Introduction
Erythropoiesis-stimulating agents (ESAs) are
commonly used to treat chemotherapy-induced anemia. The
administration of these agents has been shown to be effective for
treating anemia in patients who undergo chemotherapy. These agents
are effective as they increase hemoglobin (Hb) concentrations and
reduce or eliminate the need for red blood cell (RBC) transfusions,
thus improving quality of life (QoL) (1–3). In
anemic patients with cancer, ESAs were initially administered 3
times weekly, a schedule that had already proved effective in
patients with renal anemia (4,5).
Once-weekly (Q1W) administration with all ESAs has become the
preferred treatment modality (6,7).
Darbepoetin α has also been licensed for use once every 3 weeks
(Q3W) in cancer patients with chemotherapy-induced anemia (8). Continuous erythropoietin receptor
activator (C.E.R.A.) is an innovative agent with a prolonged
half-life compared with that of epoetin α and epoetin β in healthy
volunteers, and darbepoetin α in patients with peritoneal dialysis
(9). C.E.R.A. is a chemically
synthesized continuous erythropoietin receptor activator that
differs from erythropoietin through the integration of amide bonds
between amino groups and methoxy polyethylene glycol-succinimidyl
butanoic acid (10,11). It has been developed to provide
correction of anemia and to control Hb levels at extended
administration intervals in patients with CKD on dialysis and not
on dialysis (9). Moreover, C.E.R.A.
is currently in development for the correction of anemia and stable
control of Hb levels at Q1W and Q3W administration intervals in
cancer patients with chemotherapy-induced anemia. In preclinical
studies and studies in healthy subjects, C.E.R.A. had a lower
systemic clearance and an increased elimination half-life compared
with conventional ESAs, and superior potency in vivo with
respect to the magnitude and duration of response (12,13).
An exploratory Phase I-II dose-escalation study in anemic patients
with multiple myeloma receiving myelosuppressive chemotherapy
confirmed the long half-life of C.E.R.A. Additionally, a
dose-dependent increase in Hb response was observed with C.E.R.A.
doses up to 8.0 μg/kg when administered Q3W by subcutaneous (SC)
injection (14). Moreover, two
Phase II dose-finding studies were carried out in anemic patients
with aggressive non-Hodgkin’s lymphoma and advanced non-small cell
lung cancer (NSCLC) receiving myelosuppressive chemotherapy
(15,16). A dose-dependent increase in Hb
response was observed with C.E.R.A. doses up to 6.3 μg/kg when
administered Q3W by SC injection. Notably, there was a trend to
higher mean Hb increases and lower transfusion use in the Q3W
groups as compared to the Q1W groups with respect to the NSCLC
study. However, further dose-finding studies that use higher doses
and allow dose escalation are required to determine the optimal
C.E.R.A. Q3W dose regimen was administered in anemic cancer
patients receiving chemotherapy as a limited effect was obtained
even at the highest dose level of 6.3 μg/kg used in aggressive
non-Hodgkin’s lymphoma (15) and
advanced non-small cell lung cancer (NSCLC) (16). Additonally, with regards to safety,
neither dose-dependence in adverse events nor dose-limiting
toxicity was observed at the dose level of 8.0 μg/kg (14).
This multicenter, open-label study was designed to
evaluate the pharmacokinetic (PK)/pharmacodynamic (PD) properties
and safety of five different dose levels of C.E.R.A. administered
subcutaneously Q3W for up to 12 weeks in Japanese lung cancer
patients with anemia induced by myelosuppressive chemotherapy.
Patients and methods
Patients
A total of 47 adult patients, aged ≥20 and <80
years at the time of registration, were recruited based on the
following criteria: patient is diagnosed with lung cancer by tissue
or cytological examination, receiving cyclic chemotherapy for ≤4
weeks as 1 cycle, and capable of undergoing chemotherapy within 3
days following the start of administration of the investigational
medication. Patients were also required to have Hb levels ≤11.0
g/dl at the registration examination, a life expectancy of ≥4
months, Eastern Cooperative Oncology Group (ECOG) performance
status grades of 0–2, a satisfactory mean corpuscular volume (MCV),
and to meet transferrin saturation (Tsat), hepatic and renal
function criteria [MCV 80 fl or higher, transferrin saturation
[{Fe/(Fe + UIBC)}x100] of 15% or higher, total bilirubin value in
serum: ≤2.0 mg/dl, AST [GOT], ALT [GPT]: 80 IU/l or lower, serum
creatinine value: <2.0 mg/dl]. Exclusion criteria included
transfusion within 4 weeks prior to the planned start of
administration of the investigational medication; severe
hypertension uncontrollable by pharmaceutical products; marked
hemorrhagic lesions possibly affecting evaluation in the clinical
study or presence of serious complications; pregnant or nursing
women, who were premenopausal and tested positive for pregnancy in
a pregnancy test; expression of lack of intention to use
contraception; history/complication of cardiac infarction,
pulmonary infarction or cerebral infarction (excluding asymptomatic
cerebral infarction); and serious medication allergy including
anaphylactic shock. Patients recruited had not received treatment
with ESAs within the 4 weeks prior to registration. The design and
conduct of the study complied with the ethical principles of good
clinical practice, in accordance with the Declaration of Helsinki.
The study was approved by an independent institutional review board
at each cancer center, and all 47 patients provided written
informed consent prior to enrollment.
Study medication
Three separate strength vials were available (200,
400 or 1000 μg/ml), each containing a 1 ml solution of C.E.R.A.
Study design
This was an open-label, multicenter, clinical
pharmacology study that involved SC injections of C.E.R.A. over a
12-week treatment period, and a follow-up period for 3 weeks
following the last administration of C.E.R.A. Patients were
assigned sequentially according to increasing dose rotations to one
of five groups, receiving C.E.R.A. at 2.1, 4.2, 6.3, 9 or 12 μg/kg.
This was administered Q3W by SC injection for 12 weeks. If Hb
levels recovered to >13.0 g/dl, treatment was discontinued.
Chemotherapy and radiotherapy were used concomitantly during the
period from the day of starting administration to the time of the
last observation. Blood transfusions were performed concurrently in
patients who did not show improvement in anemia and were judged
clinically to require blood transfusion. Oral iron supplementation
was administered daily during the administration period of the
study medication if MCV was <80 fl or Tsat was <15%. Blood
samples for detection of the C.E.R.A. antibody were collected prior
to the first administration and within a maximum of 50 days
following the final administration.
