Introduction
Sentinel lymph node biopsy (SLNB) is emerging as the
new standard for axillary staging in early breast cancer, supported
by a number of randomized controlled trials (1). Intraoperative detection of sentinel
node metastases enables the surgeon to make an immediate decision
to proceed to axillary lymph node dissection (ALND), thereby
avoiding the economic and psychological costs of a second
operation. However, in order to implement the procedure in clinical
practice, a method for accurate intraoperative analysis of the SLNs
is required in order that the decision to avoid ALND can be made
during primary surgery in the case of negative SLNs. This process
can be achieved by means of frozen sectioning (FS) and touch
imprint cytology (TIC). Results of numerous studies showed that TIC
has a sensitivity equivalent to or even better than that of FS
(2–5), since TIC offers the advantages of
minimal tissue preparation, rapid staining and good cytological
detail for interpretation. No special equipment is required and no
tissue loss occurs (6). Therefore,
it is recommended by the College of American Pathologists that TIC
be applied in the intra-operative evaluation of SLNs (7).
A large series of related studies focused on the
evaluation of the sensitivity of TIC and reviewed the risk factors
associated with false-negative cases which may lead to a secondary
ALND. However, few studies have focused on false-positive cases
which result in an unnecessary ALND and potential medicolegal
repercussions that discourage both surgeons and patients (2).
The primary aim of the present study was to evaluate
the clinical value of TIC with a normative procedure of the
pathological evaluation of SLNs. The secondary aim was to
investigate the potential factors associated with the misdiagnosed
results.
Materials and methods
Patients
A consecutive series of 366 women with T1–T2
invasive breast cancer treated at the Department of Breast Surgery,
Cancer Hospital, Fudan University, China, between February 2005 and
March 2008, participated in our previous study on SLNB. Eligibility
criteria included i) diagnosis of operable primary breast cancer ≤5
cm in diameter and a unicentric lump using clinical and imaging
criteria confirmed by core or open biopsy; ii) confirmation of
negative axillary lymph nodes under clinical and imaging
examination; iii) confirmation of no previous surgery performed in
the axilla iv) non-pregnant status v) obtainment of informed
consent; and vi) the harvesting of at least one SLN during the
surgery. Certain relatively rare types of breast carcinoma were
excluded in order that the focus remain on the three most common
types of breast carcinoma: invasive ductal carcinoma (IDC), ductal
carcinoma in situ (DCIS), and ductal carcinoma in
situ with microinvasion (DCIS-Mi).
TIC procedure and pathological
evaluation
Details on the SLNB procedure at our institution
were previously reported (8). The
SLNs were cut along the long axis at a 2.0- to 3.0-mm interval
intraoperatively, and each cut surface was touched at least three
times onto a clean glass slide and stained with hematoxylin and
eosin (H&E). The slides were sent to cytopathologists
immediately after the slides were prepared. The results obtained
from the cytopathologists were used by the surgeon as the primary
intraoperative tool for determining whether ALND should be
performed. Slices were formalin-fixed and paraffin-embedded for
further evaluation.
Definitive histological assessment was performed on
the paraffin-embedded tissue. Serial sections (SS) at a 100-μm
interval with standard H&E staining were carried out
postoperatively. S-P immunohistochemical (IHC) staining (3- to 5-μm
thick) with CK-19 (clone EP1580Y; Epitomics, Burlingame, CA) and
MUC-1 (clone Ma695; Novo-Castra, Newcastle, UK) was performed
unless macrometastasis was indicated with H&E staining. The
pathological results were classified as macrometastasis (>2.0
mm), micrometastasis (0.2–2.0 mm) and isolate tumor cells (ITC,
<0.2 mm) according to the TMN staging system (9). Patients with negative TIC but positive
for either SS with H&E staining or IHC (except for
micrometastasis or ITC with IHC) required a second axillary
operation.
