Introduction
Phototherapy using ultraviolet light B (UVB, range
290–320 nm) has widespread therapeutic applications for the
treatment of dermatological diseases. Narrow-band UVB (NB-UVB),
which emits a concentrated UVB source of 311 nm, has been shown to
have a more profound therapeutic efficacy for the treatment of
psoriasis, pruritus, cutaneous T-cell lymphoma and vitiligo
(1–5). Moreover, it has been shown that NB-UVB
is as effective as psoralen in combination with ultraviolet A
(PUVA) for psoriasis without systemic toxicity (6). In normal human cells, UVB induces
inflammation and DNA damage (7).
During mild exposure to UVB, DNA repair systems are activated
(8). However, prolonged exposure to
UVB results in irreparable DNA damage, and programmed cell death,
such as apoptosis (9), results.
Thus, the mechanism of action of UVB in the clinical settings of
dermatological disease treatment is dependent on the induction of
apoptosis (10).
On the other hand, as regards cancer treatment, the
standard treatment modality for the majority of malignant tumors
includes surgical resection combined with radiotherapy and
chemotherapy, and 5-fluorouracil (5-FU) is a widely used anticancer
agent. Intracellular metabolites of 5-FU can exert cytotoxic
effects via inhibition of thymidylate synthetase, or through
incorporation into RNA and DNA, events that ultimately activate
apoptosis and cell cycle arrest (11). It is frequently used in combination
with radiotherapy, which is anticipated to improve cancer
treatment. Chemotherapy is reported to radiosensitize various types
of tumors, and may improve the outcome of cancer radiotherapy
(12,13). Multiple mechanisms appear to be
involved in the radiosensitization of tumor cells by chemotherapy,
such as the potentiation of radiation damage (base damage, DNA
single- and double-strand breaks), the inhibition of
post-irradiation DNA repair, the redistribution of the cell cycle,
and the augmentation of apoptosis (14,15).
Locoregional recurrence of breast cancer is reported
to occur in 10–13% of patients within 10 years of breast
conservation therapy and also in 3–8% of those who received
mastectomy and postoperative radiotherapy (16,17).
The general management for chest wall recurrences of breast cancer,
as well as other types of cancer, is structured on the extent and
volume of local disease, the absence of distant metastasis, general
health of the patient, and the extent of prior local therapies,
particularly radiation (18).
Surgical resection is indicated for those patients with isolated
chest wall recurrence following mastectomy. In case of unresectable
disease, neoadjuvant chemotherapy is considered, in order to render
the disease resectable. Postoperative radiotherapy involving the
chest wall and regional lymph nodes is recommended for those
patients with isolated chest wall recurrence, without a history of
prior radiotherapy. In case of patients with a history of previous
irradiation, the design of radiation fields and the radiation doses
are restricted by prior therapies (18). Occasionally, the cancer pain and
pruritus that occurs when skin is affected by a malignant tumor can
be intractable. Thus, cancer pain and pruritus are considered
difficult therapeutic issues (19).
In the situation that radiotherapy is not applicable due to prior
therapies, UVB phototherapy may be a potential optional strategy to
treat a skin lesion infiltrated by a malignant tumor.
Presently, little is known regarding the effect of
UVB phototherapy on human breast cancer cells. Thus, in the present
study we aimed to investigate the effect of UVB phototherapy, as
well as the potential effect of 5-FU, the first-line anticancer
drug for breast cancer, on radiosensitizing MCF-7 human breast
cancer cells, in an attempt to develop new therapeutic strategies
for the treatment of locoregional recurrence of breast cancer.
Materials and methods
Cell cultures and UV-irradiation
The human breast cancer cell line, MCF-7, obtained
from the Riken Cell Bank (Tsukuba, Japan), was cultured in
RPMI-1640 supplemented with 5% fetal calf serum and 1%
antibiotics/antimycotics (complete medium), and then incubated in a
5% CO2 incubator at 37˚C. Bovine serum albumin,
RPMI-1640 and 5-FU were purchased from Sigma-Aldrich (St. Louis,
MO, USA). Fetal calf serum and antibiotics/antimycotics were from
Gibco-BRL (Grand Island, NY, USA). Cells were plated at
2×105 cells/well into 6-well plates and at
5×106 into 100-mm dishes and were placed in an
incubator. To assess the effects of the co-treatment with UVB and
5-FU, cells were grown to 50% confluence and treated without or
with 5-FU (10 and 20 μM doses) for 48 h. UVB-irradiation was
administered in the midterm during the 48-h 5-FU treatment period.
MCF-7 cells were irradiated with UVB at 750 mJ/cm2 with
the use of DNA-FIX apparatus (UV lamp at 312 nm, Atto Co., Tokyo,
Japan) at room temperature (20).
Exposure time was 3 min (750 mJ/cm2), and no heat was
detectable during UV-irradiation.
Cell viability assessment
Cells were treated as described, and cell growth was
assessed by counting viable cells following trypan blue staining.
