Introduction
2′-Deoxycytidine (dCyd), a pyrimidine nucleoside, is
found at high concentrations in the plasma of poor-prognosis
patients with bladder cancer, acute lymphoma (1) or hepatoma (2), and of breast cancer patients who have
been treated with chemotherapy [cyclophosphamide, methotrexate and
5-fluorouracil (5FU)] (3).
Moreover, the anti-tumor activity of 5FU has been found to be
decreased by dCyd in SP2/0-Ag14 mouse myeloma-bearing mice
(4). However, it is unclear why
dCyd increases in cancer patients and whether the administration of
5FU affects the dCyd level in tissues. Thus, we examined free dCyd
levels in non-tumor-bearing and tumor-bearing mouse tissues, and in
tumor-bearing mice treated with 5FU or dCyd.
Moyer et al (5) studied the metabolism of radiolabeled
pyrimidine nucleosides administered to mice by intravenous
injection. They found that the half-life of dCyd was less than 9
min and that deoxyuridine (dUrd), from deamination of dCyd, was
present even at 1 min, and equaled the concentration of dCyd in the
plasma by 10 min. Additionally, they measured the radioactivity in
the acid-soluble fractions of tissues, as a nucleoside or in the
DNA fraction, after 30 min. The tissue concentration of
unmetabolized dCyd was very low. This study suggested that
administered dCyd was removed from the plasma almost immediately
and was distributed in tissues in the form of dUrd and/or DNA.
This report examined endogenous-free dCyd levels in
various tissues of mice into which SP2/0 cells had been
subcutaneously transplanted to generate solid-tumors, with or
without 5FU or dCyd administration. The free dCyd level was found
to be particularly elevated in the spleens of tumor-bearing
animals. Administration of 5FU was observed to affect liver weight
and this alteration may involve changes in the free dCyd level.
Materials and methods
Chemicals
dCyd and 5FU were obtained from Sigma-Aldrich (St.
Louis, MO, USA). RPMI-1640 medium (RPMI) was obtained from Nikken
Biomedical Laboratory (Kyoto, Japan). Fetal bovine serum (FBS) was
from Sanko Junyaku (Tokyo, Japan). Plastic tissue culture dishes,
100-mm in diameter, were from IWAKI Co. (Tokyo, Japan). Penicillin
and streptomycin were obtained from Meiji Seika Co. (Tokyo, Japan).
Phosphate-buffered saline (PBS) was from Takara Shuzo Co., Ltd.
(Shiga, Japan). Trypan blue and trichloroacetic acid (TCA) were
from Wako Pure Chemical Ind., Ltd. (Osaka, Japan). Filter units
(Millex™-GV 0.22-μm, sterile and Millex™-HV 0.45-μm, non-sterile)
were purchased from Millipore Corporation (Bedford, MA, USA).
Glycine, acetonitrile, and EDTA•2Na were obtained from Nacalai
Tesque (Kyoto, Japan) and trifluoroacetic acid (TFA) was from Merck
KGaA (Darmstadt, Germany).
Cell cultures of SP2/0 cells
SP2/0 cells were obtained from the Collection of
Cancer Cell Lines (National Institute of Hygienic Science, Tokyo,
Japan). These cells were maintained in culture dishes in RPMI-1640
containing 10% heat-inactivated FBS, penicillin at 100 U/ml and
streptomycin at 100 μU/ml, under a humidified atmosphere of 5%
CO2 at 37°C. To inoculate mice, the cells were removed
from dishes by pipetting and washed twice with PBS. The cell number
was determined during trypan blue exclusion tests (6).
Animals and dCyd and 5FU treatments
BALB/c mice (male, 5-week-old, weighing 20–25 g)
were obtained from Japan SLC (Hamamatsu, Japan). The animals were
continually cared for in a room maintained at 25±1°C and 55±5%
humidity with free access to water and food. For tumor-bearing
treatment groups, SP2/0 cell suspension (1×106
cells/mouse) was inoculated subcutaneously (s.c.) into a shaved
area on the back of each mouse on day 0. Then, 7 days later, body
weight and relative tumor volume were measured. Tumor volume was
calculated using the following formula: tumor volume
(mm3) = A × A × B /2, where A is the smallest diameter
(mm) and B is the largest diameter (mm) of solid tumor, measuring
with a vernier caliper. When tumor volume in these animals reached
approximately 100–300 mm3 at day 7, all mice were
divided into five groups as follows: normal (PBS administered to
non-tumor-bearing mice), non-tumor-dCyd (N-dCyd, 0.1 mmol/kg dCyd
administered to non-tumor-bearing mice), control (PBS administered
to tumor-bearing mice), tumor-dCyd (T-dCyd, 0.1 mmol/kg dCyd
administered to tumor-bearing mice) and tumor-5FU (T-5FU, 0.15
mmol/kg 5FU administered to tumor-bearing mice). Moreover, each
group was divided into two samples and sacrificed on different days
as described below. Each sample consisted of 2–5 mice. 5FU or dCyd
was dissolved in sterile PBS at a given concentration and filtered
through a 0.22-μm filter. On days 8 through 15, PBS, dCyd or 5FU
was administered at 0.1 ml/10 g body weight by intraperitoneal
injection (i.p.), and body weight and relative tumor volume were
recorded. Relative tumor volume was calculated by dividing the
tumor volume on a given day by that on day 7. Change in body weight
was calculated as the weight on a given day minus the weight on the
first day of treatment administration (day 8). Animals in one
sample of each group were sacrificed at 1 day after the final i.p.
injection (day 16), and the remaining animals were sacrificed at 6
days after the final i.p. injection (day 22), under diethyl ether
anesthesia. Blood was collected from the heart into test tubes
containing EDTA (1.5 mg/ml blood) and body tissues were removed and
weighed.
