Introduction
Lung cancer is one of the most common malignant
tumors worldwide, and its incidence has increased in recent years.
Current therapeutic options remain unsatisfactory for most
patients. Surgical resection has been identified as the most
effective method for the treatment of lung cancer, but it is only
available for a small number of patients (1). Therefore, it is crucial to identify a
new treatment method.
Magnetic fluid hyperthermia (MFH) is a thermal
therapy using nanotechnology and hyperthermia, first reported by
Jordan et al (2). These
authors directly injected magnetic fluids into tumors and increased
the temperature using an AMF through the Neel relaxation mechanism
(3). Since the magnetic particles
were directly injected into tumors, there was no distribution of
magnetic particles in the periphery of normal tissues and the
temperature of these tissues did not increase significantly. Thus,
the hyperthermia specifically targeted the tumors. The efficacy of
hyperthermia was demonstrated using MFH in animals with several
types of tumors, such as B16 mouse melanoma, T-9 rat glioma,
SMMC-7721 mouse hepatocarcinoma and BT-474 mouse breast cancer
(4–7). This method was found to be effective
in inducing the regression of tumors and increasing the lifespan of
the animal. As a result, MFH appears to be a promising method for
targeting malignant tumors, including lung cancer.
The present study investigated the feasibility of
MFH for the treatment of lung cancer with a focus on the antitumor
effects of hyperthermia.
Materials and methods
Magnetic fluids and AMF
Magnetic fluids (Anhui Jinke Magnetic Liquid Co.,
Ltd., Anhui, China) used in the experiment consisted of
superparamagnetic iron oxide particles (core diameter 10–40 nm)
dispersed in water. The weight fraction of the iron was 20% and the
saturation magnetization of the particles was 360 G.
A high-frequency induction heating machine (Type
SP-04AC; Shenzhen Power Supply Technology Co., Ltd., Guangdong,
China) was used to provide an electric current through copper
induction coils. The copper induction coils with a hallow structure
could be cooled down by a circulation water system. The operation
frequency of the magnetic field is 150 kHz and the power
consumption is estimated to be 4 kW.
Cell lines and animals
Human lung cancer A549 cells were purchased from the
Institute of Biochemistry and Cell Biology, Shanghai Institute of
Biological Sciences, Chinese Academy of Sciences. Cells were
cultured in RPMI-1640 medium supplemented with 10% heat-inactivated
calf serum, penicillin (100 U/ml) and streptomycin (100 mg/ml), and
grown in the presence of 5% CO2 at 37°C.
BALB/C nude mice (female, 5-week-old) were purchased
from the Shanghai Institute of Biological Sciences, Chinese Academy
of Sciences. The animal experiments were performed according to the
principles described in the Guide for the Care and Use of
Laboratory Animals as promulgated by the Zhejiang Standing
Committee of PNC.
Therapeutic effect of MFH on xenograft
lung cancer
Xenograft tumors were induced by the subcutaneous
injection of human lung cancer A549 cells under the right flank of
nude mice. When the tumor diameter reached 0.6–0.8 cm, the mice
were divided into four groups (n=8): i) control group (sterile 0.9%
NaCl), ii) low-dose group (67.5 mg/ml Fe3O4),
iii) medium-dose group (90.0 mg/ml Fe3O4),
and iv) high-dose group (112.5 mg/ml Fe3O4).
The magnetic fluid with half of the tumor volume was injected into
the tumors. Following the magnetic fluid injection (24 h), the
tumors of the mice in groups 2–4 were exposed to a high-frequency
AMF for 30 min. Tumor and rectal temperature were taken by an
optical fiber probe (YF-200). After 45 days, the animals were
sacrificed. The weight and volume inhibitory rates (IW and IV,
respectively) of the tumors were calculated as: IW = (1 - the
weight of the tumor of the experimental group/the weight of the
tumor of the control group) × 100%; IV = (1 - the volume of the
tumor of the experimental group/the volume of the tumor of the
control group).
Histological analysis
One day after hyperthermia, 2 mice were randomly
chosen from each group. After euthanizing the mice, tumors were
collected and processed for histological analysis. Briefly, the
tumors were fixed in 10% formalin, embedded in paraffin, sectioned
and stained with H&E.
The apoptosis of tumor cells was assessed under
transmission electron microscopy (TEM). Tumors were harvested and
dissected into 1-mm3 specimens. Subsequently, the
samples were fixed in 4% glutaraldehyde and cut into ultrathin
sections (70 nm) to be examined under TEM.
