes in Klebsiella pneumoniae:
Important pathways for the acquisition of virulence and resistance.
Front Microbiol. 16:15291572025. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wu C, Huang Y, Zhou P, Gao H, Wang B, Zhao
H, Zhang J, Wang L, Zhou Y and Yu F: Emergence of hypervirulent and
carbapenem-resistant Klebsiella pneumoniae from 2014–2021 in
Central and Eastern China: A molecular, biological, and
epidemiological study. BMC Microbiol. 24:4652024. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu S, Huang Z, Kong J, Zhao Y, Xu M, Zhou
B, Zheng X, Ye D, Zhou T, Cao J and Zhou C: Effects of
aerobactin-encoding gene iucB and regulator of mucoid phenotype
rmpA on the virulence of Klebsiella pneumoniae causing liver
abscess. Front Cell Infect Microbiol. 12:9689552022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chan KS, Chia CTW and Shelat VG:
Demographics, radiological findings, and clinical outcomes of
Klebsiella pneumonia vs non-Klebsiella pneumoniae pyogenic
liver abscess: A systematic review and meta-analysis with trial
sequential analysis. Pathogens. 11:9762022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shein AMS: Mechanisms of colistin
resistance and the antimicrobial effects of antibiotic and adjuvant
combination on colistin-resistant Klebsiella pneumoniae.
Chulalongkorn University Theses and Dissertations (Chula ETD); pp.
47862021
|
|
46
|
Xu L, Li J, Wu W, Wu X and Ren J:
Klebsiella pneumoniae capsular polysaccharide: Mechanism in
regulation of synthesis, virulence, and pathogenicity. Virulence.
15:24395092024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Walker KA and Miller VL: The intersection
of capsule gene expression, hypermucoviscosity and hypervirulence
in Klebsiella pneumoniae. Curr Opin Microbiol. 54:95–102.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Walker KA, Miner TA, Palacios M, Trzilova
D, Frederick DR, Broberg CA, Sepúlveda VE, Quinn JD and Miller VL:
A Klebsiella pneumoniae regulatory mutant has reduced
capsule expression but retains hypermucoviscosity. mBio.
10:e00089–19. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yu WL, Lee MF, Chang MC and Chuang YC:
Intrapersonal mutation of rmpA and rmpA2: A reason for negative
hypermucoviscosity phenotype and low virulence of rmpA-positive
Klebsiella pneumoniae isolates. J Glob Antimicrob Resist.
3:137–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Opoku-Temeng C, Kobayashi SD and DeLeo FR:
Klebsiella pneumoniae capsule polysaccharide as a target for
therapeutics and vaccines. Comput Struct Biotechnol J.
17:1360–1366. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kumar A, Chakravorty S, Yang T, Russo TA,
Newton SM and Klebba PE: Siderophore-mediated iron acquisition by
Klebsiella pneumoniae. J Bacteriol. 206:e00024242024.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Russo TA, Olson R, MacDonald U, Beanan J
and Davidson BA: Aerobactin, but not yersiniabactin, salmochelin,
or enterobactin, enables the growth/survival of hypervirulent
(hypermucoviscous) Klebsiella pneumoniae ex vivo and in
vivo. Infect Immun. 83:3325–3333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Holden VI, Breen P, Houle S, Dozois CM and
Bachman MA: Klebsiella pneumoniae siderophores induce
inflammation, bacterial dissemination, and HIF-1α stabilization
during pneumonia. mBio. 7:e01397–16. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wu J, Chen J, Wang Y, Meng Q and Zhao J:
Siderophore iucA of hypermucoviscous Klebsiella pneumoniae
promotes liver damage in mice by inducing oxidative stress. Biochem
Biophys Rep. 32:1013762022.PubMed/NCBI
|
|
55
|
Lim C, Zhang CY, Cheam G, Chu WHW, Chen Y,
Yong M, Lim KYE, Lam MMC, Teo TH and Gan YH: Essentiality of the
virulence plasmid-encoded factors in disease pathogenesis of the
major lineage of hypervirulent Klebsiella pneumoniae varies
in different infection niches. EBioMedicine. 115:1056832025.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Doorduijn DJ, Rooijakkers SH, van Schaik W
and Bardoel BW: Complement resistance mechanisms of Klebsiella
pneumoniae. Immunobiology. 221:1102–1109. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Singh S, Wilksch JJ, Dunstan RA, Mularski
