Introduction
Insulin-like growth factors (IGFs) are polypeptides
with a high sequence, similar to insulin. IGFs are part of a
complex system that cells use to communicate with their physiologic
environment. This complex system (often referred to as the IGF
‘axis’) consists of two cell-surface receptors (IGF1R and IGF2R),
two ligands (IGF-1 and IGF-2), a family of six high-affinity
IGF-binding proteins (IGFBP-1 to -6), as well as associated
IGFBP-degrading enzymes, referred to collectively as proteases.
It has become increasingly clear that the growth
hormone (GH)/IGF-1 axis plays a fundamental and obligatory role in
regulating normal somatic growth throughout fetal and childhood
development. Over the past two decades considerable evidence has
accumulated showing that these growth factors play an important
role in maintaining and supporting the progression of neoplastic
growth. A number of epidemiological reports showed that it may also
be an important determinant of cancer incidence (1).
The risk of cancer is higher among people with
elevated concentrations of IGF-1, and is lower among those with
high concentrations of IGFBP-3 (the main binding protein). The
associations are similar when people whose blood samples were taken
shortly before diagnosis are excluded from analyses, suggesting
that the observed correlations are not due to the release of the
growth factor by preclinical cancers (2).
In adults, numerous studies demonstrated that higher
IGF-1 levels are associated with a significantly increased risk of
developing a number of the most common types of cancer, such as
colon, breast, prostate and possibly lung (3). However, only a few studies have
investigated the association between IGF-1 levels and childhood
cancer risk. Higher levels of IGF-1 were shown to increase the risk
of leukemia in children (4).
Additionally, a role has been demonstrated for IGF-1 and other
components of the IGF system in the pathogenesis and progression of
other childhood malignancies (5).
IGF-1 exerts powerful effects on each of the key
stages of cancer development and behavior: cellular proliferation
and apoptosis, angiogenesis and metastasis, and more recently,
development of resistance to chemotherapeutic agents (6,7).
Given the mounting evidence of the risk of cancer,
caution should be exercised in the exogenous use of either IGF-1 or
substances that increase its concentration (8).
This study aimed to compare the IGF-1 serum level in
children with de novo malignancies to healthy children, and
to assess its relationship with cancer type, stage, metastasis and
different disease characteristics.
Subjects and methods
This study was conducted in the Pediatric Hematology
and Oncology Unit of Zagazig University Hospital and the Department
of Medical Biochemistry, Faculty of Medicine, Zagazig University,
Egypt, during the period from January 2009 to December 2009.
Subjects
The study included two groups. Group I (patient
group) consisted of 50 children with de novo malignancies,
whether hematological malignancies or solid tumors. Group II
(control group) consisted of 50 age- and gender-matched healthy
children as a control group.
Methods
Patients were subjected to a routine work-up for
their cancers according to our local standards. Estimation of the
serum level of IGF-1 in the two groups was carried out using the
DRG IGF-1 600 ELISA kit on the Sunrise Remote/Touch ELISA
analyzer.
Specimens
A total of 3 ml of blood was collected by
venipuncture and allowed to clot. The serum was then separated by
centrifugation at room temperature. Serum samples were frozen at
−20°C until the time of assay.
Principle of the test
The DRG IGF-1 600 ELISA kit is a solid phase
enzyme-linked immunosorbent assay (ELISA), based on the principle
of competitive binding. Patient samples, standards and controls
were acidified and neutralized prior to the assay procedure.
Microtiter wells were coated with a monoclonal antibody directed
towards an antigenic site on the IGF-1 molecule. The pre-treated
sample was incubated at room temperature with conjugate
(biotinylated IGF-1). The wells were washed and incubated with an
enzyme complex (streptavidin-HRP-complex). After addition of the
substrate solution, the intensity of the developed color was
reverse proportional to the concentration of IGF-1 in the patient
sample.
