Introduction
Apoptosis is a common and well-understood mechanism
of cell death resulting from the activation of caspases through two
primary pathways: i) via caspase 9 activation and a mitochondrial
route to release cytochrome c, or ii) directly via caspase 8 to
caspase 3 as a primary effector caspase. At least 14 caspases have
been identified and characterized in mammalian cells. Caspases fall
into two classes: initiator caspases (caspases 8, 9, 2 and 10) that
exclusively cleave and activate other caspases, and effector
caspases (caspases 3, 6 and 7) that cleave other proteins.
Procaspases are activated by cleavage at the same consensus site,
either by themselves or by other caspases. Other proteins targeted
by caspases include inhibitory apoptotic proteins (IAPs),
inhibitors of caspase-activated DNAase (ICADs), Bcl-2, signal
pathway proteins, cytoskeletal proteins and DNA repair enzymes,
including DNA-dependent protein kinases. These different target
proteins of caspase result in apoptotic cell death. It has been
shown more recently that one of the mechanisms of regulating the
balance between proliferation and cell death is through alternative
splicing of apoptotic genes. Deregulation of this balance
represents a pro-tumorigenic principle or neuronal death in human
carcino-genesis or neurodegenerative diseases (1).
Splicing is a cellular process that removes introns
from the pre-mRNA in eukaryotic genomes so that exons join together
to form a mature mRNA. However, when the introns of a certain
pre-mRNA are excised in more than one way, alternative splicing
occurs, resulting in several possible mature mRNAs from one gene.
Alternative pre-mRNA splicing is an essential mechanism for
generating protein diversity (2–5). It is
estimated that more than 60% of human genes undergo alternative
splicing, leading to the production of diversified functional
isoforms (6). Alternative splicing
is precisely regulated. Aberrant splicing may lead to human
disorders, including growth hormone deficiency, spinal muscular
atrophy (7,8) and possibly tumors and
neurodegenerations.
A number of pre-mRNAs for cell death signals,
including caspase 9, one of the most significant factors in the
apoptotic pathways, are alternatively spliced, yielding isoforms
with opposing functions during programmed cell death (8–10).
Findings of various reports demonstrated that the alternative
splicing of caspase 9 can be regulated by small molecules
such as ceramide in cancer cells (11), as well as by other biological
molecules SRp30a (ASF/SF2), E2F and SC35 (12,13).
To identify additional small molecules that are potentially used in
cancer treatment to regulate caspase 9 splicing, we
performed reverse transcriptase-polymerase chain reaction (RT-PCR)
experiments in cells treated with 1,040 FDA-approved drugs and
compounds. Emetine, a potent protein synthesis inhibitor in
eukaryotes (14), was found to
downregulate pro-apoptotic caspase 9 and upregulate anti-apoptotic
caspase 9b in C33A and MCF-7 cells, while in PC3 cells, an opposite
effect was observed: caspase 9b was downregulated and caspase 9 was
upregulated. We further demonstrated that this Emetine function was
mediated by phosphorylation (15).
However, the opposite effect of Emetine on caspase 9 splicing in
C33A and PC3 cells suggests that targeting alternative splicing for
cancer treatment should be cautiously assessed.
Materials and methods
Compounds
All chemicals including Emetine, calyculin A and
okadaic acid were purchased from Sigma (St. Louis, MO, USA).
Cell culture
The human cervical carcinoma C33A cells were
maintained in Dulbecco’s modified Eagle’s medium (DMEM),
supplemented with 10% (vol/vol) fetal bovine serum (FBS),
L-Glutamine and 1X penicillin-streptomycin. PC3 prostate cancer
cells were cultured in RPMI supplemented with 10% (v/v) FBS,
L-Glutamine and 1X penicillin-streptomycin. Human breast cancer
cells MCF-7 and MCF-7/Adr were cultured in RPMI supplemented with
10% (v/v) FBS, L-Glutamine and 1X penicillin-streptomycin. All
cells were maintained at less than 80% confluence under standard
incubator conditions.
