Introduction
Although equal segregation of chromosomes to the
post-mitotic daughter cells is ensured by the centrosome through
the organization of the bipolar mitotic spindle during normal cell
proliferation, multipolar mitotic spindles and a wide variety of
centrosomal anomalies are frequently observed in cancer cells
(1). The disruption of normal
chromosomal segregation may be induced by such abnormalities,
resulting in the production of aneuploid cells. The precise
molecular mechanisms associated with the segregation of chromosomes
have yet to be adequately characterized. However, a number of genes
involved in regulating centrosome duplication have been cloned and
intensively analyzed, including family members of mammalian Aurora
homologues (2).
Aurora-A is a serine/threonine protein kinase that
belongs to the Drosophila aurora and Saccharomyces
cerevisiae Ip11 kinase family and has been shown to play an
essential role in chromosome segregation and centrosome functions
(3). Aurora-A has attracted special
interest due to the localization on chromosome 20q13, a region
frequently amplified in various types of human cancer specimens
(4,5). Furthermore, results of previous
studies showed that the ectopic expression of Aurora-A in mouse
NIH/3T3 cells and Rat 1 fibroblasts causes centrosome amplification
and transformation in vitro as well as tumorigenesis in
vivo (6,7). Collectively, these findings suggested
that, if overexpressed, Aurora-A plays a role as an oncogene
through the abnormal regulation of centrosome function.
Renal cell carcinoma (RCC) is the most common
malignancy of the adult kidney and annual estimates of newly
diagnosed cases have been steadily increasing. RCC is characterized
by various features that differ from those of other malignancies
(8). In the field of RCC research,
investigators showed the potential roles of centrosomal defects and
chromosomal instability in disease progression (9). Previously, Aurora-A expression in RCC
specimens, which was markedly elevated as compared to that found in
normal kidney tissue, was found to be significantly associated with
tumor grade and cell proliferative potential (10). Thus, clarifying the role of Aurora-A
during the progression of RCC is crucial. In the present study, we
analyzed the changes in the phenotypes of human RCC cells both
in vitro and in vivo following the RNA
interference-mediated knockdown of the Aurora-A protein.
Materials and methods
Materials
Caki-2 cells derived from human RCC were purchased
from the American Type Culture Collection (Rockville, MD, USA).
Cells were maintained in RPMI (Life Technologies, Gaithersburg, MD,
USA) supplemented with 10% heat-inactivated fetal bovine serum.
A chemically synthesized oligonucleotide encoding an
Aurora-A short hairpin RNA (shRNA), including a loop motif, was
inserted downstream of the U6 promoter of the pBAsi hU6 Pur DNA
vector (Takara Bio, Otsu, Japan). The sequence of the shRNA
targeted against Aurora-A was 5′-ATGCCCTGTCTTACTGTCA-3′,
corresponding to positions 853-871 within the Aurora-A mRNA
sequence. Similarly, a control plasmid was constructed by
randomizing the sequence of shRNA (5′-TCTTAATCGCGTATAAGGC-3′).
Expression vectors were transfected into Caki-2
cells by liposome-mediated gene transfer methods as previously
described (11). Briefly, either
purified shRNA targeting Aurora-A cloned into the expression
plasmid or control plasmid was added to Caki-2 cells after
pre-incubation for 30 min with Lipofectamine (Invitrogen, San
Diego, CA, USA) and serum-free OPTI-MEM (Life Technologies). Drug
selection in 1.5 μg/ml puromycin (Sigma, St. Louis, MO, USA)
started 3 days after transfection. Colonies were then harvested and
expanded to cell lines.
Real-time RT-PCR
Total RNA (1 μg) extracted from each cell line was
reverse transcribed using an oligo dT and superscript
pre-amplification system (Life Technologies). Real-time RT-PCR was
performed using a sequence detector (Abi Prism 7700; PE Applied
Biosystems, Foster City, CA, USA) based on the Taq Man assay
according to the manufacturer’s instructions, as previously
described (12). The specimens were
analyzed in triplicate and the mean values were used for
quantification. The quantification value of Aurora-A mRNA was
described as each value relative to β-actin mRNA.
