Introduction
As a member of the epidermal growth factor receptor
family, HER-2 (also termed ErbB-2 or neu), encodes a 185-kDa type I
transmembrane receptor tyrosine kinase (1), operates a complex signal transduction
cascade and modulates proliferation, migration, adhesion,
differentiation and apoptosis of cancer cells (2,3). HER-2
protein overexpression has been identified in a number of human
carcinomas, including colorectal cancer (CRC) (4–7).
Clinical data showed that Trastuzumab (Herceptin) affected survival
in approximately 20% of patients who overexpressed HER-2 in breast
cancer (8). These data indicated
that HER-2 amplification is one of the genes to be considered in
the therapeutic management of CRC.
In China, CRC is one of the three cancers whose
incidence increased most (together with lung and female breast
cancer) between 1991 and 2005 (9,10).
Genetic and environmental factors contribute to the disease
etiology, with approximately one-third of disease variance
attributed to inherited genetic factors (11). The role of copy-number variations
(CNVs) in various types of cancer has become a hot spot over the
past few years (12,13). Studies using SNP arrays and aCGH
have suggested that DNA amplification at chromosome position 17q21,
the chromosomal locus of HER-2, is common in breast cancers
(14–17). However, the correlation between CNVs
and HER-2 overexpression in CRC has yet to be determined.
Furthermore, the majority of aCGH experiments focused on the
genome-wide screening of CNVs and the data obtained are generally
informative, but not definitive. Thus, a study comprehensively
examining CNVs in relation to HER-2 expression or prognosis should
be performed using a large number of tumors.
In this study, 134 colorectal adenocarcinoma
samples, with matched adjacent normal tissues, were collected from
the Chinese population to assess CNVs. Results showed that the copy
number gains of HER-2 were observed in a high percentage of CRC
samples. Furthermore, mRNA was overexpressed in the majority of the
CRC samples. A positive correlation was found between gains of
HER-2 and mRNA overexpression. These findings suggested the
potential role of CNV of HER-2 in CRCs.
Materials and methods
Patients and tissue collection
CRC samples were obtained from 134 surgical patients
of the Department of Gastroenterology, Shenzhen Hospital, and the
Peking University, China. Adjacent normal mucosa samples located at
least 2 cm from the macroscopically unaffected margins of the tumor
(polyporcarcinoma), were defined as the normal controls. The tumors
that were adenocarcinomas and mucinous carcinomas (when >50% of
the tumor volume was composed of mucin) were excluded. The CRC
samples were staged according to the Duke's classification system:
Duke's A (T1-T2, N0 and M0; n=41), Duke's B (T3-T4, N0 and M0;
n=36), Duke's C (any T, N1-2 and M0; n=43) and Duke's D (any T, any
N and M1; n=14). Matched samples of colorectal adenocarcinomas
(n=134) and normal colonic mucosa (n=134) were subjected to
real-time PCR analysis. CRC samples were collected from patients
undergoing bowel resection. The collected samples were stored in
liquid nitrogen. The patients were informed about the aims of
specimen collection and gave signed written consent in accordance
with the ethics guidelines of Peking University, China. Peripheral
blood samples from 152 healthy controls were collected at Peking
University People's Hospital, China. The study was approved by the
ethics committee of Peking University Shenzhen Hospital, China.
DNA extraction and quantification of copy
numbers
Genomic DNA was isolated from the tissues using the
Genomic DNA Extraction kit (Innocent, Shenzhen, China) according to
the manufacturer's instructions. Quantitative PCR was performed
using the BioRad Chromo4 real-time PCR system. The primers for
RNAse P were: forward, 5′-AGA CTA GGG TCA GAA CRCA A-3′ and
reverse, 5′-CAT TTC ACT GAA TCC GTT C-3′. The primers for HER-2
were: forward, 5′-CCA GCC TTC GAC AAC CTC TA-3′ and reverse, 5′-ACG
TCC AGA CCC AGG TAC TC-3′. Average copy numbers of RNAse P in
normal candidates (copy numbers = 2) were used as controls. The
copy numbers of HER-2 were calculated using the comparative Ct
method. Cut-off values of 0.25, 0.75, 1.25 and 1.75 were used to
define the copy numbers as 0, 1, 2 and 3, respectively. The fold
change of each sample was presented: fold change = relative
expression level/average expression level in the group with two
copies of DNA.