Statement of ethics
The present study was conducted in accordance with
the ethical standards of the Helsinki Declaration of 1964, as
revised in 2000, and was approved by our local ethics committee.
Informed consent was obtained from the study participants or their
guardians.
Statistical analysis
Data were assessed, entered and analyzed using SPSS
version 15. Data were expressed as the mean ± standard deviation
for quantitative variables, number and percentage for qualitative
variables. ANOVA (F test), the Student's t-test, Chi-square test,
Kruskall Wallis (K) test and the correlation coefficient (r) were
used when appropriate. P<0.05 was considered to indicate a
significant difference.
Results
The study was carried out on 100 children: 50
children with de novo malignancies and 50 healthy children
of matched age and gender as a control group. The age and gender of
the two groups are shown in Table
I.
 | Table IAge and gender of the patients and
controls. |
Table I
Age and gender of the patients and
controls.
| Patients n=50 | Controls n=50 | Value of the test of
significance | P-value |
|---|
| Age (years) |
| Mean ± SD | 2.5±4.9 | 2.9±4.89 | t=0.25 | 0.82 |
| Range | 1–11 | 1–11 | | |
| Gender |
| M/F (no.) | 27/23 | 25/25 |
χ2=0.16 | 0.68 |
| M/F (%) | 54.0/46.0 | 50.0/50.0 | | |
The patient group included 40 children with
hematological malignancies and 10 children with solid tumors. Acute
leukemia was the most common childhood cancer in our patients
accounting for 72.0% of all of the cancer types. Acute
lymphoblastic leukemia (ALL) accounted for approximately two thirds
of the children with acute leukemia. Lymphoma was the second most
common cancer type accounting for 8% of the cancer types. Solid
tumors collectively represented 20% of all of the cancers.
Neuroblastoma and rhabdomyosarcoma were the most common solid
tumors in our patient group. Table
II summarizes the different tumor types in our patient group
and their relative percentages.
 | Table IITumor type in the patient groups. |