Emetine treatment
Emetine dihydrochloride hydrate (Sigma) with a stock
solution concentration of 100 μM was used. Twenty-four hours prior
to Emetine treatment, the cells were plated in 2 ml medium in
6-well plates at a density of 200,000 cells/well. The cells were
treated with various concentrations of Emetine for 24 h for a
dose-dependent study. For the time course experiment, cells were
treated with 1.0 μM Emetine for various durations.
Protein phosphatase inhibitor
treatment
Cells were pretreated with calyculin A or okadaic
acid for 1 h. The media were removed. Fresh regular media with
Emetine were added to treat cells for the duration of 24 h. RT-PCR
was then carried out to examine caspase 9 splicing.
Reverse transcriptase-polymerase chain
reaction
Total RNA was extracted from cultured cells using
TRIzol reagent (Invitrogen Corp., Carlsbad, CA, USA) according to
the manufacturer’s instructions. Reverse transcription was carried
out with 1 μg total RNA using Improm II reverse transcriptase
(Promega, Madison, WI, USA) and oligo (dT) as the priming agent.
Following incubation for 1 h at 42°C, the reactions were terminated
by heating at 70°C for 15 min. To analyze alternative splicing of
the caspase 9 gene, an upstream 5′ primer
(5′-GCTCTTCCTTTGTTCATCTCC-3′) and a downstream 3′ primer
(5′-CATCTGGCTCGGGGTTACTGC-3′) were used for PCR amplification (35
cycles at 94°C, 30 sec; 55°C, 30 sec; 72°C, 1 min) with Choice Taq
Blue Mastermix (Denville Scientific Inc., South Plainfield, NJ,
USA). PCR products were separated and analyzed on agarose gels.
This study was approved by the ethics committee at
Nantong University, Nantong, Jiangsu, China.
Results
Emetine regulates the alternative
splicing of caspase 9 pre-mRNA
To identify small molecules that regulate
alternative splicing, 1,040 FDA-approved drugs and compounds were
screened using RT-PCR in C33A cells, a cervical cancer line. As
described in our previous study, Emetine regulated alternative
splicing of the Bcl-x gene (15). Notably, Emetine was also found to
increase the smaller caspase 9b mRNA with a concomitant increase of
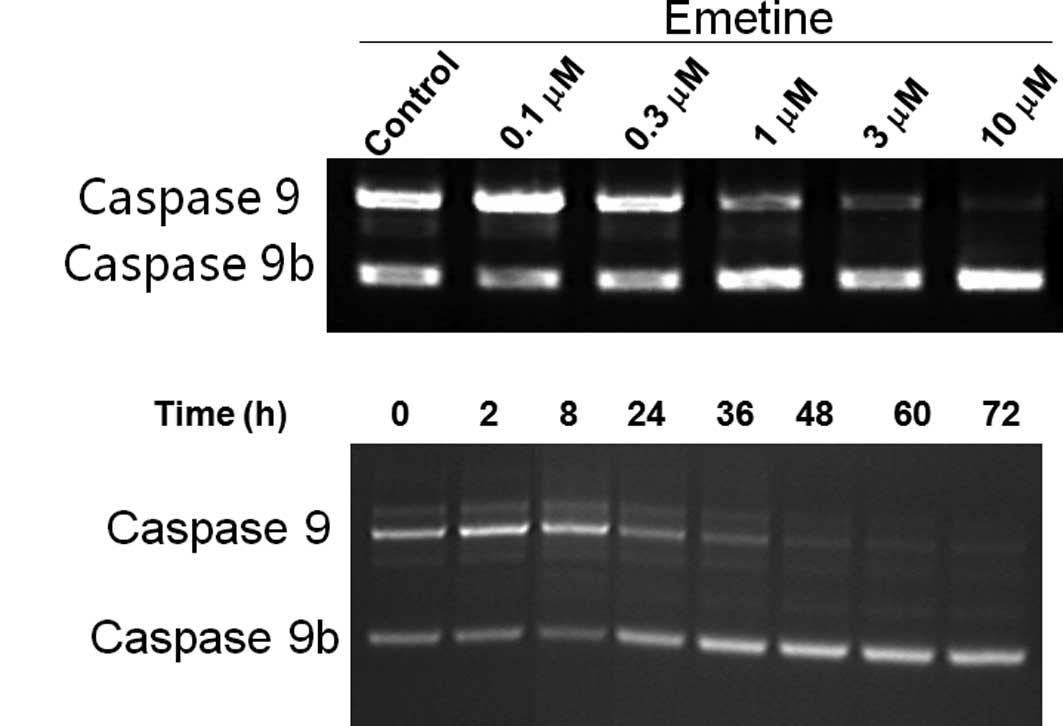
the larger caspase 9 mRNA (Fig. 1).
