Introduction
Expression of cyclooxygenase (COX)-2 is observed in
colon polyps or colon cancer tissues, and it has been confirmed
that microsomal PGES-1, which lies downstream of COX-2 and is
involved in the synthesis of prostaglandin E2
(PGE2), plays an important role in oncogenesis (1,2). It
was reported that tumor growth by PGE2 may be the result
of an increase in the production of vascular endothelial growth
factor, the growth of epithelial cells or an increase in resistance
to apoptosis (3). In particular,
PGE2 was found to induce the expression of the matrix
metalloproteinases (MMP), resulting in the release of transforming
growth factor-α (TGF-α) (4), an
EGFR ligand, and stimulating EGFR, both of which are blocked by the
COX-2 inhibitor (5). It was
clinically shown that the COX-2 inhibitor suppressed the occurrence
of colon cancer (6) in patients
treated with aspirin and decreases the recurrence of colon cancer
(7). Thus, the COX-2 inhibitor is
expected to prevent colon polyps and inhibit the metastasis of
colon cancer. It is also known to have an inhibitory effect on
cytokines such as interleuken (IL)-6.
Protein-bound polysaccharide (PSK) is a protein
polysaccharide extracted and refined from Trametes
versicolor with a molecular weight of approximately 100,000. It
is known to have pharmacologic actions including inhibition of the
production of immunosuppressive agents, enhancement of the
production or inhibition of the excessive production of cytokines,
and direct action on cancer cells. Cytokines are believed to
inhibit IL-1, IL-6 and IL-8, or the expression of TGF-β, thereby
inhibiting the expression of MMPs and suppressing the
invasion/metastasis of cancer (8).
The two drugs are based on completely different mechanisms of
pharmacological effect. However, a common effect is expected. The
anti-tumor and cytokine inhibitory effects have been demonstrated
by respective experiments, whereas an additive or synergistic
effect of the two drugs has yet to be reported. Therefore, in the
present study, the effects of the COX-2 inhibitor, etodolac, and a
non-specific immunostimulant, PSK, were investigated using in
vivo experiments.
Materials and methods
Mice
BALB/c or CDF1 male mice were purchased at 6 weeks
of age, acclimatized for 7 days, and subsequently used for the
experiments.
Preparatory experiment schedule
In the treatment experiment, a hepatic metastasis
model was constructed by infusing cancer cells in BALB/c or CDF1
male mice below the splenic capsule where etodolac and PSK were
administered. Cells (5×105) of the mouse colon cancer 26
cell line (CT26) and its highly metastatic variant were implanted
below the splenic capsule. Three days after implantation, a
spleen-removed group and a non-spleen-removed one were produced and
the hepatic metastasis model was designed. Two or 3 weeks after
cell implantation, the number of hepatic metastases in each group
was measured, and the optimal model was determined.
Using the optimal model, etodolac suspended in 1%
methyl- cellulose solution was administered at 5, 10, 20 and 30
mg/kg from day 1 after cell implantation (n=10 mice/group). From
the following day, etodolac was administered 15 times daily, and
the animals were sacrificed on day 16. At the time of sacrifice,
the inhibitory rate was determined by calculating the number of
hepatic metastases, and the optimal dosage of etodolac was
determined.
Using the optimal model, PSK was dissolved in
physiological saline. PSK was administered intraperitoneally (25 or
50 mg/kg) or orally (1000 mg/kg) (n=7 mice/group). From the day
following implantation, PSK was administered intraperitoneally on
alternate days 3 times per week, and orally on 5 consecutive days
per week. Animals were sacrificed on day 16 following cell
implantation, the number of hepatic metastases was measured, and
the optimal dosage of PSK was determined.
Experiments
The optimal dosages were determined using the
preparatory experiments, and etodolac and PSK were administered
singly or in combination, for 2 weeks from the day following cell
implantation (n=8 mice/group). The mice were sacrificed one day
after completion of the administration schedule (on day 16), and
the inhibitory rate of tumor growth compared with that of the
solvent control group was determined from the number of hepatic
metastases. Regarding the group composition, each group consisted
of 10 mice: the solvent control group, which included a forced oral
administration group of 0.5% methylcellulose solution, the etodolac
single administration group, the PSK single intraperitoneal
administration group and the group of combined administration of
etodolac + PSK.
Biochemical measurement
Each animal was sacrificed 3 h after the final
administration of each drug, and serum samples were obtained. The
samples were cryostatically preserved (−20°C or below) until
measurement of the levels of MMP-9, TGF-B, and IL-6.
Measurement of MMP-9
The Quantikine Mouse pro-MMP-9 Immunoassay (R&D
Systems, Inc., MN, USA) is a 4.5-h solid phase ELISA designed to
measure free and TIMP-1-bound mouse pro-MMP-9 in cell culture
supernatants, mouse serum and platelet-poor plasma. It contains
NS0-expressed recombinant mouse pro-MMP-9 showing dose-response
curves that are parallel to the standard curves obtained using the
recombinant Quantikine kit standards, indicating that the
Quantikine kit can be used to determine relative levels of natural
mouse pro-MMP-9. The measurement method is as follows. All of the
reagents were brought to room temperature. Reagents and samples
were prepared according to the manufacturer's instructions. Unused
components were returned to storage temperature as indicated in the
instructions. Assay diluent (50 μl) was added to each well followed
by the addition of 50 μl of the standard, control or sample to each
well. The plate was covered, and incubation was carried out for 2 h
at room temperature in a shaker. Each well was aspirated and washed
four times and substrate solution (100 μl) was then added to each
well. Incubation was carried out for 30 min at room temperature in
the dark, and 100 μl of stop solution was added to each well. The
optical density was read at 450 nm (correction wavelength set at
540 or 570 nm).
Measurement of IL-6
The Quantikine Mouse IL-6 Immunoassay (R&D
Systems) is a 4.5-h solid-phase ELISA designed to measure mouse
IL-6 in cell culture supernatants, serum and plasma. It contains
E. coli-expressed recombinant mouse IL-6 and antibodies
raised against the recombinant factors. This immunoassay has been
shown to accurately quantitate the recombinant mouse IL-6. The
results obtained using natural mouse IL-6 showed dose response
curves that are parallel to the standard curves obtained using the
Quantikine Mouse kit standards.
