Introduction
Since Gagner et al performed the first
resection of an adrenal tumor by laparoscopic adrenalectomy (LA) in
1992 (1), it has become the
standard treatment for benign adrenal tumors and has markedly
reduced the morbidity associated with this operation. A comparison
of traditional open adrenalectomy (OA) to LA showed that the length
of hospital stay and the use of post-operative analgesics in LA are
decreased, and the rate of return to normal activities is
increased.
Strong et al were able to more definitively
compare the results of LA vs. OA at the Memorial Sloan-Kettering
Cancer Center (2). These authors
showed that LA compared to OA resulted in less morbidity and
achieved similar oncological outcomes. However, applying LA for
solitary metastasis or primary adrenal carcinoma remains a matter
of considerable controversy, since port-site metastasis and
dissemination have been reported when using LA to treat adrenal
malignancies. Port-site metastasis or dissemination without
suspicion of incision or injury to the tumor has yet to be
reported. Therefore, it is important to ensure appropriate surgical
management to prevent incision or injury to the tumor in adrenal
malignancy when deciding whether to apply LA or OA.
The present study analyzed 9 consecutive patients
who underwent surgical resection of metastatic adrenal tumors in
order to clarify the decision-making factors for LA or OA.
Patients and methods
From November 2003 to November 2006, 11
adrenalectomies were performed on 9 patients for adrenal metastasis
(AM) for malignancies such as lung cancer, renal cell carcinoma
(RCC) and breast cancer at Tokai University Hospital. All patients
were treated after informed consent was obtained. Approval for the
study was obtained from the institutional review board for the
protection of human subjects at Tokai University School of
Medicine. The patients included 7 male and 2 female individuals
with a median age of 60±13 years (range 40–77). A diagnosis of AM
for the malignancies was suspected whenever a newly diagnosed
adrenal mass occurred, characterized by basal computed tomography
(CT) density superior to 10 Hounsfield units (HU), strong or
heterogeneous vascular enhancement following contrast injection
and/or increasing size in sequential imaging studies. Percutaneous
adrenal biopsy was ruled out to prevent tumor seeding. All 9
patients underwent pre-operative staging with CT, and there was no
evidence of extra-AM. The approach to surgical management using LA
or OA was determined on the basis of CT and/or magnetic resonance
imaging (MRI). Patients in the LA group were placed in the lateral
decubitus position. Four trocars were used. LA was performed with
the greatest care to prevent tumor disruption. During the
procedure, there was minimal handling of the tumor, and the adrenal
gland was resected with its surrounding fat. Specimens were
extracted intact within a bag. The patients were reviewed every 2
or 3 months by physical examination and systemic CT. The
decision-making factors based on imaging concerning surgical
management with LA or OA were analyzed according to the results of
oncological outcome, imaging, intraoperative and pathohistological
findings.
Statistical analyses were performed using
commercially available software (SPSS® 15.0J, IL, USA).
The paired t-test was used for the statistical analysis of
operative data. Distributions of biochemical disease-free survival
times were calculated according to the Kaplan-Meier curves, and the
log-rank test was used to compare curves between the two groups.
P<0.05 was considered to be statistically significant.
Results
In the present study, 9 patients underwent 11
adrenalectomies (9 laparoscopic and 2 open procedures). Patient
data and the characteristics of the primary malignancy are shown in
Table I. Operative data and
oncological outcomes of patients with AM are summarized in Table II. Non-small cell lung cancer
(NSCLC) was the most common primary malignancy (5 adrenalectomies
of 4 patients), followed by RCC (4 adrenalectomies of 4 patients)
and breast cancer (2 adrenalectomies of 1 patient). There were 1
left, 6 right and 2 bilateral adrenalectomies, with a median tumor
size of 3.9±1.4 cm (range 2.1–6.7). Of the adrenalectomies, 7
included metachronous metastases and 4 synchronous metastases.
 | Table IPatient data and characteristics of
the primary malignancy. |
Table I
Patient data and characteristics of
the primary malignancy.