Experiments were performed in triplicate wells, and the viability
of MCF-7 cells was calculated as the ratio of each experimental
condition to the control condition (untreated cells). The ratio of
cell viability was expressed as mean ± SD.
Detection of apoptosis by flow cytometry
and fluorescence microscopy
Cells were treated as described and then stained
with fluorescein isothiocyanate (FITC)-conjugated Annexin V and
propidium iodide (PI) for 5 min at room temperature according to
the manufacturer's instructions (Annexin V-FITC Apoptosis Detection
kit, BD Pharmingen, San Jose, CA, USA). The population of Annexin
V−PI− viable cells and Annexin V+
PI+ and Annexin V+/PI− apoptotic
cells was evaluated by flow cytometry, and both Annexin
V+ cell populations were considered to be apoptotic
cells. Data were collected in a FACSCalibur (Becton-Dickinson,
Mountain View, CA, USA) and analyzed using CellQuest software
(Becton-Dickinson). The experiment was performed three times, and
the ratio of apoptotic cells was expressed as mean ± SD.
To confirm the induction of apoptosis MCF-7 cells
fixed with 4% paraformaldehyde were stained with 2 μg/ml Hoechst
33258, a fluorescent DNA binding dye, for 30 min. Fluorescence of
Hoechst 33258 was captured with a charge-coupled device camera
under a fluorescent microscope. Apoptotic cells were identified by
their typical morphological appearance, including chromatin
condensation and nuclear fragmentation (21). An average of 200 nuclei from random
fields was analyzed for each data point. The experiment was
performed three times, and the ratio of apoptotic cells was
expressed as mean ± SD.
Analysis of the cell cycle by flow
cytometry
MCF-7 cells were prepared and treated as described.
The percentage of cells in each phase of the cell cycle was
analyzed by flow cytometry, using the Cycle Test Plus DNA Reagent
kit (BD Pharmingen) according to the manufacturer's instructions,
which is based on the measurement of the DNA content of nuclei
labeled with PI. Treated cells were trypsinized (250 μl of trypsin
buffer) for 10 min at room temperature, and then trypsin inhibitor
(200 μl) and RNase buffer were added and allowed to react for 10
min at room temperature. Finally, PI stain solution (200 μl) was
added and cells were incubated for 10 min in the dark on ice.
Samples were immediately analyzed in a flow cytometer
(Becton-Dickinson) and the results obtained were analyzed by
CellQuest software. The experiment was performed three times, and
the ratio of cells in the G1, intra-S and G2/M phases was expressed
as mean ± SD.
Statistical analysis
The results were expressed as mean ± SD. Statistical
analysis of all data between the control and treated groups was
performed by analysis of variance. P<0.05 was considered to be
statistically significant.
Results
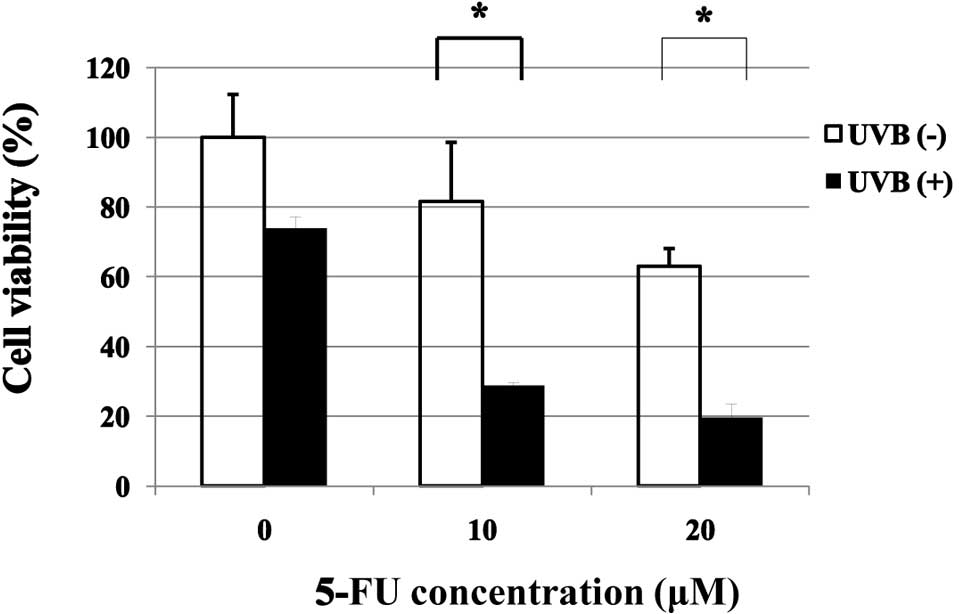
Effect of 5-FU on UVB-induced inhibition
of cell viability of MCF-7 cells
Treatment with UVB alone resulted in inhibition of
the viability of MCF-7 cells to approximately 74% of the untreated
control cells. Treatment with 5-FU alone at 10 and 20 μM resulted
in inhibition of the viability of MCF-7 cells to approximately 82
and 63%, respectively, of the untreated control cells. The
combination treatment of 5-FU and UVB resulted in a strong
potentiation of the inhibitory effect (74 vs. 29 and 19% viable
cells for UVB alone vs. UVB + 5-FU at 10 and 20 μM, respectively)
(Fig. 1).
