Introduction
Esophageal squamous cell carcinoma (ESCC) is a
malignant tumor belonging to the class of gastrointestinal
carcinomas. Patients with ESCC have a poor prognosis despite
intensive multimodality therapy such as surgery, radiation and
chemotherapy. Almost 400,000 new cases of esophageal cancer are
diagnosed annually worldwide, making it the eighth most common
cancer and the sixth most common cause of cancer-related mortality
(1). To improve patient survival,
it is important to identify those relevant biomarkers in ESCC that
are associated with adverse prognosis and to modify the therapeutic
strategy for those patients accordingly. In the present study,
expression of CD44v6 was assessed in the primary lesions of ESCC to
elucidate its significance in clinical prognosis.
CD44 is a transmembrane glycoprotein involved in
cell-cell and cell-extracellular matrix interactions, and whose
role is to maintain cellular adhesion (2,3). CD44
is encoded by a single gene on chromosome 11p13, but actually
represents a polymorphic group of transmembrane glycoproteins due
to extensive alternative splicing and post-translational
modifications (4). The human gene
is composed of 20 exons, 10 of which (exons 1–5 and 16–20) are
included in CD44 standard form (CD44s). CD44s is the smallest and
most abundant member of this polymorphic and monogenic family of
proteins. The remaining exons (exons 6–15) can be differentially
inserted into the mature mRNA via alternative splicing and may give
rise to hundreds of protein variants (5).
Overexpression of a number of CD44 variant isoforms
was associated with tumor progression, suggesting that these CD44
isoforms have unique signaling properties (6–8). In
colon cancer, CD44v3 was shown to promote invasion and resistance
to apoptosis, while CD44v6 was associated with metastasis and
decreased disease-free survival (6,7,9). In
lung cancer, preferential CD44 variant expression occurs in
squamous cell and bronchoalveolar carcinoma, where v5 and v6
variants appear to promote metastasis (8,10).
Numerous reports showed that CD44 variants promote breast cancer
progression, including the association of CD44v3-containing
isoforms and breast cancer metastasis (6,11). The
significance of CD44 variants in ESCC was discussed in previous
investigations (2,12–14).
This study aimed to elucidate the
clinicopathological significance of CD44v6 overexpression in
ESCC.
Materials and methods
Patients and tissue samples
Samples were obtained from 63 patients with primary
ESCCs. The patients had undergone radical esophagectomy without any
pre-operative induction therapy at the Department of Surgery II,
Nagoya City University Medical School, between 1997 and 2004. The
study design was approved by the institutional review board of our
university and written consent was obtained from all patients.
Tumors were classified according to the guidelines for clinical and
pathological studies on carcinoma of the esophagus established by
the Japanese Society for Esophageal Diseases. Tissue specimens were
collected from 51 males and 12 females, with a mean age of 63.5±8.3
years (range 46–78) (Table I).
Tissues for immunohistochemistry were fixed in formalin and
embedded in paraffin.
 | Table IRelationship between the
clinicopathological characteristics and immunostaining for
CD44v6. |
Table I
Relationship between the
clinicopathological characteristics and immunostaining for
CD44v6.