Serum assay
To determine PK parameters, blood samples were
collected immediately prior to and 1, 8, 15, 22, 23–26, 27, 29, 32,
36, 43, 64 and 85 days following the initial administration of
C.E.R.A. To investigate concentrations over time, samples were also
collected immediately prior to the administration of each dose.
Blood samples were allowed to stand at room temperature for 30 min
and were then centrifuged at 4°C and 3,000 rpm for 10 min to
separate the serum. The resulting serum was stored below −20°C
until used for the measurement of serum C.E.R.A. concentrations.
Serum concentrations of C.E.R.A. were measured by a validated
enzyme-linked immunosorbent assay using a primary monoclonal
antibody specific to C.E.R.A. that did not cross-react with
endogenous erythropoietin, and a secondary polyclonal
anti-immunoglobulin antibody coupled to horseradish peroxidase
(Huntingdon Life Sciences, Alconbury, UK). The assay range was 150
to 4000 pg/ml. The inter-batch assay precision was 7.8 to 11.4%,
and accuracy was −7.8 to −6.6%. The assay is specific to C.E.R.A.
and does not detect human erythropoietin, and human erythropoietin
does not interfere with the assay.
Pharmacokinetic analyses
Serum concentrations of C.E.R.A. were used to
determine the maximum serum concentration (Cmax) and
time to maximum serum concentration (Tmax). The
t1/2 was estimated for ln(2)/k, where the rate constant of
elimination (k) was determined by linear regression of the
logarithm of the serum concentration vs. time data in the
post-distribution phase. The area under the concentration-time
curve (AUC) following C.E.R.A. administration, from pre-dose on day
22 until the last sampling time at which the concentration was
measurable (day 43), was estimated by the linear trapezoidal
rule.
Pharmacodynamic analyses
The PD parameters involved the change in Hb from the
nadir value. The change in the Hb level from the nadir value was
calculated by subtraction of the nadir value over the period weeks
1–4 from the Hb value at week 7. The increase in Hb produced by
C.E.R.A. administration was estimated to occur over the 4 weeks
following first administration of C.E.R.A. and certain patients
were withdrawn from the study after 6 weeks (17). Moreover, to estimate the increase of
Hb caused by C.E.R.A., the effect of chemotherapy on Hb was
removed. Therefore, to evaluate the effect of C.E.R.A. on Hb
levels, the change in Hb was calculated between the nadir values
from weeks 1–4 and the values at week 7.
Safety
Safety endpoints included adverse events, clinical
laboratory tests, vital signs, body weight, physical examination
and ECGs. The intensities of adverse events and laboratory values
were graded according to the National Cancer Institute/Common
Terminology Criteria for Adverse Events v3.0 (CTCAE).
Statistical analysis
Mean, standard deviation, and the coefficient of
variation of the PK parameters were calculated for each treatment.
Mean, standard deviation, median, range and standard error are
provided per day for Hb levels. Descriptive statistics were
calculated using SAS (version 9.1.3). Figures were prepared with
S-Plus (version 8.1).
Results
Patients
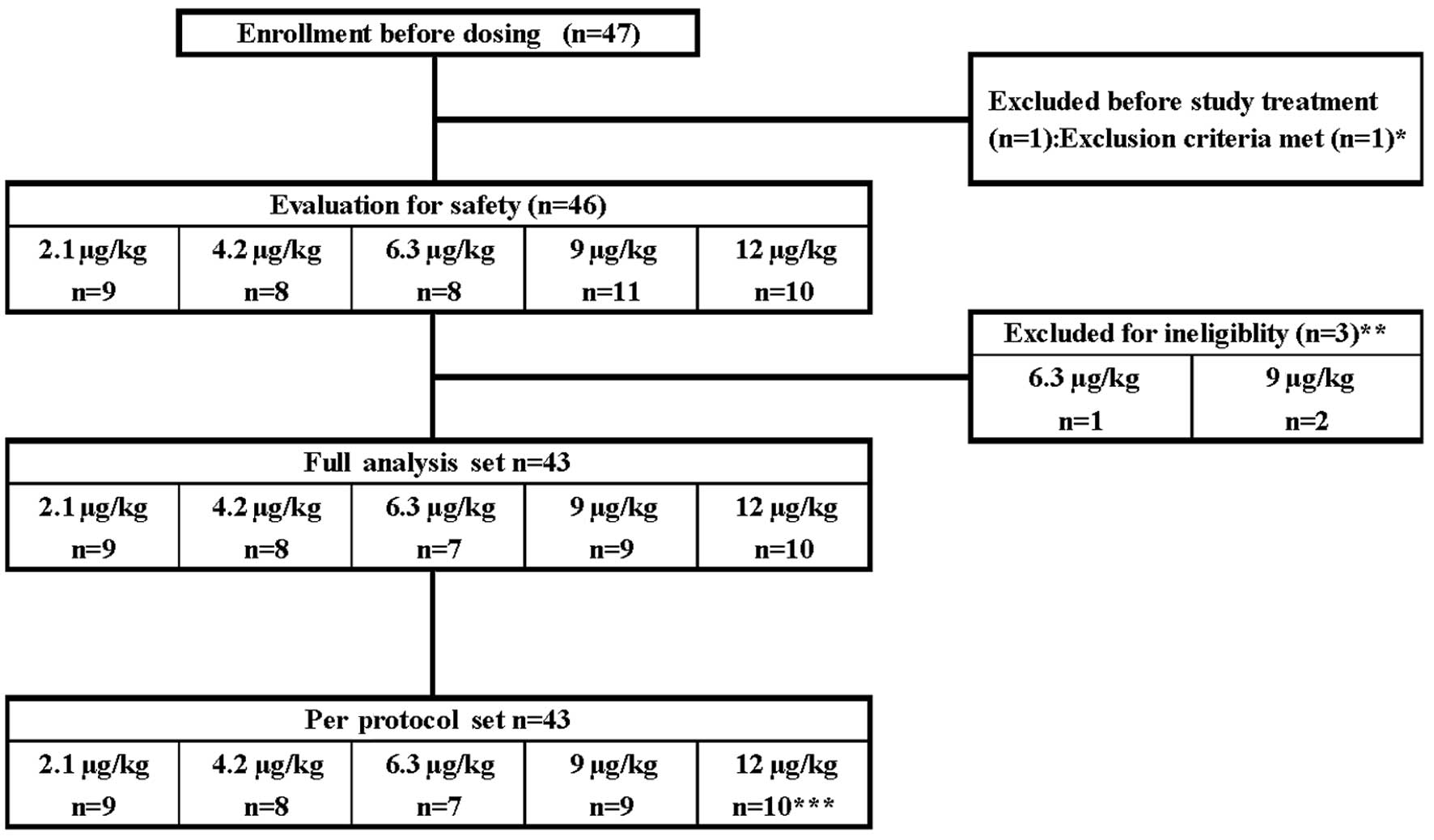
A total of 47 lung cancer patients were enrolled
(Fig. 1). Treatment of one patient
assigned to the 9 μg/kg dose group was discontinued due to the
development of ileus prior to the first administration. The
remaining 46 patients were treated with each dose of C.E.R.A. and
were included in the safety populations. In total, 46 patients
completed the study treatment period. However, one patient assigned
to the 6.3 μg/kg dose group and two patients assigned to the 9
μg/kg dose group were excluded from the PK and PD analyses due to
defects in the study medication, which included vial blistering and
contents out of specification. The remaining 43 patients were
included in the analysis of full analysis set and per protocol set
populations. However, one patient in the 12 μg/kg dose group was
excluded from the data analysis following week 7 due to a defect in
the medication vial. Doses were held for a total of 25 patients.
