Introduction
Pleomorphic carcinoma (PC) of the lung, one of the
sarcomatoid carcinomas (1),
comprises both an epithelial component and a sarcomatous component
of spindle and/or giant cells or of spindle or giant cells only and
an aggressive cancer, with the prognosis of PCs as well as
sarcomatoid carcinomas being worse than that of non-small cell
carcinomas (2,3). The epithelial component of PC shows
the same histology as poorly differentiated non-small cell
carcinoma of the lung (1).
Przygodzki et al (4),
however, showed that mutation frequencies and patterns for p53 and
K-ras were different between the epithelial components of
PCs and non-small cell carcinomas, indicating that epithelial
components of PCs are distinct from poorly differentiated non-small
cell carcinomas. However, the differences between an epithelial
component of PC and a corresponding poorly differentiated non-small
cell carcinoma have yet to be clarified.
Cell adhesion molecules are expressed in various
types of cancer and their altered expression is associated with the
dedifferentiation, invasion and metastasis of cancer cells
(5–8). E-cadherin forms a calcium-dependent
cell-cell adhesion complex together with β-catenin which binds to
the intracellular domain of E-cadherin as well as actin filaments,
connecting this adhesion complex to the cell cytoskeleton (5–8). The
E-cadherin-β-catenin complex is essential for the formation of
stable cell-cell adhesion and its reduced expression has been shown
to be associated with dedifferentiation, metastasis and poor
prognosis in various types of cancer including lung cancer
(5–8). On the other hand, the up-regulated
expression of N-cadherin, another calcium-dependent adhesion
molecule, has been shown to be associated with the invasive and
metastatic potential of cancers (9–12). It
has been shown that the overexpression of N-cadherin increases the
migration, invasion and metastasis of breast cancer cells through
the N-cadherin-mediated interaction of cancer to stromal cells
(10).
Cancer cells of an epithelial cell phenotype often
change into fibroblast-like cells expressing a mesenchymal cell
phenotype. This phenomenon, known as epithelial-mesencymal
transition, promotes the invasion and metastasis of cancer cells
(13–15). Transforming growth factor (TGF)-β is
one of the epithelial-mesencymal transition-inducing factors
(14,16,17).
TGF-β has been reported to be associated with poor prognosis of
patients with certain types of cancer including lung cancer
(18–22).
To elucidate the differences between an epithelial
component of PC and a corresponding poorly differentiated non-small
cell carcinoma, the expression of the above-mentioned molecules was
investigated immunohistochemically in poorly differentiated
adenocarcinomas of the lung and PCs with an epithelial component
exhibiting the same histology as poorly differentiated
adenocarcinoma.
Materials and methods
Subjects
A total of 14 PC specimens and 14 specimens of
poorly differentiated adenocarcinomas were used for this study. The
specimens were obtained from lung tissues resected at the Osaka
Prefectural Medical Center for Respiratory and Allergic Diseases.
Written consent was obtained from each patient prior to the
operation and the anonymous usage of tissue samples for
pathological studies was permitted. This study was approved by the
Ethics Committee of Osaka Prefectural Medical Center for
Respiratory and Allergic Diseases. The PC specimens contained
epithelial and sarcomatous components; the epithelial components
showed a histology of poorly differentiated adenocarcinoma. Poorly
differentiated adenocarcinomas 1, 6, 1, 5 and 1 were at pStages Ia,
Ib, IIa, IIb and IV, respectively.
Immunohistochemistry
Details of the primary antibodies used and their
dilutions are shown in Table I.
Immunohistochemical staining was performed using formalin-fixed,
paraffin-embedded tissue sections with an EnVision detection system
(EnVision+; Dakocytomation, Glostrup, Denmark). The
sections were deparaffinized and rehydrated, and endogenous
peroxidase activity was blocked using 0.03%
H2O2. The tissue sections were then incubated
with the primary antibody (Table
I). After washing with TBS (0.01 M Tris buffered saline, pH
7.4), the sections were incubated with the peroxidase-labeled
polymer from an EnVision+ system (Dakocytomation) and
the color reactions were obtained with 3,3′-diaminobenzidine
(Dakocytomation). For the antigen retrieval of E-cadherin,
β-catenin, N-cadherin and TGF-β, the sections were subjected to
microwave treatment for 15 min with 0.05 M Tris buffer (pH 9.0).
For the antigen retrieval of cytokeratin, the sections were
incubated with 0.05% protease (Protease type XXIV) (Sigma, St.
