Introduction
Trastuzumab is a well-known molecular-targeting drug
which provides significant clinical benefits to patients with human
epidermal growth factor (HER)2-positive breast cancer (1). In metastatic breast cancer, the
efficacy of trastuzumab was proven in terms of improved objective
responses, resulting in an increase in patient survival (2,3). The
additional or synergistic effect of trastuzumab administered in
combination with conventional types of chemotherapy has been
suggested to play a role in pre-operative treatment based on
improvement in the objective response and pathological complete
response (pCR) rates (4,5).
Although the molecular mechanism underlying its
action is poorly understood, evidence is available for the efficacy
of trastuzumab in clinical practice. Briefly, trastuzumab directly
targets the extracellular domain of HER2 and inhibits proliferation
and the anti-apoptotic potential of HER2-positive breast cancer
cells by down-regulating pathways, such as PI3K/Akt and MAPK
(1).
However, cellular heterogeneity, such as
sensitivities to cytotoxic agents, positivity for the estrogen
receptor (ER) and proliferation revealed as Ki-67 expression,
exists even in HER2-positive cancers. Furthermore, the cancer stem
cell (CSC) hypothesis, was also found to verify the existence of
intrinsic cellular and molecular heterogeneity within each tumor
(6,7).
Despite evidence for heterogeneity or the CSC
hypothesis, which HER2-positive breast cancer cells are targeted by
trastuzumab or which molecular pathways are involved in the
mechanism of trastuzumab activity has yet to be elucidated. To
resolve this issue, a retrospective analysis of HER2-positive
breast cancer patients treated with neoadjuvant chemotherapy (NAC),
with or without trastuzumab, was performed. The therapeutic
efficacies and proportional changes of positive cells of each
biomarker pre- to post-NAC for each treatment regimen were
investigated. These changes and any discrepancy in these changes
may represent treatment-resistant tumor cells in non-responders and
provide evidence of a cellular shift in target by trastuzumab
administered in combination with conventional chemotherapy as
NAC.
Patients and methods
Patients
A retrospective analysis of a prospectively
maintained clinical database was performed, including patients who
received NAC at Kumamoto City Hospital, Kumamoto City, Japan, from
March 2002 to June 2009. Of the 144 patients who received NAC
during this period, 55 patients with HER2-positive breast cancers
were enrolled in this study. A prerequisite of the hospital's NAC
inclusion criteria was invasive tumors of a diameter of ≥3 cm
and/or showing lymph node involvement. All 144 patients fulfilled
these criteria. The research protocol of the present study was
approved by the Ethics Committee of Kumamoto City Hospital, and
informed consent was obtained from all patients prior to treatment.
Certain patients had also been enrolled in a prospective
multicenter phase II trial conducted by the Kyushu Breast Cancer
Study Group or Kumamoto Breast Cancer Study Group.
Treatment regimens
The treatment regimens used in the present study
were: 18 patients were treated concurrently with epirubicin and
docetaxel (ET); 11 patients were treated with 5-fluorouracil,
epirubicin, and cyclophosphamide (FEC) followed by docetaxel (DOC);
and 26 patients were treated with anthracycline-based chemotherapy
followed by concurrent trastuzumab with taxane. All 29 patients
treated with ET or FEC followed by DOC were considered to be the
conventional chemotherapy (CT) group.
For the ET regimen, the patients received 4 cycles
of epirubicin (60 mg/m2) and docetaxel (60
mg/m2) every 3 weeks. For the FEC followed by the DOC
regimen, the patients received 4 cycles of 5-fluorouracil (500
mg/m2), epirubicin (75–100 mg/m2) and
cyclophosphamide (500 mg/m2) every 3 weeks, followed by
4 cycles of docetaxel (70–80 mg/m2) every 3 weeks. For
the trastuzumab-containing regimens, the patients received 4 cycles
of epirubicin (90 mg/m2) and cyclophosphamide (600
mg/m2) every 3 weeks, followed by concurrent weekly
trastuzumab (4 mg/kg on day 1 and subsequent infusions at a dose of
2 mg/kg) with 12 cycles of weekly paclitaxel (80 mg/m2)
or 4 cycles of FEC followed by concurrent weekly trastuzumab with 4
cycles of tri-weekly docetaxel.