A standard curve was prepared using 2 μl of crude
DNA solution, in which serially diluted samples (original, 2-, 4-,
8- and 16-diluted) were included. The Ct slopes and efficacy of
each primer were calculated via the BioRad Chromo4 real-time PCR
system and Microsoft Excel 2007 for Windows. Relative
quantification of HER-2 was performed using the 2−ΔΔCt
method.
RNA extraction and real-time PCR
Total RNA was isolated from tissues using an
AxyPrep™ Blood Total RNA MiniPrep kit (Axygen) according to the
manufacturer's instructions. First strand cDNA was synthesized with
a RevertAid™ First Stand cDNA Synthesis kit (Fermentas, Burlington,
ON, Canada). Quantitative PCR was performed via the BioRad Chromo4
real-time PCR system. At the end point of the PCR cycles, melt
curves were generated to check the product purity. Since the
efficacy of amplification of the targeted genes was almost 100%
(data not shown), the 2−Δct method was used to calculate
the real-time PCR results. The mRNA level of HER-2 was expressed as
a ratio relative to the GAPDH mRNA in each sample. An exploratory
data analysis using box plots was applied to visually identify the
expression level of target mRNA.
Statistical analysis
Statistical analysis was performed by the SPSS
Software, version 11.5. Data were analyzed by the Chi-square or
Fisher's exact tests. P<0.05 was considered to be statistically
significant. The results of HER-2 mRNA expression for normal and
tumor tissue samples were compared using two-way repeated
measurement analysis of variance (ANOVA). One-way repeated measures
analysis of variance (ANOVA-RM) was performed at a significance
level of P=0.05 to determine the differences between the controls
within each group. ANOVA-2 was performed following baseline
subtraction, at a significance level of P=0.05, to determine the
differences between the groups with amplified and unaltered HER-2
copy numbers.
Results
Gene copy number gains of HER-2 in CRC
samples
The distribution variance of HER-2 copy numbers
between the adjacent normal tissues (ANTs) from CRC patients and
peripheral blood from healthy controls was examined. As shown in
Table I, no statistical difference
of copy number distribution between ANTs and healthy normal
controls was observed. Thus, the ANTs were used as controls for the
CRC tissues in this study.
 | Table IComparison of CNVs of HER-2 between
adjacent normal tissues and healthy normal controls from peripheral
blood. |
Table I
Comparison of CNVs of HER-2 between
adjacent normal tissues and healthy normal controls from peripheral
blood.
| | Copy number | |
|---|
| |
| |
|---|
| | Deletion | | Amplification | |
|---|
| |
| |
| |
|---|
| Samples | n | 0 | 1 | 2 | 3 | >3 | P-valuea |
|---|
| ANT | 134 | 2 | 6 | 120 | 3 | 3 | 0.971 |
| HNC | 152 | 2 | 8 | 133 | 5 | 4 | - |
Table II shows the
CNVs of HER-2 in paired samples of CRCs and ANTs. A total of 134
CRC samples were examined. A relatively high percentage of CRC
samples showed amplification of HER-2 (35.1%, 47 out of 134). The
CRC tissues from patients with low-grade CRC (Duke's A and B)
comprised on average <30% of the samples that had either 3 or 4
copies or >4 copies of the HER-2 gene, whereas >30% of
samples that had either 3 or 4 copies or >4 copies of HER-2 were
observed in high-grade CRCs (Duke's C and D). A correlation was
found between the gene copy number gains and the tumor phenotypes
(P=0.011).
 | Table IICNVs of HER-2 in CRC tissues and
matched adjacent normal tissues. |
Table II
CNVs of HER-2 in CRC tissues and
matched adjacent normal tissues.
