Introduction
Endometrial stromal sarcomas (ESS) are rare
malignancies, accounting for less than 10% of uterine sarcomas
(1). The most characteristic
chromosomal aberration of this tumor type is the translocation
t(7;17)(p15-p21;q12-q21) leading to the fusion of two zinc finger
genes, JAZF1 (juxtaposed with another zinc finger) and
SUZ12 (also known as JJAZ1) (2). Recently, Li et al (3) reported the presence of the neoplastic
JAZF1/SUZ12 fusion transcript in normal cells of human
endometrium. One of the samples found to be positive for the
JAZF1/SUZ12 transcript was the immortalized T HESCs cell
line. This cell line was derived from the stromal cells obtained
from an adult female with myomas and immortalized by transfection
of a human telomerase gene (4).
Since this cell line has a normal karyotype and no fusion of the
two genes occurs at the genomic level, it was proposed that the
JAZF1/SUZ12 transcript in the T HESCs cells is generated
from regulated trans-splicing between precursor RNAs for
JAZF1 and SUZ12 (3).
However, no confirmatory reports exist thus far. To determine
whether the results obtained by Li et al (3) could be reproduced, the T HESCs cell
line was subjected to various RT-PCR for the JAZF1/SUZ12
fusion transcript.
Materials and methods
Materials
The cell line T HESCs was an American Type Culture
Collection (ATCC) culture (ATCC-CRL-4003; Lot No. 3857441)
purchased from LGC Promochem (http://www.lgcpromochem-atcc.com/) and cultured
according to ATCC recommendations. Thus, the cells were grown in
DMEM/F12 medium with 3.1 g/l glucose, 1 mM sodium pyruvate and 1.2
g/l sodium bicarbonate, without phenol red (Invitrogen Cat no.
11039). The medium was supplemented with 1% ITS + Premix (BD Cat
no. 354352) and 10% fetal bovine serum (Invitrogen Cat no.
10106-151). Prior to the molecular investigation, a cytogenetic
analysis verified the previously published karyotype of 46, XX
(3,4). An endometrial stromal sarcoma carrying
the t(7;17)(p15;q12) was used as a positive control for all
analyses.
Methods
Total RNA was extracted from the cell line using the
RNeasy micro kit (Qiagen, Hilden, Germany) and from the tumor
biopsy with TRIzol reagent (Invitrogen, Carlsbad, CA, USA)
according to the manufacturer’s instructions. cDNA was synthesized
using 2.5 μg of total RNA in a 20-μl reaction mixture containing 50
mM Tris-HCl pH 8.3 (at 25°C), 75 mM KCl, 3 mM MgCl2, 10
mM dithiothreitol, 1 mM each dNTP, 48 units RNA inhibitor (RNA
guard; GE Healthcare, Uppsala, Sweden), 25 μM random hexamers and
200 units M-MLV Reverse Transcriptase (Invitrogen). The reaction
was carried out at 37°C for 60 min, heated for 5 min at 65°C and
then maintained at 4°C until analysis. cDNA quality was checked
using ABL1-specific primers (5).
Assays
For the amplification of the JAZF1/SUZ12 fusion
transcript, the assays used were: i) the method described by Micci
et al (6). Briefly, PCR
amplification was performed using 1 μl of the cDNA as a template in
50-μl reaction volume containing 20 mM Tris-HCl (pH 8.4), 50 mM
KCl, 1.25 mM MgCl2, 0.2 mM of each dNTP, 1 unit Platinum Taq
polymerase (Invitrogen) and 0.5 μM of each of the primers
JAZF1-357F CCACAGCAGTGGAAGCCTTA and JJAZ1-843R CCGGGT
TTTGTTTGATTGAGG. To increase the sensitivity of the assay, 1 μl of
the first PCR was re-amplified using the inner primers JAZF1-390F
CCCACCCATCACCCCCTCCT and JJAZ1-827R AGGTCAGGAATCAAAGGCACCTGC in
50-μl reaction volume with the same composition as above. Both the
first and second PCR amplifications were run on a PCT-200 DNA
Engine (MJ Research, Waltham, MA, USA) with the following cycling
profile: an initial denaturation at 94°C for 5 min, followed by 30
cycles of 1 min at 94°C, 1 min at 60°C and 1 min at 72°C and a
final extension for 10 min at 72°C.