A, Wang N, Hocking D, Jebeli L, Cao H, Clements A, Jenney AWJ, et
al: LPS O antigen plays a key role in Klebsiella pneumoniae
capsule retention. Microbiol Spectr. 10:e01517212022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yang FL, Yang YL, Liao PC, Chou JC, Tsai
KC, Yang AS, Sheu F, Lin TL, Hsieh PF, Wang JT, et al: Structure
and immunological characterization of the capsular polysaccharide
of a pyrogenic liver abscess caused by Klebsiella
pneumoniae: Activation of macrophages through Toll-like
receptor 4. J Biol Chem. 286:21041–21051. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhu J, Wang T, Chen L and Du H: Virulence
factors in hypervirulent Klebsiella pneumoniae. Front
Microbiol. 12:6424842021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lam MMC, Wyres KL, Judd LM, Wick RR,
Jenney A, Brisse S and Holt KE: Tracking key virulence loci
encoding aerobactin and salmochelin siderophore synthesis in
Klebsiella pneumoniae. Genome Med. 10:772018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Shimpoh T, Hirata Y, Ihara S, Suzuki N,
Kinoshita H, Hayakawa Y, Ota Y, Narita A, Yoshida S, Yamada A and
Koike K: Prevalence of pks-positive Escherichia coli in Japanese
patients with or without colorectal cancer. Gut Pathog. 12:352017.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kaur CP, Vadivelu J and Chandramathi S:
Impact of Klebsiella pneumoniae in lower gastrointestinal
tract diseases. J Dig Dis. 19:262–271. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Paczosa MK and Mecsas J: Klebsiella
pneumoniae: going on the offense with a strong defense.
Microbiol Mol Biol Rev. 80:629–661. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chen T, Dong G, Zhang S, Zhang X, Zhao Y,
Cao J, Zhou T and Wu Q: Effects of iron on the growth, biofilm
formation and virulence of Klebsiella pneumoniae causing
liver abscess. BMC Microbiol. 20:362020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Opoku-Temeng C, Malachowa N, Kobayashi SD
and DeLeo FR: Innate host defense against Klebsiella
pneumoniae and the outlook for development of immunotherapies.
J Innate Immun. 14:167–181. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Bai R and Guo J: Interactions and
implications of Klebsiella pneumoniae with human immune
responses and metabolic pathways: A comprehensive review. Infect
Drug Resist. 17:449–462. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Han HC: Role of macrophages and
neutrophils in Klebsiella pneumoniae induced liver abscess
(KLA). National University of Singapore (Singapore); 2019
|
|
68
|
Lin SH, Chung PH, Wu YY, Fung CP, Hsu CM
and Chen LW: Inhibition of nitric oxide production reverses
diabetes-induced Kupffer cell activation and Klebsiella
pneumonia liver translocation. PLoS One. 12:e01772692017.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Chen HQ, Mo ZH and Wei WX: Case report:
Trauma-induced Klebsiella pneumoniae invasive syndrome
presenting with liver abscess, lung abscess, endophthalmitis, and
purulent meningitis. Front Med (Lausanne). 11:15138312025.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Li S, Yu S, Peng M, Qin J, Xu C, Qian J,
He M and Zhou P: Clinical features and development of sepsis in
Klebsiella pneumoniae infected liver abscess patients: A
retrospective analysis of 135 cases. BMC Infect Dis. 21:5972021.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hu Y, Anes J, Devineau S and Fanning S:
Klebsiella pneumoniae: Prevalence, reservoirs, antimicrobial
resistance, pathogenicity, and infection: A hitherto unrecognized
zoonotic bacterium. Foodborne Pathog Dis. 18:63–84. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Silvester R, Madhavan A, Kokkat A, Parolla
A, B M A, M H and Abdulla MH: Global surveillance of antimicrobial
resistance and hypervirulence in Klebsiella pneumoniae from
LMICs: An in-silico approach. Sci Total Environ. 802:1498592022.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Koli SV, Mali SV and Geevarghese J:
‘Global divide in carbapenem resistance and hypervirulence of
Klebsiella pneumonia: Comparing trends in India and
developed nations’-a comprehensive review. J Antibiot (Tokyo).