The protocol was performed according to the
guidelines of the Japanese Society for Pharmacology and was
approved by the Committee for Ethical Use of Experimental Animals
at Setsunan University.
Measurement of dCyd in tissues by
high-performance liquid chromatography (HPLC)
TCA (10 ml of a 10% solution) was added to 1 g (wet
weight) of tissue, and the tissue was homogenized. Plasma samples
were mixed with equal volumes of 10% TCA. Tissue homogenate and
plasma were centrifuged at 2,000 × g for 15 min. The supernatant
was neutralized with 2 M KOH and applied to Supelclean LC-SAX SPE
tubes (Supelco, Bellefonte, PA, USA) that had been conditioned by
washing with distilled water and 0.2 M glycine buffer (pH 3.0).
dCyd was eluted with the glycine buffer, passed through a 0.45-μm
filter and subjected to HPLC. An aliquot of the filtrate was
applied to a MIGHTYSIL RP-18 GP Aqua 250-4.6 (5 μm) column (Kanto
Kagaku Co., Tokyo, Japan). The column was maintained at 40°C. dCyd
was eluted with a linear gradient from 0.1% TFA to 20% acetonitrile
containing 0.1% TFA, at a rate of 1.0 ml/min for 30 min. The eluate
was monitored with a UV detector (875-UV detector; Jasco, Tokyo,
Japan) at 265 nm. The complete instrument consisted of an 880-PU
pump (Jasco), 880-51 degasser (Jasco), LG-980-02 ternary gradient
unit (Jasco), 860-CO column oven and Unicorder U-228 (Pantos, Uji,
Japan).
Statistical analysis of measurements
Data were analyzed using the F-test for variance and
Student’s t-test for significance.
Results
Effects of drug administration on body
weight and relative tumor volume
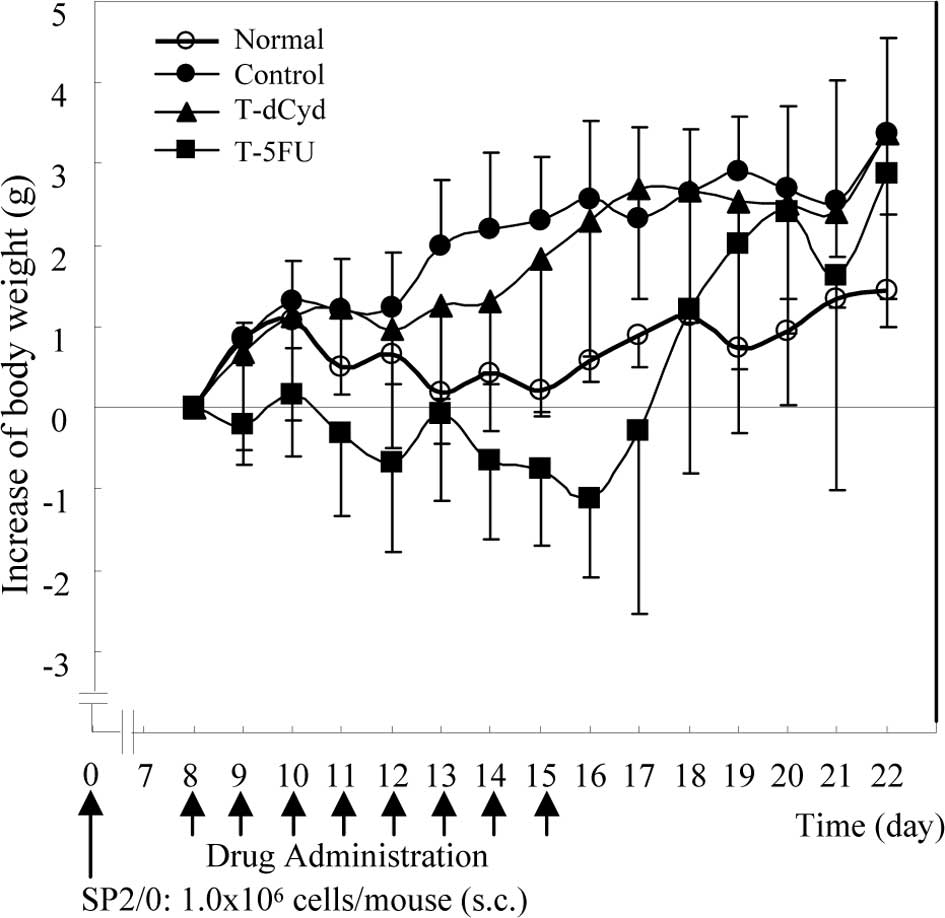
Body weight in the tumor-bearing control group was
heavier than in the normal group (Fig.
