Introduction
Foxp3 is an X-linked nuclear transcription factor
essential to the development and program of T-regulatory cells
(Tregs) that protect against the development of autoimmunity
(1,2). The tumor suppressor activity of Foxp3
was identified when it was serendipitously observed that female
mice heterozygous for the mutation in the X-linked scurfin gene
(homolog to human Foxp3) have high rates of mammary cancer as well
as other types of cancers (3).
Additional studies confirmed a relationship between mutations of
Foxp3 and breast cancer in humans, and identified Foxp3-mediated
repression of HER-2/ErbB2 and SKP2 oncogenes as a potential
mechanism for the tumor suppressor effect (3,4).
Although Tregs suppress the development of autoimmunity, they are
thought to be permissive for the development of cancer (5,6). Thus,
Foxp3 has dual but opposing roles with respect to autoimmunity and
cancer. Tregs and Foxp3 have become significant therapeutic targets
in autoimmunity and cancer. Defining Foxp3 pathways responsible for
its divergent functions should be helpful in the design of
therapies that specifically target Foxp3. Development of the Treg
function requires transcriptional activation and binding of Foxp3
to the transcription factor, NFAT (7). In this study, Foxp3 was found to
exhibit tumor suppressor activity independent of transcriptional
activation and binding NFAT.
Materials and methods
Cell lines
Human cancer cell lines Skov3 (ovarian), MDA-MB-231
(breast), MCF-7 (breast) and Jurkat (T cells) were obtained from
the American Type Tissue Collection (Rockville, MD, USA).
Antibodies
The antibodies used included mouse monoclonal IgG1
to human Foxp3 (sc-56680) and mouse anti-goat IgG-HRP (sc-2354)
(Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Plasmid constructs
Human Foxp3 cDNA was amplified by PCR from plasmid