78:457–471. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hu Y, Yang Y, Feng Y, Fang Q, Wang C, Zhao
F, McNally A and Zong Z: Prevalence and clonal diversity of
carbapenem-resistant Klebsiella pneumoniae causing neonatal
infections: A systematic review of 128 articles across 30
countries. PLoS Med. 20:e10042332023. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hu YL, Bi SL, Zhang ZY and Kong NQ:
Correlation between antibiotics-resistance, virulence genes and
genotypes among Klebsiella pneumoniae clinical strains
isolated in Guangzhou, China. Curr Microbiol. 81:2892024.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hsu JY, Chuang YC, Wang JT, Chen YC and
Hsieh SM: Healthcare-associated carbapenem-resistant Klebsiella
pneumoniae bloodstream infections: Risk factors, mortality, and
antimicrobial susceptibility, 2017–2019. J Formos Med Assoc.
120:1994–2002. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Elias R, Duarte A and Perdigão J: A
molecular perspective on colistin and Klebsiella pneumoniae:
Mode of action, resistance genetics, and phenotypic susceptibility.
Diagnostics (Basel). 11:11652021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Padmini N, Ajilda AAK, Sivakumar N and
Selvakumar G: Extended spectrum β-lactamase producing Escherichia
coli and Klebsiella pneumoniae: critical tools for
antibiotic resistance pattern. J Basic Microbiol. 57:460–470. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
da Silva Y, Ferrari R, Marin VA and Junior
CAC: A global overview of β-lactam resistance genes in
Klebsiella pneumoniae. Open Infect Dis J. 11:22–34. 2019.
View Article : Google Scholar
|
|
80
|
Li Y, Kumar S, Zhang L and Wu H and Wu H:
Characteristics of antibiotic resistance mechanisms and genes of
Klebsiella pneumoniae. Open Med (Wars). 18:202307072023.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Gogry FA, Siddiqui MT, Sultan I and Haq
QMR: Current update on intrinsic and acquired colistin resistance
mechanisms in bacteria. Front Med (Lausanne). 8:6777202021.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Sugawara E, Kojima S and Nikaido H:
Klebsiella pneumoniae major porins OmpK35 and OmpK36 allow
more efficient diffusion of β-lactams than their Escherichia coli
homologs OmpF and OmpC. J Bacteriol. 198:3200–3208. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Albarri O, AlMatar M, Var I and Köksal F:
Antimicrobial resistance of clinical Klebsiella pneumoniae
isolates: Involvement of AcrAB and OqxAB efflux pumps. Curr Mol
Pharmacol. 17:e3103232152662024.PubMed/NCBI
|
|
84
|
Liu Y, Lin Y, Wang Z, Hu N, Liu Q, Zhou W,
Li X, Hu L, Guo J, Huang X and Zeng L: Molecular mechanisms of
colistin resistance in Klebsiella pneumoniae in a tertiary
care teaching hospital. Front Cell Infect Microbiol. 11:6735032021.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Di Pilato V, Pollini S, Miriagou V,
Rossolini GM and D'Andrea MM: Carbapenem-resistant Klebsiella
pneumoniae: The role of plasmids in emergence, dissemination,
and evolution of a major clinical challenge. Expert Rev Anti Infect
Ther. 22:25–43. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Arcari G and Carattoli A: Global spread
and evolutionary convergence of multidrug-resistant and
hypervirulent Klebsiella pneumoniae high-risk clones. Pathog
Glob Health. 117:328–341. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Merla C, Kuka A, Mileto I, Petazzoni G,
Gaiarsa S, De Vitis D, Ardizzone M, Corbella M, Baldanti F and
Cambieri P: One-year surveillance for hypervirulent Klebsiella
pneumoniae detected carbapenem-resistant superbugs. Microbiol
Spectr. 12:e03292232024.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Han YL, Wen XH, Zhao W, Cao XS, Wen JX,
Wang JR, Hu ZD and Zheng WQ: Epidemiological characteristics and
molecular evolution mechanisms of carbapenem-resistant
hypervirulent Klebsiella pneumoniae. Front Microbiol.
13:10037832022. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Rahmat Ullah S, Jamal M, Rahman A and
Andleeb S: Comprehensive insights into Klebsiella
pneumoniae: Unravelling clinical impact, epidemiological trends
and antibiotic-resistance challenges. J Antimicrob Chemother.
79:1484–1492. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Grégoire N, Aranzana-Climent V, Magréault
S, Marchand S and Couet W: Clinical pharmacokinetics and
pharmacodynamics of colistin. Clin Pharmacokinet. 56:1441–1460.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zha L, Pan L, Guo J, French N, Villanueva
EV and Tefsen B: Effectiveness and safety of high dose tigecycline
for the treatment of severe infections: A systematic review and
meta-analysis. Adv Ther. 37:1049–1064. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zhao T, Chen N, Zhang M, Lin L, Lin B,
Fang Y, Hua Z and Liang C: Bibliometric analysis of global research
on the clinical applications of aminoglycoside antibiotics:
Improving efficacy and decreasing risk. Front Microbiol.