1). In the T-5FU group, body weight at day 16 was lower than
the baseline, but increased to the level of the tumor-bearing
controls at day 22. Body weight in the N-dCyd group was similar to
that in the non-tumor-bearing normal group (data not shown).
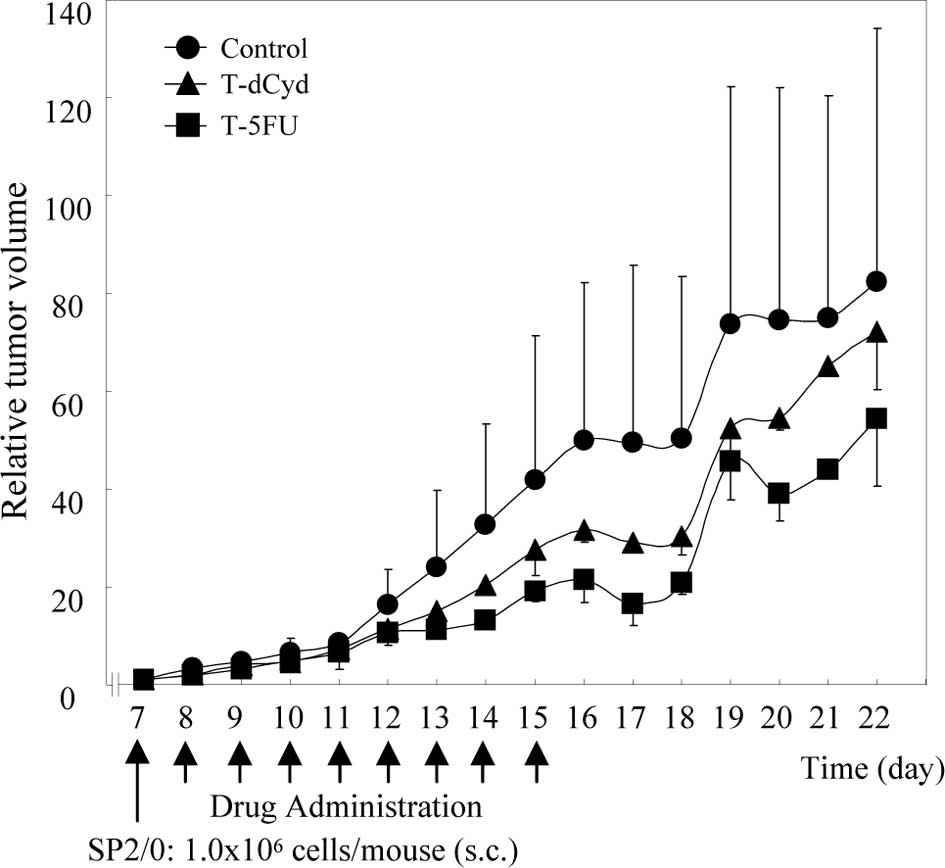
The relative tumor volume of the tumor-bearing
control group was 50 on day 16, while that of the T-dCyd group was
30 (Fig. 2). In the T-5FU group,
the relative tumor volume increased less than in the control
group.
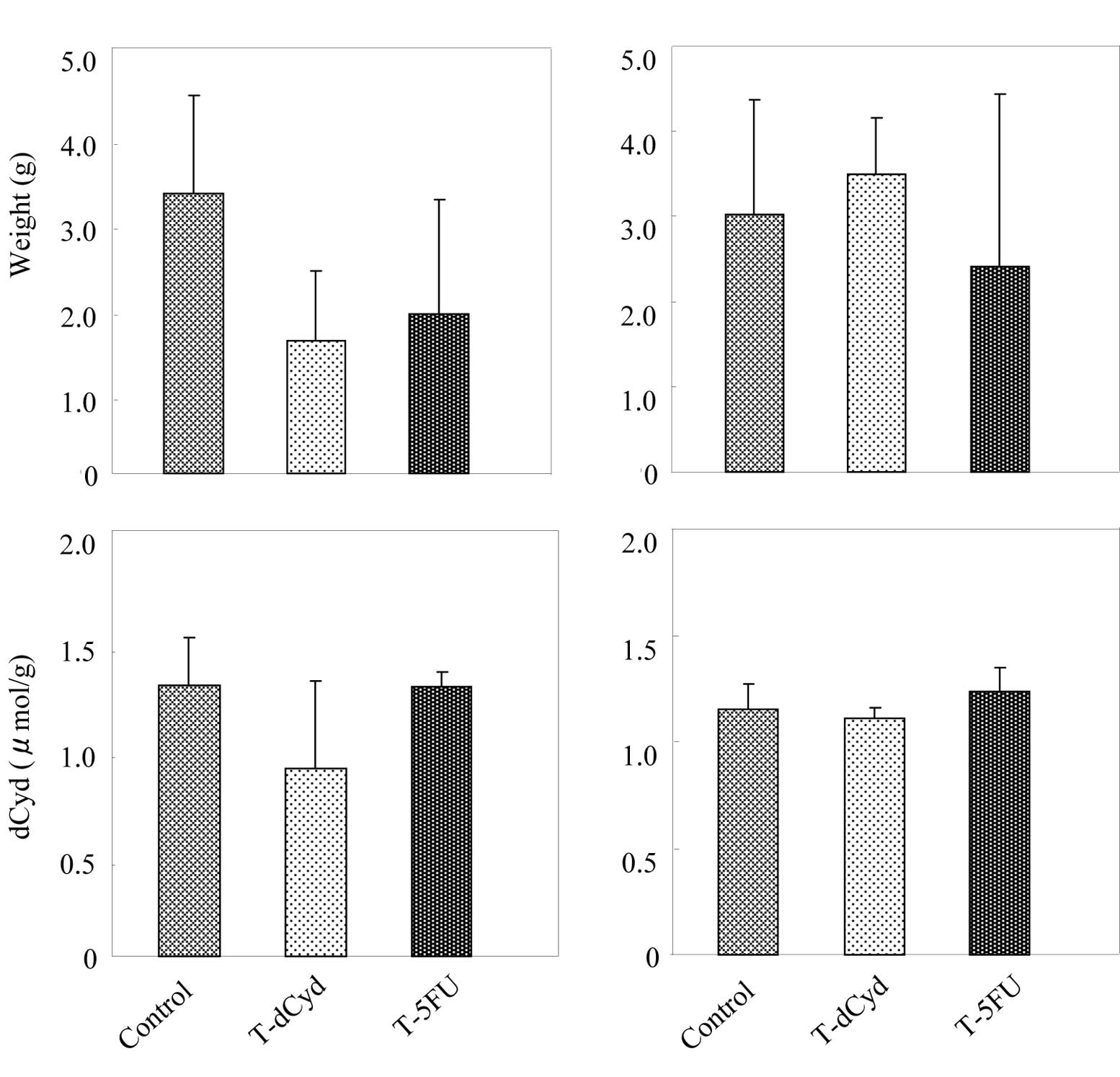
Tissue weight and dCyd level