13250 (Addgene, Cambridge, MA, USA) and ligated into
pIRES2-ZsGreen1 (Clontech, Mountain View, CA, USA) to
produce the plasmid pIRES2-ZsGreen1-Foxp3,
pIRES2-ZsGreen1-Foxp3C (C-terminal deletion of residues
328–431) and pIRES2-ZsGreen1-Foxp3N (N-terminal deletion of
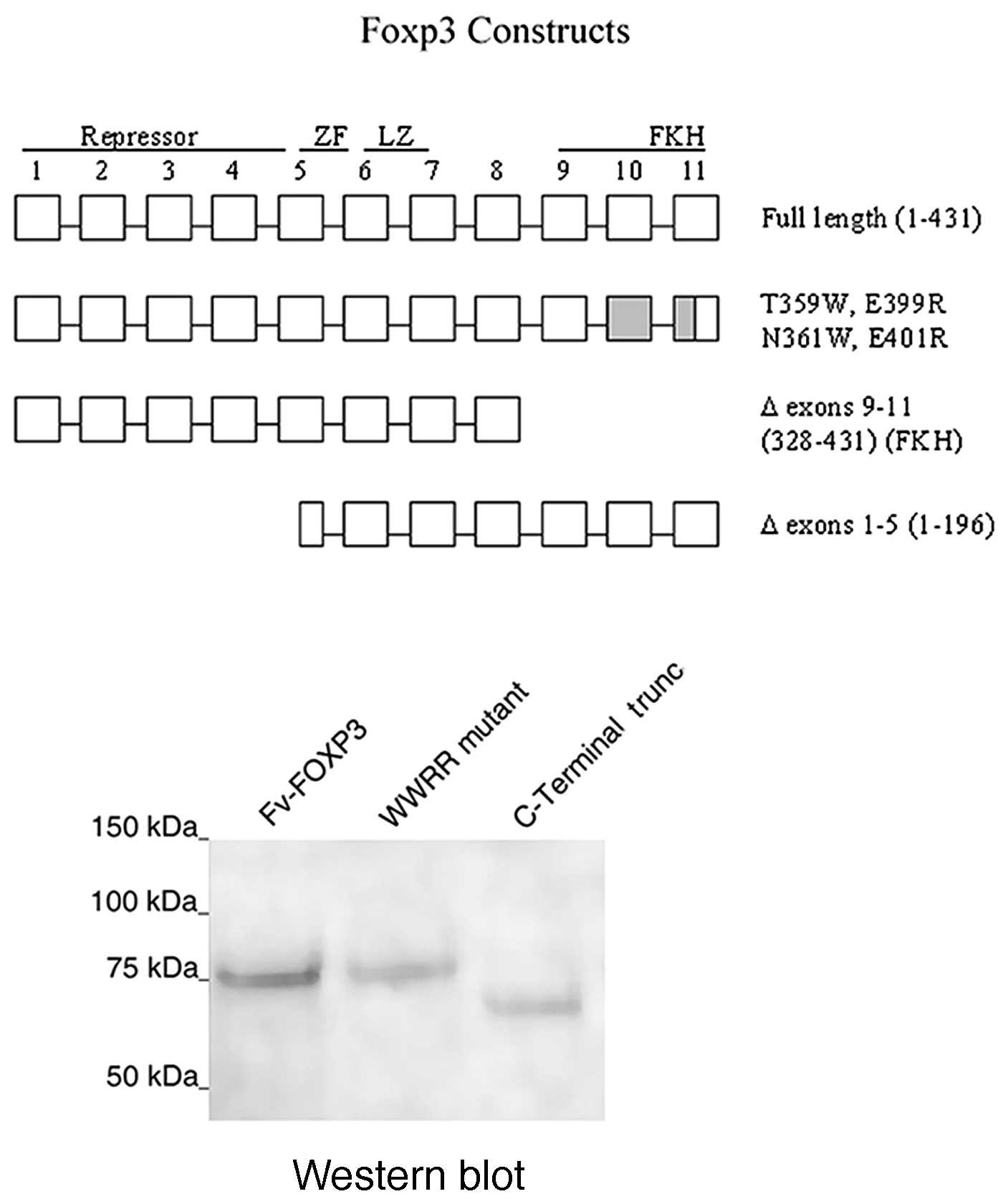
residues 1–196) for direct transfection into cancer cells (Fig. 1A). Molecular fusions of mAb 3E10 Fv
were also generated as pPicZA-Fv-Foxp3,
pPicZA-Fv-Foxp3-WWRR (mutations T359W, N361W, E399 and E401R
of Foxp3), pPicZA-Fv-Foxp3C (C-terminal deletion of residues
328-431) and pPicZA-Fv-Foxp3N (N-terminal deletion of
residues 1–196) (Fig. 1A).
Recombinant Foxp3 mutants were constructed using a QuikChange II
site-directed mutagenesis kit (Stratagene, TX, USA). Constructs
were confirmed by DNA sequencing.
Expression and purification of Foxp3 and
Fv-Foxp3 recombinant proteins
Recombinant proteins were expressed in X-33 cells,
lysed by passage through a French Cell Press, and purified by
immobilized metal ion affinity chromatography (IMAC) on Ni-NTA
Agarose (Qiagen, Valencia, CA, USA) as previously described
(8). Eluted protein was
concentrated to 10 μg/ml, reconstituted with fetal calf serum (FCS)
to 5%, and, the protein was exchanged dialyzed 100-fold in 30,000
MWCO spin filters (Millipore Corp., Billerica, MA, USA) against
McCoy’s medium (Mediatech, Inc., Herndon, VA, USA) containing 5%
glycerol.
Cytotoxicity of Foxp3 constructs in
vitro
Foxp3 and Foxp3 mutant cDNA were ligated into the
pIRES2-ZsGreen1 expression vector (Clontech, Mountain View,
CA, USA) that co-expresses a green fluorescent protein with the
cloned insert. Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA)
was used according to the manufacturer’s protocol for plasmid
transfection into Skov-3 cells. Following transfection, Skov-3
cells were incubated for 7 days at 37°C in 24-well plates.