Statistical analysis
The results of TIC were compared with those of the
final pathology and were classified as true-positive (TP),
true-negative (TN), false-negative (FN) or false-positive (FP),
both on a patient and node basis. True-positive cases were those
that were found to contain carcinoma both on TIC and on subsequent
H&E and IHC staining. The formulas used to calculate
statistical parameters were: Sensitivity = TP/(TP + FN);
specificity = TN/(TN + FP); overall accuracy = (TP + TN)/(TP + FP +
TN + FN); negative predictive value (NPV) = TN/(TN + FN) and
positive predictive value (PPV) = TP/(TP + FP). For statistical
analysis, Fisher’s exact test was performed with a cut-off point of
P<0.05 using the SPSS statistical analysis program, version 16.0
(SPSS Inc., Chicago, IL, USA).
Results
Pricipal observations
The mean age of the 366 patients was 49.9 years
(range 24–81). The median size of the measured primary tumors was
22 mm (4–5 mm). The mean number of the SLNs was 2.75 per patient.
For all surgeries, the results of the intraoperative assessment of
the SLNs were available prior to completion of the lumpectomy or
mastectomy.
Based on the final pathology, among the 107 cases in
which positive lymph nodes were present, 74 (69.2%) had at least
one sentinel lymph node that was positive. Overall, 149 positive
lymph nodes were removed. TIC identified 82/107 patients and a
total 119/149 nodes, as well as 3 false-positive patients (9
nodes), resulting in a sensitivity, specificity and overall
accuracy rate of 76.6, 98.8 and 92.3%, respectively, on a per
patient basis, and 79.9, 98.9 and 96.1%, respectively, on a per
node basis (Table I).
Micrometastases were found in 19 SLNs, of which 6 nodes were
identified by TIC. Only 7/107 (6.5%) patients had SLNs that were
positive for micrometastasis but no macrometastasis in any other
SLNs, and 5/7 were overlooked by TIC, resulting in a sensitivity
for micrometastasis of 28.6% on a per patient basis and 31.6% on a
per node basis. Sensitivity for macrometastasis was 80.0% on a per
patient basis and 86.9% on a per node basis (Table II).
 | Table IThe total sensitivity, specificity,
overall accuracy rate, negative and positive predictive value of
touch imprint cytology for the studied cases. |
Table I
The total sensitivity, specificity,
overall accuracy rate, negative and positive predictive value of
touch imprint cytology for the studied cases.
| Final
histopathological diagnosisa |
|---|
|
|
|---|
| TIC | By case | By node |
|---|
| Sensitivity | 76.6% (82/107) | 79.9% (119/149) |
| Specificity | 98.8% (256/259) | 98.9% (834/843) |
| Overall accuracy | 92.3% (338/366) | 96.1% (953/992) |
| NPV | 91.1% (256/281) | 96.5% (834/864) |
| PPV | 96.5% (82/85) | 93.0% (119/128) |
 | Table IIAnalysis of touch imprint cytology
(TIC) of the metastatic deposit with regard to its size. |
Table II
Analysis of touch imprint cytology
(TIC) of the metastatic deposit with regard to its size.
| SLN metastasis size
at final histopathology | TIC | n | Sensitivity (%) |
|---|
| Macrometastases
(n=100) | Positive | 80 | 80.0% |
| Negative | 20 | |
| Micrometastases or
ITC (n=7) | Positive | 2 | 28.6% |
| Negative | 5a | |
| No. of metastases
(n=259) | Positive | 3 | |
| Negative | 256 | |
Metastatic foci of 83/107 (77.6%) patients were
identified in the initial SLN captured. Additionally, all of the
patients that had metastatic SLNs were confirmed to be node
involved within the first four SLNs harvested.
Among the 366 patients, the postoperative
pathological evaluation determined DCIS in 33 patients, DCIS-Mi in
25 patients and IDC in 308 patients. The metastatic rates of the
patients with DCIS, DCIS-Mi and IDC were 3.0% (1 case), 16.0% (4
cases) and 33.1% (102 cases), respectively. For the patients with
DCIS, DCIS-Mi and IDC, 1.0% (1/97 nodes), 10.0% (6/60 nodes) and
34.1% (285/835 nodes) SLNs captured, respectively, were confirmed
to contain cancer cells. The sensitivity of TIC for patients with
DCIS, DCIS-Mi and IDC was 0, 50.0 and 78.4%, respectively, on a per
patient basis, and 0, 66.7 and 90.5%, respectively, on a per node
basis.