ii) A one-step PCR using the primers described by Li
et al (3). The 50-μl
reaction had the same composition as the one mentioned above,
except that the forward primer Fusion-286F AAGATTCAGCCGAAGCTCTCG
and the reverse primer Fusion-541R TGTTTGTTCTGGAGTTTCGATGAGACA were
used.
iii) The method described by Li et al
(3). For the detection of the
JAZF1/SUZ12 fusion transcript, PCR was performed with
Platinum Taq DNA Polymerase High Fidelity (Invitrogen). The 50-μl
reaction volume contained 1X High Fidelity PCR buffer, 0.2 mM of
each dNTP, 2 mM MgSO4, 0.2 μM of each of the forward and
reverse primers, 1 unit Platinum Taq DNA Polymerase High Fidelity
and 1 μl of the cDNA. Four primer combinations were used: i) The
human-JAZF1-284 primer AGAAGATTCAGCCGAAGCTC with Fusion-541R; ii)
the Rhesus-JAZF1-284 primer AGAAGATTCAGCCGAAGCTG with Fusion-541R;
iii) the human-JAZF1-286F primer AAGATTCAGCCGAAGCTCTCG with
Fusion-541R; and iv) the Rhesus-JAZF1-286F AAGATTCAGCCGAAGCTGTCA
with Fusion-541R. The above amplifications were performed using the
touchdown protocol described by Li et al (3). The cycle profile was an initial
denaturation step at 94°C for 30 sec followed by 2 cycles of 30 sec
at 94°C, 30 sec at 66°C and 1 min at 68°C, 2 cycles of 30 sec at
94°C, 30 sec at 64°C and 1 min at 68°C, 4 cycles of 30 sec at 94°C,
30 sec at 62°C and 1 min at 68°C and 37 cycles 30 sec at 94°C, 30
sec at 60°C and 1 min at 68°C.
Amplification of the wild-type and SUZ12
transcripts
For the detection of the wild-type normal JAZF1 and
SUZ12 transcripts, PCR was performed with Platinum Taq DNA
Polymerase High Fidelity (Invitrogen). The 50-μl reaction volume
contained 1X High Fidelity PCR buffer, 0.2 mM of each dNTP, 2 mM
MgSO4, 0.2 μM of each of the forward and reverse
primers, 1 unit Platinum Taq DNA Polymerase High Fidelity and 1 μl
of the cDNA. The JAZF1 wild-type transcript was amplified
using the primers JAZF1-12F GGCTCTCGATGTAGCACCATGACAG and
JAZF1-776R GCTGGTGAGGATTTCTTGGCACAG. The SUZ12 transcript
was amplified using the primers JJAZ1-329F CTGTGGAGGGGGTGGCAGTTACTC
and JJAZ1-827R AGGTCAGGAATCAAAGGCACCTGC.
The cycle profile was an initial denaturation step
at 94°C for 30 sec followed by 35 cycles of 30 sec at 94°C, 30 sec
at 60°C and 1 min at 68°C and a final extension for 5 min at
68°C.
For sequence analyses, the RT-PCR-amplified
fragments were run on 1.5% agarose gels, purified using the Qiagen
gel extraction kit (Qiagen) and directly sequenced using the
dideoxy procedure with an ABI Prism BigDye terminator v1.1 cycle
sequencing kit (PE Applied Biosystems, Foster City, CA, USA) on the
Applied Biosystems Model 3100-Avant DNA sequencing system. The
BLAST software (http://www.ncbi.nlm.nih.gov/BLAST/) was used for the
computer analysis of the sequence data. For comparison, the
reference sequences with accession numbers NM_175061 (JAZF1)
and NM_015355 (SUZ12) were used.