Western blot analysis
Western blot analysis was performed as previously
described (11). Briefly, samples
containing equal amounts of protein (15 μg) from the lysates of
cultured cells were electrophoresed on an SDS-polyacrylamide gel
and transferred to a nitrocellulose filter. The filters were
blocked in phosphate-buffered saline (PBS) containing 5% non-fat
milk powder at 4°C overnight and then incubated for 1 h with
antibodies against Aurora-A (TransGenic Inc., Kumamoto, Japan),
total and phosphorylated Akt, PARP (Cell Signaling Technology,
Danvers, MA, USA), p53 (Epitomics Inc., Burlingame, CA, USA) and
β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The
filters were incubated for 30 min with horseradish
peroxidase-conjugated secondary antibodies and specific proteins
were then detected using an enhanced chemiluminescence Western
blotting system (Amersham Pharmacia Biotech, Arlington Heights, IL,
USA).
MTT assay
The inhibitory effects of chemotherapeutic agents on
the in vitro growth of each cell line were assessed using MTT
(Sigma) as previously described (11). Briefly, 1×104 cells were
seeded in each well of 96-well microtiter plates and allowed to
attach overnight. Cells were then treated with various
concentrations of docetaxel, cisplatin, etoposide or doxorubicin.
After 48 h of incubation, 20 μl of 5 mg/ml MTT in PBS was added to
each well, followed by incubation for 4 h at 37°C. Formazan
crystals were dissolved in DMSO. The optical density was determined
with a microculture plate reader (Becton Dickinson Labware, Lincoln
Park, NJ, USA) at an absorbance of 540 nm. Absorbance values were
normalized to the values obtained for the vehicle-treated cells to
determine the percentage of surviving cells. Each assay was
performed in triplicate.
Flow cytometric analysis and DNA
degradation assay
The cell cycle phase distribution was assessed by a
flow cytometric analysis of DNA content. Cells were fixed in 70%
ethanol, and incubated with 1 μg/ml RNaseA for 30 min. Following
centrifugation, the cells were resuspended in 1 ml of 50 μg/ml
propidium iodide (Sigma) and incubated for 30 min. DNA content was
evaluated using a FACScan flow cytometer and quantified using
CellQuest software (Becton Dickinson). Each experiment was
performed in triplicate.
Nucleosomal DNA degradation was quantified by a Cell
Death Detection ELISA kit using antihistone antibody (Roche,
Mannheim, Germany) as previously described (13). Briefly, 1×105 cells were
seeded in 5-cm culture dishes and allowed to adhere overnight.
Following treatment with docetaxel under the same schedule as
described above, cells were harvested and assays were performed in
triplicate according to the manufacturer’s instructions.
Statistical analysis
Differences between the two groups were compared
using the unpaired t-test. Statistical calculations were performed
using Statview 5.0 software (Abacus Concepts, Inc., Berkley, CA,
USA). P<0.05 was considered to be statistically significant.
Results
Cell lines
Caki-2 cells were transfected with an expression
vector containing the shRNA targeted against Aurora-A or vector
alone. After drug selection, a number of stable independent clones
were established and two of these clones (Caki-2/sh-A#1 and
Caki-2/sh-A#2), in which the vector containing Aurora-A shRNA had
been introduced, and control vector-transfected Caki-2 cells
(Caki-2/C) were selected for further studies.