Consequently, these patients were not administered the full four
doses of the study medication during the 12-week period. The
reasons for discontinuation of the study medication were
progressive disease, Hb levels >13 g/dl, Hb level elevation of
≥2.0 g/dl from previous treatment to next treatment and termination
of concomitant chemotherapy.
Baseline characteristics and demographics are shown
in Table I. Patients had received
chemotherapy prior to the start of this study. A total of 11, 13,
25, 18 and 10% of patients had undergone surgery and 33, 50, 13, 18
and 20% of patients had undergone radiotherapy in the 2.1, 4.2,
6.3, 9 and 12 μg/kg dose groups, respectively. Median Hb levels
ranged from 8.9 to 10.5 g/dl across all dose groups. The values for
iron parameters in the 9 and 12 μg/kg dose groups at baseline were
lower than those in the 2.1, 4.2 and 6.3 μg/kg dose groups.
 | Table ISummary of patient’s baseline
characteristics (range or %): safety population. |
Table I
Summary of patient’s baseline
characteristics (range or %): safety population.
| C.E.R.A. dose group
(μg/kg Q3W) |
|---|
|
|
|---|
| 2.1 μg/kg
n=9 | 4.2 μg/kg
n=8 | 6.3 μg/kg
n=8 | 9 μg/kg
n=11 | 12 μg/kg
n=10 |
|---|
| Gender (%) |
| Male | 6 (67) | 8 (100) | 4 (50) | 8 (73) | 8 (80) |
| Female | 3 (33) | 0 | 4 (50) | 3 (27) | 2 (20) |
| Median age,
years | 59 (53–79) | 67 (59–76) | 64 (34–72) | 67 (50–77) | 71 (56–79) |
| Median body weight
(kg) | 60 (53–75) | 56 (47–66) | 58 (48–61) | 56 (44–75) | 52 (44–66) |
| ECOG PS (%) |
| 0 | 5 (56) | 1 (12) | 6 (75) | 2 (18) | 3 (30) |
| 1 | 4 (44) | 7 (88) | 2 (25) | 9 (82) | 7 (70) |
| 2 | 0 | 0 | 0 | 0 | 0 |
| History of lung
cancer |
| Small cell | 2 (22) | 3 (38) | 5 (63) | 2 (18) | 6 (60) |
| Non-small cell | 7 (78) | 5 (62) | 3 (37) | 8 (73) | 4 (40) |
| Mixed type | 0 | 0 | 0 | 1 (9) | 0 |
| Previous treatment
(%) |
| Chemotherapy | 9 (100) | 8 (100) | 8 (100) | 11 (100) | 10 (100) |
| Surgery | 1 (11) | 1 (13) | 2 (25) | 2 (18) | 1 (10) |
| Radiotherapy | 3 (33) | 4 (50) | 1 (13) | 2 (18) | 2 (20) |
| Transfusion | 0 | 1 (13) | 1 (13) | 0 | 0 |
| Median Hb level
(g/dl) | 9.2 (7.7–10.5) | 10.0
(9.3–10.8) | 10.5
(8.8–10.9) | 8.9 (7.2–10.4) | 10.0
(8.1–11.8) |
| Median serum
ferritin (ng/ml) | 516 (85–800) | 392 (147–771) | 139 (10–2100) | 340 (13–647) | 286 (55–651) |
| Median serum Fe
(μg/dl) | 72 (33–111) | 68 (22–75) | 76 (19–123) | 49 (19–70) | 47 (15–69) |
| Median Tsat
(%) | 24.3
(11.0–46.3) | 25.8
(10.0–35.4) | 28.0
(6.7–42.7) | 18.7
(10.0–31.0) | 16.3
(8.4–27.6) |
Patients received at least one chemotherapy cycle
within 3 days following the initial C.E.R.A. administration, which
was most commonly platinum-based chemotherapy (the percentage of
carboplatin or cisplatin treatments: 78, 63, 43, 78 and 40% in the
2.1, 4.2, 6.3, 9 and 12 μg/kg dose groups, respectively). Other
common chemotherapies included amrubicin hydrochloride (11, 13, 29,
0 and 60% in the 2.1, 4.2, 6.3, 9, and 12 μg/kg dose groups,
respectively) and docetaxel hydrate (11, 25, 29, 22 and 0% in the
2.1, 4.2, 6.3, 9 and 12 μg/kg dose groups, respectively) (Table II).
 | Table IISummary of patient’s baseline Hb
level, reticulocyte counts and oral supplementation and combination
chemotherapy (range or %): PPS population. |
Table II
Summary of patient’s baseline Hb
level, reticulocyte counts and oral supplementation and combination
chemotherapy (range or %): PPS population.
| C.E.R.A. dose group
(μg/kg Q3W) |
|---|
|
|
|---|
| 2.1 μg/kg
n=9 | 4.2 μg/kg
n=8 | 6.3 μg/kg
n=7 | 9 μg/kg
n=9 | 12 μg/kg
n=10 |
|---|
| Reticulocyte counts
×104/mm3 | 9.4 (3.3–12.5) | 8.0a (4.3–15.0) | 9.4 (3.3–12.5) | 6.6 (3.2–11.2) | 6.0 (2.6–10.4) |
| Oral iron
supplementation during treatment (%) | 3 (33) | 3 (38) | 3 (33) | 8 (89) | 5 (50) |
| Chemotherapy, n,
(%) |
| Platinumsb | 4 (44) | 4 (50) | 4 (44) | 5 (55) | 3 (30) |
| Platinums +
taxanes | | 3 (33) | 0 | 2 (22) | 1 (10) |
| Non-platinums | 2 (22) | 3 (38) | 2 (22) | 2 (22) | 6 (60) |
| Taxanes | 1 (11) | 2 (25) | 1 (11) | 2 (22) | 0 |
|
Anthracyclines | 1 (11) | 1 (13) | 1 (11) | 0 | 6 (60) |
Overall, 22 patients received oral iron therapy. The
percentage of patients receiving concomitant oral iron
supplementation was higher in the 9 and 12 μg/kg dose groups (89
and 50%, respectively) compared with the 2.1, 4.2 and 6.3 μg/kg
dose groups (33, 38 and 43%, respectively) (Table II).