Statistical analysis
To evaluate the significance of the overall
differences in tumor volumes and tumor weights among the in
vivo groups, statistical analysis was performed by analysis of
variance (ANOVA). P<0.05 was considered to be statistically
significant. The tumor volume and weight data were expressed as the
means ± standard error on graphs.
Results
Heat generation by magnetic fluids in
AMF
The temperature rose rapidly within 5 min and
remained stable after 10 min. The average plateau temperature of
groups 2–4 was 41.3, 44.5 and 46.8°C, respectively. The temperature
of each group was maintained for 30 min. The rectum temperature was
maintained at ~30.0±0.5°C. These data demonstrate a specific
magnetic hyperthermia.
Effects of hyperthermia on the
tumors
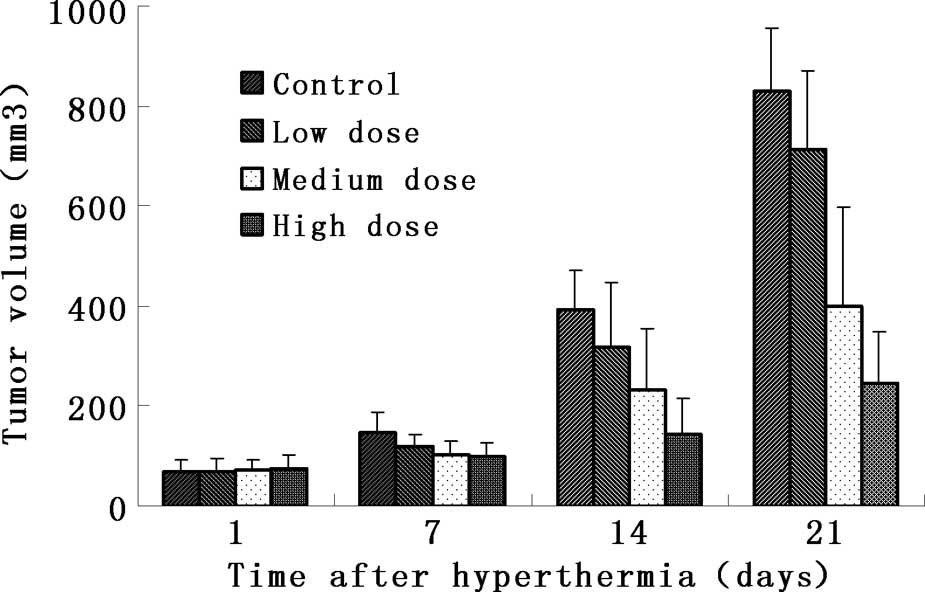
Anatomical analysis showed that the tumors in the
experimental groups became smaller (Fig. 1). The mass and volume inhibitory
ratio of group 4 was 62.0 and 70.2%, respectively, which was much
higher than that of the remaining groups (Table I). Comparisons between the
experimental and control groups revealed that groups 3 and 4 were
markedly different from the controls (p<0.01), while there was
no significant difference between group 2 and the control group
(p>0.05).
 | Table IVolume and mass inhibitory rates of
lung cancer A549 cells in nude mice after treatment. |
Table I
Volume and mass inhibitory rates of
lung cancer A549 cells in nude mice after treatment.