These results indicate that the Quantikine Mouse
IL-6 Immunoassay kit can be used to determine relative mass values
for natural mouse IL-6. The measurement method is as follows.
Reagents, samples and standard dilutions were prepared as described
in the previous sections. Excess microplate strips were removed
from the plate frame, returned to the folic pouch containing the
dessicant pack and resealed. Assay diluent RD1-14 (50 μl) was added
to the center of each well. Notably, RD1-14 contains undissolved
material even when mixed well before and during use. Standard,
control or sample (50 μl) was added to the center of each well,
which was covered with the adhesive strip provided and mixed by
gently tapping the plate frame for 1 min. Incubation was carried
out for 2 h at room temperature. Plate layouts were provided to
record standards and samples assayed. Each well was aspirated and
washed, repeating the process four times for a total of five
washes. Washing was performed by filling each well with wash buffer
(400 μl) using a squirt bottle, manifold dispenser or autowasher.
The complete removal of liquid at each step is essential to good
performance. After the last wash, any remaining wash buffer was
removed by aspirating or decanting. The plate was inverted and
blotted against clean paper towels. Mouse IL-6 conjugate (100 μl)
was added to each well, which was covered with a new adhesive
strip. Incubation was carried out for 2 h at room temperature.
Aspiration/wash was repeated as in step 5, and 100 μl of stop
solution was added to each well. Incubation was carried out for 30
min at room temperature in the dark, and then 100 μl of stop
solution was added to each well. The plate was gently tapped to
ensure thorough mixing. The optical density of each well was
determined within 30 min, using a microplate reader set to 450 nm.
When the wavelength correction was available, it was set to 540 or
570 nm. When the wavelength correction was not available, the
readings at 540 or 570 nm were subtracted from the readings at 450
nm to correct for optical imperfections in the plate. Readings
taken directly at 450 nm without correction may be higher and less
accurate.
Measurement of TGF-β
The Quantikine Mouse TGF-β Immunoassay (R&D
Systems) measures TGF-β in cell culture supernatants, mouse serum
and platelet-poor plasma. The measurement method is as follows.
Sample or standard (100 μl) was added to reagent diluent or an
appropriate diluent per well, which was covered with an adhesive
strip and incubated for 2 h at room temperature. Aspiration/wash
was repeated as in step 2 of plate preparation, and 100 μl of the
substrate dilution of Streptavidin-HRP was added to each well. The
plate was covered and incubated for 20 min at room temperature in
the dark. Stop solution was added to each well. The plate was
gently tapped to ensure thorough mixing. The optical density of
each well was determined immediately using a microplate reader set
to 450 nm. When the wavelength correction was available, it was set
to 540 or 570 nm. When the wavelength correction was not available,
the readings at 450 nm were substracted to correct for optical
imperfections in the plate. Readings made directly at 450 nm
without correction may be higher and less accurate.
The animal experiments were conducted in accordance
with the Guideline for Animal Experiments of Tokyo Medical
University.
Statistical analysis
Statistical analysis was conducted using the
Student's t-test. P≤0.05 was considered to be a statistically
significant difference. The results are provided as mean ± SD.
Results
Number of hepatic metastases
BALB/c mice infused with a highly
metastatic variant of the colon 26 cell line (CT26)
The number of hepatic metastases was 5.0±7.0 in the
non-spleen-removed group at 2 weeks, 68.3±54.8 in the
spleen-removed group at 2 weeks, 51.0±42.4 in the
non-spleen-removed group at 3 weeks and 100±0 in the spleen-removed
group at 3 weeks.
BALB/c mice infused with CT26
cells
The number of hepatic metastases was 8.7±6.1 in the
non-spleen-removed group at 2 weeks, 8.0±12.1 in the spleen-removed
group at 2 weeks, 89.7±17.9 in the non-spleen-removed group at 3
weeks and 41.7±34.4 in the spleen-removed group at 3 weeks.
CDF1 mice infused with a highly
metastatic variant of CT26 cells
The number of hepatic metastases was 1.0±1.0 in the
non-spleen-removed group at 2 weeks, 21.0±15.4 in the
spleen-removed group at 2 weeks, 74.7±26.6 in the
non-spleen-removed group at 3 weeks, and 51.0±43.7 in the
spleen-removed group at 3 weeks.
CDF1 mice infused with CT26 cells
The number of hepatic metastases was 43.3±28.5 in
the non-spleen-removed group at 2 weeks, 30.7±31.7 in the
spleen-removed group at 2 weeks, 88.0±25.2 in the
non-spleen-removed group at 3 weeks and 96.0±6.9 in the
spleen-removed group at 3 weeks.
Some individual mice had >100 metastases, which
were judged to be impossible to evaluate. In BALB/c and CDF1 mice
without removal of the spleen, the highly metastatic variant of the
colon 26 cell line and CT26 cells at 2 weeks had ≤100 metastases.
However, in the CDF1 mice, a large number of hepatic metastatic
lesions of CT26 cells were observed, which was regarded as the
optimal model (Table I).
 | Table INumber of hepatic metastases in each
group. |
Table I
Number of hepatic metastases in each
group.