Table II
Tumor type in the patient groups.
| Tumor type | No. | % |
|---|
| Hematological
malignancies | 40 | 80.0 |
| ALL | 23 | 46.0 |
| AML | 11 | 22.0 |
| Biphenotypic AL | 2 | 4.0 |
| Hodgkin's
lymphoma | 2 | 4.0 |
| Non-Hodgkin's
lymphoma | 2 | 4.0 |
| Solid tumors | 10 | 20.0 |
| Neuroblastoma | 3 | 6.0 |
|
Ganglioneuroblastoma | 1 | 2.0 |
|
Rhabdomyosarcoma | 3 | 6.0 |
| PNET | 1 | 2.0 |
| Wilms' tumor | 1 | 2.0 |
| Duodenal
adenocarcinoma | 1 | 2.0 |
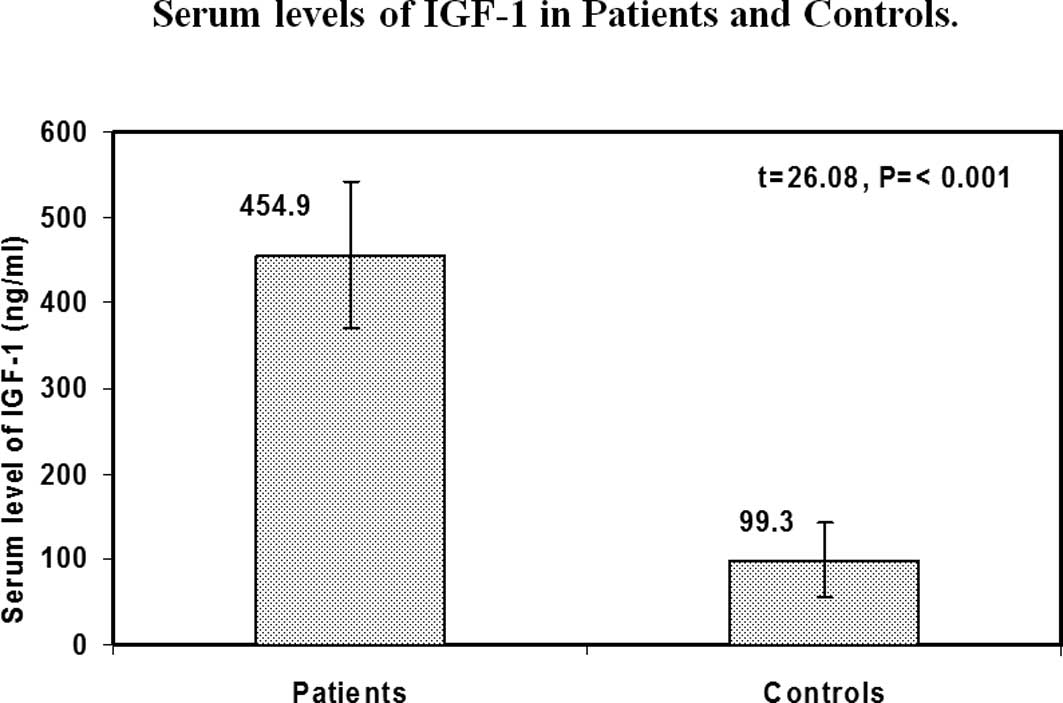
IGF-1 serum levels in the patients and
controls
Our results showed that the children with cancer had
significantly higher levels of IGF-1 than the healthy controls
(mean ± SD was 454.9±85.7 ng/ml for patients and 99.3±44.1 ng/ml
for controls (p<0.001) (Fig. 1).
Gender and age are significant factors affecting the serum level of
IGF-1. Subsequently, we divided our patients into different age and
gender groups and found that the cancer patients still had
significantly higher serum levels of IGF-1 than the healthy
controls of the same age and gender group (Tables III and IV).
 | Table IIIComparison between different age
groups of patients and controls with regard to serum levels of
IGF-1. |
Table III
Comparison between different age
groups of patients and controls with regard to serum levels of
IGF-1.
| Serum levels of IGF-1
(ng/ml) | | |
|---|
|
| | |
|---|
| Age group
(years) | Patients (mean ±
SD) (Range) | Controls (mean ±
SD) (Range) | t-value | P-value |
| <2 | 458.75±63.3
(401–539) | 92.8±47.7
(45–173) | 10.90 | <0.001 |
| 2–5 | 448.2±84.2
(285–605) | 97.0±41.5
(40–200) | 18.90 | <0.001 |
| 6–9 | 457.6±85.7
(299–632) | 112.3±52.4
(52–192) | 12.38 | <0.001 |
| 9–11 | 501±150.9
(331–619) | 119.2±32.9
(85–156) | 5.73 | <0.010 |
 | Table IVComparison between different gender
groups of patients and controls with regard to serum levels of
IGF-1. |
Table IV
Comparison between different gender
groups of patients and controls with regard to serum levels of
IGF-1.