16:15322312025. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Edwards F, MacGowan A and Macnaughton E:
Antimicrobial therapy: Principles of use. Medicine. 49:624–631.
2021. View Article : Google Scholar
|
|
94
|
Riccobono E, Bussini L, Giannella M, Viale
P and Rossolini GM: Rapid diagnostic tests in the management of
pneumonia. Expert Rev Mol Diagn. 22:49–60. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Nayeem A, Suresh AS, Vellapandian C, Singh
S, Elossaily GM and Prajapati BG: Comprehensive insights into
cephalosporins: Spectrum, generations, and clinical applications.
Curr Drug Ther. 2024. View Article : Google Scholar
|
|
96
|
Kaiser P, Wächter J and Windbergs M:
Therapy of infected wounds: Overcoming clinical challenges by
advanced drug delivery systems. Drug Deliv Transl Res.
11:1545–1567. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Trillos-Almanza MC and Gutierrez JCR: How
to manage: Liver abscess. Frontline Gastroenterol. 12:225–231.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Chang D, Sharma L, Dela Cruz CS and Zhang
D: Clinical epidemiology, risk factors, and control strategies of
Klebsiella pneumoniae infection. Front Microbiol.
12:7506622021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Li F, Zhang H, Xu Y, Eresen A, Zhang Z and
Liu J: Clinical and CT comparative study of invasive and
non-invasive Klebsiella pneumoniae liver abscesses. Clin.
Radiol. 78:40–46. 2023.
|
|
100
|
Nojima H, Shimizu H, Murakami T, Yamazaki
M, Yamazaki K, Suzuki S, Shuto K, Kosugi C, Usui A and Koda K:
Successful hepatic resection for invasive Klebsiella
pneumoniae large multiloculated liver abscesses with
percutaneous drainage failure: A case report. Front Med (Lausanne).
9:10928792023. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Russo A, Fusco P, Morrone HL, Trecarichi
EM and Torti C: New advances in management and treatment of
multidrug-resistant Klebsiella pneumoniae. Expert Rev Anti
Infect Ther. 21:41–55. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Wang Q, Yan T, Ma C, Teng X, Shen C, Wang
N, Yu K, Chu W, Zhou Q and Liu Z: Poor glycemic control in
carbapenem-resistant Klebsiella pneumoniae infections:
Impact on epidemiological features, mortality risks, and polymyxin
resistance. Infect Drug Resist. 18:647–660. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Li Y, Kumar S and Zhang L: Mechanisms of
antibiotic resistance and developments in therapeutic strategies to
combat Klebsiella pneumoniae infection. Infect Drug Resist.
17:1107–1119. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Karaiskos I, Galani I, Papoutsaki V,
Galani L and Giamarellou H: Carbapenemase producing Klebsiella
pneumoniae: Implication on future therapeutic strategies.
Expert Rev Anti Infect Ther. 20:53–69. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Miller JC, Cross AS, Tennant SM and
Baliban SM: Klebsiella pneumoniae lipopolysaccharide as a
vaccine target and the role of antibodies in protection from
disease. Vaccines (Basel). 12:11772024. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Tiria FR and Musila L: A review of the
innate immune evasion mechanisms and status of vaccine development
of Klebsiella pneumonia. Microbiol Res J Int. 31:33–47.
2021. View Article : Google Scholar
|
|
107
|
Wantuch PL, Knoot CJ, Marino EC, Harding
CM and Rosen DA: Klebsiella pneumoniae bioconjugate vaccine
functional durability in mice. Vaccine. 43:1265362025. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Jiao X, Wang M, Liu Y, Yang S, Yu Q and
Qiao J: Bacteriophage-derived depolymerase: A review on prospective
antibacterial agents to combat Klebsiella pneumoniae. Arch
Virol. 170:702025. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Gholizadeh O, Ghaleh HEG, Tat M, Ranjbar R
and Dorostkar R: The potential use of bacteriophages as
antibacterial agents against Klebsiella pneumoniae. Virol J.
21:1912024. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Yoo S, Lee KM, Kim N, Vu TN, Abadie R and
Yong D: Designing phage cocktails to combat the emergence of
bacteriophage-resistant mutants in multidrug-resistant
Klebsiella pneumoniae. Microbiol Spectr. 12:e01258232024.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Noor K and Shehzad MI: Gene-targeted
antimicrobial strategies using CRISPR-cas systems against
multidrug-resistant bacterial pathogens. Int J Appl Clin Res.
