Introduction
Nordihydroguaiaretic acid (NDGA) is a non-specific
inhibitor of lipoxygenase. NDGA and its derivatives exhibit
anti-cancer effect on various types of cancer, including cervical
cancer (1–3). High-risk human papillomavirus (HPV)
infection is strongly associated with cervical cancer. The
persistent expression of HPV oncogenes E6 and E7 is crucial for the
induction and maintenance of malignant phenotypes (4). HPV E6 and E7 are well-known for their
ability to inhibit the activity of tumor suppressors p53 and pRb,
respectively (5). Studies have
shown that derivatives of NDGA inhibit the growth of cervical
cancer cells by stabilizing p53 as a result of down-regulated E6
expression, which may be partially responsible for apoptosis
induction or cell cycle arrest at the G1 phase found in
NDGA-treated cells (1,6).
p21 is a key mediator of G1/S transition,
the expression of which is frequently absent or at low levels in
cervical cancer (7), and the
restoration of p21 expression suppresses the growth of cervical
cancer cells (8,9). It is known that regulation of p21
expression occurs primarily at the transcription level in
p53-dependent or p53-independent manners (10) and acetylation of histone H3 within
the p21 promoter is also a key event for effective p21
transcription (11,12). With respect to cervical cancer, the
results of certain studies showed that the p53 state is correlated
with p21 expression (13) and that
restoration of p53 function, either by RNAi or drug treatment
against HPV E6, induces p21 expression (14,15).
This study aimed to investigate the effect of NDGA
on the growth and p21 expression of cervical cancer SiHa cells.
Moreover, the effect of NDGA on levels of histone H3 acetylation,
p53 protein and other factors was investigated in order to
determine the mechanism by which NDGA regulated the p21 gene
expression.
Materials and methods
Cell line
The human cervical cancer cell line SiHa was
obtained from the Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences (Shanghai Institute of Cell Biology,
Chinese Academy of Sciences, China), cultured in Dulbecco’s
modified Eagle’s medium (DMEM; 4.5 g/l glucose; Invitrogen, Grand
Island, NY, USA) supplemented with 10% fetal bovine serum, 100 U/ml
penicillin and 100 μg/ml streptomycin in a 5% CO2
incubator at 37°C.
Reagents
NDGA (Sigma-Aldrich, St. Louis, MO, USA) was
dissolved in dimethyl sulfoxide (DMSO) and the stock solution was
diluted to the required concentrations in DMEM prior to being added
to the cell culture medium. Final DMSO concentrations were ≤0.2%.
Antibodies against Ac-Histone H3 (Lys 9/14) and β-actin were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Antibodies against p53 were purchased from Signalway Antibody
(Pearland, TX, USA). All other chemicals were of reagent grade.
Cell proliferation assay
Cell proliferation was measured by the MTT assay.
SiHa cells were seeded into 96-well culture plates at
5×103 cells per well. Each group consisted of six
parallel wells and was subsequently treated with various
concentrations of NDGA for 24, 48 and 72 h. The control cells were
treated with 2% DMSO in culture medium. Following treatment, the
cells were incubated with MTT reagent (0.5 mg/ml) at 37°C for 4 h.
The resulting formazan crystals were solubilized by adding 150 μl
DMSO to each well. The optical density (OD) at 492 nm was measured
and cell viability was determined using the formula: cell viability
(%) = (absorbance of the treated wells - absorbance of the blank
control wells)/(absorbance of the negative control wells -
absorbance of the blank control wells) × 100%. The MTT experiments
were repeated at least three times.
Flow cytometry analysis
The cell cycle was analyzed using flow cytometry.
Briefly, cells (1×106) were collected and washed twice
in phosphate-buffered saline (PBS), then fixed in 70% alcohol for
30 min at 4°C. After being washed in cold PBS three times, the
cells were resuspended in 1 ml of PBS solution with 40 μg of
propidium iodide (Sigma) and 100 μg of RNase A (Sigma) for 30 min
at 37°C. The samples were then analyzed for their DNA content by
fluorescence activated cell sorting (BD Immunocytometry Systems,
San Jose, CA, USA). Each experiment was repeated at least three
times.