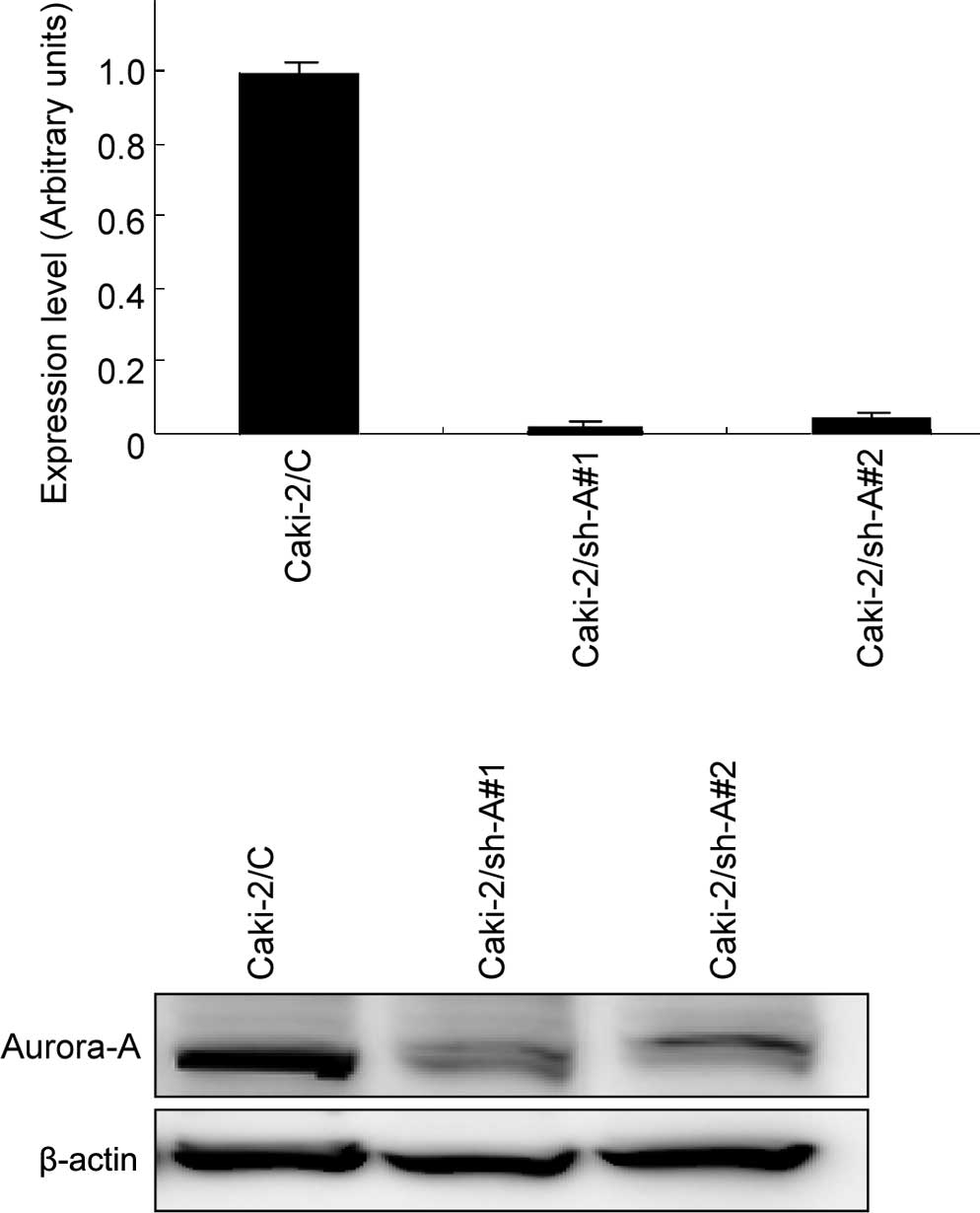
Expression levels of Aurora-A in Caki-2
sublines
Real-time RT-PCR and Western blotting were
subsequently performed to evaluate the expression levels of
Aurora-A mRNA and protein, respectively, in Caki-2/C, Caki-2/sh-A#1
and Caki-2/sh-A#2 cells. As shown in Fig. 1, abundant levels of Aurora-A
expression were observed in Caki-2/C cells at both the mRNA and
protein levels, whereas Aurora-A expression levels in Caki-2/sh-A#1
and Caki-2/sh-A#2 cells were inhibited to <10% compared to those
in Caki-2/C cells.
