Introduction
In previous studies, we provided the first evidence
for the anti-colon tumor effect of dietary vitamin B6 in
azoxymethane (AOM)-treated mice (1,2). In
accordance with this finding, accumulating epidemiological evidence
indicates an inverse association between vitamin B6 status and
colon cancer (3–5). It has been suggested that the
anti-tumor effect of vitamin B6 is mediated by lowering cell
proliferation, inflammation, angiogenesis, oxidative stress and DNA
damage (6–10). However, the underlying mechanism has
yet to be clarified.
Colon damage and loss of epithelial cells was
considered to be a risk factor for colon carcinogenesis (11,12).
Epidemiological studies indicated that the association between
vitamin B6 status and colon cancer is particularly significant in
males consuming alcohol (3).
Consumption of alcohol has been reported to cause colonic
epithelium damage, which has been considered to promote colon
carcinogenesis (13). Evidence
suggests that vitamin B6 has a protective role against cytotoxicity
in cell culture studies (14,15),
although the mechanism remains unclear. Thus, the protective effect
of vitamin B6 on colonic damage caused by a carcinogen should be
determined.
In general, tissue damage caused by stress exposure
(heat shock, oxidative stress and hypoxia) is associated with a
higher expression of heat shock proteins (HSPs, molecular
chaperones) (16–18). HSPs are overexpressed in a variety
of human cancers, and are involved in tumor cell proliferation,
differentiation, invasion, metastasis, death and recognition by the
immune system (16–18). Conversely, HSPs are well known for
their cytoprotective factor in response to cell injury.
Accordingly, it is of interest to examine whether dietary vitamin
B6 modulates the expression of colonic HSPs. The present study was
performed to examine the effect of dietary vitamin B6 on colonic
damage and expression of HSPs in 1,2-dimethylhydrazine
(DMH)-treated rats.
Materials and methods
Animals
Male Sprague Dawley rats (4 weeks of age) were
purchased from the Hiroshima Laboratory Animal Center (Hiroshima,
Japan) and maintained according to the Guide for the Care and Use
of Laboratory Animals established by Hiroshima University. The
study was approved by the ethics committee of the same university.
The animals were individually housed in an air-conditioned room at
23–24°C with a 12 h light cycle (light, 8:00–20:00). The animals
received two intraperitoneal injections of DMH (20 mg/kg) and were
fed a stock diet (MF, Oriental Yeast Co., Ltd., Tokyo) for 2 weeks.
The rats were then divided into three groups of 12–13 rats per
group and provided free access to experimental diets and tap water.
The composition of the basal diet (% w/w) was corn oil, 20; casein,
24; L-cystine, 0.2; cellulose, 5; sucrose, 20; corn starch, 26.3;
vitamin mixture (vitamin B6-free), 1 (19); and salt mixture, 3.5 (19). Pyridoxine (PN) HCl was supplemented
to the basal diet at a dose of 1, 7 or 35 mg/kg. The level of PN
HCl recommended in the AIN-93 diets was 7 mg/kg. A 1-mg/kg dose was
reported to be the minimum level required for preventing growth
depression caused by vitamin B6 deficiency (19). Feces were collected for the final 3
days. After 22 weeks of administering the experimental diets, the
animals were sacrificed by decapitation with an anesthetic of
diethyl ether. The colon was removed and stored at −70°C until
analysis.
Materials
The activity of intestinal-derived alkaline
phosphatase in feces was determined according to the method
described elsewhere (20).
p-Nitrophenyl phosphate was used as the substrate, and the
absorbance of the reaction product p-nitrophenol was
determined spectrophotometrically at 405 nm. Intestinal alkaline
phosphatase activity was inhibited using 60 mM L-phenylalanine,
which acts as a specific non-competitive inhibitor of the
intestinal isozyme. The difference between total activity
(non-inhibited) and the activity following inhibition with
L-phenylalanine is the activity of the intestinal isoenzyme. One
unit of the activity was defined as μmol p-nitrophenol/min,
and the fecal activity was expressed as units/3 days of feces
collected. The colonic epithelium PCNA labeling index was
determined according to the method described elsewhere (21).
Protein and mRNA analyses
Colon protein levels of HSP70 and heme oxygenase-1
(HO-1) were determined by ELISA kits (Stressgen, MI, USA). Protein
concentration was determined by the Bradford method with a standard
of bovine serum albumin (22).