False-positive results based on serial
sections with hematoxylin and eosin
A total of 9 SLNs from 3 patients were positive upon
TIC and negative using SS with H&E staining; the 3 patients
received an unnecessary ALND. Four of the 9 ‘false-positive’ nodes
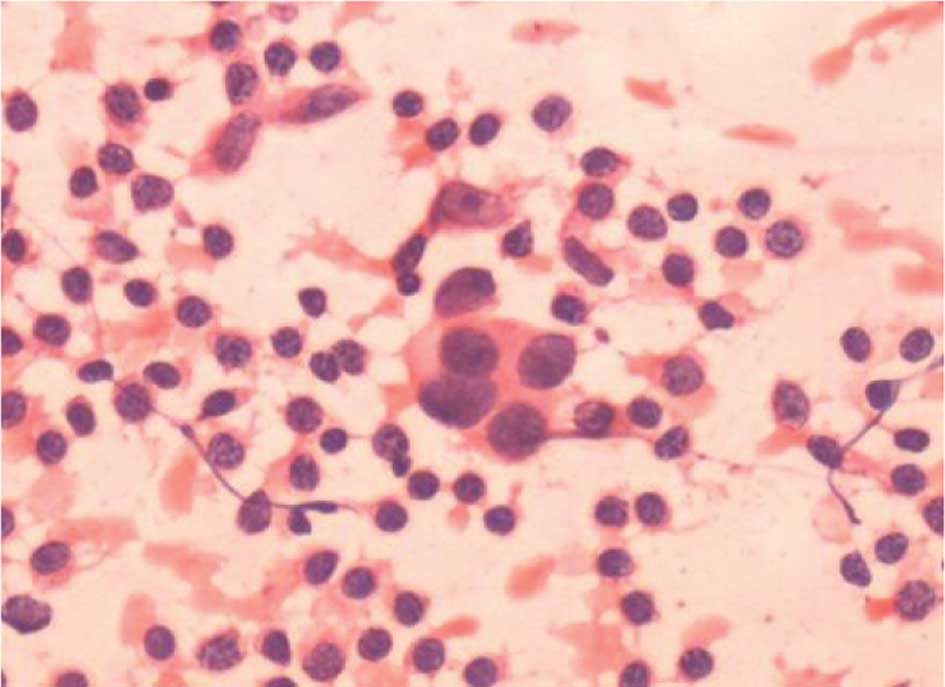
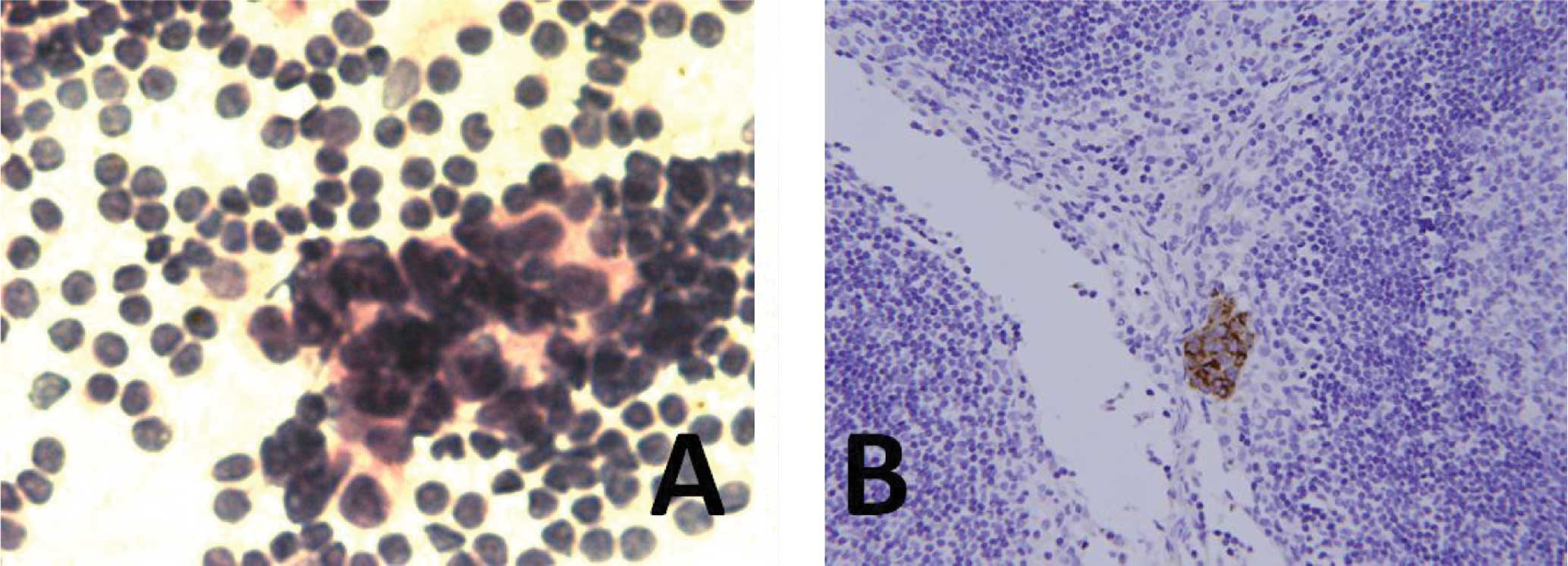
were considered to be atypical when reviewed (Fig. 1). Subsequent IHC staining identified
three micrometastasis-positive SLNs (Fig. 2). On reviewing the remaining 2
‘false positive’ SLNs, malignant cells were found in the two cases
that were considered to be positive by the cytopathologists.
False-negative results based on serial
sections with hematoxylin and eosin staining
A total of 30 SLNs from 25 patients were
false-negative, and micrometastases were detected in 13/30 (43.3%)
SLNs based on SS with H&E staining. The result of the IHC
staining showed 2 micrometastasis-positive SLNs using SS with
H&E staining that were assessed to be macrometastatic. The
false-negatives were examined to determine whether the misdiagnoses
were due to interpretation or sampling error. Two imprints showed
cytological evidence of metastases (interpretation error), whereas
the remaining 28 imprints were due to sampling error. All of the 25
false-negative patients underwent a second axillary operation.
Discussion
The results from the present study are in accordance
with previously published data indicating that the TIC technique
provides acceptable accuracy in detecting macrometastasis, but not
micrometastasis (2,6,10,11).
The sensitivity of TIC in published studies varies widely from 34%
(11) to 96% (9). The sensitivity in our study was
moderate (76.6%) by taking 2- to 3-mm serial slices of the sentinel
node. Studies have found that this cutting interval may increase