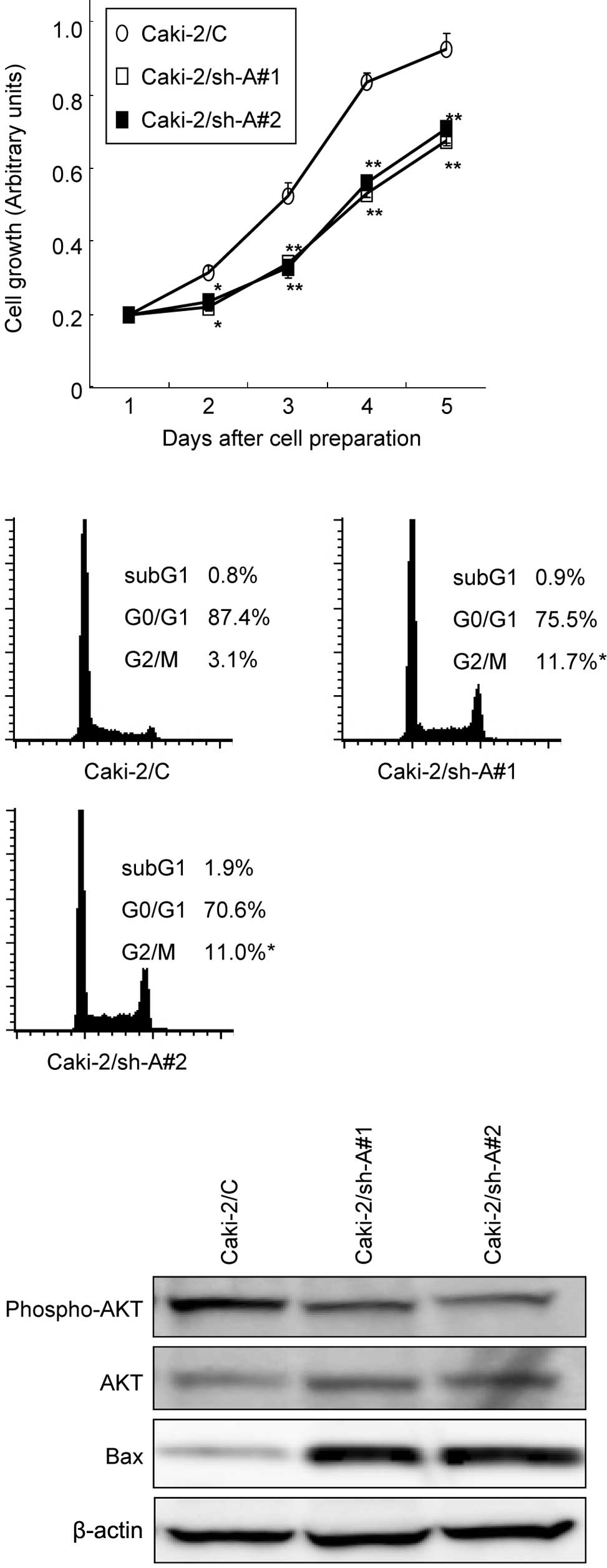
Effect of Aurrora-A suppression on the
growth of Caki-2 sublines
The MTT assay was performed to compare the growth
patterns of the Caki-2 sublines. As shown in Fig. 2A, the growth of Caki-2/sh-A#1 and
Caki-2/sh-A#2 cells was significantly inhibited compared to that of
Caki-2/C cells, showing growth inhibition by ~40% on day 5. We then
investigated the cell cycle distributions in Caki-2 cell sublines
by flow cytometry. The proportions of Caki-2/sh-A#1 and
Caki-2/sh-A#2 cells in the G2-M phase were significantly greater
than those of Caki-2/C cells (Fig.
2B).
Western blot analysis was used to determine whether
the decrease in Aurora-A production by Caki-2 cells modulates the
expression levels of various molecules involved in signal
transduction pathways as well as apoptosis. Of these molecules, the
expression levels of phosphorylated Akt in Caki-2/sh-A#1 and
Caki-2/sh-A#2 cells were markedly down-regulated compared to those
in Caki-2/C cells, while the Bax expression levels in Caki-2/sh-A#1
and Caki-2/sh-A#2 cells were significantly higher than those in
Caki-2/C cells (Fig. 2C).
To determine whether the inhibition of Aurora-A
expression results in enhanced chemosensitivity in RCC, the Caki-2
sublines were treated with various doses of chemotherapeutic
agents, such as docetaxel, cisplatin, etoposide and doxorubicin.