| Patient | Age at surgery for AM
(years) | Gender | Primary
malignancy | History of other
organ metastases |
|---|
|
|---|
| Pathological
findings | Site |
|---|
| 1 | 74 | M | RCC (clear-cell
carcinoma) | Right | None |
| 2 | 47 | F | Breast cancer
(mammary carcinoma) | Left | Axillary lymph
node |
| 3 | 49 | M | RCC (clear-cell
carcinoma) | Left | None |
| 4 | 40 | M | Lung cancer (poorly
differentiated adenocarcinoma) | Right | None |
| 5 | 60 | F | RCC (clear-cell
carcinoma) | Left | Thyroid gland |
| 6 | 74 | M | RCC (clear-cell
carcinoma) | Left | Lung |
| 7 | 77 | M | Lung cancer (mixture
of large-cell carcinoma, spindle carcinoma, giant-cell carcinoma
and adenocarcinoma) | Left | None |
| 8 | 56 | M | Lung cancer
(large-cell carcinoma) | Right | None |
| 9 | 58 | M | Lung cancer
(well-differentiated adenocarcinoma) | Left | None |
 | Table IIOperative data and oncological
outcomes of the patients with adrenal metastasis. |
Table II
Operative data and oncological
outcomes of the patients with adrenal metastasis.
| Patient | Time | Side | Interval to diagnosis
of AM (months) | Operative
procedure | Operating time
(min) | Blood loss (ml) | Hospital stay
(days) | Tumor size (cm) | Follow-up
(months) | Status |
|---|
| 1 | Metachronous | Right | 16 | LA | 103 | 19 | 8 | 2.5 | 12 | NEMD (dead due to
pulmonary emphysema) |
| 2 | Metachronous | Bilateral | 108 | LA | 100 (right)
90 (left) | 3 (right)
20 (left) | 11 | 3.3 (right)
2.1 (left) | 13 | Recurrence, but
alive |
| 3 | Synchronous | Right | 0 | LA | 215 | 207 | 6 | 4.0 | 38 | NEMD |
| 4 | Synchronous | Left | 0 | LA | 105 | 14 | 7 | 2.6 | 7 | Recurrence and dead
due to primary malignancy |
| 5 | Metachronous | Right | 106 | OA | 190 | 347 | 9 | 5.5 | 29 | Recurrence, but
alive |
| 6 | Metachronous | Right | 84 | LA | 92 | 31 | 5 | 3.5 | 20 | Recurrence and dead
due to primary malignancy |
| 7 | Synchronous | Right | 0 | OA | 257 | 333 | 8 | 6.7 | 45 | NEMD |
| 8 | Synchronous | Right | 0 | LA | 170 | 83 | 6 | 4.3 | 31 | NEMD |
| 9 | Metachronous | Bilateral | 70 | LA | 130 (right)
144 (left) | 43 (right)
24 (left) | 10 | 2.6 (right)
3.3 (left) | 25 | NEMD |
The median tumor size for the LA group was 3.1±0.7
cm (range 2.1–4.3) and for the OA group, 6.1±0.8 cm (5.5 and 6.7
cm) (p=0.001). The operative time for the LA group was 127±42 min
(range 90–215) and for the OA group, 224±47 min (190 and 257 min)
(p=0.018). Blood loss for the LA group was 49±63 g (range 3–207)
and for the OA group, 340±10 g (333 and 347 g) (p<0.001). Among
the patients with AM from RCC, 4 patients underwent contralateral
LA (n=3) and ipsilateral LA (n=1). The median operative time for
the contralateral group was 166±65 min (range 92–215) and for the
ipsilateral case, 103 min. Blood loss for the contralateral group
was 163±62 g (range 92–207) and for the ipsilateral case, 17 g. No
complications were noted and no conversion of LA to OA occurred.
All 9 adrenal tumors selected for LA were removed safely by LA
without strong adhesion to the surrounding tissue. Two adrenal
tumors removed by OA had strong adhesion to the surrounding tissue.
In all cases, pathological examination confirmed the diagnosis of
metastasis related to the primary malignancy. All of the patients
had complete resection without capsular disruption and a negative
margin in the pathological findings. Moreover, no port-site and
local recurrence occurred.