| Serum levels of
IGF-1 (ng/ml) | | |
|---|
|
| | |
|---|
| Gender | Patients (mean ±
SD) (Range) | Controls (mean ±
SD) (Range) | t-value | P-value |
|---|
| Male | 457.4±91.0
(285–632) | 113.0±48.8
(45–200) | 16.80 | <0.001 |
| Female | 451.4±80.8
(299–619) | 92.2±37.5
(40–189) | 20.02 | <0.001 |
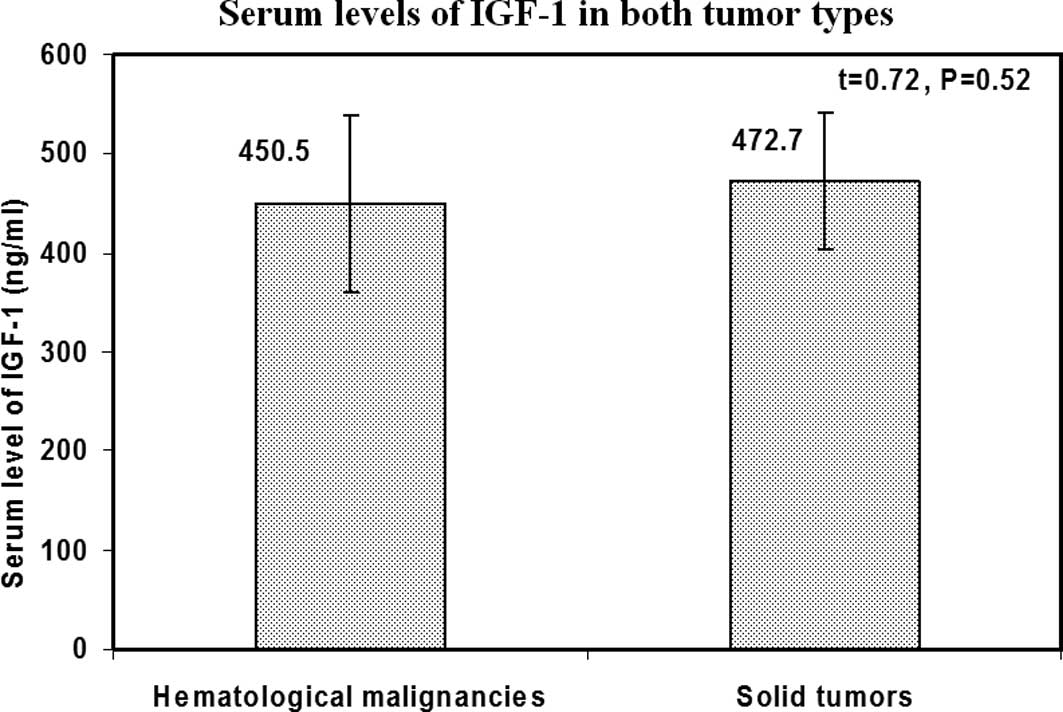
IGF-1 serum levels, tumor type, and
initial clinical and laboratory data
No statistically significant difference was noted
between patients with hematological malignancies and those with
solid tumors with regard to the serum levels of IGF-1 (P=0.52)
(Fig. 2). Additionally, no
relationship was found between IGF-1 levels and any of the initial
clinical and laboratory data including the overall risk of the
patients.
IGF-1 serum levels and different ALL
immunophenotypes
Although T-ALL patients had higher serum levels of
IGF-1 than those with other immunophenotypes, the difference was
not statistically significant (P=0.61).
IGF-1 serum levels and different
French-American-British (FAB) subtypes in ALL and acute myeloid
leukemia (AML)
No significant relationship was noted between the
serum levels of IGF-1 and FAB subtypes in the patients with acute
leukemia (P=0.18 and 0.45, respectively).