Reverse transcriptase -polymerase chain
reaction (RT-PCR)
Total RNA was isolated from cells using RNAiso Plus
(Takara, Japan) and reverse-transcribed using a reverse
transcription system (Promega, Madison, WI, USA) according to the
manufacturer’s instructions. PCR amplification of different genes
was performed using Easy Taq DNA Polymerase (TransGen Biotech,
China). Table I shows the primer
sequences used for RT-PCR and the product sizes. GAPDH served as an
internal control. The optimal reaction conditions for PCR were
determined for each primer pair. Denaturation was at 94°C for 40
sec and annealing at 56°C for 40 sec, followed by elongation at
72°C for 40 sec. PCR products were analyzed by 1.5%
agarose/ethidium bromide gel electrophoresis. OD values of mRNA
were analyzed with Pro-Gel 4.0 Software and GAPDH was used to
normalize mRNA. Each experiment was repeated at least three
times.
 | Table IPrimer sequences for RT-PCR. |
Table I
Primer sequences for RT-PCR.
| Gene | Sense primer
(5′-3′) | Anti-sense primer
(5′-3′) | Tm (cycles) | Size (bp) |
|---|
| p21 |
TAGCAGCGGAACAAGGAGTCAG |
CAGTCTAGGTGGAGAAACGGG | 56 (30) | 263 |
| SMRT |
GGAATCACGCTCGGAAACAATG |
GGCGGTCTTTGTACAACCTTCA | 58 (30) | 420 |
| GAPDH |
ACCACAGTCCATGCCATCAC |
TCCACCACCCTGTTGCTGTA | 56 (30) | 450 |
Western blot analysis
Nuclear extracts from SiHa cells were prepared using
a Nuclear Protein Extraction kit (BestBio, China). The protein
concentrations were determined using an Enhanced BCA Protein Assay
kit (Beyotime, China). The nuclear proteins (40 μg) were
fractionated using sodium dodecyl sulfate-10% polyacrylamide gels
for electrophoresis (SDS-PAGE) and the proteins were transferred
onto a polyvinylidene fluoride (PVDF) membrane. The membrane was
blocked at room temperature for 4 h with 5% bovine serum albumin in
Tris-buffered saline containing 0.05% Tween-20, 10 mmol/l Tris-HCl
pH 7.5 and 150 mmol/l NaCl, and then incubated with the appropriate
primary antibodies overnight at 4°C. Horseradish
peroxidase-conjugated anti-goat IgG was used as the secondary
antibody, and the protein bands were detected using the enhanced
chemiluminescence method. The relative protein levels were
normalized to β-actin and expressed as percentages of the control.
The membrane was incubated for 30 min at 50°C in buffer that
contained 2% SDS, 62.5 mmol/l Tris (pH 6.7) and 100 mmol/l
2-mercaptoethanol. The membrane was then washed and incubated with
the other primary antibody.
Chromatin immunoprecipitation (ChIP)
assay
ChIP assays were performed using a ChIP Assay kit
(Beyotime) according to the manufacturer’s instructions. In brief,
SiHa cells were fixed by 1% formaldehyde for 10 min at 37°C.
Following washing with cold PBS, the cells were lysed in SDS lysis
buffer and sonicated to shear the DNA to an average fragment size
of 600 bp. The lysates were cleared by centrifugation and
Ac-Histone H3 (Lys 9/14) antibody was added to the supernatants.
Immunoprecipitation from soluble chromatin was conducted overnight
at 4°C. Immune complexes were collected with protein A+G
agarose/salmon sperm DNA for 60 min at 4°C and then extensively
washed. The samples were extracted with elution buffer (1% SDS, 0.1
mol/l NaHCO3) and heated at 65°C for 4 h to reverse
cross links. The DNA was purified and used for amplification of the
p21 or p27 promoter DNA. The primers used for p21 were: forward,
5′-ACCAACGCAGGCGAGGGACT-3′; and reverse, 5′-CCGG
CTCCACAAGGAACTGA-3′ (12). The
primers used for p27 were: forward, 5′-TGGAGAAGCACTGCAGAGAC-3′; and
reverse, 5′-GCGTGTCCTCAGAGTTAGCC-3′ (12). The products were separated by
agarose gel electrophoresis and visualized by ethidium bromide
staining. Each experiment was repeated at least three times.
Statistical analysis
The quantitative variables are presented as means ±
SD. The difference between two groups was assessed using an
independent t-test. The differences among ≥3 groups were compared
using one-way ANOVA. P<0.05 was considered to be statistically
significant.