| | CD44v6
expression | |
|---|
| |
| |
|---|
| Total | High (n=35) | Low (n=28) | p-value |
|---|
| Gender |
| Male | 51 | 27 | 24 | |
| Female | 12 | 8 | 4 | 0.3893 |
| Age |
| <65 | 35 | 22 | 13 | |
| ≥65 | 28 | 13 | 15 | 0.1922 |
| T factor |
| T1 | 21 | 11 | 10 | |
| T2 | 10 | 5 | 5 | |
| T3 | 21 | 12 | 9 | |
| T4 | 11 | 7 | 4 | 0.9138 |
| T1 | 21 | 11 | 10 | |
| T2–4 | 42 | 24 | 18 | 0.7199 |
| N factor |
| Negative | 20 | 10 | 10 | |
| Positive | 43 | 25 | 18 | 0.5450 |
| Stage |
| 0 | 6 | 2 | 4 | |
| I | 11 | 6 | 5 | |
| II | 13 | 7 | 6 | |
| III | 16 | 9 | 7 | |
| IV | 17 | 11 | 6 | 0.7726 |
| 0–I | 17 | 8 | 9 | |
| II–IV | 46 | 27 | 19 | 0.4093 |
| Lymphatic
invasion |
| Negative | 19 | 9 | 10 | |
| Positive | 44 | 26 | 18 | 0.3901 |
| Vein invasion |
| Negative | 28 | 16 | 12 | |
| Positive | 35 | 19 | 16 | 0.8206 |
|
Differentiation |
| Well | 18 | 10 | 8 | |
| Moderate | 40 | 24 | 16 | |
| Poor | 5 | 1 | 4 | 0.2369 |
Immunohistochemistry
Immunohistochemical staining was performed on
formalin-fixed, paraffin-embedded ESCC tissues. Paraffin-embedded
tumor sections were deparaffinized, rehydrated, heat-treated by
microwaving in 10 mM citrate buffer for 15 min for antigen
retrieval and cooled to room temperature. Sections were then
treated with 0.3% H2O2 in methanol for 30 min
to neutralize endogenous peroxidases, blocked with non-specific
goat serum for 10 min and incubated with the primary monoclonal