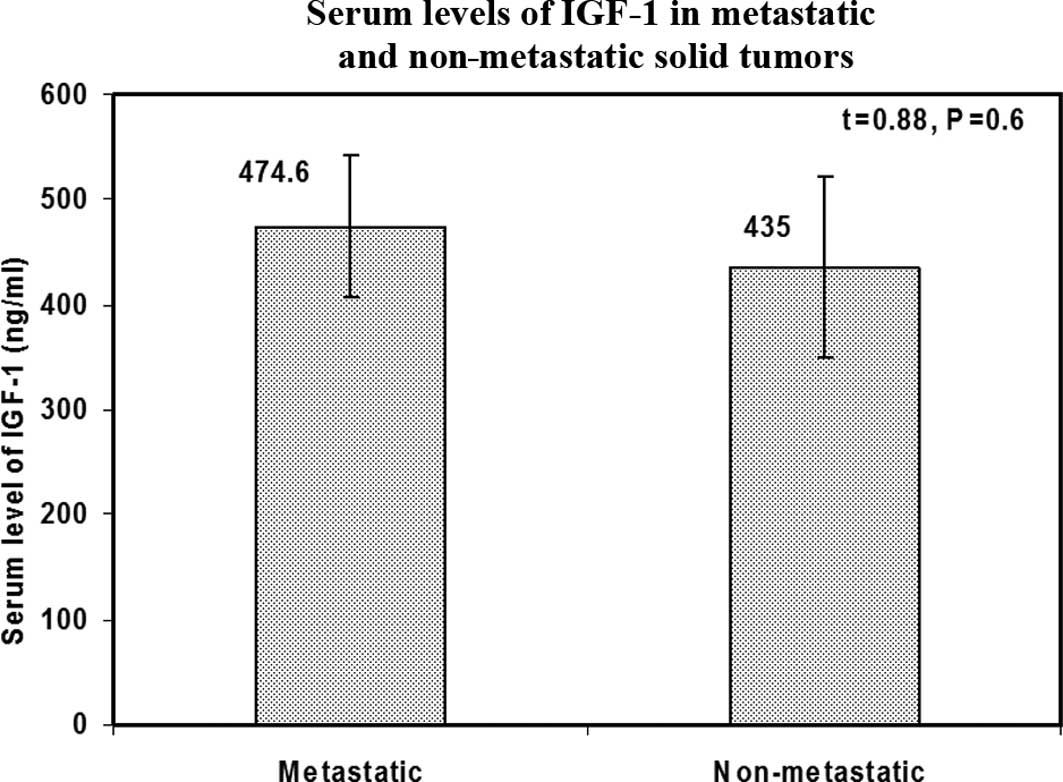
IGF-1 serum levels and metastasis in
solid tumors
Although patients with metastatic solid tumors had
higher serum levels of IGF-1 as compared to those without
metastasis, the difference was not statistically significant
(P=0.6) (Fig. 3).
IGF-1 serum levels and different stages
of solid tumors
No significant relationship was found between the
serum levels of IGF-1 and different stages of solid tumors
(P=0.23).
Discussion
Since its identification, there have been unresolved
concerns about the potential cancer-enhancing properties of IGF-1.
Circumstantial evidence in support stems from various sources:
in vitro studies, animal studies, epidemiological
observations within the general population and patients with growth
hormone (GH) excess and deficiency, as well as from the therapeutic
manipulation of GH and IGF-1 actions (9).
Considerable epidemiological data have suggested a
possible link between circulating GH and/or IGF-1 levels and the
development of a variety of different cancer types. Numerous
studies suggested that subjects with serum IGF-1 levels that are in
the higher percentiles of the normal range have a significantly
increased risk of developing a number of the most common types of
cancer, such as colon, breast, prostate and possibly lung (3). However, only a few studies have
investigated the correlation between IGF-1 levels and childhood
cancer risk.
Our results showed that children with cancer had
significantly higher levels of IGF-1 than healthy controls. Based
on the fact that serum levels of IGF-1 are affected by age and
gender, this relationship was examined in different age and gender
groups. We found that cancer patients showed significantly higher
serum levels of IGF-1 than healthy controls of the same age and
gender group.
The relationship between higher levels of IGF-1 and
cancer can be explained by the fact that IGF-1 is a potent
proliferative agent affecting almost every cell type and also a
powerful antiapoptotic agent affecting apoptotic responses to a
variety of agents of numerous cell types. These two effects result
in a state of hyperproliferation. Such an imbalance between cell
proliferation and death would favor, even slightly, the survival of
stem cells that had undergone early genetic ‘hits’. Thus, the pool
of damaged cells available for second and subsequent hits are
likely to increase (10).
Whether IGF-1 is a causal factor or simply a
surrogate measure of the malignant process remains unknown. A study
by Ma et al (2) showed an
association between colorectal cancer risk in men and elevated
plasma levels of IGF-1 by using plasma samples drawn over a long
period of time prior to the clinical appearance of the tumors.
