Introduction
Neuroglioma, or glioma, is the most common primary
tumor of the central nervous system, accounting for approximately
40–50% of all intracranial tumors (1). These tumors are characterized by a
high invasive potential and a wide diversity of histological
appearance. As with other tumors, one of the crucial steps in
invasion is angiogenesis of the peritumoral tissues (2,3). Two
primary factors that mediate tumor angiogenesis, vascular
endothelial growth factor (VEGF) and matrix metalloproteinase-9
(MMP-9), have been researched in various tumor types (1–3). These
studies show that VEGF and MMP-9 expression varies in the majority
of tumor cells and is directly associated with tumor invasion.
Recent advances in imaging techniques have increased
the use of non-invasive examination in the diagnosis and treatment
of glioma. Magnetic resonance imaging (MRI) is one such valuable
imaging technique, which has the advantages of non-traumatic,
non-ionizing radiation and multiple planar imaging. In combination
with other approaches, MRI is capable of visualizing various
intracranial lesions (both structural and functional) and detecting
the correlation between the major white matter fiber bundle and
glioma lesions. Additionally, the complexity of these tumors has
generated interest in identifying biomarkers that are capable of
aiding in the diagnosis and treatment of gliomas (4). The current study used MRI to determine
the correlation of expression of VEGF and MMP-9 with MRI
characteristics and clinical pathological grades of cerebral
gliomas to aid in clinical treatment and prognosis assessment.
Materials and methods
Study subjects
The study involved 45 cerebral glioma patients from
the Department of Neurosurgery, the Third Affiliated Hospital of
Harbin Medical University, China, between September 2008 and July
2010. The population included 26 males and 19 females aged 18–65
years, with a mean age of 52.6 years. The patients were divided
into two groups: a low-grade group (grades I and II, n=21) and a
high-grade group (grades III and IV, n=24), according to diagnostic
gradation criteria for gliomas from the 2007 World Health
Organization (WHO) classification of tumors of the central nervous
system (5).
MRI scan
MRI scans were performed by a Philips Gyroscan Intra
1.5T superconductor MRI scanner. A standard quadrature head coil
was used. SE sequences of MR scans included axial and sagittal T1WI
and axial T2WI. A contrast agent (Gd-DAPA, 0.1 mM/kg) was
administered by fast injection (1–2 min for completion) at a dose
of 0.1 mM/kg via the elbow vein to enhance axial and sagittal T1WI
scans. Capture settings were as follows: TlWI, TR/TE 431 ms/11 ms;
T2WI, TR/TE 4850 ms/120 ms; FOV 24×24 cm; matrix 256×256; NEX 2;
band width 12.5 kHz; slice thickness, 5 mm; interval, 1 mm.
Enhanced scan parameters were identical to T1WI.
Immunohistochemical examination of VEGF
and MMP-9
Fresh tumor specimens were obtained during surgery
and fixed with 10% formalin solution. Tumor tissues were embedded
in paraffin, cut into 5 μm serial sections, and placed onto glass
slides. One slide was routinely stained with hematoxylin and eosin
(H&E) to confirm the pathological results. Other sections were
used to perform immunohistochemical staining with mouse anti-human
VEGF monoclonal antibody and mouse anti-human MMP-9 monoclonal
antibody (Wuhan Boster Biological Technology Co., Ltd., China)
detected by SP or DAB kits, according to the manufacturer’s
instructions (Wuhan Boster Biological Technology Co., Ltd.). VEGF
was visualized by brown or dark brown staining in the cytoplasm or
cell membrane of tumor cells; MMP-9 appeared as brown or
brown-yellow particles in the cytoplasm of tumor cells. We assessed
the distribution, intensity and location of staining in positive
cells. Some sections were incubated with phosphate-buffered saline
(PBS) instead of primary antibody as a negative control and known
positive breast cancer sections were used as a positive
control.
MRI detection for intracranial edema
index (EI)
The edge clarity and uniformity of the tumor signal
were evaluated by two highly qualified imaging physicians.