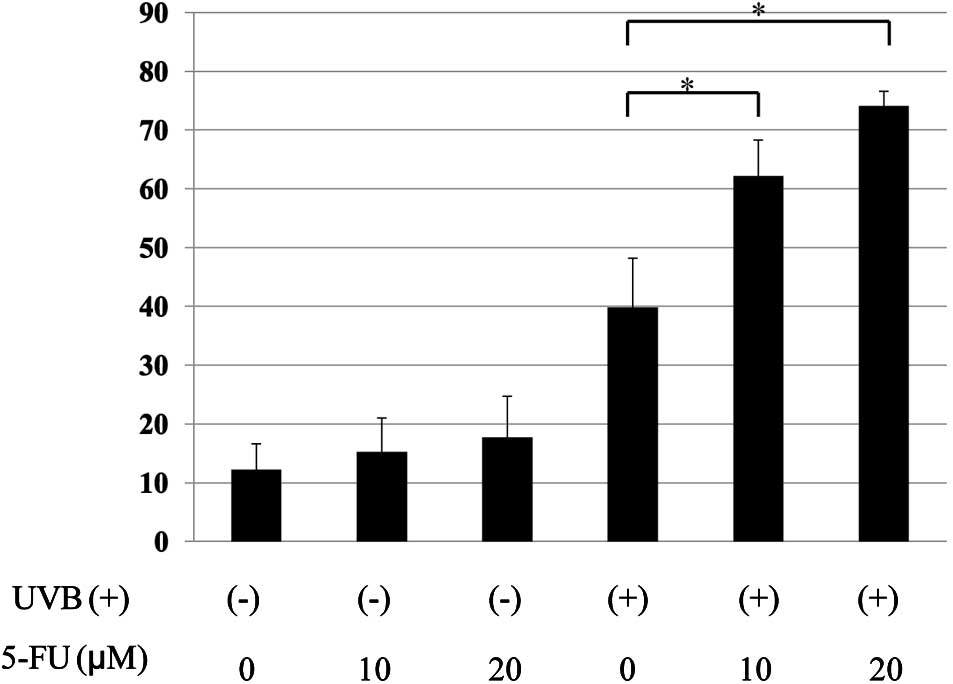
5-FU potentiated UVB-induced apoptosis in
MCF-7 cells
Treatment of cells with 5-FU alone resulted in a
slight increase in apoptotic cells (12.2 vs. 15.2 and 17.2%;
untreated vs. 5-FU at 10 and 20 μM). UVB-therapy alone also induced
apoptosis in MCF-7 cells (12.2 vs. 39.8%; untreated vs. UVB alone).
When the cells were treated with the combination of 5-FU and UVB, a
significant increase in the percentage of apoptotic cells was
observed (39.8 vs. 62.2 and 74.1%; UVB alone vs. UVB + 5-FU at 10
and 20 μM) (Fig. 2).
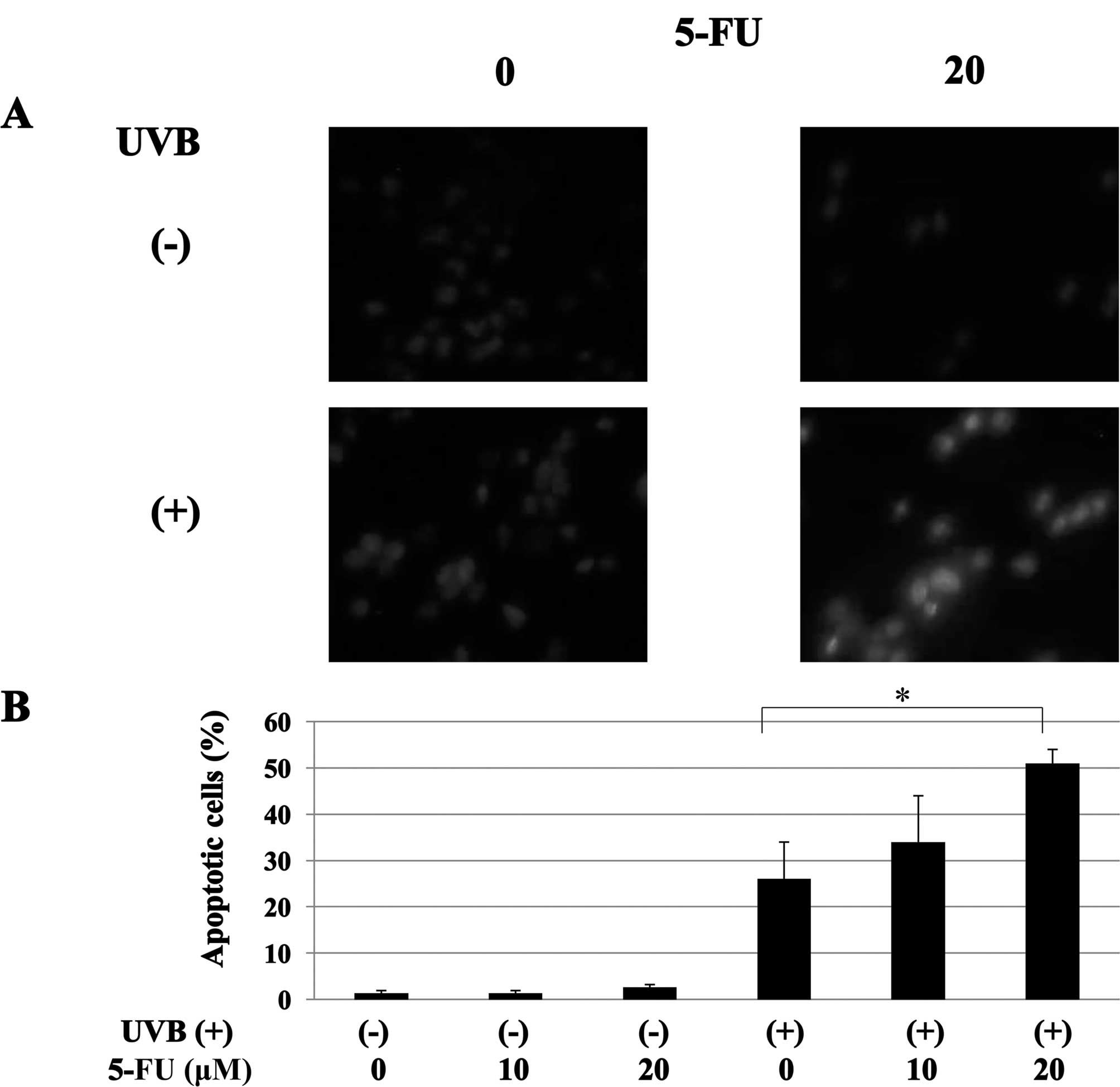
The induction of apoptosis was also confirmed by
Hoechst 33258 staining. UVB-treated cells exhibited highly
condensed and fragmented nuclei morphology, which are the typical
features of apoptosis (1.3 vs. 26.0%; untreated vs. UVB alone).
5-FU treatment strongly enhanced UVB-induced apoptosis in MCF-7
cells (26.0 vs. 34.3 and 50.6%; UVB alone vs. UVB + 5-FU at 10 and
20 μM) (Fig. 3).
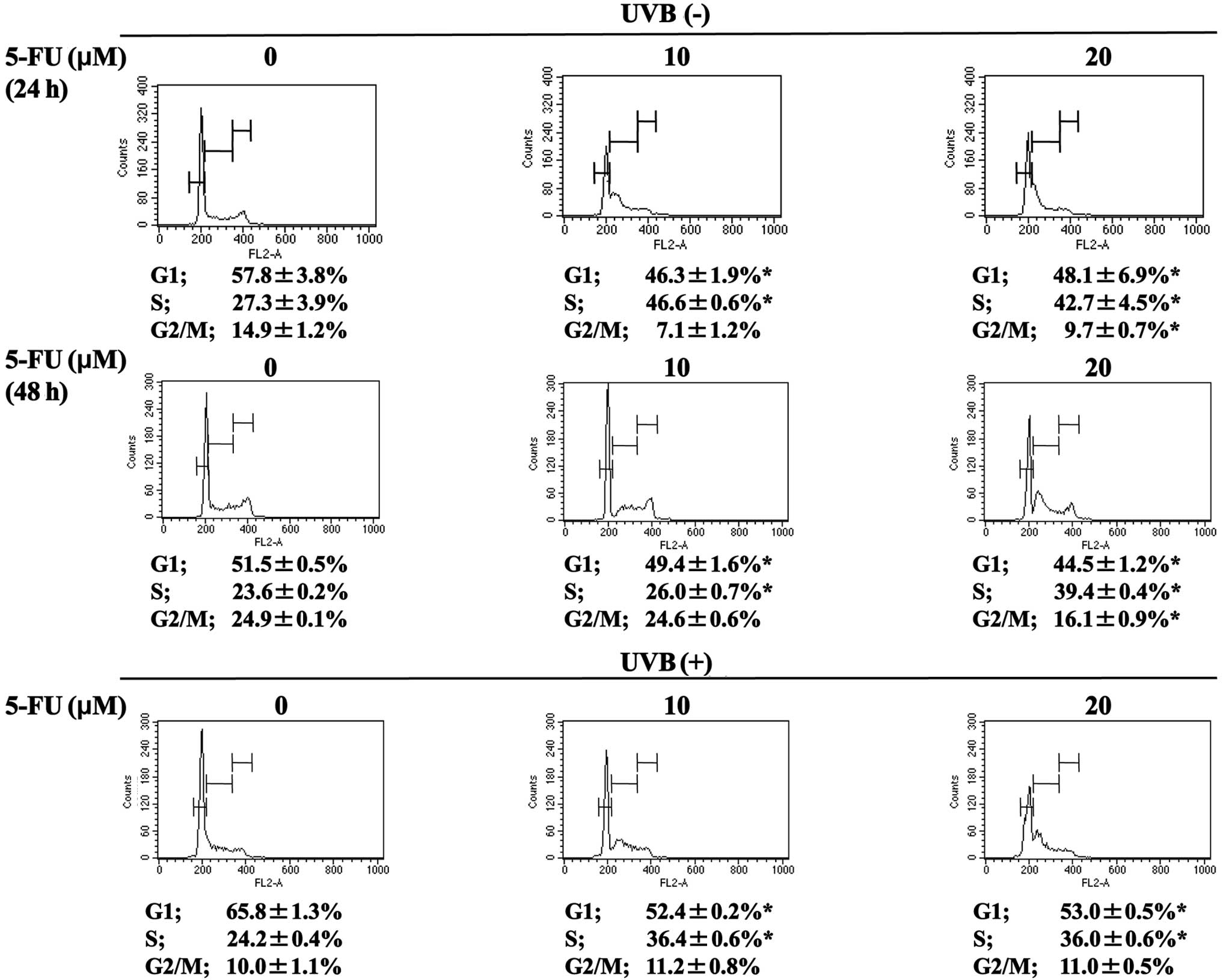
5-FU potentiated the arrest of MCF-7
cells treated with UVB in the intra-S phase of the cell cycle
Treatment of MCF-7 cells with 5-FU alone for 24 h
resulted in a significant intra-S cell cycle arrest (percentage of
cells in S phase: 27.3±3.9 vs. 46.6±0.6 and 42.7±4.5%; untreated