Thus, the possibility that plasma levels were affected by the
disease process was minimized, thereby confirming the causal
relationship between IGF-1 and cancer.
Numerous studies have investigated the role of IGF-1
in hematological malignancies and solid tumors (5). However, no study thus far has compared
the levels of IGF-1 in the two tumor types.
In our study, no differences were noted between
hematological malignancies and solid tumors and between ALL and AML
patients with regard to the serum level of IGF-1, confirming that
IGF-1 levels are higher in cancer patients regardless of their
cancer type. Additionally, no association was found between IGF-1
levels and different FAB subtypes in ALL and AML patients. In ALL
patients, no relationship was found between IGF-1 levels and
different immunophenotypic subtypes.
High levels of IGF-1 have been shown to increase
proliferative stress on progenitor cells in bone marrow (4,11),
increasing the number of cell divisions and, in turn, the risk of
leukemia. Further evidence supports a role for IGF-1 in the
pathogenesis of leukemia: IGF-1 receptors were found to be
expressed on leukemic lymphoblasts; IGF-1 stimulated the growth of
leukemic cells in vitro (12); IGF-1 has been shown to protect
hematopoietic cells from apoptosis (13); and the administration of GH, the
effect of which is mediated through the IGF-1 system (14), has been reported to increase the
risk of childhood acute leukemia (15).
Accelerated fetal growth may be a risk factor for
childhood ALL, a tenet supported by evidence that is related to
increased levels of IGF-1. In a recent study, McLaughlin et
al (16) reported an increased
risk of ALL among children with birth weights greater than 3,500
g.
Rangel et al (17) also showed that the estimated risk
for certain types of cancer has been found to be statistically and
significantly higher with a birth weight of more than 4,000 g (The
estimated risk was 1.86 for leukemia, 1.99 for non-Hodgkin's
lymphoma and 4.76 for Wilms' tumor).
Contrary to our results, Petridou et al
(14) reported that there was no
significant association between IGF-1 and the likelihood of
childhood leukemia. However, these authors found that an increment
of 1 μg/ml of IGFBP-3 was associated with a substantial and
statistically significant reduction in childhood leukemia by 28%.
Since IGFBP-3 is essentially a binding protein, these findings
suggest that bioavailable IGF-1 plays an important role in the
etiology of childhood leukemia.
The role of IGF-1 and other components of the IGF
system in the pathogenesis and progression of other childhood
malignancies have been investigated (5). In lymphoma, IGF-1 has been shown to
stimulate the proliferation of T-cell lymphoma lines that have
phenotypic characteristics of thymic pre-T cells and to inhibit
cell differentiation, which may crucial in early lymphoma
tumorigenesis (18). Moreover,
IGF-1 causes a significant increase in the proliferation of
Burkitt's lymphoma cells, which may be blocked by antiserum against
IGF-1 (19).
IGF-2 plays a role as both an autocrine and a
paracrine growth factor in neuroblastoma (20,21).
High levels of IGF-1 have been shown to play an important role in
the pathogenesis of osteosarcoma (22). Northern blot analysis of tumor
biopsy specimens from patients with alveolar and embryonal
rhabdomyosarcoma showed high levels of IGF-2 mRNA expression,
suggesting the possibility that the upregulation of IGF-2 plays a
role in the unregulated growth of these tumors (23). The role of IGF-1R signaling in
Ewing's sarcoma family of tumors (ESFT), including primitive
neuroectodermal tumors, has been evaluated extensively. EFST cell
lines were shown to express IGF-1R and secrete IGF-1.
IGF-1R-blocking antibodies were shown to be successful in the
interruption of this autocrine loop (24,25).
Inactivation of WT1, a tumor-suppressor gene, has been shown to
up-regulate a number of components of the IGF system that
contribute to inappropriate proliferation and development of Wilms'
tumor (26–28).