Specifically, the largest lesion entity area on the enhanced
horizontal axis TIWI was used to measure the maximum diameter of
the tumors. Additionally, vertical diameter line length was
measured on the enhanced horizontal axis of the MRI, and the
maximum height of the tumor body was measured on the sagittal MRI.
Thus, the volume of the tumor body was determined by multiplying
these three parameters. Edema volume (including tumor body) and
maximum edema height were measured according to standard methods
(6) on T2WI to calculate the layer
number occupied by T2WI edema. The layer number was also multiplied
by the layer thickness and interval to obtain the edema
volume/tumor body volume (EI) ratio.
MRI detection for the enhancement
percentage (EP) of the intracranial lesions
On MRI contrast images, a region of interest (ROI)
within the lesion entity area of the T1WI scan was selected, and
the signal intensity was measured. The EP, which is used to reflect
the degree of contrast enhancement in lesions, was calculated as EP
= difference value of unenhanced and enhanced signal
intensity/unenhanced signal intensity × 100.
Statistical analysis
Values are expressed as the mean ± standard
deviation (x±s). Data were analyzed with SPSS 12.0 software.
Comparisons of parameters among different groups were performed
using t-tests and chi-square tests. Analysis was performed using
the Pearson’s correlation method. P<0.05 was considered to be
statistically significant.
Results
Immunohistochemical examination of
VEGF
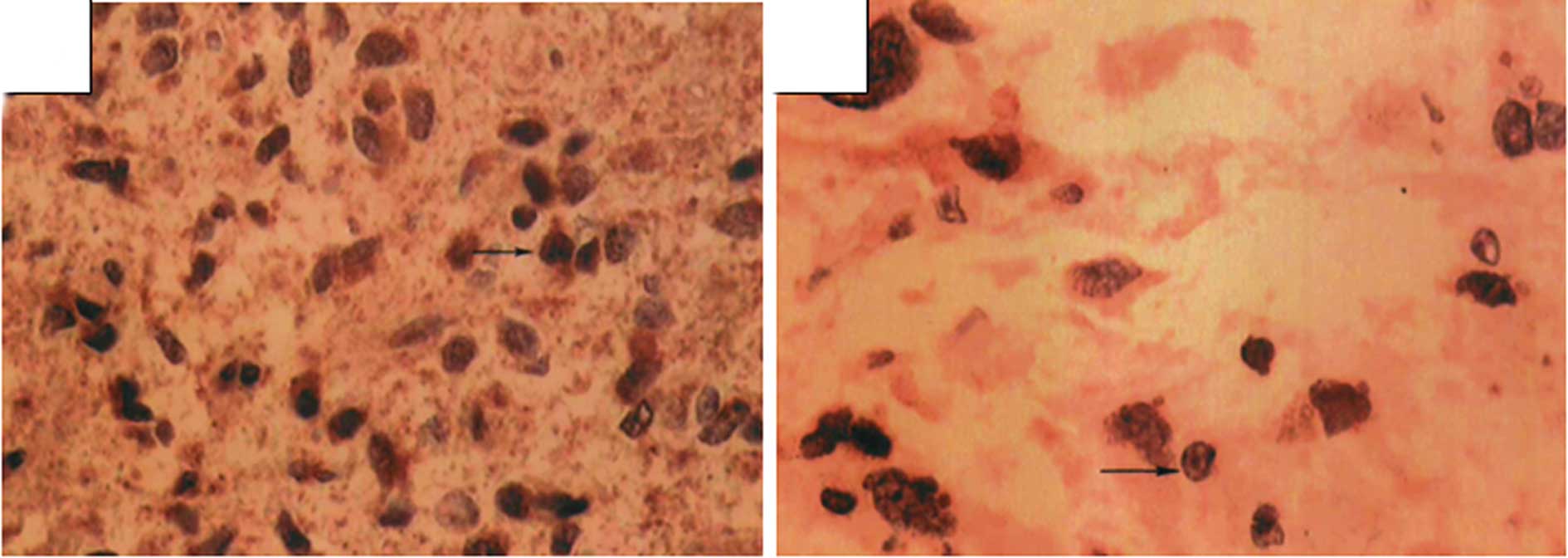
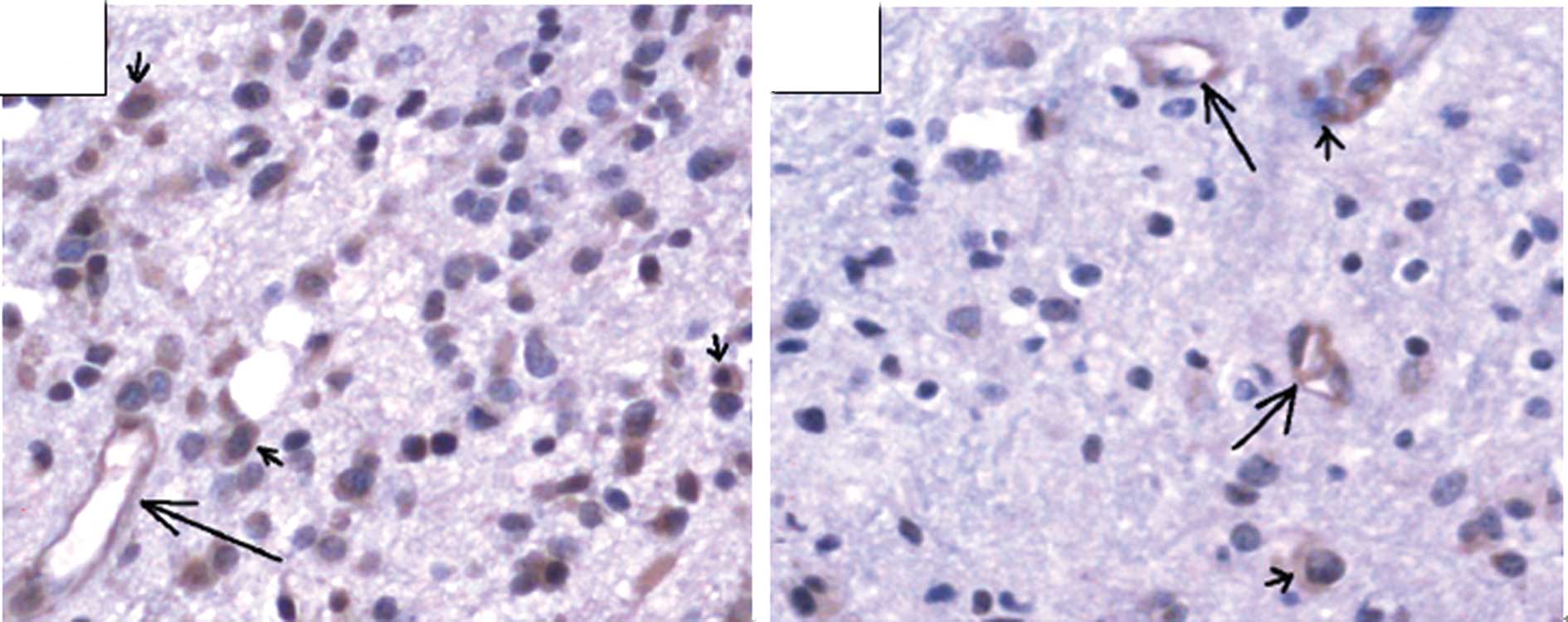
VEGF expression was detected in the cytoplasm of
tumor cells or occasionally in cell membranes. Cell nucleus
staining was also occasionally observed as yellow or yellow-brown
in color, mostly in tumor cells and in a few interstitial cells.
VEGF was detected in 82.2% of the cells (37/45). However,
expression varied by tumor grade, with a positive expression in
66.67% (14/21) of the low-grade tumors and 95.83% (23/24) of the
high-grade tumors. This expression difference was significant
between the two groups (P<0.05, Table I and Fig. 1).
 | Table ICorrelation between the expression of
VEGF and MMP-9 in cerebral gliomas and clinical pathological grade
of the tumor. |
Table I
Correlation between the expression of
VEGF and MMP-9 in cerebral gliomas and clinical pathological grade
of the tumor.