Pathological findings indicated fibrosis and the
infiltration of inflammatory cells in the fatty tissue around the
tumors removed by OA, while there was no fibrosis and infiltration
of inflammatory cells in the fatty tissue surrounding the tumors
removed by LA. The durations of the hospital stay after LA and OA
were 8±2 (range 5–11) and 9±0.5 days (range 8–9), respectively
(p=0.595). The observed follow-up term was 24±12 months (range
12–45). No patients presented with local relapse or port-site
metastasis. Median overall disease-free survival (MODFS) was 29±7
months (range 7–38) and the disease-free survival rate (DFSR) was
55.6%. DFSR for the LA group was 57% and for the OA group, 50%
(p=0.661). MODFS for the group with no history of other metastases
was 39±6 months (range 7–45) and for the group with a history of
other organ metastases, 21±5 months (range 13–29). DFSR for the
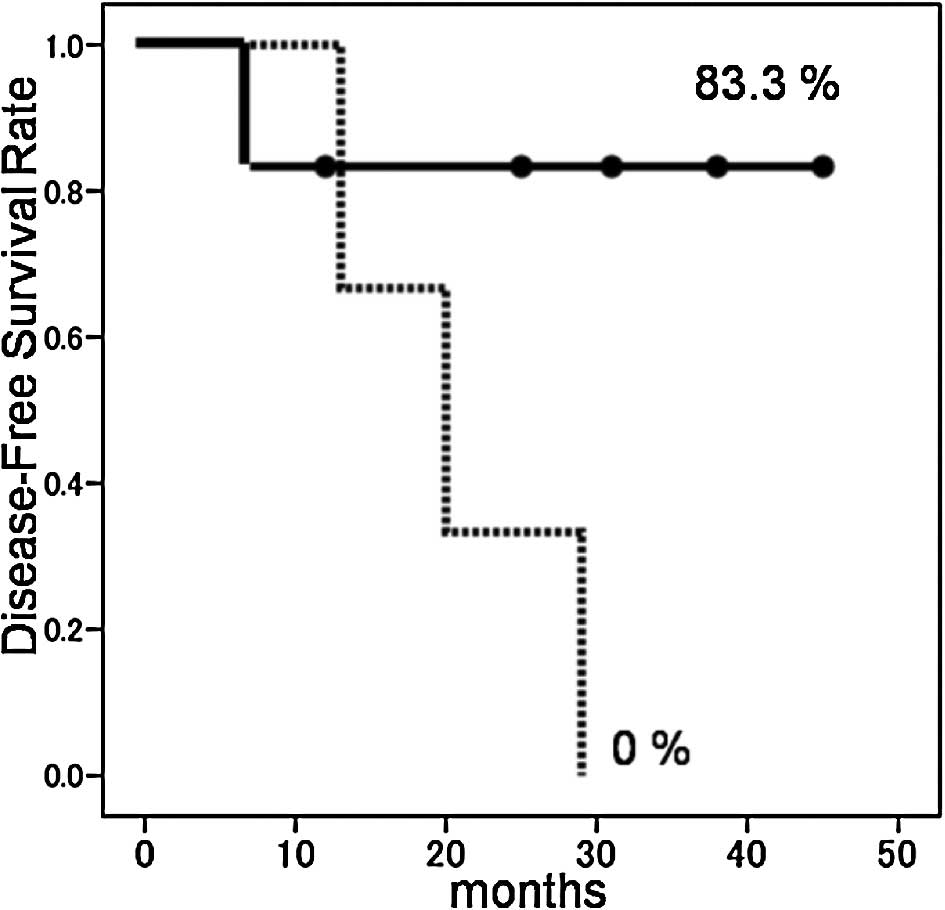
group with isolated AM was 83.3%, and DFSR for those with a history
of other organ metastases was 0% (p=0.077) (Fig. 1). MODFS for the metachronous group
was 23±4 months (range 13–29) and for the synchronous group, 30±7
months (range 7–38). DFSR for the metachronous group was 40% and
for the synchronous group, 75% (p=0.435).