vs. 5-FU at 10 and 20 μM), which became less evident after 48 h of
treatment with 5-FU alone (percentage of cells in S phase: 23.6±0.2
vs. 26.0±0.7, and 39.4±0.4%; untreated vs. 5-FU at 10 and 20 μM)
(Fig. 4). Concomitant with intra-S
phase arrest, a significant decrease was noted in both G1 and G2/M
phases. In contrast, treatment of MCF-7 cells with UVB alone
resulted in cell cycle arrest in the G1 phase. In combination with
5-FU, compared with cells treated with UVB alone, an increase was
noted in the percentage of cells in the intra-S phase, (percentage
of cells in S phase: 24.2±0.4 vs. 36.4±0.6 and 36.0±0.6% for UVB
alone vs. UVB + 5-FU at 10 and 20 μM, respectively). When compared
with cells treated with 5-FU alone at 10 μM for 48 h, a significant
increase was noted in cells in the intra-S phase with a concomitant
decrease in cells in the G2/M phase [percentage of cells in S phase
and G2/M phase: 26.0±0.7 vs. 36.4±0.6 and 24.6±0.6% vs. 11.2±0.8%
for 5-FU alone (10 μM) vs. UVB + 5-FU (10 μM), respectively]
(Fig. 4).
Discussion
Phototherapy has emerged as a promising therapeutic
tool for the treatment of dermatologic conditions such as
psoriasis, pruritus, cutaneous T-cell lymphoma, superficial basal
cell carcinoma and malignant melanoma (1,2,5,22,23).
Phototherapy requires the simultaneous presence of