| Groups | Cases | VEGF | MMP-9 |
|---|
| |
|
|
|---|
| | Positive | Negative | χ2 | P-value | Positive | Negative | χ2 | P-value |
|---|
| Low-grade | 21 | 14 | 7 | 6.98 | <0.05 | 5 | 16 | 11.24 | <0.01 |
| High-grade | 24 | 23 | 1 | | | 18 | 6 | | |
Immunohistochemical examination of
MMP-9
MMP-9 was detected in the cytoplasm of tumor cells,
vascular endothelial cells and basal membranes. The percentage of
tumors expressing MMP-9 was 51.1% (23/45), but, similar to VEGF,
this expression varied by tumor grade: 23.81% (5/21) of low-grade
tumors expressed MMP-9 versus 75% (18/24) of high-grade tumors.
These differences were statistically significant (P<0.01;
Table I and Fig. 2).
MRI scanning results
Standard T1WI scans of high-grade gliomas showed low
signals or equal mixed signals; only a few showed low, equal and
high mixed signals. T2WI scans of high-grade gliomas showed equal
and high mixed signals, with clear boundaries and observable
necrosis or bleeding, and the majority of the gliomas showed uneven
and marked enhancement. By contrast, low-grade gliomas showed low
or slightly low signals in T1WI. The majority of signals were
relatively uniform, but a few signals were mixed. T2WI scans of
low-grade gliomas showed high or slightly high, signals, and the
majority of the signals were extremely uniform, with clear
boundaries, usually not enhanced or slightly enhanced, with rare
bleeding. Gliomas were observed invading the corpus callosum
to the contralateral hemisphere through the midline, and a few of
the gliomas affected multiple sites, showing multiple lesions. EI,
EP and the maximum tumor diameter were (1.23±0.78), (4.57±3.79)%
and (36.2±11.17) mm, respectively, for low-grade tumors, and
(7.75±6.61), (35.46±18.78)% and (56.22±10.12) mm, respectively, for
high-grade tumors.
Correlation between VEGF and MMP-9
expression in gliomas and peritumoral EI, EP and tumor size
The correlation between EI, EP and the maximum tumor
diameter are shown in Table II.
EI, EP and the maximum diameter were all significantly higher in
the high-grade tumor patients than in the low-grade patients.
Pearson’s correlation analysis showed that the expression of VEGF
and MMP-9 was positively correlated with EI (r=0.516, r=0.626,
P<0.05), EP (r=0.567, r=0.549, P<0.05) and maximum tumor
diameter (r=0.655, r=0.506, P<0.05).
 | Table IICorrelation between EI, EP, and
maximum tumor diameter. |
Table II
Correlation between EI, EP, and
maximum tumor diameter.
| Groups | EI | EP (%) | Max tumor diameter
(mm) |
|---|
| Low-grade | 1.23±0.78 | 4.57±3.79 | 36.2±11.17 |
| High-grade | 7.75±6.61 | 35.46±18.78 | 56.22±10.12 |
| t | 6.32 | 9.65 | 5.23 |
| P-value | <0.05 | <0.01 | <0.05 |
Discussion
Glioblastoma is the most common cancer in the
central nervous system, and preoperative examinations depend mainly
on CT and MRI (7,8). The properties of tumors are usually
determined by gross specimen observation and microscopic
observation of their shape and arrangement. The type and degree of
differentiation of the tumor tissues may be determined by
immunohistochemical markers (9,10).
Prompt and accurate diagnosis and treatment of
cerebral neoplasms are critical for decreasing morbidity and
mortality. New therapeutic modalities, such as image-guided surgery
and anti-angiogenic agents, are becoming increasingly reliant on
high-quality imaging for diagnostic evaluation, treatment planning
and post-treatment follow-up. CT and MRI are the mainstays of
imaging in current practice. MRI, with its multiplanar abilities
and superior contrast resolution, is now the modality of choice.
MRI is accurate in preoperative diagnosis and assessing the
characteristics of primary intra-axial brain tumors. This technique
is extremely accurate in assessing the grade of gliomas. Tumor
necrosis, irregular margins and peritumoral edema are the most
significant indicators of tumor grade (11,12).