Three to four weeks after completion of NAC,
breast-conserving surgery or mastectomy with sentinel node biopsy
or axillary lymph node dissection was performed with intent to
cure. Patients who underwent breast-conserving surgeries received
radiotherapy to the conserved breast. Patients with hormone
receptor (HR)-positive cancers received adjuvant endocrine therapy
according to the patient's menopausal status.
Evaluation of clinical and pathological
responses
Definitive diagnoses of invasive breast cancers were
performed by the hospital's pathologist on hematoxylin and
eosin-stained sections obtained from core needle biopsy samples.
Biological markers were examined both at diagnosis and surgery
using the samples of core needle biopsy and residual tumor in the
operated breast, respectively. The variables of interest were tumor
size, lymph node involvement, nuclear grade, proportions of
ER-/progesterone receptor (PgR)-positive cells, HER2 status and
Ki-67 labeling index. Immunohistochemical staining was performed
according to a protocol described previously (8).
At least 5 microscopic fields (x40) were counted to
determine the proportion of ER- and PgR-positive tumor cells, and
the observed proportions were calculated as a percentage of all
counted cells. The hormone receptor status was determined based on
the dichotomic criteria as: positive if the proportion of cells was
≥10% and negative if the proportion was <10%. ER- and/or
PgR-positive cells were rated as HR-positive. Proliferation
activity was judged by immunostaining with the Ki-67 antibody
(Dako, Glostrup, Denmark). The proportion of proliferating cells
was determined by counting at least 500 tumor cells. HER2
expression was initially evaluated using the Hercep Test (Dako).
HER2 positivity was indicated by 3+ staining intensity. The HER2
equivocal (2+ staining) was tested using fluorescence in
situ hybridization with a threshold ratio of >2.0 for
positive HER2:CEP17.
Tumor burden was measured by caliper and ultrasound
after each cycle and evaluated by the Response Evaluation Criteria
in Solid Tumors (9). The
pathological responses were assessed in surgical specimens of the
breast with reference to the standards of the Japanese Breast
Cancer Society (10). Based on this
criterion, pathological responses were evaluated based on invasive
components and not carcinoma in situ. Thus, tumors with
residual ductal carcinoma in situ were included in the pCR
group. Furthermore, marked changes approaching a complete response
in only a few remaining invasive tumor cells were classified as
near-pCR (11). Patients with pCR
and near-pCR were classified as pathological responders, and their
pathological responses were defined as quasi-pCR (12). The remaining patients were
designated as pathological non-responders.
Evaluation of changes in the proportion
of positive cells for each biomarker
To assess the biological mechanism of the additional
effect of trastuzumab administered in combination with conventional
types of chemotherapy, the proportion of positive cells for each
biomarker was determined at diagnosis and surgery. Using
immunohistochemical staining, the tumor defined as pCR was excluded
from the analysis, but the tumor defined as near-pCR with evaluable
cells was included. To focus on ER, PgR and Ki-67 independently, a
subgroup analysis was performed for breast tumors with 10% or
higher proportional positivity for ER and PgR, which indicate
HR-/HER2-positive breast cancer.
Statistical analysis
Statistical comparisons were performed using the
Chi-square test and the paired Student's t-test. A two-sided
P-value of <0.05 was considered to be statistically significant.
JMP 8.0 software package (SAS Institute Inc., Cary, NC, USA) was
used for statistical analysis.
Results
Clinical and pathological responses in
HER2-positive breast cancer
The patient characteristics at diagnosis are shown
in Table I. No significant
differences were noted in patient characteristics between the CT
group and the trastuzumab-containing (CT+T) group. Clinical
complete response (cCR) rates were 6.9% for NAC without trastuzumab
and 46.2% for NAC with trastuzumab. The pCR rates were 13.8% for
NAC without trastuzumab and 26.9% for NAC with trastuzumab.