HSP70, HO-1 and β-actin gene primers were purchased from Qiagen
(QT00187397, QT00175994 and QT00193473, respectively; Helden,
Germany). A single melt curve was observed for each primer set in
all real-time RT-PCR reactions. Real-time RT-PCR was conducted
using an iQ5 real-time PCR detection system (Bio-Rad Laboratories,
CA, USA). Gene expressions were quantitated using Bio-Rad iQ5
optical system software (Bio-Rad Laboratories).
Statistical analysis
Statistical analysis was conducted by one-way
analysis of variation (ANOVA) and Scheffe’s multiple range test
(Excel Statistics 2006 for Windows, Social Survey Research
Information Co., Ltd., Tokyo, Japan). P<0.05 was considered to
be statistically significant.
Results
Growth was unaffected by dietary treatment (data not
shown). Food intake (for 3 days) at 8, 15 and 22 weeks was also not
affected by the dietary level of vitamin B6. Two rats in the 1 mg
vitamin B6/kg group had an adenoma in the colon, and were not used
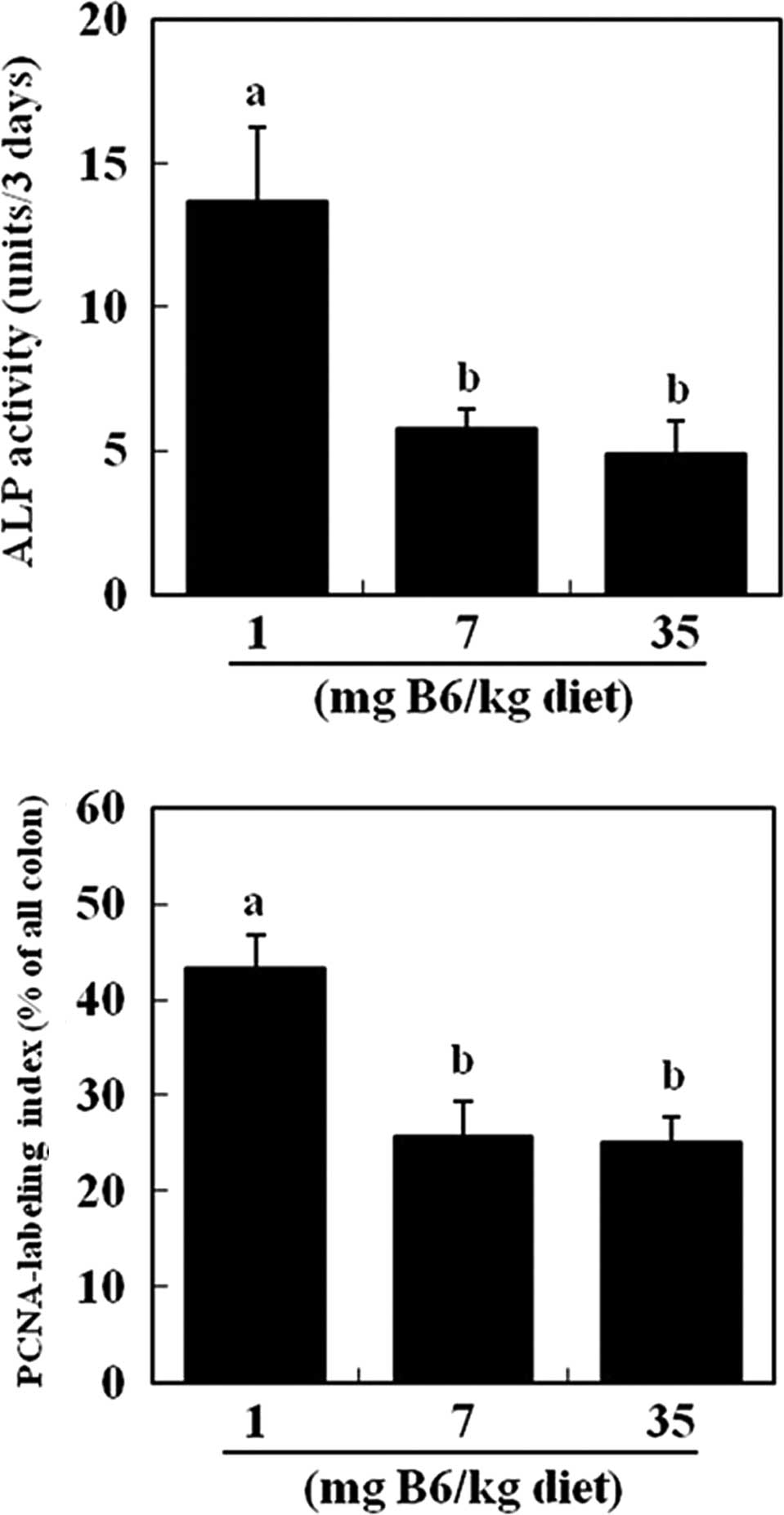
for the further analysis of colon parameters. Fecal activity of
intestinal alkaline phosphatase was significantly lower in the 7
and 35 mg PN HCl/kg diet groups (−58 and −64%, respectively) as