To further validate our findings, C33A cells were treated with
various concentrations of Emetine or for different time durations.
A decrease in the ratio of caspase 9/ caspase 9b was observed
(Fig. 1A). In our previous studies,
Emetine was also found to alter splicing of the Bcl-x gene
(15) but not the tau, SMN or
BACE1 genes, indicating its relative specificity on caspase 9
and Bcl-x splicing. To further validate whether Emetine regulates
splicing in the caspase 9 gene and to evaluate whether
regulation of caspase 9 splicing has potential relevance to
cancer therapy, we examined the effects of Emetine on the pre-mRNA
processing of caspase 9 in several tumor cell lines. Cells
were treated over various time durations and/or with various
concentrations of Emetine. Semi-quantitative RT-PCR was used to
determine the effects of Emetine. In MCF-7 and MCF-7/Adr cells
(breast cancer cell lines) (Fig. 1C and
D), regulation of caspase 9 splicing was found to be
slightly less pronounced than in C33A cells. However, the pattern
of the regulation was similar to that observed in C33A cells; i.e.,
an increase of caspase 9b and a decrease of caspase 9. Conversely,
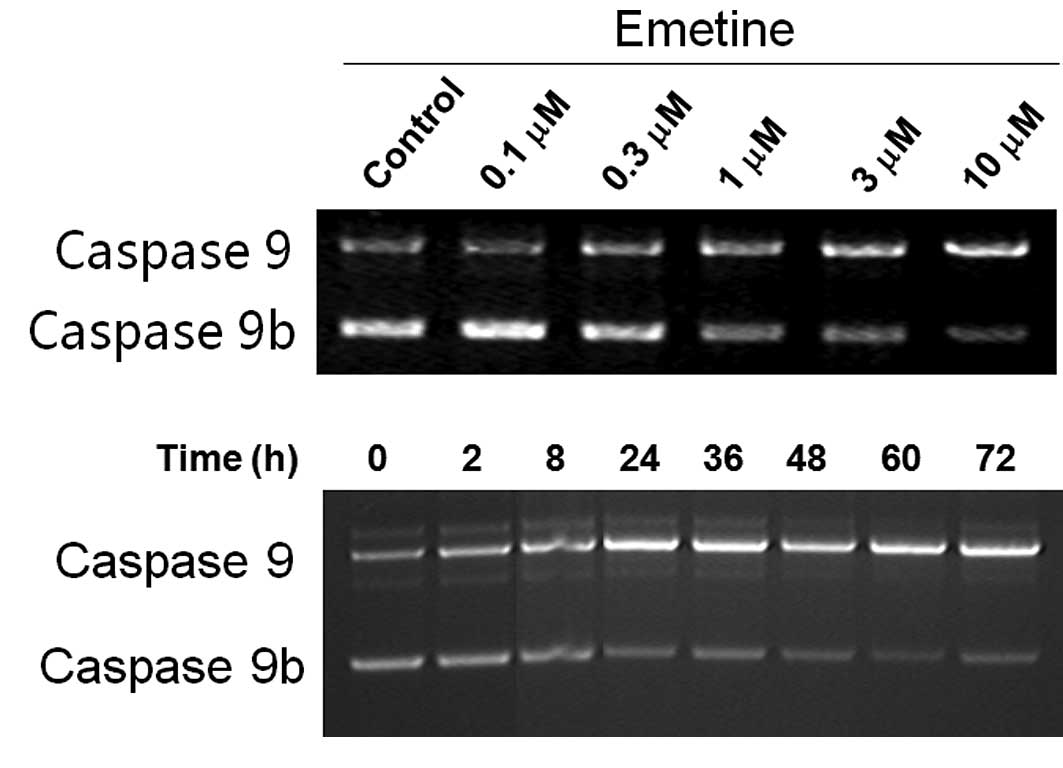
in PC3 (a prostate cancer cell line) cells (Fig. 2), an opposite effect of Emetine on
caspase 9 splicing was noted: an increase of caspase 9 and a
decrease of caspase 9b. The effects of Emetine on the pre-mRNA
processing of caspase 9 in PC3 cells were time course- and
dose-dependent (Fig. 2A and B).