Louis, MO, USA) at 37˚C for 30 min and then boiled twice for 5 min
each time in 0.01% citrate buffer (pH 6.0). The immunostain was
graded according to the proportion of positive cells (p), as
follows: (−), p≤5%; (1+), 5%<p≤30%; (2+), 30%<p≤70%; (3+),
p>70%.
 | Table IAntibodies used for the
immunohistochemistry analysis. |
Table I
Antibodies used for the
immunohistochemistry analysis.
| Antigen | Clone | Source | Dilution | Antigen
retrieval |
|---|
| Pankeratin | Polyclonal | Dakocytomation,
Glostrup, Denmark | 1:500 | Protease |
| EMA | E29 | Dakocytomation,
Glostrup, Denmark | 1:80 | None |
| CEA | Polyclonal | Dakocytomation,
Glostrup, Denmark | 1:1000 | None |
| E-cadherin | 36B5 | Novocastra, Newcastle
upon Tyne, UK | 1:50 | MW |
| β-catenin | 17C2 | Novocastra, Newcastle
upon Tyne, UK | 1:80 | MW |
| N-cadherin | IAR06 | Novocastra, Newcastle
upon Tyne, UK | 1:100 | MW |
| TGF-β | TGFB17 | Novocastra, Newcastle
upon Tyne, UK | 1:40 | MW |
Statistical analysis
Immunohistochemical positive staining was analyzed
using the λ2 test using Stat Mate III for Windows
software (ATMS, Tokyo, Japan). Significance was defined as
p<0.05.
Results
Clinical findings
Table II shows a
clinical summary of the PC patients. The patients were male,
ranging in age from 41 to 76 years (mean 62.6). PCs 1, 9, 3 and 1
were at pStages Ib, IIb, IIIa and IV, respectively. The patients
underwent lobectomy, with 3 and 4 patients receiving radiation
therapy and chemotherapy, respectively, in addition to the
lobectomy. Of the 14 patients, 11 succumbed to the disease during
the follow-up period, 10 patients succumbed within 11 months after
the lobectomy regardless of pStage and 1 patient succumbed 11 years
after the lobectomy.
 | Table IIClinical findings of 14 patients with
pleomorphic carcinomas. |
Table II
Clinical findings of 14 patients with
pleomorphic carcinomas.
| Patient | Age (years) | Gender | Location | Size (mm) | pStage | Treatment | Histology | E/S percentage | Follow-up |
|---|
|
|---|
| E | S |
|---|
| 1 | 74 | M | LLL | 47×63×70 | IIIa | Lob.+Rad. | PDA | Spindle | 20/60 | 11 mo.dead |
| 2 | 71 | M | RUL | 42×23 | IV | Lob. | PDA | Spindle | 80/20 | 5 mo.dead |
