Introduction
Pituicytoma is a rare benign neoplasm occurring in
the sellar and suprasellar regions and it originates in the
neurohypophysis or infundibulum. Scothorne reported the first case
in 1955 (1). Since then, 28
additional cases (not including the present two cases) of this
neoplasm have been reported (1–14),
including one autopsy case (5). It
is difficult to identify pituicytomas from other sellar and
suprasellar neoplasms, such as granular cell tumors and pilocytic
astrocytomas. The diagnosis of the neoplasms was based on a
pathological examination. The neoplasms were found to be
histologically benign, but their hypervascular nature makes
surgical resection difficult (2).
Local recurrence following subtotal resection is common (3). The clinical and surgical features of
two cases of pituicytomas and the relevant literature is reviewed
and evaluated.
Case report
Case one
A 47-year-old female without prior medical history
described a six-month history of headaches and menstrual disorder.
Visual function was normal, and preoperative neuroendocrine studies
were normal apart from a slightly elevated prolactin level of 45
ng/ml, thought to be secondary to the pituitary stalk disinhibition
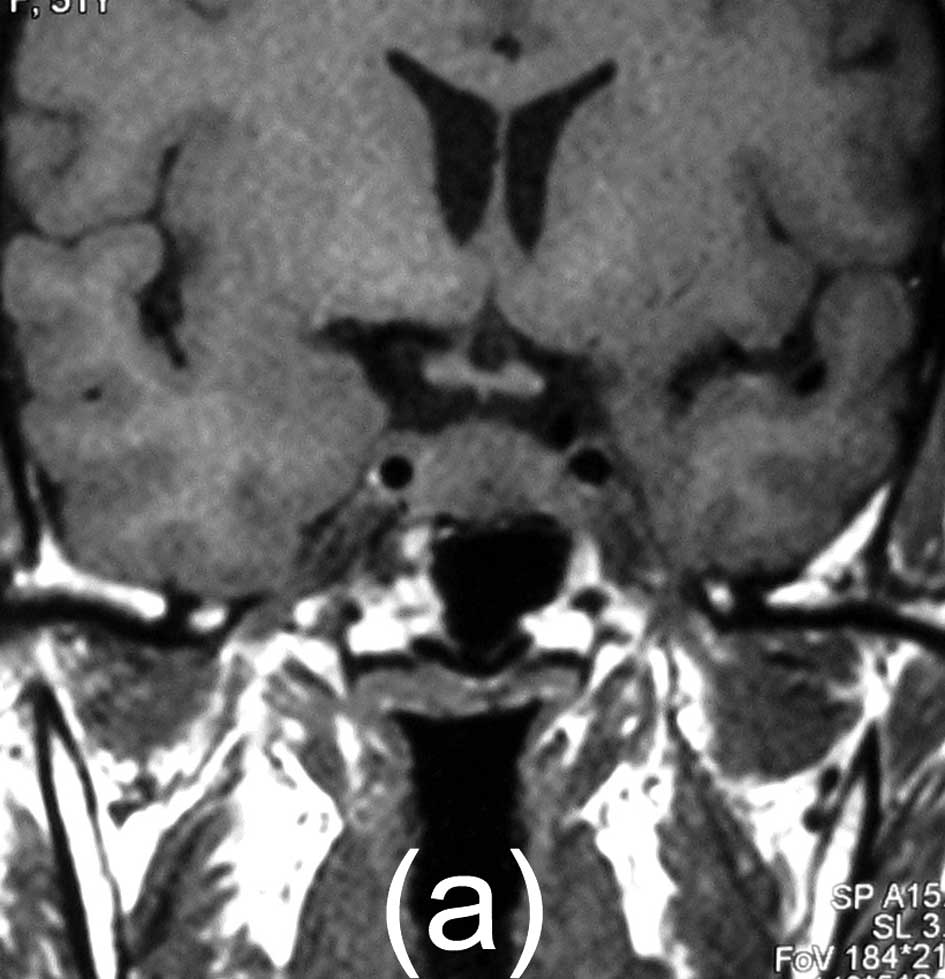
effect. Magnetic resonance imaging (MRI) scans showed a 16×13×10 mm
solid mass. The mass exhibited intermediate signal intensity on
T1-weighted images, intermediate, slightly increased signal
intensity on T2-weighted images, and marked homogeneous enhancement
with contrast administration, and the neurohypophysis signal had
disappeared. It appeared to be centered on the posterior lobe. The
computed tomography (CT) images did not reveal calcifications,
necrosis, bone destruction or hyperostosis.
