Introduction
Fibrous dysplasia (FD) of bone is an osteoblastic
lineage disease that is caused by a post-zygotic activating
mutation in the gene that encodes the α-subunit of the stimulatory
G-protein (1). The mutated
osteoblasts lead to excessive osteoclastic activity and increased
bone resorption in FD, similar to that observed in high-turnover
bone diseases including Paget's disease and osteoporosis, and this
pathological hallmark provides a rationale for the use of
bisphosphonates, such as dronate and alendronate, potent
antiresorptive drugs, in patients with FD (2). Numerous studies have noted that
intravenous pamidronate therapy alone or in combination with oral
alendronate markedly relieved bone pain, improved the radiological
aspects, increased bone density and decreased bone turnover in
children or adults with polyostotic fibrous dysplasia (PFD)
(3–6). A number of studies have documented
that oral alendronate alone had similar positive results in
patients with PFD or McCune-Albright syndrome (MAS) (3,7–10). In
this case report, we describe a 79-year-old postmenopausal female
with PFD, who had severe lower limb deformities and had experienced
multiple sites of chronic bone pain for over 55 years. This patient
demonstrated a significant improvement in pain relief and a
reduction in bone turnover markers following 102 months of daily
oral alendronate treatment alone.
Case report
A female patient had developed a limp in her right
leg at 12 years of age (1943). At the age of 16 (1947), moderate
bone pain had started in her right lower leg, and subsequently,
chronic bone pain developed in her bilateral hip, thigh and left
lower leg. At 27 years of age (1958), she first presented to
Niigata University due to persistent severe bone pain and a
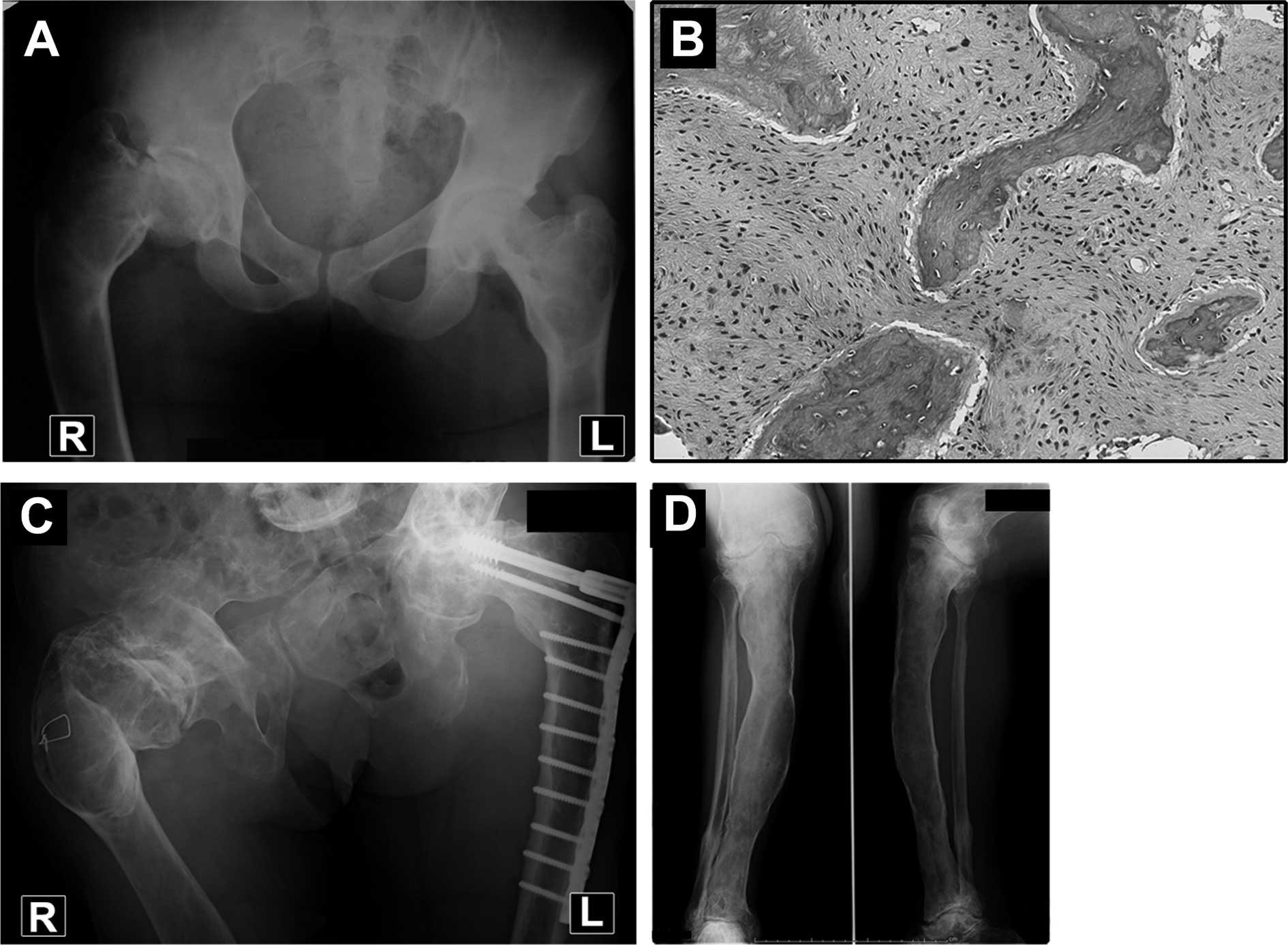
distinct deformity of her right hip (Fig. 1A). She was diagnosed as having PFD
and received a subtrochanteric osteotomy of her right femur. At the
age of 33 (1964), the patient sustained a pathological fracture of
her right proximal femur and was successfully treated by cast
immobilization. At the age of 37 (1969), she experienced another
fracture in the left femoral neck, and this fracture was treated by
nail plate fixation with a fibular bone graft. The pathological
examination of biopsy tissues showed the characteristic pattern of
FD. Mild to moderate (sometimes severe) bone pain in multiple sites