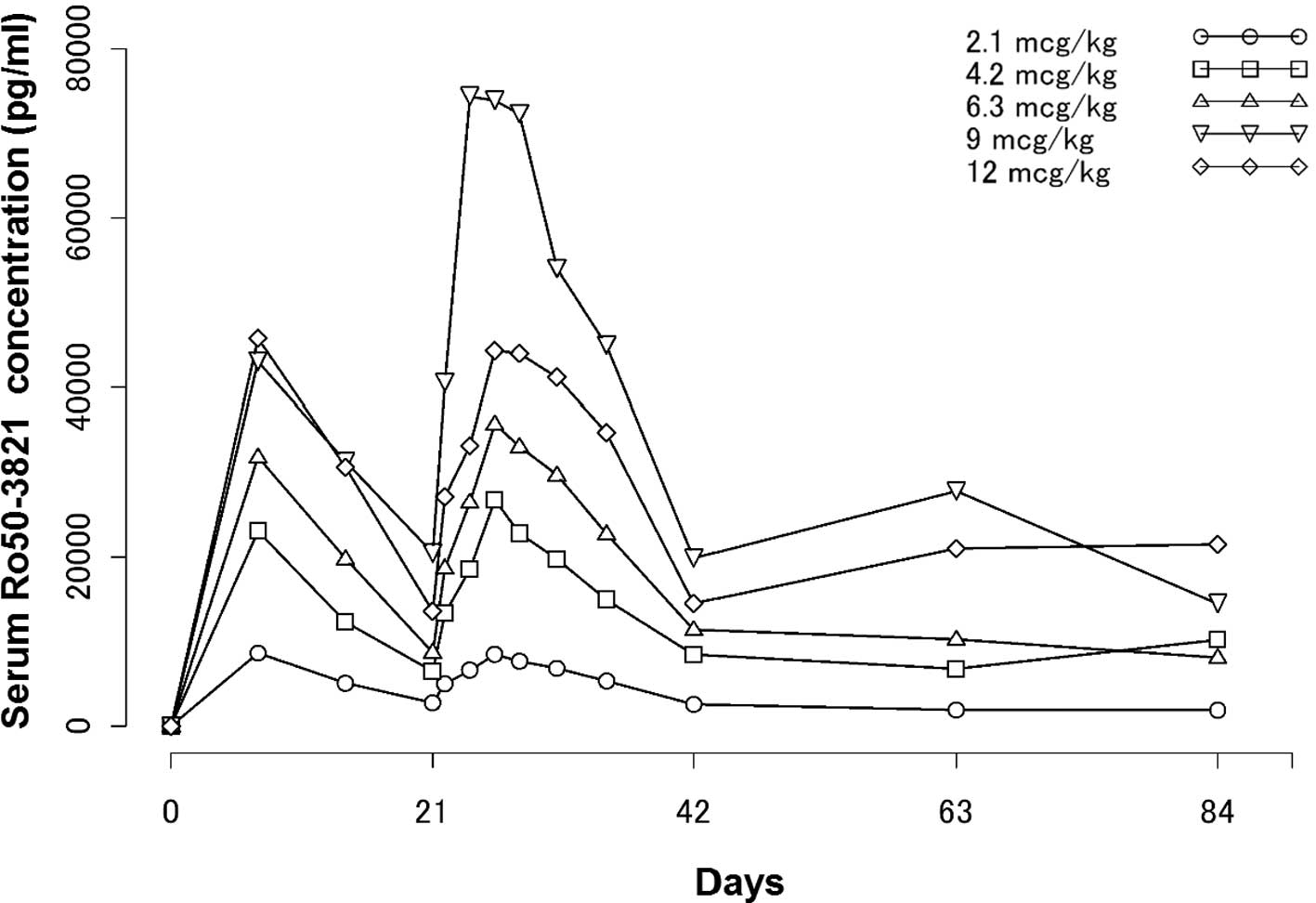
Pharmacokinetic analyses
Serum C.E.R.A. concentration- time profiles from
weeks 1–13 are shown in Fig. 2. PK
parameters were estimated following the second dosing and the
correlation between dose and Cmax was estimated
following the initial dosing. PK parameter values are shown in
Table III. Serum concentrations
of C.E.R.A. following the second administration in the 2.1, 4.2,
6.3, 9 and 12 μg/kg dose groups reached Cmax at 119 to
167 h (mean); the drug was then eliminated with half-lives
(t1/2) of 143 to 217 h, respectively (median).
Tmax values were similar among the dose groups.
T1/2 values were shown to be high across the dose groups
and appeared to show no major differences among the dose
groups.
 | Table IIIPK parameters following the second
dosing. |
Table III
PK parameters following the second
dosing.
