Introduction
Elongator protein 3 (ELP3), the catalytic subunit of
the elongator complex of RNA polymerase II, is involved in
transcriptional elongation (1,2).
Besides transcriptional elongation, ELP3 possesses various
functions, including chromatin modification by acetylation of
histones (2) and demethylation of
paternal DNA in zygotes (3). ELP3
is required for cell cycle progression in the presence of
DNA-damaging agents in yeast (4).
However, the opposite effect was reported in humans; ELP3
overexpression causes cell cycle arrest in a human embryonic kidney
cell line (5). ELP3 also acetylates
actin or tubulin in the microtubules of neurons (6–9). ELP3
mutation results in the degeneration of motor neurons in humans,
suggesting a role for ELP3 in the migration and differentiation of
neurons (10). Although a number of
functions of ELP3 are known, the role of ELP3 in cancers and its
clinical implications have yet to be studied.
Endometrioid adenocarcinoma is the most common
invasive malignancy of the female genital system (11,12).
Despite advances in the methods of detection and treatment, the
prognosis of patients with endometrioid adenocarcinoma remains
unfavorable. We examined the clinical implications of the
expression of a number of markers, including CDCP1 and ALDH1, in
endometrioid adenocarcinoma. ELP3 expression has not been studied
in endometrial tissue of the uterus. In the present study, ELP3
expression was immunohistochemically examined in normal endometrium
and clinical samples with endometrioid adenocarcinoma, and its
clinical implications were evaluated.
Materials and methods
Patients and methods
One hundred patients who underwent surgery for
endometrioid adenocarcinoma at Osaka University Hospital, Japan,
during the period between January 1998 and January 2007 were
examined. Clinicopathological findings of the patients are shown in
Table I. Patient ages ranged from
22 to 75 years (median 54.7). Resected specimens were
macroscopically examined to determine the location and size of the
tumors. Normal endometrial tissue (6 cases in the proliferative and
4 in the secretory phase), collected from patients with functional
bleeding, was included as a control. Histological specimens were
fixed in 10% formalin and paraffin-embedded. Paraffin-embedded
specimens were stored in a dark room in the Department of Pathology
of Osaka University Hospital at room temperature, sectioned at 4-μm
at the time of staining, and stained with H&E and an
immunoperoxidase procedure. The histological stage was determined
according to the International Federation of Obstetricians and
Gynecologists (FIGO) staging system (15). The patients were followed up with
laboratory examinations, including routine peripheral blood cell
counts at 1- to 6-month intervals, X-ray, computed tomographic scan
and pelvic examination at 6- to 12-month intervals. The follow-up
period for survivors ranged from 7 to 122 months (median 82). The
study was approved by the ethics review board of the Graduate
School of Medicine, Osaka University.
 | Table ISummary of characteristics of 100
patients with endometrioid adenocarcinoma. |
Table I
Summary of characteristics of 100
patients with endometrioid adenocarcinoma.
| Characteristics | No. of patients |
|---|
| Tumor |
| T1 | 72 |
| T2 | 8 |
| T3 | 20 |
| Stage |
| I | 63 |
| II | 4 |
| III | 27 |
| IV | 6 |
| Histological
grade |
| 1 | 40 |
| 2 | 39 |
| 3 | 21 |
| Lymph node
metastasis |
| Negative | 75 |
| Positive | 25 |
| MIB-1 labeling index
(%) |
| <20 | 16 |
| ≥20 | 84 |
| Response to
chemotherapy |
| Response | 21 |
| No response | 17 |
| Recurrence |
| Negative | 80 |
| Positive | 20 |
| Survival |
| Yes (with no
recurrence) | 79 |
| Yes (with
recurrence) | 6 |
| No | 15 |
Immunohistochemistry for ELP3 and
MIB-1
ELP3 expression was immunohistochemically examined
with anti-ELP3 antibody (Sigma, St. Louis, MO, USA). The
proliferative activity of cancer cells was examined with monoclonal
antibody MIB-1 (Immunotech, Marseilles, France), thereby
identifying the proliferation-associated antigen Ki-67. Following
antigen retrieval using a Pascal pressurized heating chamber (Dako
A/S, Glostrup, Denmark), the sections were incubated with anti-ELP3
and MIB-1, diluted at ×250 and ×100 magnification, respectively,
and then treated with a ChemMate EnVision kit (Dako). DAB (Dako)
was used as a chromogen. As the negative control, staining was
carried out in the absence of the primary antibody. Positive
staining of endometrioid glandular epithelium was used as a
positive control. Scoring for ELP3 staining was performed
independently by two pathologists (J.I. and E.M.) who examined the
samples in a blinded manner with respect to the clinical
information of the subjects. Tumor cells expressing ELP3 revealed
clear staining in the cytoplasm (Fig.