Pathological response rates (i.e., quasi-pCR) were 31.0% for NAC
without trastuzumab and 65.4% for NAC with trastuzumab (Table II).
 | Table IPatient characteristics at
diagnosis. |
Table I
Patient characteristics at
diagnosis.
| Chemotherapy alone
(n=29) |
Chemotherapy+Trastuzumab (n=29) | |
|---|
|
|
| |
|---|
| Characteristics | No. of patients
(%) | No. of patients
(%) | P-value |
|---|
| Age (years) | | | 0.62 |
| Median | 53 | 51 | |
| Range | 29–67 | 32–67 | |
| Tumor size | | | 0.89 |
| T1 | 2 (7) | 2 (8) | |
| T2 | 15 (52) | 14 (54) | |
| T3 | 10 (35) | 7 (27) | |
| T4 | 2 (7) | 3 (12) | |
| Total | 29 (100) | 26 (100) | |
| Axillary nodes | | | 0.46 |
| N0 | 12 (41) | 7 (27) | |
| N1 | 14 (48) | 15 (58) | |
| N2 | 3 (10) | 3 (12) | |
| N3 | 0 (0) | 1 (4) | |
| Total | 29 (100) | 26 (100) | |
| Hormone receptor | | | 0.32 |
| ER- and/or
PgR-positive | 14 (48) | 16 (62) | |
| ER- and
PgR-negative | 15 (52) | 10 (38) | |
| Total | 29 (100) | 26 (100) | |
| Menopausal
status | | | 0.53 |
| Pre-menopausal | 11 (38) | 12 (46) | |
| Post-menopausal | 18 (29) | 12 (54) | |
| Total | 29 (100) | 26 (100) | |
 | Table IIClinical and pathological responses
according to treatment regimens. |
Table II
Clinical and pathological responses
according to treatment regimens.
| Chemotherapy alone
(n=29) | Chemotherapy +
Trastuzumab (n=29) | |
|---|
|
|
| |
|---|
| Responses | No. of patients
(%) | No. of patients
(%) | P-value |
|---|
| Clinical
response | | | 0.002 |
| cCR | 2 (7) | 12 (46) | |
| cPR | 24 (83) | 11 (42) | |
| cSD | 3 (10) | 3 (12) | |
| cPD | 0 (0) | 0 (0) | |
| Total | 29 (100) | 26 (100) | |
| Pathological
response | | | 0.010 |
| Quasi-pCR | 9 (31) | 17 (65) | |
| Non-quasi-pCR | 20 (69) | 9 (35) | |
| Total | 29 (100) | 26 (100) | |
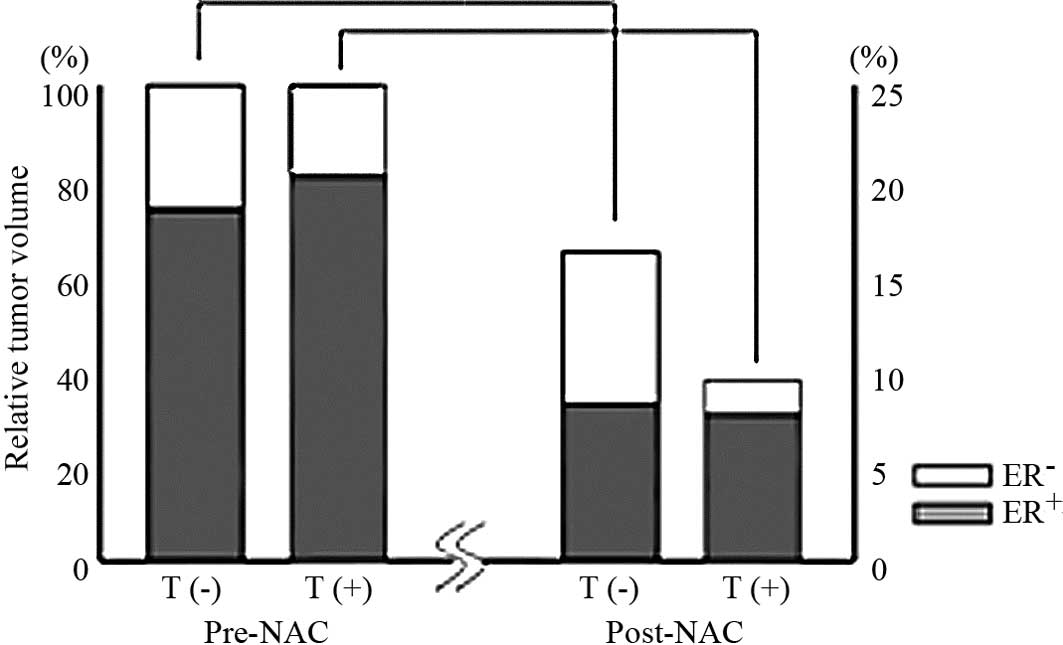
Changes in the biological markers of
HR-/HER2-positive breast cancer
No significant differences with respect to the mean
proportion of ER-, PgR-, and Ki-67-positive cells at diagnosis were
noted between the CT and CT+T groups. The CT group showed a
significantly decreased proportion of ER-positive cells at surgery
compared to the proportion prior to NAC (50.9 vs. 73.5%, P=0.02)
(Table III). On the other hand,
no changes were found in the proportion of ER-positive cells at
surgery compared to that prior to NAC (80.3 vs. 81.9%, P=0.61) for
the CT+T group (Table III). As
noted above, treatment responses were significantly improved, and
the tumor reduction ratio was significantly higher in the CT+T
group than that in the CT group. Consequently, ER-negative cells