Despite the lack of significant differences in sensitivities to
cisplatin and doxorubicin in the Caki-2 sublines, Caki-2/sh-A#1 and
Caki-2/sh-A#2 cells were more sensitive to docetaxel and etoposide
than Caki-2/C cells. In other words, the IC50 value of
docetaxel and that of etoposide in both Caki-2/sh-A#1 and
Caki-2/sh-A#2 cells were reduced by ~90 and 50%, respectively, as
compared to those in Caki-2/C cells (data not shown). Accordingly,
docetaxel, which was shown to have the most powerful cytotoxic
effect on Caki-2/sh-A#1 and Caki-2/sh-A#2 cells, was used in the
subsequent study associated with chemosensitivity.
Effect of Aurora-A suppression on the
sensitivity of Caki-2 sublines to docetaxel
As shown in Fig. 3A,
docetaxel showed dose-dependent cytotoxicity in all Caki-2
sublines. However, the growth of Caki-2/sh-A#1 and Caki-2/sh-A#2
cells was significantly inhibited by docetaxel relative to the
Caki-2/C cells in a dose-dependent manner. Furthermore, apoptotic
changes in the Caki-2 sublines following treatment with various
concentrations of docetaxel were investigated. The proportions of
cells undergoing apoptotic cell death in Caki-2/sh-A#1 and
Caki-2sh-A#2 cells were significantly greater compared to those in
Caki-2/C cells (Fig. 3B). In
addition, treatment of Caki-2/sh-A#1 and Caki-2sh-A#2 cells with
docetaxel resulted in the induction of p53 and the cleavage of
PARP, but not of Caki-2/C cells (Fig.
3C).
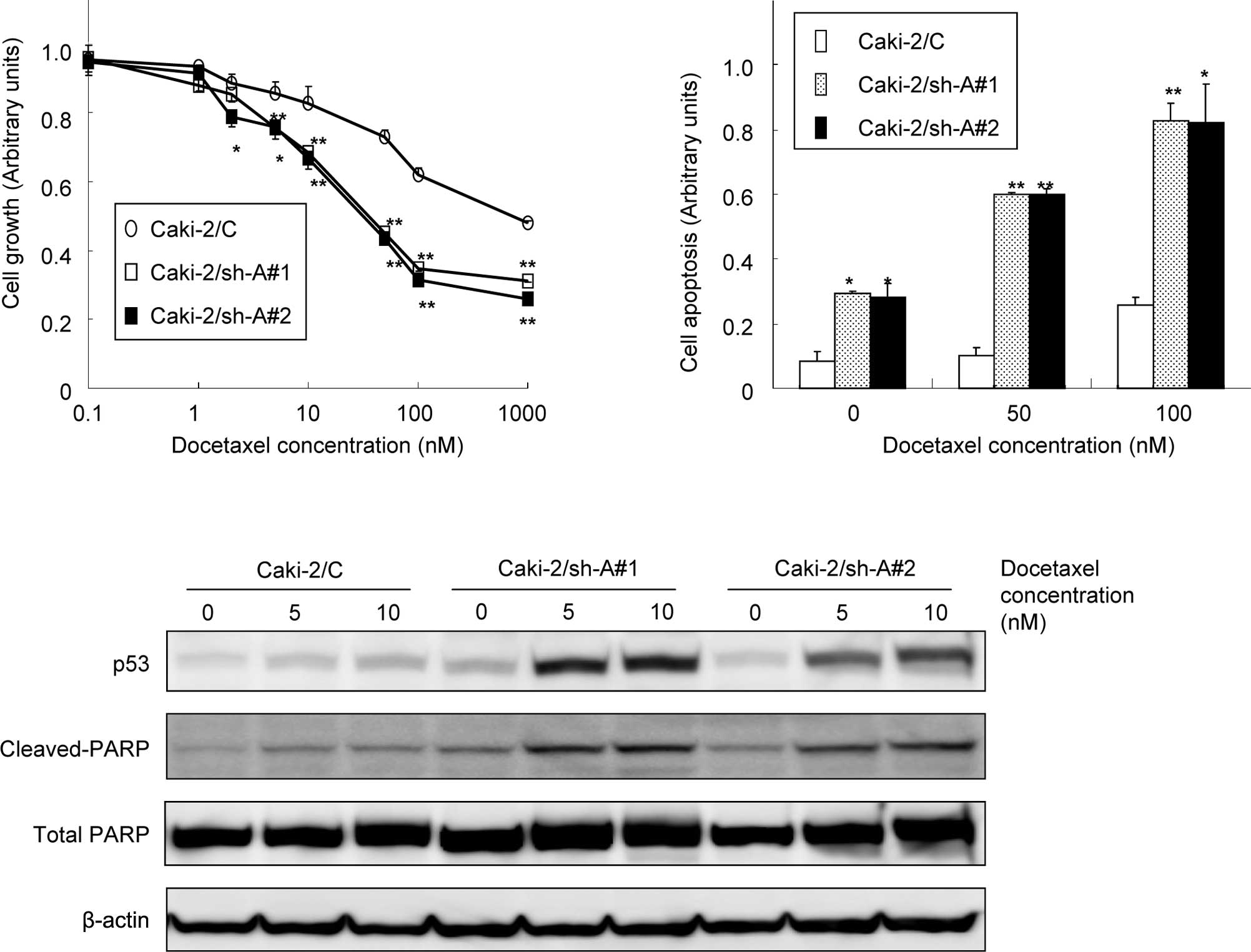 | Figure 3Effect of Aurora-A suppression on the
sensitivity of Caki-2 sublines to docetaxel. (A) Caki-2 sublines
(Caki-2/C, control vector-only transfected cell line; Caki-2/sh-A#1
and Caki-2/sh-A#2, Aurora-A short hairpin RNA-transfected cell
lines) were treated with the indicated doses of docetaxel. After 48
h of incubation, cell growth was determined in triplicate by MTT
assay. Bars, SD. ** and *, significantly
different from control (p<0.01 and p<0.05, respectively). (B)
Caki-2 sublines were treated with the indicated doses of docetaxel.
After 48 h of incubation, cell apoptosis was determined in
triplicate cultures by an ELISA kit using antihistone antibody.
Bars, SD. ** and *, significantly different
from control (p<0.01 and p<0.05, respectively). (C) Proteins
were extracted from the Caki-2 sublines treated with 5 and 10 nM of
docetaxel for 48 h. Then, p53 and both total and cleaved PARP
protein levels were analyzed by Western blotting. |
Discussion
Considering the frequent observation of chromosomal