the sensitivity of TIC by increasing the effective surface area for
analysis, allowing for the detection of small deposits and
micrometastasis while increasing the pathological reporting time
(2,11–16).
In our institution, intraoperative assessment is accomplished
during primary surgery and does not appear to prolong the surgical
procedure.
It should be noted that the metastastic rate of
patients is as low as 3.0% for patients with DCIS, whereas the
sensitivity for TIC in patients with DCIS is 0 and 50% for DCIS-Mi
patients. Despite these results, intraoperative evaluation in
patients with DCIS or DCIS-Mi diagnosed by core needle biopsy was
performed, since these preoperative histopathological assessments
did not reach a high enough specificity to accurately diagnose the
majority of the pure DCIS cases. Ultrasound-guided core needle
biopsy is considered to be a reliable non-invasive alternative to
surgical biopsy for obtaining a histopathological diagnosis with
considerable sensitivity and specificity. However, its limitations
include relatively high false-negative results (range 0.9–9.0%) and
underestimation of disease with a wide range (17), suggesting that it is necessary to
perform intraoperative assessment for patients with DCIS diagnosed
by core needle biopsy who may need SLNB.
Studies have indicated that lobular histology may be
more difficult to interpret using touch imprint cytology, leading
to a higher false-negative rate (18). Lobular carcinomas were excluded in
our study due to the limited number of cases (4 cases), and we
focused on the ductal carcinoma type. We found 25 false-negative
and 3 false-positive patients using TIC, and aimed to identify the
potential and common factors associated with the misdiagnosed
results.