| 3 | 58 | M | RML | 90×76 | IIb | Lob. | PDA | Spindle+giant | 40/60 | 3 mo.alive |
| 4 | 52 | M | LUL | 32×30 | IIIa | Lob.+Che. | PDA | Spindle | 80/20 | 11 mo.dead |
| 5 | 70 | M | RLL | 45×23 | Ib | Lob. | PDA | Spindle+giant | 20/80 | 2 mo.dead |
| 6 | 75 | M | RUL | 60×50 | IIb | Lob. | PDA | Spindle+giant | 10/90 | 6 mo.dead |
| 7 | 68 | M | LUL | 80 | IIb | Lob.+Che. | PDA | Giant | 10/90 | 3 mo.dead |
| 8 | 76 | M | LUL | 40×30×35 | IIIa | Lob.+Rad. | PDA | Giant | 10/90 | 11 yr.dead |
| 9 | 67 | M | RUL | 45×30×48 | IIb | Lob. | PDA | Spindle | 20/80 | 5 mo.dead |
| 10 | 69 | M | LLL | 50×30 | IIb | Lob. | PDA | Spindle+giant | 10/90 | 3 mo.alive |
| 11 | 44 | M | LUL | 72×65×70 | IIb | Lob.+Rad. | PDA | Spindle+giant | 20/80 | 9 mo.dead |
| 12 | 48 | M | LUL | 60×55×80 | IIb | Lob.+Che | PDA | Spindle+giant | 10/90 | 7 mo.alive |
| 13 | 64 | M | LUL | 72×46 | IIb | Lob. | PDA | Spindle+giant | 20/80 | 6 mo.dead |
| 14 | 41 | M | LUL | 35×25×30 | IIb | Lob.+Che. | PDA | Giant | 20/80 | 6 mo.dead |
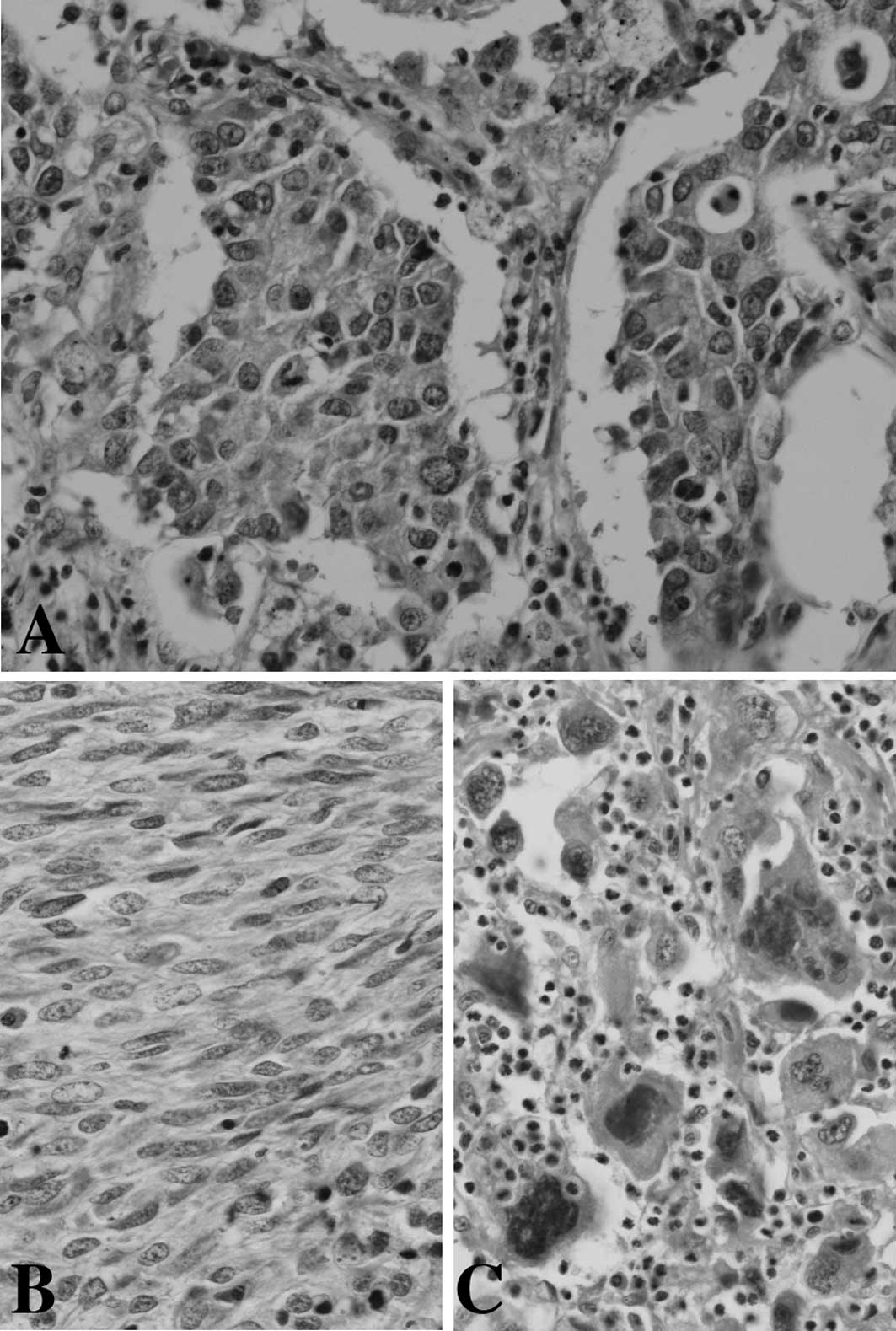
Histological findings
The histology of the PCs is shown in Table II. Epithelial components of the PCs
consisted of poorly differentiated adenocarcinoma (Fig. 1A). Sarcomatous components of 4, 3
and 7 PCs contained spindle tumor cells only (Fig. 1B), giant tumor cells only (Fig. 1C) and spindle and giant tumor cells,
respectively.
Immunohistochemical findings
Table III shows
the expression of epithelial markers such as cytokeratin, EMA and
CEA, as well as a mesenchymal marker, vimentin, in PCs and poorly
differentiated adenocarcinomas. Cytokeratin, EMA and CEA were
expressed in epithelial components of 12, 11 and 7 of 14 PCs,
respectively. The epithelial components of the PCs expressed
cytokeratin or EMA. The expression of vimentin was found in the
epithelial component of 1 of 14 PCs. Cytokeratin, EMA and CEA were
expressed in sarcomatous components of 10, 10 and 4 of 14 PCs.
Sarcomatous components of 12 of 14 PCs expressed cytokeration or
EMA. Sarcomatous components of PCs except one PC expressed
cytokeration, EMA or CEA. Cytokeratin, EMA and CEA were expressed
in 14, 13 and 12 of 14 poorly differentiated adenocarcinomas.