antibodies for CD44v6 (1:200; Serotec, UK) and CD44s (1:500; Enzo,
Miami, FL, USA) overnight at 4°C. Immunoreactive protein was
detected with a Dako Envision™ + System, HRP (DAB), and the
sections were then counterstained with hematoxylin.
The immunostaining of CD44v6 and CD44s was
subjectively assessed by two independent investigators (M.S. and
H.I.) using light microscopy. The CD44v6-positive cells were
counted and the positive expression was classified as:
low-expression group with positive cells <50% and
high-expression group with positive cells >50%.
Statistical analysis
Statistical analysis was performed using the
Stat-View software package (Abacus Concepts, Berkeley, CA, USA).
The Chi-square test was used to analyze the association between the
immunohistochemical analysis and the clinical histopathological
parameters of the patients. The survival of ESCC patients following
surgery was assessed using the Kaplan-Meier method and survival
times were compared using the log-rank test. The data are expressed
as the mean ± SD. Multivariate analysis was performed using the Cox
regression model and logistic multivariate regression model. In all
analyses, p<0.05 was considered to be statistically
significant.
Results
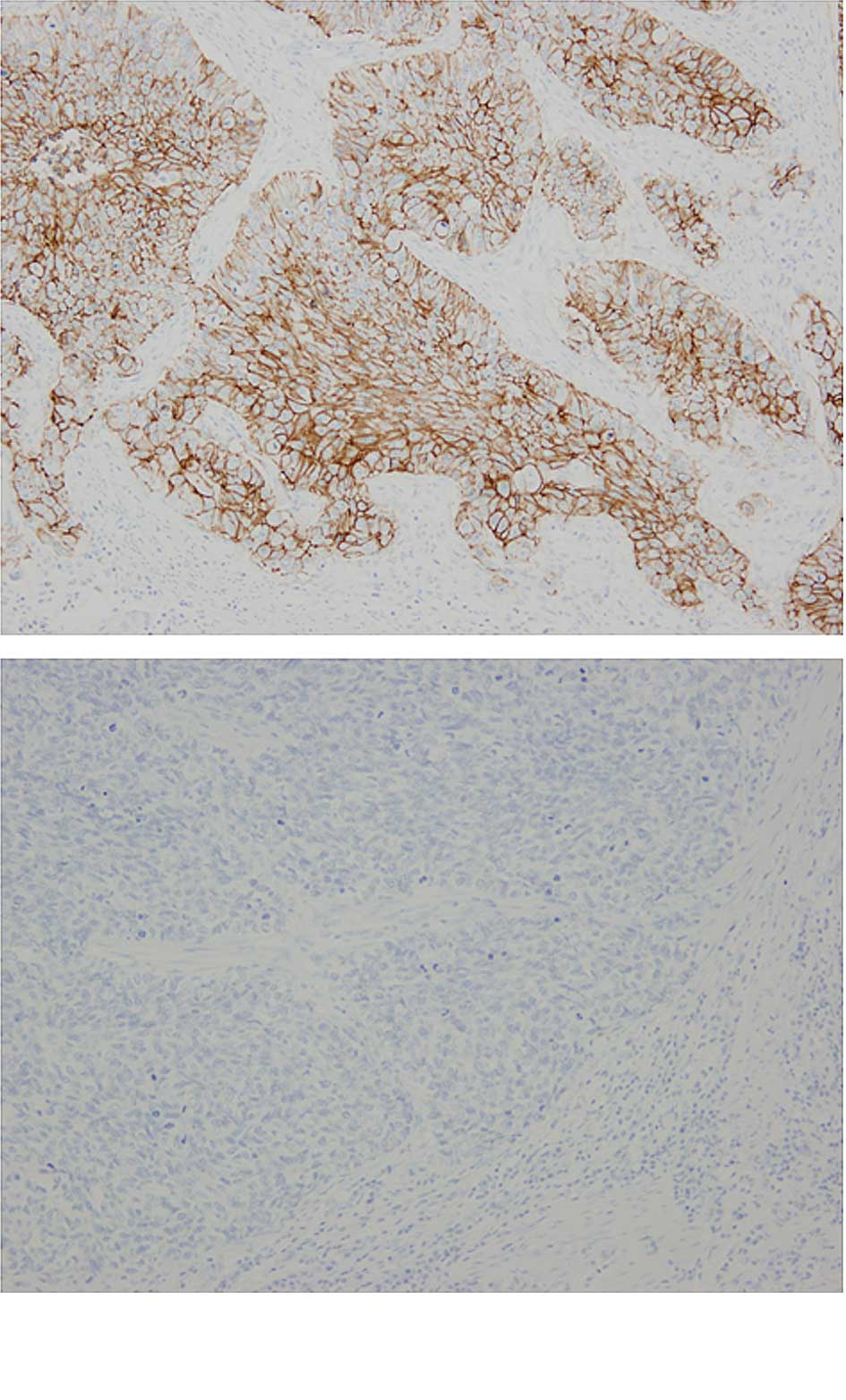
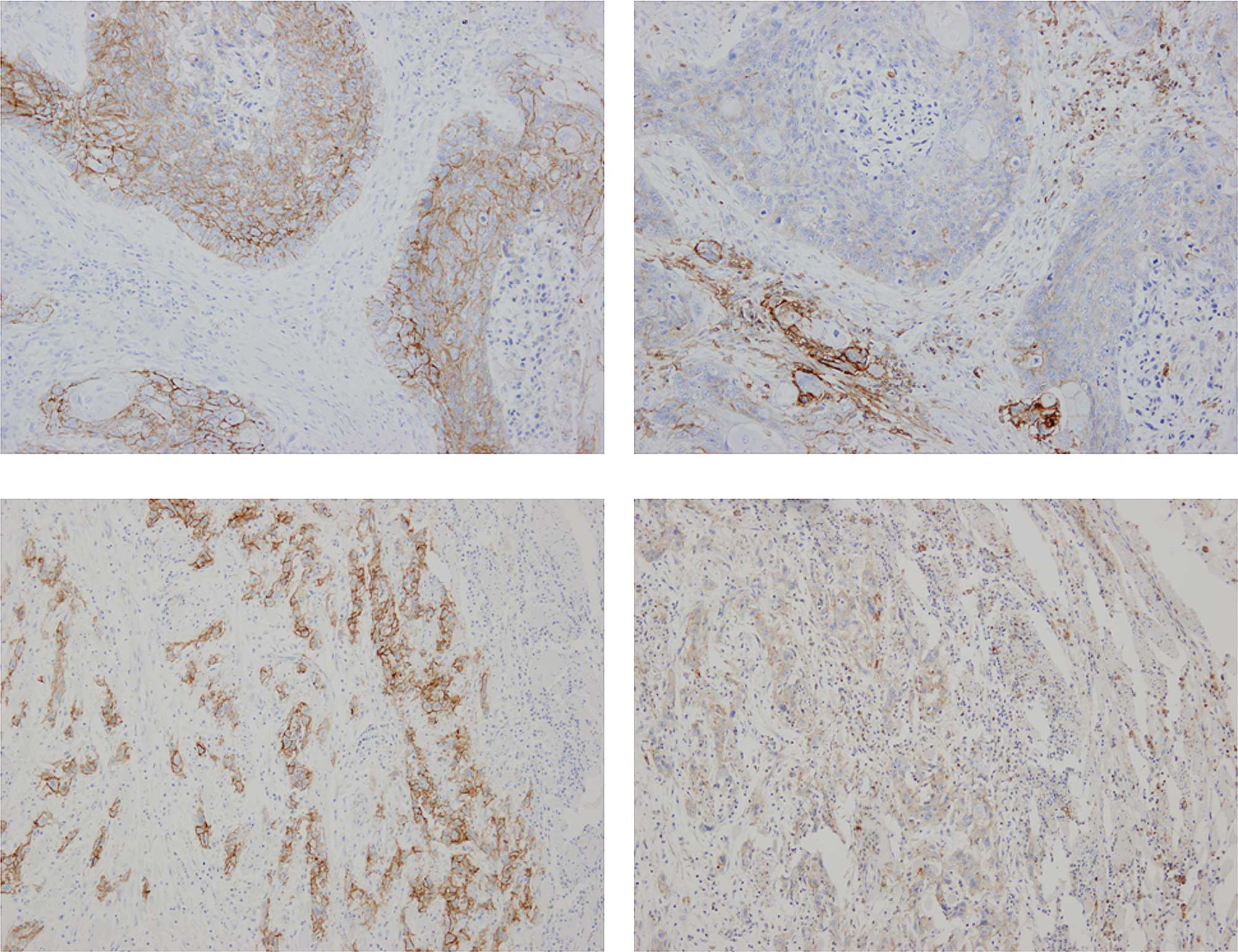
The frequency of CD44v6 expression was 90.5%
(57/63). Representative cases of immunostaining are shown in
Fig. 1. The CD44v6 high-expression
