Introduction
Breast cancer is a significant threat to women’s
health, and with increasing rates of incidence, it is currently one
of the most common malignancies in women. However, as diagnostic
tools and treatment methods continue to improve, a decrease in the
mortality rate for breast cancer patients has been observed
(1). Currently, the clinical
treatment for breast cancer includes surgery, chemotherapy,
radiotherapy and endocrine therapy to provide a comprehensive
treatment regimen. The therapeutic effect of this combination of
treatments has also become more effective with the introduction of
new therapeutic drugs (2). In
particular, the availability of taxane drugs has improved the
effectiveness of chemotherapy treatments for breast cancer.
Taxol (paclitaxel) was identified as a drug that is
capable of inhibiting microtubule depolymerization, and as such,
may prevent spindle formation, cause cell cycle arrest in the G2/M
phase and terminate mitosis (3). In
the clinic, application of taxol was shown to have a significant
therapeutic effect on ovarian, breast, non-small cell lung and head
and neck cancer (4). However, not
all cell types were sensitive to the effects of taxol. Multidrug
resistance (MDR) is currently a leading cause of insensitivity to
chemotherapy, and taxol resistance was observed shortly after its
application (5,6). Additional studies indicated that
molecules which interfered with the binding of taxol to
microtubules (7) or the increased
expression of the drug transporter protein, P-glycoprotein
(8), may result in taxol resistance
(9). More recently, a series of
apoptosis-related genes, including bcl-2, p53 and erbB2, and
related enzymes such as PKC and telomerase, have been found to play
key roles in taxol resistance (10). Despite these valuable insights, the
study of taxol and other anti-cancer drug resistance mechanisms are
being investigated to identify specific mechanisms for taxol
resistance (11).
Cohen et al (12) identified the taxol resistance gene 1
(TXR1) and showed that it reduced the secretion of the
apoptosis-inducing protein, thrombospondin-1 (TSP1). TSP1 has been
shown to induce apoptosis and inhibit angiogenesis (13), roles that are consistent with a
taxol-resistant phenotype. Furthermore, Papadaki et al
detected the transcription of TXR1 and TSP1 in fresh tumor tissues
obtained from 96 patients with non-small cell lung cancer that had
not received pre-operative chemotherapy. For this cohort, a high
expression of TSP1 and a low expression of TXR1 were associated
with an improved prognosis (14).
In the present study, expression of TXR1 and TSP1 were monitored in
the taxol-resistant breast cancer cell line, MCF-7, to determine
the role of TXR1 in taxol resistance.
Materials and methods
Reagents and cell lines
Taxo1 (Concord Pharmaceutical, China), TRIzol,
Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA, USA), MMLV reverse
transcriptase (Promega, Madison, WI, USA) and BCA protein assay
kits (Pierce, Rockford, IL, USA) were purchased as listed. Methyl
thiazolyl tetrazolium (MTT), dimethyl sulfoxide (DMSO) and
propidium iodide (PI) were purchased from Sigma (St. Louis, MO,
USA). Antibodies used included TXR1 rabbit anti-human polyclonal
antibody (a gift from Professor S. Cohen, Stanford University, USA)
(12), TSP1 rabbit anti-human
polyclonal antibody (BA2130, Wuhan Boster) and mouse anti-human
β-actin monoclonal antibody (Sigma). The human breast cancer cell
lines, MCF-7 and MCF-7/ADR, were purchased from American Type
Culture Collection (ATCC) and were cultured in complete Dulbecco’s
modified Eagle’s medium (DMEM) (Hyclone) containing 10% fetal
bovine serum (FBS) (Gibco, Carlsbad, CA, USA) at 37°C in a
humidified atmosphere containing 5% CO2.
Constructed plasmids
For TXR1-GFP, the human TXR1 (NM_018457.2) cDNA
clone (OriGene clone SC113472) was subcloned by PCR into pEGFP-C3
(Clontech) using the primers (5′-XhoI and 3′-BamHI
sites are underlined): 5′-CCG CTC GAG ATG TGG AAT CCC AAT
GCC-3′ (forward) and 5′-CGC GGA TCC TCA GTC AGA ATC ACT GCT
GGA-3′ (reverse). Sequencing confirmed the clones were
generated.