Results
RT-PCR analysis of the T HESCs cell line
for the detection of JAZF1/SUZ12 chimeric transcript
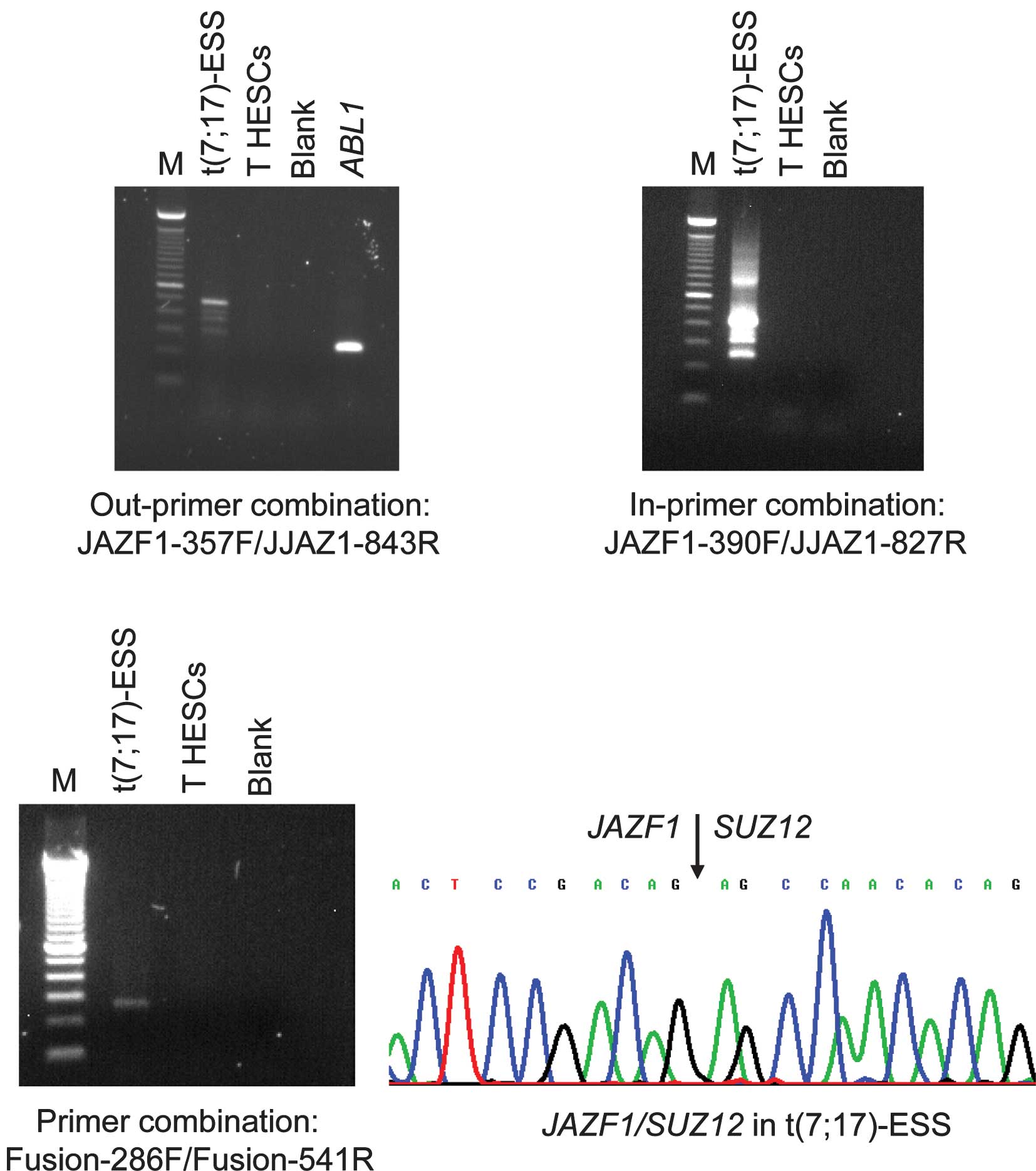
RT-PCR with the JAZF1-357F forward and JJAZ1-843R
reverse primers amplified a 480-bp cDNA fragment from endometrial
stromal sarcoma carrying the t(7;17)(p15;q12), but not from the
immortalized normal T HESCs cell line (Fig. 1). To verify the presence of the
JAZF1/SUZ12 chimeric transcript, the amplified products were
analyzed by direct sequencing (Fig.
