Introduction
Gastric cancer occurs with a variable geographic
distribution worldwide, and the incidence is high in China
(1). Helicobacter pylori
(H. pylori) infection is an established risk factor in
gastric cancer, triggering chronic inflammation of the stomach and
leading to stepwise development of the malignancy (2,3).
However, a number of previous studies have demonstrated that H.
pylori is involved in gastric cancer tumorigenesis in a small
proportion of individuals infected with the organism, indicating
that individual genetic susceptibility may also play a critical
role in gastric cancer (4).
Subsequent studies further indicated that single nucleotide
polymorphism (SNP) may have an effect on gastric tumorigenesis
(5). Determinants of the host
response to H. pylori infection continue to focus on
polymorphisms in genes related to innate and acquired immune
responses, including TLR-4, NOD2 and COX-2
(5–7).
Cyclooxygenase-2 (COX-2) is known as an
inducible enzyme that catalyzes conversion of arachidonic acid to
prostaglandins in response to various inflammatory stimuli
(8). Progression from initial
gastric lesions to gastric cancer has been correlated with
COX-2 over-expression, providing evidence that its activity
may be involved in the onset of gastric carcinogenesis (9). Previous studies demonstrated that
COX-2 is also associated with proliferation and apoptosis
markers and that it is an independent prognostic factor in gastric
cancer (10). At present, 2
polymorphisms in COX-2 have been reported, which are G>A
and G>C base transitions, at positions -1195(rs689466) and
-765(rs20417) base pairs (bp) from the transcriptional start site.
The promoter region polymorphic variant of -1195G>A and
-765G>C has been demonstrated to have a functional effect on
COX-2 transcription (11,12),
which may cause gastric cancer. Although a number of studies have
been carried out to examine the manner in which the two SNPs
increase the risk of developing gastric cancer, the results from
various groups have not been consistent (13–15).
In the current literature, few studies have examined
the role of COX-2 polymorphisms in gastric cancer using
gastric cancer case and control family members from the Chinese Han
population. The current study was performed to evaluate the manner
in which the COX-2 polymorphisms and H. pylori
infection synergistically increased the risk of gastric cancer
patients with various degrees of relationship in the Chinese Han
population.
Materials and methods
Study population
A total of 296 subjects from 90 gastric cancer case
families, and 319 subjects from 90 control families were included.
The individuals in this study were selected from four villages of
two counties in the Henan Province of China. Samples were obtained
through cluster sampling in this highly cancer-prevalent area
following a previous cancer screening. The gastric cancer patient
samples were collected from these four villages between August 2003
and March 2007, including samples obtained from patients who had
suffered from non-cardiac adenocarcinoma of the stomach during the
five years prior to the screening. The patients were
histopathologically diagnosed, and were considered as the probands.
Their first- and second-degree relatives were further investigated.
The control families were selected to correspond to the number of
case family members and the age of the probands (±5 years), gender
and address. Controls were healthy community-based individuals
without any similar digestive diseases and had no marital ties with
the case family. Subjects in this study provided informed consent
prior to participating in the study, and the private data were kept
as coded data.
DNA extraction
Blood samples were collected from the subjects and
DNA was extracted using the salting-out procedure and stored in a
−80°C freezer for subsequent studies.
Genotyping analysis
The COX-2-1195G>A and -765G>C
polymorphisms were genotyped using polymerase chain
reaction-restriction fragment length polymorphism. Primers were
designed and synthesized by Beijing SBS Genetech Co., Ltd. The
sequences were as follows: COX-2-1195G>A:
5′-CCCTGAGCACTACCCATGAT-3′ (forward) and 5′-GCC
CTTCATAGGAGATACTGG-3′ (reverse). COX-2-765G>C:
5′-TATTATGAGGAGAATTTACCTTTCGC-3′ (forward) and
5′-GCTAAGTTGCTTTCAACAGAAGAAAT-3′ (reverse). Thermo cycling
conditions of PCR were: COX-2-1195G>A: 95°C for 5 min, 35
cycles of 95°C for 30 sec, 54°C for 40 sec, 72 °C for 45 sec and
finally 72°C for 10 min; COX-2-765G>C: 95°C for 5 min, 35
cycles of 95°C s for 30 sec, 55.5°C for 40 sec, 72°C for 45 sec and
finally 72°C for 10 min. The PCR products were digested with
restriction enzymes PvuII (Takara Biotechnology Co., Ltd.)