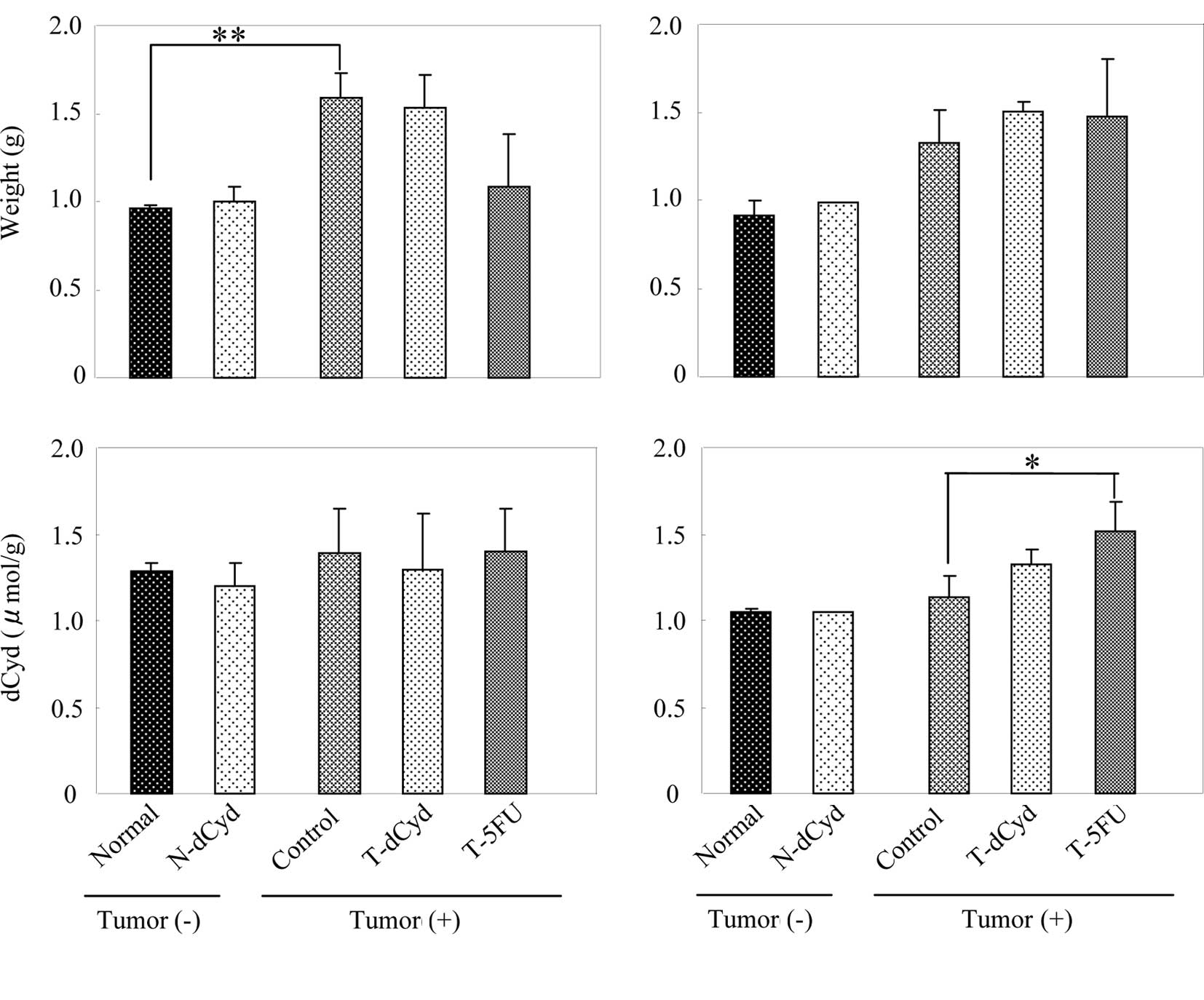
At day 16, liver weight in the tumor-bearing control
group was 1.4-fold heavier than in the normal group (Fig. 3A). However, liver weight of the
T-5FU group decreased in comparison to its control group. The level
of dCyd in the liver was not affected by tumor-bearing status or by
administration of dCyd or 5FU. At day 22, liver weight in the
tumor-bearing control group was 1.6-fold heavier than in the normal
group (Fig. 3B), and that in the
T-5FU group was similar to the controls. The level of dCyd in the
liver was increased by administration of 5FU.