group comprised 55.6% (n=35) of the patients and the low expression
group included 44.4% of the patients (n=28). CD44v6 was observed
only in epithelial and tumor cells. By contrast, CD44s was not
solely expressed in epithelial and tumor cells in ESCCs. CD44s was
also strongly present in lymphocytic cells and was loosely stained
in the interstitium. Therefore, we could not evaluate the
immunohistochemical expression for CD44s in the same manner. CD44v6
and CD44s staining was shown directly in consecutive serial
sections of the same samples (Fig.
2).
The correlation between immunostaining for CD44v6
and the clinicopathological characteristics of the patients are
shown in Table I. No significant
correlation was observed between clinicopathological
characteristics such as age, gender, T factor, N factor, stage,
lymphatic invasion, venous invasion and differentiation, and the
immunohistochemical expression of CD44v6 (Table I).
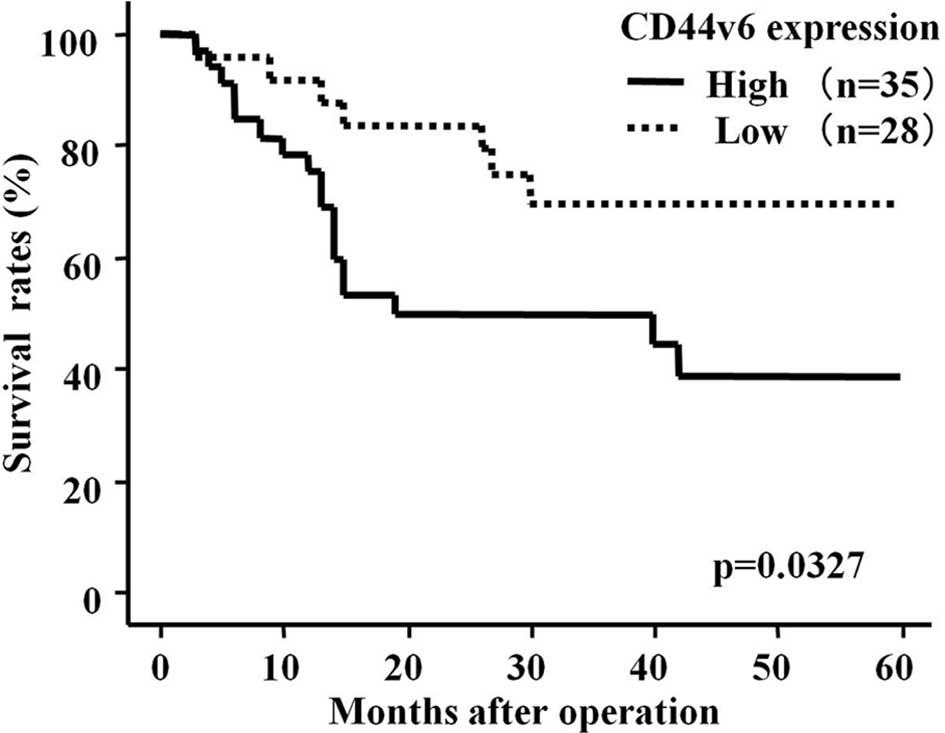
We subsequently investigated the correlation between
immunostaining for CD44v6 and survival in ESCC patients after
surgery (median follow-up, 28 months). The patients in the CD44v6
high-expression group had a significantly shorter survival
following surgery than patients whose expression was low (p=0.0301,
log-rank test) (Fig. 3). Moreover,
favorable outcomes were noted for the clinicopathological
characteristics of 6 patients whose expression of CD44v6 was not
detected, (Table II).