photosensitizers, which accumulate in target cells resulting in the
induction of apoptosis or necrosis. A variety of anti-cancer drugs,
such as 5-FU, cisplatin and taxol, have been accepted as clinically
useful radiosensitizers (24–26).
Therefore, we hypothesized that 5-FU is a potential radiosensitizer
in potentiating the anti-cancer effect of UVB phototherapy in
breast cancer cells.
In preliminary experiments, we tested the effect of
UVB-irradiation on MCF-7 human breast cancer cells and observed
that UVB at a dose of 500, 750 and 1000 mJ/cm2 had an
aproximately 15–30% inhibitory effect in the cell viability assay.
The doses of UVB are used in the clinical treatment of cutaneous
T-cell lymphoma (5), and the dose
of 750 mJ/cm2, which had minimal inhibitory effect on
cell viability, was selected for subsequent experiments. In
constrast, treatment of MCF-7 with 5-FU alone at concentrations of
10 and 20 μM for 48 h resulted in 18 and 37% inhibition of the
proliferative activity of MCF-7 cells, respectively. In addition,
the combination of 5-FU (10 and 20 μM) and UVB (750
mJ/cm2) significantly potentiated an inhibitory effect
on MCF-7 cell growth. Consequently, 5-FU at 10 and 20 μM for 48 h
and UVB-irradiation at 750 mJ/cm2 in the midterm of 5-FU
treatment were selected for subsequent experiments.