abnormalities in human cancer cells, malignant disease progression
may be associated with changes in the phenotype induced by genes
involved in chromosomal segregation (1). Among such genes, Aurora-A has
attracted great interest, since various studies have shown that the
overexpression of Aurora-A induces disruption of normal cell cycle
progression, resulting in the promotion of oncogenic transformation
(2,3,6,7).
Furthermore, the up-regulation of Aurora-A expression and its
significant correlation to conventional prognostic factors in a
variety of human malignancies has been demonstrated (3,14,15).
We also previously reported that the expression of Aurora-A in RCC
tissue is significantly stronger than that in normal kidney tissue,
and that the Aurora-A expression level in RCC appears to be
significantly correlated to tumor grade and cell proliferative
activity (10). However, the role
of Aurora-A during the progression of RCC has yet to be determined.
Subsequently, this study investigated the effect of suppressed
Aurora-A expression in human RCC Caki-2 cells on changes in their
growth and chemosensitivity.
Initially, Caki-2 sublines were established in which
Aurora-A expression was decreased by approximately 90% as compared
to that in control Caki-2 cells by introducing an expression
plasmid containing shRNA targeted against Aurora-A. Using the
Caki-2 sublines, it has subsequently been shown that the
suppression of Aurora-A causes a significant accumulation of cells
in the G2-M phase, resulting in the marked inhibition of Caki-2
cell proliferation. These findings suggest that Aurora-A is an
essential molecule for regulating the growth of RCC cells. Thus,
Aurora-A may be a suitable molecular target for the inhibition of
cell proliferation in RCC.
Of note is the mechanism involved in
Aurora-A-mediated growth regulation in Caki-2 sublines. Of several
molecules implicated in major signal transduction pathways, Akt
pathway appeared to be markedly inactivated by the down-regulation
of Aurora-A in Caki-2 cells. We showed a significant induction of
Bax protein in Caki-2 cells by suppressing the expression of
Aurora-A. These findings have been supported by various previous
studies showing activation of the Akt pathway characterized by the
exertion of an anti-apoptotic impact on various types of cancer
cells (16,17). In their study, Cha et al
reported treatment with histone deacetylase inhibitor resulting in
the induction of G2-M arrest and apoptosis through the degradation
of Aurora-A (18).