| | Copy number | | |
|---|
| |
| | |
|---|
| | Deletion | Amplification
no. | | |
|---|
| |
|
| | |
|---|
| Samples | n | ≤2 | >2 | P-valuea | P-valueb |
|---|
| Total | 134 | | | 3.22E-10 | - |
| CRC | | 87 | 47 | | |
| ANT | | 128 | 6 | | |
| Duke's A and B | 77 | | | 6.56E-05 | - |
| CRC | | 58 | 19 | | |
| ANT | | 75 | 2 | | |
| Duke's C and D | 57 | | | 2.88E-06 | 0.011 |
| CRC | | 31 | 26 | | |
| ANT | | 53 | 4 | | |
Positive correlation between copy number
increases and mRNA overexpression of HER-2 in CRCs
To determine whether a genotype-phenotype
correlation exists in CNVs of HER-2, the mRNA expression levels of
HER-2 between the CRC samples and paired ANTs by quantitative
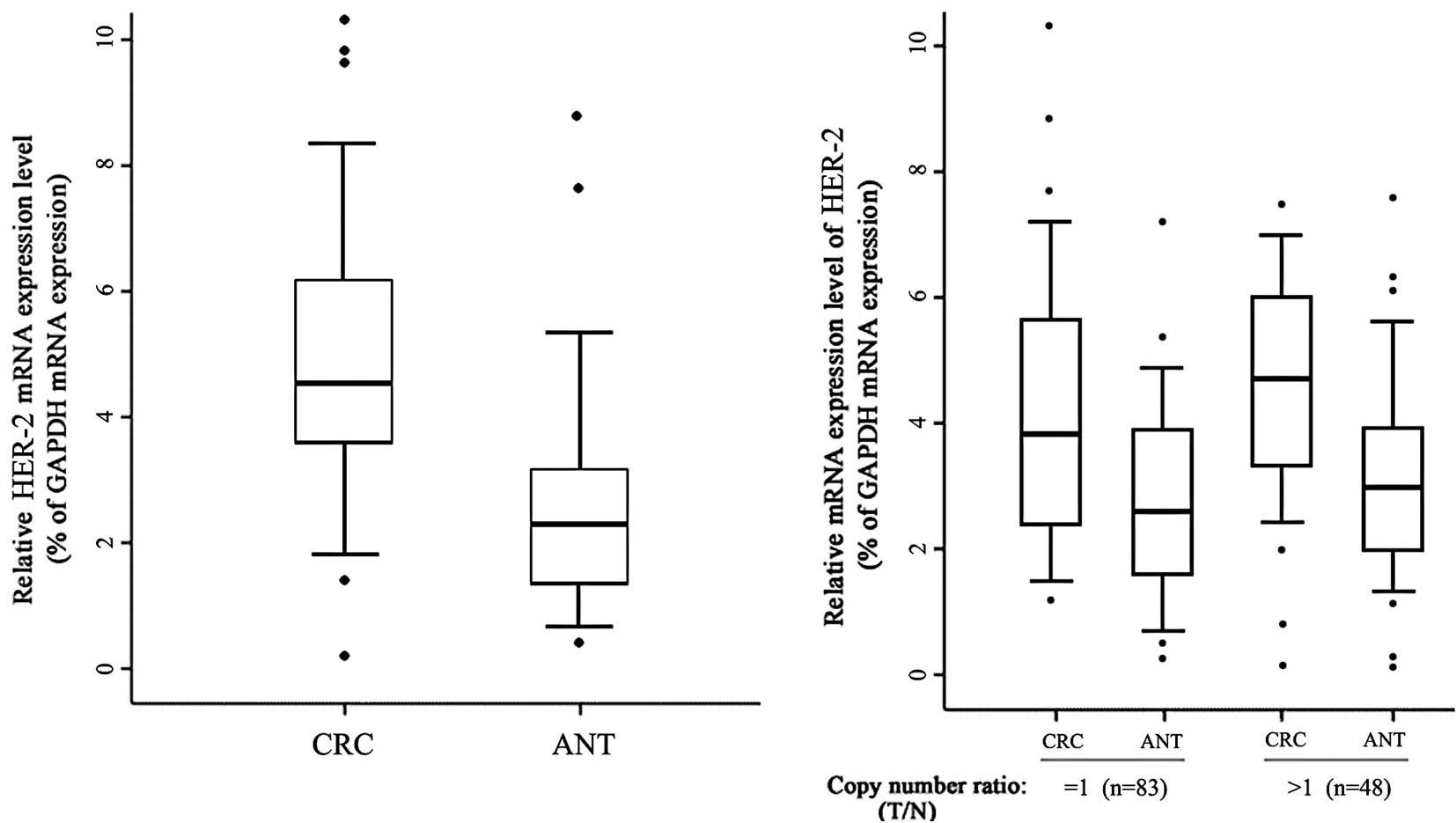
real-time RT-PCR were compared. As shown in Fig. 1A, an increased mRNA expression level
of HER-2 was observed in the CRC tissues compared to that of the
ANTs (P<0.001). Gene CNVs contribute to qualitative and
quantitative diversities in their gene products. Samples with
increased or unaltered copies of HER-2 were selected for testing to
determine whether the HER-2 mRNA expression was correlated with the
copy numbers. The samples with decreased copies of HER-2 (3 out of
134) were not included due to the small sample size. As shown in
Fig. 1B, the CRC samples in the
group with amplified or unaltered copies of HER-2 showed an
increased expression of mRNA compared to the ANTs (P<0.01). A
significant statistical difference was noted between the CRC
samples in the groups with amplified and unchanged copies of HER-2
(P=0.023). Thus, DNA copy amplification plays at least a partial
role in the overexpression of HER-2 in CRCs.
Discussion
CNVs have recently gained considerable interest as a
source of genetic variation likely to play a role in phenotypic
diversity and evolution. They have been clearly shown to directly
or indirectly affect a healthy individual's susceptibility to