A transsphenoidal microsurgical approach was
selected for tumor resection. A soft, reddish, minor vascular mass
was found in the neurohypophysis. A total resection was performed,
in order topreserve the pituitary stalk, and normal hypophysis was
observed during the resection. The final pathological diagnosis was
pituicytoma. No adjuvant treatment was administered. No residual or
regrowth of the tumor was noted, as shown by MRI scans, over a
follow-up period of 18 months (Fig.
1).
Case two
A 51-year-old female presented with one year of
visual complaints, six months of diabetes insipidus and two months
of headaches. Visual field testing showed left- temporal
hemianopsia. The results of preoperative neuroendocrine studies
were normal apart from an elevated prolactin level of 188 ng/ml.
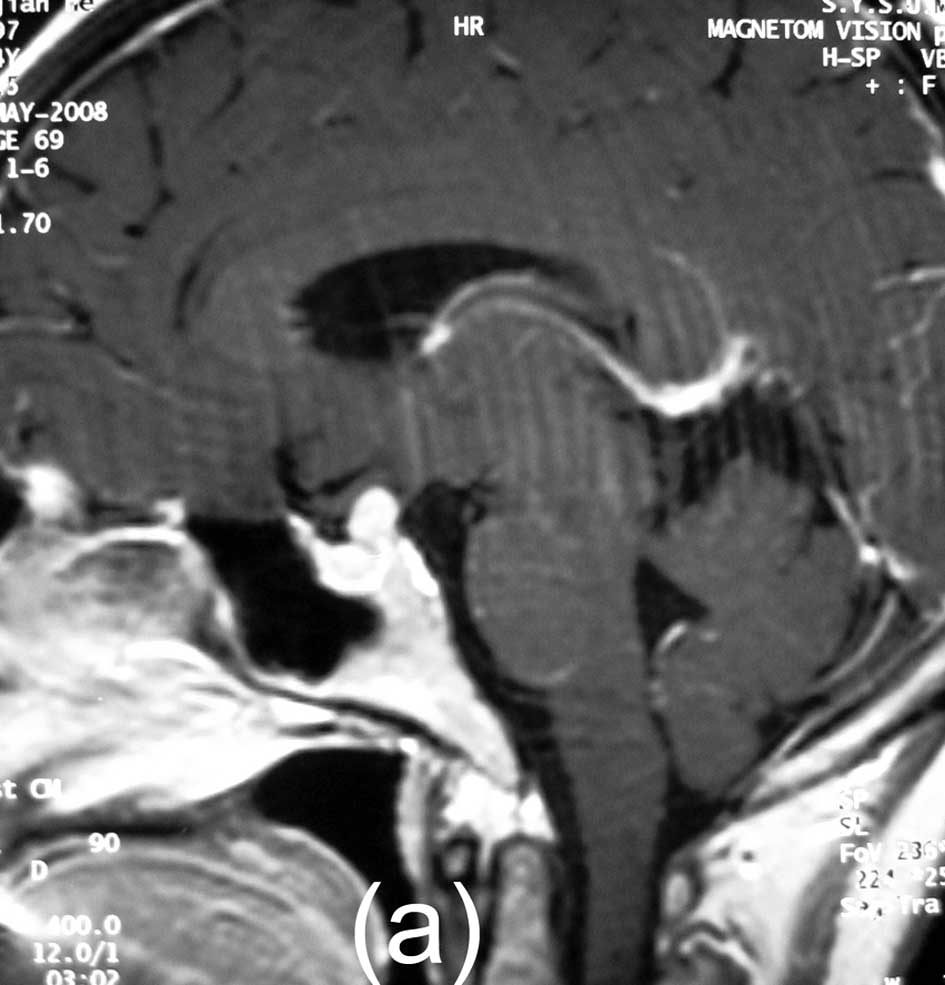
The MRI scan showed a suprasellar and post-chiasmatic mass, and the
tumor measured 9×7×6 mm. The mass exhibited intermediate signal
intensity on T1-weighted images, intermediate, slightly increased
signal intensity on T2-weighted images, and marked homogeneous
enhancement with contrast administration. The CT images showed no
calcifications, necrosis, bone destruction or hyperostosis. The
mass was attached to the pituitary stalk and infundibulum, and
appeared to have originated from the pituitary stalk, causing
compression of the left optic chiasm.