Transfection
Cells were plated and grown to 70–90% confluence
without antibiotics. Transfections were then performed using
Lipofectamine™ 2000 (Invitrogen) according to the manufacturer’s
instructions. To obtain stable cell lines, cells were transiently
transfected with TXR1-expressing vectors and selected with 400
μg/ml G418 for 28 days. Experiments using transiently transfected
cells were performed 48 h following transfection.
Small interfering RNA (siRNA)-mediated
downregulation of gene expression
Two sets of siRNA duplexes specific for TXR1 were
chemically synthesized (Invitrogen): siRNA duplex 1, (sense) 5′-CAG
UGA UAG UAG ACA AGA ATT-3, (anti-sense) 5′-UUC UUG UCU ACU AUC ACU
GTT-3; siRNA duplex 2, (sense) 5′-GGU UAG AUC AUA UAG CUA ATT-3′,
(anti-sense) 5′-UUA GCU AUA UGA UCU AAC CTT-3′. These duplexes were
designed based on sequences at nucleotide (nt) 469 and nt 813 of
the TXR1 mRNA sequence, NM_018457, respectively. A chemically
synthesized mock siRNA (fluorescein-labeled, non-silencing) was
also purchased from Invitrogen. Transfection of these oligos (50
nM) was performed using Lipofectamine™ 2000 (Invitrogen). For RNA
extraction, cells were harvested 48 h following transfection. To
measure drug cytotoxicity, cells were grown in 6-well plates and
sub-cultured into 96-well plates 24 h following transfection.
Selection of a taxol-resistant cell
line
The human breast cancer cell line, MCF-7, was
maintained in RPMI-1640 containing 1% penicillin-streptomycin
(Gibco) and 10% FBS. MCF-7 cells were selected for resistance to
taxol in a stepwise manner as previously described (15). Briefly, MCF-7 cells were exposed to
0.001 μg/ml taxol. Once normal growth was achieved, the cells were
maintained at this concentration for 21 days prior to the drug dose
being increased. MCF-7/T cells were maintained at a final
concentration of 0.06 μg/ml taxol.
Growth inhibition assays
Growth rates of cells in culture were determined
using MTT assays. Briefly, MCF-7 cells were plated
(2xl04/200 μl medium) in 96-well plates and grown under
normal conditions for 24 h. Various concentrations of taxol (0.006,
0.06, 0.6, 6 and 60 μg/ml) were then added, and 24, 48, 72 and 96 h
following the addition of taxol, 20 μl MTT (5 mg/ml) was added.
Four hours later, 100 μl DMSO was added to each well and absorbance
values were determined using a microplate reader (Bio-Rad,
Hercules, CA, USA) at 570 nm. The IC50 value was estimated using
the SPSS software.
Flow cytometry
Cells (5×105) were harvested and washed
with phosphate-buffered saline (PBS), then fixed in cold 75%
ethanol overnight at 4°C. Following staining with PI solution for
30 min, cells were detected on a FACScan flow cytometer and
analyzed using CellQuest software (Becton-Dickinson, San Jose, CA,
USA).
Reverse transcription PCR
Total RNA from cells was extracted using TRIzol
reagent (Invitrogen), and cDNA libraries were generated using
reverse transcription reactions containing M-MLV reverse
transcriptase and oligo dT primers. The primers used for gene
amplification are listed in Table
I. PCR assays included a 5 min incubation at 94°C, 30–32 cycles
of 94°C for 30 sec, 60°C for 30 sec, and 72°C for 30 sec, followed
by 5 min at 72°C. The β-actin gene was used as an internal
control.
 | Table IPrimer sequences for real-time
PCR. |
Table I
Primer sequences for real-time
PCR.