| Parameter | Dose (μg/kg) | Mean ± SD | No. |
|---|
| Cmax
(ng/ml) | 2.1 | 9.09±5.26 | 9 |
| 4.2 | 27.4±7.63 | 8 |
| 6.3 | 38.9±13.9 | 7 |
| 9 | 84.6±36.0 | 9 |
| 12 | 47.8±14.3 | 10 |
| Tmax
(h) | 2.1 | 146±43.1 | 9 |
| 4.2 | 130±23.1 | 8 |
| 6.3 | 119±43.6 | 7 |
| 9 | 149±80.2 | 9 |
| 12 | 167±42.6 | 10 |
| AUCt
(ng*h/ml) | 2.1 | 2730±1680 | 9 |
| 4.2 | 8540±2430 | 8 |
| 6.3 | 12100±4630 | 7 |
| 9 | 23700±9470 | 9 |
| 12 | 16200±5520 | 10 |
| t1/2
(h) | 2.1 | 185 (152–276) | 6 |
| 4.2 | 217 (176–279) | 8 |
| 6.3 | 175 (165–182) | 6 |
| 9 | 143 (126–163) | 7 |
| 12 | 162 (159–237) | 7 |
| AUCinf
(ng*h/ml) | 2.1 | 4060±2380 | 6 |
| 4.2 | 12000±5580 | 8 |
| 6.3 | 16100±7220 | 6 |
| 9 | 30100±12500 | 7 |
| 12 | 23400±8080 | 7 |
| Cmin
(pg/ml) | 2.1 | 2900±1680 | 8 |
| 4.2 | 8460±4000 | 8 |
| 6.3 | 11400±5480 | 7 |
| 9 | 19900±15200 | 9 |
| 12 | 14500±7130 | 10 |
| Cav
(ng/ml) | 2.1 | 3.74±2.85 | 9 |
| 4.2 | 8.92±2.22 | 8 |
| 6.3 | 12.3±4.4 | 7 |
| 9 | 27.4±13.4 | 9 |
| 12 | 19.5±6.68 | 10 |
| MRT (day) | 2.1 | 15.9±5.87 | 6 |
| 4.2 | 16.7±5.74 | 8 |
| 6.3 | 15±5.59 | 6 |
| 9 | 10.9±1.74 | 7 |
| 12 | 15.5±6.89 | 7 |
AUCinf in the 2.1, 4.2, 6.3, 9 and 12
μg/kg dose groups were 4060±2380, 12000±5580, 16100±7220,
30100±12500 and 23400±8080 ng•h/ml, respectively. Cmax
values were 9.09±5.26, 27.4±7.63, 38.9±13.9, 84.6±36.0 and
47.8±14.3 ng/ml, respectively.
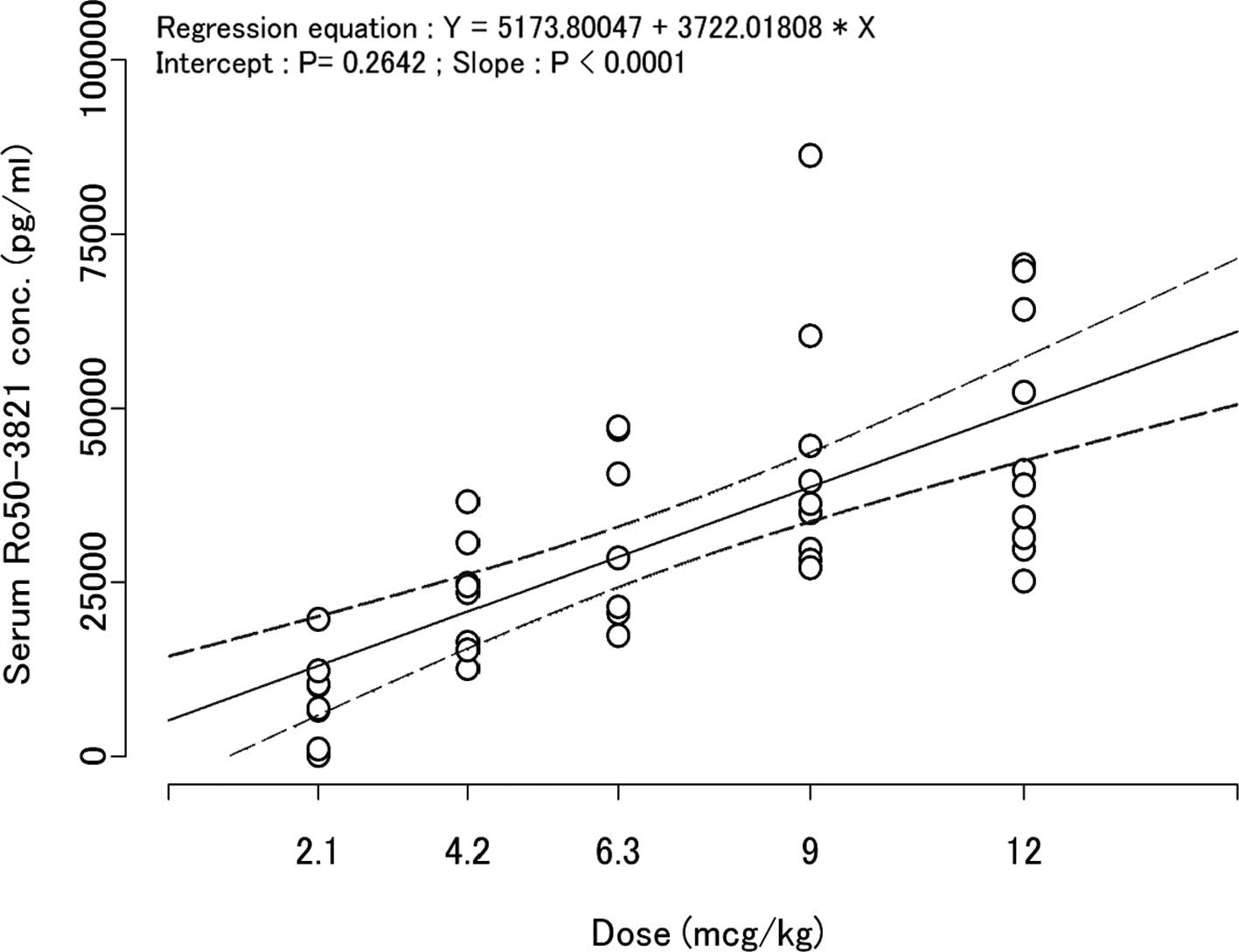
Exposure (AUCinf, Cmax) was
increased in proportion to the dose from the 2.1 μg/kg to the 9
μg/kg dose groups. On the other hand, no such increases were
observed between the 9 μg/kg and 12 μg/kg dose groups. The 95%
confidence interval for the y-intercept of the regression line
between dose and Cmax was 0 (zero) (Fig. 3).