Changes in chemosensitivity following the
down-regulation of Aurora-A expression in Caki-2 cells were then
investigated. Of several chemotherapeutic agents examined in this
study, docetaxel has shown the most potent cytotoxic effect on
Aurora-A shRNA-transfected Caki-2 cells by reducing the
IC50 value by approximately 90%. Furthermore, the degree
of chemosensitivity to docetaxel in the Caki-2 sublines is closely
proportional to that of cells undergoing apoptosis, accompanying
the induction of p53 and the cleavage of PARP. Taxanes, including
docetaxel, have been shown to stabilize microtubules by binding
tubulin and to interfere with microtubule disassembly. In other
words, these agents are capable of stopping cell cycle progression,
causing cells in the M phase to accumulate at the
metaphase-anaphase transition and subsequently promote apoptosis
(19). Taken together, these
findings indicate that the synergistic induction of apoptosis in
cancer cells may be achieved by the suppression of Aurora-A
expression combined with the use of docetaxel. Similarly, the
enhancement of docetaxel-induced apoptosis by the inhibition of
Aurora-A expression using a variety of cancer cell lines, such as
head and neck, esophageal and breast cancer cell lines has also
been reported (20–22).
In conclusion, the suppression of Aurora-A
expression by human RCC Caki-2 cells using shRNA inhibits their
growth through the modulation of signal transduction and apoptotic
pathways. Additionally, a synergistic cytotoxic effect is achieved
in Caki-2 cells by combining the suppression of Aurora-A expression
with docetaxel treatment. Collectively, these findings suggest that
targeting Aurora-A using RNA- interfering technology in combination
with docetaxel is a novel promising strategy for the treatment of
patients with advanced RCC.
References
|
1
|
Nigg EA: Mitotic kinases as regulators of
cell division and its checkpoints. Nat Rev Mol Cell Biol. 2:21–32.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carmena M and Earnshaw WC: The cellular
geography of aurora kinase. Nat Rev Mol Cell Biol. 4:842–854. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Warner SL, Bearss DJ, Han H and von Hoff
DD: Targeting aurora-2 kinase in cancer. Mol Cancer Ther.
2:589–595. 2003.PubMed/NCBI
|
|
4
|
Fukushige S, Waldman FM, Kimura M, Abe T,
Furukawa T, Sunamura M, Kobari M and Horii A: Frequent gain of copy
number on the long arm of chromosome 20 in human pancreatic
adenocarcinoma. Genes Chromosomes Cancer. 19:161–169. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Isola JJ, Kallioniemi OP, Chu LW, Fuqua
SA, Hilsenbeck SG, Osborne CK and Waldman FM: Genetic aberrations
detected by comparative genomic hybridization predict outcome in
node-negative breast cancer. Am J Pathol. 147:905–911.
1995.PubMed/NCBI
|
|
6
|
Zhou H, Kuang J, Zhong L, Kuo WL, Gray JW,
Sahin A, Brinkley BR and Sen S: Tumor amplification kinase
STK15/BTAK induces centrosome amplification, aneuploidy and
transformation. Nat Genet. 20:189–193. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bischoff JR, Anderson L, Zhu Y, Mossie K,
Ng L, Souza B, Schryver B, Flanagan P, Clairvoyant F, Ginther C,
Chan CS, Novotny M, Slamon DJ and Plowman GD: A homologue of
Drosophila aurora kinase is oncogenic and amplifies in human
colorectal cancers. EMBO J. 17:3052–3065. 1998.PubMed/NCBI
|
|
8
|
Drucker BJ: Renal cell carcinoma: current
status and future prospects. Cancer Treat Rev. 31:536–545. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bodmer D, van den Hurk W, van Groningen
JJ, Eleveld MJ, Martens GJ, Weterman MA and van Kessel AG:
Understanding familial and non-familial renal cell cancer. Hum Mol
Genet. 11:2489–2498. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kurahashi T, Miyake H, Hara I and Fujisawa
M: Significance of Aurora-A expression in renal cell carcinoma.