| Gene target | Primer | Sequence (5′→3′) | Product size
(bp) |
|---|
| TXR1 | Foward |
GGACCCTTCCCTCAAGTCTC | 157 |
| Reverse |
CTCTTCCCATTTCCCCTAGC | |
| TSP1 | Foward |
GCTGGTGGTAGACTAGGGTTGTTT | 143 |
| Reverse |
CCAGAAGGTGCAATACCAGCAT | |
| BCRP | Foward |
TGAGCCTTTGGTTAAGACCG | 107 |
| Reverse |
TGGTGTTTCCTTGTGACACTG | |
| GSTP1 | Foward |
ACCTCCGCTGCAAATACATC | 98 |
| Reverse |
CTCAAAAGGCTTCAGTTGCC | |
| MDR1 | Foward |
GGCTCCGATACATGGTTTTCC | 76 |
| Reverse |
CCAGTGGTGTTTTTAGGGTCATC | |
| MRP1 | Foward |
GTTTCTCAGATCGCTCACCC | 102 |
| Reverse |
TCCACCAGAAGGTGATCCTC | |
| MRP2 | Foward |
CCAGCTCTATGGCTGCTAGAA | 181 |
| Reverse |
CCACTTTGTTTTGAGCAAACTGT | |
| MVP | Foward |
CTGGGAGTTGGTGGTGATCT | 379 |
| Reverse |
CAACTGGCACTTTGAGGTGA | |
| TOP2A | Foward |
TTTGACCACGCGGAGAAG | 170 |
| Reverse |
GAGTCCATCAGATTTGTGGAA | |
| β-actin | Foward |
TCCCTGGAGAAGAGCTACGA | 509 |
| Reverse |
AAAGCCATGCCAATCTCATC | |
| GAPDH | Foward |
CCTGCCAAGTATGATGACATCAAGA | 66 |
| Reverse |
GTAGCCCAGGATGCCCTTTAGT | |
Quantitative real-time PCR
Quantitative PCR was performed using the Applied
Biosystems Sequence Detection System 7900 (Applied Biosystems
Company, CA, USA). A 10 μl mixture containing Power SYBR-Green PCR
MasterMix (Applied Biosystems Company), 500 nmol for each primer
and 300 ng cDNA templates were incubated at 95°C for 5 min,
followed by 50 cycles of 94°C for 20 sec, 60°C for 20 sec, and 72°C
for 40 sec, with a final extension at 72°C for 5 min. A temperature
ramp was then performed from 72 to 95°C at a rate of 0.1°C/sec with
continuous fluorescent acquisition. Copy numbers of the mRNAs
detected were then quantified by calculating the CT values from the
melt curves generated.