3:1–7. 2025.
|
|
112
|
Tang M, Kong X, Hao J and Liu J:
Epidemiological characteristics and formation mechanisms of
multidrug-resistant hypervirulent Klebsiella pneumoniae.
Front Microbiol. 11:5815432020. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Lindstedt K: Human gut colonisation by the
Klebsiella pneumoniae species complex: Detection, duration,
dynamics, and microbiota associations. 2024.
|
|
114
|
Elbehiry A and Abalkhail A: Spectral
precision: Recent advances in matrix-assisted laser
desorption/ionization time-of-flight mass spectrometry for pathogen
detection and resistance profiling. Microorganisms. 13:14732025.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Miller S and Chiu C: The role of
metagenomics and next-generation sequencing in infectious disease
diagnosis. Clin Chem. 68:115–124. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Setterfield MA: Biomarker identification
and multiplex assay development for the detection of antimicrobial
resistance. Newcastle University; 2022
|
|
117
|
Heffernan AJ: Dose optimisation of
intravenous and nebulised antibiotics for the treatment of
Pseudomonas aeruginosa pneumonia. Griffith University; 2023
|
|
118
|
Budia-Silva M, Kostyanev T, Ayala-Montaño
S, Bravo-Ferrer Acosta J, Garcia-Castillo M, Cantón R, Goossens H,
Rodriguez-Baño J, Grundmann H and Reuter S: International and
regional spread of carbapenem-resistant Klebsiella
pneumoniae in Europe. Nat Commun. 15:50922024. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Zhang L, Chen J, Qu Y, Cao X, Cui J, Li J
and Yu A: Development and validation of a predictive model for
invasive syndrome in patients with Klebsiella pneumoniae
liver abscess. Front Med (Lausanne). 12:16634072025. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Pope JL, Yang Y, Newsome RC, Sun W, Sun X,
Ukhanova M, Neu J, Issa JP, Mai V and Jobin C: Microbial
colonization coordinates the pathogenesis of a Klebsiella
pneumoniae infant isolate. Sci Rep. 9:33802019. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Lin YT, Siu LK, Lin JC, Chen TL, Tseng CP,
Yeh KM, Chang FY and Fung CP: Seroepidemiology of Klebsiella
pneumoniae colonizing the intestinal tract of healthy Chinese
and overseas Chinese adults in Asian countries. BMC Microbiol.
12:132012. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
AL-Busaidi BN, AL-Muzahmi M, AL-Shabibi Z,
Rizvi M, AL-Rashdi A, AL-Jardani A, Farzand R and AL-Jabri Z:
klebsiella pneumoniae clinical strains of hypervirulent
capsular serotypes K1 and K2 demonstrating resistance against human
serum bactericidal activity and virulence in galleria mellonella
model. 2024. View Article : Google Scholar
|
|
123
|
Verani JR, Blau DM, Gurley ES, Akelo V,
Assefa N, Baillie V, Bassat Q, Berhane M, Bunn J, Cossa ACA, et al:
Child deaths caused by Klebsiella pneumoniae in sub-Saharan
Africa and south Asia: A secondary analysis of child health and
mortality prevention surveillance (CHAMPS) data. Lancet Microbe.
5:e131–e141. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Monteiro AdSS, Cordeiro SM and Reis JN:
Virulence factors in Klebsiella pneumoniae: A literature
review. Indian J Microbiol. 64:389–401. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Liao Y, Gong J, Yuan X, Wang X, Huang Y
and Chen X: Virulence factors and carbapenem-resistance mechanisms
in hypervirulent Klebsiella pneumoniae. Infect Drug Resist.
17:1551–1559. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Lam MMC, Wick RR, Wyres KL, Gorrie CL,
Judd LM, Jenney AWJ, Brisse S and Holt KE: Genetic diversity,
mobilisation and spread of the yersiniabactin-encoding mobile
element ICEKp in Klebsiella pneumoniae populations. Microb
Genom. 4:e0001962018.PubMed/NCBI
|
|
127
|
Bossuet-Greif N, Vignard J, Taieb F, Mirey
G, Dubois D, Petit C, Oswald E and Nougayrède JP: The colibactin
genotoxin generates DNA interstrand cross-links in infected cells.
mBio. 9:e02393–17. 2018. View Article : Google Scholar : PubMed/NCBI
|