Introduction
Gastric cancer (GC) is one of the most common types
of cancer in East Asia and the second leading cause of
cancer-related death worldwide, with over 730,000 deaths per year
(1). Although noteworthy advances
in the early diagnosis and endoscopic or surgical resection of this
entity have occurred in recent years in Japan and Korea, advanced
stage is achieved prior to detection when curative resection may no
longer be a viable option. When GC is advanced, systemic
chemotherapy is regarded as crucial in improving patient quality of
life and prolonging survival (2–4).
However, GC causes a deficiency in nutrition due to peritoneal
metastasis, rapidly depriving patients of their well-being and
favorable performance status (PS). It is difficult to control this
entity by first-line chemotherapy alone, despite the availability
of numerous cytotoxic agents that are active against GC. Currently,
a 5-fluorouracil (FU)-based regimen in combination with cisplatin
(CDDP) is the most commonly used first-line therapy in the
treatment of advanced GC (5–7).
However, the optimal regimen for a second-line chemotherapy for GC
remains to be determined. Although the SPIRITS trial reported an
overall survival (OS) of more than 1 year, progression-free
survival (PFS) with S-1 plus CDDP in a first-line setting was only
6 months, and 74% of the patients received second-line chemotherapy
(8). Moreover, in the JCOG9912
trial, OS and PFS were 11.4 and 4.2 months, respectively, in the
S-1 alone arm, and 74% of the patients received second-line
chemotherapy (9). By contrast, in
the FLAGS global trial, OS and PFS were 8.6 and 4.8 months,
respectively, in the S-1 plus CDDP arm, and only 31% of the
patients received second-line chemotherapy (10). In the V-325 trial (6), OS and time-to-progression were 9.2 and
5.6 months, respectively, in the DCF (docetaxel, CDDP and FU) arm,
and only 32% of the patients received further chemotherapy. This
indicates that, in certain cases, optimal second-line chemotherapy
may contribute to the favorable OS observed with first-line
treatment.
Recently, Thuss-Patience et al reported that
irinotecan (CPT-11) alone (250 mg/m2 every 3 weeks, to
be increased to 350 mg/m2 depending on toxicity)
significantly improved OS compared to best supportive care
[HR=0.48; 95% confidence interval (CI), 0.25–0.92; P=0.023] in a
second-line setting in patients previously treated with only one
regimen (11). Consequently, CPT-11
may be a key novel agent for the treatment of advanced GC in a
second-line setting. On the other hand, the weekly administration
of paclitaxel (PAC) for GC following the failure of prior
chemotherapy has become standard practice in Japan, as it offers
milder toxicities than a tri-weekly schedule but with equivalent
activity (12,13). In previous phase II trials, it was
found that PAC was active against GC with peritoneal metastasis
that was refractory to regimens containing 5-FU (14,15).
However, only a few phase II trials involving weekly PAC regimen
for GC have been reported in a second-line setting.
At our institute, CPT-11 is the preferred agent in a
second-line setting, unless contraindicated due to peritoneal
metastasis or inadequate liver function, in which case taxane
agents are selected. The purpose of the present retrospective study
was to compare CPT-11 alone and weekly PAC in a second-line setting
following the failure of a fluoropyrimidine-based regimen in terms
of survival benefit and safety in the treatment of recurrent or
unresectable GC.
Patients and methods
Patients
Between April, 2004 and March, 2009, 179 patients
received second-line chemotherapy with CPT-11 monotherapy or weekly
PAC at Division of Medical Oncology, Cancer Institute Hospital of
the Japanese Foundation for Cancer Research, Tokyo, Japan. Of
these, 92 patients with no findings of peritoneal metastasis,
radiologically confirmed intestinal stenosis, or massive ascites
(beyond the pelvic cavity) received CPT-11 alone (Cohort I). The
remaining 87 patients received weekly PAC as they did not meet the
safety criteria for administration of CPT-11 (Cohort P). Patients
were selected according to the following criteria: i)
histologically confirmed GC with metastatic or recurrent disease;
ii) an Eastern Cooperative Oncology Group (ECOG) PS of 0–2; iii)
failure of prior chemotherapy with a fluoropyrimidine-based
regimen, either in an adjuvant setting or for metastatic disease;
iv) age ≥18 years; v) adequate function of bone marrow and
preserved organ function; vi) no synchronous double cancer or other
serious disease; and vii) availability of written informed consent
prior to administration of the treatment.