Vimentin was not expressed in all poorly differentiated
adenocarcinomas. The expression of adhesion molecules and TGF-β in
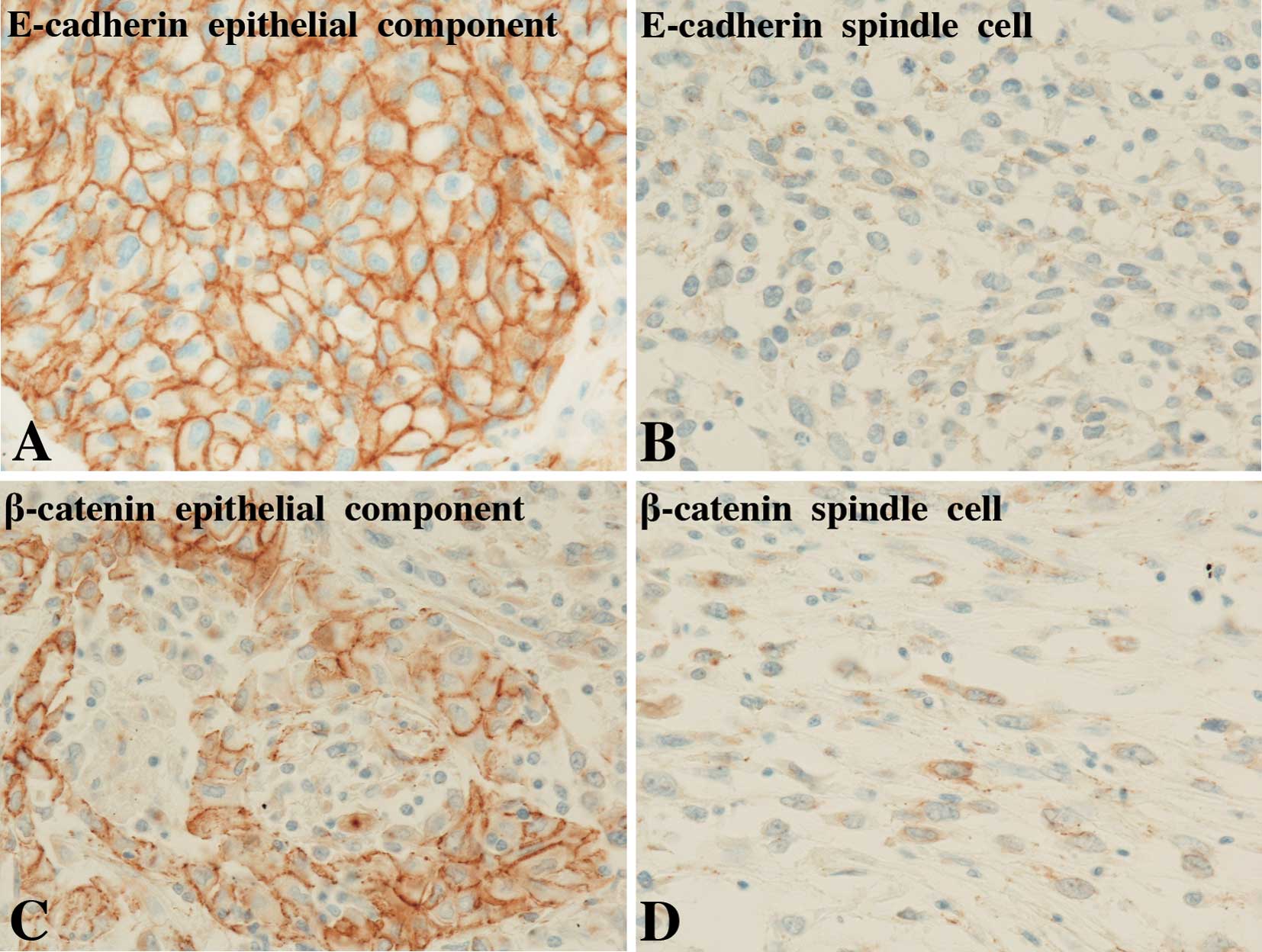
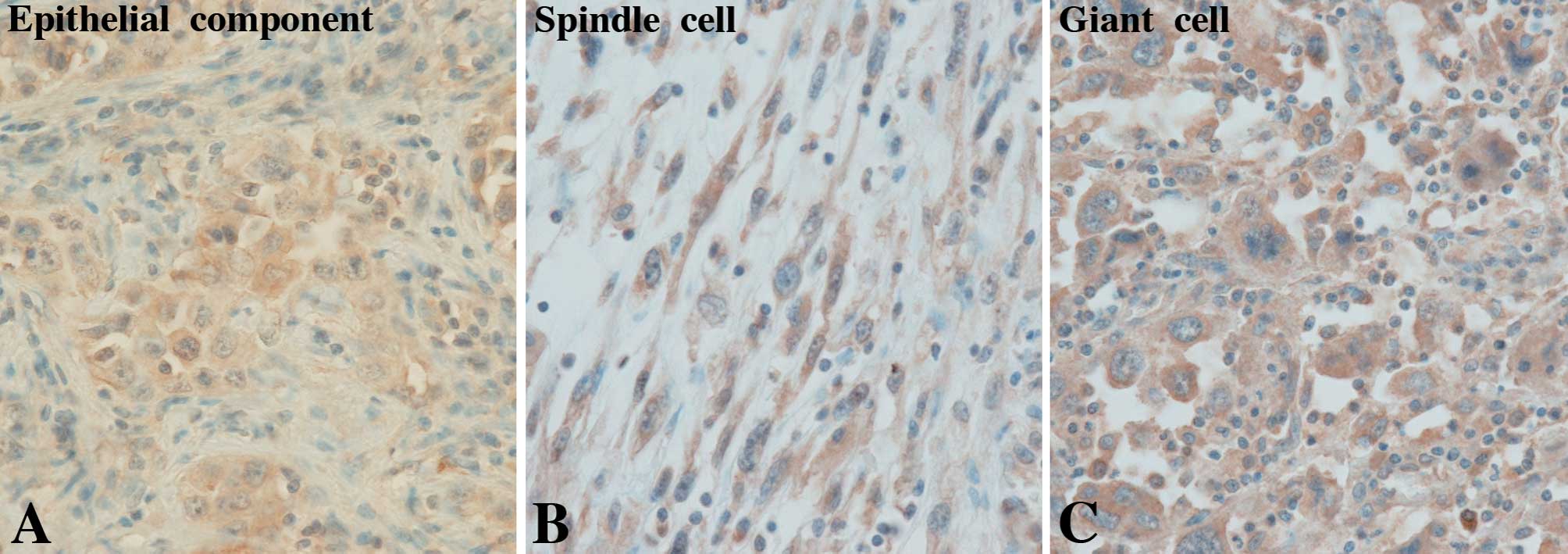
PCs and poorly differentiated adenocarcinomas is shown in Table IV. Positive staining of E-cadherin,
β-catenin and N-cadherin was found on the membrane of tumor cells
(Fig. 2), whereas TGF-β was
positively stained in the cytoplasm of tumor cells (Fig. 3). A sarcomatous component of one PC
only showed both membranous and nuclear staining of β-catenin.
Table V shows the comparison of
incidences of the expression of adhesion molecules and TGF-β at the
2+ and 3+ staining grades among epithelial and sarcomataous
components of PCs and poorly differentiated adenocarcinomas. The
incidences of the expression of E-cadherin and β-catenin at the 2+
and 3+ staining grade are significantly higher in poorly
differentiated adenocarcinomas than in epithelial or sarcomatous
components of PCs. The incidence of the expression of TGF-β at the
2+ and 3+ staining grades was significantly lower in poorly
differentiated adenocarcinomas than in epithelial components of
PCs. No significant difference in the expression of E-cadherin,
β-catenin and TGF-β at the 2+ and 3+ staining grades was found
between sarcomatous and epithelial components of PCs. No
significant difference was noted in an incidence of the expression
of N-cadherin at the 2+ and 3+ staining grades among poorly
differentiated adenocarcinomas and epithelial and sarcomatous
components of PCs.
 | Table IIIExpression of epithelial markers and
vimentin in pleomorphic carcinomas and poorly differentiated
adenocarcinomas of the lung. |
Table III
Expression of epithelial markers and
vimentin in pleomorphic carcinomas and poorly differentiated
adenocarcinomas of the lung.
| Cytokeratin | EMA | CEA | Vimentin |
|---|
|
|
|
|
|
|---|
| Case | E | S | E | S | E | S | E | S |
|---|
| Pleomoprhic