The major biological characteristics of tumors
include uncontrollable growth, metastatic potential, serious harm
to the body and mortality. Spreading of tumors involves early
growth of the primary tumor, angiogenesis, tumor cell detachment
and invasion of matrix, invasion of the vascular system, thrombus
formation and secondary growth location in tissues and organs
(13). Throughout this process,
tumor angiogenesis and matrix destruction are key events and have
been central to oncology research in recent years.
During tumor growth, angiogenesis factors, including
VEGF, are secreted to promote tumor vascularization, which leads to
the continuous growth of tumor cells and invasion into adjacent
tissues and structures (14).
Additionally, the interaction between tumor cells and the
extracellular matrix is a critical step of invasion and metastasis.
Matrix metalloproteinases cause degradation of the extracellular
matrix and regulate cell adhesion, aiding these steps (15,16).
MMP-9, in particular, degrades and destroys the adjacent matrix and
creates tumor vascular endothelial barrier damage, leading to a
decreased steric hindrance, an increased vascular permeability and
the extravasation of nutrients (17). These changes provide matrix and
space for the neovascularization of gliomas and promote tumor
growth and invasion.
Results of our study show that glioma tissues with a
higher degree of malignancy are more likely to express VEGF and
MMP-9, indicating that VEGF and MMP-9 play significant roles in the
progression of gliomas. Thus, the expression of VEGF and MMP-9
shows the malignant phenotype of gliomas, and serves as an
indicator of glioma invasion.
CT and MRI have great significance in localization
diagnosis and qualitative diagnosis of gliomas (18). CT is extremely accurate and reliable
in the localization of diagnosis of gliomas with a certain
reference value for qualitative diagnosis. However, MRI is not
affected by the artifact of posterior cranial fossa, has a more
vivid black and white contrast, is capable of simultaneously
running transverse, coronal and sagittal scans, and thus is better
than pure transverse CT. The biggest advantage of a brain MRI is
that it provides a good anatomical background without bone artifact
and is also capable of exhibiting the panorama and
three-dimensional location of the tumor. This feature is an
improvement on the CT for tumors of the cerebellum, brain stem,
saddle area and craniocervical junction. Information provided by
conventional MRI includes enhancement degree, enhancement form,
peritumoral edema scope, tumor size, clarity of the boundaries,
uniformity of the signals, mass effect, midline shift, lesion
sites, necrotic cyst and bleeding, flowing void effect and T1WI and
T2WI signal strength. MRI features correlate with glioma
pathological stages (19). In
particular, the EP, peritumoral edema scope, tumor diameter,
unclear boundaries, signal asymmetry, mass effect and midline shift
are positively correlated with pathological grade; in particular,
peritumoral edema and EP were highly correlated (20). MRI studies show unclear enhancement
in brain-tumor interfaces and partial gliosis, indicating low-grade
glioma. Notably, with increasing pathological grade the brain-tumor
interfaces become irregular, with uneven enhancement in wall
thickness, and the enhancement gradually becomes clear. Since VEGF
and MMP-9 were capable of promoting tumor angiogenesis, and
high-grade gliomas have significant angiogenesis, EP may be a
significant reference index for determining tumor malignancy
through imaging (21). In this
study, the expression of VEGF and MMP-9 were positively correlated
with EI, EP and maximum tumor diameter. These findings suggest that
the expression of VEGF and MMP-9 may be used as a reference factor
in judging tumor malignancy in combination with intracranial MRI
examination.
In conclusion, the expression of VEGF and MMP-9 in
tumor tissues, in combination with the peritumoral EI, EP and tumor
size, as well as uniformity of the signal and other characteristics
of MRI, may be used as indicators of malignant behavior, key to
diagnosing brain gliomas early. Thus, patients are able to receive
timely surgical treatment, in combination with radiation therapy
and chemotherapy, which is significant in improving the quality of
life of patients.
References
|
1
|
Weller M, Felsberg J, Hartmann C, et al:
Molecular predictors of progression-free and overall survival in
patients with newly diagnosed glioblastoma: a prospective
translational study of the German Glioma Network. J Clin Oncol.