The findings showed 9 false-positive SLNs in our
series. The 9 SLNs were re-examined, and positive results for the 5
imprints were confirmed following revision; 3 of the 5 were
identified as micrometastasis-positive SLNs by subsequent IHC
staining. Four of the 9 false-positive nodes were considered to be
atypical when reviewed. Three possible reasons exist for the
false-positive result. Firstly, micrometastatic foci which were
overlooked by SS with H&E may have been detected by TIC. This
is rare and cases should be considered true positive when
subsequent IHC staining indicates micrometastasis. Therefore, for
the false-positive cases based on SS with H&E staining, SS with
IHC staining should be performed to avoid misdiagnosis. Secondly,
the imprint cytology specimen may have contained the only
metastatic deposit in the part of the lymph node that was lost in
the deeper sections of the lymph node and, consequently, was not
located on final histopathology. Finally, the clinical impression
of the surgeon regarding the appearance of the nodes may affect the
decision of the cytopathologists. Cytopathologists may be required
to examine the nodes thoroughly when the nodes are pathologically
palpable or the cut surfaces of the SLNs are suspicious. This
requirement may lead to cytopathologists being overly cautious when
deciding on a negative result when the imprints appear to be
atypical. In the present study, the surgeon’s clinical impression
was that 4 false-positive SLNs, using SS with H&E staining,
were atypical. However, these SLNs were determined to be carcinoma
cells by the cytopathologists.
In the present study, 30 false-negative SLNs were
found, and micrometastasis was detected in 13/30 (43.3%) SLNs,
using SS with H&E staining. We conclude that the main reason
for the false-negative results of the imprint cytology was poor
quality of the imprint samples due to sampling error. One of the
significant factors impacting sampling error is micrometastasis in
sentinel nodes. SLNs were cut along the long axis at a 2.0- to
3.0-mm interval to prepare imprints while SLNs were cut at a 100-μm
interval to perform the final histopathological diagnosis.
Therefore, small metastatic foci may be not be detected by TIC but
identified by final histopathological diagnosis due to the more
precise cutting method. The intraoperative detection of
micrometastatic disease is a well-known issue, leading to high
false-negative rates. A meta-analysis (19) of 31 related studies found a pooled
sensitivity of 63% (95% CI, 57–69) and specificity of 99% (95% CI,
98–99) for TIC; the pooled sensitivity for macrometastasis was 81%
and that for micrometastasis was 22%. The only statistically
significant predictor for a false-negative evaluation of axillary
node involvement was found to be the proportion of micrometastasis
in multivariable analysis (19). In
the present study, TIC was significantly more sensitive for
macrometastasis than micrometastasis; 80.0 vs. 28.6%, respectively,
(P=0.001). It has been suggested that shortening the cutting
interval may be useful for improving the quality of the imprint
samples. Therefore, a prospective controlled study commenced in
March 2008 at our institution to compare the clinical value of the
cutting method in preparing imprints between the routine protocol
(to cut along the long axis at a 2.0- to 3.0-mm interval) and the
new protocol (to cut along the short axis at a 1.5-mm
interval).
In conclusion, at our institution, TIC is considered
an accurate, practical, time-and cost-efficient procedure with
minimal tissue preparation for intraoperatively evaluating SLNs. It
is feasible for clinical use and is able to detect macrometastasis
in SLNs intraoperatively with an acceptable accuracy early stage
invasive breast cancer patients, although its ability to detect
micrometastasis is limited. Furthermore, micrometastasis in SLNs
and interpretation error may be the key reasons for false-positive
and false-negative results in the intraoperative evaluation of
SLNs.
Acknowledgements
The authors thank the patients and family members
for their willingness to cooperate in our study. Grants were
endowed by the Research Foundation of Sci-Tech Committee of
Shanghai City (no. 54119524) and the ‘Shu Guang’ Project Foundation
of the Education Committee of Shanghai City (no. 05SG04).