were more commonly eradicated in the CT+T group (Fig. 1).
 | Table IIIChanges in biological markers prior to
neoadjuvant chemotherapy and at surgery in HER2/HR-positive breast
cancer. |
Table III
Changes in biological markers prior to
neoadjuvant chemotherapy and at surgery in HER2/HR-positive breast
cancer.
| Chemotherapy
alone | Chemotherapy +
Trastuzumab |
|---|
|
|
|
|---|
| % (±SD) | | % change | % (±SD) | | % change |
|---|
| % of ER-positive
cells |
| At diagnosis | 73.5 (±24.2) | ] | −22.6
(P=0.02) | 81.9 (±25.6) | ] | −1.6
(P=0.61) |
| After NAC | 50.9 (±39.6) | 80.3 (±29.3) |
| % of PgR-positive
cells |
| At diagnosis | 55.4 (±30.5) | ] | −35.3
(P=0.01) | 44.7 (±33.3) | ] | −9.2
(P=0.40) |
| After NAC | 20.1 (±33.2) | 35.5 (±18.2) |
| Ki-67 labeling
index |
| At diagnosis | 45.1 (±20.1) | ] | −13.7
(P=0.004) | 45.4 (±17.1) | ] | −26.5
(P=<0.0001) |
| After NAC | 31.4 (±23.3) | 18.9 (±19.4) |
Similar findings were observed for the PgR-positive
tumors. In the CT group, the proportion of PgR-positive cells was
significantly decreased at surgery compared to the proportion prior
to NAC (20.1 vs. 55.4%, P=0.01) (Table III). In contrast, in the CT+T
group, no changes were observed in the proportion of PgR-positive
cells in the ER-positive subset at surgery compared to that prior
to NAC (35.5 vs. 44.7%, P=0.40) (Table III).
Although a significant decrease in Ki-67-positive
cells was observed in the two treatment groups, the change in
proportion was much greater in the CT+T group than the change in
proportion in the CT group (−26.5 vs. −13.7%) (Table III). This result suggests that
trastuzumab sensitizes proliferative cancer cells to taxane-based
cytotoxic chemotherapy.
Discussion
The present study showed that HER2-positive breast
cancers treated with anthracycline-based chemotherapy followed by
taxane with trastuzumab showed a higher pathological response rate
than those treated with conventional chemotherapy. Moreover, a
discrepancy in changes in the proportion of ER-, PgR- and
Ki-67-positive cells from pre- to post-NAC was noted in the two
groups. Through retrospective cellular insight into HER2-positive
cancers, we showed direct evidence for a shift in target in
eradicated tumor cells from ER-positive to ER-negative by the
addition of trastuzumab to taxane-based chemotherapies. This
observation was based on the findings of improved objective
responses and a significant reduction in total counts of
ER-negative HER2-positive cancer cells following treatment with
trastuzumab in combination with chemotherapy compared to treatment
with chemotherapy alone.