Treatment
In Cohort I, patients received infusional 150
mg/m2 CPT-11 on days 1 and 15 every 4 weeks following
pre-medication with an intravenous 5-HT3 blocker and
dexamethasone. In Cohort P, patients received infusional 80
mg/m2 PAC on days 1, 8, and 15 every 4 weeks after
pre-medication with an intravenous 5-HT3 blocker.
Diphenhydramine, dexamethasone and ranitidine were also
administered to prevent hypersensitivity reactions. Chemotherapy
was continued until the event of disease progression, unacceptable
toxicities, or the patient's refusal of treatment.
Response and toxicity evaluation
Each patient underwent CT scan after every two
cycles of therapy to document the extent of disease and evaluate
response to treatment. Objective responses for measurable lesions
were assessed according to the guidelines of the Response
Evaluation Criteria in Solid Tumors Committee (RECIST 1.0).
Symptomatic toxicity and laboratory data were noted every 2 weeks
at the outpatient department. Toxicities were assessed using the
Common Toxicity Criteria for Adverse Events, version 3.0 (CTCAE
v.3.0). Dose reduction and treatment delays were advocated
according to the extent of hematological and non-hematological
toxicities.
Statistical analysis
Progression-free survival was calculated from
initiation of the second-line treatment until the time of
progression occurred, the patient succumbed to any cause, or when
neither of these events occurred, the date of the last follow-up.
Overall survival was calculated from the time the second-line
treatment commenced to the date of death or the last follow-up
visit. Survival curves were obtained using the Kaplan-Meier method.
Univariate analysis of OS and PFS was performed using the log-rank
test. Statistical analyses were performed using SPSS (SPSS Inc.,
Chicago, IL, USA). P-values were two-sided; P<0.05 was regarded
as statistically significant.
Results
Patient characteristics
Table I shows the
patient characteristics. The median age of the patients was 61
years (range 19–82) in Cohort I and 62 years (range 28–73) in
Cohort P, and the majority of the study population was male
(64.2%). A total of 164 (91.6%) patients had an ECOG PS of 0 or 1.
One of the 92 (1.1%) patients in Cohort I and 14 of the 87 (16.1%)
patients in Cohort P had a PS of 2. Primary lesions were present in
41 of 92 (44.6%) patients in Cohort I and in 43 of 87 (49.4%)
patients in Cohort P. The patients had previously received
chemotherapy with fluoropyrimidine-based regimens containing S-1,
S-1 plus CDDP, capecitabine plus CDDP, or 5-FU plus methotrexate.
Tumor response was evaluated in 125 of the 179 (69.8%) patients
with measurable target lesions. The lymph nodes (Cohorts I and P:
44.6 and 39.1%, respectively), peritoneum (Cohorts I and P: 39.1
and 85.1%, respectively) and the liver (Cohorts I and P: 39.1 and
24.1%, respectively) were the most common metastatic sites. Among
the 179 patients, histological type was intestinal in 50 (27.9%)
patients and diffuse in 129 (72.1%) patients, according to the
Lauren classification. Chemotherapy was administered in a
third-line setting in 72 of 92 (78.3%) patients in Cohort I and in
15 of 87 (17.2%) patients in Cohort P. Of the 72 patients who
received third-line chemotherapy in Cohort I, 53 (73.6%) patients
received weekly PAC and 14 (19.4%) patients received tri-weekly
docetaxel (DOC).
 | Table IPatient characteristics (n=177). |
Table I
Patient characteristics (n=177).