 | Table IIClinicopathological characteristics
of 6 patients. |
Table II
Clinicopathological characteristics
of 6 patients.
| Patient | Gender | Age | T factor | N factor | Stage | Lympatic
invasion | Vein invasion | Differrentiation
(months) | Follow-up | Outcome |
|---|
| 1 | Male | 50 | T3 | n2 | III | + | + | Poor | 60 | Alive |
| 2 | Male | 74 | T1 | n1 | II | + | + | Well | 30 | Dead |
| 3 | Male | 67 | T1 | n0 | 0 | − | − | Moderate | 44 | Alive |
| 4 | Female | 57 | T3 | n1 | III | + | + | Well | 59 | Alive |
| 5 | Male | 62 | T1 | n0 | 0 | − | − | Moderate | 58 | Alive |
| 6 | Male | 72 | T1 | n0 | I | + | − | Moderate | 55 | Alive |
Univariate analysis revealed that among the
clinicopathological factors, tumor status [risk ratio (RR)=9.346;
p=0.0025], lymph node status (RR=6.211; p=0.0001), lymphatic
invasion (RR=6.623; p=0.0105), venous invasion (RR=2.809; p=0.0209)
and CD44v6 expression (RR=2.491; p=0.0410) were all statistically
significant prognostic factors (Table
III).
 | Table IIIUnivariate analysis. |
Table III
Univariate analysis.
|
Characteristics | Risk ratio | 95% CI | p-value |
|---|
| Age at surgery |
| <65 | 1 | | |
| ≥65 | 0.915 | 0.415–2.018 | 0.8261 |
| Gender |
| Female | 1 | | |
| Male | 0.872 | 0.326–2.331 | 0.7843 |
| Histological
grade |
| Well | 1 | | |
|
Moderately/poorly | 0.642 | 0.288–1.431 | 0.2784 |
| Tumor status |
| T1 | 1 | | |
| T2–4 | 9.346 | 2.193–40.000 | 0.0025 |
| Lymph node
status |
| n0–1 | 1 | | |
| n2–4 | 6.211 | 2.222–15.625 | 0.0001 |
| Lymphatic
invasion |
| Negative | 1 | | |
| Positive | 6.623 | 1.558–28.571 | 0.0105 |
| Vein invasion |
| Negative | 1 | | |
| Positive | 2.809 | 1.170–6.757 | 0.0209 |
| CD44v6
expression |
| Low | 1 | | |
| High | 2.491 | 1.038–5.975 | 0.0410 |
Multivariate analysis revealed that CD44v6
overexpression (RR=2.793; p=0.0301) as well as tumor status
(RR=9.259; p=0.0275) and lymph node status (RR=4.785; p=0.0023)
were factors independently associated with an unfavorable prognosis
of patients with ESCC (Table
IV).
 | Table IVMultivariate analysis. |
Table IV
Multivariate analysis.