At day 16, spleen weight and the level of dCyd in
the spleen of the tumor-bearing control group increased 4.3-fold,
1.4-fold above the normal group (Fig.
4A). At day 22, spleen weight and the level of dCyd in the
spleen of the tumor-bearing control group was increased 4.2-fold,
1.4-fold above the normal group (Fig.
4B). Administration of dCyd did not affect the level of dCyd
detected in the liver or spleen of tumor-bearing or
non-tumor-bearing mice on either day.
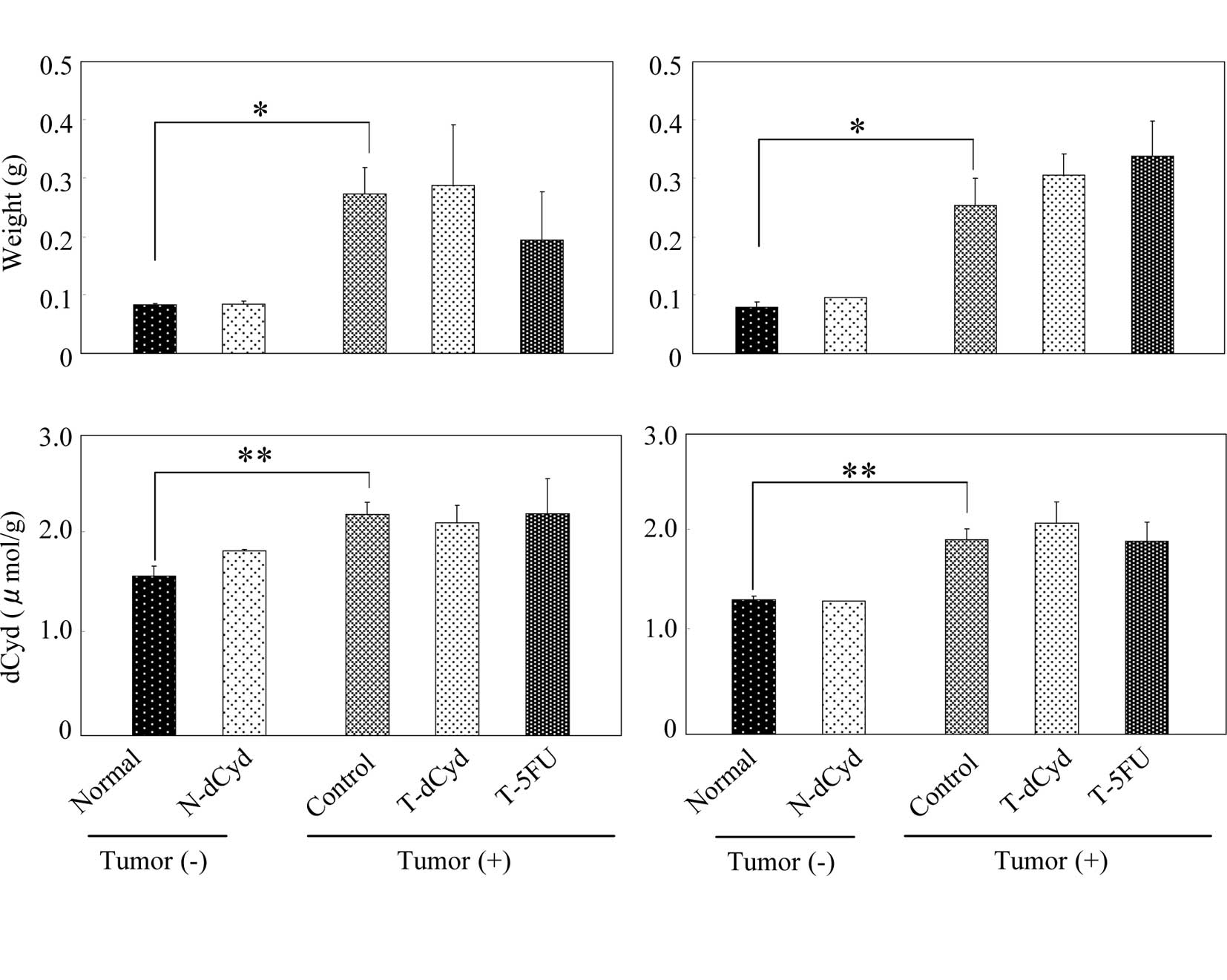
At day 16, tumor weight appeared to decrease in the
T-dCyd and T-5FU groups (Fig. 5A).