| Characteristics | Irinotecan, n (%)
(n=92) | Paclitaxel, n (%)
(n=87) |
|---|
| Gender |
| Male | 67 (72.8) | 48 (55.2) |
| Female | 25 (27.2) | 39 (44.8) |
| Median age
(range) | 61 (19–82) | 62 (28–73) |
| ECOG PS |
| 0 | 73 (79.3) | 37 (42.5) |
| 1 | 18 (19.6) | 36 (41.4) |
| 2 | 1 (1.1) | 14 (16.1) |
| Previous gastrectomy
(+) | 51 (55.4) | 44 (50.6) |
| Histological
typea |
| Intestinal | 37 (40.2) | 13 (14.9) |
| Diffuse | 55 (59.8) | 74 (85.1) |
| Target lesions
(+) | 74 (80.4) | 35 (83.3) |
| Massive ascites
(+) | 0 (0.0) | 33 (37.9) |
| No. of involved
organs |
| 1 | 37 (40.2) | 30 (34.5) |
| ≥2 | 55 (59.8) | 57 (65.5) |
| Metastatic sites |
| Lymph nodes | 41 (44.6) | 34 (39.1) |
| Peritoneum | 36 (39.1) | 74 (85.1) |
| Liver | 36 (39.1) | 21 (24.1) |
| Lung | 6 (6.5) | 2 (2.3) |
| Bone | 1 (1.1) | 9 (10.3) |
| Ovary | 5 (5.4) | 11 (12.6) |
| Adrenal gland | 0 (0.0) | 4 (4.6) |
| Portal embolism | 2 (2.2) | 2 (2.3) |
| Other | 1 (1.1) | 3 (3.4) |
| Prior
chemotherapy |
| S-1 | 21 (22.8) | 44 (50.6) |
| S-1 plus CDDP | 55 (59.8) | 21 (24.1) |