compared to the 1 mg PN HCl/kg group (P<0.05, Fig. 1A). The activity of intestinal
microbial-derived alkaline phosphatase was unaffected by dietary
vitamin B6 (data not shown). The PCNA labeling index of the colonic
epithelium was also significantly lower in the 7 and 35 mg PN
HCl/kg diet groups (−38 and −42%, respectively) as compared to the
1 mg PN HCl/kg group (P<0.05, Fig.
1B).
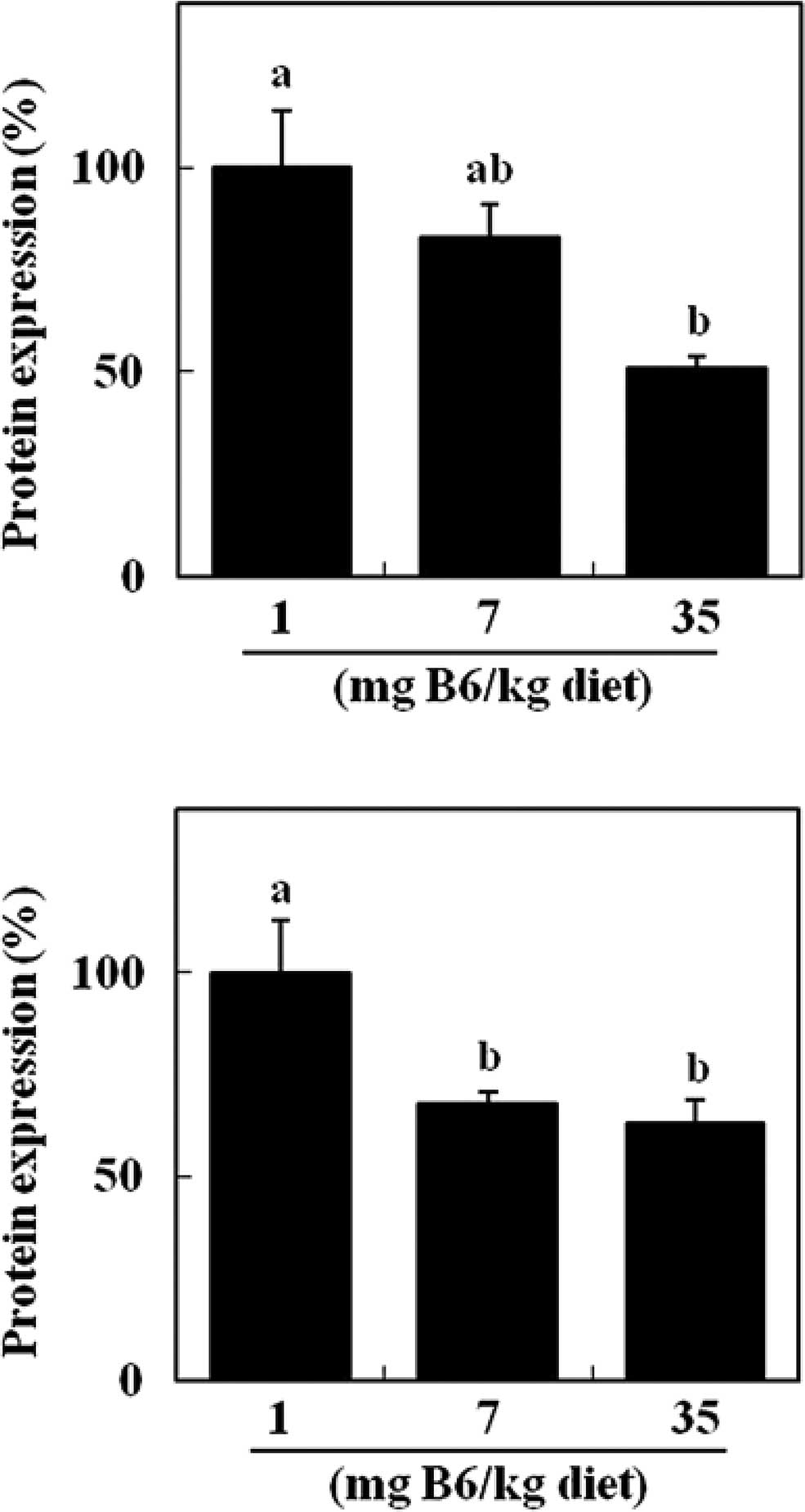
Dietary supplemental vitamin B6 appeared to decrease
colonic HSP70 protein in a dose-dependent manner (Fig. 2A). Compared to the 1 mg PN HCl/kg
diet, the colonic level of HSP70 protein was significantly reduced
in the 35 mg PN HCl/kg diet group (−49%) (P<0.05, Fig. 2A). Colonic levels of HO-1 protein
were significantly lower in the 7 and 35 mg PN HCl/kg diet groups
(−32 and −37%, respectively) compared to the 1 mg PN HCl/kg diet
group (P<0.05, Fig. 2B). Colonic
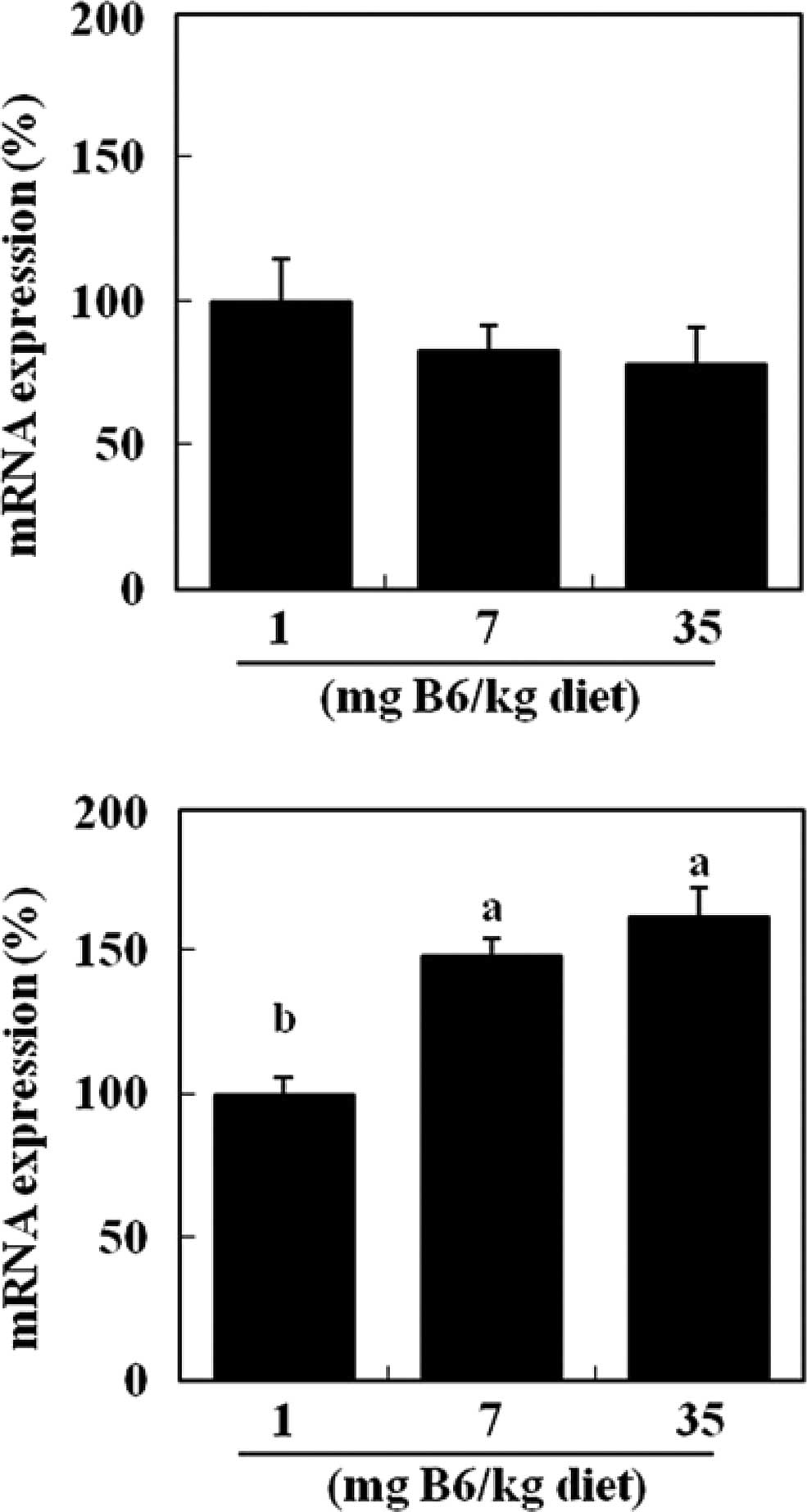
mRNA levels of HSP70 were unaffected by dietary levels of vitamin
B6 (Fig. 3A). Colonic levels of
HO-1 mRNA were significantly elevated in the 7 and 35 mg PN HCl/kg
diet groups (+48 and +62%, respectively) compared to the 1 mg PN
HCl/kg diet group (P<0.05, Fig.
3B).
Discussion