Results
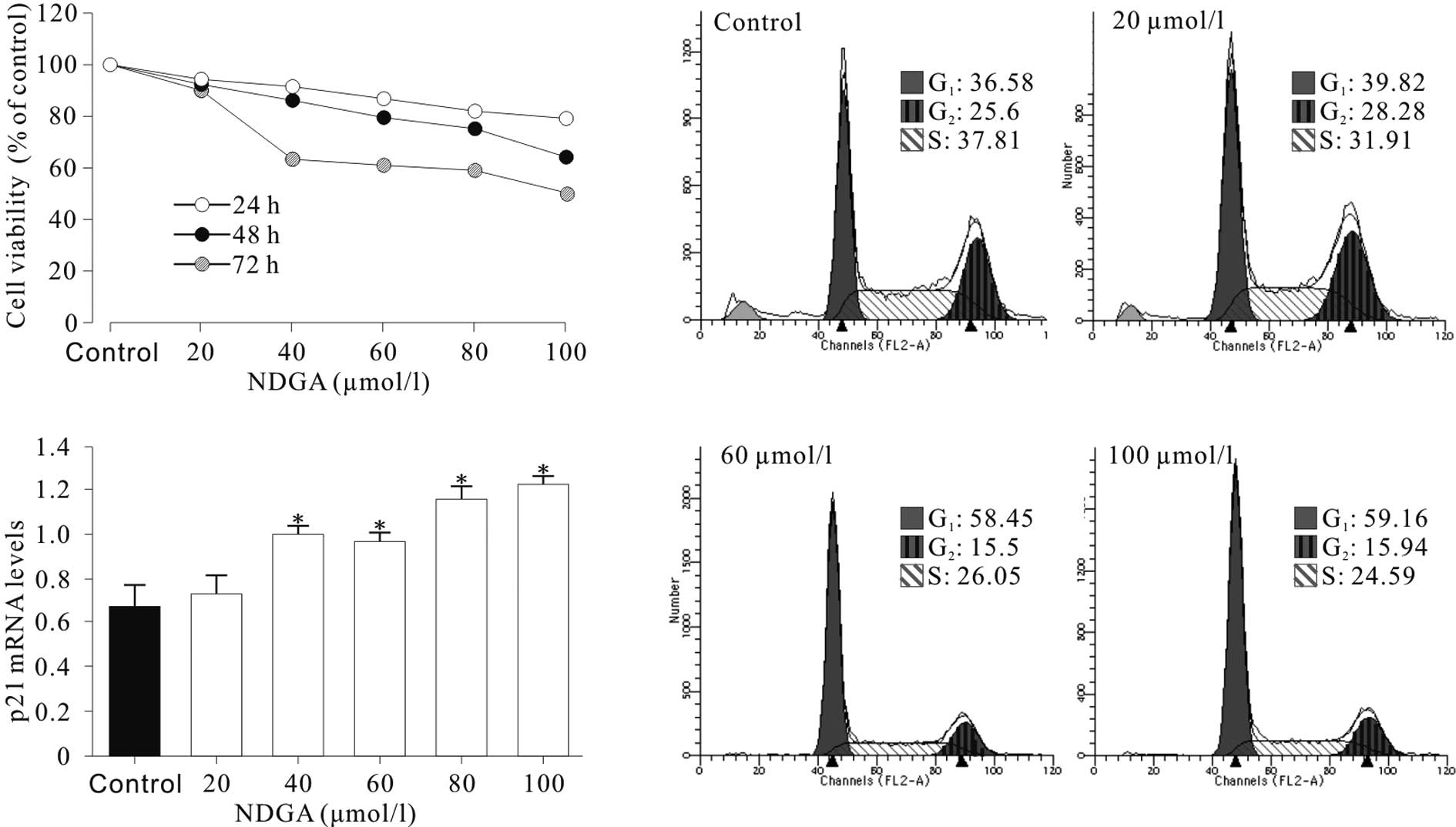
NDGA inhibited SiHa cell growth and
induced cell cycle arrest at G1 phase
The treatment of SiHa cells with various
concentrations of NDGA for 24–72 h resulted in cell growth
inhibition in a dose- and time-dependent manner (Fig. 1A). A cell cycle assay was performed
and it was found that cell cycle progression of SiHa cells was
significantly inhibited at the G1 phase. As shown in
Fig. 1B, the SiHa cells showed a
higher proportion of cells at the G1 phase and a
decrease in the proportion of cells at the G2 phase,
compared to the control cells. Cell cycle distribution analysis
showed that the increase in G1-phase cells observed in
SiHa was significant (P<0.05) and the induction of cell cycle
arrest was dose-dependent. These results demonstrated that NDGA
substantially inhibited the proliferation of SiHa cells and induced
G1 phase arrest in vitro.