Discussion
LA has been acknowledged as the gold standard
treatment for small benign secreting tumors, while it remains a
subject of debate for large and potentially malignant adrenal
tumors and AM. Long-term survival after resection of isolated
adrenal metastatic tumors was first reported in 1982 by Twomey
et al (3). The 2 patients in
their study were disease-free for 6 and 14 years following
resection of isolated adrenal metastases from large-cell lung
cancer. Since then, a number of series, including several from the
Memorial Sloan-Kettering Cancer Center, confirmed that when
metastasis is isolated to the adrenal gland, adrenalectomy achieves
long-term survival (2). These
series showed that LA, compared to OA, resulted in less morbidity
and achieved similar oncological outcomes. Evaluation of survival
time points demonstrated that patients with synchronous metastasis
had 73 and 38% 1- and 3-year survival following adrenal resection,
respectively, while patients with metachronous lesions had 86 and
46% 1- and 3-year survival, respectively (p=0.62). No significant
difference in survival between patients treated with LA and those
treated with OA (p=0.43). Suzuki reported that survival ranged from
20 to 30 months after surgical removal of AM, compared to a mean
survival of 6–8 months for patients whose AM was not resected
(4).
In the present study, the 7 adrenalectomies of 6
patients were for isolated AM and the other 4 adrenalectomies were
performed for 3 patients who had histories of other organ
metastases. Adrenalectomy is a more effective treatment for
isolated AM than for AM associated with a history of other organ
metastases, as there were significant differences for DFSR in our
study between the group of patients with isolated AM and patients
with AM associated with a history of other organ metastases.
However, this does not mean that adrenalectomy is not effective for
patients with AM associated with a history of other organ
metastases. Among the patients who had histories of other organ
metastases, 2 adrenalectomies were performed for 2 patients who had
metastasis from RCC and 2 adrenalectomies were perfomed for 1
patient who had metastasis from breast cancer. Certain patients
with metastatic RCC may be treated with radical nephrectomy and