Emetine exerts its effect on caspase 9
splicing, via protein phosphatase 1 (PP1)
Previous studies showed that ceramide and Emetine
affect the splicing of Bcl-x in a phosphorylation-dependent
pathway. To examine whether Emetine exerts the effects on
caspase 9 splicing in a similar manner, C33A and PC3 cells
were treated with phosphatase inhibitors calyculin A and okadaic
acid. We found that 5 μM calyculin A, an inhibitor of both protein
phosphatase 1 (PP1) and protein phosphatase 2A (PP2A), blocked the
Emetine effects on caspase 9 alternative splicing in C33A
and PC3 cells (Fig. 3, comparing
+Emetine with +Emetine/calyculin A). To establish whether PP1 or
PP2A was the Emetine-responsive protein phosphatase that regulates
caspase 9 alternative splicing, C33A and PC3 cells were
pretreated for 1 h with 5 μM okadaic acid, a selective PP2A
inhibitor. Pretreatment with okadaic acid had no effect on
caspase 9 alternative splicing (Fig. 3, comparing +Emetine with
+Emetine/okadaic acid). Taken together, these results suggest that
PP1 mediates the effects of Emetine on the alternative splicing of
caspase 9.
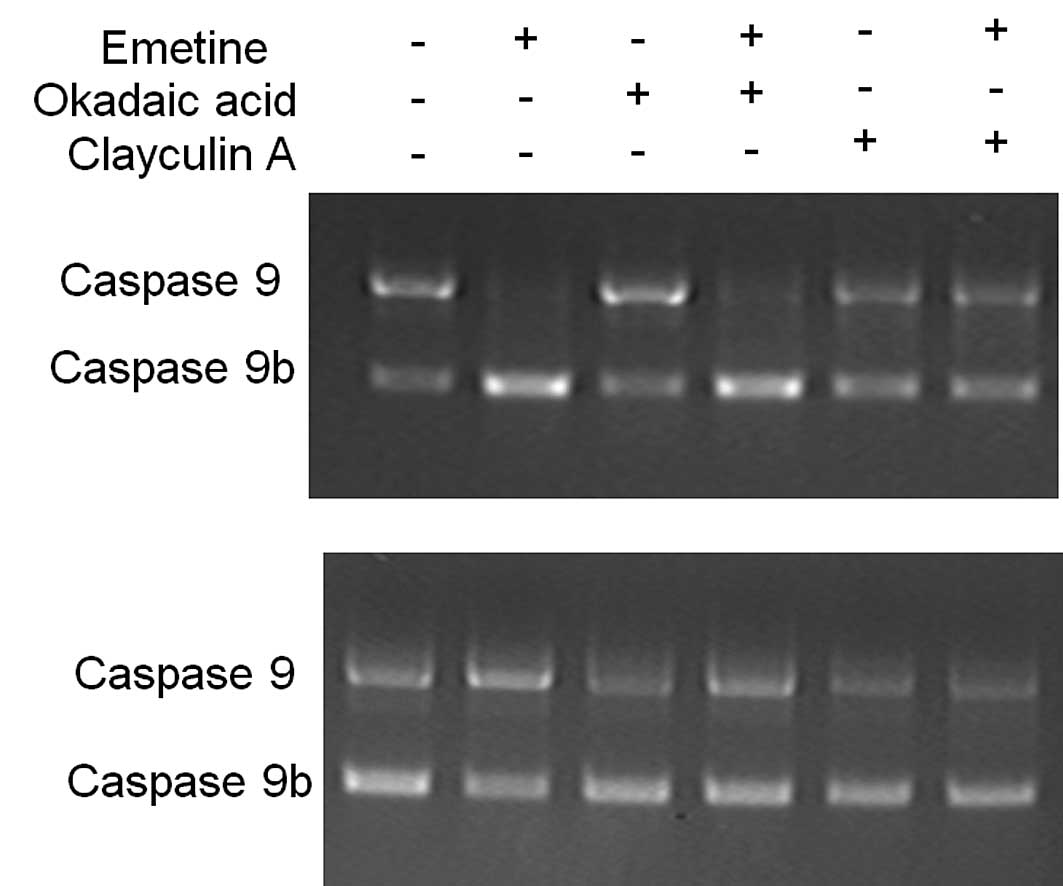 | Figure 3Calyculin A, but not okadaic acid,
blocks the effect of Emetine on caspase 9 splicing. Cells
were pretreated with either 5 μM calyculin A, an inhibitor of both
protein phosphatase 1 (PP1) and protein phosphatase 2A (PP2A), or
with 5 μM okadaic acid, a selective PP2A inhibitor, and then
exposed to 1.0 μM Emetine for 24 h. RT-PCR was carried out. The
results suggest that PP1, but not PP2A, mediates the effects of
Emetine on the alternative splicing of caspase 9. (A)
Effects of calyculin A in C33A cells. (B) Effects of calyculin A in
PC3 cells. All experiments were repeated at least three times. +,
with Emetine, okadaic acid or calyculin A; -, without Emetine,
okadaic acid or calyculin A. |
Discussion
We previously described that Emetine regulated
alternative splicing of Bcl-x, increasing the smaller pro-apoptotic
Bcl-xS isoform and decreasing the larger anti-apoptotic Bcl-xL
isoform, resulting in the possible sensitization of tumor cells to
chemotherapy (15). We further
demonstrated that Emetine modulated Bcl-x splicing by affecting PP1
phosphatase. These results were consistent with an earlier study
(11) that a second chemotherapy
agent, ceramide, also affected Bcl-x splicing through PP1. Notably,