| Parameter | Risk ratio | 95% CI | p-value |
|---|
| Tumor status |
| T1 | 1 | | |
| T2–4 | 9.259 | 1.279–66.667 | 0.0275 |
| Lymph node
status |
| n0–1 | 1 | | |
| n2–4 | 4.785 | 1.748–13.158 | 0.0023 |
| Lymphatic
invasion |
| Negative | 1 | | |
| Positive | 0.369 | 0.038–3.559 | 0.3880 |
| Vein invasion |
| Negative | 1 | | |
| Positive | 2.037 | 0.718–5.780 | 0.1809 |
| CD44v6
expression |
| Low | 1 | | |
| High | 2.793 | 1.104–7.066 | 0.0301 |
Discussion
The present study aimed to determine the
clinicopathological significance of CD44v6 expression in ESCC. In
this study, three main findings were noted. First, CD44v6 was
expressed at a high frequency in patients with ESCC. Of the 63 ESCC
cases, positivity for CD44v6 was observed in 57 cases (90.5%)
(Table I). Second, the survival of
patients whose expression of CD44v6 was high was significantly less
favorable than that of patients whose expression was low (p=0.0301;
Fig. 3). Third, immunohistochemical
overexpression of CD44v6 was an independent prognostic indicator of
patients with ESCC (Table
III).
The first major finding involving CD44 in the
metastatic process was the identification of a CD44 variant isoform
containing exons v4-v7 in a highly metastasizing rat pancreatic
carcinoma cell line (BSp73ASML). Transfection of this specific
variant into related BSp73AS cells that did not metastasize
conferred metastatic potential to those cells upon injection into
syngeneic rats (15). Moreover, a
CD44 exon v6-specific antibody blocked the metastatic propensity of
these cells. When animals injected with metastatic BSpASv4-v7 cells
were treated with anti-CD44v6 antibody, lymph node and lung
metastases were blocked (16).
Since these findings, CD44v6 has attracted increasing interest and
investigations are currently underway regarding the physiological
significance of CD44v6 as a prognostic factor for tumor
progression. The exact role that CD44v6 plays in ESCC has yet to be
elucidated, but has become an area of active study.
In previous studies, high expression frequencies
exceeding 90% have been found in squamous cell carcinomas (SCCs)
derived from head and neck, esophagus, skin and lung (10, 17–20).
Our studies are in agreement with those results. In addition to
CD44v6, CD44s was expressed in epithelial and tumor cells in ESCCs
at high frequencies. CD44s stained strongly in lymphocytic cells
and was loosely stained in the stroma. On the other hand, CD44v6
was expressed only in carcinoma and non-cancerous epithelial cells.
Therefore, CD44v6 appears to be an ideal target antigen for the
majority of SCCs. This frequent and homogeneous expression of
CD44v6 renders SCC a suitable target for therapeutic approaches
using CD44v6-specific antibodies.
There has been some controversy regarding the
relationship of CD44v6 to the prognoses of various malignancies. It
was previously reported that overexpression of CD44v6 is associated
with metastasis of prostate cancer (21) and the prognosis of thymic epithelial
neoplasms (22). In breast cancer,
Kaufmann et al proposed that CD44v6 is a good marker for
prognosis independent of progesterone receptor, lymph node status,
tumor size and grade (23). By
contrast, it has been suggested that CD44v6 is negatively
associated with the progression of various malignancies. Lipponen
et al studied 173 patients with bladder tumors and found
that those with negative CD44v6 immunoreactivity had poorer
prognoses (24). We showed the
survival rates of ESCC patients with overexpression of CD44v6 to be
markedly worse and that CD44v6 expression was a significant
independent prognostic factor for patients with ESCC. Notably, in 6
patients lacking CD44v6 expression, favorable outcomes were found
for their clinicopathological characteristics (Table II). These results suggest that ESCC
patients lacking CD44v6 expression have more favorable prognoses,
even when disease is advanced. CD44v6 is potentially a co-receptor
for c-Met and VEGFR-2 (28,29). Further studies are required in order
to clarify the role of CD44v6 protein in ESCC.
CD44v6 is a good prognostic marker and a suitable
target for anticancer therapy for ESCC patients. Consequently,
patients suffering from head and neck squamous cell carcinoma have
entered phase I clinical trials utilizing CD44v6 antibodies that
were either radiolabeled or covalently linked to a toxin (25,26).
Although the phase I clinical trials appeared to be promising, 1
patient developed toxic epidermal necrolysis and succumbed to the
disease. For this reason, the development of this drug has been
terminated (27). Despite the
termination of the trials, CD44v6 remains a valid target for
anticancer therapy. Alternative strategies targeting CD44v6
functions have been presented.