NDGA induced cell cycle arrest by
up-regulating p21
To determine the mechanisms of NDGA-induced cell
cycle arrest, the effect of NDGA on the expression of p21, a key
mediator of G1/S transition, was examined. It was found
that NDGA treatment for 72 h significantly increased the levels of
p21 mRNA (Fig. 1C) by up to nearly
2-fold.
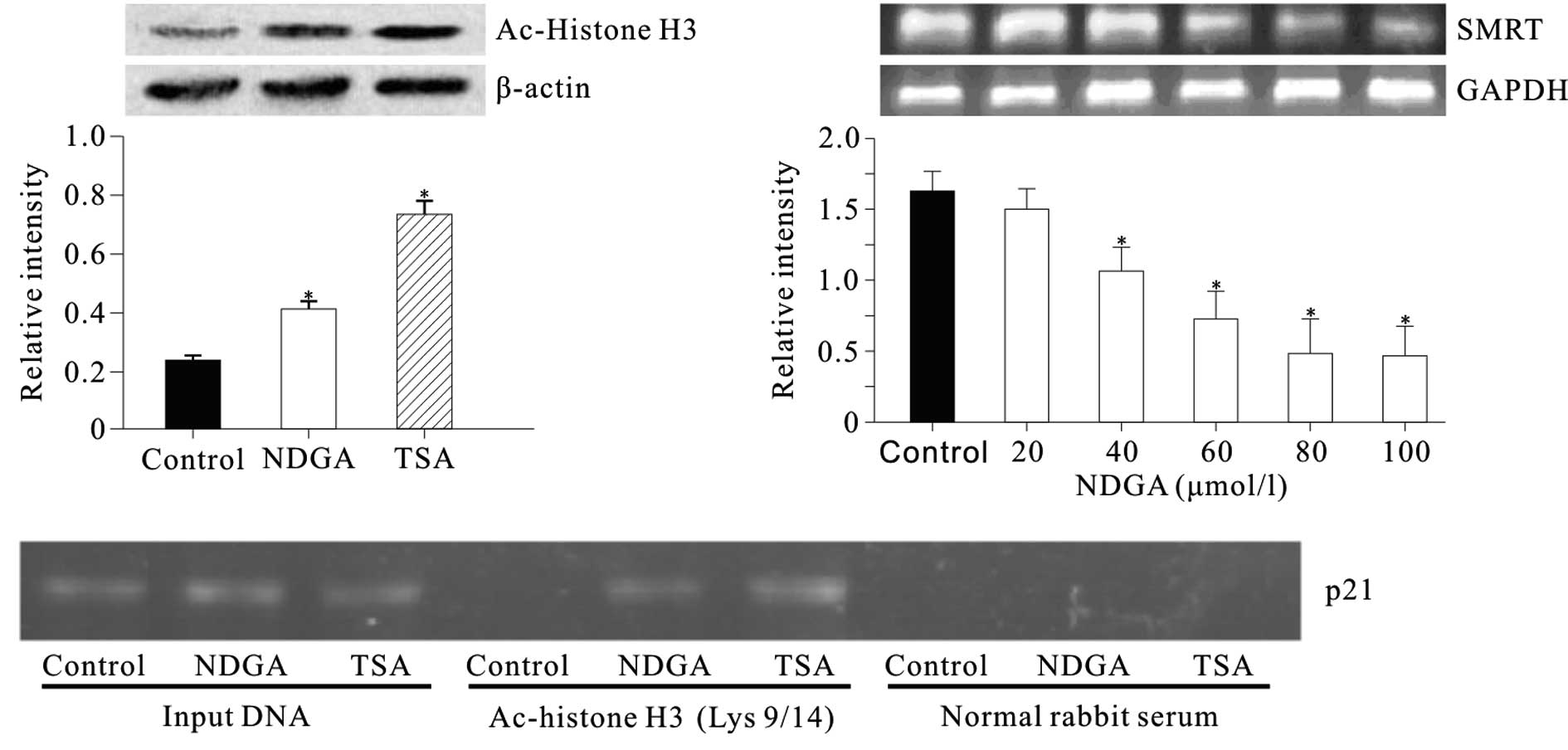
NDGA induced accumulation of acetylated
histone H3
The level of histone H3 acetylation was determined
at Lys 9 and 14 in SiHa cells treated with NDGA. Nucleic proteins
were isolated from cells treated with NDGA (80 μmol/l) for 72 h.
Cells were treated with histone deacetylase inhibitor (HDACi)
trichostatin A (TSA) (300 nmol/l) as a positive control. Western
blot analysis showed that prior to incubation with NDGA, the level
of acetylated histone H3 in SiHa cell was low (Fig. 2A). Incubation with NDGA for 72 h
resulted in the accumulation of acetylated histone H3.