at 37°C overnight for analysis of COX-2-1195G>A and with
HhaI (Takara Biotechnology Co., Ltd.) for -765G>C. The
digested products were separated using 3% agarose gel
electrophoresis, stained with ethidium bromide and visualized under
ultraviolet light. Subsequently, an altered pattern of bands was
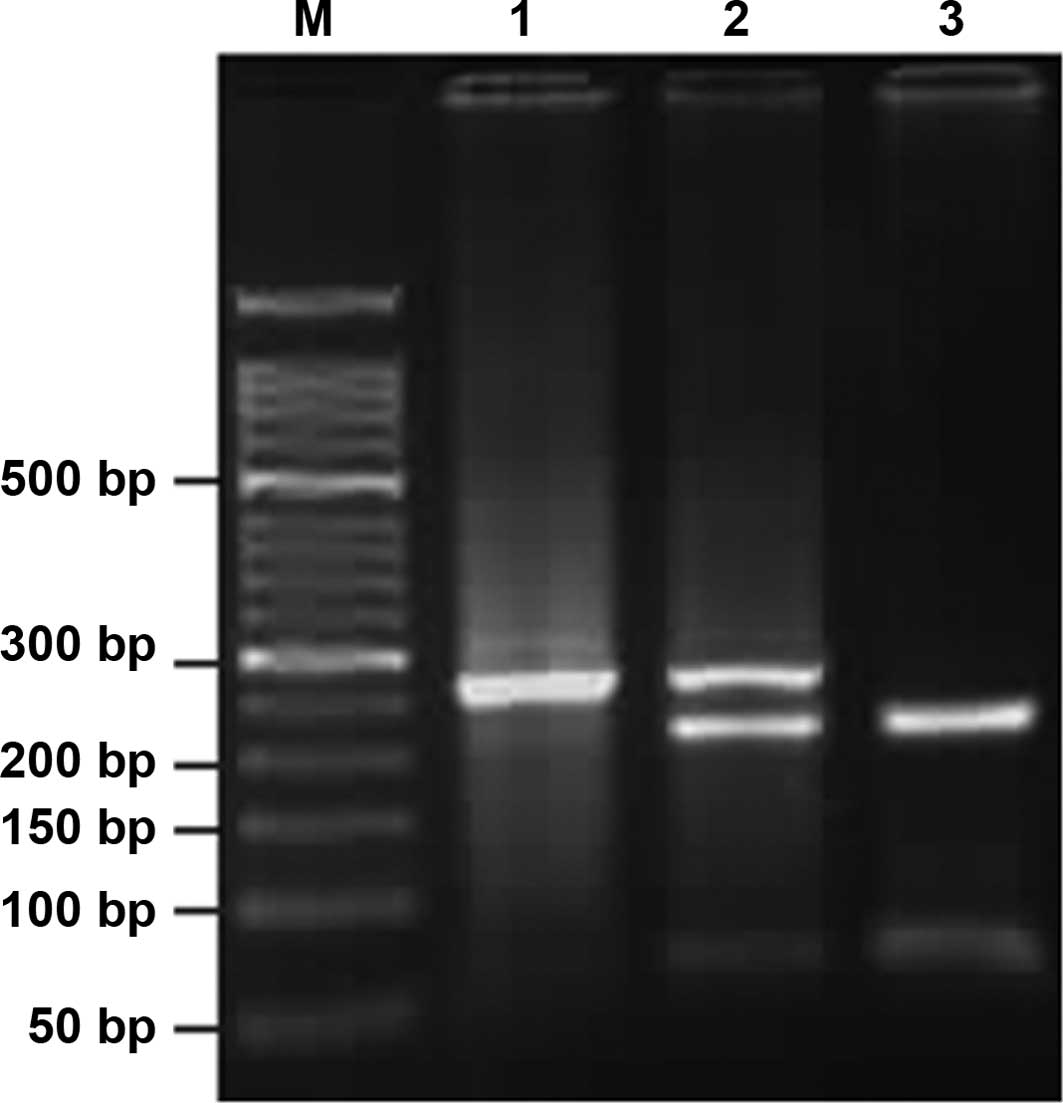
observed: a single band of 273 bp indicated that
COX-2-1195A/A was not digested; three bands of 273, 220 and