UVB alone inhibited the viability of MCF-7 cells by
approximately 26% and was dependent on the induction of apoptosis
and cell cycle arrest in the G1 phase. As indicated by the Annexin
V/PI assay, UVB alone induced an increase in apoptotic cells to
approximately 40 and to approximately 25% as indicated by the
Hoechst 33258 staining assay. This difference was attributed to the
detection ability of these methods; the Annexin V/PI assay is able
to detect cells in the early phase of apoptosis, i.e., when
membrane changes occur without nuclear fragmentation, whereas the
Hoechst 33258 staining assay detects only cells with nuclear
fragmentation and chromatin condensation. Moreover, analysis of the
cell cycle revealed that treatment with UVB alone resulted in a
significant increase in the percentage of cells in the G1 phase of
the cell cycle.
In contrast, the inhibitory effect of 5-FU alone (10
and 20 μM) on cell viability, as evaluated by the trypan blue
staining, was found to be 18 and 36%, and this effect was also
dependent partially on the induction of apoptosis but mostly on
intra-S phase cell cycle arrest. The percentage of apoptotic cells
increased by approximately 3 and 5% following 5-FU treatment at 10
and 20 μM, respectively, as indicated by the Annexin V/PI assay,
and approximately 1 and 2% as indicated by the Hoechst 33258
staining assay. Notably, a significant intra-S phase arrest was
observed upon cell cycle analysis. The percentage of cells in the S
phase increased by 19.3 and 15.4% after a 24-h treatment with 5-FU
at 10 and 20 μM, respectively, and by 2.4 and 15.8%, respectively,
following a 48-h treatment. Therefore, the effect of 5-FU alone on
MCF-7 cells was mostly dependent on intra-S phase cell cycle
arrest, particularly at the time UVB-irradiation was performed.
Following a 48-h treatment, intra-S arrest was observed only with
treatment of 5-FU at 20 μM, which is probably dependent on the
metabolic degradation of the drug in in vitro settings.
When UVB-irradiation was combined with 5-FU, a
significant potentiation of the inhibitory effect on MCF-7 cells
was observed. Cell viability decreased by 70 and 80%, respectively,
with 5-FU at 10 and 20 μM, and the percentage of apoptotic cells
increased by 22 and 34%, respectively, following Annexin V/PI
staining, and by 8 and 26%, respectively, following Hoechst 33258
staining assay. A significant increase in the percentage of intra-S
cells was also observed. When compared to cells treated without
5-FU and without UVB-irradiation, there was a 12.8 and 12.4%
increase in the percentage of cells in the intra-S phase,
respectively, with 5-FU at 10 and 20 μM. Of note is that this
increase was not accompanied by cell arrest in the G1 phase;
however, a simultaneous was noted decrease in cells in the G2/M
phase.
Previously, Hamaoka et al (27) reported that a combination treatment
of 5-FU and UV-irradiation resulted in an additive inhibitory
effect on the cell growth of MCF-7 cells and was hypothesized to be
dependent on rRNA inhibition. Although the present findings are
similar, we found that the effect was not merely additive, but that
synergism occurred between 5-FU and UVB with regards to the
inhibitory effect on cell viability. These data are strongly
suggestive of the radiosensitization of human breast cancer cells
by 5-FU. The difference between our findings and those of the
previous report was possibly dependent on the time point
UVB-irradiation was applied during 5-FU treatment. Our treatment
protocol was based on the continuous treatment of cells with 5-FU
for 24 h, after which UVB-irradiation was administered, and the
cells were then treated for a further 24 h in the presence of 5-FU.
When translating this protocol to the clinical setting, a patient
should receive continuous administration of 5-FU, either orally or
intravenously, and UVB-irradiation should be administered in the
midterm. The optimal protocol remains to be confirmed in animal
experiments before clinical trials are initiated.
The cytotoxic effect of 5-FU is caused by the
inhibition of thymidylate synthase and incorporation into RNA and
DNA (11). The exact mechanism by
which 5-FU increases radiation sensitivity remains to be
elucidated, but a number of mechanisms at the cellular level have
been reported (28). One mechanism
involves the killing of cells in the S phase of the cell cycle,
which are relatively radioresistant compared to cells in the G2/M
phase (29). McKay et al
(30) reported that UV-induced
apoptosis was greatly reduced by inhibiting S-phase progression,
suggesting that the induction of apoptosis requires S-phase
progression following UV-irradiation. Notably, as was the case in
our previous report using human colon cancer cells (31), 5-FU treatment significantly
increased intra-S cell cycle arrest in human breast cancer MCF-7
cells as well. The inappropriate progression through the S phase,
such as disordered S-phase checkpoints, induced by drugs, is a
possible mechanism of the enhancement of radiation sensitivity. A
previous report (32) found that
human colon cancer HT-29 cells, which express activated G1/S
cyclins, but not SW620 cells, which do not show activated cyclins,
are radiosensitized by fluorodeoxyuridine under identical drug
treatment conditions. The blockade of S phase entry or inhibition
of progression into the S phase has also been reported to inhibit
sensitization (33,34). Collectively, these reports and our
findings suggest that the enhancement of the sensitization of MCF-7
breast cancer cells to UVB, induced by 5-FU is, at least partly,
dependent on the induction of cell cycle arrest during the S
phase.