References
|
1
|
Veronesi U, Paganelli G, Viale G, et al: A
randomized comparison of sentinel-node biopsy with routine axillary
dissection in breast cancer. N Engl J Med. 349:546–553. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Creager AJ, Geisinger KR, Shiver SA, et
al: Intraoperative evaluation of sentinel lymph nodes for
metastatic breast carcinoma by imprint cytology. Mod Pathol.
15:1140–1147. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liang R, Craik J, Juhasz ES and Harman CR:
Imprint cytology versus frozen section: intraoperative analysis of
sentinel lymph nodes in breast cancer. ANZ J Surg. 73:597–599.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aihara T, Munakata S, Morino H and
Takatsuka Y: Comparison of frozen section and touch imprint
cytology for evaluation of sentinel lymph node metastasis in breast
cancer. Ann Surg Oncol. 11:747–750. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Motomura K, Inaji H, Komoike Y, et al:
Intraoperative sentinel lymph node examination by imprint cytology
and frozen sectioning during breast surgery. Br J Surg. 87:597–601.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Creager AJ, Geisinger KR, Perrier ND, et
al: Intraoperative imprint cytologic evaluation of sentinel lymph
nodes for lobular carcinoma of the breast. Ann Surg. 239:61–66.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fitzgibbons PL, Page DL, Weaver D, et al:
Prognostic factors in breast cancer. College of American
Pathologists Consensus Statement 1999. Arch Pathol Lab Med.
124:966–978. 2000.PubMed/NCBI
|
|
8
|
Chen JJ, Huang XY, Liu ZB, et al: Sentinel
node biopsy and quality of life measures in a Chinese population.
Eur J Surg Oncol. 35:921–927. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rubio IT, Korourian S, Cowan C, Krag DN,
Colvert M and Klimberg VS: Use of touch preps for intraoperative
diagnosis of sentinel lymph node metastases in breast cancer. Ann
Surg Oncol. 5:689–694. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Menes TS, Tartter PI, Mizrachi H, et al:
Touch preparation or frozen section for intraoperative detection of
sentinel lymph node metastases from breast cancer. Ann Surg Oncol.
10:1166–1170. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zgajnar J, Frkovic-Grazio S, Besic N, et
al: Low sensitivity of the touch imprint cytology of the sentinel
lymph node in breast cancer patients – results of a large series. J
Surg Oncol. 85:82–86; discussion 8. 2004.
|
|
12
|
Mullenix PS, Carter PL, Martin MJ, et al:
Predictive value of intra-operative touch preparation analysis of
sentinel lymph nodes for axillary metastasis in breast cancer. Am J
Surg. 185:420–424. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bochner MA, Farshid G, Dodd TJ, Kollias J
and Gill PG: Intraoperative imprint cytologic assessment of the
sentinel node for early breast cancer. World J Surg. 27:430–432.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Karamlou T, Johnson NM, Chan B, Franzini D
and Mahin D: Accuracy of intraoperative touch imprint cytologic
analysis of sentinel lymph nodes in breast cancer. Am J Surg.
185:425–428. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baitchev G, Gortchev G and Todorova A:
Intraoperative sentinel lymph node examination by imprint cytology
during breast surgery. Curr Med Res Opin. 18:185–187. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Llatjos M, Castella E, Fraile M, et al:
Intraoperative assessment of sentinel lymph nodes in patients with
breast carcinoma: accuracy of rapid imprint cytology compared with
definitive histologic workup. Cancer. 96:150–156. 2002. View Article : Google Scholar
|
|
17
|
Schueller G, Schueller-Weidekamm C and
Helbich TH: Accuracy of ultrasound-guided, large-core needle breast
biopsy. Eur Radiol. 18:1761–1773. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cox C, Centeno B, Dickson D, et al:
Accuracy of intraoperative imprint cytology for sentinel lymph node
evaluation in the treatment of breast carcinoma. Cancer. 105:13–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tew K, Irwig L, Matthews A, Crowe P and
Macaskill P: Meta-analysis of sentinel node imprint cytology in
breast cancer. Br J Surg. 92:1068–1080. 2005. View Article : Google Scholar : PubMed/NCBI
|