The effect of NDGA on the acetylation of histone H3
associated with the p21 gene promoter was examined by ChIP.
Chromatin fragments from cells cultured with and without NDGA (80
μmol/l) for 72 h were immunoprecipitated with an antibody against
acetylated histone H3 at Lys 9 and Lys 14. DNA from the
immunoprecipitated DNA-protein complex was isolated. From this DNA,
a 324-bp fragment of the p21 promoter region was amplified. In the
control, the acetylated histone H3 was nearly undetectable. After
treatment with NDGA, we observed a significant increase in
acetylated histone H3 within p21 promoter (Fig. 2B).
NDGA down-regulated silencing mediator
for retinoid and thyroid hormone receptors (SMRT) level
To determine the mechanism by which NDGA induced
histone H3 acetylation, the effect of NDGA on SMRT transcription
was detected. The results showed that NDGA inhibited the SMRT mRNA
level in a dose-dependent manner (Fig.
2C).
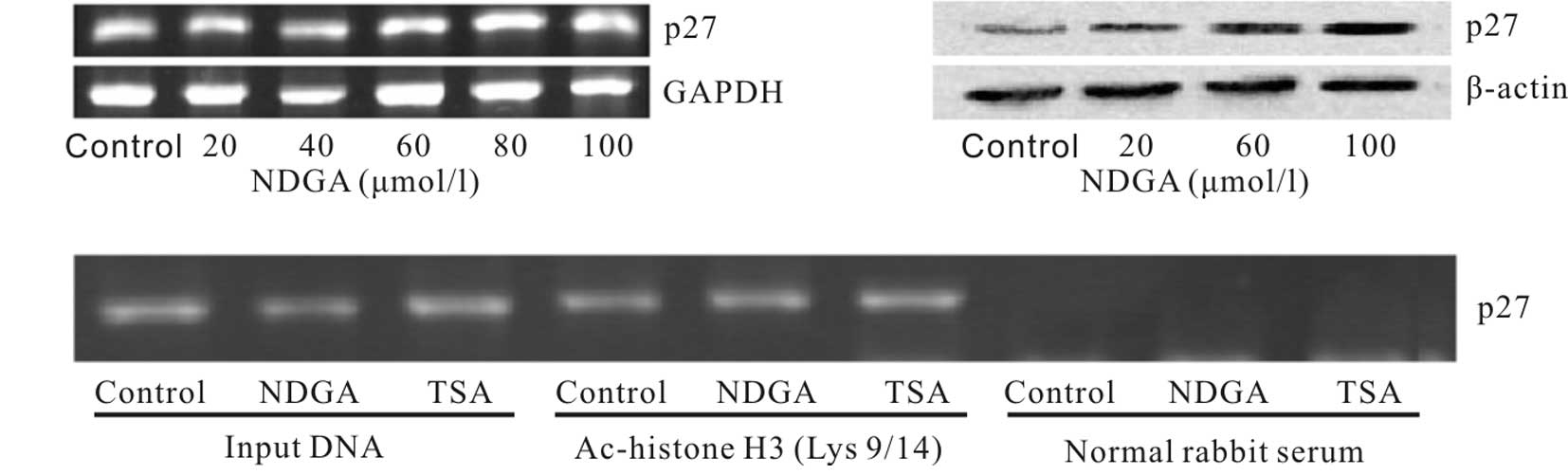
NDGA had no effect on the acetylation of
histones H3 in chromatin associated with p27 gene
To determine whether this effect was selective for a
limited number of genes, the level of histone H3 acetylation in the
p27 gene was examined. The expression of p27 mRNA increased no more
than doubled, while the p27 protein level up-regulated
significantly (Fig. 3A and B). No
change in the levels of histone H3 acetylation was detected
following treatment with NDGA (Fig.
3C). The results suggest that the NDGA-induced changes in
histone acetylation were localized to specific areas of the
chromatin.