| CAP plus CDDP | 4 (4.3) | 2 (2.3) |
| 5-FU plus MTX | 2 (2.2) | 14 (16.1) |
| Other | 0 (0.0) | 2 (2.3) |
| Adjuvant S-1 | 10 (10.9) | 4 (4.6) |
| Third-line
chemotherapy | 72 (78.2) | 15 (17.2) |
| Weekly PAC | 53 (57.6) | 0 (0.0) |
| Tri-weekly DOC | 14 (15.2) | 0 (0.0) |
| 5-FU plus MTX | 1 (1.1) | 5 (5.7) |
| CPT-11 | 0 (0.0) | 6 (6.9) |
| Other | 4 (4.3) | 4 (4.6) |
Response and survival
No complete response was observed among the 179
patients, and overall response rate (ORR) was 6.5% in Cohort I and
9.8% in Cohort P. Disease control (partial response and stable
disease) rate (DCR) was 43.5% in Cohort I and 54.9% in Cohort P.
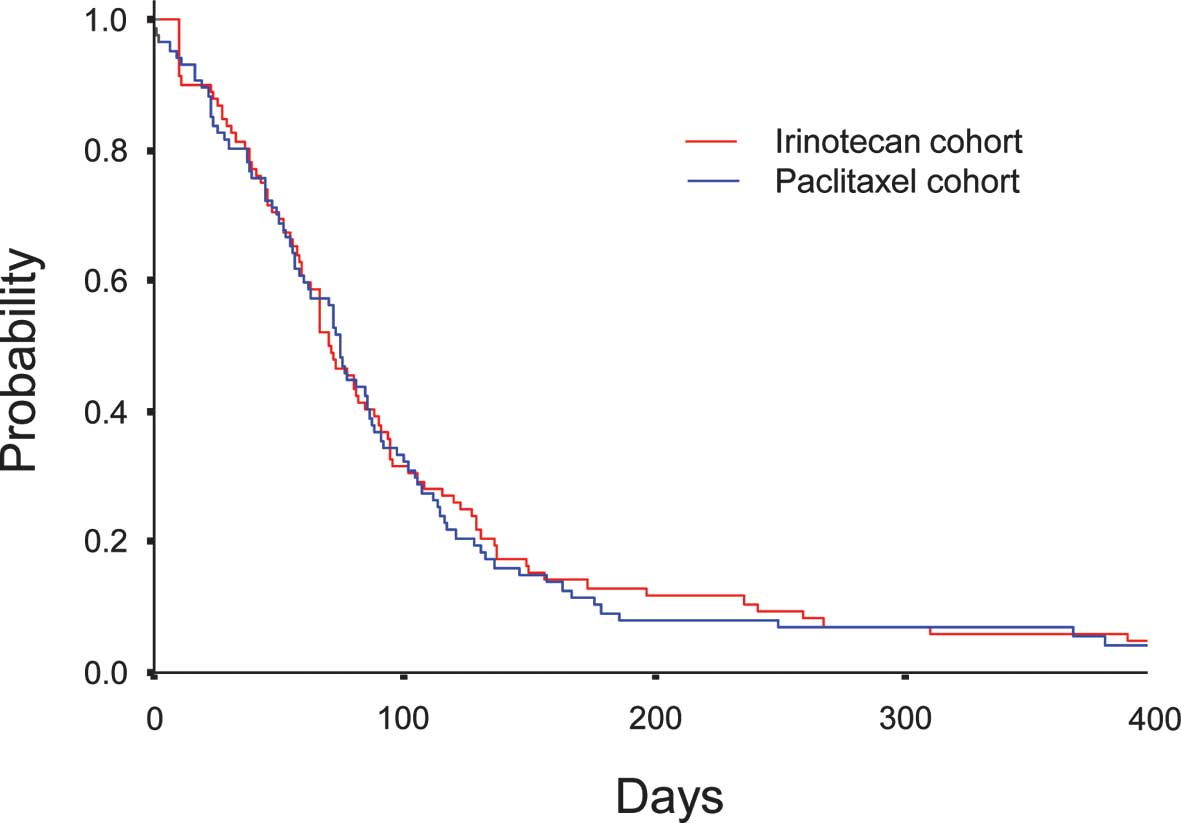
Median PFS was comparable between 2.6 months (95% CI, 2.2–3.0) in
Cohort I and 2.8 months (95% CI, 2.5–3.0) in Cohort P (P=0.812)
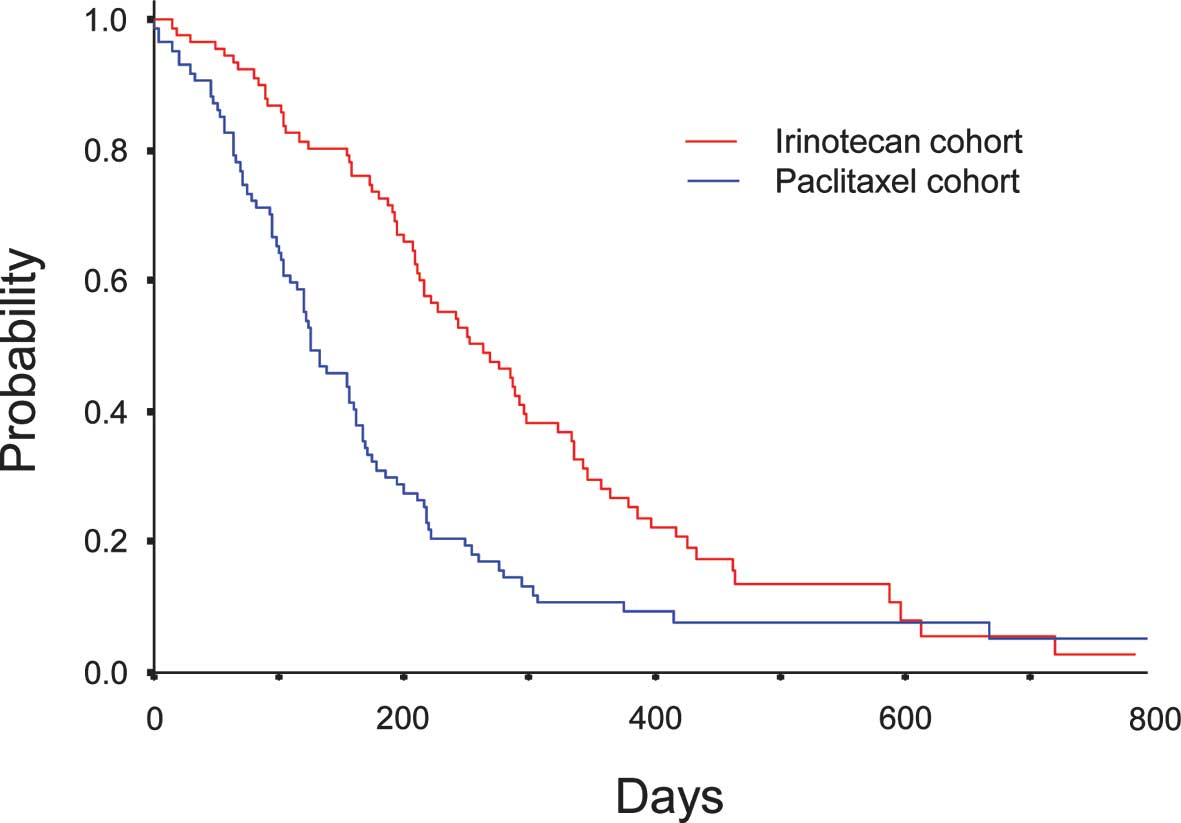
(Fig. 1). Median OS was 9.8 months
(95% CI, 7.8–11.8) in Cohort I and 4.9 months (95% CI, 3.8–5.9) in
Cohort P (P<0.0001) (Fig. 2). A
significant difference was observed in OS between Cohort I and
Cohort P.