| Non-spleen-removed (2
weeks) | Spleen-removed (2
weeks) | Non-spleen-removed (3
weeks) | Spleen-removed (3
weeks) |
|---|
| BALB/c mice infused
with a highly metastatic variant of the CT26a cell line | 5.0±7.0 | 63.8±54.8 | 51.0±42.4 | 100±0 |
| BALB/c mice infused
with the CT26 cell line | 8.7±6.1 | 8.0±12.1 | 89.7±17.9 | 41.7±34.4 |
| CDF1 mice infused
with a highly metastatic variant of the CT26 cell line | 1.0±1.0 | 21.0±15.4 | 74.7±26.6 | 51.0±43.7 |
| CDF1 mice infused
with the CT26 cell line | 43.3±28.5 | 30.7±31.7 | 88.0±25.2 | 96.0±6.9 |
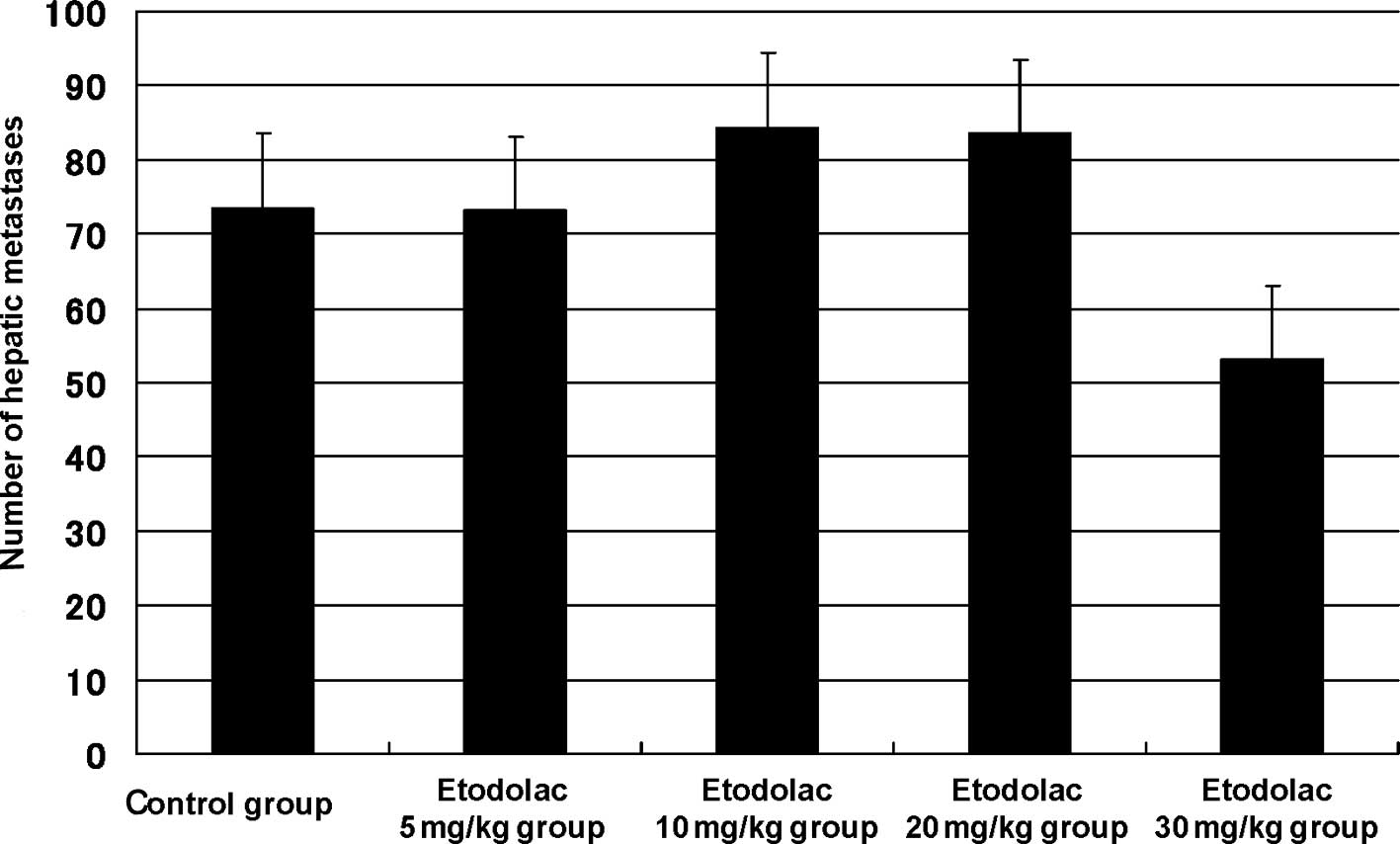
Effect of etodolac treatment
Regarding the effect of etodolac on the hepatic
metastatic model using CT26 cells, the number of hepatic metastases
was 73.5±10.2 in the control group, 73.1±13.3 in the 5 mg/kg
etodolac group, 84.4±10.7 in the 10 mg/kg group, 83.5±9.3 in the 20
mg/kg group and 53.1±13.2 in the 30 mg/kg group. The inhibitory
rate was 0.7, −14.8, −13.6 and 27.8% (p=0.2), respectively.
Although no significant difference was observed, the highest
inhibitory effect was observed at 30 mg/kg etodolac, which was
regarded as the optimal dosage (Fig.
1).