The patient underwent a right pterional craniotomy
resection. A soft, reddish, moderately vascular mass was adherent
to the pituitary stalk. A total resection was performed, to
preserve the pituitary stalk. The visual acuity and visual field
defects improved after surgery. The diabetes insipidus was
moderated after surgery, and desmopressin was prescribed at a dose
of 0.1 mg/day. The patient was discharged on a low dose of
dexamethasone. Endocrinological studies after discharge revealed
panhypopituitarism requiring thyroid and adrenal hormone
replacement. A follow-up MRI scan obtained with gadolinium showed
no residual mass 11 months after the surgery (Fig. 2).
Pathological findings
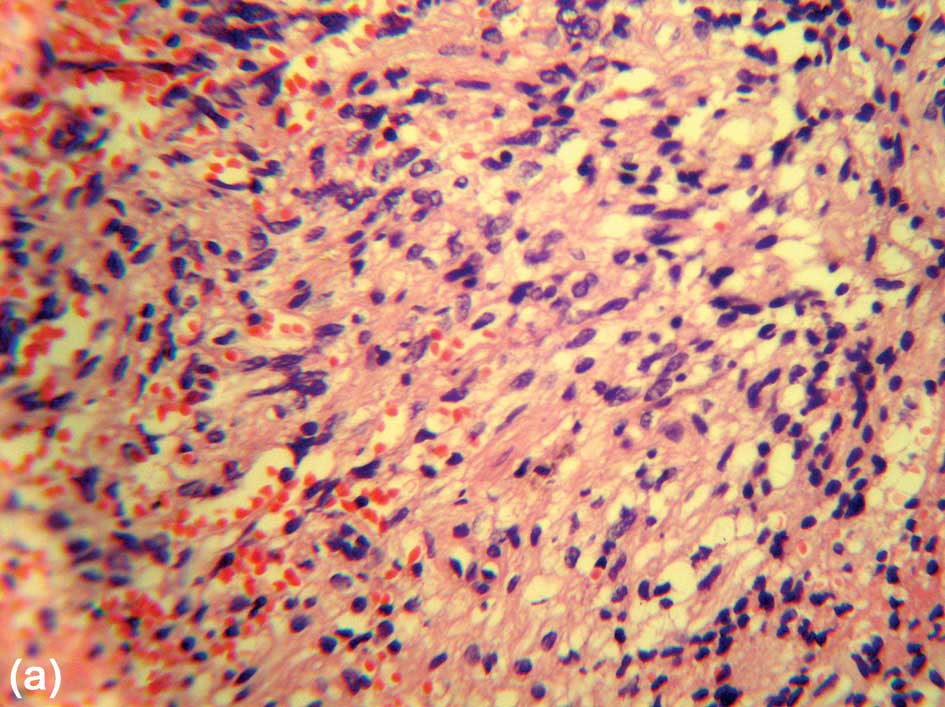
A hematoxylin and eosin (H&E) stain showed that
the tumor was predominantly a compact structure consisting of
elongated, bipolar spindle cells arranged in interlacing fascicles
or assuming a storiform pattern (Fig.
3). The cytoplasm was lightly eosinophilic. No significant
cellular or nuclear pleomorphism, mitotic activity, necrosis,
cytoplasmic granules, eosinophilic granular bodies or evidence of
brain invasion were observed.