Adverse events
Table II shows the
adverse effects observed in this study. The most frequent grade 3/4
adverse hematological event was neutropenia, which was observed in
7 of 92 (7.6%) patients in Cohort I and in 13 of 87 (14.9%)
patients in Cohort P. Leukopenia was found in 4 of 92 (4.3%)
patients in Cohort I and in 4 of 87 (4.6%) patients in Cohort P.
Anemia was noted in 3 of 92 (3.3%) patients in Cohort I and in 10
of 87 (11.5%) patients in Cohort P. Septic shock occurred in one
(1.1%) patient, who died within 30 days of the last administration
of the CPT-11 alone regimen. The most common grade 3/4 adverse
non-hematological event was anorexia, which was found in 5 of 92
(5.4%) patients in Cohort I and in 4 of 87 (4.6%) patients in
Cohort P.
 | Table IIFrequency of grade 3/4 adverse
events. |
Table II
Frequency of grade 3/4 adverse
events.
| Adverse events
(AEs) | Irinotecan, n (%)
(n=92) | Paclitaxel, n (%)
(n=87) |
|---|
| Hematological
AEs |
| Leukopenia | 4 (4.3) | 4 (4.6) |
| Neutropenia | 7 (7.6) | 13 (14.9) |
| Anemia | 3 (3.3) | 10 (11.5) |
|
Thrombocytopenia | 1 (1.1) | 1 (1.1) |
| Febrile
neutropenia | 1 (1.1) | 2 (2.3) |
| Non-hematological
AEs |
| Anorexia | 5 (5.4) | 4 (4.6) |
| Nausea | 2 (2.2) | 0 (0.0) |
| Fatigue | 1 (1.1) | 3 (3.4) |
| Diarrhea | 3 (3.3) | 1 (1.1) |
| Hyponatremia | 0 (0.0) | 4 (4.6) |
| Hyperkalemia | 1 (1.1) | 1 (1.1) |
| Hypokalemia | 0 (0.0) | 3 (3.4) |
| Increased
transaminase | 0 (0.0) | 4 (4.6) |
| Neuropathy
(sensory) | 0 (0.0) | 2 (2.3) |
| Treatment-related
deaths | 1 (1.1) | 0 (0.0) |
Discussion
First-line chemotherapy is considered to confer a
survival benefit in the treatment of advanced GC. However, optimal
second-line chemotherapy contributes to the favorable OS observed
in first-line treatment. Few well-designed, randomized trials of
chemotherapy in a second-line setting have been conducted and the
optimal treatment regimen following the failure of first-line
chemotherapy remains to be determined (16). The results of the AIO phase III
trial suggest that CPT-11 is a key novel agent in a second-line
setting (11).
In advanced colorectal cancer, the availability of
the three active cytotoxic agents of FU-leucovorin, CPT-11 and
oxaliplatin during the treatment course is crucial as it maximizes
the OS of patients (17). In the
treatment of advanced GC, combination regimens using FU with CDDP
are currently regarded as standard first-line therapy. However, the
importance to OS of the availability of two active cytotoxic
agents, CPT-11 and the taxane agent PAC or DOC, during further
treatment remains to be determined. CPT-11 often cannot be used
following the failure of second-line treatment since severe
toxicities may occur, in that GC progressively develops clinical
symptoms of intestinal stenosis, massive ascites and obstructive
jaundice due to dissemination. Therefore, CPT-11 is the treatment
of choice in a second-line setting, unless contraindicated due to
peritoneal metastasis or inadequate liver function.