Photodynamic analyses
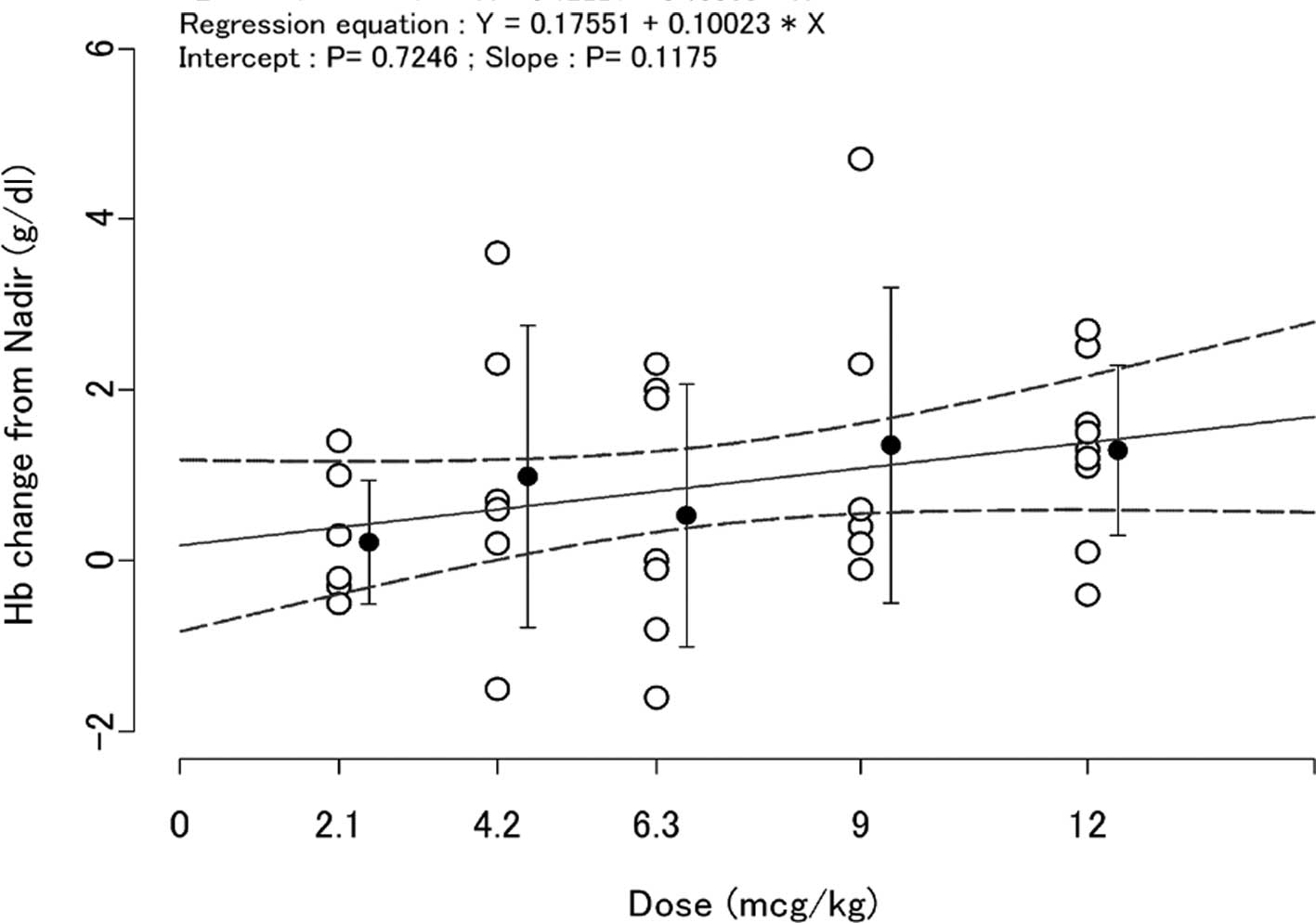
The change in Hb levels from the nadir value over
the period weeks 1–4 to week 7 values is shown in Fig. 4. The changes in Hb levels were above
0 (zero) in the dosing groups and the slope between the dose of
C.E.R.A. and the change in Hb was greater than 0 (zero) (Fig. 4).
Safety and tolerability
Q3W administration of C.E.R.A. was generally well
tolerated across the dose groups. All of the patients reported at
least one adverse event. Most adverse events were those expected in
cancer patients receiving chemotherapy, and none occurred in a
manner dependent on the C.E.R.A. dose. The percentage of patients
reporting adverse events in which a causal correlation with the
study medication cannot be completely excluded were 89, 75, 88, 64
and 40% in the 2.1, 4.2, 6.3, 9 and 12 μg/kg dose groups,
respectively. The most common adverse events (incidence rate ≥10%)
related to the study medication were potassium increase (17%),
neutrophil decrease (13%), WBC decrease (11%), diarrhea (11%),
constipation (11%) and headache (11%). A total of 10 patients
experienced grade 3–4 adverse events related to the study treatment
including WBC decrease, febrile neutropenia, neutrophil decrease,
Hb decrease, platelet decrease, lymphocyte decrease, potassium
increase and sodium decrease (Table
IV). No clinically significant changes occurred from baseline
in laboratory values and vital signs during the study period in the
dose groups, or dose-dependent correlations with increased blood
pressure. One patient in the 6.3 μg/kg dose group experienced grade
2 hypertension possibly associated with C.E.R.A. treatment from the
1st to the 14th day of the second administration. An
antihypertensive medication was administered following the onset of
hypertension. Blood pressure was then stabilized.
 | Table IVMost common reported adverse events
related to study treatment (in ≥10% of patients in any treatment
group). |
Table IV
Most common reported adverse events
related to study treatment (in ≥10% of patients in any treatment
group).
| Adverse event, n
(%) | 2.1 μg/kg
(n=9) | 4.2 μg/kg
(n=8) | 6.3 μg/kg
(n=8) | 9 μg/kg (n=11) | 12 μg/kg
(n=10) | Total (n=46) |
|---|
| Potassium
increase | 2 (22.2) | 3 (37.5) | - | 1 (9.1) | 2 (20.0) | 8 (17) |
| Neutrophils
decrease | 2 (22.2) | 3 (37.5) | 1 (12.5) | - | - | 6 (13) |
| WBC decrease | 3 (33.3) | 3 (37.5) | - | - | - | 6 (13) |
| Lymphocytes
decrease | 1 (11.1) | 3 (37.5) | 1 (12.5) | - | - | 5 (11) |
| Sodium
increase | 2 (22.2) | - | - | 1 (9.1) | 2 (20.0) | 5 (11) |
| Diarrhea | 1 (11.1) | - | 1 (12.5) | - | 3 (30.0) | 5 (11) |
| Constipation | 3 (33.3) | 2 (25.0) | - | - | - | 5 (11) |
| Headache | 1 (11.1) | - | 3 (37.5) | 1 (9.1) | - | 5 (11) |
Serious adverse events were observed in one patient
from each of the 4.2 and 6.3 μg/kg dose groups, and three patients
in the 12 μg/kg dose group. Serious adverse events were evaluated
as not related to the study medication.
Thrombovascular adverse events were not observed.
Withdrawal of one patient in the 12 μg/kg dose group was attributed
to disease progression and no mortality was reported during the
study period. Moreover, no anti-C.E.R.A. antibodies were detected
in any of the patients.
Discussion
Anemia is a frequent complication in patients with
lung cancer who are administered chemotherapy (18,19).