| Groups | Tumor volume
(mm3, mean ± SD) | Volume inhibitory
rate (%) | Tumor mass (g, mean ±
SD) | Mass inhibitory rate
(%) |
|---|
| Control | 831.0±126.2 | - | 1.71±1.11 | - |
| Low-dose | 713.2±157.1 | 14.1a | 1.51±0.25 | 11.7a |
| Medium-dose | 399.2±199.2 | 51.9b | 0.93±0.40 | 45.6b |
| High-dose | 247.3±102.0 | 70.2b | 0.65±0.21 | 62.0b |
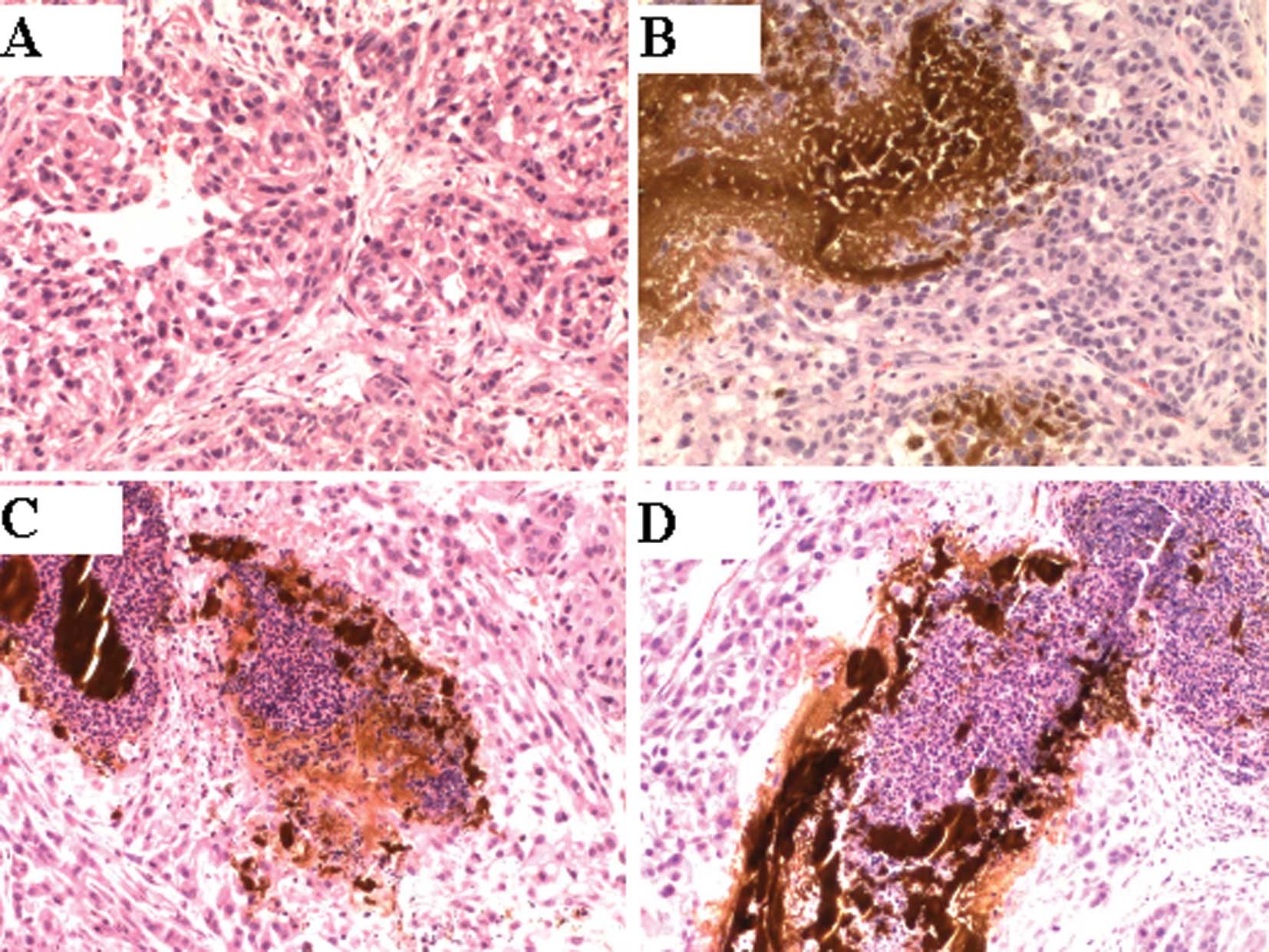
Histological analysis
Histological examination in groups 3 and 4 revealed
that there was an accumulation of black nanosized
Fe3O4 particles at the stroma in the margin
of the tumors. A number of tumor cells disappeared at the site
adjacent to this accumulation, and a necrotic zone was found
surrounding the material (Fig.
2).