Various molecular and cellular effects of
trastuzumab, such as the activation of antibody-dependent cellular
cytotoxicity, prevention of shedding of the extracellular domain
(ECD) of HER2, inhibition of cell proliferation by preventing
HER2-activated intracellular signaling and inhibition of
HER2-regulated angiogenesis, were previously reported (1,13–15).
Trastuzumab administered in combination with conventional types of
chemotherapy has been shown to exhibit an additional effect
involving improvement of the pCR rate for NACs (4,5).
However, eradication of targeted cells by the additional effect of
trastuzumab administered in combination with conventional types of
chemotherapy has yet to be investigated. In the present study, the
additional effect of trastuzumab administered in combination with
conventional types of chemotherapy was targeted mainly at
ER-negative cells. In addition, PgR-negative and proliferative
cells (Ki-67-positive cells) were preferably eradicated with
trastuzumab-containing chemotherapies. Trastuzumab is thus
considered to sensitize proliferating tumor cells independently of
the ER genomic pathway when administered in combination with
taxane-based chemotherapies.
ER genomic pathway-independent proliferating tumor
cells are considered to be responsible for disease recurrence and
resistance to various types of chemotherapy. The CSC hypothesis
similarly asserts that disease recurrence and treatment resistance
to various types of chemotherapy and irradiation can be attributed
to this type of tumor cell. Thus, ER pathway-independent
proliferating cells may merge with treatment-resistant tumor cells,
such as CSCs. Concerning the association between CSCs and the
clinicopathological characteristics of breast cancer,
ALDH-1-positive breast cancer cells, which are putative breast
CSCs, are more likely to be ER-negative, Ki-67-positive and
HER2-positive (16,17). Moreover, the proportion of cells
with these markers is closely related with poor prognosis in breast
cancer. Li et al showed that tumorigenic cancer cells, which
are phenotypically determined as CD44-positive/CD24-negative, are
intrinsically resistant to conventional chemotherapy. These authors
also showed that a dual inhibitor of HER1/HER2, lapatinib,
regulates the proportion of tumorigenic cells in a tumor (18). Thus, the CSC hypothesis has been
accepted as clinically relevant and is applied to the treatment of
breast cancer. Previous findings and the results of the present
study indicate that further investigation of the molecular
rationale of molecular-targeting therapies used to overcome
chemotherapy resistance in breast cancer is required.
The decision to define quasi-pCR by grouping bona
fide pCR and near-pCR in this study was justified by the
evidence that following pre-operative chemotherapy quasi-pCR
predicts favorable disease-free survival (12). This criterion was applied to
determine the type of tumor cell that was likely to be eradicated
by the addition of trastuzumab to taxane-based chemotherapies.
Findings showed the pathological response rate or quasi-pCR to be
65.4% in the CT+T group. These data are comparable with those of
previous reports involving NAC with or without trastuzumab
(4,19–21),
confirming our analysis.
In conclusion, the insight gained from the present
study may provide a biological rationale of the additional clinical
efficacy of trastuzumab administered in combination with
conventional types of chemotherapy. However, the molecular
mechanism for this shift in target from ER-positive to ER-negative
breast cancer cells in the treatment of trastuzumab-containing
chemotherapies should be investigated. Furthermore, prospective
studies testing the hypothesis that trastuzumab sensitizes
treatment-resistant breast cancer cells or those cells responsible
for recurrence to conventional chemotherapies are warranted.
Acknowledgements
We thank the staff at the Department of Clinical
Pathology of Kumamoto City Hospital for their technical
assistance.
References
|
1
|
Hudis CA: Trastuzumab – mechanism of
action and use in clinical practice. N Engl J Med. 357:39–51.
2007.
|
|
2
|
Slamon DJ, Leyland-Jones B, Shak S, et al:
Use of chemotherapy plus a monoclonal antibody against HER2 for
metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marty M, Cognetti F, Maraninchi D, et al:
Randomized phase II trial of the efficacy and safety of trastuzumab
combined with docetaxel in patients with human epidermal growth
factor receptor 2-positive metastatic breast cancer administered as
first-line treatment: the M77001 study group. J Clin Oncol.