Anemia has a profound impact on QoL, with fatigue being one of its
common symptoms (20–22). Furthermore, the widespread use of
platinum-based chemotherapy contributes further to the development
of anemia in patients with lung cancer (23).
C.E.R.A. is a chemically synthesized continuous
erythropoietin receptor activator with a prolonged serum half-life
that has been shown to be safe and effective for the treatment of
chemotherapy-induced anemia when administered using Q1W or Q3W
administration schedules (16).
This is the first study to examine the PK, PD and
safety profiles of C.E.R.A. treatment subcutaneously Q3W in
Japanese patients with lung cancer and anemia induced by
chemotherapy. This study demonstrated that C.E.R.A. subcutaneously
administered to Japanese lung cancer patients showed exposure in
accordance with dose increase and a long half-life. Exposure
(AUCinf, Cmax) following second
administration increased with dose proportionality in the 2.1 to 9
μg/kg dose groups. On the other hand, exposure was not increased
across the 9 to 12 μg/kg dose groups. The reason for this
non-linearity between dose and exposure may be due to the small
number of patients, the verification of patient characteristics at
baseline and the fact that the information was limited. It was
considered to be reduced bioavailability from the SC injection site
in the 12 μg/kg dose group, as the Tmax values (an
indicator of SC absorption rate) were similar among the dose groups
and the t1/2 values (indicator of elimination rate) also
showed no major differences among the dose groups. A similar
phenomenon was observed following SC injection of epoetin β
(24). Nakagawa et al
reported that Cmax and AUCinf following SC
injection of epoetin β to lung cancer patients increased with
dose-proportionality from the 9000 to 36000 IU dose groups and were
similar between the 36000 and 54000 IU dose groups (25). These authors also suggested that
declining bioavailability of epoetin β at higher doses is partially
due to absorption following SC injection of this drug into the
lymphatic system (26). The same
mechanism may explain the reason for non-linearity between the dose
of C.E.R.A. and levels of exposure, but further studies are
required to clarify this non-linearity.
Median values for t1/2 ranged from 143 to
217 h in this study. These results showed that the values for
t1/2 of C.E.R.A. were prolonged by 5–10 times compared
with those reported for epoetin β in patients with lung cancer
(24,25) or 2–4 times compared with those
reported for darbepoetin α in patients with cancer (27).
Agoram et al suggested that the
platinum-containing chemotherapy cycle count affected the clearance
of darbepoetin α (28); thus, the
PK parameters following the initial administration of C.E.R.A. were
assessed to estimate the parameters under the same conditions of
chemotherapy. Following the first C.E.R.A. administration,
Cmax showed dose-proportionality as the 95% confidence
interval for the y-intercept of the regression line between dose
and Cmax was 0 (zero) (Fig.
3). These results suggested linear PKs of C.E.R.A. following SC
administration, above the range of 2.1 to 12 μg/kg.
In addition, changes in Hb levels from the nadir
value over the period weeks 1–4 to week 7 values were observed
(Fig. 4). The change in Hb levels
was above 0 (zero) in all dose groups and the slope of the line
relating dose of C.E.R.A. to the change of Hb was over 0 (zero).
These results suggest that the SC administration of C.E.R.A. above
the range of 2.1 to 12 μg/kg Q3W increased Hb levels in these
patients and these increases were somewhat dose-dependent. The Hb
responses supported results previously observed in studies with
C.E.R.A. in cancer patients following SC Q3W administration
(15,16). Furthermore, the responses observed
at doses of 9 and 12 μg/kg do not appear to have previously been
reported.
All 46 patients receiving C.E.R.A. administration at
least once were included in the safety analyses. C.E.R.A. was
generally well tolerated across the dose groups, with adverse
events that may be expected for patients with lung cancer receiving
chemotherapy (e.g., neutrophil decrease), and these were similar to
those reported for epoetin β (24).
The most common adverse events were those expected in a cancer
population receiving chemotherapy. Adverse events reported with
regards to the study medication were observed in 32 (69.6%)
patients. C.E.R.A. did not appear to have any adverse effects
involving occurrence of thrombovascular events.
In conclusion, the dose proportionality observed
regarding the PK and PD profiles and the good tolerability and
safety profile in this study involving a small number of patients
with lung cancer suggested that extended Q3W administration
intervals are feasible in the clinic. However, further dose-finding
studies may be required to determine the optimal C.E.R.A. dose
regimen at Q3W in cancer patients with anemia induced by
chemotherapy.
Acknowledgements
This study was funded by Chugai Pharmaceuticals Co.
Ltd., Tokyo, Japan. Study medications were provided by Hoffmann-La
Roche Inc. Responsibility for opinions, conclusions and
interpretation of data lies with the authors.
References
|
1
|
Oberhoff C, Neri B, Amadori D, Perty KU,
Gamucci T, Rebmann U, et al: Recombinant human erythropoietin in
the treatment of chemotherapy induced anemia and prevention of
transfusion requirement associated with solid tumors: a randomized,
controlled study. Ann Oncol. 9:239–241. 1998. View Article : Google Scholar
|
|
2
|
Littlewood TJ, Bajetta E, Nortier JW,
Vercammen E and Rapoport B; Epoetin Alfa Study Group. Effects of
epoetin alfa on hematologic parameters and quality of life in
cancer patients receiving nonplatinum chemotherapy; results of a
randomized, double-blind, placebo-controlled trial. J Clin Oncol.
19:2865–2874. 2001.
|
|
3
|
Vansteenkiste J, Pirker R, Massuti B,
Barata F, Font A, Fiegl M, Siena S, Gateley J, Tomita D, Colowick
AB and Musil J: Double-blind placebo-controlled, randomized phase
III trial of darbepoetin alfa in lung cancer patients receiving
chemotherapy. J Natl Cancer Inst. 94:1211–1220. 2002. View Article : Google Scholar
|
|
4
|
Ludwig H, Fritz E, Kotzmann H, Hocker P,
Gisslinger H and Barnus U: Erythropoietin treatment of anemia
associated with multiple myeloma. N Engl J Med. 322:1693–1699.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eshbach JW, Egrie JC, Dowing MR, Browne JK
and Adamson JW: Correction of the anemia of end-stage renal disease
with recombinant human erythropoietin. Results of a combined phases
I and II clinical trial. N Engl J Med. 316:73–78. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cazzola M, Beguin Y, Kloczko J, Spicka I
and Coiffier B: Once-weekly epoetin beta is highly effective in
treating anaemic patients with lymphoproliferative malignancy and
defective endogenous erythropoietin production. Br J Haematol.