Western blot analysis
Total protein was extracted from cells in the
logarithmic phase of growth and quantified using the BCA method.
Equal amounts of protein were electrophoresed on 12–15% SDS-PAGE
and electro-transferred to PVDF membranes using Mini-PROTEAN 3
systems (Bio-Rad). PVDF membranes were blocked with PBS containing
5% fat-free milk powder for 2 h, and then incubated with primary
antibodies overnight at 4°C. Anti-mouse or anti-rabbit IgG
secondary antibodies conjugated to horseradish peroxidase (HRP)
were incubated with the membranes, and peroxidase activity was
visualized using enhanced chemiluminescence (GE Healthcare,
UK).
Statistical analysis
Data were analyzed by a paired-sample t-test or an
independent sample t-test, using SPSS 11.0 software. P<0.05 was
considered to be statistically significant.
Results
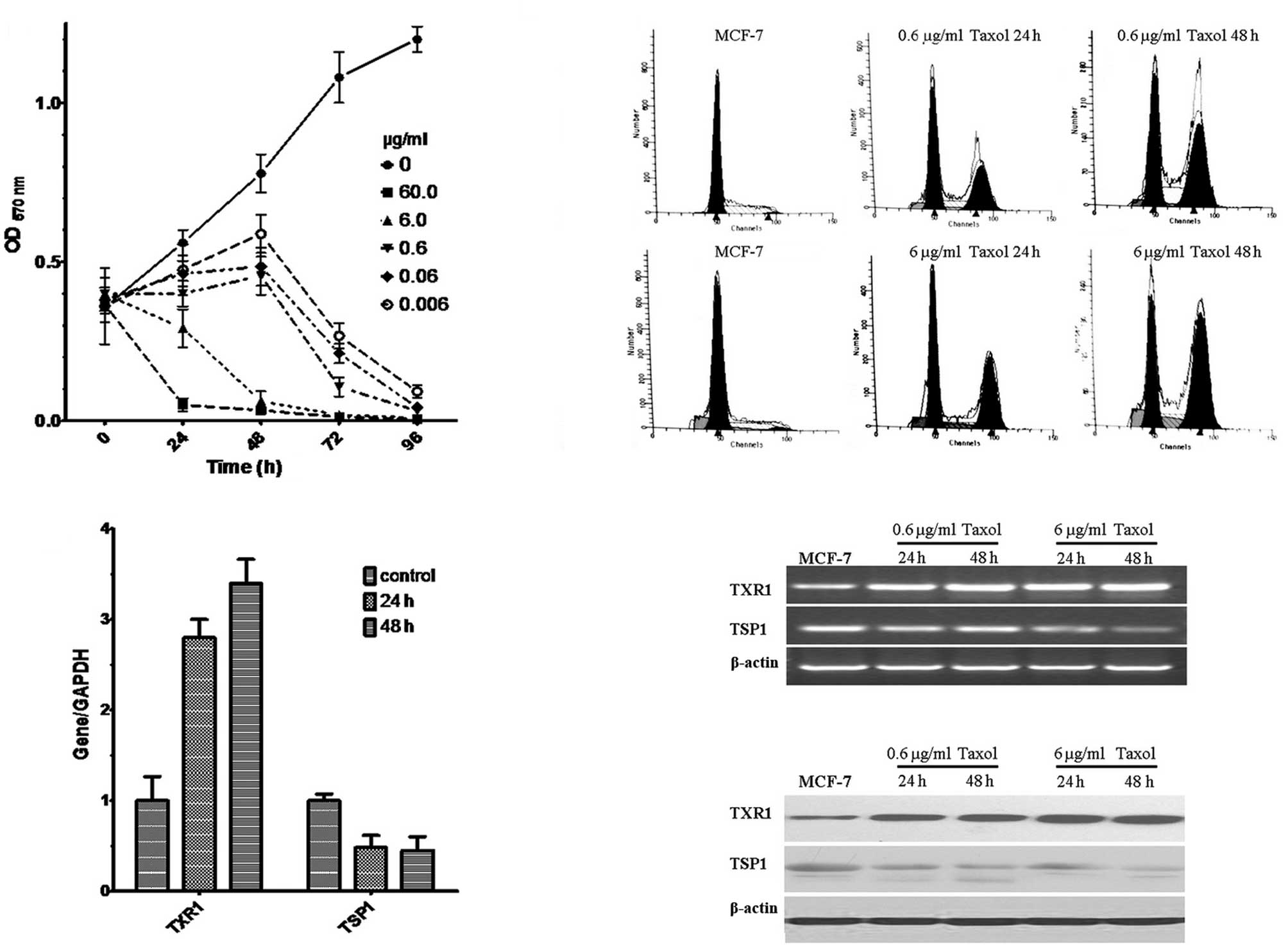
Taxol affects TXR1 response in MCF7
breast cancer cells
Various concentrations of taxol (60, 6, 0.6, 0.06
and 0.006 μg/ml) were assayed for their ability to affect the
growth of MCF-7 cells in MTT assays. Cell viability was observed to
decrease with higher concentrations of taxol and longer incubation
periods (P<0.01, Fig. 1A). Based
on these experiments, taxol concentrations of 6 and 0.6 μg/ml were
selected for further study. Under these conditions FACS analysis
revealed the induction of G2/M arrest (Fig. 1B). Of note, taxol treatment (0.6
μg/ml for 24 and 48 h) resulted in an induction of the TXR1 gene
and a downregulation of its downstream target TSP1, as shown by
real-time PCR (Fig. 1C), RT-PCR
(Fig. 1D) and Western blot analysis
(Fig. 1E). These data suggest that
TXR1 is a taxol-inducible gene in MCF7 cells.