Immunohistochemical stain was performed on
formalin-fixed paraffin-embedded tissue using the Envision system
(DakoCytomation, Carpinteria, CA, USA). Antigen retrieval was
performed by utilizing steam heat. Primary antibodies against the
following antigens were used: S-100 protein (polyclonal,
ready-to-use, Code no. IR504, Dako), vimentin (monoclonal,
ready-to-use, N-Series primary antibody, Code no. 1583, Dako),
epithelial membrane antigen (EMA, monoclonal, ready-to-use, Dako),
glial fibrillary acidic protein (GFAP, polyclonal, 1:500, Dako),
Ki-67 (monoclonal MIB-1, 1:50, Code no. M7248, Dako),
neuron-specific enolase (NSE, Code no. IR612, ready-to-use, Dako),
neurofilament (monoclonal, Code no. IR607, ready-to-use, Dako ),
chromogranin A (polyclonal, Code no. A0430, 1:500, Dako), prolactin
(polyclonal, Code no. A0569, 1:200, Dako), catabolite gene
activator (CgA) (polyclonal, Code no. A0569, 1:200, Dako), growth
hormone (polyclonal, Code no. A0570, 1:400, Dako), follicle
stimulating hormone (monoclonal, Code no. M3504, 1:50, Dako),
luteinizing hormone (monoclonal, Code no. M3502, 1:50, Dako),
thyroid stimulating hormone (monoclonal, Code no. M3503, 1:50,
Dako) and adrenocorticotropin (monoclonal, Code no. M3501, 1:50,
Dako). The tumor showed diffuse strong immunoreactivity of S-100
protein, vimentin and EMA, and GFAP showed a focal, weak positive
reaction. Negative reactions were noted for NSE, neurofilament,
chromogranin A, CgA and synaptophysin. Ki-67 (MIB-1) labeling was
<1%. The anterior pituitary hormone was also negative.
Discussion
A total of 1,490 consecutive patients underwent
transsphenoidal resections of the pituitary adenomas in our
hospital between January 1996 and December 2008. Approximately half
of the adenomas were macroadenomas (15). Among these, we identified two
pituicytomas. A total of 30 bona fide examples have been
described thus far, often as case reports. Pituicytomas are rare
and little is known about the epidemiology (4). There appears to be a slight
predominance in males (14,16). Since the cell of origin remained
undetermined, nosological confusion surrounded the use of the term
pituicytoma, which is also termed infundibuloma, granular cell
myoblastoma, choristoma and granular cell tumor (16). Pituicytoma is now included and
described as an indolent World Health Organization grade 1 tumor
that involves the posterior pituitary and/or its stalk, is composed
of spindle cells, and is derived from pituicytes (17). Pituicytomas appear to be benign,
slow-growing tumors presenting as the result of symptoms from mass
effect.
As previously reported (1–13), 12
of the 30 patients (40%) presented with decreased visual acuity or
visual field defects, caused by compression of the optic nerves
and/or chiasm. A total of 16 of the 30 patients (53%) presented
with signs of pituitary insufficiency, with the most common
endocrinopathy being decreased libido, amenorrhea, hypopituitarism
and hypogonadism. A total of 5 patients presented with both visual
and endocrinopathic symptoms. A total of 7 of the 30 patients (23%)
presented with headaches. A number of patients also had vague
complaints of memory changes, dizziness and fatigue. Only 1 patient
presented with tumor apoplexy (6).
Of our cases, only 1 patient presented with diabetes insipidus,
which was uncommon. These clinical presentations are in concordance
with a sellar or suprasellar area mass.
The CT findings are not well reported, as the CT
contrast-enhanced axial scans revealed heterogeneous enhancement in
1 patient (Fig. 2). The majority of
the reported MRI findings of pituicytomas are isointense on
T1-weighted images and hyperintense on T2-weighted images, with
homogeneous enhancement following the administration of gadolinium
(4,12). This presentation was different from
the inhomogeneity of granular cell tumors (4). The rapidity of enhancement correlates
with the increased vascularity of the lesions, as reported by Gibbs
et al (11), Wolfe et al (4) and Uesaka et al (9), and the angiogram revealed a
significant vascular blush (4,9).