Fluorescent cells represent cells expressing Foxp3. Subsequently,
fluorescent cells were examined for the number of dead vs. viable
cells. One hundred transfected cells were counted in each of the
triplicate wells, and the results were expressed as the mean
percent ± SD (standard deviation). In studies of antibody-mediated
protein transduction, cancer cells in the medium containing 10% FCS
were added to each of the multiple wells in 96-well plates and
grown to 40% confluence overnight in the presence of 5%
CO2. Fv-Foxp3 recombinant proteins were added undiluted
(100 nmol/l) to duplicate wells and in serial dilutions with the
X-33 lysate as a control. Cell death was measured by the nuclear
uptake of propidium iodide, and the results were expressed as the
mean percent ± SD by counting 100 cells in 3 separate areas of
duplicate wells.
Statistical analysis
A p-value was determined by a two-tailed Student’s
t-test for non-paired samples assuming equal variances.
Results
Recombinant Fv-Foxp3 proteins
To determine whether Foxp3-mediated cytotoxicity
requires NFAT, we produced and purified Foxp3 constructs shown to
eliminate NFAT binding. In their study, Wu et al previously
showed that the combination of selected mutations of Foxp3
C-terminal forkhead domain (FKH), T359W, N361W, E399 and E401R
(WWRR) prevented NFAT binding (7).
We produced Foxp3, Foxp3 WWRR mutant, and Foxp3 with C-terminal