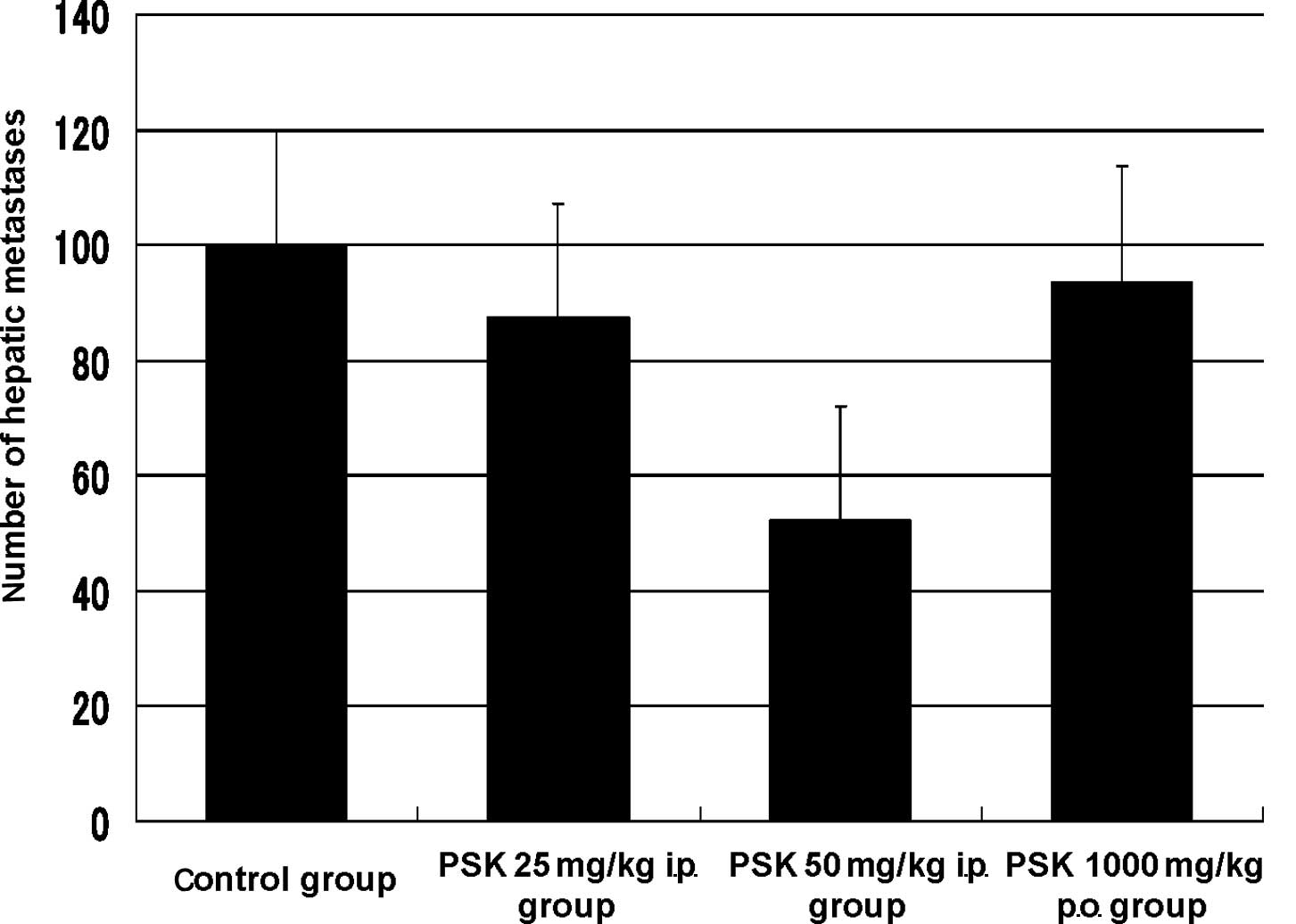
Effect of PSK treatment
Regarding the effect of PSK on the hepatic
metastatic model using CT26 cells, the number of hepatic metastases
was 100.0±0 in the control group (n=7), 87.3±12.7 in the 25 mg/kg
intraperitoneal administration PSK group, 52.2±18.0 in the 50 mg/kg
intraperitoneal administration PSK group and 93.0±7.0 in the 1000
mg/kg oral administration PSK group. The inhibitory rate was 12.7,
47.8 (p=0.3) and 7.0%, respectively. Although no significant
difference was noted, the highest inhibitory effect was observed at
50 mg/kg intraperitoneal administration of PSK, which was regarded
as the optimal dosage (Fig. 2).
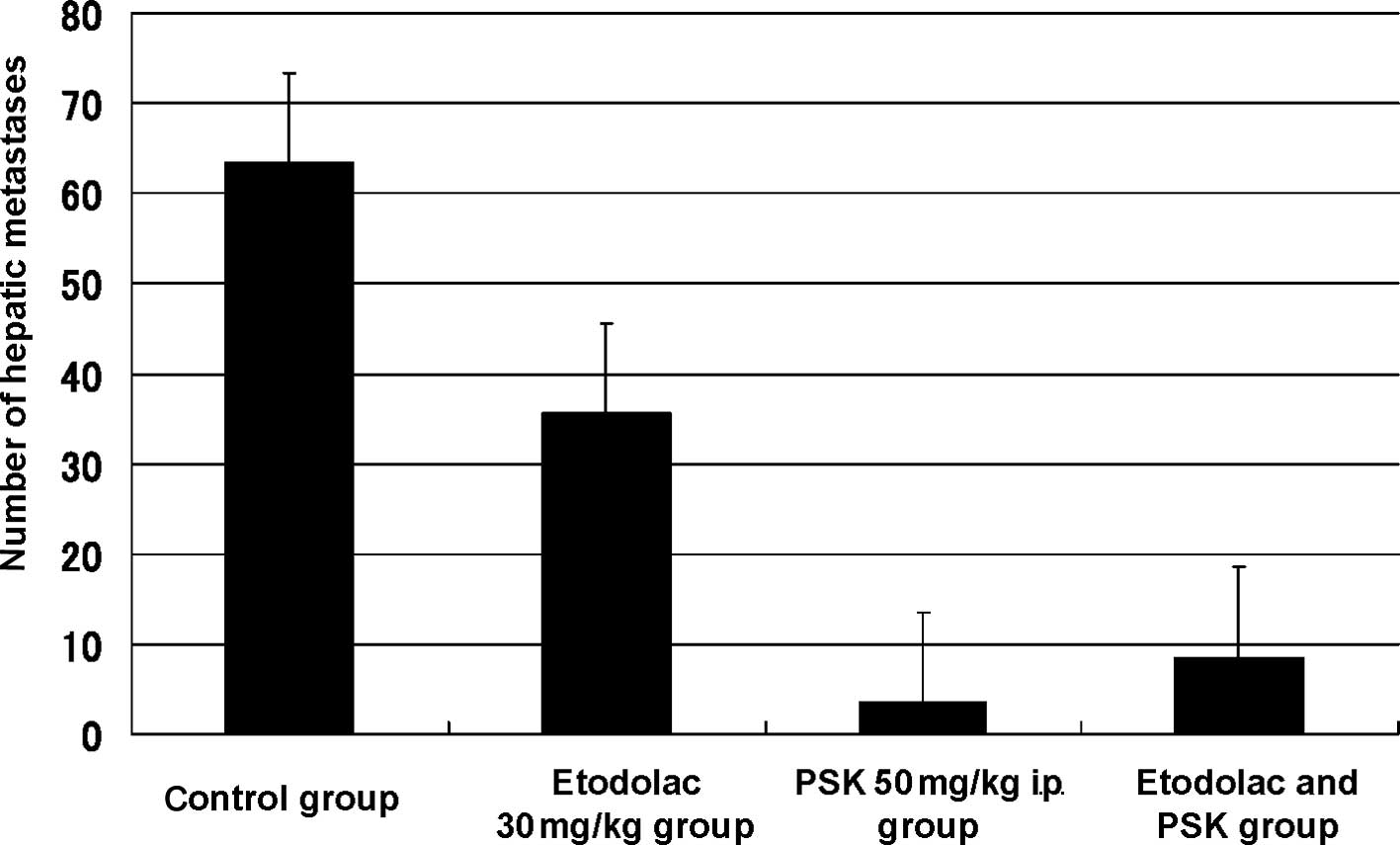
Combination of etodolac and PSK
Regarding the number of hepatic metastases following
administration of 30 mg/kg etodolac, 50 mg/kg intraperitoneal
administration of PSK, and the combination of etodolac and PSK, the
number of hepatic metastases was 63.3±12.9 in the control group,
35.6±12.0 in the etodolac group, 3.5±1.0 in the PSK group, and
8.5±4.6 in the etodolac and PSK group. The inhibitory rate of
hepatic metastasis was 2.2% in the etodolac group, 94.5% (p=0.002)
in the PSK group and 86.6% in the etodolac and PSK group (p=0.001)
(Fig. 3).