As previously described (4), the characteristic features of
pituicytomas that distinguish them from pilocytic astrocytomas or
schwannomas include strongly positive staining for S-100 and
vimentin, and focally positive staining for GFAP. EMA showed a
strong expression in the 2 patients. The transmembrane glycoprotein
of EMA is encoded by the MUC1 gene, located on chromosome 1
in the 1q21–24 region. EMA is present in numerous epithelial cells.
EMA, an integral mucin complex, is representative of the antigens
involved in the secretory processes of glandular epithelia, which
change quantitatively during specialization, differentiation and
neoplastic transformation (18).
However, the role of EMA in pituicytomas requires further study.
GFAP is a 54 kDa, type III intermediate filament protein that is
the major constituent of glial filaments in astrocytes (19). GFAP is considered to be a general
marker for astrocytes in the central nervous system (20). The accurate diagnosis of brain
invasion is therefore critical and, in borderline cases, an
immunohistochemical stain for GFAP aids in the delineation of
entrapped glial elements within the substance of a brain-invasive
meningioma (21).
Our cases are noteworthy for various reasons.
Firstly, they add another possible entity to the differential
diagnosis of a pituitary stalk and diabetes insipidus mass. The
tumor originated in the pituitary stalk in one of our patients.
Notably, fewer symptoms of diabetes insipidus were found at
presentation than anticipated and were clearly reported in only one
case (7). Pituitary stalk lesions
are often identified to investigate symptoms such as diabetes
insipidus. As noted in the literature, patients who develop
pathology involving the stalk commonly present with varying degrees
of hypopituitarism, diabetes insipidus and hyperprolactinemia
(22).
On the other hand, the two tumors were not highly
vascular, and the patients exhibited moderate bleeding from the
tumors, which were completely resected during the surgery. Another
reason that the two tumors may have been much easier to excise
without significant bleeding was due to their smaller size. These
findings are not in agreement with some authors. Ulm et al
reported that pituicytomas generally were highly vascular, and
significant bleeding is often encountered during resection
(3,7–10).
These authors correctly call attention to the fact that surgery may
be difficult due to the location and marked vascularity of the
tumors. Heavy intraoperative bleeding hindered a complete resection
in certain patients.
No adjuvant treatment was administered to the 2
patients. Follow-up MRI scanning obtained with gadolinium showed no
residual or regrowth mass 18 and 11 months post-operatively.
Recurrence following a subtotal resection is common, occurring in 4
of the 7 cases reported in the literature for which a follow-up of
greater than six months was available (2,3,7–9).
With total resection, the prognosis is favorable. None of the
patients receiving total resection had recurrences at the reported
follow-ups. The highly vascular nature of the tumor and its
potential for infiltration can make total resection difficult.
Subtotal resection due to extensive bleeding occurred in the two
cases reported by Ulm et al (3). A total of 3 patients with subtotal
resection underwent postoperative radiation, including 2 with
radiosurgery and 1 with fractionated radiation. The role of
radiation therapy is uncertain in this rare lesion. Since few cases
of pituicytoma have been treated with radiation, and given that the
natural history appears similar to that of granular cell tumor, the
data available currently should be utilized. The authors are in
agreement with Brat et al, who noted that completeness of
excision was the most significant predictor of recurrence (2), and that surgery is the first choice of
treatment for patients with a pituicytoma.
References
|
1
|
Scothorne CM: A glioma of the posterior
lobe of the pituitary gland. J Pathol Bacteriol. 69:109–112. 1955.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brat DJ, Scheithauer BW, Staugaitis SM,
Holtzman RN, Morgello S and Burger PC: Pituicytoma: a distinctive
lowgrade glioma of the neurohypophysis. Am J Surg Pathol.