53 bp indicated that G/A was only partially digested; two bands of
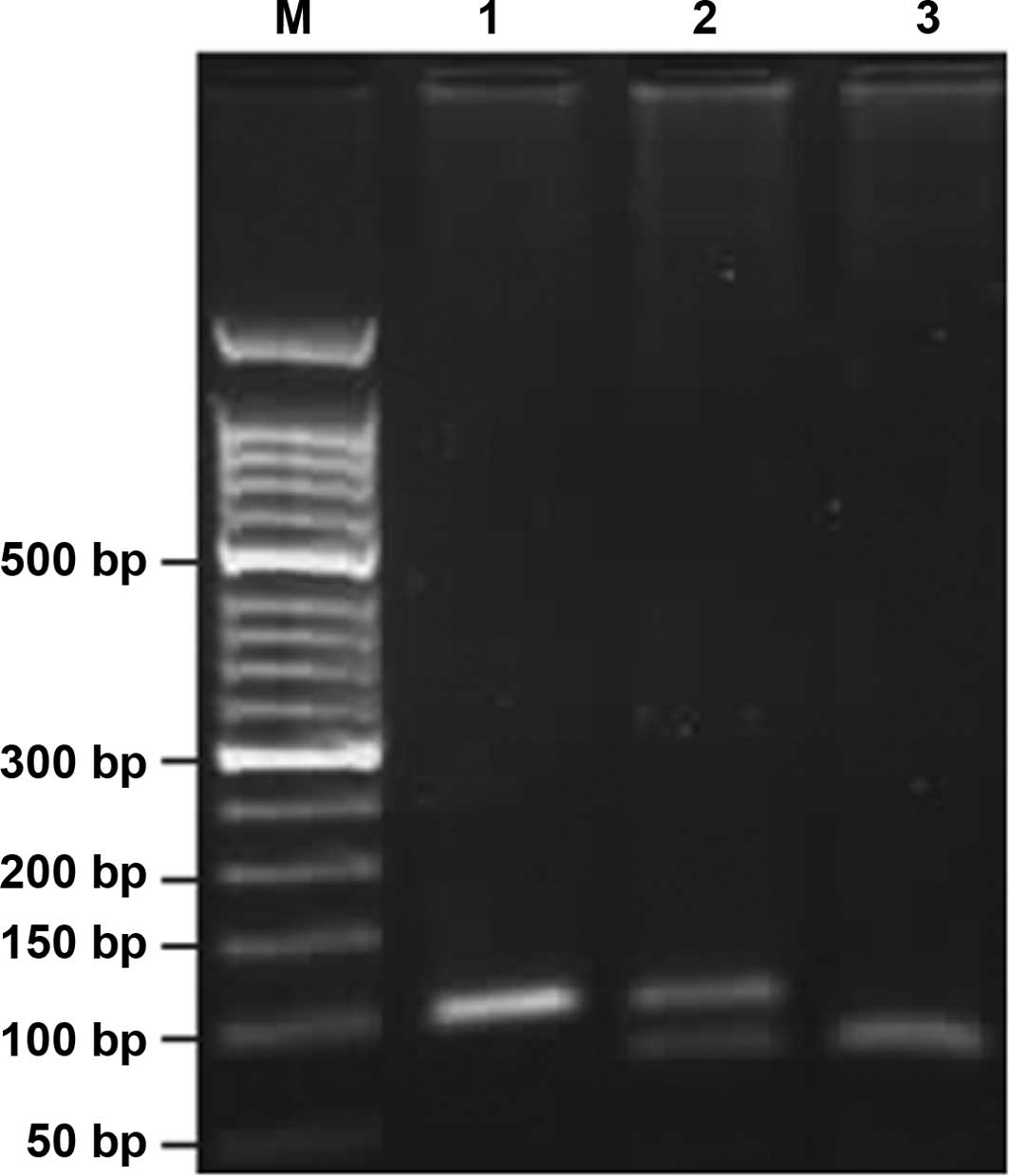
220 and 53 bp indicated that G/G was completely digested (Fig. 1). Analogically, COX-2-765C/C
appeared as a single band of 100 bp; G/C, three bands of 100, 75
and 25 bp, and G/G, two bands of 75 and 25 bp (Fig. 2). The 25-bp fragment was not
distinguishable from the primer-dimer band in the agarose gel. Each
type of PCR product was sequenced to ascertain the genotype.