1), and the staining intensities were comparable to those of
normal epithelia. Cases with <10% ELP3-positive cells among
tumor cells were regarded as ELP3-low, those with 10–25% cells as
ELP3-intermediate and those with >25% cells as ELP3-high. The
MIB-1 labeling index was defined as the percentage of stained
nuclei per 1,000 cells.
Double staining of ELP3 with MIB-1
Double staining of ELP3 with MIB-1 was carried out
using the EnVision G/2 Doublestain system (Dako) according
to the manufacturer’s instructions. Sections were initially
incubated with anti-ELP3 antibody (1:250), colored with DAB and,
subsequently, Ki-67 expression was detected by MIB-1 (1:100),
colored with Permanent Red (Dako).
Statistical analysis
Statistical analyses were performed using StatView
software (SAS Institute Inc., Cary, NC, USA). The Chi-square and
Fisher’s exact probability tests were used to analyze the
correlation between ELP3 expression and clinicopathological factors
in endometrioid adenocarcinoma. Overall survival (OS) was measured
from the time of diagnosis. Disease-free survival (DFS) was
measured from the time of diagnosis until recurrence of the
disease. The Kaplan-Meier method was used to calculate the OS and
DFS rate, and differences in survival curves were evaluated with
the log-rank test. Cox’s proportional hazard regression model was
used in a stepwise manner to analyze the independent prognostic
factors. P≤0.05 was considered to be statistically significant.
Results
Immunohistochemical findings
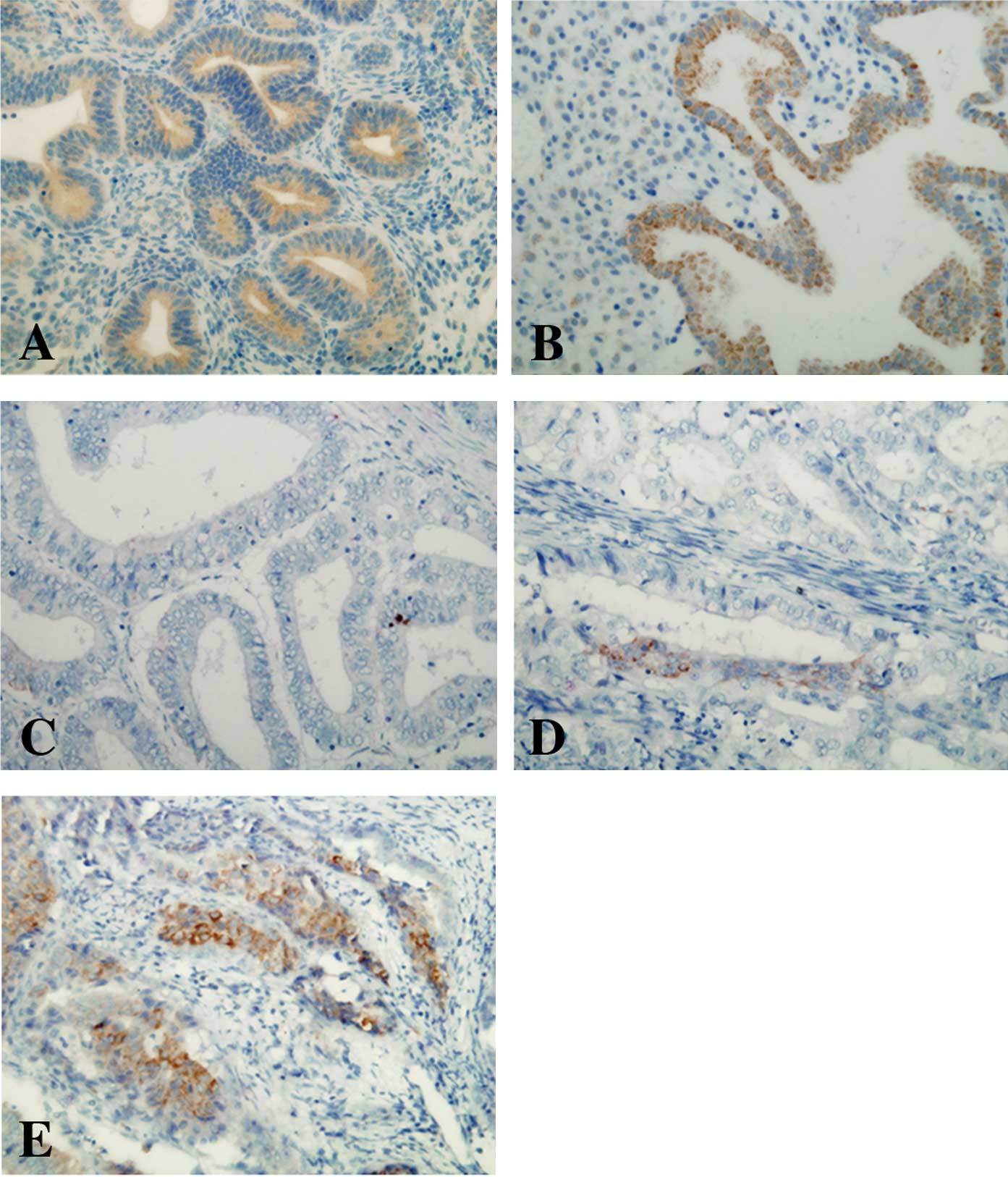
ELP3 expression was examined in normal endometrium.
A strong ELP3 expression was detected in the endometrial glands of
all the examined tissues (proliferative and secretory phases,
Fig. 1A and B). The expression of
ELP3 was then examined in 100 samples of endometrioid
adenocarcinoma tissue. Tumor cells revealed variable ELP3
expression levels. Of the 100 cases, 13% were classified as
ELP3-high, 56 (56%) as ELP3-intermediate and the remaining 31 cases
(31%) as ELP3-low (Figs. 1C-E).
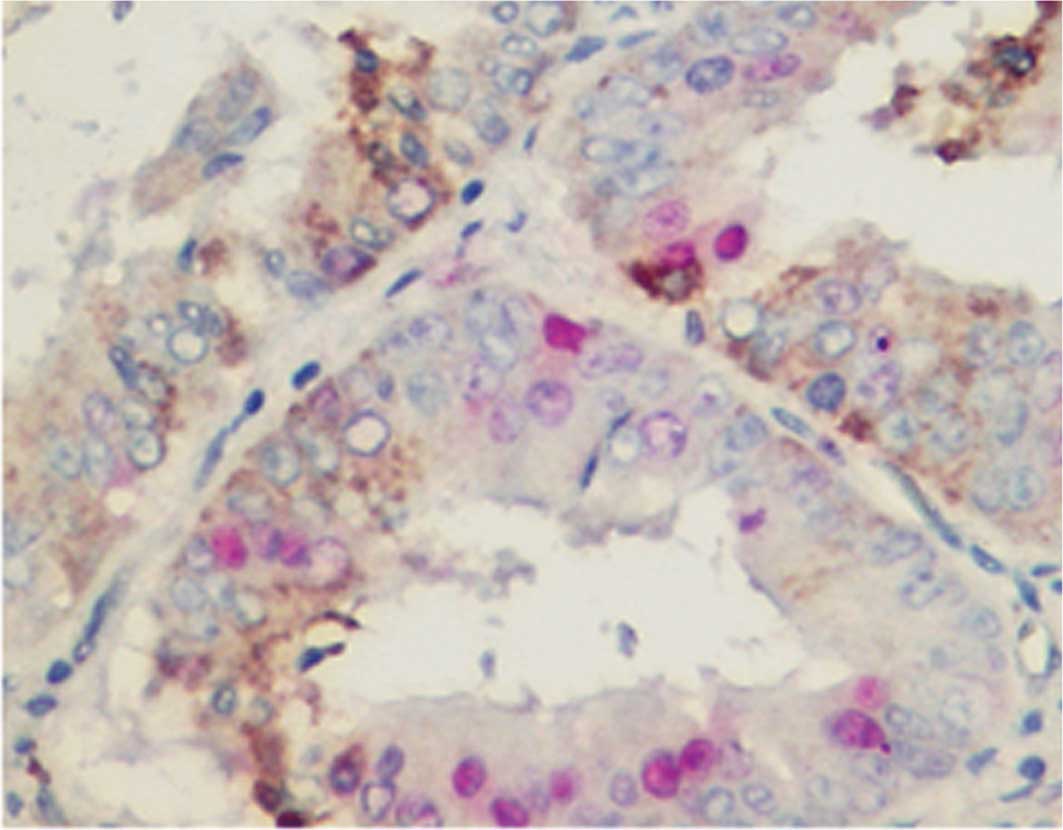
Double staining of ELP3 with MIB-1
To examine the proliferation status of
ELP3-expressing cells, double staining of ELP3 was carried out with
MIB-1. ELP3-expressing and MIB-1-stained cells were almost mutually
exclusive; most of the ELP3-expressing cells were negative for
MIB-1, whereas most MIB-1-positive cells revealed no ELP3
expression (Fig. 2).