Notably, in their study, Panno et al
(35) showed that psoralen alone,
or in combination with ultraviolet A (UVA, range 320–400 nm) (PUVA)
significantly affected the proliferation of breast cancer cells,
including MCF-7. The inhibitory effect of PUVA was mostly dependent
on induction of apoptosis, as detected by an increase in p53,
caspase activation and DNA ladder. The use of ultraviolet
irradiation at different ranges, with psoralen as the
photosensitizer, corroborates our findings and potentially allows
for the use of photochemotherapy in the clinical setting of breast
cancer treatment.
Since UVB phototherapy and 5-FU are already
clinically available, and the doses of 5-FU and UVB-irradiation
tested in the present study are relatively close to those
physiologically achievable in human plasma and human skin,
respectively, their use in clinical settings of anti-cancer therapy
may be feasible (5,36). In light of our results, the
combination therapy of UVB phototherapy and 5-FU should be
considered as a promising new candidate therapeutic modality for
patients with locoregional recurrence of breast cancer, as well as
other skin lesions infiltrated by malignant tumors, particularly
those for whom the use of radiotherapy is limited by prior
therapies.
In conclusion, we demonstrated that the combination
therapy of UVB and 5-FU is an effective and promising strategy for
the treatment of breast cancer, particularly for locoregional
recurrence. 5-FU enhanced UVB-induced apoptosis in MCF-7 cells by
the induction of cell cycle arrest during the S phase. Since UVB
phototherapy and 5-FU are already in clinical use as anti-cancer
agents, application of the combination treatment for breast cancer
may be feasible without the need for phase I studies.
Acknowledgements
The authors thank Ms. Mika Matsuhashi, Ms. Michiru
Kawabata and Mr. Naoyuki Yoshikawa of the Department of Transfusion
Medicine (University of Tokyo) for their kind advisory and
technical assistance.
References
|
1
|
Lui H: Phototherapy of psoriasis: update
with practical pearls. J Cutan Med Surg. 6:17–21. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reynolds NJ, Franklin V, Gray JC, Diffey
BL and Farr PM: Narrow-band ultraviolet B and broad-band
ultraviolet A phototherapy in adult atopic eczema: a randomised
controlled trial. Lancet. 357:2012–2016. 2001. View Article : Google Scholar
|
|
3
|
Hamzavi I, Jain H, McLean D, Shapiro J,
Zeng H and Lui H: Parametric modeling of narrowband UV-B
phototherapy for vitiligo using a novel quantitative tool: the
Vitiligo Area Scoring Index. Arch Dermatol. 140:677–683. 2004.
View Article : Google Scholar
|
|
4
|
Gathers RC, Scherschun L, Malick F,
Fivenson DP and Lim HW: Narrowband UVB phototherapy for early-stage
mycosis fungoides. J Am Acad Dermatol. 47:191–197. 2002. View Article : Google Scholar
|
|
5
|
Matsuoka Y, Yoneda K, Katsuura J, et al:
Successful treatment of follicular cutaneous T-cell lymphoma
without mucinosis with narrow-band UVB-irradiation. J Eur Acad
Dermatol Venereol. 21:1121–1122. 2007. View Article : Google Scholar
|
|
6
|
Snellman E, Klimenko T and Rantanen T:
Randomized half-side comparison of narrowband UVB and
trimethylpsoralen bath plus UVA treatments for psoriasis. Acta Derm
Venereol. 84:132–137. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Halliday GM: Inflammation, gene mutation
and photoimmuno-suppression in response to UVR-induced oxidative
damage contributes to photocarcinogenesis. Mutat Res. 571:107–120.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Griffiths HR, Mistry P, Herbert KE and
Lunec J: Molecular and cellular effects of ultraviolet
light-induced genotoxicity. Crit Rev Clin Lab Sci. 35:189–237.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cotton J and Spandau DF: Ultraviolet
B-radiation dose influences the induction of apoptosis and p53 in
human keratinocytes. Radiat Res. 147:148–155. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aufiero BM, Talwar H, Young C, et al:
Narrow-band UVB induces apoptosis in human keratinocytes. J
Photochem Photobiol B. 82:132–139. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Longley DB, Harkin DP and Johnston PG:
5-Fluorouracil: mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huerta S, Gao X, Livingston EH, Kapur P,
Sun H and Anthony T: In vitro and in vivo radiosensitization of
colorectal cancer HT-29 cells by the smac mimetic JP-1201. Surgery.