The level of dCyd was similar among the groups. At day 22, tumor
weights and levels of dCyd in tumors were unaffected by the
administration of dCyd or 5FU (Fig.
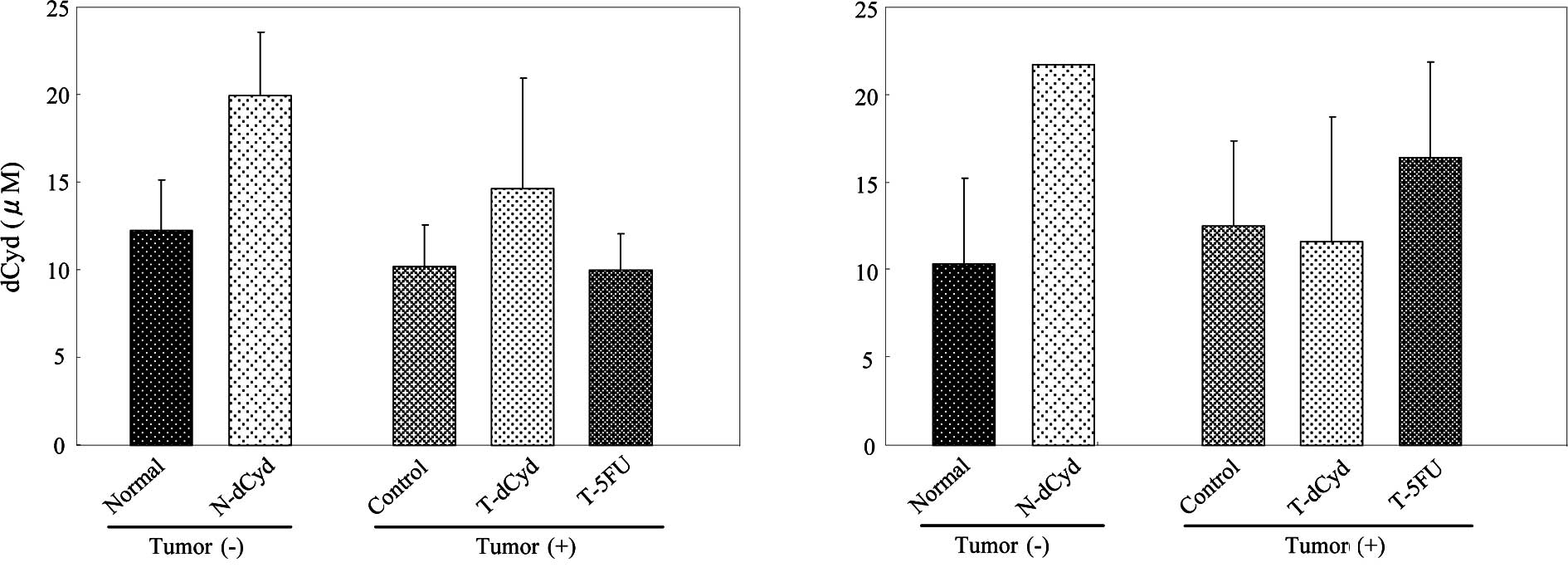
5B). At day 16, the level of dCyd in the plasma was
approximately 10 μM and was increased by administration of dCyd
(Fig. 6A). No difference in plasma
dCyd was observed between tumor-bearing and non-tumor-bearing mice
on days 16 and 22 (Fig. 6A and
B).
Table I shows the
weight, level of dCyd and net amount of dCyd in various tissues of
the normal and control groups on days 16 and 22. The weights of
kidney and lungs were not different between groups. On the other
hand, the weights of liver and spleen were markedly increased in
the tumor-bearing control group. The level of dCyd in liver,
kidney, spleen and tumor was approximately 1.0–2.0 μmol/g tissue,
whereas that in the heart and lungs was 0.2–0.8 μmol/g tissue. The
net amount of dCyd in liver and spleen was markedly increased in
the control group.
 | Table IdCyd levels in various tissues and
plasma of BALB/c mice, at days 16 and 22 after inoculation with (+)
or without (−) SP2/0 mouse myeloma cells. |
Table I
dCyd levels in various tissues and
plasma of BALB/c mice, at days 16 and 22 after inoculation with (+)
or without (−) SP2/0 mouse myeloma cells.