This study has shown that dietary supplemental
vitamin B6 suppressed DMH-induced colon damage as evidenced by the
data pertaining to fecal alkaline phosphatase activity (Fig. 1). Our previous study indicated that
dietary vitamin B6 lowered colonic levels of oxidative stress
markers, including 8-hydroxyguanosine and 4-hydroxynonenal, and
inflammatory mediators, such as COX-2 and iNOS in AOM-treated mice
(10). Therefore, the protective
effect of vitamin B6 against colon damage appears to be, at least
in part, explained by the lower oxidative stress and inflammation.
Further study is in progress to fully elucidate the molecular basis
of the underlying mechanisms of the protective role of vitamin
B6.
The cytotoxicity of colonic components, including
secondary bile acids and oxygen radicals, may cause epithelial cell
loss in the large bowel, leading to a compensatory crypt cell
proliferation and the development of colon cancer (13,14).
In the DMH-treated rats, supplemental vitamin B6 caused a
significant reduction in the PCNA labeling index, an index of cell
proliferation as well as fecal intestinal phosphatase activity
(Fig. 1). This finding suggests
that the reduced intestinal damage by supplemental vitamin B6
decreases cell proliferation in the colonic epithelium. Previously,
we reported that dietary supplemental vitamin B6 caused reduced
colonic epithelial cell proliferation despite no alteration in
apoptosis and the total cell number in the epithelium of
AOM-treated mice (1). The reduced
colonic cell proliferation by dietary vitamin B6 may be
counteracted by the reduced loss of the epithelial cells.
Notably, this study has demonstrated that dietary
supplemental vitamin B6 reduced the levels of colonic HSP70 and
HO-1 proteins in DMH-treated rats (Fig.
2). Since tissue damage by stress exposure induces a higher
expression of HSPs, reduced colon damage may result in a lower
protein expression of HSP70 and HO-1. HSPs are considered to be
novel targets for anti-tumor agents (16–18).
HSP70 promotes cancer cell growth and prevents apoptosis, thereby
increasing the survival of cells exposed to a wide range of
otherwise lethal stimuli. HSP70 neutralization has been found to
exert potent anti-tumor effects in animal models of colon cancer
(23). It has been suggested that
HO-1 protects tumor cells against oxidative stress and is regarded
as an enzyme facilitating tumor progression (18,19).