Correlation of ELP3 expression with
clinical variables
The correlation of ELP3 expression levels
(ELP3-high, ELP3-intermediate and ELP3-low) with
clinicopathological characteristics was evaluated (Table II). A low ELP3 expression was
correlated with a high T-factor (p=0.036), stage (p=0.001), lymph
node metastasis (p<0.001), resistance to chemotherapy (p=0.045),
recurrence (p=0.004) and poor prognosis (p=0.003). Other
parameters, including the histological grade of the tumor and the
MIB-1 labeling index, did not correlate with ELP3 expression
(Table II).
 | Table IICorrelation between ELP3 expression
and clinicopathological parameters. |
Table II
Correlation between ELP3 expression
and clinicopathological parameters.
| ELP3 expression | p-value |
|---|
|
| |
|---|
| Low | Intermediate | High | |
|---|
| Tumor | | | | 0.036 |
| T1 | 17 | 42 | 13 | |
| T2 | 4 | 4 | 0 | |
| T3 | 11 | 9 | 0 | |
| Stage | | | | 0.001 |
| I | 11 | 39 | 13 | |
| II | 1 | 3 | 0 | |
| III | 17 | 10 | 0 | |
| IV | 2 | 4 | 0 | |
| Histological
grade | | | | 0.379 |
| 1 | 10 | 24 | 6 | |
| 2 | 11 | 22 | 6 | |
| 3 | 10 | 10 | 1 | |
| Lymph node
metastasis | | | | <0.001 |
| Negative | 15 | 47 | 13 | |
| Positive | 17 | 8 | 0 | |
| MIB-1 labeling index
(%) | | | | 0.703 |
| <20 | 4 | 9 | 3 | |
| ≥20 | 28 | 46 | 10 | |
| Response to
chemotherapy | | | | 0.045 |
| Response | 7 | 11 | 3 | |
| No response | 12 | 5 | 0 | |
| Recurrence | | | | 0.004 |
| Negative | 19 | 48 | 13 | |
| Positive | 12 | 8 | 0 | |
| Survival | | | | 0.003 |
| Yes (with no
recurrence) | 19 | 48 | 12 | |
| Yes (with
recurrence) | 1 | 5 | 0 | |
| No | 11 | 3 | 1 | |
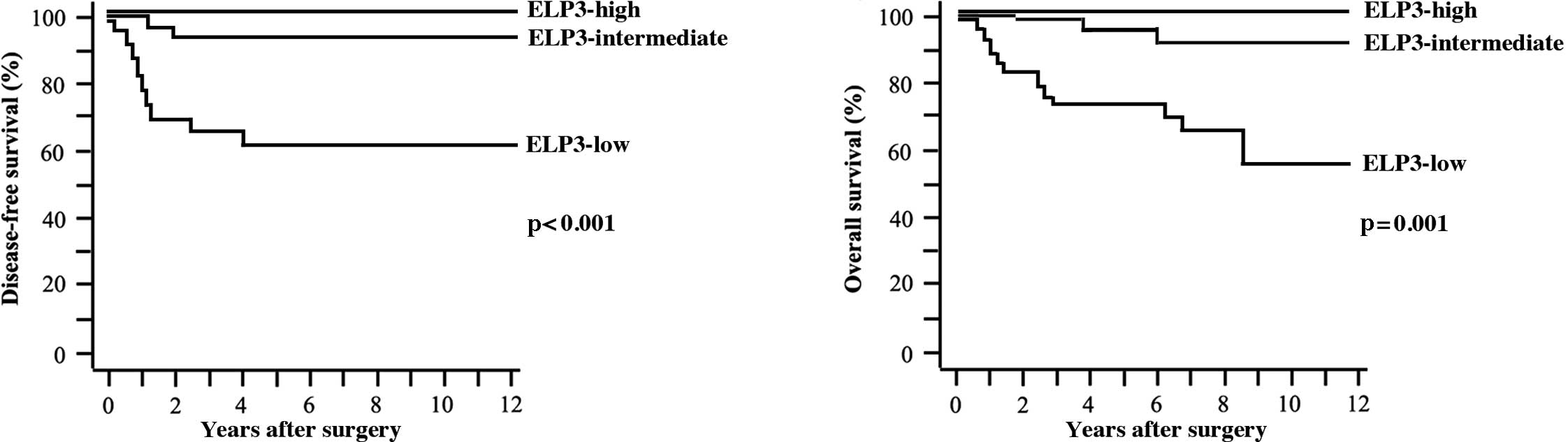
Five-year DFS and OS rates were 86.7 and 90.6%,
respectively. Tumors recurred in 21 patients. Of these, 15 patients
succumbed to the disease. A statistically significant difference
was found in DFS (p<0.001) and OS rates (p=0.001) among patients
with ELP3-high, ELP3-intermediate and ELP3-low tumors (Fig. 3A and B).