23:4265–4274. 2005. View Article : Google Scholar
|
|
4
|
Buzdar AU, Ibrahim NK, Francis D, et al:
Significantly higher pathologic complete remission rate after
neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin
chemotherapy: results of a randomized trial in human epidermal
growth factor receptor 2-positive operable breast cancer. J Clin
Oncol. 23:3676–3685. 2005. View Article : Google Scholar
|
|
5
|
Gianni L, Eiermann W, Semiglazov V, et al:
Neoadjuvant chemotherapy with trastuzumab followed by adjuvant
trastuzumab versus neoadjuvant chemotherapy alone, in patients with
HER2-positive locally advanced breast cancer (the NOAH trial): a
randomised controlled superiority trial with a parallel
HER2-negative cohort. Lancet. 375:377–384. 2010.
|
|
6
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu S, Dontu G and Wicha MS: Mammary stem
cells, self-renewal pathways, and carcinogenesis. Breast Cancer
Res. 7:86–95. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kai K, Nishimura R, Arima N, Miyayama H
and Iwase H: p53 expression status is a significant molecular
marker in predicting the time to endocrine therapy failure in
recurrent breast cancer: a cohort study. Int J Clin Oncol.
11:426–433. 2006. View Article : Google Scholar
|
|
9
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar
|
|
10
|
Kurosumi M, Akashi-Tanaka S, Akiyama F, et
al: Histo-pathological criteria for assessment of therapeutic
response in breast cancer (2007 version). Breast Cancer. 15:5–7.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kuroi K, Toi M, Tsuda H, Kurosumi M and
Akiyama F: Issues in the assessment of the pathologic effect of
primary systemic therapy for breast cancer. Breast Cancer.
13:38–48. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Toi M, Nakamura S, Kuroi K, et al: Phase
II study of preoperative sequential FEC and docetaxel predicts of
pathological response and disease-free survival. Breast Cancer Res
Treat. 110:531–539. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Molina MA, Codony-Servat J, Albanell J,
Rojo F, Arribas J and Baselga J: Trastuzumab (herceptin), a
humanized anti-Her2 receptor monoclonal antibody, inhibits basal
and activated Her2 ectodomain cleavage in breast cancer cells.
Cancer Res. 61:4744–4749. 2001.PubMed/NCBI
|
|
14
|
Gennari R, Menard S, Fagnoni F, et al:
Pilot study of the mechanism of action of preoperative trastuzumab
in patients with primary operable breast tumors overexpressing
HER2. Clin Cancer Res. 10:5650–5655. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nahta R and Esteva FJ: HER2 therapy:
molecular mechanisms of trastuzumab resistance. Breast Cancer Res.
8:2152006. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ginestier C, Hur MH, Charafe-Jauffret E,
et al: ALDH1 is a marker of normal and malignant human mammary stem
cells and a predictor of poor clinical outcome. Cell Stem Cell.
1:555–567. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morimoto K, Kim SJ, Tanei T, et al: Stem
cell marker aldehyde dehydrogenase 1-positive breast cancers are
characterized by negative estrogen receptor, positive human
epidermal growth factor receptor type 2, and high Ki67 expression.
Cancer Sci. 100:1062–1068. 2009. View Article : Google Scholar
|
|
18
|
Li X, Lewis MT, Huang J, et al: Intrinsic
resistance of tumorigenic breast cancer cells to chemotherapy. J
Natl Cancer Inst. 100:672–679. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Paluch-Shimon S, Wolf I, Goldberg H, et
al: High efficacy of pre-operative trastuzumab combined with
paclitaxel following doxorubicin & cyclophosphamide in operable
breast cancer. Acta Oncol. 47:1564–1569. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chumsri S, Jeter S, Jacobs LK, et al:
Pathologic complete response to preoperative sequential
doxorubicin/cyclophosphamide and single-agent taxane with or
without trastuzumab in stage II/III HER2-positive breast cancer.
Clin Breast Cancer. 10:40–45. 2010. View Article : Google Scholar
|
|
21
|
Chen XS, Wu JY, Huang O, et al: Molecular
subtype can predict the response and outcome of Chinese locally
advanced breast cancer patients treated with preoperative therapy.
Oncol Rep. 23:1213–1220. 2010.PubMed/NCBI
|