deletion of the entire FKH (Δ aa 328–431) as molecular fusion
proteins with mAb 3E10 cell-penetrating Fv fragment (Fig. 1A). The FKH is involved in DNA
binding and nuclear localization, and enables transcriptional
activation or repression (9). A
Western blot analyis of the purified proteins developed with
antibodies to Foxp3 showed recombinant proteins of the expected
sizes (Fig. 1B).
Foxp3 cytotoxicity independent of
NFAT
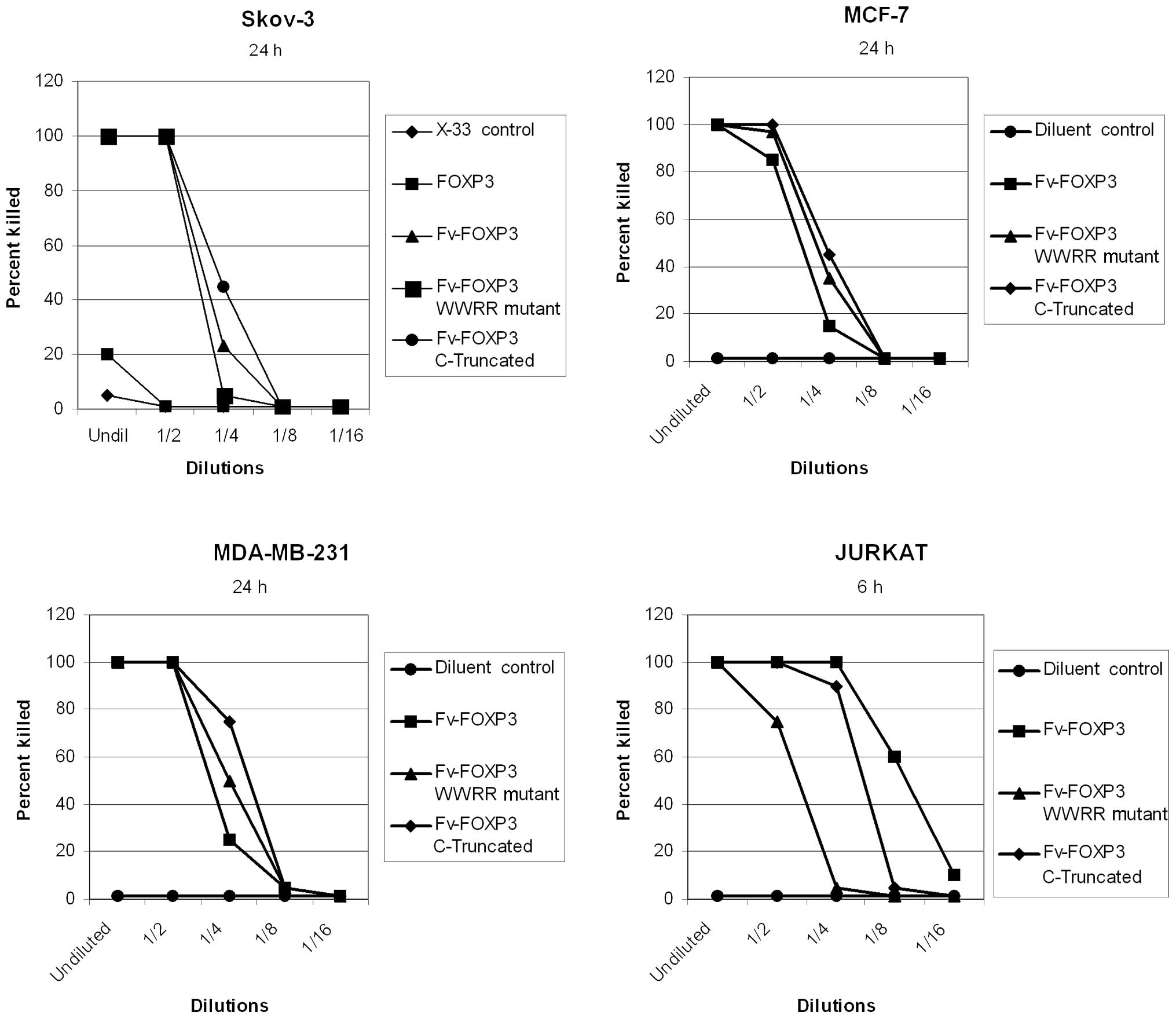
Cytotoxicity of Foxp3 in cancer cells was previously
demonstrated by antibody- mediated protein transduction (8,10,11).
In the present study, Fv-Foxp3 was cytotoxic in four cancer cell
lines, but Foxp3 alone and the X-33 cell lysate as controls were
not cytotoxic (Fig. 2). However,
Fv-Foxp3 with WWRR mutations that prevent NFAT binding was also
cytotoxic in these cancer cell lines (Fig. 2). Moreover, deletion of the entire
Foxp3 FKH failed to eliminate Foxp3 cytotoxicity in cancer cells
(Fig. 2). These results were
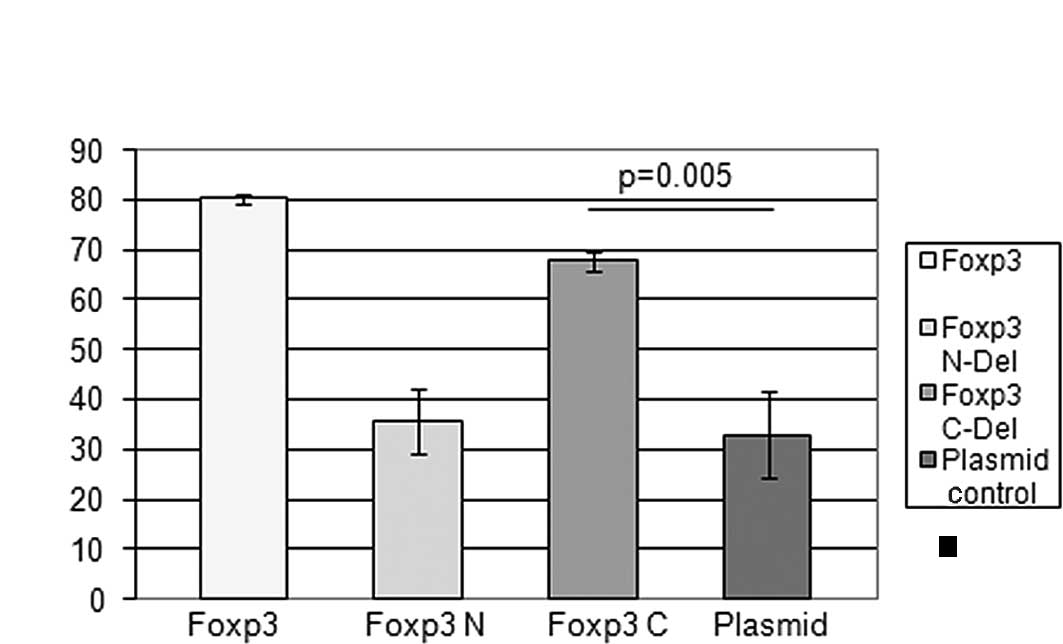
confirmed by the direct transfection of Foxp3 cDNA into Skov-3
cells. Both full-length and C-truncated Foxp3 were cytotoxic
(Fig. 3). However, an N-terminal
deletion mutant of Foxp3 (Fig. 1A)
completely abrogated Foxp3 cytotoxicity, indicating that the
N-terminus of Foxp3 is required for Foxp3 tumor suppressor activity
(Fig. 3).