Finally, 10% of the specimens were genotyped in duplicate using the
same assay and the results were 100% concordant.
H. pylori status analysis
The H. pylori immunoglobulin G antibody was
determined on the serum specimens of all case and control family
members using ELISA. The relevant reagents were provided by the
National Institute for Communicable Disease Control and Prevention
of the Chinese Center for Disease Control and Prevention.
Statistical analysis
The Hardy-Weinberg equilibrium equation was used to
determine whether the proportion of each genotype obtained was in
agreement with the expected values as calculated from allele
frequencies. The Chi-square test was used to examine the
differences in demographic variables. Mean ages of case and control
family members were compared using the t-test. Unconditional
logistic regression analysis was used to estimate the odds ratio
(OR) and its 95% confidence interval (CI) as a measure of the
association between the genotype carrier and risk of gastric
cancer. The interaction between H. pylori infection and
polymorphisms of COX-2 was analyzed using stratification
analysis. P-values were two-sided, and P<0.05 was considered as
statistically significant. Data analysis was performed by
Statistical Product and Service Solutions software (Version 15.0,
SPSS, Inc., Chicago, IL, USA) unless otherwise specified.
Results
Demographic characteristics
The demographic distribution of case family members
and healthy controls is shown in Table
I. The mean age of the gastric cancer case families was
44.0±16.6 years [mean ± standard deviation (SD)] and the controls
was 44.3±15.9 years (mean ± SD, P=0.81), indicating that there was
no difference between the two groups. Males comprised 42.9% of case
family members compared with 43.6% among controls (P=0.87).
Regarding H. pylori infection, there was a significant
difference between the case family groups and the controls (72.3%
versus 62.7%; P=0.01). With regard to familial history of gastric
cancer, case groups exhibited a higher freqeuncy than the controls
(19.6% versus 5.3%; P<0.01).
 | Table ISelected characteristic distribution
in case and control family group. |
Table I
Selected characteristic distribution
in case and control family group.
| Variables | Case family group
(%)(n=296) | Control family
group (%)(n=319) | P-value |
|---|
| Mean age (SD) | 44.0 (16.6) | 44.3 (15.9) | 0.81 |
| Sex |
| Male | 127 (42.9) | 139 (43.6) | 0.87 |
| Female | 169 (57.1) | 180 (56.4) | |
| H.
pylori |
| Yes | 214 (72.3) | 200 (62.7) | 0.01a |
| No | 82 (27.7) | 119 (37.3) | |
| Family
carcinomas |
| Yes | 58 (19.6) | 17 (5.3) | <0.01a |
| No | 238 (80.4) | 302 (94.7) | |
Genotype frequencies and gastric cancer
risk
The genotype distribution and allele frequencies of
COX-2 polymorphisms in gastric cancer case and control
families are shown in Table II.
The genotype distribution of COX-2-1195G>A and -765G>C
in the case and control family members did not significantly
deviate from the expected Hardy-Weinberg equilibrium (P>0.05).
For COX-2-1195G>A, the frequencies of GG, GA and AA
genotypes were 17.9, 49 and 33.1% in the case family group and
25.1, 52.0 and 22.9% in the control group, respectively. Compared
with the frequency of the GG genotype, an association was found
between the COX-2-1195AA genotype and the risk of gastric
cancer (AA genotype: OR = 2.03; 95% CI, 1.27–3.22). A statistical
difference was observed between the A carrier and A allele
frequencies among the case and control family members (A carriers:
OR = 1.55; 95% CI, 1.02–2.28; A allele: OR = 1.42; 95% CI,
1.09–1.81). With regard to COX-2-765G>C, the frequencies
of GG, GC and CC genotypes in the case and control family group
were 81.4, 17.9 and 0.7%, and 86.2, 13.5 and 0.3%, respectively.