the authors of that study also demonstrated that in addition to
Bcl-x, ceramide regulated alternative splicing of the caspase
9 gene through a similar mechanism, leading to an increase of
the larger pro-apoptotic caspase 9 and a decrease of the smaller
anti-apoptotic caspase 9b. Based on their studies, it appears that
there is a synergetic effect on splicing of Bcl-x and caspase 9 in
A549 cells, leading to an increase of pro-apoptotic isoforms for
the two genes and to sensitization of A549 cells to death
inducers.
However, in contrast to the effects of ceramide on
the splicing of Bcl-x and caspase 9 in A549 cells, i.e., an
increase of the pro-apoptotic forms of Bcl-x (Bcl-xS) and
caspase 9 (caspase 9) genes, this study revealed that
Emetine has an opposite effect on the alternative splicing of
caspase 9 in different tumor cell lines. In PC3 cells,
Emetine increased pro-apoptotic caspase 9 (full-length) with a
concomitant decrease of anti-apoptotic smaller caspase 9b, while in
C33A and MCF-7 cells Emetine increased anti-apoptotic caspase 9b
with a decrease of the full-length pro-apoptotic caspase 9
(Figs. 1-3). The results in C33A and MCF-7 cells
were unexpected, as Emetine had been considered to be one of the
potential chemotherapeutic agents assumed to induce cell death. As
discussed in our earlier study (15), Emetine acts as an effective
chemotherapeutic agent by increasing the lifespan of tumor-bearing
mice (16,17) and thus has the possibility for
clinical advantage (18,19). However, although no previous study
explains the mechanism responsible for the anti-tumor effect of
Emetine, our results regarding the effects of Emetine on Bcl-x
splicing (15) suggested that
Emetine promoted the expression of pro-apoptotic proteins by
regulating alternative splicing. However, based on the results in
this study, we deduce that Emetine sensitizes cells to chemotherapy
in certain types of tumors but does not sensitize them in others by
regulating alternative splicing of caspase 9 in various
ways. It is possible that a balance exists among apoptotic genes
including Bcl-x and caspase 9. In addition, it appears that
chemotherapy that is effective for one type of cancer may not be
effective for others, although the target is the same (such as
caspase 9). Our studies provide insights into the future treatment
of cancers by targeting alternative splicing.