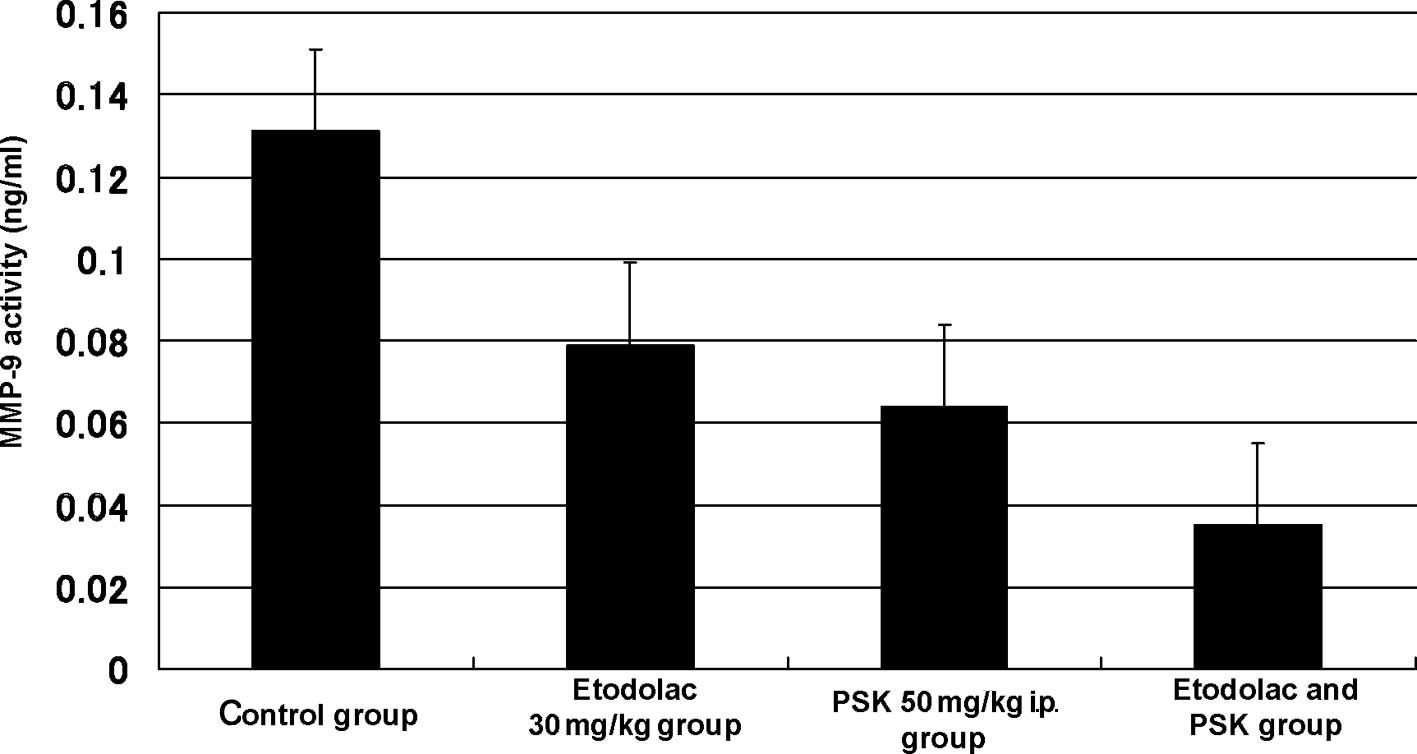
Biochemical measurement
The MMP-9 level was 0.131±0.049 ng/ml in the control
group, 0.079±0.030 ng/ml (p=0.022) in the 30 mg/kg oral
administration etodolac group, 0.064±0.021 ng/ml (p=0.003) in the
50 mg/kg intraperitoneal administration PSK group, and 0.035±0.009
ng/ml (p=0.001) in the intraperitoneal administration of the
combination of etodolac (30 mg/kg) and PSK (50 mg/kg) group
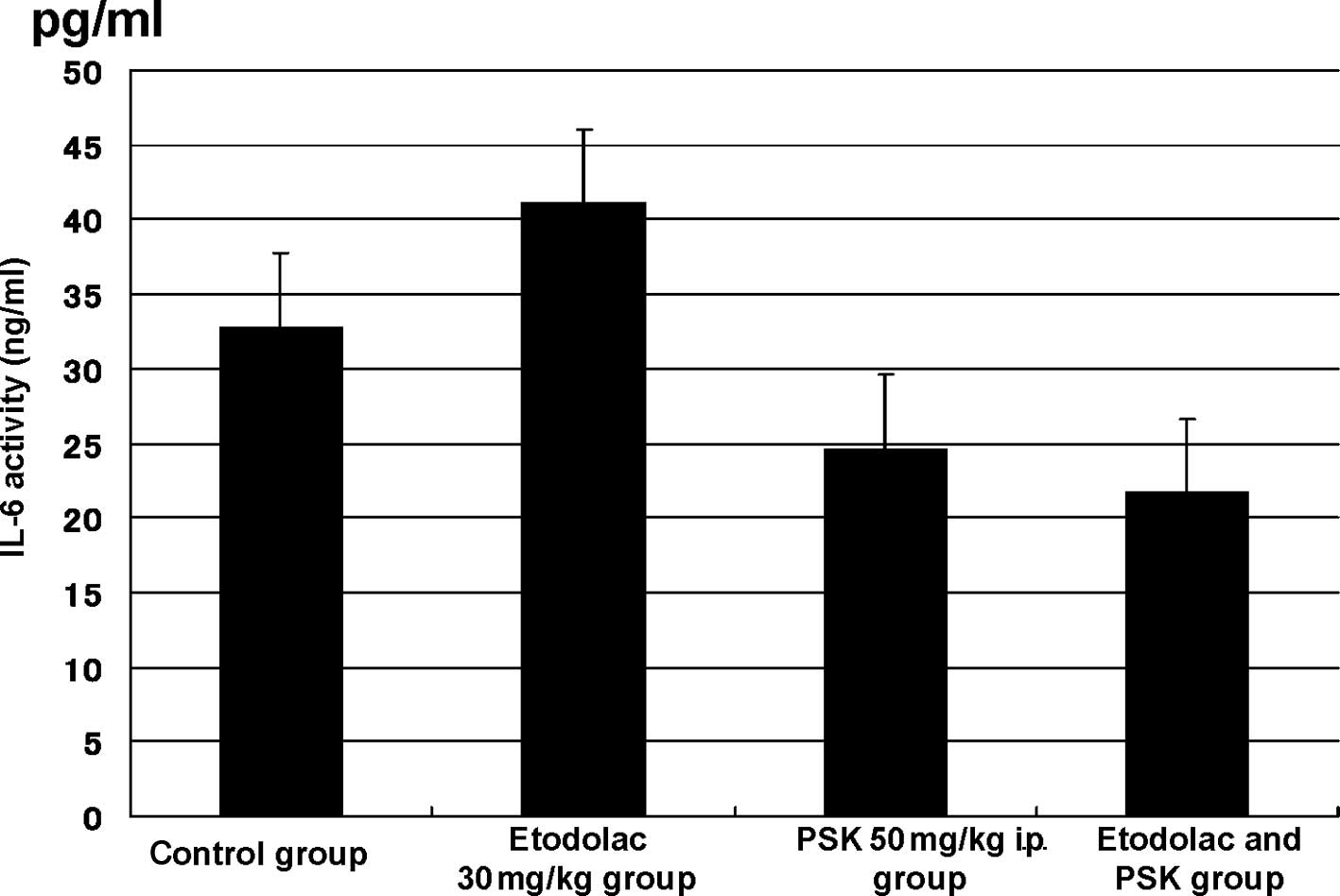
(Fig. 4). The IL-6 level was
32.75±10.028 pg/ml in the control group, 41.095±14.534 pg/ml in the
30 mg/kg oral administration etodolac group, 24.556±5.739 pg/ml in