lasted for over 33 years.
In 2001, at the age of 70, the patient developed
dysbasia and severe bone pain again in her left hip that lasted
more than 4 months due to the subcutaneous prominence of the nail
and an incomplete cortical fracture. The original plate was
replaced by an AO condyle dynamic screw plate. Tissue from the
intraoperative biopsy showed an activating mutation of the GNAS1
(Gsα) gene, and there was no maturation in the non-lamellar woven
bone or in the fibrous matrix (Fig.
1B). Following the last surgery the patient was able to walk
outside with a T-cane, although the multiple sites of bone pain
continued to interfere with her daily activities. Since 2002, at 71
years of age, she has been receiving oral alendronate sodium at 5
mg/day 102 months as of the writing of this manuscript). The
changes in bone pain were evaluated using the visual pain analog
scale (0–10) at every visit. Biochemical measurements, including
serum total alkaline phosphatase (ALP), calcium and phosphate, were
performed at approximately 2-month intervals. Serum bone alkaline
phosphatase (BAP) and urine N-terminal cross-linked telopeptide of
type I collagen (NTX) were also examined 79–102 months following
initiation of therapy. Radiographs of the involved bone lesions
were taken at one-year intervals. Changes in the lumber spine
(L2–L4) bone mineral density (BMD) were measured using dual-energy
X-ray absorptiometry (DEXA).
On physical examination prior to alendronate
treatment, there were moderate restrictions of the patient's range
of motion in both sides of her hip joints, but she had no reduction
in muscle force of the extremities. She had tenderness in her
bilateral proximal femora and right tibia. The pain levels were
moderate in the bilateral hips and right lower leg, and were mild
in the left knee and the right ankle (Table I).
 | Table IChanges in pain levels after 102
months of oral alendronate treatment. |
Table I
Changes in pain levels after 102
months of oral alendronate treatment.
| Visual analog pain
scale |
|---|
|
|
|---|
| Sites | Before treatment | Posttreatment |
|---|
| Left hip | 6 | 1 |
| Right hip | 5 | 1 |
| Left knee | 3 | 0 |
| Right lower leg | 5 | 1 |
| Right ankle | 3 | 0 |
Radiological examinations at pretreatment indicated
that the deformity of the right femur and the radiolucent lesions
in multiple sites had progressed compared to her first visit.