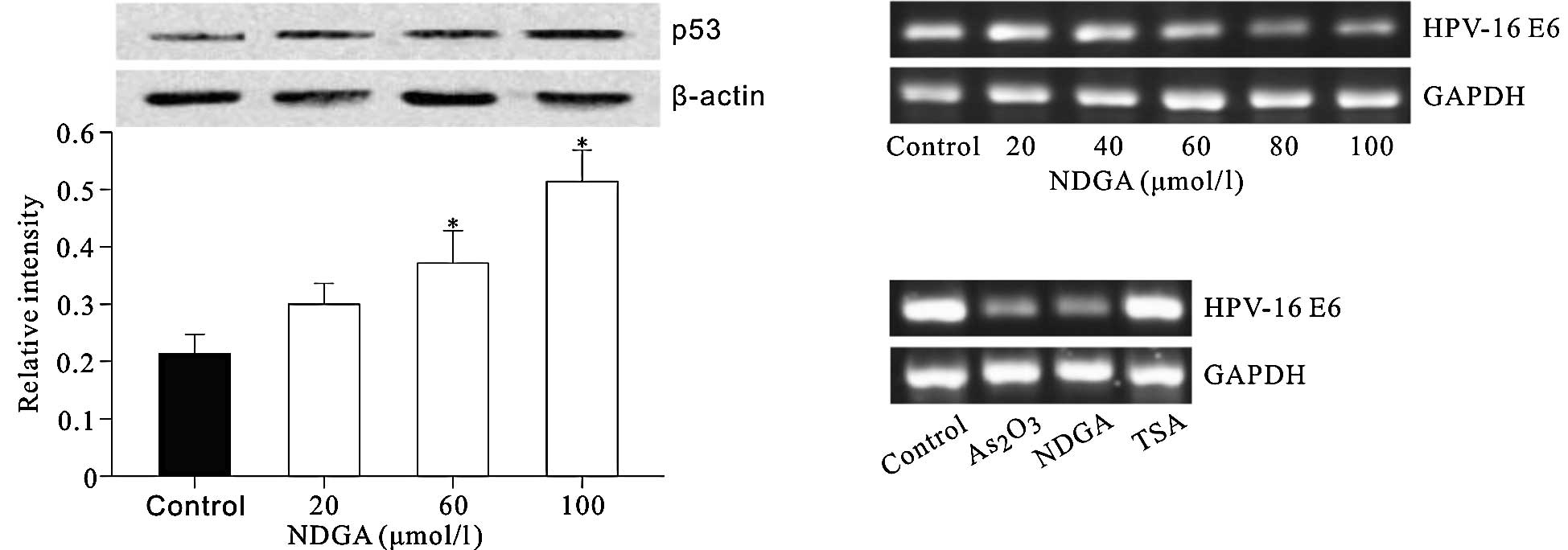
NDGA restored the protein level of p53 by
inhibiting HPV16 E6 expression
The effect of NDGA on the protein level of p53, a
tumor suppressor which is also a critical regulator of p21
transcription, was detected. The results showed that NDGA elevated
the p53 protein level in a dose-dependent manner (Fig. 4A). The effect of NDGA and TSA on the
transcription of HPV16 E6, which induces degradation of p53 was
also investigated. As2O3 was used as a
positive control, as it inhibits the expression of HPV16 E6/E7
(16). The results showed that NDGA
significantly inhibited the HPV16 E6 mRNA level in a dose-dependent
manner (Fig. 4B and C), whereas TSA
had no such effect.
Discussion
NDGA and its derivatives are potentially useful
drugs due to their significant inhibitory effects on various types
of cancer. However, detailed mechanisms that underlie their
anti-cancer roles remain unclear. For this reason, we studied the
effect of NDGA on the growth of cervical cancer SiHa cells and its
mechanism. We found that NDGA treatment significantly inhibited the
growth of SiHa cells and induced cell cycle arrest at the
G1 phase, which may be a result of the up- regulation of
p21, a key mediator of G1/S transition. Based on these
observations, we studied the effect of NDGA on the p53 protein
level and histone H3 acetylation, two key factors for p21
transcription (10).
In cervical cancer cells, HPV E6 abrogates p53
function (5) and the restoration of
p53 activity in SiHa cells increases p21 mRNA (14,17).
As with 3-O-methyl-NDGA, a derivative of NDGA (1), we found that NDGA stabilizes p53
protein by suppressing HPV-16 E6 expression. p53 forms a complex
with Sp1 and simultaneously dissociates HDAC1 from the C terminus
of Sp1, which, in turn, induces p21 expression (18). Moreover, since HPV E6 and E7 are
expressed as bicistronic mRNA, the restoration of p53 function may
be a result of down-regulated HPV E7, the silencing of which
decreases histone deacetylase SIRT1 in SiHa cells (19). Reciprocal regulation occurs between
SIRT1 and p53 in which SIRT1 binds and de-acetylates activated p53
(20), whereas activated p53
down-regulates SIRT1 translation via miR-34a (21).