27:5743–50. 2009. View Article : Google Scholar
|
|
2
|
Arany Z, Foo SY, Ma Y, et al:
HIF-independent regulation of VEGF and angiogenesis by the
transcriptional coactivator PGC-1 alpha. Nature. 451:1008–1012.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zheng SQ, Huang RQ and Zhang YJ: Role of
matrix metalloproteinase (MMP)-2 and -9 and vascular endothelial
growth factor C in lymph node metastasis of breast cancer [J].
Zhonghua Bing Li Xue Za Zhi. 39:240–244. 2010.PubMed/NCBI
|
|
4
|
Sulman EP and Aldape K: The use of global
profiling in biomarker development of gliomas. Brain Pathol.
21:88–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Inamura T, Nishio S, Takeshita I, Fujiwara
S and Fukui M: Peritumoral brain edema in meningiomas influence of
vascular supply on its development. Neurosurgery. 31:179–185. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Piroth MD, Pinkawa M, Holy R, Stoffels G,
Demirel C, Attieh C, Kaiser HJ, Langen KJ and Eble MJ:
Integrated-boost IMRT or 3-D-CRT using PET-PET based auto-contoured
target volume delineation for glioblastoma multiforme - a
dosimetric comparison. Radiat Oncol. 4:572009.
|
|
8
|
Brandes AA, Tosoni A, Franceschi E, Reni
M, Gatta G and Vecht C: Glioblastoma in adults. Crit Rev Oncol
Hematol. 67:139–152. 2008. View Article : Google Scholar
|
|
9
|
Chan ES and Yeh MM: The use of
immunohistochemistry in liver tumors. Clin Liver Dis. 14:687–703.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sethi K, Sarkar S, Das S, Mohanty B and
Mandal M: Biomarkers for the diagnosis of thyroid cancer. J Exp
Ther Oncol. 8:341–352. 2010.PubMed/NCBI
|
|
11
|
Chen W and Silverman DH: Advances in
evaluation of primary brain tumors. Semin Nucl Med. 38:240–250.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Costello JF: DNA methylation in brain
development and gliomagenesis. Front Biosci. 8:s175–s184. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Diamandis EP, Fritsche HA, Lilja H, Daniel
WC and Schwartz MK: Tumor Markers: Physiology, Pathobiology,
Technology, and Clinical Applications. AACC press; Washington DC:
pp. 172002
|
|
14
|
Puduvalli VK and Sawaga R:
Antiangiogenesis therapeutic stragegios and clinical implications
for brain tumors. J Neurooncol. 50:189–200. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Amãlinei C, Cãruntu ID, Giuscã SE and
Bãlan RA: Matrix metalloproteinases involvement in pathologic
conditions. Rom J Morphol Embryol. 51:215–228. 2010.
|
|
16
|
Savinov AY and Strongin AY: Matrix
metalloproteinases, T cell homing and beta-cell mass in type 1
diabetes. Vitam Horm. 80:541–562. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Martin MD and Matrisian LM: The other side
of MMPs: protective roles in tumor progression. Cancer Metastasis
Rev. 26:717–724. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Langer A: A systematic review of PET and
PET/CT in oncology: a way to personalize cancer treatment in a
cost-effective manner? BMC Health Serv Res. 10:283–299. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen CQ, Liao WH and Chen CY: Correlation
between peritumoral edema on MRI, VEGF expression, MVD and
pathological grading in astrocytomas. Chin J Mod Med. 14:38–43.
2004.
|
|
20
|
Deng GX, Zhang H and Qian LX: Ordinal
logistic regression analysis of MRl and pathological grade in
astrocytoma. Chin Remed Clin. 5:680–682. 2005.
|
|
21
|
Anderson JC, Grammer JR, Wang W, Nabors
LB, Henkin J, Stewart JE Jr and Gladson CL: ABT-510, a modified
type 1 repeat peptide of thrombospondin, inhibits malignant glioma
growth in vivo by inhibiting angiogenesis. Cancer Biol Ther.
6:454–462. 2007. View Article : Google Scholar : PubMed/NCBI
|