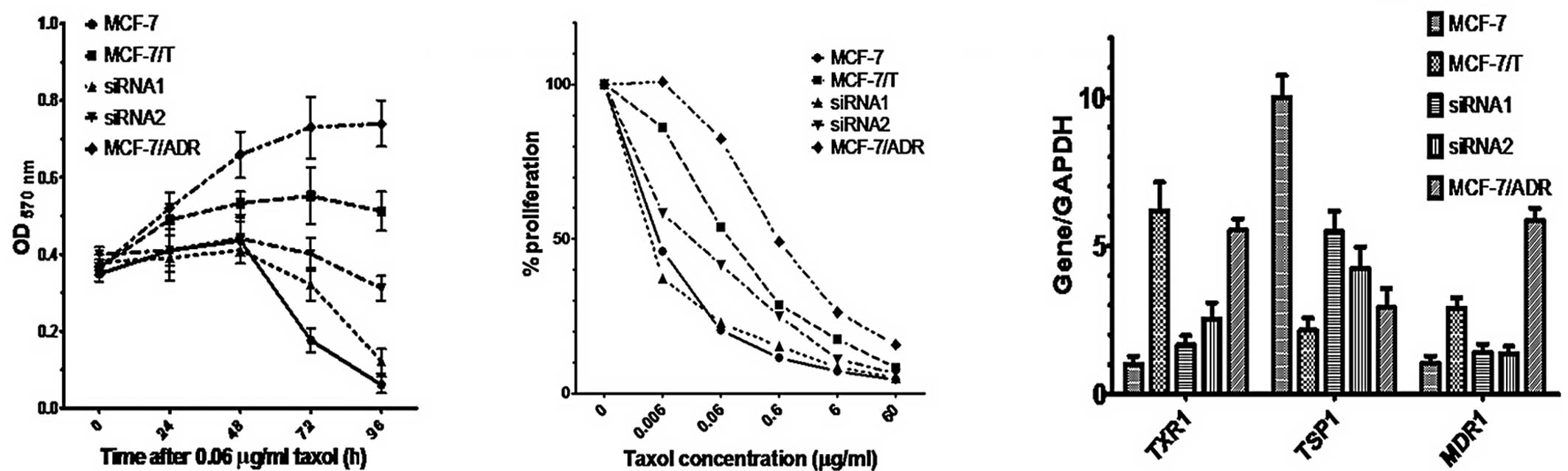
Taxol resistance in MCF7 correlates with
upregulation of TXR1
To confirm the role of TXR1 in taxol sensitivity of
MCF7 cells, we established a taxol-resistant MCF-7 cell line,
MCF-7/T, by exposing MCF-7 cells to stepwise increases in taxol
concentrations (0.01 to 0.2 μg/ml for 5 months). No difference in
the proliferation rate was observed for MCF-7/T vs. the parental
cell line under standard culture conditions (data not shown). By
contrast, under taxol challenge (0.06 μg/ml for 0–96 h or 0–60
μg/ml for 48 h), MCF-7/T cells were significantly more resistant to
taxol-induced toxicity compared to the parental cell line as
assessed by MTT assay (Figs. 2A and
B). This resistant phenotype was associated with a constitutive
overexpression of TXR1 and MDR1 genes, and downregulation of TSP1
(Fig. 2C), supporting the
hypothesis that, besides MDR1, TXR1 may play a significant role in
taxol resistance in breast cancer cells.
Downregulation of TXR1 reverses the
taxol-resistant phenotype in MCF7 cells
To confirm this conclusion, two different siRNAs
(siRNA1, siRNA2; 25 nM) directed against TXR1 were singularly
transfected into MCF-7/T cells. An irrelevant siRNA sequence was
used as a control. A 60–80% decrease in TXR1 expression and an
increase in TSP1 (200–250%) expression were detected in siRNA1- and
siRNA2-transfected cells, compared to siRNA control-transfected
cells, as assessed by real-time PCR 48 h post-transfection. A
downregulation of MDR1 was also observed (Fig. 2C). Accordingly, a significant
enhancement in chemosensitivity (taxol, 0.06 μg/ml for 0–96 h or
0–60 μg/ml taxol for 48 h), and therefore a rescue of the resistant
phenotype was observed in MCF-7/T cells following TXR1 silencing,
as assessed by the MTT assay (Figs. 2A
and B, P<0.01 for the two siRNAs). Thus, TXR1 expression
plays a significant role in the taxol resistance of MCF7 cells.
Ectopic TXR1 expression induces taxol
resistance in MCF7 cells
To confirm the role of TXR1 in modulating the
response to taxol in breast cancer cells, TXR1 (pEGFP-TXR1) was
ectopically expressed by stable transfection in MCF7 cells
(MCF-7-TXR1). As a control, cells were transfected with the
corresponding empty vector (pEGFP) (MCF-7-EGFP). While MCF-7-TXR1
and MCF-7-EGFP cells exhibited no difference in terms of morphology
or the cell proliferation rate under standard culture conditions
(data not shown), enforced TXR1 expression correlated with an
increased drug resistance under taxol challenge (0.06 μg/ml for
0–96 h) (Fig. 3A, P<0.01).