Urol Oncol. 25:128–133. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miyake H, Nelson C, Rennie PS and Gleave
ME: Overexpression of insulin-like growth factor binding protein-5
helps accelerate progression to androgen-independence in the human
prostate LNCaP tumor model through activation of
phosphatidylinositol 3′-kinase pathway. Endocrinology.
141:2257–2265. 2000.PubMed/NCBI
|
|
12
|
Miyake H, Hara I, Kurahashi T, Inoue TA,
Eto H and Fujisawa M: Quantitative detection of micrometastases in
pelvic lymph nodes in patients with clinically localized prostate
cancer by real-time reverse transcriptase-PCR. Clin Cancer Res.
13:1192–1197. 2007. View Article : Google Scholar
|
|
13
|
Miyake H, Yamanaka K, Muramaki M, Hara I
and Gleave ME: Therapeutic efficacy of adenoviral-mediated p53 gene
transfer is synergistically enhanced by combined use of antisense
oligodeoxynucleotide targeting clusterin gene in a human bladder
cancer model. Neoplasia. 7:171–179. 2005. View Article : Google Scholar
|
|
14
|
Tanaka E, Hashimoto Y, Ito T, Okumura T,
Kan T, Watanabe G, Imamura M, Inazawa J and Shimada Y: The clinical
significance of Aurora-A/STK15/BTAK expression in human esophageal
squamous cell carcinoma. Clin Cancer Res. 11:1827–1834. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gritsko TM, Coppola D, Paciga JE, Yang L,
Sun M, Shelley SA, Fiorica JV, Nicosia SV and Cheng JQ: Activation
and overexpression of centrosome kinase BTAK/Aurora-A in human
ovarian cancer. Clin Cancer Res. 9:1420–1426. 2003.PubMed/NCBI
|
|
16
|
Guan Z, Wang XR, Zhu XF, Huang XF, Xu J,
Wang LH, Wan XB, Long ZJ, Liu JN, Feng GK, Huang W, Zeng YX, Chen
FJ and Liu Q: Aurora-A, a negative prognostic marker, increases
migration and decreases radiosensitivity in cancer cells. Cancer
Res. 67:10436–10444. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu X, Shi Y, Woods KW, Hessler P, Kroeger
P, Wilsbacher J, Wang J, Wang JY, Li C, Li Q, Rosenberg SH, Giranda
VL and Luo Y: Akt inhibitor a-443654 interferes with mitotic
progression by regulating aurora a kinase expression. Neoplasia.
10:828–837. 2008.PubMed/NCBI
|
|
18
|
Cha TL, Chuang MJ, Wu ST, Sun GH, Chang
SY, Yu DS, Huang SM, Huan SK, Cheng TC, Chen TT, Fan PL and Hsiao
PW: Dual degradation of aurora A and B kinases by the histone
deacetylase inhibitor LBH589 induces G2-M arrest and apoptosis of
renal cancer cells. Clin Cancer Res. 15:840–850. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou J and Giannakakou P: Targeting
microtubules for cancer chemotherapy. Current Med Chem. 5:665–671.
2005.
|
|
20
|
Mazumdar A, Henderson YC, El-Naggar AK,
Sen S and Clayman GL: Aurora kinase A inhibition and paclitaxel as
targeted combination therapy for head and neck squamous cell
carcinoma. Head Neck. 31:625–634. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tanaka E, Hashimoto Y, Ito T, Kondo K,
Higashiyama M, Tsunoda S, Ortiz C, Sakai Y, Inazawa J and Shimada
Y: The suppression of aurora-A/STK15/BTAK expression enhances
chemosensitivity to docetaxel in human esophageal squamous cell
carcinoma. Clin Cancer Res. 13:1331–1340. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee HH, Zhu Y, Govindasamy KM and Gopalan
G: Downregulation of Aurora-A overrides estrogen-mediated growth
and chemoresistance in breast cancer cells. Endocr Relat Cancer.
15:765–775. 2008. View Article : Google Scholar : PubMed/NCBI
|