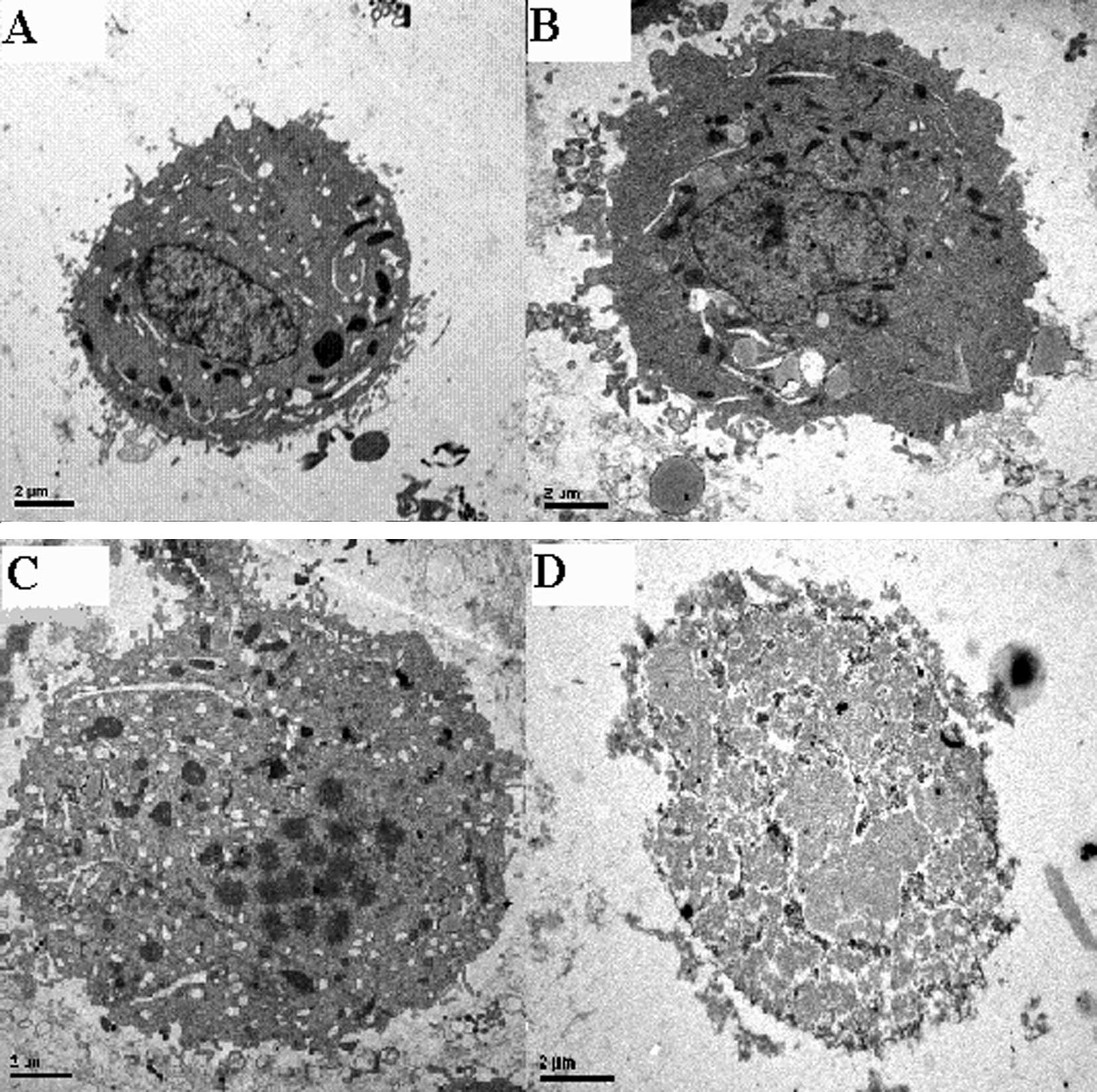
Tumor apoptosis was observed under TEM. Apoptotic
tumor cells were identified using characteristics such as
karyopycnosis, chromatin margination and condensation. In group 4,
the normal tumor cell structure disappeared. Karyorrhexis and
karyolysis were evident (Fig.
3).
Discussion
Empirical and clinical studies have indicated that
heat plays a crucial role in human lung cancer (8). Despite the promising results,
hyperthermia has yet to be established as a clinical routine
treatment. This is not due to a general lack of efficacy, but
rather due to the limitations of the current available techniques
with respect to selectively targeting the tumor region and
homogeneously distributing the heat within the tumor tissue.
Magnetically induced interstitial hyperthermia
addresses these shortcomings, especially for deep-seated and poorly
accessible tumors. Superparamagnetic biocompatible nanoparticles
are directly injected into the tumor tissue where they are
stimulated by an AMF to produce heat due to Brownian and Neel
relaxation processes. Our method of MFH is capable of heating the
tumor directly. During the hyperthermia, the average temperature of
groups 2–4 rose from 41.3 to 46.8°C, and the rectum temperature was
approximately 30.0°C, allowing the operator to induce tumor cell
apoptosis and necrosis without damaging the adjacent normal
tissue.