Moreover, TXR1-expressing cells exhibited downregulation of TSP1,
but also increased levels of other drug resistance genes including
BCRP, GSTP1 and MVP, as determined by real-time PCR (Fig. 3B).
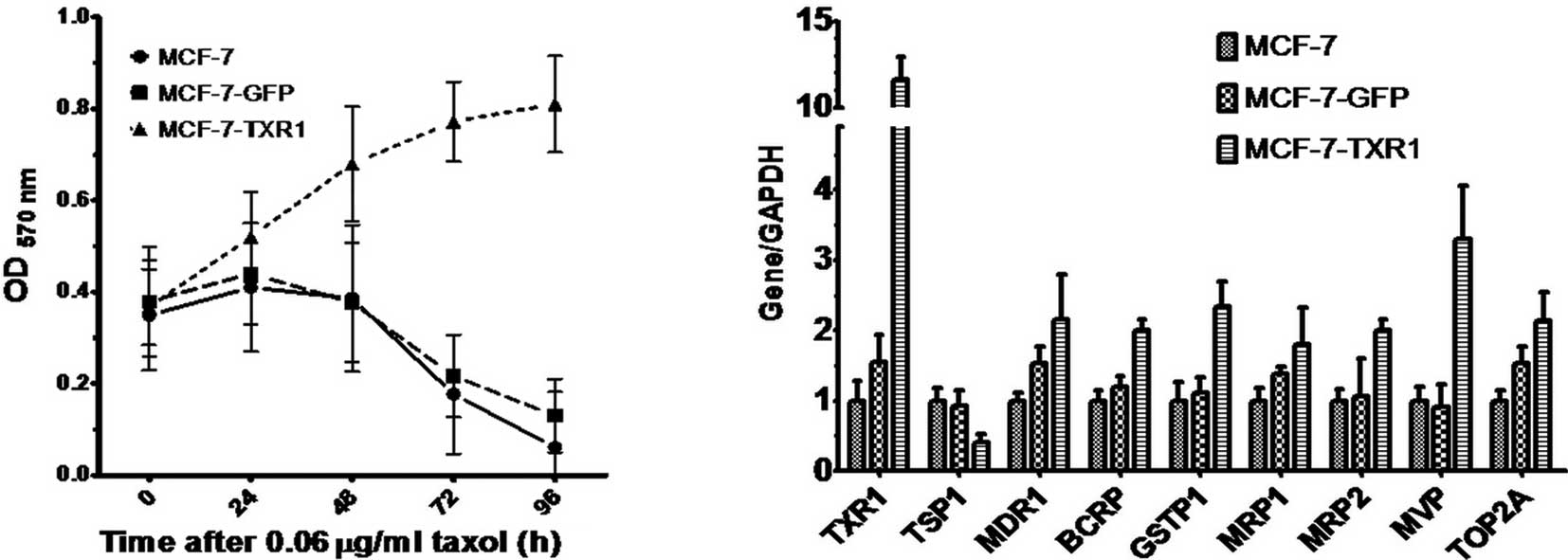 | Figure 3Transcription of drug resistance genes
with TXR1 overexpression and treatment with taxol. (A) MTT assays
of MCF-7, MCF-7-EGFP, and MCF-7-TXR1 cell lines treated with 0.06
μg/ml taxol. Taxol did not inhibit the growth of MCF-7-TXR1 cells
and overexpression of TXR1 enhanced the taxol resistance of MCF-7
cells. (B) mRNA levels of TXR1, TSP1, BCRP, GSTP1, MDR1, MRP1,
MRP2, MVP and TOP2A were detected using real-time PCR and
normalized to GAPDH for MCF-7, MCF-7-EGFP and MCF-7-TXR1 cell
lines. Overexpression of TXR1 was associated with a relative
increase in TXR1 mRNA, a decrease in TSP1 mRNA and an increase in
the drug resistance genes BCRP, GSTP1 and MVP. |
Discussion
In this study, MCF-7 cells that were resistant to
low doses of taxol had higher levels of TXR1, and MCF-7 cells with
an exogenous expression of TXR1 exhibited an increase in taxol
resistance. By contrast, when TXR1 expression was reduced by the
transient transfection of TXR1-targeted siRNAs, the
chemosensitivity of the transfected cells increased significantly,
leading to the hypothesis that TXR1 has a significant role in
mediating taxol resistance and complements mechanisms of MDR.