the 50 mg/kg intraperitoneal administration PSK group, and
21.648±5.733 pg/ml (p=0.023) in the intraperitoneal administration
of the combination of etodolac (30 mg/kg) and PSK (50 mg/kg) group
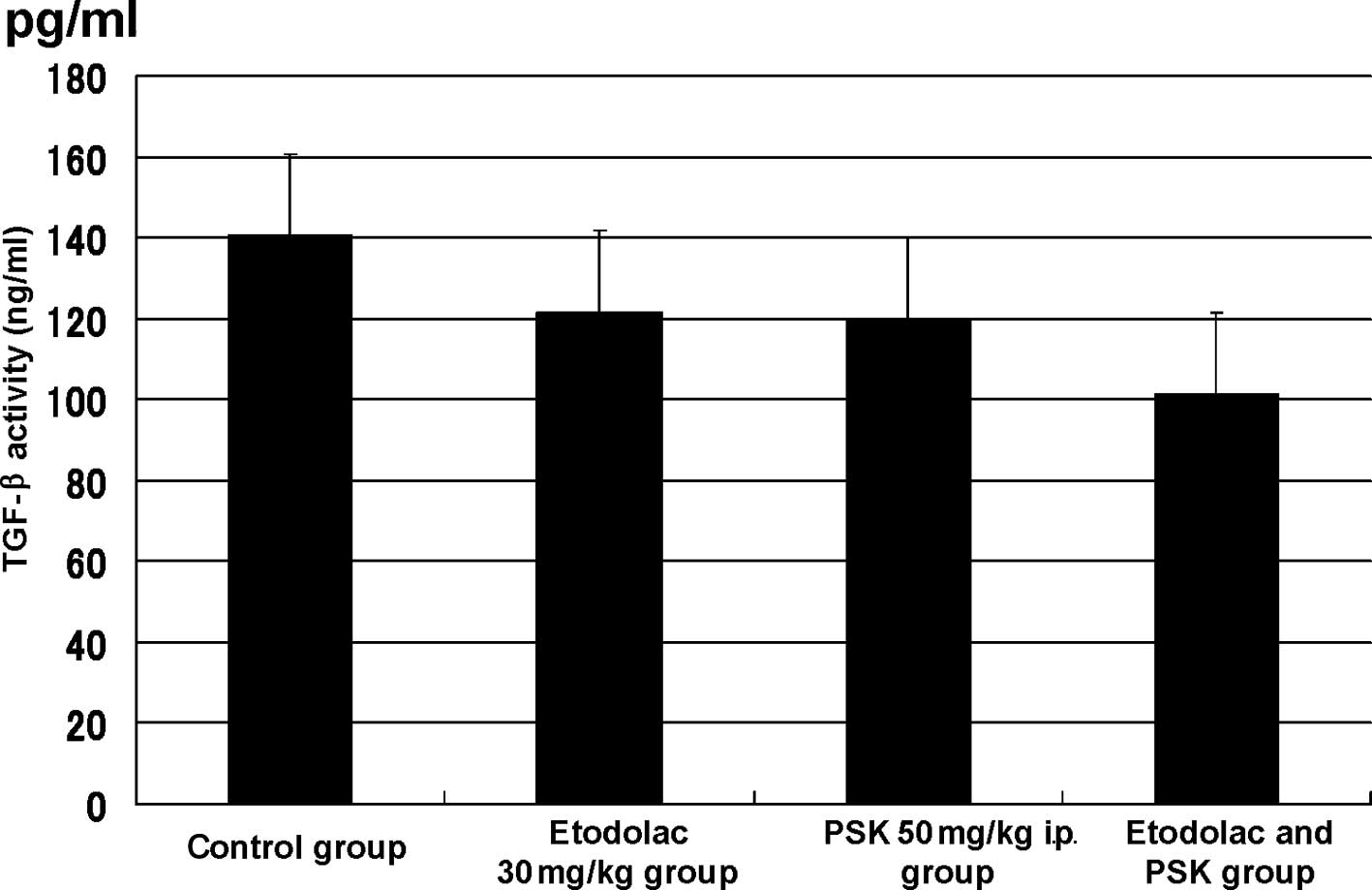
(Fig. 5). The TGF-β level was
140.700±27.455 pg/ml in the control group, 121.713±44.992 pg/ml in
the 30 mg/kg oral administration etodolac group, 120.015±29.928
pg/ml in the 50 mg/kg intraperitoneal administration PSK group and
101.441±28.909 pg/ml (p=0.015) in the intraperitoneal
administration of the combination of etodolac (30 mg/kg) and PSK
(50 mg/kg) group (Fig. 6).