carcinoma |
| 1 | ( − ) | ( − ) | (3+) | (3+) | (3+) | ( − ) | (2+) | (3+) |
| 2 | (3+) | (3+) | (3+) | (1+) | ( − ) | ( − ) | ( − ) | (2+) |
| 3 | (3+) | ( − ) | ( − ) | ( − ) | ( − ) | ( − ) | ( − ) | (3+) |
| 4 | (3+) | (3+) | (3+) | (3+) | (3+) | ( − ) | ( − ) | (3+) |
| 5 | (3+) | (3+) | ( − ) | ( − ) | (3+) | (2+) | ( − ) | (3+) |
| 6 | (3+) | (3+) | (3+) | ( − ) | (3+) | (2+) | ( − ) | (3+) |
| 7 | (3+) | (2+) | (3+) | (3+) | ( − ) | ( − ) | ( − ) | (3+) |
| 8 | (3+) | ( − ) | (3+) | (2+) | (1+) | ( − ) | ( − ) | (2+) |
| 9 | (3+) | ( − ) | ( − ) | ( − ) | (2+) | (1+) | ( − ) | (3+) |
| 10 | (3+) | (3+) | (3+) | (3+) | ( − ) | ( − ) | ( − ) | (3+) |
| 11 | (3+) | (2+) | (3+) | (2+) | ( − ) | ( − ) | ( − ) | (3+) |
| 12 | (3+) | (2+) | (3+) | (2+) | ( − ) | ( − ) | ( − ) | (3+) |
| 13 | (3+) | (3+) | (3+) | (3+) | (3+) | (2+) | ( − ) | (3+) |
| 14 | ( − ) | (3+) | (3+) | (3+) | ( − ) | ( − ) | ( − ) | ( − ) |
| Poorly
differentiated adenocarcinoma |
| 1 | (1+) | | (3+) | | (1+) | | ( − ) | |
| 2 | (3+) | | (3+) | | (3+) | | ( − ) | |
| 3 | (3+) | | (3+) | | (3+) | | ( − ) | |
| 4 | (3+) | | (3+) | | (2+) | | ( − ) | |
| 5 | (3+) | | (3+) | | (2+) | | ( − ) | |
| 6 | (3+) | | (3+) | | (1+) | | ( − ) | |
| 7 | (3+) | | (3+) | | ( − ) | | ( − ) | |
| 8 | (3+) | | (3+) | | (1+) | | ( − ) | |
| 9 | (3+) | | ( − ) | | ( − ) | | ( − ) | |
| 10 | (3+) | | (3+) | | (3+) | | ( − ) | |
| 11 | (3+) | | (3+) | | (2+) | | ( − ) | |
| 12 | (3+) | | (3+) | | (3+) | | ( − ) | |
| 13 | (3+) | | (3+) | | (1+) | | ( − ) | |
| 14 | (3+) | | (3+) | | (3+) | | ( − ) | |
 | Table IVExpression of adhesion molecules and
TGF-β in pleomorphic carcinomas and poorly-differentiated
adenocarcinomas of the lung. |
Table IV
Expression of adhesion molecules and
TGF-β in pleomorphic carcinomas and poorly-differentiated
adenocarcinomas of the lung.
| E-cadherin | β-catenin | N-cadherin | TGF-β |
|---|
|
|
|
|
|
|---|
| Case | E | S | E | S | E | S | E | S |
|---|