In conclusion, overexpression of CD44v6 is an
independent prognostic indicator for patients with ESCC. CD44v6 may
be used as a prognostic marker for various malignancies, including
ESCC. Although the precise molecular mechanism of up-regulated
CD44v6 expression has yet to be clarified, our data have clearly
indicated that CD44v6 may be a favorable candidate as a prognostic
marker as well as a molecular target for the development of an
effective therapeutic reagent for patients with esophageal
cancer.
Acknowledgements
The authors would like to thank Ms. Shinobu Makino
for the excellent technical assistance.
References
|
1
|
Parkin DM and Bray F: Evaluation of data
quality in the cancer registry: principles and methods Part II.
Completeness. Eur J Cancer. 45:756–764. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lyons AJ and Jones J: Cell adhesion
molecules, the extracellular matrix and oral squamous carcinoma.
Int J Oral Maxillofac Surg. 36:671–679. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rautava J, Soukka T, Inki P,
Leimola-Virtanen R, Saloniemi I, Happonen RP and Heikinheimo K:
CD44v6 in developing, dysplastic and malignant oral epithelia. Oral
Oncol. 39:373–379. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Naor D, Wallach-Dayan SB, Zahalka MA and
Sionov RV: Involvement of CD44, a molecule with a thousand faces,
in cancer dissemination. Semin Cancer Biol. 18:260–267. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Van Weering DH, Baas PD and Bos JL: A
PCR-based method for the analysis of human CD44 splice products.
PCR Methods Appl. 3:100–106. 1993.PubMed/NCBI
|
|
6
|
Iida N and Bourguignon LY: New CD44 splice
variants associated with human breast cancers. J Cell Physiol.
162:127–133. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuniyasu H, Oue N, Tsutsumi M, Tahara E
and Yasui W: Heparan sulfate enhances invasion by human colon
carcinoma cell lines through expression of CD44 variant exon 3.
Clin Cancer Res. 7:4067–4072. 2001.PubMed/NCBI
|
|
8
|
Pirinen R, Hirvikoski P, Bohm J,
Kellokoski J, Moisio K, Viren M, Johansson R, Hollmen S and Kosma
VM: Reduced expression of CD44v3 variant isoform is associated with
unfavorable outcome in non-small cell lung carcinoma. Hum Pathol.
31:1088–1095. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kuhn S, Koch M, Nubel T, Ladwein M,
Antolovic D, Klingbeil P, Hildebrand D, Moldenhauer G, Langbein L,
Franke WW, Weitz J and Zoller M: A complex of EpCAM, claudin-7,
CD44 variant isoforms, and tetraspanins promotes colorectal cancer
progression. Mol Cancer Res. 5:553–567. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mizera-Nyczak E, Dyszkiewicz W, Heider KH
and Zeromski J: Isoform expression of CD44 adhesion molecules,
Bcl-2, p53 and Ki-67 proteins in lung cancer. Tumour Biol.
22:45–53. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iida N and Bourguignon LY: Coexpression of
CD44 variant (v10/ex14) and CD44S in human mammary epithelial cells
promotes tumorigenesis. J Cell Physiol. 171:152–160. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koyama S, Maruyama T and Adachi S:
Expression of epidermal growth factor receptor and CD44 splicing
variants sharing exons 6 and 9 on gastric and esophageal
carcinomas: a two-color flow-cytometric analysis. J Cancer Res Clin
Oncol. 125:47–54. 1999. View Article : Google Scholar
|
|
13
|
Gotoda T, Matsumura Y, Kondo H, Ono H,
Kanamoto A, Kato H, Watanabe H, Tachimori Y, Nakanishi Y and
Kakizoe T: Expression of CD44 variants and prognosis in oesophageal
squamous cell carcinoma. Gut. 46:14–19. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li DM, Li SS, Zhang YH, Zhang HJ, Gao DL
and Wang YX: Expression of human chorionic gonadotropin, CD44v6 and
CD44v4/5 in esophageal squamous cell carcinoma. World J
Gastroenterol. 11:7401–7404. 2005.PubMed/NCBI
|
|
15
|
Gunthert U, Hofmann M, Rudy W, Reber S,
Zoller M, Haussmann I, Matzku S, Wenzel A, Ponta H and Herrlich P:
A new variant of glycoprotein CD44 confers metastatic potential to
rat carcinoma cells. Cell. 65:13–24. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seiter S, Arch R, Reber S, Komitowski D,
Hofmann M, Ponta H, Herrlich P, Matzku S and Zoller M: Prevention
of tumor metastasis formation by anti-variant CD44. J Exp Med.