For the first time, we found that NDGA promoted the
acetylation of histone H3 in total and p21 gene-associated
chromatin, with the latter effect being gene selective, since NDGA
has no impact on the p27 gene. However, NDGA promoted the levels of
p27 mRNA and particularly that of p27 protein, which, together with
p21 and p53, contribute to NDGA-induced cell cycle arrest. It is
already established that acetylation at K9 and K14 of histone H3 is
associated with the relaxation of chromatin for transcription
(22), whereas the acetylation
levels at these sites were very low in the SiHa cells (19,23).
Although wild-type p53 does not induce acetylation at K9 and K14 of
histone H3 (24), it was reported
that the presence of p53 likely inhibits K9 deacetylation and
facilitates K14 acetylation in response to UV irradiation (23). Therefore, other mechanisms exist by
which NDGA induces histone H3 acetylation.
The histone acetylation state is affected by the
balance between histone deacetylases (HDACs) and histone acetyl
transferases (HATs), both of which have various members (25). Of the numerous HATs that exist, p300
plays a crucial role in the regulation of p21 expression (26). However, in SiHa cells p300 loses
function for mutation (27). A
review of the literature showed that HDAC3-HDAC4-N-CoR/SMRT is a
complex that plays a critical role in the regulation of histone
acetylation associated with the p21 promoter, as HDAC4 or SMRT
down-regulation results in increased histone H3 acetylation at the
proximal p21 promoter (28). Our
preliminary result proved that NDGA significantly down-regulated
SMRT transcription. However, whether NDGA affects other components
of this complex remains to be determined.
In conclusion, our results suggest that NDGA induces
p21 transcription by selectively elevating histone H3 acetylation
associated with p21 gene and p53 protein levels via inhibition of
HPV-16 E6 expression.
Acknowledgements
This study was supported by the Scientific Research
Foundation of the Graduate School of Southeast University, P.R.
China (No. YBJJ0915).
References
|
1
|
Allen KL, Tschantz DR, Awad KS, Lynch WP
and DeLucia AL: A plant lignan, 3′-O-methyl-nordihydroguaiaretic
acid, suppresses papillomavirus E6 protein function, stabilizes p53
protein, and induces apoptosis in cervical tumor cells. Mol
Carcinog. 46:564–575. 2007.
|
|
2
|
Meyer GE, Chesler L, Liu D, Gable K,
Maddux BA, Goldenberg DD, Youngren JF, Goldfine ID, Weiss WA,
Matthay KK and Rosenthal SM: Nordihydroguaiaretic acid inhibits
insulin-like growth factor signaling, growth, and survival in human
neuroblastoma cells. J Cell Biochem. 102:1529–1541. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meyers RO, Lambert JD, Hajicek N, Pourpak
A, Kalaitzis JA and Dorr RT: Synthesis, characterization, and
anti-melanoma activity of tetra-O-substituted analogs of
nordihydroguaiaretic acid. Bioorg Med Chem Lett. 19:4752–4755.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zur Hausen H: Papillomavirus infections –
a major cause of human cancers. Biochim Biophys Acta. 1288:F55–F78.
1996.
|
|
5
|
Narisawa-Saito M and Kiyono T: Basic
mechanisms of high-risk human papillomavirus-induced
carcinogenesis: roles of E6 and E7 proteins. Cancer Sci.
98:1505–1511. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao J, Zhao Y, Chen W, Li YM and Bian XW:
The differentiation- inducing effect of Nordy on HPV-16
subgenes-immortalized human endocervical cells H8. Anticancer
Drugs. 19:713–719. 2008. View Article : Google Scholar
|
|
7
|
Bahnassy AA, Zekri AR, Alam El-Din HM,
Aboubakr AA, Kamel K, El-Sabah MT and Mokhtar NM: The role of
cyclins and cyclins inhibitors in the multistep process of
HPV-associated cervical carcinoma. J Egypt Natl Canc Inst.
18:292–302. 2006.PubMed/NCBI
|
|
8
|
Tsao YP, Huang SJ, Chang JL, Hsieh JT,
Pong RC and Chen SL: Adenovirus-mediated p21(WAF1/SDII/CIP1) gene
transfer induces apoptosis of human cervical cancer cell lines. J
Virol. 73:4983–4990. 1999.PubMed/NCBI
|
|
9
|
Hirano K, Hirano M, Zeng Y, Nishimura J,
Hara K, Muta K, Nawata H and Kanaide H: Cloning and functional
expression of a degradation-resistant novel isoform of p27Kip1.