| Pleomorphic
carcinoma |
| 1 | ( − ) | ( − ) | (3+) | ( − ) | ( − ) | ( − ) | (3+) | (2+) |
| 2 | ( − ) | ( − ) | ( − ) | ( − ) | ( − ) | ( − ) | (2+) | ( + ) |
| 3 | ( − ) | ( − ) | (3+) | (1+) | (2+) | (1+) | ( − ) | ( − ) |
| 4 | (2+) | (1+) | ( − ) | ( − ) | ( − ) | ( − ) | (3+) | (3+) |
| 5 | (3+) | (1+) | (3+) | (1+) | ( − ) | (2+) | (2+) | (2+) |
| 6 | ( − ) | ( − ) | ( − ) | (1+)a | ( − ) | (1+) | ( − ) | ( − ) |
| 7 | ( − ) | ( − ) | ( − ) | ( − ) | ( − ) | ( − ) | (2+) | (2+) |
| 8 | (2+) | ( − ) | ( − ) | ( − ) | ( − ) | ( − ) | (3+) | (1+) |
| 9 | ( − ) | ( − ) | ( − ) | ( − ) | (3+) | (3+) | ( − ) | (1+) |
| 10 | (1+) | ( − ) | ( − ) | ( − ) | (2+) | (2+) | ( − ) | ( − ) |
| 11 | ( − ) | ( − ) | ( − ) | ( − ) | ( − ) | ( − ) | (2+) | ( − ) |
| 12 | (1+) | ( − ) | ( − ) | ( − ) | ( − ) | ( − ) | (2+) | (2+) |
| 13 | (3+) | ( − ) | ( − ) | ( − ) | ( − ) | ( − ) | (1+) | ( − ) |
| 14 | ( − ) | ( − ) | (3+) | ( − ) | (1+) | ( − ) | (3+) | (2+) |
| Poorly
differentiated adenocarcinoma |
| 1 | (3+) | | (3+) | | (2+) | | (2+) | |
| 2 | (3+) | | (3+) | | ( − ) | | ( − ) | |
| 3 | (3+) | | (3+) | | ( − ) | | ( − ) | |
| 4 | (2+) | | (3+) | | ( − ) | | ( − ) | |
| 5 | (3+) | | (2+) | | ( − ) | | ( − ) | |
| 6 | (3+) | | (3+) | | ( − ) | | (2+) | |
| 7 | ( − ) | | (3+) | | ( − ) | | (1+) | |
| 8 | (3+) | | (2+) | | ( − ) | | (1+) | |
| 9 | (3+) | | (3+) | | ( − ) | | ( − ) | |
| 10 | (3+) | | (3+) | | ( − ) | | ( − ) | |
| 11 | (3+) | | (3+) | | ( − ) | | ( − ) | |
| 12 | (3+) | | (3+) | | ( − ) | | ( − ) | |
| 13 | (3+) | | (3+) | | ( − ) | | ( − ) | |
| 14 | (3+) | | (3+) | | ( − ) | | (1+) | |
 | Table VExpression of adhesion molecules and
TGF-β at 2+ and 3+ staining grades in pleomorphic carcinomas and
poorly differerentiated adenocarcinomas of the lung. |
Table V
Expression of adhesion molecules and
TGF-β at 2+ and 3+ staining grades in pleomorphic carcinomas and
poorly differerentiated adenocarcinomas of the lung.