148:346–353. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dai Y, Liu M, Tang W, et al: Molecularly
targeted radiosensitization of human prostate cancer by modulating
inhibitor of apoptosis. Clin Cancer Res. 14:7701–7710. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Adamsen BL, Kravik L and De Angelis PM:
Cellular response to chemoradiotherapy, radiotherapy and
chemotherapy in two colorectal cancer cell lines. Radiat Res.
171:562–571. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wilson GD, Bentzen SM and Harari PM:
Biologic basis for combining drugs with radiation. Semin Radiat
Oncol. 16:2–9. 2006. View Article : Google Scholar
|
|
16
|
Clarke M, Collins R, Darby S, et al:
Effects of radiotherapy and of differences in the extent of surgery
for early breast cancer on local recurrence and 15-year survival:
an overview of the randomised trials. Lancet. 366:2087–2106.
2005.PubMed/NCBI
|
|
17
|
Shikama N, Sekiguchi K and Nakamura N:
Management of locoregional recurrence of breast cancer. Breast
Cancer. May 7–2010.(Epub ahead of print).
|
|
18
|
Taylor ME: Breast cancer: chest wall
recurrences. Curr Treat Options Oncol. 3:175–177. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Holme SA and Mills CM: Crotamiton and
narrow-band UVB phototherapy: novel approaches to alleviate
pruritus of breast carcinoma skin infiltration. J Pain Symptom
Manage. 22:803–805. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakayama S, Katoh EM, Tsuzuki T and
Kobayashi S: Protective effect of alpha-tocopherol-6-O-phosphate
against ultraviolet B-induced damage in cultured mouse skin. J
Invest Dermatol. 121:406–411. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cai BX, Luo D, Lin XF and Gao J: Compound
K suppresses ultraviolet radiation-induced apoptosis by inducing
DNA repair in human keratinocytes. Arch Pharm Res. 31:1483–1488.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fergin P: Photodynamic therapy of
dermatoses other than non-melanoma skin cancer. Australas J
Dermatol. 46(Suppl 3): S272005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Babilas P, Karrer S, Sidoroff A,
Landthaler M and Szeimies RM: Photodynamic therapy in dermatology –
an update. Photodermatol Photoimmunol Photomed. 21:142–149.
2005.
|
|
24
|
Saad ED and Hoff PM: UFT and oral
leucovorin as radiation sensitizers in rectal and other
gastrointestinal malignancies. Cancer Invest. 21:624–629. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zenda S, Onozawa Y, Tahara M, et al:
Feasibility study of single agent cisplatin and concurrent
radiotherapy in Japanese patients with squamous cell carcinoma of
the head and neck: preliminary results. Jpn J Clin Oncol.
37:725–729. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Choy H, Rodriguez FF, Koester S,
Hilsenbeck S and von Hoff DD: Investigation of taxol as a potential
radiation sensitizer. Cancer. 71:3774–3778. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hamaoka T, Furuya Y, Yamamoto K and Kuroda
Y: MCF-7 growth inhibition by ultraviolet radiation and
5-fluorouracil: the importance of treatment sequence. Cancer Lett.
154:183–187. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lawrence TS, Blackstock AW and McGinn C:
The mechanism of action of radiosensitization of conventional
chemotherapeutic agents. Semin Radiat Oncol. 13:13–21. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Byfield J: Useful interactions between
5-fluorouracil and radiation in man: 5-fluorouracil as a
radiosensitizer. CRC Press; Boca Raton, FL: 1990
|
|
30
|
McKay BC, Becerril C, Spronck JC and
Ljungman M: Ultraviolet light-induced apoptosis is associated with
S-phase in primary human fibroblasts. DNA Repair (Amst). 1:811–820.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sasaki K, Tsuno NH, Sunami E, et al:
Chloroquine potentiates the anti-cancer effect of 5-fluorouracil on
colon cancer cells. BMC Cancer. 10:3702010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lawrence TS, Davis MA and Loney TL:
Fluoropyrimidine-mediated radiosensitization depends on cyclin
E-dependent kinase activation. Cancer Res. 56:3203–3206.
1996.PubMed/NCBI
|
|
33
|
Naida JD, Davis MA and Lawrence TS: The
effect of activation of wild-type p53 function on
fluoropyrimidine-mediated radiosensitization. Int J Radiat Oncol
Biol Phys. 41:675–680. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lawrence TS, Davis MA, Tang HY and Maybaum
J: Fluoro-deoxyuridine-mediated cytotoxicity and radiosensitization
require S phase progression. Int J Radiat Biol. 70:273–280. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Panno ML, Giordano F, Mastroianni F, et
al: Breast cancer cell survival signal is affected by bergapten
combined with an ultraviolet irradiation. FEBS Lett. 584:2321–2326.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fraile RJ, Baker LH, Buroker TR, Horwitz J
and Vaitkevicius VK: Pharmacokinetics of 5-fluorouracil
administered orally, by rapid intravenous and by slow infusion.
Cancer Res. 40:2223–2228. 1980.PubMed/NCBI
|