Discussion
Transcription factors form large complexes of
interacting proteins that regulate gene activation pathways.
Dissecting functional activation pathways is crucial to the design
of therapeutic agents that target transcription factors. Tregs and
Foxp3 have become significant therapeutic targets both in
autoimmunity and cancer (13).
However, inhibiting Treg function to enhance tumor rejection
carries risks associated with developing autoimmunity. On the other
hand, enhancing Treg function to control autoimmunity may
predispose to the development of cancer. More studies are therefore
required to better define the divergent signaling pathways of
Foxp3, thereby aiding in the design of target-specific
therapies.
We have investigated the use of a cell-penetrating
antibody, mAb 3E10, and its Fv fragment as an intracellular and
intranuclear delivery vehicle for the delivery of transcription
factor proteins, such as p53 and Foxp3 (8,12). The
up-regulation of Foxp3 has been shown to be cytotoxic in cancer
cells in vitro and in vivo (3,8).
However, it is of concern that up-regulating Foxp3 may enhance Treg
function in vivo, thereby abrogating its clinical
effectiveness as a tumor suppressor in vivo. The results of
our study show that Foxp3 cytotoxicity can be separated from its
effect on Treg cell function by eliminating NFAT binding. Although
the repressor and FKHs of Foxp3 are critical for its Treg function
(14), our study shows that
cytotoxic activity is preserved in the absence of the FKH. Previous
studies identified HER-2 and SKP2 oncogenes as
potential targets suppressed by Foxp3 (3,4). Our
unexpected finding that the N-terminal, but not the C-terminal,
deleted Foxp3 results in loss of cytotoxicity indicates that there
may be additional mechanisms for Foxp3 cytotoxicity independent of
the FKH. This is not unprecedented, as the p53 tumor suppressor has
been shown to mediate apoptosis by both transcription-dependent and
-independent pathways (15,16). In our study, the role of Foxp3
N-terminus in cellular cytotoxicity may be related to its potential
binding to a number of auxiliary transcription factors or
chromatin-modifying proteins (17).
Further studies are required to define the role of N-terminal Foxp3
in cell toxicity. However, our finding that the Treg and tumor
suppressor functions of Foxp3 are mediated through separate
signaling pathways enables us to develop methods for the
up-regulation of Foxp3-induced cancer cell cytotoxicity without
enhancing Treg function.
Acknowledgements
This study was supported by a grant from the
Veterans Affairs (RHW).
Abbreviations:
References
|
1
|
Hori S, Nomura T and Sakaguchi S: Control
of regulatory T cell development by the transcription factor Foxp3.
Science. 299:1030–1031. 2003. View Article : Google Scholar
|
|
2
|
Schubert LA, Jeffery E, Zhang Y, Ramsdell
F and Ziegler SF: Scurfin (FOXP3) acts as a repressor of
transcription and regulates T cell activation. J Biol Chem.