It has been demonstrated that pegylated zinc protoporphyrin, a
potent HO inhibitor, administered in vitro induced apoptosis
of human colon carcinoma SW480 cells and inhibited the growth of
murine colon carcinoma in vivo (24). Thus, the results of the present
study suggest that the anti-colon tumor effect of vitamin B6 is, at
least in part, mediated by reducing the protein expression of HSP70
and HO-1. To the best of our knowledge, this is the first study to
demonstrate the possible involvement of HSPs in the mechanism of
anti-tumorigenesis by dietary nutrients. The expression of
proinflammatory cytokines including TNF-α, IL-1α and IL-6 was
up-regulated, and NF-κB was activated in monocytes by HSP70
(25). Our previous study indicated
that dietary vitamin B6 decreased the colonic expression of COX-2
and iNOS in AOM-treated mice (10).
Thus, it is of interest to test whether a reduced HSP70 protein
expression by dietary vitamin B6 results in lower inflammatory
mediators.
In the present study, colonic HSP70 and HO-1 mRNA
levels were not reduced by dietary vitamin B6 (Fig. 3). Thus, the lower protein expression
of HSP70 and HO-1 by dietary B6 appears not to be mediated by
lowering the mRNA expression. These results are expected since a
previous study has shown that HSP70 protein expression is regulated
by the degradation of HSP70 proteins as well as transcriptional
regulation (26). Further studies
are required to examine the underlying mechanisms of a reduced
protein expression of the HSPs by vitamin B6. In our preliminary
study, no effect of dietary vitamin B6 was observed on the colonic
protein expression of HSP90, HSP60 and HSP25 (Kayashima et
al; unpublished data). At present, it is not known why the
protein expression of HSP70 and HO-1 was specifically affected by
dietary vitamin B6.
HSP70 has been suggested to be a cytoprotective
factor by modulating protein unfolding and protein degradation
(16). HO-1 has also been suggested
to prevent cell damage and exhibit marked antioxidant activity
(17). Thus, prior to this study,
we considered the possibility that dietary vitamin B6 causes a
higher expression of HSPs, leading to the protective function of
vitamin B6 against colon damage by DMH. However, this possibility
appears to be negated.
In conclusion, the present study provided the first
evidence that dietary vitamin B6 lowers colonic damage and protein
expression of HSP70 and HO-1 in rats exposed to DMH. These findings
suggest novel mechanisms of an anti-colon tumor effect of dietary
vitamin B6.
Acknowledgements
This study was supported in part by a Grant-in-Aid
for Scientific Research from the Ministry of Education, Culture,
Sports, Science and Technology of Japan.
Abbreviations:
|
PN
|
pyridoxine
|
|
HSP
|
heat shock protein
|
|
HO-1
|
heme oxygenase-1
|
|
DMH
|
1,2-dimethylhydrazine
|
|
AOM
|
azoxymethane
|
References
|
1
|
Komatsu SI, Watanabe H, Oka T, Tsuge H,
Nii H and Kato N: Vitamin B-6-supplemented diets compared with a
low vitamin B-6 diet suppress azoxymethane-induced colon
tumorigenesis in mice by reducing cell proliferation. J Nutr.
131:2204–2207. 2001.PubMed/NCBI
|
|
2
|
Komatsu S, Isobe M, Yanaka N and Kato N: A
high-fat diet enhances the inhibitory effect of dietary vitamin B6
on colon cell proliferation in mice. Oncol Rep. 14:265–269.
2005.PubMed/NCBI
|
|
3
|
Ishihara J, Otani T, Inoue M, Iwasaki M,
Sasazuki S and Tsugane S: Low intake of vitamin B-6 is associated
with increased risk of colorectal cancer in Japanese men. J Nutr.
137:1808–1814. 2007.PubMed/NCBI
|
|
4
|
Theodoratou E, Farrington SM, Tenesa A, et
al: Dietary vitamin B6 intake and the risk of colorectal cancer.
Cancer Epidemiol Biomarkers Prev. 17:171–182. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Larsson SC, Orsini N and Wolk A: Vitamin
B6 and risk of colorectal cancer: a meta-analysis of prospective
studies. JAMA. 303:1077–1082. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsubara K, Mori M, Matsuura Y and Kato
N: Pyridoxal 5′-phosphate and pyridoxal inhibit angiogenesis in
serum-free rat aortic ring assay. Int J Mol Med. 8:505–508.