1). The fusion was identical to that previously reported, i.e.,
junction of exon 3 of JAZF1 with exon 2 of SUZ12
(2,6). Similarly, nested PCR with the inner
primer combination JAZF1-390F/JJAZ1-827R amplified
JAZF1/SUZ12 cDNA fragments from endometrial stromal sarcoma,
but not from the T HESCs cell line (Fig. 1). The same results were obtained in
PCR with the primer set Fusion-286F/Fusion-541R. To elucidate, the
JAZF1/SUZ12 chimeric transcript was amplified from the
endometrial stromal sarcoma cDNA, but no JAZF1/SUZ12 was
found in the T HESCs cell line (Fig.
1).
The quality of the cDNA synthesis was examined by
amplification of a cDNA fragment of the ABL1 gene, used as
an internal control. A 300-bp ABL1 cDNA fragment was
amplified in the T HESCs cell line, indicating the good quality of
the synthesized cDNA.
Using Platinum Taq DNA Polymerase High Fidelity,
JAZF1/SUZ12 chimeric transcripts were amplified with the
human-JAZF1-284/Fusion-541R and human-JAZF1-286F/Fusion-541R primer
combinations in the positive endometrial stromal sarcoma sample,
but not in the T HESCs cell line (Fig.
2A). The Rhesus-JAZF1-284/Fusion-541R and the
Rhesus-JAZF1-286F/Fusion-541R did not amplify any cDNA fragments
(Fig. 2B). RT-PCR analysis showed
that in the T HESCs cell line, both the normal JAZF1 and
SUZ12 are expressed. Subsequently, the primers JAZF1-12F and
JAZF1-776R amplified a 788-bp cDNA fragment, which corresponds to
the entire open reading of JAZF1. Additionally, the primer
set JJAZ1-329F and JJAZ1-827R generated a 522-bp SUZ12 cDNA
fragment that contained part of exon 1, exons 2-6 and part of exon
7 (Fig. 2).
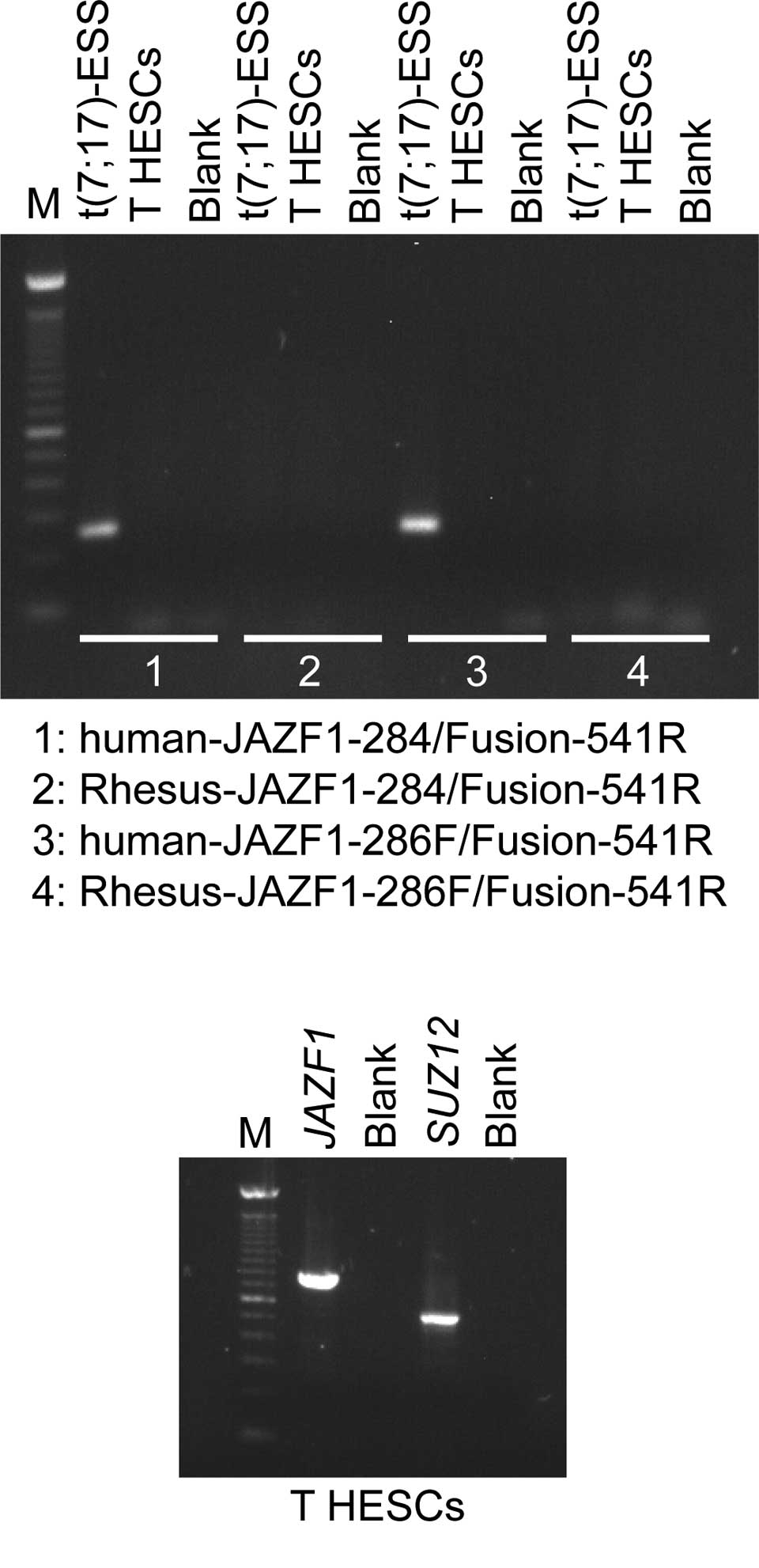 | Figure 2RT-PCR analysis of the T HESCs cell
line. (A) For the detection of the JAZF1/SUZ12 chimeric
transcript, 1 μl cDNA was used as a template in PCR amplification
with the primer combinations: human-JAZF1-284/Fusion-541R,
Rhesus-JAZF1-284/Fusion-541R, human-JAZF1-286F/Fusion-541R and