Fibrous dysplastic lesions were also detected in her skull, right
first rib, right humerus, right ulna, right hand, pubic bones, left
tibia and right foot by 99mTc-methylene diphosphonate (MDP) bone
scan. The lumber spine BMD value was 0.900 g/cm2
(T-score, −0.4) at pretreatment (Table
II).
 | Table IIChanges in the lumbar spine (L2–L4)
BMD after 89 months of oral alendronate treatment. |
Table II
Changes in the lumbar spine (L2–L4)
BMD after 89 months of oral alendronate treatment.
| Age (years) | BMD
(g/cm2) | Mean T-score | BMD changes vs.
baseline |
|---|
| Pretreatment | 71 | 0.900 | −0.4 | |
| Posttreatment (6
months) | 71 | 0.971 | −0.1 | 7.9% |
| Posttreatment (89
months) | 78 | 1.135 | 1.1 | 26.2% |
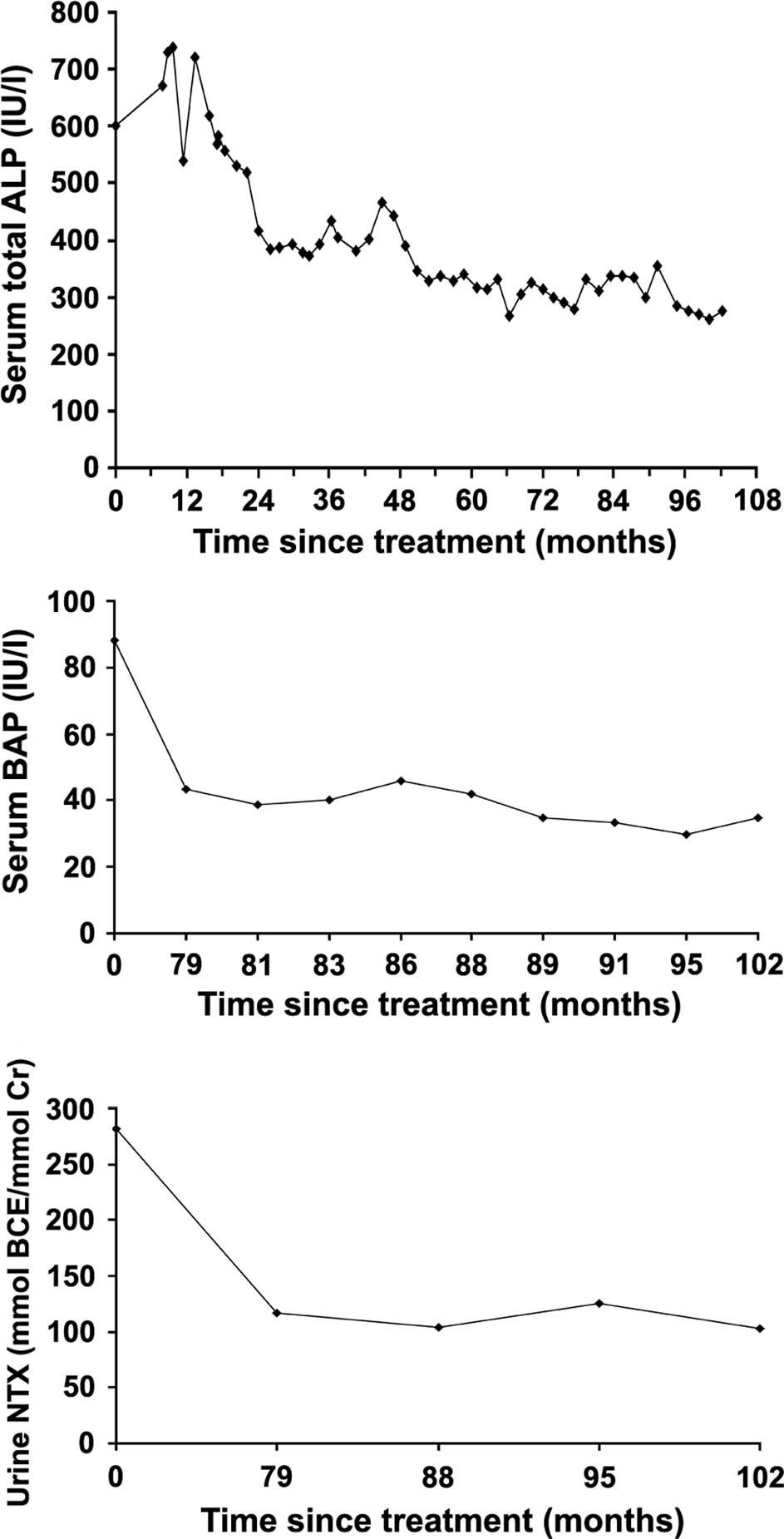
The patient's bone turnover markers prior to
treatment showed high levels of serum ALP, 602 IU/l (reference
range 125–259 by the JSCC method); BAP, 88.3 U/l (reference range
9.6–35.4); and urine NTX, 281.7 nmol BCE/mmol Cr (reference range
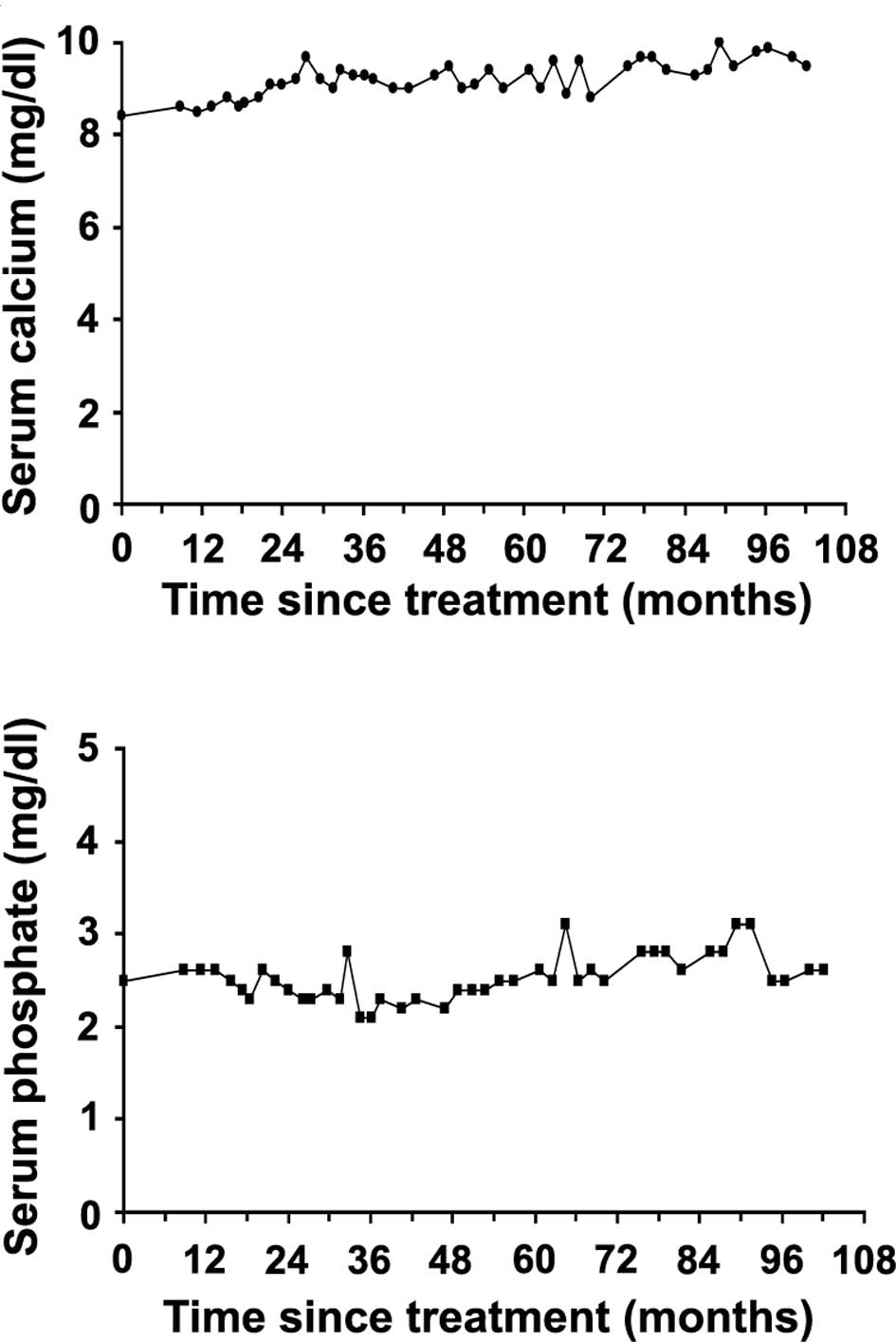
8.3–69.9 nmol BCE/mmol Cr). The levels of serum calcium and serum
phosphate at pretreatment were slightly lower than the normal
range, 8.4 mg/dl (normal range 8.7–10.0 mg/dl) and 2.4 mg/dl
(normal range 2.5–4.6 mg/dl), respectively. No other abnormalities
were found during the biochemical analysis.