Univariate analysis showed that the T-factor, stage,
histological grade, lymph node metastasis and ELP3 expression were
significant factors for both OS and DFS (Table III). The multivariate analysis
revealed that ELP3 expression and histological grade were
independent factors for OS. None of the factors were significant
for DFS (Table III).
 | Table IIIUnivariate and multivariate analyses
of prognostic factors for overall survival (OS) and disease-free
survival (DFS). |
Table III
Univariate and multivariate analyses
of prognostic factors for overall survival (OS) and disease-free
survival (DFS).
| OS | DFS |
|---|
|
|
|
|---|
| Univariate | Multivariate | Univariate | Multivariate |
|---|
|
|
|
|
|
|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value |
|---|
| Tumor | 1.54
(1.23–1.93) | 0.002 | 1.21
(0.82–1.80) | 0.337 | 1.59
(1.26–2.00) | <0.001 | 1.44
(0.94–2.22) | 0.094 |
| Stage | 2.60
(1.62–4.17) | <0.001 | 0.79
(0.27–2.30) | 0.660 | 2.63
(1.63–4.25) | <0.001 | 0.57
(0.18–1.77) | 0.329 |
| Histological
grade | 3.32
(1.58–7.00) | 0.002 | 3.10
(1.20–8.01) | 0.020 | 3.29
(1.58–6.83) | 0.002 | 2.38
(0.89–6.37) | 0.084 |
| Lymph node
metastasis | 8.34
(2.75–25.3) | 0.002 | 2.47
(0.60–10.1) | 0.210 | 7.83
(2.61–23.5) | <0.001 | 2.45
(0.55–10.9) | 0.240 |
| MIB-1 labeling
index | 2.56
(0.34–19.5) | 0.364 | | | 2.85
(0.38–21.7) | 0.311 | | |
| ELP3
expression | 0.19
(0.07–0.52) | 0.001 | 0.24
(0.07–0.79) | 0.019 | 0.21
(0.08–0.57) | 0.002 | 0.33
(0.10–1.11) | 0.073 |
Discussion
In the present study, the characteristics of
patients, such as age and tumor stage, were similar to those in a
previous study by Steiner et al, indicating that our results
are commonly applicable to endometrioid adenocarcinoma worldwide
(16).
ELP3 expression was detected in non-cancerous
endometrial glands of the proliferative and secretory phases. This
is the first study showing ELP3 expression in the endometrium.
Although the role of ELP3 in normal endometrium remains to be
elucidated, ELP3 may function as a tumor-suppressor in endometrioid
adenocarcinoma, since a reduced expression of ELP3 correlated with
a poor prognosis for patients. Low ELP3 expression was correlated
with a high T-factor, an advanced stage, the occurrence of lymph
node metastasis, resistance to chemotherapy and a high recurrence
rate. Recently, Gu et al reported that ELP3 overexpression
inhibits cell growth and causes cell cycle arrest in a human
embryonic kidney cell line (5). In
the present study, ELP3-expressing cells stained negative with
MIB-1, which is consistent with the findings of Gu et
al.
Li et al reported that ELP3 is required for
S-phase progression in yeast in the presence of DNA-damaging
agents, such as hydroxyurea (4). By
contrast, a high expression of ELP3 in humans was reported to
correlate with vulnerability to anticancer drugs. This may be
attributable to the difference in species. ELP3 is known to
regulate the structure of chromatin and the methylation of genomes
(2,17). Although target genes epigenetically
regulated by ELP3 have not yet been reported, the identification of
target genes of ELP3 may aid in understanding the various effects
ELP3 has on the human and yeast cell cycle.