resection of the metastatic lesions. A recent study found that
metastasectomy was of clinical benefit across a range of prognostic
groups and was independently associated with survival (5). Regarding AM from breast cancer, the
optimal treatment is unclear, but early recognition and
adrenalectomy of the tumor has potential survival benefits
(6). For isolated AM in our study,
only 1 patient (patient 4) relapsed in relation to the opposite
adrenal gland at 11 months after LA. The primary malignancy was
poorly differentiated adenocarcinoma of lung cancer. We considered
that the case of isolated AM from high-grade malignancy had potent
metastasis. Such a case requires careful follow-up.
We noted 4 patterns of pre-operative imaging and
adrenalectomy for AM in this study. The first pattern was AM whose
size was small with fatty tissue detected surrounding the tumor by
imaging. The second pattern noted was the size of the AM reduced by
chemotherapy. The third pattern was AM with a large size and
irregular contour. The fourth pattern was AM of a large size and
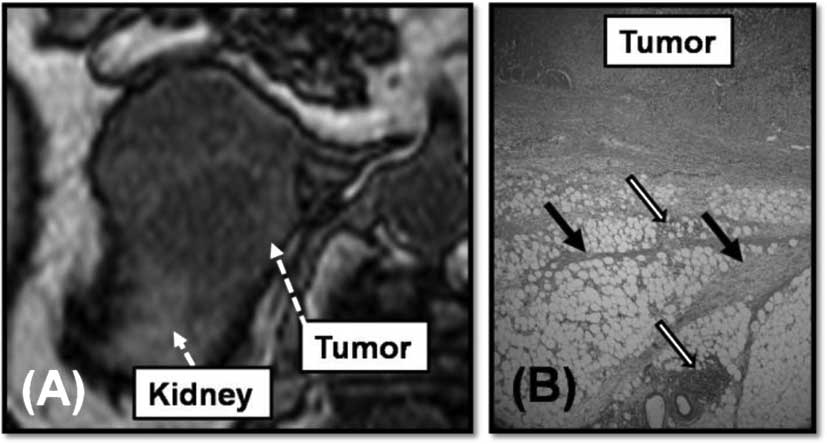
with a cystic component. A representative case of the first pattern
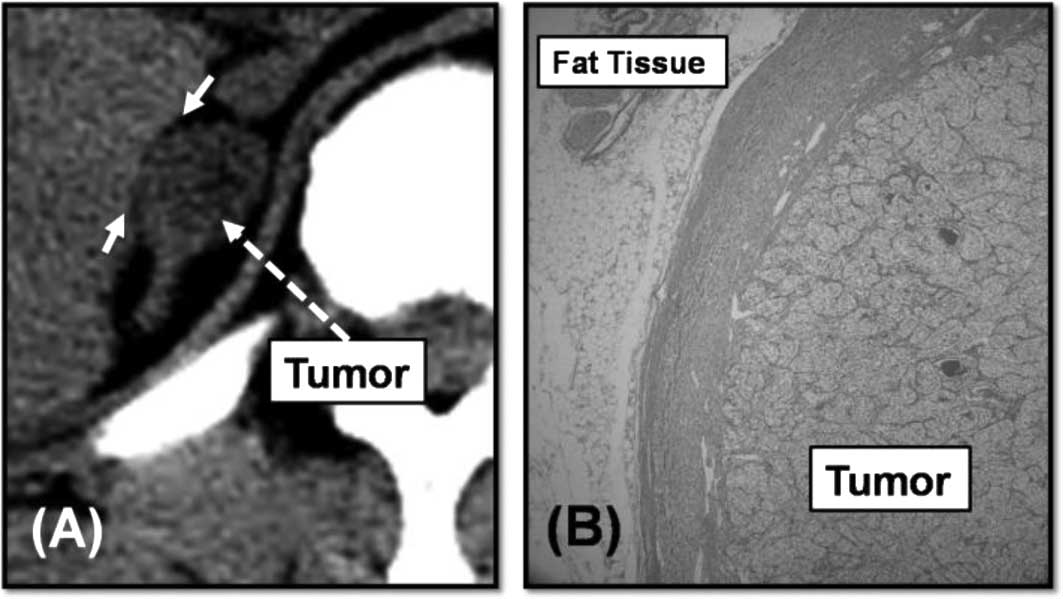
was patient 1. Patient 1 received radical nephrectomy for right
RCC. AM was diagnosed at 16 months after radical nephrectomy. The
tumor size of the AM was 2.5 cm. The tumor shape was round and
smooth and fatty tissue was detected surrounding the tumor by CT
(Fig. 2A). We performed resection
including the surrounding fat. Fatty tissue is required to detach
the tumor and proximal organs. We performed LA for the tumor
without any adhesion. According to the pathological findings, the
adrenal tumor was diagnosed as clear-cell carcinoma, and as the
primary malignancy, and the border between the tumor and the
surrounding fatty tissue was evident (Fig. 2B).
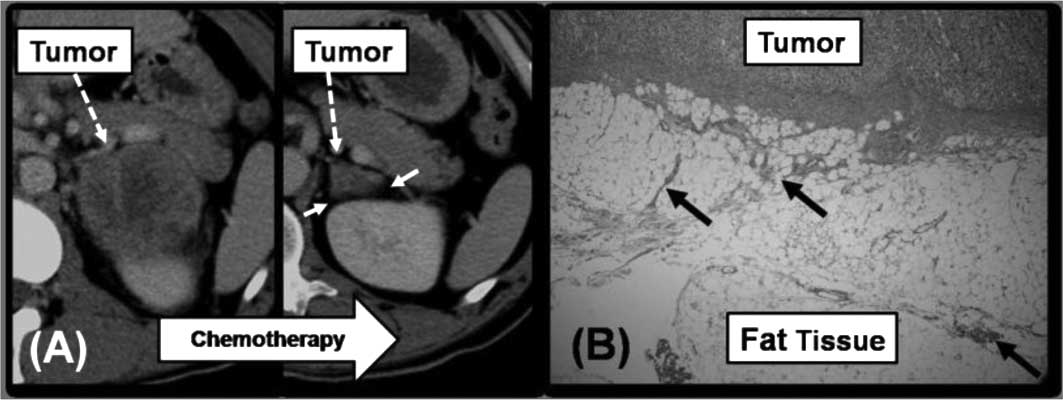
The second pattern was exemplified by patient 4.
Patient 4 received video-assisted thoracic surgery (VATS) for right
lung cancer. The pathological finding of the lung cancer indicated
poorly differentiated adenocarcinoma. Lung cancer and left AM were
diagnosed at a specific point in time with no evidence of extra-AM.