Discussion
Regarding the involvement of COX-2 in
carcinogenesis, the contribution of Bcl-2 by the PGE2
production pathway, an increase in the invasiveness of cancer
cells/vascularization by the production of vascular growth factors,
VEGF and bFGF, and inhibition of the immunological surveillance
mechanism have been suggested (9).
The expression of COX-2 in colon polyps, colon cancer tissue, and
interstitial cells has been confirmed, and it is known that
microsomal PGES-1, which lies downstream of COX-2 and is involved
in the synthesis of PGE2, plays an important role in
oncogenesis. In particular, PGE2 is believed to induce
the expression of matrix metalloproteinases (MMP), resulting in the
release of transforming growth factor-α (TGF-α), an EGFR ligand,
thus stimulating EGFR (4). It has
been reported that tumor growth caused by PGE2 may
increase the production of vascular endothelial growth factor and
may cause the growth of epidermal cells or an increase in
resistance to apoptosis. It has been clinically demonstrated that
COX-2 inhibitor suppresses the occurrence of colon polyps in
patients treated with aspirin (5)
and inhibits the recurrence of colon cancer (6). COX-2 also causes chronic inflammation
and inhibits the production of IL-6, which increases as cancer
advances (10). A COX-2 inhibitor,
based on the inhibitory effect of such actions, is expected to
prevent colon polyps and inhibit the metastasis of colon cancer.
The APPROVe clinical trial was conducted to investigate the
prevention of colon polyps, while the VICTOR clinical trial was
conducted to evaluate the inhibition of the recurrence of colon
cancer of the COX-2 inhibitor. However, both clinical trials ended
without obtaining signficant results due to adverse cardiovascular
side effects (11,12).
Protein-bound polysaccharide, PSK, is a protein
polysaccharide extracted and refined from Trametes
versicolor with a molecular weight of approximately 100,000.
PSK is known to have pharmacologic actions including the inhibition
of the production of immunosuppressive agents, an increase in the
production or inhibition of the excessive production of cytokines,
and direct action on cancer cells. It is considered that cytokines
inhibit IL-1, IL-6, and IL-8 or the expression of TGF-β, thereby
inhibiting the expression of MMPs, and suppressing the
invasion/metastasis of cancer (8).
Although the two drugs are based on a completely
different pharmacological effect and mechanism of action, a common
effect is expected. Although their anti-tumor and cytokine
inhibitory effects have been proven by respective experiments, an
additive or synergistic effect of the two drugs has yet to be
reported. Therefore, in the present study, the effects of the COX-2
inhibitor, etodolac, and the non-specific immunostimulant, PSK,
were evaluated through in vivo experiments.
Animal experiments were conducted using a COX-2
inhibitor. In their study, Ogata et al measured and reviewed
subcutaneously implanted tumors. Nimesulid was found to induce
apoptosis, but did not show any tumor reduction effect, although it
had an effect on weight gain and prolongation of survival. When
5-FU was used in combination with nimesulid, a tumor reduction
effect, weight gain, and prolongation of survival were observed in
the 20 mg/kg/day administration group (13).
Ishizaki et al infused cancer cells into the
spleen, and nimesulid or etodolac was administered, respectively.
The number of hepatic metastatic lesions were then assessed.
Etodolac reduced the number of hepatic metastatic lesions, whereas
nimesulid did not show such a tendency. As regards the cause, the
authors demonstrated that etodolac, compared with nimesulid,
inhibits the expression of COX-2 within the tumor more strongly,
and inhibits the expression of MMP-9 (14).
As shown above, the reproducibility of the effects
of a COX-2 inhibitor was confirmed by the experiments conducted
using etodolac with tumor-reducing effect.
In Japan, PSK is commonly administered as a
non-specific immunostimulant. PSK is known to have effects such as
an increase in immune capacity, an increase in ability to produce
TNF-α, inhibition of excessive production of IL-6, inhibition of
TGF-β, and inhibition of MMP activity. It is also known to have
clinical effects in combined use for postoperative adjuvant
chemotherapy for stomach (15,16)
and colon (17–19) cancers.
The two drugs, PSK and etodolac, exhibited common
pharmacological effects such as the inhibition of cytokines and MMP
activity, although their mechanism of action varied. Thus,
experiments to were conducted ascertain whether the combined use of
the two drugs had particular effects or not and examined the number
of hepatic metastases and serological factors.
In this experiment, etodolac produced a decrease in
the number of hepatic metastases, but no significant difference was
observed. Thus, the reproducibility of the findings of Ishizaki
et al was not confirmed. In contrast, the number of hepatic
metastases following treatment with PSK was significantly reduced,
and combined use showed an inhibitory but not a synergistic
effect.