Due to the low occurrence (<1%) of the CC genotype, heterozygous
and homozygous variants were combined for the analysis (C carriers:
G/C + C/C). Compared with the GG homozygous variant or G allele, C
carriers or C allele were not shown to be statistically different
between the two groups (C carriers: OR=1.44; 95% CI, 0.91–2.23; C
allele: OR = 1.45; 95% CI, 0. 92–2.25).
 | Table IIGenotype distribution of
COX-2-1195G>A and -765G>C in case and control family
groups. |
Table II
Genotype distribution of
COX-2-1195G>A and -765G>C in case and control family
groups.
| Locus genotype | Case family group
(%)(n=296) | Control family
group (%)(n=319) | OR (95% CI) |
|---|
|
COX-2-1195 |
| G/G | 53 (17.9) | 80 (25.1) | 1.0 |
| G/A | 145 (49.0) | 166 (52.0) | 1.32
(0.86–2.01) |
| A/A | 98 (33.1) | 73 (22.9) | 2.03
(1.27–3.22)a |
| A carriers | 243 (82.1) | 239 (74.9) | 1.55
(1.02–2.28)a |
| G allele | 251 (42.4) | 326 (51.1) | 1.0 |
| A allele | 341 (57.6) | 312 (48.9) | 1.42
(1.09–1.81)a |
|
COX-2-765 |
| G/G | 241 (81.4) | 275 (86.2) | 1.0 |
| G/C | 53 (17.9) | 43 (13.5) | 1.41
(0.91–2.20) |
| C/C | 2 (0.70) | 1 (0.30) | 2.28
(0.20–25.38) |
| C carriers | 55 (18.6) | 44 (13.8) | 1.44
(0.91–2.23) |
| G allele | 535 (90.4) | 593 (92.9) | 1.0 |
| C allele | 57 (9.60) | 45 (7.10) | 1.45
(0.92–2.25) |
Genotype distribution of COX-2-1195G>A
and -765G>C between various degrees of relationship
As shown in Table
III, the genotype distribution of COX-2-1195G>A and
-765G>C in different relative grades between the case and
control family members was further examined. Since the spouse had
no genetic affinity with the host of the family, the spouse was
disregarded while analyzing the gastric cancer genetic
susceptibility. The OR value of COX-2-1195 AA genotype in
the first-degree relatives (OR=3.35; 95% CI, 1.69–6.12) was higher
than that in the second-degree relatives (OR=2.66; 95% CI,
1.06–6.82). Similarly, the OR values of A carriers (GA + AA) and A
allele exhibited a diminished trend from closer degrees of
relationship (A carriers: first-degree relatives: OR=2.11; 95% CI,
1.19–3.67; second-degree relatives: OR=1.98; 95% CI, 0.89–4.63; A
allele: first-degree relatives: OR=1.77; 95% CI, 1.29–2.41;
second-degree relatives: OR=1.69; 95% CI, 0.99–2.68). Compared with
the GG genotype, the mutant genotype distribution of -765G>C was
not correlated with gastric cancer in the first- or second-degree
relatives, due to the fact that the OR values included 1.00 (C
carriers: first-degree relatives: OR=1.62; 95% CI, 0.89–2.87;
second-degree relatives: OR=1.35; 95% CI, 0.54–3.22).
 | Table IIIGenotype distribution of
COX-2-1195G>A and -765G>C between various degrees of
relationship (disregarding spouse). |
Table III
Genotype distribution of
COX-2-1195G>A and -765G>C between various degrees of
relationship (disregarding spouse).
| First-degree
relatives | | Second-degree
relatives | |
|---|
|
| |
| |
|---|
| Genotype | Case families
(n=173) | Control families
(n=190) | OR (95% CI) | Cases families
(n=71) | Control families
(n=77) | OR (95% CI) |
|---|
|
COX-2-1195 |
| G/G | 24 (13.9) | 48 (25.3) | 1.0 | 10 (14.1) | 19 (24.7) | 1.0 |
| G/A | 84 (48.6) | 102 (53.7) | 1.66
(0.91–3.01) | 30 (42.3) | 36 (46.8) | 1.58
(0.65–3.87) |
| A/A | 65 (37.6) | 40 (21.1) | 3.35
(1.69–6.12)a | 31 (43.7) | 22 (28.6) | 2.66
(1.06–6.82)a |
| A carriers | 149 (86.1) | 142 (74.7) | 2.11
(1.19–3.67)a | 61 (85.9) | 58 (75.3) | 1.98
(0.89–4.63) |
| G allele | 132 (38.2) | 198 (52.1) | 1.0 | 50 (35.2) | 74 (48.1) | 1.0 |
| A allele | 214 (61.8) | 182 (47.9) | 1.77
(1.29–2.41)a | 92 (64.8) | 80 (51.9) | 1.69
(0.99–2.68) |
|
COX-2-765 |
| G/G | 139 (80.3) | 165 (86.8) | 1.0 | 58 (81.7) | 66 (85.7) | 1.0 |
| C carriers | 34 (19.7) | 25 (13.2) | 1.62
(0.89–2.87) | 13 (18.3) | 11 (14.3) | 1.35
(0.54–3.22) |
The interaction of COX-2-1195G>A
polymorphism and H. pylori infection status
As COX-2-1195G>A and H. pylori were
involved in gastric cancer, the interaction between this functional
SNP and H. pylori infection status was examined using
stratified analysis (Table IV).