cancer, for example by varying the gene dosage of tumor suppressors
or oncogenes (18,19). However, numerous discrepancies are
found among previous studies that used high-resolution approaches
to screen CNVs (20–24). Thus, validation of such CNVs by a
substantial amount of clinical samples is required.
It is suggested that the genes present in very small
regions of CNVs are ideal candidates for evaluation in cancer
pathogenesis. Evaluation of the CNVs for such genes is a starting
point for investigations into the role of gene amplification in the
colorectal carcinogenic process. Although a number of studies
showed that the chromosome 17q21 region encompassing HER-2 was
amplified in breast cancer samples (14–17),
the correlation between CNVs and HER-2 overexpression in CRC has
yet to be elucidated. In this study, 134 CRC (adenocarcinomas)
samples were collected for CNV analysis of HER-2. Since no
statistical difference not noted in CNVs between the healthy normal
controls and normal tissues from CRC patients, the CNVs of HER-2 in
CRCs were presumably acquired DNA aberrations. The amplification of
HER-2 (35.1%, 47 out of 134) was found in the collected CRC
samples. Results of the present study showed that the frequency of
DNA copy number gains of HER-2 in advanced CRCs was significantly
more than in early-stage CRC (Table
II), suggesting that copy number gains played a role in CRC
progression and may contribute to tumor aggressiveness. However, we
found that copy numbers of HER-2 were also deleted in a small
percentage of samples (2.24%, 3 out of 134). This discrepancy may
be due to the various ethnicities and populations used in the
studies. Another reason involves the various methodologies used in
the studies. We used a gene-specific strategy to target short
fragments (several hundred base pairs) and the sensitivity was
increased.
CNVs have also been shown to induce phenotypes.