A role for the IGF system in cancer metastasis has
recently been documented in various types of human cancer. Barozzi
et al (29) found that the
overexpression of IGF-2 was predictive of liver metastasis in
patients with colorectal cancer. Hakam et al (30) showed a stepwise increase in the
expression of IGF-1R during the progression from colonic adenomas
towards primary colorectal adenocarcinomas and metastases. In our
study, although the serum levels of IGF-1 were higher in metastatic
solid tumors than non-metastatic ones, the difference was
statistically non-significant.
In conclusion, the IGF-1 serum level is an important
indicator of risk for the most prevalent forms of childhood cancer
and may be used to both identify children at the highest risk for
these cancers and, aid in determining who may benefit most from
preventive strategies. Given the small number of children in our
study, studies with larger populations are required to confirm
these results.
References
|
1
|
Holly JM, Gunnell DJ and Smith DG: Growth
hormone, IGF-I and cancer. Less intervention to avoid cancer? More
intervention to prevent cancer? J Endocrinol. 162:321–330. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ma P, Pollak MN, Giovannucci E, Chan JM,
Tao Y, Hennekens CH and Stampfer MJ: Prospective study of
colorectal cancer risk in men and plasma levels of insulin-like
growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer
Inst. 91:620–625. 1999. View Article : Google Scholar
|
|
3
|
Burroughs KD, Dunn SE, Barrett JC and
Taylor JA: Insulin-like growth factor-I: a key regulator of human
cancer risk? J Natl Cancer Inst. 91:579–581. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ross JA, Perentesis JP, Robison LL and
Davies SM: Big babies and infant leukemia: a role for insulin-like
growth factor-1? Cancer Causes Control. 7:553–559. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim SY, Toretsky JA, Scher D and Helman
LJ: The role of IGF-1R in pediatric malignancies. Oncologist.
14:83–91. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Laban C, Bustin SA and Jenkins PJ: The
GH-IGF-I axis and breast cancer. Trends Endocrinol Metab. 14:28–34.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bustin SA and Jenkins PJ: The growth
hormone-insulin-like growth factor-I axis and colorectal cancer.
Trends Mol Med. 7:447–454. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Smith GD, Gunnell DJ and Holly JM: Cancer
and insulin-like growth factor-1: a potential mechanism linking the
environment with cancer risk. BMJ. 321:847–848. 2000.PubMed/NCBI
|
|
9
|
Jenkins PJ, Mukherjee A and Shalet SM:
Does growth hormone cause cancer? Clin Endocrinol. 64:115–121.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pollak MN, Schernhammer ES and Hankinson
SE: Insulin-like growth factors and neoplasia. Nat Rev Cancer.
4:505–518. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Albanes D and Winick M: Are cell number
and cell proliferation risk factors for cancer? J Natl Cancer Inst.
80:772–774. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sanders M, Sorba S and Dainiak N:
Insulin-like growth factors stimulate erythropoiesis in
serum-substituted umbilical cord blood cultures. Exp Hematol.
21:25–30. 1999.PubMed/NCBI
|
|
13
|
Williams GT and Smith CA: Molecular
regulation of apoptosis: genetic controls on cell death. Cell.
74:777–779. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Petridou E, Dessypris N, Spanos E,
Mantzoros C, Skalkidou A, Kalmanti M, Koliouskas D, Kosmidis H,
Panagiotou JP, Piperopoulou F, Tzortzatou F and Trichopoulos D:
Insulin-like growth factor-I and binding protein-3 in relation to
childhood leukemia. Int J Cancer. 80:494–496. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ritzen EM: Does growth hormone increase
the risk of malignancies? Horm Res. 39:99–101. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McLaughlin CC, Baptiste MS, Schymura MJ,
Nasca PC and Zdeb MS: Birth weight, maternal weight and childhood
leukemia. Br J Cancer. 94:1738–1744. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rangel M, Cypriano M, de Martino Lee ML,
Luisi FA, Petrilli AS, Strufaldi MW and Franco MD: Leukemia,
non-Hodgkin's lymphoma, and Wilms tumor in childhood: the role of
birth weight. Eur J Pediatr. 169:875–881. 2010.