Taking H. pylori-seronegative AA genotype and A carriers
together as a reference, H. pylori-seropositive
COX-2-1195 AA genotype and A carriers exhibited
significantly increased susceptibility to gastric cancer (AA
genotype: OR=2.96; 95% CI, 1.57–5.58; A carriers: OR=2.04; 95% CI,
1.18–3.52).
 | Table IVThe interaction of
COX-2-1195G>A polymorphism and H. pylori infection
status. |
Table IV
The interaction of
COX-2-1195G>A polymorphism and H. pylori infection
status.
| H. pylori
status | Genotype | Case family group
(n=296) (%) | Control family
group (n=319) (%) | OR (95% CI) |
|---|
|
COX-2-1195 | | | |
| Negative | GG | 29 (9.8) | 39 (12.2) | 1.0 |
| GA | 26 (8.8) | 48 (15.0) | 0.73
(0.37–1.43) |
| AA | 27 (9.1) | 32 (10.0) | 1.14
(0.56–2.29) |
| A carriers | 53 (17.9) | 80 (25.1) | 0.89
(0.49–1.61) |
| Positive | GG | 24 (8.10) | 41 (12.9) | 1.0 |
| GA | 119 (40.2) | 118 (37.0) | 1.72
(0.98–3.03) |
| AA | 71 (24.0) | 41 (12.9) | 2.96
(1.57–5.58)a |
| A carriers | 190 (64.2) | 159 (49.8) | 2.04
(1.18–3.52)a |
Discussion
On the basis of genotyping 296 gastric cancer case
family members and 319 control family members in the Chinese Han
population, the COX-2-1195AA genotype was found to be
associated with an increased risk of gastric cancer (OR=2.03; 95%
CI, 1.27–3.22). The results from our study were consistent with
those obtained by Tan et al, Shi et al and
Coskunpinar et al (16–18),
which indicated that the COX-2-1195AA genotype was
associated with advanced colorectal cancer, asthma and lung
carcinoma, respectively. However, Peters et al (19) reported that there was no significant
difference in the genotype distribution of COX-2-1195A>G
polymorphism between patients and controls in the risk of head and
neck cancer in Caucasian individuals. We have traced the various
factors contributing to the contradictory results and found that
there were two major reasons for this inconsistency. One reason is
that the subjects used in the above studies were selected from
different cancer categories. Another reason may be that the
frequency of the COX-2-1195 genotype varies between
populations. Our comparative study of the genotype distribution of
COX-2-1195 polymorphism has demonstrated the clear
differences between the Chinese and Caucasian populations.
Frequencies of 25.1, 52.0 and 22.9% for 1195GG, 1195GA, and 1195AA
genotypes were found in the Chinese control group, whereas
corresponding frequencies of 3, 37 and 59% were found in Caucasian
controls.
Another finding of note in the present study is that
the COX-2-1195 AA genotype exhibited a positive correlation
with the risk of gastric cancer, and the OR value in the
first-degree relatives was higher than that in the second
(first-degree relatives OR=3.35; 95% CI, 1.69–6.12; second-degree
relatives: OR=2.66; 95% CI, 1.06–6.82). It has been proposed that
gastric cancer has a familial genetic tendency, and the risk may be
reduced according to the decreasing degree of relationship. These
results have demonstrated that polymorphisms may play a significant
role in the development of gastric cancer. The COX-2-1195 A
carriers and A allele exhibit similar trends. However, the OR value
of the second-degree relatives had no statistical significance.
The COX-2-765G/C change appears to be
functionally relevant. However, COX-2-765G>/C
polymorphism failed to exhibit an association with gastric cancer
in this study, and there was no significant correlation between the
first- and second-degree relatives and COX-2-765 genotypes.