Phenotypic effects of genetic variations, both at the single
nucleotide polymorphisms and CNVs, are presumably caused by changes
in the expression levels, either by directly affecting the genes
concerned via the genetic change, or indirectly through position
effects or downstream pathways and regulatory networks (25). In the present study, the correlation
between the HER-2 mRNA expression and the copy numbers of its DNA
was investigated. The correlation was not as positive as
anticipated, although a statistical difference was obtained.
Furthermore, the mRNA expression of HER-2 was increased in both the
groups of amplified and unchanged DNA copies. A statistical
difference was noted in the mRNA expression between the groups of
amplified and unaltered DNA copies. Thus, CNVs played a role in the
overexpression of the HER-2 mRNA in CRCs, while another mechanism
was also involved. This is consistent with two recent studies which
assessed an over-representation of differentially expressed genes
among CNV-mapping transcripts and observed a weak yet significant
positive correlation between the relative expression level and gene
dosage (26,27). However, in certain samples the
number of copies had no effect on the relative expression levels,
suggesting either dosage compensation mechanisms or the incomplete
inclusion of regulatory elements in the deletion/duplication event
(26,27). The mechanism of this phenomenon has
yet to be elucidated. Investigators determined that immediate early
genes may play a role in this process. These genes are initially
expressed at levels proportional to the number of copies by
directly or indirectly inducing the expression of a repressor,
which reduces or even abolishes the expression of the CNV gene
(26,27). In the second model, the extra copies
of a gene impair, through steric hinderance, the access of the
copies to a specific transcription factory, where this particular
locus should be transcribed (28).
In conclusion, the findings suggest that copy number
increasing of HER-2 serves as a diagnostic indicator for CRC,
whether alone or in combination with other markers. The mechanism
of the heterogeneous expression levels, however, has yet to be
elucidated. Whether methylation of DNA or immediate early genes
plays a role requires extensive investigation in the future.
Acknowledgements
This study was supported by grants from the China
National Natural Science Foundation (30900774), the
Shenzhen-Hongkong Innovation Foundation (08fz-08) and the China
Postdoctoral Science Foundation (20090460788).
References
|
1
|
Schechter AL, Stern DF, Vaidyanathan L, et
al: The neu oncogene: an erb-B-related gene encoding a 185,000-Mr
tumour antigen. Nature. 312:513–516. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Prenzel N, Fischer OM, Streit S, Hart S
and Ullrich A: The epidermal growth factor receptor family as a
central element for cellular signal transduction and
diversification. Endocr Relat Cancer. 8:11–31. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siddiqa A, Long LM, Li L, Marciniak RA and
Kazhdan I: Expression of HER-2 in MCF-7 breast cancer cells
modulates anti-apoptotic proteins Survivin and Bcl-2 via the
extracellular signal-related kinase (ERK) and phosphoinositide-3
kinase (PI3K) signalling pathways. BMC Cancer. 8:1292008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pohl M, Stricker I, Schoeneck A, et al:
Antitumor activity of the HER2 dimerization inhibitor pertuzumab on
human colon cancer cells in vitro and in vivo. J Cancer Res Clin
Oncol. 135:1377–1386. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Al-Kuraya K, Novotny H, Bavi P, et al:
HER2, TOP2A, CCND1, EGFR and C-MYC oncogene amplification in
colorectal cancer. J Clin Pathol. 60:768–772. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Herreros-Villanueva M, Rodrigo M, Claver
M, et al: KRAS, BRAF, EGFR and HER2 gene status in a Spanish
population of colorectal cancer. Mol Biol Rep. June;2010.(E-pub
ahead of print).