Following oral alendronate treatment, the bone pain
in her multiple sites was markedly reduced during the first 8
months, and was then completely relieved or has remained at a mild
level up to the most recent follow-up (Table I). The high pretreatment level of
serum ALP decreased by 24 months after treatment initiation and
then decreased gradually to a near normal level (275 IU/l at the
final examination) (Fig. 2A). The
high pretreatment level of serum BAP was decreased to within the
normal range after 102 months of initiating the treatment (35 U/l
at the final examination) (Fig.
2B). The urine NTX also markedly decreased from a high
pretreatment level to a lower one at 102 months posttreatment
(102.5 nmol BCE/mmol Cr at the final examination) (Fig. 2C). The serum calcium level was
slightly lower than the normal level during the first 17 months of
treatment, but was then maintained consistently at a normal level
(Fig. 3A). The serum phosphate
level oscillated between normal and slightly low levels between 17
and 55 months after treatment was initiated, but remained stable
within the normal range following treatment (Fig. 3B).
Following 89 months of alendronate treatment, the
value of BMD had increased by 26.2% (mean BMD, 1.135
g/cm2; T-score, 1.1) when compared with the pretreatment
value (Table II). Although there
was no clear evidence of cortical thickening or refilling of the
fibrocystic bone lesions during the 102 months of alendronate
treatment (Fig. 1C and D), the
patient has not suffered from any pathological fractures during the
treatment, and is capable of walking outside with a T-cane for
several hours without resting. No adverse drug reactions occurred
following oral alendronate administration, and the medication is
currently being continued.
Discussion
Few case reports exist on the long-term follow-up of
elderly patients with long-standing symptomatic PFD (11–13),
and there are also few studies on the ambulation of elderly PFD
patients with severe lower extremity deformities (11,13).
Although some of these patients had fairly severe deformities, they
were functionally active and were able to walk with aids (11,13).
In the present case, total follow-up has continued for over 52
years since the first diagnosis of PFD and, in spite of the severe
shepherd's crook deformity in her later years, the patient was able
to walk outside using a cane for a considerable amount of time.
However, multiple sites of chronic bone pain had lasted for over 55
years until she received oral bisphosphonate therapy.
Nitrogen-containing bisphosphonates such as
pamidronate and alendronate are drugs that prevent
osteoclast-mediated bone resorption, and are widely used to treat
osteoporosis and similar diseases, such as Paget's disease of the
bone. Numerous open clinical studies with high-dose intravenous
infusions of pamidronate have shown that they may yield favorable
results in patients with symptomatic FD (3–6).
Successful outcomes have also been reported for the use of oral
alendronate without combination with intravenous infusion in a few
patients with PFD (3,7–10).
These patients were treated with different doses of alendronate: 70
mg weekly in a 10.5-year-old female (10); 20 mg daily in a 22-year-old male
(7); 10 mg daily in 34- and
39-year-old females (3); 5 mg daily
in a 3-year-old female (8) and a
45-year-old premenopausal female (9). These regimens led to significant
improvements in pain relief and substantial reductions in the
levels of bone turnover markers, with the exception of 2 adult
patients who did not show any changes in urine NTX (3,9). A
distinct increase in BMD by bone scanning was also noted (7,8).
However, improvements in cortical thickening and filling of the
bone lesions were not apparent (3,9). In
the present study, the patient was treated with low-dose (5 mg/day)
oral alendronate alone for more than 102 months (8.5 years) since
the age of 71, and this treatment period was far longer than that
of previous studies, in which a maximum period of 2 years was
recorded. To the best of our knowledge, this is the first report of
the use of long-term oral alendronate treatment in a postmenopausal
elderly patient with PFD. The patient's long-standing bone pain was
almost completely relieved during the period beginning 8 months
after starting oral alendronate therapy.
The levels of total serum ALP and serum BAP are
frequently elevated in patients with PFD and MAS, and in certain
cases, these bone formation markers are significantly higher than
in normal subjects (4,11). In the present case, the pretreatment
levels of serum ALP and BAP were also relatively high, but the
levels markedly declined to near normal and normal ranges,
respectively, during oral alendronate treatment. The urinary NTX,
which is known to be one of the most sensitive predictors of bone
resorption in bisphosphonate therapy, also decreased significantly
following alendronate treatment. Along with the decrease in the
levels of bone turnover markers, the mean BMD value at the lumbar
spine was increased markedly by 26.2%. However, the BMD at the
involved lesions was not available in this study. By contrast, no
clear effects were noted on cortical thickening and filling of
lytic lesions in the radiographic aspects. This negative
radiological finding following long-term alendronate treatment in
our case was similar to the findings described in previous studies,
indicating that the intravenous administration of pamidronate did
not result in any clear improvement in the radiological appearance
(14) and had no effect on the
skeletal burden (15). However, our
patient has not suffered from any pathological fractures during the
alendronate treatment period.