Following adjuvant chemotherapy, the tumor size of AM was reduced
to 2.6 cm, and the fatty tissue surrounding the tumor was detected
by CT (Fig. 3A). We performed LA
for the tumor with slight adhesion. According to the pathological
findings, the adrenal tumor was diagnosed as poorly differentiated
adenocarcinoma and as the primary malignancy, and fibrosis was
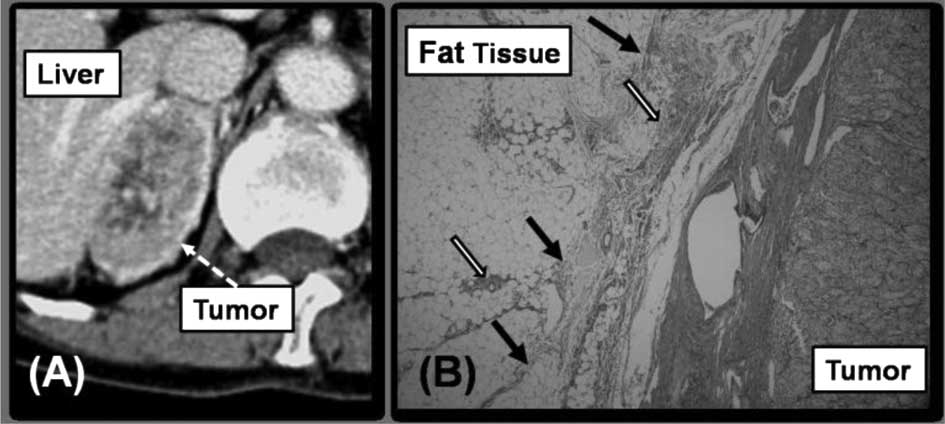
evident in the fatty tissue surrounding the tumor (Fig. 3B). The third pattern was noted in
patient 5 who received radical nephrectomy for right RCC. The
pathological finding of RCC indicated clear-cell carcinoma. AM was
diagnosed 103 months after radical nephrectomy. The tumor size of
AM was 5.5 cm. Fatty tissue surrounding the tumor was not detected
between the tumor and liver, and the contour of the tumor was
irregular in CT (Fig. 4A).
Detaching the tumor and liver was determined to be difficult and an
OA was performed. During the surgical operation, we encountered
strong adhesion between the tumor and the liver. According to the
pathological findings, the adrenal tumor was diagnosed as
clear-cell carcinoma, and as the primary malignancy. Fibrosis and
the infiltration of inflammatory cells were evident in the fatty
tissue between the tumor and the liver (Fig. 4B). Fibrosis and inflammatory cells
play a role in adhesiogenesis (7).
Therefore, the pathological findings of fibrosis and the
infiltration of inflammatory cells in fatty tissue were in
accordance with adhesion in the operative findings.
The fourth pattern was exemplified by patient 7.
This patient was diagnosed with left lung cancer and a right
adrenal tumor, and VATS was performed for the left lung cancer. The
pathological finding of lung cancer indicated a combination of
large-cell carcinoma, spindle carcinoma, giant-cell carcinoma and
adenocarcinoma. The tumor size of AM was 6.7 cm. Fatty tissue
surrounding the tumor was not detected between the right kidney and
the tumor, and MRI revelaed the tumor to have an irregular contour
and to be cystic (Fig. 5A).
Detaching the tumor and kidney was determined to be difficult and a
risk of perforating the cystic component was identified. We
performed OA with nephrectomy as strong adhesion was detected
between AM and the right kidney as expected. According to the
pathological findings, the adrenal tumor was diagnosed as the
primary malignancy, and fibrosis and the infiltration of
inflammatory cells were evident in the fatty tissue between AM and
the right kidney (Fig. 5B). The
pathological findings were also in accordance with the operative
findings for patient 5. We anticipated adhesion between the tumor
and proximal organs from an imaging study. However, adhesion should
be considered as in the case of patient 4. Fatty tissue between the
tumor and proximal organs was detected after chemotherapy, but
there was adhesion in the operative findings, and fibrosis was
noted in the fatty tissue around the tumor in the pathological
findings.
In the present study, no port-site and local
recurrence occurred. Port-site recurrence is reported rarely
(8,9). Local recurrence and port-site
metastases are associated with four factors: natural tumor
behavior, local wound factors, immune and stress responses, and
laparoscopic-related factors such as surgical manipulation
(10). Great care must be taken in
the surgical manipulation to prevent dissemination. We performed
adrenalectomy with minimal handling of the tumor. The adrenal gland
was resected with its surrounding fat to prevent tumor disruption
and there was no port-site and local recurrence; minimal handling
of the tumor and tumor resection with its surrounding fat are
crucial in the prevention of port-site and local recurrence.