Based on reports of its activity against other types
of cancer (18,19), PAC, a taxane agent, is frequently
used in a fractionated weekly schedule at 80 mg/m2 in a
second-line or further setting for GC. In previous phase II trials,
weekly PAC administration was found to be active against GC with
peritoneal metastasis (14,15). Moreover, it has been suggested that
the regimen is feasible, safe and shows consistent activity against
heavily treated GC in a second-line or further setting (12,20).
Neutropenia (14.9%), anemia (10%), leukopenia (4%), and anorexia
(4%) were the most common grade 3/4 adverse events observed in this
study, occurring less frequently than in previous reports. In
Cohort P patients who were unable to receive CPT-11 in a
second-line setting, the median PFS was 77 days and median survival
time was 136 days from initiation of PAC. This supports the
feasibility of using PAC in patients contraindicated for CPT-11 due
to evidence of peritoneal metastasis.
In this retrospective study, ORR and DCR in Cohort I
were 6.5 and 43.5%, respectively, compared with 9.8 and 54.9%,
respectively, in Cohort P. The median PFS (Cohort I vs. P, 2.6 vs.
2.8 months) did not differ between the two treatment cohorts (HR,
0.97; 95% CI, 0.72–1.30; P=0.812). However, the median OS (Cohort I
vs. P, 9.8 vs. 4.9 months) differed significantly between the two
treatment cohorts (HR, 0.50; 95% CI, 0.36–0.69; P<0.0001). PFS
in Cohort I was comparable with that in Cohort P, but OS in Cohort
I was much longer. Third-line chemotherapy was administered in 72
of 92 (78.3%) patients in Cohort I and in 15 of 87 (17.2%) patients
in Cohort P. The high frequency of third-line chemotherapy in
Cohort I may explain the discrepancy in OS between the two cohorts.
Among the 72 patients who received third-line chemotherapy in
Cohort I, 53 (73.6%) received weekly PAC and 14 (19.4%) received
tri-weekly DOC. This course of treatment indicates that
availability of CPT-11 in a second-line setting prolongs the
survival of patients with advanced GC.
It is difficult to confirm the optimal second-line
regimen from the results of the present study, and it appears that
in the course of treatment, the availability of the four active
cytotoxic agents, FU, CDDP, CPT-11 and the taxane agent PAC or DOC,
prolong the lives of patients with advanced GC. However, the
availability of CPT-11 in a second-line setting, in particular,
appears to beneficial in terms of survival. Although this was a
retrospective study, the results provide evidence that the
availability of CPT-11 in a second-line setting is beneficial to
advanced GC patients in whom fluoropyrimidine-based first-line
chemotherapy proved unsuccessful.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. June 17–2010.(Epub ahead of
print).
|
|
2
|
Murad AM, Santiago FF, Petroianu A, Rocha
PR, Rodrigues MA and Rausch M: Modified therapy with
5-fluorouracil, doxorubicin, and methotrexate in advanced gastric
cancer. Cancer. 72:37–41. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pyrhönen S, Kuitunen T, Nyandoto P and
Kouri M: Randomised comparison of fluorouracil, epidoxorubicin and
methotrexate (FEMTX) plus supportive care with supportive care
alone in patients with non-resectable gastric cancer. Br J Cancer.
71:587–591. 1995.PubMed/NCBI
|
|
4
|
Wagner AD, Grothe W, Haerting J, Kleber G,
Grothey A and Fleig WE: Chemotherapy in advanced gastric cancer: a
systematic review and meta-analysis based on aggregate data. J Clin
Oncol. 24:2903–2909. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vanhoefer U, Rougier P, Wilke H, et al:
Final results of a randomized phase III trial of sequential
high-dose methotrexate, fluorouracil, and doxorubicin versus
etoposide, leucovorin, and fluorouracil versus infusional
fluorouracil and cisplatin in advanced gastric cancer: A trial of
the European Organization for Research and Treatment of Cancer
Gastrointestinal Tract Cancer Cooperative Group. J Clin Oncol.