Paclitaxel affects tubulin polymerization to promote
the stability of microtubules (16), resulting in the accumulation of
intracellular microtubules and the breakdown of mitotic spindles to
arrest cell cycle progression in the G2/M phase, or cause apoptosis
(17). Similar to other anti-cancer
drugs, tumor cells have also been found to become taxane-resistant
(18). In these cells, an increased
capacity to break down drugs, as well as an increase in expression
of the MDR protein, P-glycoprotein (P-gp), is most frequently
observed (19). Other
characteristics of multi-drug resistant cells include activation of
drug efflux pumps, increased drug transport, metabolic changes and
increased DNA repair due to drug damage (20,21). A
variety of specific molecules have been identified that promote
drug resistance in breast cancer cells, including MDR1, MRP, GSTP1
and Topo II (22). However, the
expression levels of a number of these molecules are not high,
suggesting that there are multiple pathways that have a role in
mediating drug resistance.
Cohen et al first characterized the txr1 gene
as encoding an 18 kDa proline and serine-rich protein with nuclear
expression and a role in taxol resistance. These authors also
demonstrated that TXR1 is capable of inhibiting TSP1 expression at
the transcriptional level, thereby preventing taxol-induced
apoptosis in human tumor cells. Inactivations of TXR1 by CD47, or
activation of TSP1, were also shown to enhance taxol-induced
cytotoxicity in tumor cells. In combination, results by these
authors suggested that TXR1, as a thrombin-mediated regulator, was
a potential therapeutic target (12). However, the correlation between TXR1
and drug resistance in breast cancer remained to be investigated.
Results of this study have shown TXR1 to be highly expressed in the
taxol-resistant breast cancer cell line MCF-7/T. Additionally,
results of the MTT assay show that these high expression levels
decreased the sensitivity of the breast cancer cells to taxol.
Furthermore, when TXR1-targeted siRNAs were transiently expressed,
the taxol sensitivity of the MCF-7/T cells was reversed, thereby
supporting a role for TXR1 in the taxol-resistant phenotype of
breast cancer cells. Based on these results, we hypothesize that
TXR1-targeted siRNAs are also capable of increasing the
chemosensitivity of other types of breast cancer cells.
While transcription and translation of TXR1 were
found to increase with exposure of MCF-7 cells to taxol, mRNA and
protein levels of TSP1 have been found to decrease. Accordingly,
when TXR1 was overexpressed, the taxol resistance of MCF-7 cells
increased, and expression of TSP1 significantly decreased. Further
experiments to modulate TXR1 expression using siRNA demonstrated
have shown that MCF-7/T cells are capable of having their
sensitivity to taxol restored, suggesting that the increase in
taxol resistance mediated by TXR1 is due to the inhibition of TSP1
expression. However, despite this valuable insight into a possible
mechanism for taxol-induced drug resistance in breast cancer cells,
the interactions between TSP1 and TXR1 remain unclear. On the other
hand, the ectopic expression of TXR1 is associated with the
upregulation of a number of genes associated with drug resistance
(BCRP, GSTP1 and MVP), which reflects the complexity of the TXR1
resistance mechanism, and not only with apoptosis. Further
elucidation of the interactions between these molecules and their
regulation would not only contribute to our understanding of taxol
resistance but also identify novel therapeutic targets that may
restore the therapeutic effect of taxol and improve the prognosis
of patients with breast cancer.
References
|
1
|
Marsh S and McLeod HL: Pharmacogenetics
and oncology treatment for breast cancer. Expert Opin Pharmacother.
8:119–127. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moore A: Breast-cancer therapy-looking
back to the future. N Engl J Med. 357:1547–1549. 2007. View Article : Google Scholar
|
|
3
|
Horwitz SB: Mechanism of action of taxol.