In conclusion, a low ELP3 expression is a poor
prognostic factor in endometrioid adenocarcinoma. Further studies
are required to clarify whether ELP3 expression would be a useful
prognostic marker in other types of cancer.
Acknowledgements
The authors thank Ms. Megumi Sugano, Ms. Etsuko
Maeno and Ms. Takako Sawamura for their technical assistance. This
study was supported by grants from the Ministry of Education,
Culture, Sports, Science and Technology, Japan (no. 20590364 and
no. 20014010).
References
|
1
|
Wittschieben BO, Otero G, de Bizemont T,
et al: A novel histone acetyltransferase is an integral subunit of
elongating RNA polymerase II holoenzyme. Mol Cell. 4:123–128. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hawkes NA, Otero G, Winkler GS, et al:
Purification and characterization of the human elongator complex. J
Biol Chem. 277:3047–3052. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Okada Y, Yamagata K, Hong K, Wakayama T
and Zhang Y: A role for the elongator complex in zygotic paternal
genome demethylation. Nature. 463:554–558. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Q, Fazly AM, Zhou H, Huang S, Zhang Z
and Stillman B: The elongator complex interacts with PCNA and
modulates transcriptional silencing and sensitivity to DNA damage
agents. PLoS Genet. 5:e10006842009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gu J, Sun D, Zheng Q, Wang X, Yang H, Miao
J, Jiang J and Wei W: Human elongator complex is involved in cell
cycle and suppresses cell growth in 293T human embryonic kidney
cells. Acta Biochim Biophys Sin. 41:831–838. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Solinger JA, Paolinelli R, Klöss H, et al:
The Caenorhabditis elegans elongator complex regulates
neuronal alpha-tubulin acetylation. PLoS Genet.
6:e10008202010.PubMed/NCBI
|
|
7
|
Gardiner J, Barton D, Marc J and Overall
R: Potential role of tubulin acetylation and microtubule-based
protein trafficking in familial dysautonomia. Traffic. 8:1145–1149.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barton D, Braet F, Marc J, Overall R and
Gardiner J: ELP3 localises to mitochondria and actin-rich domains
at edges of HeLa cells. Neurosci Lett. 455:60–64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Creppe C, Malinouskaya L, Volvert ML, et
al: Elongator controls the migration and differentiation of
cortical neurons through acetylation of alpha-tubulin. Cell.
136:551–564. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Simpson CL, Lemmens R, Miskiewicz K, et
al: Variants of the elongator protein 3 (ELP3) gene are associated
with motor neuron degeneration. Hum Mol Genet. 18:472–481. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer Statistics. CA Cancer J Clin. 59:225–249. 2009.
|
|
12
|
Horn LC, Meinel A, Handzel R and Einenkel
J: Histopathology of endometrial hyperplasia and endometrial
carcinoma: an update. Ann Diagn Pathol. 1:297–311. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mamat S, Ikeda J, Enomoto T, Ueda Y,
Rahadiani N, Tian T, Wang Y, Qiu Y, Kimura T, Aozasa K and Morii E:
Prognostic significance of CUB domain containing protein expression
in endometrioid adenocarcinoma. Oncol Rep. 23:1221–1227.
2010.PubMed/NCBI
|
|
14
|
Rahadiani N, Ikeda JI, Mamat S, et al:
Expression of aldehyde dehydrogenase 1 (ALDH1) in endometrioid
adenocarcinoma and its clinical implications. Cancer Sci.
102:903–908. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zaino RJ: FIGO staging of endometrial
adenocarcinoma: a critical review and proposal. Int J Gynecol
Pathol. 28:1–9. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Steiner E, Eicher O, Sagemüller J, et al:
Multivariate independent prognostic factors in endometrial
carcinoma: a clinicopathologic study in 181 patients: 10 years
experience at the Department of Obstetrics and Gynecology of the
Mainz University. Int J Gynecol Cancer. 13:197–203. 2003.
|
|
17
|
Chinenov Y: A second catalytic domain in
the Elp3 histone acetyltransferases: a candidate for histone
demethylase activity? Trends Biochem Sci. 27:115–117. 2002.
View Article : Google Scholar : PubMed/NCBI
|