18:2648–2657. 2000.
|
|
6
|
Van Cutsem E, Moiseyenko VM, Tjulandin S,
et al: Phase III study of docetaxel and cisplatin plus fluorouracil
compared with cisplatin and fluorouracil as first-line therapy for
advanced gastric cancer: a report of the V325 Study Group. J Clin
Oncol. 24:4991–4997. 2006.PubMed/NCBI
|
|
7
|
Cunningham D, Starling N, Rao S, et al;
Upper Gastrointestinal Clinical Studies Group of the National
Cancer Research Institute of the United Kingdom. Capecitabine and
oxaliplatin for advanced esophagogastric cancer. N Engl J Med.
358:36–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koizumi W, Narahara H, Hara T, et al: S-1
plus cisplatin versus S-1 alone for first-line treatment of
advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet
Oncol. 9:215–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boku N, Yamamoto S, Fukuda H, et al:
Fluorouracil versus combination of irinotecan plus cisplatin versus
S-1 in metastatic gastric cancer: a randomised phase 3 study.
Lancet Oncol. 10:1063–1069. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ajani JA, Rodriguez W, Bodoky G, et al:
Multicenter phase III comparison of cisplatin/S-1 with
cisplatin/infusional fluorouracil in advanced gastric or
gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin
Oncol. 28:1547–1553. 2010. View Article : Google Scholar
|
|
11
|
Thuss-Patience PC, Kretzschmar A, Deist T,
et al: Irinotecan versus best supportive care (BSC) as second-line
therapy in gastric cancer. A randomized phase III study of the
Arbeitsgemeinschaft Internistische Onkologie (AIO). J Clin Oncol.
27(Suppl 15): abs. 4540. 2009.PubMed/NCBI
|
|
12
|
Hironaka S, Zenda S, Boku N, Fukutomi A,
Yoshino T and Onozawa Y: Weekly paclitaxel as second-line
chemotherapy for advanced or recurrent gastric cancer. Gastric
Cancer. 9:14–18. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kodera Y, Ito S, Mochizuki Y, et al: A
phase II study of weekly paclitaxel as second-line chemotherapy for
advanced gastric Cancer (CCOG0302 study). Anticancer Res.
27:2667–2671. 2007.PubMed/NCBI
|
|
14
|
Yamada Y, Shirao K, Ohtsu A, et al: Phase
II trial of paclitaxel by three-hour infusion for advanced gastric
cancer with short premedication for prophylaxis against
paclitaxel-associated hypersensitivity reactions. Ann Oncol.
12:1133–1137. 2001. View Article : Google Scholar
|
|
15
|
Yamaguchi K, Tada M, Horikoshi N, et al:
Phase II study of paclitaxel with 3-h infusion in patients with
advanced gastric cancer. Gastric Cancer. 5:90–95. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wesolowski R, Lee C and Kim R: Is there a
role for second-line chemotherapy in advanced gastric cancer?
(Review). Lancet Oncol. 10:903–912. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grothey A, Sargent D, Goldberg RM and
Schmoll HJ: Survival of patients with advanced colorectal cancer
improves with the availability of fluorouracil-leucovorin,
irinotecan, and oxaliplatin in the course of treatment. J Clin
Oncol. 22:1209–1214. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rosenberg P, Andersson H, Boman K, et al:
Randomized trial of single agent paclitaxel given weekly versus
every three weeks and with peroral versus intravenous steroid
premedication to patients with ovarian cancer previously treated
with platinum. Acta Oncol. 41:418–424. 2002. View Article : Google Scholar
|
|
19
|
Sparano JA, Wang M, Martino S, et al:
Weekly paclitaxel in the adjuvant treatment of breast cancer. N
Engl J Med. 358:1663–1671. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shimoyama R, Yasui H, Boku N, et al:
Weekly paclitaxel for heavily treated advanced or recurrent gastric
cancer refractory to fluorouracil, irinotecan, and cisplatin.
Gastric Cancer. 12:206–211. 2009. View Article : Google Scholar : PubMed/NCBI
|