Acknowledgements
Dr Kritsanapol Boon-Unge was partly funded by the
Royal Thai Government Staff Development Scholarship. JZ was funded
by a Nantong University start-up fund and by the Priority Academic
Program Development of Jiangsu Higher Education Institution
(PAPD).
References
|
1
|
Mercatante D and Kole R: Modification of
alternative splicing pathways as a potential approach to
chemotherapy. Pharmacol Ther. 85:237–243. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Black DL: Protein diversity from
alternative splicing: a challenge for bioinformatics and
post-genome biology. Cell. 103:367–370. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Graveley BR: Alternative splicing:
increasing diversity in the proteomic world. Trends Genet.
17:100–107. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goldstrohm AC, Greenleaf AL and
Garcia-Blanco MA: Co-transcriptional splicing of pre-messenger
RNAs: considerations for the mechanism of alternative splicing.
Gene. 277:31–47. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Caceres JF and Kornblihtt AR: Alternative
splicing: multiple control mechanisms and involvement in human
disease. Trends Genet. 18:186–193. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Modrek B and Lee C: A genomic view of
alternative splicing. Nat Genet. 30:13–19. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Krawczak M, Reiss J and Cooper DN: The
mutational spectrum of single base-pair substitutions in mRNA
splice junctions of human genes: causes and consequences. Hum
Genet. 90:41–54. 1992. View Article : Google Scholar
|
|
8
|
Schwerk C and Schulze-Osthoff K:
Regulation of apoptosis by alternative pre-mRNA splicing. Mol Cell.
19:1–13. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Johnson CR and Jarvis WD: Caspase-9
regulation: an update. Apoptosis. 9:423–427. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moore MJ, Wang Q, Kennedy CJ and Silver
PA: An alternative splicing network links cell-cycle control to
apoptosis. Cell. 142:625–636
|
|
11
|
Chalfant CE, Rathman K, Pinkerman RL, et
al: De novo ceramide regulates the alternative splicing of caspase
9 and Bcl-x in A549 lung adenocarcinoma cells. Dependence on
protein phosphatase-1. J Biol Chem. 277:12587–12595. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Massiello A and Chalfant CE: SRp30a
(ASF/SF2) regulates the alternative splicing of caspase-9 pre-mRNA
and is required for ceramide-responsiveness. J Lipid Res.
47:892–897. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Merdzhanova G, Edmond V, De Seranno S, et
al: E2F1 controls alternative splicing pattern of genes involved in
apoptosis through upregulation of the splicing factor SC35. Cell
Death Differ. 15:1815–1823. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grollman AP: Inhibitors of protein
biosynthesis. V Effects of emetine on protein and nucleic acid
biosynthesis in HeLa cells. J Biol Chem. 243:4089–4094.
1968.PubMed/NCBI
|
|
15
|
Boon-Unge K, Yu Q, Zou T, Zhou A,
Govitrapong P and Zhou J: Emetine regulates the alternative
splicing of Bcl-x through a protein phosphatase 1-dependent
mechanism. Chem Biol. 14:1386–1392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jondorf WR, Abbott BJ, Greenberg NH and
Mead JA: Increased lifespan of leukemic mice treated with drugs
related to (-)-emetine. Chemotherapy. 16:109–129. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Johnson RK and Jondorf WR: Some inhibitory
effects of (--)-emetine on growth of Ehrlich ascites carcinoma.
Biochem J. 140:87–94. 1974.PubMed/NCBI
|
|
18
|
Panettiere F and Coltman CA Jr: Experience
with emetine hydrochloride (NSC 33669) as an antitumor agent.
Cancer. 27:835–841. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Siddiqui S, Firat D and Olshin S: Phase II
study of emetine (NSC-33669) in the treatment of solid tumors.
Cancer Chemother Rep. 57:423–428. 1973.PubMed/NCBI
|