Furthermore, the ultrasonically activated scalpel or ultrasonic
endoaspirator should be carefully handled so that it does not touch
the tumor surface as this may create a risk of tumor cell
dissemination.
Various factors aid in the decision-making process
when determining whether the most appropriate approach is LA or OA.
When imaging did not detect fatty tissue around the tumor, we
predicted an irregular contour and adhesion. When fat tissue is not
clearly detected around the tumor, MRI may be more effective due to
its high ability to detect adipose. Detecting adipose may help
avoid incomplete resection, which often means converting LA to OA
(8). Moreover, it was reported that
the volume of blood loss from large adrenal tumors is significantly
greater than that from small tumors (4). Therefore, a large AM has a risk of
extensive blood loss. A tumor with a cystic component that is
difficult to detach due to adhesion presents a risk of perforation
and dissemination.
Moreover, in the case of AM from RCC, a
contralateral case involves a longer operative time and more blood
loss than an ipsilateral case. Therefore, contralateral AM from RCC
is difficult to treat with adrenalectomy. Thus, decision making for
the appropriate surgical management, whether to apply OA or LA, is
crucial.
In conclusion, LA is a less invasive treatment than
OA for AM. However, for complete resection, OA should be selected
for cases where resection by LA is difficult. Therefore, in
decision making regarding the appropriate surgical management with
LA or OA, it is crucial to closely assess pre-operative imaging.
Imaging features supporting OA include: no detection of fatty
tissue between the tumor and proximal organs, tumors with an
irregular contour, large tumors and tumors with a cystic
component.
Abbreviations:
|
AM
|
adrenal metastasis
|
|
LA
|
laparoscopic adrenalectomy
|
|
OA
|
open adrenalectomy
|
|
CT
|
computed tomography
|
|
MRI
|
magnetic resonance imaging
|
|
HU
|
Hounsfield units
|
|
RCC
|
renal cell carcinoma
|
|
NSCLC
|
non-small cell lung cancer
|
|
MODFS
|
median overall disease-free
survival
|
|
DFSR
|
disease-free survival rate
|
References
|
1
|
Gagner M, Lacroix A and Bolté E:
Laparoscopic adrenalectomy in Cushing’s syndrome and
pheochromocytoma. N Engl J Med. 327:10331992.
|
|
2
|
Strong V, D’Angelica M, Tang L, et al:
Laparoscopic adrenalectomy for isolated adrenal metastasis. Ann
Surg Oncol. 14:3392–3400. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Twomey P, Montgomery C and Clark O:
Successful treatment of adrenal metastases from large-cell
carcinoma of the lung. JAMA. 248:581–583. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Suzuki H: Laparoscopic adrenalectomy for
adrenal carcinoma and metastases. Curr Opin Urol. 16:47–53. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eggener E, Yossepowitch O, Kundu S, et al:
Risk score and metastasectomy independently impact prognosis of
patients with recurrent renal cell carcinoma. J Urol. 180:873–878.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu J, Shen P, Wang F, et al: Solitary
adrenal metastasis from invasive ductal breast cancer: an uncommon
finding. World J Surg Oncol. 28:7–10. 2010. View Article : Google Scholar
|
|
7
|
Liakakos T, Thomakos N, Fine M, et al:
Peritoneal adhesions: etiology, pathophysiology, and clinical
significance. Recent advances in prevention and management. Dig
Surg. 18:260–273. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sebag F, Calzolari F, Harding J, et al:
Isolated adrenal metastasis: the role of laparoscopic surgery.
World J Surg. 30:888–892. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weyhe D, Belyaev O, Skawran S, et al: A
case of port-site recurrence after laparoscopic adrenalectomy for
solitary adrenal metastasis. Surg Laparosc Endosc Percutan Tech.
17:218–220. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tanaka K, Hara I, Takenaka A, et al:
Incidence of local and port site recurrence of urologic cancer
after laparoscopic surgery. Urology. 71:728–734. 2008. View Article : Google Scholar : PubMed/NCBI
|