In conclusion, the data from the current case
suggest that long-term daily administration of low-dose alendronate
alone is capable of providing satisfactory results with regard to
reduction of bone pain and bone turnover, and may increase the BMD
in patients with PFD. Although it appears likely that oral
alendronate alone cannot cure FD itself, this regimen is a
preferred option for the treatment of patients exhibiting PFD
symptoms, particularly those patients with long-standing bone
pain.
References
|
1
|
Marie PJ, de Pollak C, Chanson P and Lomri
A: Increased proliferation of osteoblastic cells expressing the
activating Gs alpha mutation in monostotic and polyostotic fibrous
dysplasia. Am J Pathol. 150:1059–1069. 1997.
|
|
2
|
Riminucci M, Kuznetsov SA, Cherman N,
Corsi A, Bianco P and Gehron Robey P: Osteoclastogenesis in fibrous
dysplasia of bone: In situ and in vitro analysis of IL-6
expression. Bone. 33:434–442. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lane JM, Khan SN, O'Connor WJ, et al:
Bisphosphonate therapy in fibrous dysplasia. Clin Orthop. 382:6–12.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Isaia GC, Lala R, Defilippi C, et al: Bone
turnover in children and adolescents with McCune-Albright syndrome
treated with pamidronate for bone fibrous dysplasia. Calcif Tissue
Int. 71:121–128. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chapurlat RD, Hugueny P, Delmas PD and
Meunier PJ: Treatment of fibrous dysplasia of bone with intravenous
pamidronate: long-term effectiveness and evaluation of predictors
of response to treatment. Bone. 35:235–242. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chapurlat RD: Medical therapy in adults
with fibrous dysplasia of bone. J Bone Miner Res. 21(Suppl 2):
114–119. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamomoto T, Ozono K, Shima M, Yoshikawa H
and Okada S: Alendronate and pharmacological doses of 1 alpha OHD3
therapy in a patient with McCune-Albright syndrome and accompanying
hypophosphatemia. J Bone Miner Metab. 20:170–173. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khadilkar VV, Khadilkar AV and Maskati GB:
Oral bisphosphonates in polyostotic fibrous dysplasia. Indian
Pediatr. 40:894–896. 2003.PubMed/NCBI
|
|
9
|
Kitagawa Y, Tamai K and Ito H: Oral
alendronate treatment for polyostotic fibrous dysplasia: a case
report. J Orthop Sci. 9:521–525. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aragão AL and Silva IN: Oral Alendronate
Treatment for Severe Polyostotic Fibrous Dysplasia due to
McCune-Albright Syndrome in a Child: A Case Report. Int J Pediatr
Endocrinol. Oct 4–2010.(E-pub ahead of print).
|
|
11
|
Harris WH, Dudley HR Jr and Barry RJ: The
natural history of fibrous dysplasia. An orthopaedic, pathological,
and roentgenographic study. J Bone Joint Surg Am. 44:A207–A233.
1962.PubMed/NCBI
|
|
12
|
Sissons HA and Malcolm AJ: Fibrous
dysplasia of bone: case report with autopsy study 80 years after
the original clinical recognition of the bone lesions. Skeletal
Radiol. 26:177–183. 1997.PubMed/NCBI
|
|
13
|
Szendrói M, Rahóty P, Antal I and Kiss J:
Fibrous dysplasia associated with intramuscular myxoma (Mazabraud's
syndrome): a long-term follow-up of three cases. J Cancer Res Clin
Oncol. 124:401–406. 1998.
|
|
14
|
Plotkin H, Rauch F, Zeitlin L, Munns C,
Travers R and Glorieux FH: Effect of pamidronate treatment in
children with polyostotic fibrous dysplasia of bone. J Clin
Endocrinol Metab. 88:4569–4575. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Collins MT, Kushner H, Reynolds JC, et al:
An instrument to measure skeletal burden and predict functional
outcome in fibrous dysplasia of bone. J Bone Miner Res. 20:219–226.
2005. View Article : Google Scholar : PubMed/NCBI
|