Discussion
The follow-up data of the PC patients showed that 10
of 11 patients who died of PC succumbed to the disease within a
year after the lobectomy and one patient succumbed 11 years after
the lobectomy. The follow-up data confirmed poor prognosis of
patients with PCs, as previously reported (2,3).
A sarcomatous component of PC is considered to
represent the sarcomatous change of cancer cells in an epithelial
component (1). This change is
supported by the immunohistochemical and ultrastructural
identification of epithelial features in a sarcomatous component
and by identical gene mutations in epithelial and sarcomatous
components (1,23). The immunohistochemical results of
the present study also confirmed the expression of epithelial
markers, such as cytokeratin, EMA and CEA, in sarcomatous
components in 13 of 14 PCs, thus supporting the epithelial origin
of tumor cells in a sarcomatous component of PC.
It has been reported that the N-cadherin expression
is uncommon in adenocarcinomas of the lung and is not a prognostic
factor thereof (24,25). In the present study, incidences of
N-cadherin expression were also low in poorly differentiated
adenocarcinomas, as well as in epithelial and sarcomatous
components of PCs. Additionally, no significant difference was
noted in the N-cadherin expression among the adenocarcinomas and
components of PC. These results suggest that the aggressiveness of
PCs is not associated with N-cadherin expression.
The E-cadherin and β-catenin expression was reduced,
while the TGF-β expression was increased in epithelial and
sarcomatous components of PCs compared to poorly differentiated
adenocarcimonas, although the epithelial components of PCs showed
the same histology as poorly differentiated adenocarcinomas.
Numerous studies showed that the loss or reduction of the
E-cadherin-β-catenin complex is associated with metastasis and a
poor prognosis in non-small cell carcinomas of the lung (5,6,26,27).
Moreover, the TGF-β expression was reported to be associated with
poor prognosis of patients with adenocarcinomas of the lung
(18,19). Therefore, the reduced expression of
E-cadherin and β-catenin and the increased expression of TGF-β in
PCs appear to be associated with the aggressiveness of PCs.
Furthermore, although the histology is the same, the different
expression of the three molecules between epithelial components of
PCs and poorly differentiated adenocarcinomas appears to reflect
their different nature. On the other hand, TGF-β has been reported
to be an inducing factor of the epithelial-mesenchymal transition
in which cancer cells lose the epithelial phenotype and acquire the
mesenchymal phenotype, thereby becoming more aggressive (13–17).
Therefore, TGF-β may play a role in the generation of spindle or
giant cells in a sarcomatous component from cancer cells in an
epithelial component in PCs.
Przygodzki et al (4) showed that mutation frequencies and
patterns for p53 and K-ras were different between the
epithelial components of PCs and non-small cell carcinomas,
indicating that PC is distinct from non-small cell carcinoma of the
lung. In agreement with this study, the present results showed that
the expression of E-cadherin, β-catenin and TGF-β was different
between poorly differentiated adenocarcinomas and the epithelial
components of PCs showing the same histology as
poorly-differentiated adenocarcinoma. It is generally accepted that
a sarcomatous component of PC develops from an epithelial component
of PC (1,23). However, PC is not a tumor of
non-small cell carcinoma of the lung with a sarcomatous change.
References
|
1
|
Corrin B, Wick MR, Chang YL, et al:
Sarcomatoid carcinoma. Pathology and Genetics, Tumors of the Lung,
Pleura, Thymus and Heart, World Health Organization Classification
of Tumours. Travis WD, Brambilla E, Müller-Hermelink HK and Harris
CC: IARC Press; Lyon: pp. 53–58. 2004
|
|
2
|
Wick MR, Ritter JH and Humphrey PA:
Sarcomatoid carcinomas of the lung: a clinicopathologic review. Am
J Clin Pathol. 108:40–53. 1997.PubMed/NCBI
|
|
3
|
Mochizuki T, Ishii G, Nagai K, et al:
Pleomorphic carcinoma of the lung. Clinicopathologic
characteristics of 70 cases. Am J Surg Pathol. 32:1727–1735. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Przygodzki RM, Koss MN, Moran CA, et al:
Pleomorphic (giant and spindle cell) carcinoma is genetically
distinct from adenocarcinoma and squamous cell carcinoma by K-ras-2
and p53 analysis. Am J Clin Pathol. 106:487–492. 1996.PubMed/NCBI
|
|
5
|
Bremnes RM, Veve R, Hirsch FR and Franklin
WA: The E-cadherin cell-cell adhesion complex and lung cancer
invasion, metastasis, and prognosis. Lung Cancer. 36:115–124. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Charalabopoulos K, Gogali A, Kostoula OK
and Constantopoulos SH: Cadherin superfamily of adhesion molecules
in primary lung cancer. Exp Oncol. 26:256–260. 2004.PubMed/NCBI
|
|
7
|
Jeanes A, Gottardi CJ and Yap AS:
Cadherins and cancer: how does cadherin dysfunction promote tumor
progression? Oncogene. 27:6920–6929. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Makrilla N, Kollias A, Manolopoulos L and
Syrigos K: Cell adhesion molecules: role and clinical significance
in cancer. Cancer Invest. 27:1023–1037. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nieman MT, Prudoff RS, Johnson KR and
Wheelock MJ: N-cadherin promotes motility in human breast cancer
cells regardless of their E-cadherin expression. J Cell Biol.