|
|
7
|
Yamashita H, Nishio M, Toyama T, et al:
Coexistence of HER2 over-expression and p53 protein accumulation is
a strong prognostic molecular marker in breast cancer. Breast
Cancer Res. 6:R24–R30. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Leonard DS, Hill AD, Kelly L, Dijkstra B,
McDermott E and O'Higgins NJ: Anti-human epidermal growth factor
receptor 2 monoclonal antibody therapy for breast cancer. Br J
Surg. 89:262–271. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sung JJ, Lau JY, Goh KL and Leung WK:
Increasing incidence of colorectal cancer in Asia: implications for
screening. Lancet Oncol. 6:871–876. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu JB, Sun XB, Dai DX, et al: Epidemiology
of gastroenterologic cancer in Henan Province, China. World J
Gastroenterol. 9:2400–2403. 2003.PubMed/NCBI
|
|
11
|
Lichtenstein P, Holm NV, Verkasalo PK, et
al: Environmental and heritable factors in the causation of cancer
– analyses of cohorts of twins from Sweden, Denmark, and Finland. N
Engl J Med. 343:78–85. 2000.
|
|
12
|
Andrews J, Kennette W, Pilon J, et al:
Multi-platform whole-genome microarray analyses refine the
epigenetic signature of breast cancer metastasis with gene
expression and copy number. PLoS One. 5:e86652010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cho EK, Tchinda J, Freeman JL, Chung YJ,
Cai WW and Lee C: Array-based comparative genomic hybridization and
copy number variation in cancer research. Cytogenet Genome Res.
115:262–272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Orsetti B, Courjal F, Cuny M, Rodriguez C
and Theillet C: 17q21–q25 aberrations in breast cancer: combined
allelotyping and CGH analysis reveals 5 regions of allelic
imbalance among which two correspond to DNA amplification.
Oncogene. 18:6262–6270. 1999.
|
|
15
|
Buness A, Kuner R, Ruschhaupt M, Poustka
A, Sultmann H and Tresch A: Identification of aberrant chromosomal
regions from gene expression microarray studies applied to human
breast cancer. Bioinformatics. 23:2273–2280. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yao J, Weremowicz S, Feng B, et al:
Combined cDNA array comparative genomic hybridization and serial
analysis of gene expression analysis of breast tumor progression.
Cancer Res. 66:4065–4078. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Benusiglio PR, Pharoah PD, Smith PL, et
al: HapMap-based study of the 17q21 ERBB2 amplicon in
susceptibility to breast cancer. Br J Cancer. 95:1689–1695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dear PH: Copy-number variation: the end of
the human genome? Trends Biotechnol. 27:448–454. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shlien A, Tabori U, Marshall CR, et al:
Excessive genomic DNA copy number variation in the Li-Fraumeni
cancer predisposition syndrome. Proc Natl Acad Sci USA.
105:11264–11269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Divne AM and Allen M: A DNA microarray
system for forensic SNP analysis. Forensic Sci Int. 154:111–121.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Macconaill LE, Aldred MA, Lu X and
Laframboise T: Toward accurate high-throughput SNP genotyping in
the presence of inherited copy number variation. BMC Genomics.
8:2112007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gorringe KL and Campbell IG:
High-resolution copy number arrays in cancer and the problem of
normal genome copy number variation. Genes Chromosomes Cancer.
47:933–938. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mitra A, Liu G and Song J: A genome-wide
analysis of array-based comparative genomic hybridization (CGH)
data to detect intra-species variations and evolutionary
relationships. PLoS One. 4:e79782009. View Article : Google Scholar
|
|
24
|
Malan V, Chevallier S, Soler G, et al:
Array-based comparative genomic hybridization identifies a high
frequency of copy number variations in patients with syndromic
overgrowth. Eur J Hum Genet. 18:227–232. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dermitzakis ET and Stranger BE: Genetic
variation in human gene expression. Mamm Genome. 17:503–508. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guryev V, Saar K, Adamovic T, et al:
Distribution and functional impact of DNA copy number variation in
the rat. Nat Genet. 40:538–545. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Henrichsen CN, Vinckenbosch N, Zollner S,
et al: Segmental copy number variation shapes tissue
transcriptomes. Nat Genet. 41:424–429. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sexton T, Umlauf D, Kurukuti S and Fraser
P: The role of transcription factories in large-scale structure and
dynamics of interphase chromatin. Semin Cell Dev Biol. 18:691–697.
2007. View Article : Google Scholar : PubMed/NCBI
|