177:443–455. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Van Hal NL, van Dongen GA, Stigter-van
Walsum M, Snow GB and Brakenhoff RH: Characterization of CD44v6
isoforms in head-and-neck squamous-cell carcinoma. Int J Cancer.
82:837–845. 1999.PubMed/NCBI
|
|
18
|
Kanke M, Fujii M, Kameyama K, Kanzaki J,
Tokumaru Y, Imanishi Y, Tomita T and Matsumura Y:
Clinicopathological significance of expression of CD44 variants in
head and neck squamous cell carcinoma. Jpn J Cancer Res.
91:410–415. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Heider KH, Sproll M, Susani S, Patzelt E,
Beaumier P, Ostermann E, Ahorn H and Adolf GR: Characterization of
a high-affinity monoclonal antibody specific for CD44v6 as
candidate for immunotherapy of squamous cell carcinomas. Cancer
Immunol Immunother. 43:245–253. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Simon JC, Heider KH, Dietrich A, Wuttig C,
Schopf E, Adolf GR, Ponta H and Herrlich P: Expression of CD44
isoforms in human skin cancer. Eur J Cancer. 32A:1394–1400. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ekici S, Cerwinka WH, Duncan R, Gomez P,
Civantos F, Soloway MS and Lokeshwar VB: Comparison of the
prognostic potential of hyaluronic acid, hyaluronidase (HYAL-1),
CD44v6 and microvessel density for prostate cancer. Int J Cancer.
112:121–129. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sonobe S, Miyamoto H, Nobukawa B, Izumi H,
Futagawa T, Ishikawa N, Yamazaki A, Uekusa T, Abe H and Suda K:
Prognostic value of CD44 isoform expression in thymic epithelial
neoplasms. Cancer. 103:2015–2022. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kaufmann M, Heider KH, Sinn HP, von
Minckwitz G, Ponta H and Herrlich P: CD44 variant exon epitopes in
primary breast cancer and length of survival. Lancet. 345:615–619.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lipponen P, Aaltoma S, Kosma VM, Ala-Opas
M and Eskelinen M: Expression of CD44 standard and variant-v6
proteins in transitional cell bladder tumours and their relation to
prognosis during a long-term follow-up. J Pathol. 186:157–164.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Heider KH, Kuthan H, Stehle G and Munzert
G: CD44v6: a target for antibody-based cancer therapy. Cancer
Immunol Immunother. 53:567–579. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stroomer JW, Roos JC, Sproll M, Quak JJ,
Heider KH, Wilhelm BJ, Castelijns JA, Meyer R, Kwakkelstein MO,
Snow GB, Adolf GR and van Dongen GA: Safety and biodistribution of
99mTechnetium-labeled anti-CD44v6 monoclonal antibody BIWA 1 in
head and neck cancer patients. Clin Cancer Res. 6:3046–3055.
2000.PubMed/NCBI
|
|
27
|
Riechelmann H, Sauter A, Golze W, Hanft G,
Schroen C, Hoermann K, Erhardt T and Gronau S: Phase I trial with
the CD44v6-targeting immunoconjugate bivatuzumab mertansine in head
and neck squamous cell carcinoma. Oral Oncol. 44:823–829. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Orian-Rousseau V, Chen L, Sleeman JP,
Herrlich P and Ponta H: CD44 is required for two consecutive steps
in HGF/c-Met signaling. Genes Dev. 16:3074–3086. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tremmel M, Matzke A, Albrecht I, Laib AM,
Olaku V, Ballmer-Hofer K, Christofori G, Heroult M, Augustin HG,
Ponta H and Orian-Rousseau V: A CD44v6 peptide reveals a role of
CD44 in VEGFR-2 signaling and angiogenesis. Blood. 114:5236–5244.
2009. View Article : Google Scholar : PubMed/NCBI
|