147:631–644. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hazan RB, Phillips GR, Qiao RF, Norton L
and Aaronson SA: Exogenous expression of N-cadherin in breast
cancer cells induces cell migration, invasion, and metastasis. J
Cell Biol. 148:779–790. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li G, Satyamoorthy K and Herlyn M:
N-cadherin-mediated intercellular interactions promote survival and
migration of melanoma cells. Cancer Res. 61:3819–3825.
2001.PubMed/NCBI
|
|
12
|
Cavallaro U: N-cadherin as an invasion
promoter: a novel target for antitumor therapy? Curr Opin Investig
Drugs. 5:1274–1278. 2004.PubMed/NCBI
|
|
13
|
Voulgari A and Pintzas A:
Epithelial-mesenchymal transition in cancer metastasis: mechanisms,
markers and strategies to overcome drug resistance in the clinic.
Biochim Biophys Acta. 1976:75–90. 2009.PubMed/NCBI
|
|
14
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iwatsuki M, Mimori K, Yokobori T, et al:
Epithelial-mesenchymal transition in cancer development and its
clinical significance. Cancer Sci. 101:293–299. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rees JRE, Onwuegbusi BA, Save VE, Alderson
D and Fitzgerald RC: In vivo and in vitro evidence for transforming
growth factor-β1-mediated epithelial to mesenchymal transition in
esophageal adenocarcinoma. Cancer Res. 66:9583–9590. 2006.
|
|
17
|
Kaimori A, Potter J, Kaimori JY, Wang C,
Mezey E and Koteish A: Transforming growth factor-β1 induces an
epithelial-to-mesenchymal transition state in mouse hepatocytes in
vitro. J Biol Chem. 282:22089–22101. 2007.
|
|
18
|
Takanami I, Imamura T, Hashizume T,
Kikuchi K, Yamamoto Y and Kodaira S: Transforming growth factor-β1
as a prognostic factor in pulmonary adenocarcinoma. J Clin Pathol.
47:1098–1100. 1994.
|
|
19
|
Takanami I, Imamura T, Hashizume T,
Kikuchi K, Yamamoto Y, Yamamoto T and Kodaira S: Expression of
PDGF, IGF-II and TGF-β1 in pulmonary adnocarcinoma. Pathol Res
Pract. 192:1113–1120. 1996.
|
|
20
|
Robson H, Anderson E, James RD and
Schofield RF: Transforming growth factor β1 expression in human
colorectal tumours: an independent prognostic marker in a subgroup
of poor prognosis patients. Br J Cancer. 74:753–758. 1996.
|
|
21
|
Saito H, Tsujitani S, Oka S, Kondo A,
Ikeguchi M, Maeta M and Kaibara N: The expression of transforming
growth factor-β1 is significantly correlated with the expression of
vascular endothelial growth factor and poor prognosis of patients
with advanced gastric carcinoma. Cancer. 86:1455–1462. 1999.
|
|
22
|
Desruisseau S, Palmari J, Giusti C, Romain
S, Martin PM and Berthois Y: Determination of TGF β1 protein level
in human primary breast cancers and its relationship with survival.
Br J Cancer. 94:239–246. 2006.
|
|
23
|
Pelosi G, Scarpa A, Manzotti M, et al:
K-ras gene mutational analysis supports a monoclonal origin of
biphasic pleomorphic carcinoma of the lung. Mod Pathol. 17:538–546.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakashima T, Huang C, Liu D, et al:
Neural-cadherin expression associated with angiogenesis in
non-small-cell lung cancer patients. Br J Cancer. 88:1727–1733.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zynger DL, Dimov ND, Ho LC, Laskin WB and
Yeldandi AV: Differential expression of neural-cadherin in
pulmonary epithelial tumours. Histopathology. 52:348–354. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kase S, Sugio K, Yamazaki K, Okamoto T,
Yano T and Sugimachi K: Expression of E-cadherin and β-catenin in
human non-small cell lung cancer and the clinical significance.
Clin Cancer Res. 6:4789–4796. 2000.
|
|
27
|
Nozawa N, Hashimoto S, Nakashima Y, et al:
Immunohistochemical α- and β-catenin and E-cadherin expression and
their clinicopathological significance in human lung
adenocarcinoma. Pathol Res Pract. 202:639–650. 2006.
|