The important finding from our study was the effect
of MFH on lung cancer nodules in a murine model. A higher
temperature potentially improved the therapeutic effect on lung
cancer. When the strength of the magnetic field was kept stable, it
resulted in a higher dose of the magnetic fluid, resulting in a
more rapid increase in temperature and a higher maximum
temperature. Comparisons between the experimental and control
groups revealed that hyperthermia with intensity over a threshold
level markedly reduced the mass and volume of the tumors, which
could not be achieved by hyperthermia with intensity below the
threshold level. Our results strongly suggest that the intensity of
hyperthermia is crucial for the efficacy of the MFH therapy and a
threshold level has to be achieved to obtain the expected efficacy
of the therapy.
Notably, we found that the apoptotic rate increases
with a higher temperature (9).
Apoptosis is programmed cell death and is an important indicator of
successful therapy for various types of cancer. Cells undergoing
apoptosis demonstrate certain characteristic changes, including the
formation of DNA fragments, chromatin margination, vacuolization of
cytoplasm, shrinkage of cells and formation of apoptotic bodies
(10,11). The temperature of 42–46°C disrupts
the enzymatic system and structure in tumor cells, induces
apoptosis and subsequent necrosis. When the temperature was above
46°C, numerous large necrotic areas were observed, and the
nanometer granules were distributed in the interstitial matrix of
tumors or were phagocytized by tumor cells. Necrosis, apoptosis,
karyopycnosis, disintegration, karyorrhexis and karyolysis, as well
as vacant nuclei were found in the tumor cells surrounding the
granules.
In conclusion, MFH yielded favorable temperature
elevation in mouse xenograft lung cancer tumors, created
well-defined areas of apoptosis and necrosis and inhibited tumor
growth. Further optimization of magnetic fluid and AMF parameters
may allow for the testing of other animal models. This
mini-invasive hyperthermia therapy appears promising for the
treatment of lung cancer.
Acknowledgements
This study was supported by grants from the Medicine
and Health Foundation of Zhejiang Province (Grant no. 2009A163),
and the Science and Technology Development Foundation of Hangzhou
(Grant no. 20080333B02).
References
|
1
|
Hammerschmidt S and Wirtz H: Lung cancer:
current diagnosis and treatment. Dtsch Arztebl Int. 106:809–818.
2009.PubMed/NCBI
|
|
2
|
Jordan A, Wust P, Scholz R, et al: Effects
of magnetic fluid hyperthermia (MFH) on C3H mannary carcinoma in
vivo. Int J Hyperthermia. 13:587–605. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hildebrandt B, Wust P, Ahlers O, et al:
The cellular and molecular basis of hyperthermia. Crit Rev Oncol
Hematol. 43:33–56. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ito A, Matsuoka F, Honda H and Kobayashi
T: Antitumor effects of combined therapy of recombinant heat shock
protein 70 and hyperthermia using magnetic nanoparticles in an
experimental subcutaneousmurine melanoma. Cancer Immunol
Immunother. 53:26–32. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yanase M, Shinkai M, Honda H, Wakabayashi
T, Yoshida J and Kobayashi T: Antitumor immunity induction by
intracellular hyperthermia using magnetite cationic liposomes. Jpn
J Cancer Res. 89:775–782. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang ZY, Song J and Zhang DS: Nanosized
As2O3/Fe2O3 complexes combined with magnetic fluid hyperthermia
selectively target liver cancer cells. World J Gastroenterol.
15:2995–3002. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kikumori T, Kobayashi T, Sawaki M, et al:
Anti-cancer effect of hyperthermia on breast cancer by magnetite
nanoparticle-loaded anti-HER2 immunoliposomes. Breast Cancer Res
Treat. 113:435–441. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang Z, Yan W, Ming J and Yu Y: Docetaxel
weekly regimen in conjunction with RF hyperthermia for pretreated
locally advanced non-small cell lung cancer: a preliminary study.
BMC Cancer. 7:1892007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen GZ, Luo BD, Wang HQ, et al: Effect of
hyperthermia on rat hipocampal pyramidal cell apoptosis in vitro.
Di Yi Jun Yi Da Xue Xue Bao. 23:233–235. 2003.PubMed/NCBI
|
|
10
|
Mathiasen I, Sergeev I, Bastholm L, Elling
F, Norman A and Jäättelä M: Calcium and calpain as key mediators of
apoptosis-like death induced by vitamin D compounds in breast
cancer cells. J Biol Chem. 277:30738–30745. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barni S, Pontiggia P, Bertone V, Vaccarone
R, Silvotti MG, Pontiggia E and Mathe G: Hyperthermia-induced cell
death by apoptosis in myeloma cells. Biomed Pharmacother.
55:170–173. 2001. View Article : Google Scholar : PubMed/NCBI
|