Regarding serological factors, the MMP-9 level was
significantly inhibited by any of the drugs administered singly,
while combined use did not show any synergistic effect. Etodolac
and PSK strongly showed an inhibitory effect on MMP-9.
When used as a single drug, a weak effect on IL-6
and TGF-β was observed, and an inhibitory effect was confirmed only
when used in combination. Thus, the clinical significance of the
combined use of the two drugs was identified.
Yoshino et al investigated the combined use
with 5-FU, and found that nimesulid has a combined inhibitory
effect on tumor growth. Therefore, weight gain and survival were
affected due to the inhibition of hypercytokinemia caused by the
side effects of 5-FU. In contrast, it was reported that
postoperative adjuvant chemotherapy when combined with PSK for
stomach or colon cancers improved the immune capacity decreased by
surgery or anti-tumor drugs, while a reduction in side effects by
the inhibition of hypercytokinemia was considered (20). Findings of the present study showed
that the combined use of PSK or COX-2 is likely to increase this
effect and may be used in the clinical setting in the future.
References
|
1
|
Yoshimatsu K, Golijanin D, Paty PB, et al:
Inducible microsomal prostaglandin E synthase is overexpressed in
colorectal adenomas and cancer. Clin Cancer Res. 7:3971–3976.
2001.PubMed/NCBI
|
|
2
|
Takeda H, Sonoshita M, Oshima H, et al:
Cooperation of cyclooxygenase 1 and cyclooxygenase 2 in intestinal
polyposis. Cancer Res. 63:4872–4877. 2003.PubMed/NCBI
|
|
3
|
Seno H, Oshima M, Ishikawa T, et al:
Cyclooxygenase-2 and prostaglandin E2 receptor
EP2-dependent angiogenesis in ApcΔ716 mouse
intestinal polyps. Cancer Res. 62:506–511. 2002.PubMed/NCBI
|
|
4
|
Tsujii M, Kawano S and DuBois RN:
Cyclooxygenase-2 expression in human colon cancer cells increase
metastatic potential. Proc Natl Acad Sci USA. 94:3336–3340. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tomozawa S, Nagawa H, Tsuno N, et al:
Inhibition of haematogenous metastasis of colon cancer in mice by a
selective COX-2 inhibitor, JTE-522. Br J Cancer. 81:1274–1279.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kune GA, Kune S and Watson LF: Colorectal
cancer risk, chronic illness, operations, and medications: case
control results from the Melbourne Colorectal Cancer Study. Cancer
Res. 48:4399–4404. 1988.PubMed/NCBI
|
|
7
|
Matsunaga N, Yamada N and Hirakawa K:
Combined treatment with fluorinated pyrimidines and selective
cyclooxygenase-2 inhibitor for liver metastasis of colon cancer.
Nippon Rinsho. 61:487–489. 2003.(In Japanese).
|
|
8
|
Zhang H, Morisaki T, Matsunaga H, et al:
Protein-bound polysaccharide PSK inhibits tumor invasiveness by
down-regulation of TGF-β1 and MMPs. Clin Exp Metastasis.
18:343–352. 2000.PubMed/NCBI
|
|
9
|
Irie T, Tsujii M and Tsuji S: The effect
of COX on tumor progression. Ganchiryou to Shukushu Frontiers in
Cancer Treatment. 16:17–21. 2004.
|
|
10
|
Peluffo GD, Stillitani I, Rodriguez VA, et
al: Reduction of tumor progression and paraneoplastic syndrome
development in murine lung adenocarcinoma by nonsteroidal
anti-inflammatory drugs. Int J Cancer. 110:825–830. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bresalier RS, Sandler RS, Quan H, et al:
Cardiovascular events associated with rofecoxib in a colorectal
adenoma chemoprevention trial. N Engl J Med. 352:1092–1102. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kerr DJ, Dunn JA, Langman MJ, et al:
Rofecoxib and cardiovascular adverse events in adjuvant treatment
of colorectal cancer. N Engl J Med. 357:360–369. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ogata J, Sakamoto N, Hisada M, Wada T,
Kato K, Aoki T and Koyanagi Y: Experimental study on the antitumor
effect of a selective cyclooxygenase-2 inhibitor combined with
5-fluorouracil in a mouse model of colon cancer. J Tokyo Med Univ.
61:336–345. 2003.
|
|
14
|
Ishizaki T, Katsumata K, Tsuchida A, et
al: Etodolac, a selective cyclooxygenase-2 inhibitor, inhibits
liver metastasis of colorectal cancer cells via the suppression of
MMP-9 activity. Int J Mol Med. 17:357–362. 2006.PubMed/NCBI
|
|
15
|
Nakazato H, Koike A, Saji S, Ogawa N and
Sakamoto J: Efficacy of immunochemotherapy as adjuvant treatment
after curative resection of gastric cancer. Lancet. 343:1122–1126.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Toge T and Yamaguchi Y: Protein-bound
polysaccharide increases survival in resected gastric cancer cases
stratified with a preoperative granulocyte and lymphocyte count.
Oncology. 7:1157–1161. 2000.
|
|
17
|
Ohwada S, Ikeya T, Yokomori T, et al:
Adjuvant immunochemotherapy with oral Tegafur/Uracil plus PSK in
patients with stage II or III colorectal cancer: a randomized
controlled study. Br J Cancer. 90:1003–1010. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoshitani S and Takashima S: Efficacy of
postoperative UFT (Tegafur/Uracil) plus PSK therapies in elderly
patients with resected colorectal cancer. Cancer Biother
Radiopharm. 24:35–39. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sakamoto J, Morita S, Oba K, Matsui T,
Kobayashi M, Nakazato H and Ohashi Y: Efficacy of adjuvant
immunochemotherapy with polysaccharide K for patients with
curatively resected colorectal cancer: a meta-analysis of centrally
randomized controlled clinical trials. Cancer Immunol Immunother.
55:404–411. 2006. View Article : Google Scholar
|
|
20
|
Yoshino S, Hazama S, Shimizu R, et al:
Immunoregulatory effects of PSK on the balance between Th1 and Th2
in patients with colorectal cancer. Biotherapy. 17:26–31. 2003.
|