122:386–393. 2003. View Article : Google Scholar
|
|
7
|
Waltzman R, Croot C, Justice GR, Fesen MR,
Charu V and Williams D: Randomized comparison of epoetin alfa
(40,000 U weekly) and darbepoetin alfa (200 microg every 2 weeks)
in anemic patients with cancer receiving chemotherapy. Oncologist.
10:642–650. 2005. View Article : Google Scholar
|
|
8
|
Canon JL, Vansteenkiste J, Bodoky G,
Mateos MV, Bastit L, Ferreira I, Rossi G and Amado RG: Randomized,
double-blind, active-controlled trial of every-3-week darbepoetin
alfa for the treatment of chemotherapy-induced anemia. J Natl
Cancer Inst. 98:273–284. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Locatelli F and Reigner B: C.E.R.A.
pharmacodynamics, pharmacokinetics and efficacy in patients with
chronic kidney disease. Expert Opin Investig Drugs. 16:1649–1661.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Macdougall IC, Bailon P, Tare T and Pahlke
W: CERA (Continuous Erythropoiesis Receptor Activator) for the
treatment of renal anemia: an innovative agent with unique receptor
binding characteristics and prolonged serum half-life. J Am Soc
Nephrol. 14:769A(Abstract SU-PO 1063)2003.
|
|
11
|
Macdougall IC: CERA (Continuous
Erythropoietin Receptor Activator): a new
erythropoiesis-stimulating agent for the treatment of anemia. Curr
Hematol Rep. 4:436–440. 2005.PubMed/NCBI
|
|
12
|
Haselbeck A, Reigner B, Jordan B, Pannier
A and Glaspy J: Pre-clinical and Phase I pharmacokinetic and
mode-of-action studies of CERA (Continuous Erythropoiesis Receptor
Activator), an innovative erythropoietic agent with an extended
serum half-life. Proc Am Soc Clin Oncol. 22:748(Abstract
3006)2003.
|
|
13
|
Dougherty FC, Reigner B, Jordan P and
Pannier A: Continuous Erythropoiesis Receptor Activator (CERA)
provides dose-dependent erythropoietic activity with a prolonged
half-life in healthy volunteers. Ann Oncol. 15:iii157(Abstract
592)2004.
|
|
14
|
Dmoszynska A, Kloczko J, Rokicka M,
Hellmann A, Spicka I and Eid JE: A dose exploration, phase I/II
study of administration of continuous erythropoietin receptor
activator once every 3 weeks in anemic patients with multiple
myeloma receiving chemotherapy. Haematologica. 92:493–501. 2007.
View Article : Google Scholar
|
|
15
|
Osterborg A, Steegmann JL, Hellmann A,
Couban S, Mayer J and Eid JE: Phase II study of three dose levels
of continuous erythropoietin receptor activator (C.E.R.A.) in
anemic patients with aggressive non-Hodgkin’s lymphoma receiving
combination chemotherapy. Br J Haematol. 136:736–744.
2007.PubMed/NCBI
|
|
16
|
Hirsh V, Glaspy J, Mainwaring P, Manegold
C, Ramlau R and Eid JE: Phase II study of two dose schedules of
C.E.R.A. (Continuous Erythropoiesis Receptor Activator) in anemic
patients with advanced non-small cell lung cancer (NSCLC) receiving
chemotherapy. Trials. 8:82007. View Article : Google Scholar
|
|
17
|
Osterborg A, Brandberg Y, Molostova V,
Losava G, Abdalkadyrov K, Hedenus M and Messinger D: Randomized,
double-blind, placebo-controlled trial of recombinant human
erythropoietin, epoetin β, in hematologic malignancies. Journal of
Clinical Oncology. 20:2486–2494. 2002.
|
|
18
|
Crawford J: Anemia and lung cancer. Lung
Cancer. 38:S75–S78. 2002. View Article : Google Scholar
|
|
19
|
Langer CJ, Choy H, Glaspy JA and Colowick
A: Standards of care for anemia management in oncology; focus on
lung carcinoma. Cancer. 95:613–623. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cella D: Factors influencing quality of
life in cancer patients: anemia and fatigue. Semin Oncol. 25:43–46.
1998.PubMed/NCBI
|
|
21
|
Pirker R, Wiesenberger K, Pohl G and Minar
W: Anemia in lung cancer: clinical impact and management. Clin Lung
Cancer. 5:90–97. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morère JF: Role of epoetin in the
management of anemia in patients with lung cancer. Lung Cancer.
46:149–156. 2004.PubMed/NCBI
|
|
23
|
Ludwig H, Van Belle S, Barett-Lee P,
Birgegard G, Bokemeyer C, Gascón P, Kosmidis P, Krzakowski M,
Nortier J, Olmi P, Schneider M and Schrijvers D: The European
Cancer Anemia Survey (ECAS): a large, multinational, prospective
survey defining the prevalence, incidence, and treatment of anemia
in cancer patients. Eur J Cancer. 40:2293–2306. 2004. View Article : Google Scholar
|
|
24
|
Fujisaka Y, Tamura T, Ohe Y, Kunitoh H,
Sekine I, Yamamoto N, Nokihara H, Horiike A, Kodama T and Saijo N:
Pharmacokinetics and Pharmacodynamics of Weekly Epoetin Beta in
Lung Cancer Patients. Jpn J Oncol. 36:477–482. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakagawa K, Ozaki T, Satoh T, Miyazaki M,
Akashi Y, Terashima M, Fujisaka Y, Tamura T, Fukuoka M and Saijo N:
Epoetin Beta Subcutaneous Administration to Lung Cancer Patients
with Anemia; Results of Pharmacokinetics/Pharmacodynamics Study.
Jpn J Lung Cancer. 47:313–322. 2007. View Article : Google Scholar
|
|
26
|
Tang L, Persky AM, Hochhaus G and Meibohm
B: Pharmacokinetic aspects of biotechnology products. J Pharm Sci.
93:2184–2204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Glaspy J, Henry D, Patel R, et al: Effects
of chemotherapy on endogenous erythropoietin levels and the
pharmacokinetics and erythropoietic response of darbepoetin alfa: a
randomized clinical trial of synchronous versus asynchronous dosing
of darbepoetin alfa. Eur J Cancer. 41:1140–1149, Epub 2005 Apr
8.
|
|
28
|
Agoram B, Heatherington AC and Gastonguay
MR: Development and evaluation of a population
pharmacokinetic-pharmacodynamic model of darbepoetin alfa in
patients with nonmyeloid malignancies undergoing multicycle
chemotherapy. AAPS J. 8:E552–E563. 2006. View Article : Google Scholar
|