| | | dCyd |
|---|
| | |
|
|---|
| Tissue | Tumor | Tissue weight
(g) | Level (μmol/g) | Net amount
(μmol/tissue) |
|---|
| |
|
|
|
|---|
| | 16th | 22nd | 16th | 22nd | 16th | 22nd |
|---|
| Heart |
| − | 0.109 | 0.111 | 0.617 | 0.418 | 0.067 | 0.046 |
| + | 0.107 |
0.092a | 0.764 | 0.464 | 0.062 | 0.043 |
| Liver |
| − | 0.966 | 0.912 | 1.269 | 1.045 | 1.245 | 0.952 |
| + |
1.593b | 1.328 | 1.369 | 1.133 | 2.229 | 1.510 |
| Kidney |
| − | 0.152 | 0.149 | 1.815 | 1.213 | 0.275 | 0.179 |
| + | 0.168 | 0.152 | 1.724 | 1.345 | 0.290 | 0.205 |
| Lung |
| − | 0.142 | 0.136 | 0.188 | 0.410 | 0.027 | 0.056 |
| + | 0.158 | 0.147 | 0.248 | 0.424 | 0.040 | 0.062 |
| Spleen |
| − | 0.064 | 0.060 | 1.594 | 1.344 | 0.133 | 0.107 |
| + |
0.273a |
0.254a |
2.212b |
1.947b |
0.605b |
0.492b |
| Tumor | + | 3.279 | 3.020 | 1.271 | 1.154 | 4.050 | 3.546 |
| Plasma |
| − | | | 12.3 | 10.3 | | |
| + | | | 10.2 | 12.5 | | |
Discussion
We investigated the free dCyd level in various
tissues of mice, with and without tumors, and in mice with and
without 5FU or dCyd administration. The free dCyd level was
measured at 1 day after the last drug administration (day 16), when
the body weight of mice administered 5FU was maximally decreased,
and at 1 week after the last administration (day 22), when weights
had returned to the level of the controls. It is likely that the
increasing body weight was influenced by increasing tumor
volumes.
Liver weights had decreased with 5FU treatment at
day 16 and were similar to those of the tumor-bearing control group
at day 22. At day 22, dCyd in liver in the T-5FU group was higher
than in the control group; this difference appeared to correlate
with the increase in plasma dCyd.
Berlinger (7)
reported that the concentration of dCyd was increased in plasma and
in cerebrospinal fluid after hepatectomy. Schneider et al
(8) reported that the activity of
the enzyme that synthesizes dCyd diphosphate choline was increased
in rat hepatomas and in regenerating liver after partial
hepatectomy and was related to increased DNA synthesis. These
reports may be significant for our study, in which the level of
dCyd in the liver increased with the recovery of liver weight after
decreases caused by administration of the anti-tumor agent 5FU.
Thus, it is suggested that the free dCyd level is influenced by
changes in tissue weight or tissue regeneration.
In this study, spleen had the highest level of dCyd
among the measured tissues. In spleen, the weight, level of dCyd
and net amount of dCyd were all significantly increased in
tumor-bearing mice, compared to the non-tumor groups, on both day
16 and day 22. Osogoe et al reported that after tritiated
dCyd was administered to mice intraperitoneally, radioactivity was
strongly detected in the germinal center cells of the spleen and in
Peyer’s patches of the intestine in both mouse (9) and rat (10). We speculate whether dCyd may act as
a defense mechanism in the body, given the spleen’s role among the
lymphatic tissues and its involvement in the reticuloendothelial
system. The spleen contains plasma cells, B lymphocytes, T
lymphocytes, antigen-presenting cells and macrophages, which all
function in immunity. Recent studies have focused on
activation-induced cytidine deaminase (AID), a member of the
cytidine-deaminase family, for which cytidine and dCyd are
substrates. AID is essential for somatic hypermutation (SHM) and
class-switch recombination in the immunoglobulin genes (11), and the AID gene is specifically
expressed in germinal center B cells (12) in the spleen, in its role as a
secondary lymphoid organ. Moreover, the inappropriate expression of
AID causes DNA and/or RNA editing (13,14)
and is involved in human malignancies via genomic DNA cleavage,
which contributes to tumorigenesis (15). The increase in free dCyd in the body
may inhibit mutations caused by AID through an effect on the
production or repair of DNA and/or RNA.
Of the tissues measured, the highest net amount of
dCyd was found in tumors. We think this high level reflects the
dCyd level in plasma, where it can be used as prognostic marker for
cancer patients (1). However, the
dCyd level in plasma was unaffected by tumor-bearing status in this
study. These results suggest that the dCyd level in plasma depends
indirectly on tumor size. For example, it is possible that dCyd
level is affected by changes in the activity of enzymes that
metabolize dCyd. We aim to examine the activities of such enzymes,
e.g., deoxycytidine deaminase (16), cytidine deaminase (17) and deoxycytidine kinase (18,19),
in tissues of non-tumor-bearing and tumor-bearing mice.
Notably, the administration of dCyd to tumor-bearing
mice seemed to decrease the relative tumor volume and the tumor
weight in this study. This suggests that dCyd has the ability to
inhibit tumor growth, at least somewhat. However, in our previous
study (4), dCyd had no effect on
survival in tumor-bearing mice. Differences between the previous
and the present study in the tumor inoculation protocol may be
responsible for this. In the previous experiments, the tumor was
implanted and dCyd was administered intraperitoneally, whereas in
the present study, the tumor was implanted subcutaneously on the
back of the mice, with dCyd administered intraperitoneally. Since
dCyd must travel in the blood circulation to reach the solid tumor,
metabolites or anabolites of dCyd may have anti-tumor activity.
Additionally, the ability of dCyd to inhibit tumor growth may be
indirect (e.g., by activating defensive factors in lymphatic
tissues).