24:362–368. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ulm AJ, Yachnis AT, Brat DJ and Rhoton AL
Jr: Pituicytoma: reports of two cases and clues regarding
histogenesis. Neurosurgery. 54:753–757. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wolfe SQ, Bruce J and Morcos JJ:
Pituicytoma: case report. Neurosurgery. 63:E173–E174. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takei H, Goodman JC, Tanaka S,
Bhattacharjee MB, Bahrami A and Powell SZ: Pituicytoma incidentally
found at autopsy. Pathol Int. 55:745–749. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Benveniste RJ, Purohit D and Byun H:
Pituicytoma presenting with spontaneous hemorrhage. Pituitary.
9:53–58. 2006. View Article : Google Scholar
|
|
7
|
Figarella-branger D, Dufour H, Femandez C,
Bouvier-Labit C, Grisoli F and Pellissier JF: Pituicytomas, a
mis-diagnosed benign tumor of the neurohypophysis: report of three
cases. Acta Neuropathol. 104:313–319. 2002.PubMed/NCBI
|
|
8
|
Kowalski RJ, Prayson RA and Mayberg MR:
Pituicytoma. Ann Diagn Pathol. 8:290–294. 2004. View Article : Google Scholar
|
|
9
|
Uesaka T, Miyazono M, Nishio S and Iwaki
T: Astrocytoma of the pituitary gland (pituicytoma): case report.
Neuroradiology. 44:123–125. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Katsuta T, Inoue T, Nakagaki H, Takeshita
M, Morimoto K and Iwaki T: Distinctions between pituicytoma and
ordinary pilocytic astrocytoma. J Neurosurg. 98:404–406. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gibbs WN, Monukie S, Linskey ME and Hasso
AN: Pituicytoma: diagnostic features on selective carotid
angiography and MR imaging. Am J Neuroradiol. 27:1639–1642.
2006.PubMed/NCBI
|
|
12
|
Thiryayi WA, Gnanalingham KK, Reid H,
Heald A and Kearney T: Pituicytoma: a misdiagnosed benign tumour of
the posterior pituitary. Br J Neurosurg. 21:47–48. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakasu Y, Nakasu S, Saito A, Horiguchi S
and Kameya T: Pituicytoma: two case reports. Neurol Med Chir
(Tokyo). 46:152–156. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shah B, Lipper MH, Laws ER, Lopes B and
Spellman MJ: Posterior pituitary astrocytoma: a rare tumor of the
neurohypophysis: a case report. Am J Neuroradiol. 26:1858–1861.
2005.PubMed/NCBI
|
|
15
|
Han ZL, He DS, Mao ZG and Wang HJ:
Cerebrospinal fluid rhinorrhea following trans-sphenoidal pituitary
macroadenoma surgery: experience from 592 patients. Clin Neurol
Neurosurg. 110:570–579. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rossi ML, Bevan JS, Esiri MM, Hughes JT
and Adams CB: Pituicytoma (pilocytic astrocytoma). Case report J
Neurosurg. 67:768–772. 1987.PubMed/NCBI
|
|
17
|
Rousseaua A, Mokhtaria K and Duyckaertsa
C: The 2007 WHO classification of tumors of the central nervous
system – what has changed? Curr Opin Neurol. 21:720–727. 2008.
|
|
18
|
Shashikant C, Talal AS and Georges D:
Epithelial membrane antigen in hematolymphoid neoplasms: a review.
Appl Immunohistochem. 5:203–215. 1997. View Article : Google Scholar
|
|
19
|
Eng LF, Ghirnikar RS and Lee YL: Glial
fibrillary acidic protein: GFAP-thirty-one-years (1969–2000).
Neurochem Res. 25:1439–1451. 2000.PubMed/NCBI
|
|
20
|
Huang QL, Zhao SF, Gaudin A, Quennedey B
and Gascuel J: Glial fibrillary acidic protein and vimentin
expression in the frog olfactory system during metamorphosis. Neuro
Report. 16:1439–1442. 2005.
|
|
21
|
Trembath D, Miller CR and Perry A: Gray
zones in brain tumor classification evolving concepts. Adv Anat
Pathol. 15:287–297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rupp D and Molitch M: Pituitary stalk
lesions. Curr Opin Endocrinol Diabetes Obes. 15:339–345. 2008.
View Article : Google Scholar
|