Biochem J. 353:51–57. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abbas T and Dutta A: p21 in cancer:
intricate networks and multiple activities. Nat Rev Cancer.
9:400–414. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen YX, Fang JY, Lu R and Qiu DK:
Expression of p21(WAF1) is related to acetylation of histone H3 in
total chromatin in human colorectal cancer. World J Gastroenterol.
13:2209–2213. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Richon VM, Sandhoff TW, Rifkind RA and
Marks PA: Histone deacetylase inhibitor selectively induces p21WAF1
expression and gene-associated histone acetylation. Proc Natl Acad
Sci USA. 97:10014–10019. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Giannoudis A and Herrington CS:
Differential expression of p53 and p21 in low grade cervical
squamous intraepithelial lesions infected with low, intermediate,
and high risk human papillomaviruses. Cancer. 89:1300–1307. 2000.
View Article : Google Scholar
|
|
14
|
Hietanen S, Lain S, Krausz E, Blattner C
and Lane DP: Activation of p53 in cervical carcinoma cells by small
molecules. Proc Natl Acad Sci USA. 97:8501–8506. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
De-Castro Arce J, Göckel-Krzikalla E and
Rösl F: Retinoic acid receptor beta silences human
papillomavirus-18 oncogene expression by induction of de novo
methylation and heterochromatinization of the viral control region.
J Biol Chem. 282:28520–28529. 2007.PubMed/NCBI
|
|
16
|
Deng Y, Lin C, Zheng J, Fu M, Liang X,
Chen J, Xiao P and Wu M: Overexpression of Bcl-2 partly inhibits
apoptosis of human cervical cancer SiHa cells induced by arsenic
trioxide. Chin Med J (In English). 113:84–88. 2000.PubMed/NCBI
|
|
17
|
Yoshinouchi M, Yamada T, Kizaki M, Fen J,
Koseki T, Ikeda Y, Nishihara T and Yamato K: In vitro and in vivo
growth suppression of human papillomavirus 16-positive cervical
cancer cells by E6 siRNA. Mol Ther. 8:762–768. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lagger G, Doetzlhofer A, Schuettengruber
B, Haidweger E, Simboeck E, Tischler J, Chiocca S, Suske G,
Rotheneder H, Wintersberger E and Seiser C: The tumor suppressor
p53 and histone deacetylase 1 are antagonistic regulators of the
cyclin-dependent kinase inhibitor p21/WAF1/CIP1 gene. Mol Cell
Biol. 23:2669–2679. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Allison SJ, Jiang M and Milner J:
Oncogenic viral protein HPV E7 up-regulates the SIRT1 longevity
protein in human cervical cancer cells. Aging. 1:316–327.
2009.PubMed/NCBI
|
|
20
|
Vaziri H, Dessain SK, Ng Eaton E, Imai SI,
Frye RA, Pandita TK, Guarente L and Weinberg RA: hSIR2(SIRT1)
functions as an NAD-dependent p53 deacetylase. Cell. 107:149–159.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamakuchi M, Ferlito M and Lowenstein CJ:
miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci
USA. 105:13421–13426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jenuwein T and Allis CD: Translating the
histone code. Science. 293:1074–1080. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Allison SJ and Milner J: Loss of p53 has
site-specific effects on histone H3 modification, including serine
10 phosphorylation important for maintenance of ploidy. Cancer Res.
63:6674–6679. 2003.PubMed/NCBI
|
|
24
|
Warnock LJ, Adamson R, Lynch CJ and Milner
J: Crosstalk between site-specific modifications on p53 and histone
H3. Oncogene. 27:1639–1644. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eberharter A and Becker PB: Histone
acetylation: a switch between repressive and permissive chromatin.
Second in review series on chromatin dynamics. EMBO Rep. 3:224–229.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xiao H, Hasegawa T and Isobe K: p300
collaborates with Sp1 and Sp3 in p21(waf1/cip1) promoter activation
induced by histone deacetylase inhibitor. J Biol Chem.
275:1371–1376. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ohshima T, Suganuma T and Ikeda M: A novel
mutation lacking the bromodomain of the transcriptional coactivator
p300 in the SiHa cervical carcinoma cell line. Biochem Biophys Res
Commun. 281:569–575. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wilson AJ, Byun DS, Nasser S, Murray LB,
Ayyanar K, Arango D, Figueroa M, Melnick A, Kao GD, Augenlicht LH
and Mariadason JM: HDAC4 promotes growth of colon cancer cells via
repression of p21. Mol Biol Cell. 19:4062–4075. 2008. View Article : Google Scholar : PubMed/NCBI
|