Rhesus-JAZF1-286F/Fusion-541R, the enzyme Platinum Taq DNA
Polymerase High Fidelity and a touchdown PCR cycling protocol.
Using the human-JAZF1-284/Fusion-541R and
human-JAZF1-286F/Fusion-541R, JAZF1/SUZ12 chimeric
transcripts were amplified in an endometrial stromal sarcoma
carrying the t(7;17) chromosomal aberration [t(7;17)-ESS], whereas
no JAZF1/SUZ12 chimeric transcripts were amplified in the T
HESCs cell line. The Rhesus-JAZF1-284/Fusion-541R and
Rhesus-JAZF1-286F/Fusion-541R did not generate any PCR products.
(B) RT-PCR analysis showed that in the T HESCs cell line, both the
normal JAZF1 and SUZ12 genes are expressed. The
primer set for JAZF1 amplified a cDNA fragment that
corresponds to the entire open reading of JAZF1. The
amplified cDNA fragment of SUZ12 contained part of exon 1,
exons 2–6 and part of exon 7. M, 100-bp DNA ladder. Blank, no RNA
in the cDNA synthesis. |
Discussion
The present results do not support the previous
finding by Li et al (3),
that is the presence of a JUZF1/SUZ12 chimeric transcript in
the immortalized normal T HESCs cell line derived from endometrial
stromal cells. Various RT-PCR assays did not amplify
JUZF1/SUZ12 cDNA fragments in the T HESCs cell line, whereas
the same assays easily generated JUZF1/SUZ12-amplified
transcripts in an endometrial stromal cell sarcoma carrying the
t(7;17) chromosomal aberration (Figs.
1 and 2). The discrepancy
between the present and the previous findings (3) may be due to a different group of cells
examined, different culturing conditions or difference in the PCR
routine. The formation of chimeric cDNA fragments has been reported
as artefacts of the PCR (7,8). The commercially available AMV and MMLV
reverse transcriptase have the ability to switch from one template
to another resulting in recombinant DNA artefacts (9,10). In
addition, thermostable polymerases generate artificial recombinant
fragments by template switching (11–14).