This result is consistent with that of Liu et al (13) but differs from findings of studies
performed in the populations of The Netherlands and India (14,15).
In a study by Sitarz et al, COX-2-765G allele
promoter polymorphism was found to be significantly associated with
gastric cancer in The Netherlands (14). However, Saxena et al
(15) reported that the frequency
of the C carrier has a significant association with gastric
adenocarcinoma. This inconsistent result may be due to the markedly
different allele frequencies of COX-2-765G/C. The
frequencies of the GG, GC and CC genotypes of COX-2-765G/C
were 25.1, 52.0 and 22.9%, respectively, in the present study,
whereas Sitarz et al (14)
reported the frequencies to be 59, 32 and 9%, respectively, in The
Netherlands. In the study by Saxena et al (15), the Indian and Chinese distributions
of 765GG, 765GC and 765CC genotypes were 71.0 versus 86.2%, 25.7
versus 13.5% and 3.3 versus 0.3%, respectively. Differences in
dietary habits or environmental factors (smoking or consumption of
alcohol) may be another explanation for these observations. Studies
are required to examine the effect of COX-2-765G>C
polymorphism on gastric cancer among various ethnic
backgrounds.
H. pylori infection and polymorphism are
regarded as major pathogenic factors in gastric cancer development
(2). Interaction of
COX-2-1195G>A and H. pylori infection status
between the case and control family groups was also examined by
stratification analysis. As a result, H. pylori-seropositive
COX-2-1195AA homozygote and A carriers may exhibit increased
susceptibility to gastric cancer (AA homozygote: OR=2.96; 95% CI,
1.57–5.58; A carriers: OR=2.04; 95% CI, 1.18–3.52). This indicates
that H. pylori infection and polymorphism may have a
synergistic effect. H. pylori infection-induced the
COX-2 expression has been previously well evaluated in
gastric mucosa (20). H.
pylori infection induces up-regulated activation of
inflammatory cytokines, which may lead to further alteration of
COX-2 expression (21,22).
The bacterial virulence factors located outside the H.
pylori cag Pathogenicity Island (cagPAI) activate
MEK/ERK1/-2 signaling to mediate bacterial effects on the
COX-2 promoter, an effect that has also been observed in
vitro (23). The
above-mentioned description may facilitate an explanation for the
manner in which H. pylori infection and
COX-2-1195G>A polymorphism have a combined effect on
gastric cancer. However, the exact mechanism underlying the
development of gastric cancer remains to be investigated.
In conclusion, this study has demonstrated that the
COX-2-1195AA genotype is a genetic susceptibility factor of
gastric cancer in various degrees of relationship in the Chinese
Han population. H. pylori infection may enhance the risk of
gastric cancer in individuals with the COX-2-1195AA
genotype. Further epidemiologic studies are required to determine
the interaction between pro-inflammatory genotypes, dietary habits
and exposure to environmental carcinogens.
Acknowledgements
This study was supported by grants from National
Natural Science Foundation of China (No. 30972547). We thank staff
members in Xin’an and Xinxiang County Health Bureau and CDC for
their support to our epidemiological study.
References
|
1
|
Hussain SK, Mu LN, Cai L, et al: Genetic
variation in immune regulation and DNA repair pathways and stomach
cancer in China. Cancer Epidemiol Biomarkers Prev. 18:2304–2309.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li C, Xia HH, Xie W, et al: Association
between interleukin-1 gene polymorphisms and Helicobacter
pylori infection in gastric carcinogenesis in a Chinese
population. J Gastroenterol Hepatol. 2:234–239. 2007. View Article : Google Scholar
|
|
3
|
Correa P: New strategies for the
prevention of gastric cancer: Helicobacter pylori and
genetic susceptibility. J Surg Oncol. 90:134–138. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peek RM Jr and Blaser MJ: Helicobacter
pylori and gastrointestinal tract adenocarcinomas. Nat Rev
Cancer. 2:28–37. 2002. View
Article : Google Scholar
|
|
5
|
Macarthur M, Hold GL and El-Omar EM:
Inflammation and Cancer II. Role of chronic inflammation and
cytokine gene polymorphisms in the pathogenesis of gastrointestinal
malignancy. Am J Physiol Gastrointest Liver Physiol. 286:515–520.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rosenstiel P, Hellmig S, Hampe J, et al:
Influence of polymorphisms in the NOD1/CARD4 and NOD2/CARD15 genes
on the clinical outcome of Helicobacter pylori infection.