276:37672–37679. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zuo T, Wang L, Morrison C, Chang X, Zhang
H, Li W and Liu Y, Wang Y, Liu X, Chan MW, Liu JQ, Love R, Liu CG,
Godfrey V, Shen R, Huang TH, Yang T, Park BK, Wang CY, Zheng P and
Liu Y: FOXP3 is an X-linked breast cancer suppressor gene and an
important repressor of HER-2/ErbB2 oncogene. Cell. 129:1275–1286.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zuo T, Liu R, Zhang H, Chang X and Liu Y,
Wang L, Zheng P and Liu Y: FOXP3 is a novel transcriptional
repressor for the breast cancer oncogene SKP2. J Clin Invest.
117:3765–3773. 2007.PubMed/NCBI
|
|
5
|
Kim J, Lahl K, Hori S, Loddenkemper C,
Chardhry A, deRoos P, Rudensky A and Sparwasser T: Depletion of
Foxp3+ cells leads to induction of autoimmunity by
specific ablation of regulatory T cells in genetically targeted
mice. J Immunol. 183:7631–7634. 2009.PubMed/NCBI
|
|
6
|
Hinz S, Pagerols-Raluy L, Oberg H,
Ammerpohl O, Grüssel S, Sipos B, Grützmann R, Pilarsky C,
Ungefroren H, Saeger H, Klöppel G, Kabelitz D and Kalthoff H: Foxp3
expression in pancreatic carcinoma cells as a novel mechanism of
immune evasion in cancer. Can Res. 67:8344–8350. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu Y, Borde M, Heissmeyer V, Feuerer M,
Lapan AD, Stroud JC, Bates DL, Guo L, Han A, Ziegler SF, Mathis D,
Benoist C, Chen L and Rao A: FOXP3 controls regulatory T cell
function through cooperation with NFAT. Cell. 126:375–387. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Heinze E, Baldwin S, Chan G, Hansen J,
Song J, Clements D, Aragon R, Nishimura R, Reeves M and Weisbart R:
Antibody-mediated protein therapy induces apoptosis in cancer cells
in vitro and inhibits metastasis in vivo. Int J
Oncol. 35:167–173. 2009.PubMed/NCBI
|
|
9
|
Ziegler SF: FOXP3: Of mice and men. Annu
Rev Immunol. 24:209–226. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weisbart RH, Stempniak M, Harris S, Zack
DJ and Ferreri K: An autoantibody is modified for use as a delivery
system to target the cell nucleus: Therapeutic implications. J
Autoimmun. 11:539–546. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hansen JE, Tse CM, Chan G, Heinze ER,
Nishimura RN and Weisbart RH: Intranuclear protein transduction
through a nucleoside salvage pathway. J Biol Chem. 282:20790–20793.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hansen JE, Fischer LK, Chan G, Chang SS,
Baldwin SW, Aragon RJ, Carter JJ, Lilly M, Nishimura RN, Reeves ME
and Weisbart RH: Antibody-mediated p53 protein therapy prevents
liver metastasis in vivo. Cancer Res. 67:1769–1774. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Katoh H, Zheng P and Liu Y: Signalling
through FOXP3 as an X-Linked tumor suppressor. Int J Biochem Cell
Biol. 42:1784–1787. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lopes JE, Torgerson TR, Schubert LA,
Anover SD, Ocheltree EL, Ochs HD and Ziegler SF: Analysis of FOXP2
reveals multiple domains required for its function as a
transcriptional repressor. J Immunol. 177:3133–3142. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matas D, Sigal A, Stambolsky P, Milyavsky
M, Weisz L, Schwartz D, Goldfinger N and Rotter V: Integrity of the
N-terminal transcription domain of p53 is required for mutant p53
interference with drug-induced apoptosis. EMBO J. 20:4163–4172.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Haupt S, Berger M, Goldberg Z and Haupt Y:
Apoptosis – the p53 network. J Cell Sci. 116:4077–4085. 2003.
|
|
17
|
Zhou Z, Song X, Li B and Greene MI: FOXP3
and its partners: structural and biochemical insights into the
regulation of FOXP3 activity. Immunol Res. 42:19–28. 2008.
View Article : Google Scholar : PubMed/NCBI
|