Thus, any amplified PCR product deviated from the anticipated ones
should be considered with great caution.
The inherent RT-PCR risk of cross contamination or
plasmid contamination may be another explanation for the difference
between the findings of the present study and the results of Li
et al (3). In their original
study, in which the fusion JAZF1/SUZ12 was reported for the
first time, Koontz et al examined 10 normal endometria. None
of the endometria had the JAZF1/SUZ12 fusion. (2). In another study, Hrzenjak et al
examined 10 additional normal endometria, but again, none of the
endometria had the JAZF1/SUZ12 fusion (15).
In conclusion, the presence, if any, of a
JUZF1/SUZ12 chimeric transcript, in human normal endometrial
stromal cells is not a constant, reproducible result.
Acknowledgements
This study was supported by The Swedish Childhood
Cancer Foundation.
References
|
1
|
Baker P and Oliva E: Endometrial stromal
tumours of the uterus: a practical approach using conventional
morphology and ancillary techniques. J Clin Pathol. 60:235–243.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Koontz JI, Soreng AL, Nucci M, et al:
Frequent fusion of the JAZF1 and JJAZ1 genes in endometrial stromal
tumors. Proc Natl Acad Sci USA. 98:6348–6353. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li H, Wang J, Mor G and Sklar J: A
neoplastic gene fusion mimics trans-splicing of RNAs in normal
human cells. Science. 321:1357–1361. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Krikun G, Mor G, Alvero A, et al: A novel
immortalized human endometrial stromal cell line with normal
progestational response. Endocrinology. 145:2291–2296. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Panagopoulos I, Mertens F, Domanski HA, et
al: No EWS/FLI1 fusion transcripts in giant-cell tumors of bone.
Int J Cancer. 93:769–772. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Micci F, Walter CU, Teixeira MR, et al:
Cytogenetic and molecular genetic analyses of endometrial stromal
sarcoma: nonrandom involvement of chromosome arms 6p and 7p and
confirmation of JAZF1/JJAZ1 gene fusion in t(7;17). Cancer Genet
Cytogenet. 144:119–124. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brakenhoff RH, Schoenmakers JG and Lubsen
NH: Chimeric cDNA clones: a novel PCR artifact. Nucleic Acids Res.
19:19491991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zeng XC and Wang SX: Evidence that BmTXK
beta-BmKCT cDNA from Chinese scorpion Buthus martensii Karsch is an
artifact generated in the reverse transcription process. FEBS Lett.
520:183–185. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ouhammouch M and Brody EN:
Temperature-dependent template switching during in vitro cDNA
synthesis by the AMV-reverse transcriptase. Nucleic Acids Res.
20:5443–5450. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zaphiropoulos PG: Template switching
generated during reverse transcription? FEBS Lett. 527:3262002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Judo MS, Wedel AB and Wilson C:
Stimulation and suppression of PCR-mediated recombination. Nucleic
Acids Res. 26:1819–1825. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Odelberg SJ, Weiss RB, Hata A and White R:
Template-switching during DNA synthesis by Thermus aquaticus
DNA polymerase I. Nucleic Acids Res. 23:2049–2057. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shammas FV, Heikkila R and Osland A:
Fluorescence-based method for measuring and determining the
mechanisms of recombination in quantitative PCR. Clin Chim Acta.
304:19–28. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zaphiropoulos PG: Non-homologous
recombination mediated by Thermus aquaticus DNA polymerase
I. Evidence supporting a copy choice mechanism. Nucleic Acids Res.
26:2843–2848. 1998.PubMed/NCBI
|
|
15
|
Hrzenjak A, Moinfar F, Tavassoli FA, et
al: JAZF1/JJAZ1 gene fusion in endometrial stromal sarcomas:
molecular analysis by reverse transcriptase-polymerase chain
reaction optimized for paraffin-embedded tissue. J Mol Diagn.
7:388–395. 2005. View Article : Google Scholar
|