2001.
|
|
7
|
Komatsu S, Watanabe H, Oka T, Tsuge H and
Kato N: Dietary vitamin B6 suppresses colon tumorigenesis,
8-hydroxyguanosine, 4-hydroxynonenal, and inducible nitric oxide
synthase protein in azoxymethane-treated mice. J Nutr Sci
Vitaminol. 48:65–68. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Komatsu S, Yanaka N, Matsubara K and Kato
N: Antitumor effect of vitamin B6 and its mechanisms. Biochim
Biophys Acta. 1647:127–130. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yanaka N, Koyama TA, Komatsu S, Nakamura
E, Kanda M and Kato N: Vitamin B6 suppresses NF-κB activation in
LPS-stimulated mouse macrophages. Int J Mol Med. 16:1071–1075.
2005.
|
|
10
|
Kanellis P, Gagliardi M, Banath JP, et al:
A screen for suppressors of gross chromosomal rearrangements
identifies a conserved role for PLP in preventing DNA lesions. PLoS
Genet. 3:1438–1453. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lapre JA and van der Meer R: Diet-induced
increase of colonic bile acids stimulates lytic activity of fecal
water and proliferation of colonic cells. Carcinogenesis. 13:41–44.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lapre JA, De Vries HT and van der Meer R:
Cytotoxicity of fecal water is dependent on the type of dietary fat
and is reduced by supplemental calcium phosphate in rats. J Nutr.
123:578–585. 1993.PubMed/NCBI
|
|
13
|
Wallace JL, Whittle BJ and Boughton-Smith
NK: Prostaglandin protection of rat colonic mucosa from damage
induced by ethanol. Dig Dis Sci. 30:866–876. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Geng M-Y, Saito H and Katsuki H: Effects
of vitamin B6 and its related compounds on survival of cultured
brain neurons. Neurosci Res. 24:61–65. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Geng M-Y, Saito H and Nishiyama N:
Protective effects of pyridoxal phosphate against glucose
deprivation-induced damage in cultured hippocampal neurons. J
Neurochem. 68:2500–2506. 1997. View Article : Google Scholar
|
|
16
|
Calderwood SK, Khaleque MA, Sawyer DB and
Ciocca DR: Heat shock proteins in cancer: chaperones of
tumorigenesis. Trends in Biochem Sci. 31:164–172. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Was H, Dulak J and Jozkowicz A: Heme
oxygenase-1 in tumor biology and therapy. Current Drug Targets.
11:1551–1570. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu X, Fan WG, Li DP, Lin MCM and Kung H:
Heme oxygenase-1 system and gastrointestinal tumors. World J
Gastroenterol. 16:2633–2637. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reeves PG, Nielsen FH and Fahey GC Jr:
AIN-93 purified diets for laboratory rodents: final report of the
American Institute of Nutrition ad hoc writing committee on the
reformulation of the AIN-76A rodent diet. J Nutr. 123:1939–1951.
1993.
|
|
20
|
Davis CD: Low dietary copper increases
fecal free radical production, fecal water alkaline phosphatase
activity and cytotoxicity in healthy men. J Nutr. 133:522–527.
2003.
|
|
21
|
Teixeira CR, Tanaka S, Haruma K, Yoshihara
M, Sumii K and Kajiyama G: Proliferating cell nuclear antigen
expression at the invasive tumor margin predicts malignant
potential of colorectal carcinomas. Cancer. 73:575–579. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schmitt E, Maingret L, Puig PE, et al:
Heat shock protein 70 neutralization exerts potent antitumor
effects in animal models of colon cancer and melanoma. Cancer Res.
66:4191–4197. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hirai K, Sasahira T, Ohmori H, Fujii K and
Kuniyasu H: Inhibition of heme oxygenase-1 by zinc protoporphyrin
IX reduces tumor growth of LL/2 lung cancer in C57BL mice. Int J
Cancer. 120:500–505. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Asea A, Kraeft SK, Kurt-Jones EA, et al:
HSP70 stimulates cytokine production through a CD14-dependant
pathway, demonstrating its dual role as a chaperone and cytokine.
Nat Med. 6:435–442. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qian S, McDonough H, Boellmann F, Cyr DM
and Patterson C: CHIP-mediated stress recovery by sequential
ubiquitination of substrates and Hsp70. Nature. 440:551–555. 2006.
View Article : Google Scholar : PubMed/NCBI
|