Cellular Microbiology. 8:1188–1198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hold GL, Rabkin CS, Chow WH, et al: A
functional polymorphism of toll-like receptor 4 gene increases risk
of gastric carcinoma and its precursors. Gastroenterology.
132:905–912. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Simmons DL, Botting RM and Hla T:
Cyclooxygenase isozymes: the biology of prostaglandin synthesis and
inhibition. Pharmacol Rev. 56:387–437. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun WH, Yu Q, Shen H, et al: Roles of
Helicobacter pylori infection and cyclooxygennase-2
expression in gastric carcinogenesis. World J Gastroenterol.
10:2809–2813. 2004.PubMed/NCBI
|
|
10
|
Mrena J, Wiksten JP, Kokkola A, Nordling
S, Ristimäki A and Haglund C: COX-2 is associated with
proliferation and apoptosis markers and serves as an independent
prognostic factor in gastric cancer. Tumor Biol. 31:1–7. 2010.
View Article : Google Scholar
|
|
11
|
Zhang X, Miao X, Tan W, et al:
Identification of functional genetic variants in cyclooxygenase-2
and their association with risk of esophageal cancer.
Gastroenterology. 129:565–76. 2005.PubMed/NCBI
|
|
12
|
Szczeklik W, Sanak M and Szczeklik A:
Functional effects and gender association of COX-2 gene
polymorphism G-765C in bronchial asthma. J Allergy Clin
Immunol. 114:248–253. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu F, Pan K, Zhang X, et al: Genetic
variants in cyclooxygenase-2: expression and risk of gastric cancer
and its precursors in a Chinese population. Gastroenterology.
130:1975–1984. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sitarz R, Leguit RJ, de Leng WW, et al:
The COX-2 promoter polymorphism -765 G>C is associated
with early-onset, conventional and stump gastric cancers. Modern
Pathology. 21:685–690. 2008.
|
|
15
|
Saxena A, Prasad KN, Ghoshal UC, Bhagat
MR, Krishnani N and Husain N: Polymorphism of -765G>C
COX-2 is a risk factor for gastric adenocarcinoma and peptic
ulcer disease in addition to H. pylori infection: a study
from northern India. World J Gastroenterol. 14:1498–1503. 2008.
|
|
16
|
Tan W, Wu J, Zhang X, et al: Associations
of functional polymorphisms in cyclooxygenase-2 and platelet
12-lipoxygenase with risk of occurrence and advanced disease status
of colorectal cancer. Carcinogenesis. 28:1197–1201. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi J, Misso NL, Kedda MA, et al:
Cyclooxygenase-2 gene polymorphisms in an Australian population:
association of the -1195G>A promoter polymorphism with mild
asthma. Clin Exp Allergy. 38:913–920. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Coskunpinar E, Eraltan IY, Turna A and
Agachan B: Cyclooxygenase-2 gene and lung carcinoma risk. Med
Oncol. View Article : Google Scholar : 2010.
|
|
19
|
Peters WH, Lacko M, te Morsche RH, Voogd
AC, Oude Ophuis MB and Manni JJ: COX-2 polymorphisms and the
risk for head and neck cancer in white patients. Head Neck.
31:938–943. 2009. View Article : Google Scholar
|
|
20
|
McCarthy CJ, Crofford LJ, Greenson J and
Scheiman JM: Cyclooxygenase-2 expression in gastric antral mucosa
before and after eradication of Helicobacter pylori infection. Am J
Gastroenterol. 94:1218–1223. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim SS, Ruiz VE, Carroll JD and Moss SF:
Helicobacter pylori in the pathogenesis of gastric cancer
and gastric lymphoma. Cancer Lett. View Article : Google Scholar : 2011.
|
|
22
|
Di JM, Pang J, Sun QP, et al: Toll-like
receptor 9 agonists up-regulates the expression of cyclooxygenase-2
via activation of NF-kappaB in prostate cancer cells. Mol Biol Rep.
37:1849–1855. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jüttner S, Cramer T, Wessler S, et al:
Helicobacter pylori stimulates host cyclooxygenase-2 gene
transcription: critical importance of MEK/ERK-dependent activation
of USF1/-2 and CREB transcription factors. Cell Microbiol.
5:821–834. 2003.
|