|
|
18
|
Gjerset RA, Yeargin J, Volkman SK, Vila V,
Arya J and Haas M: Insulin-like growth factor-I supports
proliferation of autocrine thymic lymphoma cells with a pre-T cell
phenotype. J Immunol. 145:3497–3501. 1990.PubMed/NCBI
|
|
19
|
Estrov Z, Meir R, Barak Y, Zaizov R and
Zadik Z: Human growth hormone and insulin-like growth factor-1
enhance the proliferation of human leukemic blasts. J Clin Oncol.
9:394–399. 1991.PubMed/NCBI
|
|
20
|
El-Badry OM, Romanus JA, Helman LJ, Cooper
MJ, Rechler MM and Israel MA: Autonomous growth of a human
neuroblastoma cell line is mediated by insulin-like growth factor
II. J Clin Invest. 84:829–839. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
El-Badry OM, Helman LJ, Chatten J,
Steinberg SM, Evans AE and Israel MA: Insulin-like growth factor
II-mediated proliferation of human neuroblastoma. J Clin Invest.
87:648–657. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Calle EE and Kaaks R: Overweight, obesity
and cancer: epidemiological evidence and proposed mechanisms. Nat
Rev Cancer. 4:579–591. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Minniti CP, Tsokos M, Newton WA Jr and
Helman LJ: Specific expression of insulin-like growth factor-II in
rhabdomyosarcoma tumor cells. Am J Clin Pathol. 101:198–203.
1994.PubMed/NCBI
|
|
24
|
Scotlandi K, Benini S, Sarti M, Serra M,
Lollini PL, Maurici D, Picci P, Manara MC and Baldini N:
Insulin-like growth factor I receptor-mediated circuit in Ewing's
sarcoma/peripheral neuroectodermal tumor: a possible therapeutic
target. Cancer Res. 56:4570–4574. 1996.PubMed/NCBI
|
|
25
|
Yee D, Favoni RE, Lebovic GS, Lombana F,
Powell DR, Reynolds CP and Rosen N: Insulin-like growth factor I
expression by tumors of neuroectodermal origin with the t (11; 22)
chromosomal translocation: a potential autocrine growth factor. J
Clin Invest. 86:1806–1814. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Drummond IA, Madden SL, Rohwer-Nutter P,
Bell GI, Sukhatme VP and Rauscher FJ III: Repression of the
insulin-like growth factor II gene by the Wilms tumor suppressor
WT1. Science. 257:674–677. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Werner H, Re GG, Drummond IA, Sukhatme VP,
Rauscher FJ III, Sens DA, Garvin AJ, LeRoith D and Roberts CT Jr:
Increased expression of the insulin-like growth factor I receptor
gene, IGF1R, in Wilms tumor is correlated with modulation of IGF1R
promoter activity by the WT1 Wilms tumor gene product. Proc Natl
Acad Sci USA. 90:5828–5832. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zumkeller W, Schwander J, Mitchell CD,
Morrell DJ, Schofield PN and Preece MA: Insulin-like growth factor
(IGF)-I, -II and IGF binding protein-2 (IGFBP-2) in the plasma of
children with Wilms' tumor. Eur J Cancer. 29A:1973–1977. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Barozzi C, Ravaioli M, D'Errico A, Grazi
GL, Poggioli G, Cavrini G, Mazziotti A and Grigioni WF: Relevance
of biologic markers in colorectal carcinoma: a comparative study of
a broad panel. Cancer. 94:647–657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hakam A, Yeatman TJ, Lu L, Mora L, Marcet
G, Nicosia SV, Karl RC and Coppola D: Expression of insulin-like
growth factor-1 receptor in human colorectal cancer. Hum Pathol.
30:1128–1133. 1999. View Article : Google Scholar : PubMed/NCBI
|