From the results of this study, we found that i) the
administration of 5FU had an effect on liver weight, which
correlated with tissue dCyd level, ii) free dCyd levels were high
in the spleens of tumor-bearing mice and iii) the administration of
dCyd had the ability to inhibit solid tumor growth. Further studies
are required to clarify the role of dCyd in the tumor-bearing
body.
Acknowledgements
The authors thank Ms. R. Ikenaga for the technical
assistance.
References
|
1
|
Ahmed WA, Ali-Din NH, Yoshioka M and
EL-Merzabani M: 2′-Deoxycytidine (DCYD) as a potential biological
marker for detecting acute lymphocytic leukemia and bladder cancer.
J Union Arab Biol Cairo. 20A:97–111. 2003.
|
|
2
|
Ahmed WA, Moneer M, Abo-Shady MM, Mansour
HH, Abd-El-Wahab N, Yoshioka M and EL-Merzabani M: 2′-Deoxycytidine
as a potential biomarker for detection of hepatocellular carcinoma.
Egyptian J Hosp Med. 21:191–201. 2005.
|
|
3
|
Yoshioka M, Abu-Zeid M, Kubo T and
El-Merzabani M: Identification of a previously unknown compound as
2′-deoxycytidine found in the plasma of breast cancer patients
under combined chemotherapy. Biol Pharm Bull. 17:169–174. 1994.
|
|
4
|
Iwazaki A and Yoshioka M: 2′-Deoxycytidine
decreases the anti-tumor effects of 5-fluorouracil on mouse myeloma
cells. Biol Pharm Bull. 33:1024–1027. 2010.
|
|
5
|
Moyer JD, Malinowski N and Ayers O:
Salvage of circulating pyrimidine nucleosides by tissues of the
mouse. J Biol Chem. 260:2812–2818. 1985.PubMed/NCBI
|
|
6
|
Rossi L, Serafini S, Schiavano GF,
Casabianca A, Vallanti G, Chiarantini L and Magnani M: Metabolism,
mitochondrial uptake and toxicity of 2′, 3′-dideoxycytidine.
Biochem J. 344:915–920. 1999.
|
|
7
|
Berlinger WG, Stene RA, Spector R and
Al-Jurf AS: Plasma and cerebrospinal fluid nucleosides and
oxypurines in acute liver failure. J Lab Clin Med. 110:137–144.
1987.PubMed/NCBI
|
|
8
|
Schneider WC and Behki RM: Phosphorus
compounds in animal tissues. VII Enzymatic formation of
deoxycytidine diphosphate choline and lecithin by tissue
homogenates. J Biol Chem. 238:3565–3571. 1963.
|
|
9
|
Osogoe B and Ueki A: A radioautographic
study of the utilization of deoxycytidine for the formation of
deoxyribonucleic acid-thymine in lymphocytes. J Cell Biol.
46:403–405. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Osogoe B, Tyler RW and Everett NB: The
patterns of labeling of germinal-center cells with tritiated
deoxycytidine. J Cell Biol. 57:215–220. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Muramatsu M, Kinoshita K, Fagarasan S,
Yamada S, Shinkai Y and Honjo T: Class switch recombination and
hypermutation require activation-induced cytidine deaminase (AID),
a potential RNA editing enzyme. Cell. 102:553–563. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Muramatsu M, Sankaranand VS, Anant S,
Sugai M, Kinoshita K, Davidson NO and Honjo T: Specific expression
of activation-induced cytidine deaminase (AID), a novel member of
the RNA-editing deaminase family in germinal center B cells. J Biol
Cell. 274:18470–18476. 1999.
|
|
13
|
Kotani A, Okazaki I, Muramatsu M,
Kinoshita K, Begum NA, Nakajima T, Saito H and Honjo T: A target
selection of somatic hypermutations is regulated similarly between
T and B cells upon activation-induced cytidine deaminase
expression. Proc Natl Acad Sci USA. 102:4506–4511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harris RS, Petersen-Mahrt SK and Neuberger
MS: RNA editing enzyme APOBEC1 and some of its homologs can act as
DNA mutators. Mol Cell. 10:1247–1253. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Okazaki IM, Hiai H, Kakazu N, Yamada S,
Muramatsu M, Kinoshita K and Honjo T: Constitutive expression of
AID leads to tumorigenesis. J Exp Med. 197:1173–1118. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chan TS, Lakhchaura BD and Hsu TF:
Differences in deoxycytidine metabolism in mouse and rat. Biochem
J. 210:367–371. 1983.PubMed/NCBI
|
|
17
|
Miwa M, Eda H, Ura M, Ouchi KF, Keith DD,
Foley LH and Ishitsuka H: High susceptibility of human cancer
xenografts with higher levels of cytidine deaminase to a
2′-deoxycytidine antimetabolite, 2′-deoxy-2′-methylidenecytidine.
Clin Cancer Res. 4:493–497. 1998.PubMed/NCBI
|
|
18
|
Chen EH, Johnson EE II, Vetter SM and
Mitchell BS: Characterization of the deoxycytidine kinase promoter
in human lymphoblast cell lines. J Clin Invest. 95:1660–1668. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hapke DM, Stegmann AP and Mitchell BS:
Retroviral transfer of deoxycytidine kinase into tumor cell lines
enhances nucleoside toxicity. Cancer Res. 56:2343–2347.
1996.PubMed/NCBI
|