Trends Pharmacol Sci. 13:134–136. 1992. View Article : Google Scholar
|
|
4
|
Sparano JA, Wang M, Martino S, Jones V,
Perez EA, Saphner T, Wolff AC, Sledge GW Jr, Wood WC and Davidson
NE: Weekly paclitaxel in the adjuvant treatment of breast cancer.
New Engl J Med. 358:1663–1671. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Broxterman HJ, Gotink KJ and Verheul HM:
Understanding the causes of multidrug resistance in cancer: a
comparison of doxorubicin and sunitinib. Drug Resist Updat.
12:114–126. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sangrajrang S and Fellous A: Taxol
resistance. Chemotherapy. 46:327–334. 2000. View Article : Google Scholar
|
|
7
|
Martello LA, Verdier-Pinard P, Shen HJ, He
L, Torres K, Orr GA and Horwitz SB: Elevated levels of microtubule
destabilizing factors in a taxol-resistant/dependent A549 cell line
with an alpha-tubulin mutation. Cancer Res. 63:1207–1213.
2003.PubMed/NCBI
|
|
8
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ferguson T, Wilcken N, Vagg R, Ghersi D
and Nowak AK: Taxanes for adjuvant treatment of early breast
cancer. Cochrane Database Syst Rev. 17:CD0044212007.PubMed/NCBI
|
|
10
|
Kutuk O and Letai A: Alteration of the
mitochondrial apoptotic pathway is key to acquired paclitaxel
resistance and can be reversed by ABT-737. Cancer Res.
68:7985–7994. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hudis C and Dang C: The taxane limbo: how
low can we go? J Natl Cancer Inst. 100:761–763. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lih CJ, Wei W and Cohen SN: Txr1: a
transcriptional regulator of thrombospondin-1 that modulates
cellular sensitivity to taxanes. Genes Dev. 20:2082–2095. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nor JE, Mitra RS, Sutorik MM, Mooney DJ,
Castle VP and Polverini PJ: Thrombospondin-1 induces endothelial
cell apoptosis and inhibits angiogenesis by activating the caspase
death pathway. J Vasc Res. 37:209–218. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Papadaki C, Mavroudis D, Trypaki M,
Koutsopoulos A, Stathopoulos E, Hatzidaki D, Tsakalaki E,
Georgoulias V and Souglakos J: Tumoral Expression of TXR1 and TSP1
predicts overall survival of patients with lung adenocarcinoma
treated with first-line docetaxel-gemcitabine regimen. Clin Cancer
Res. 15:3827–3833. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Greenberger LM, Lothstein L, Williams SS
and Horwitz SB: Distinct P-glycoprotein precursors are overproduced
in independently isolated drug-resistant cell lines. Proc Natl Acad
Sci USA. 85:3762–3766. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jordan MA and Wilson L: Microtubules as a
target for anticancer drugs. Nat Rev Cancer. 4:253–265. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Van Amerongen R and Berns A: TXR1-mediated
thrombospondin repression: a novel mechanism of resistance to
taxanes? Genes Dev. 20:1975–1981. 2006.PubMed/NCBI
|
|
18
|
Orr GA, Verdier-Pinard P, McDaid H and
Horwitz SB: Mechanisms of taxol resistance related to microtubules.
Oncogene. 22:7280–7295. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ark-Otte J, Samelis G, Rubio G, Lopez Saez
JB, Pinedo HM and Giaccone G: Effects of tubulin-inhibiting agents
in human lung and breast cancer cell lines with different multidrug
resistance phenotypes. Oncol Rep. 5:249–255. 1998.PubMed/NCBI
|
|
20
|
McGrogan BT, Gilmartin B, Carney DN and
McCann A: Taxanes, microtubules and chemoresistant breast cancer.
Biochim Biophys Acta. 1785:96–132. 2008.PubMed/NCBI
|
|
21
|
Lavie Y, Cao H, Volner A, Lucci A, Han TY,
Geffen V, Giuliano AE and Cabot MC: Agents that reverse multidrug
resistance, tamoxifen, verapamil, and cyclosporin A, block
glycosphingolipid metabolism by inhibiting ceramide glycosylation
in human cancer cells. J Biol Chem. 272:1682–1687. 1997. View Article : Google Scholar
|
|
22
|
Longley D and Johnston P: Molecular
mechanisms of drug resistance. J Pathol. 205:275–292. 2005.
View Article : Google Scholar
|