Introduction
Findings of recent studies have shown that there is
a possible correlation of estrogen with the biological activity of
gastric cancer cells (1), and that
the expression of estrogen receptor α66 (ERα66) may correlate with
poorer prognosis among patients with gastric cancer (2).
ERα36, a novel variant of the full-length 66 kDa
ERα66, has one of the most crucial roles in cell growth and
differentiation in various types of cancer (3). This variant differs from ERα66 by
lacking the transcriptional activation domains (AF-1 and AF-2), but
retains the partial dimerization and ligand-binding domains and
DNA-binding domain.
ERα36 enhances oncogenesis, and promotes cell growth
and survival during endocrine therapy in breast cancer (4). The expression of ERα36 was
subsequently detected in breast (5), colorectal (6) and endometrial cancer (7). Furthermore, unlike ERα66, which is
often detected in the cell nucleus, ERα36 is located in the
cytoplasm and plasma membrane. As a result, ERα36 mediates the
membrane-initiated effects of estrogen signaling cascades and
stimulates cell growth (3,8). These features make ERα36 an attractive
target for antibody-based therapy.
The expression of ERα66 has been detected in gastric
cancer cell lines as well as in normal and cancer tissues. However,
the physiological role of ERα66’s possible involvement in the
etiology of gastric cancer remains to be clarified. Recently, it
was reported that the effect of tamoxifen treatment in
ERα66-positive breast tumors could be prevented by ERα36. A similar
event may occur in other types of cancer, including gastric cancer.
Therefore, understanding the existence and expression status of
ERα36 may have significant implications in the prognosis and
treatment of gastric cancer.
Although ERα36 has been extensively studied in other
types of cancer, no investigation has been conducted in gastric
cancer. We hypothesize that ERα66 and its splicing variant ERα36
may play a role in the oncogenesis of gastric cancer. The present
study was undertaken to examine the expression of ERα36 and ERα66
in gastric cancer tissues by using a validated specific and
sensitive real-time quantitative PCR assay. In this study, we
examined tissue from 45 cases of gastric cancer to observe the
potential difference of ERα66 and ERα36 expression in gastric
cancer tissues and their matched normal tissues, and to assess the
correlation between ERα66 and ERα36 expression and
clinicopathological characteristics in gastric cancer patients.
Materials and methods
Case selection
Specimens were obtained from 45 patients who
underwent curative resection of gastric cancer at the Department of
Surgical Oncology of the Sir Run Run Shaw Hospital, Zhejiang
University College of Medicine, China, between July 2007 and
November 2009. Informed consent was obtained from all patients, and
the study was conducted according to the guidelines of the Hospital
Ethics Committee. The patients comprised 26 males and 19 females,
aged 35–81 years (mean 60.0). The correlation between the
expression of ERα36 and ERα66 and clinicopathological parameters
including age, gender, differentiation state, location and pTNM
pathological classification according to the International Union
against Cancer (UICC) (9) were
evaluated. The clinicopathological characteristics of the 45 cases
are shown in Table I.
 | Table IClinicopathological characteristics of
45 patients with gastric cancer. |
Table I
Clinicopathological characteristics of
45 patients with gastric cancer.
| Clinicopathological
characteristics | Case (n) |
|---|
| Age |
| ≤60 | 25 |
| >60 | 20 |
| Gender |
| Male | 26 |
| Female | 19 |
| Histological
type |
| Differentiated | 22 |
|
Undifferentiated | 23 |
| Location |
| Upper or whole | 15 |
| Middle or lower | 30 |
| Tumor size (cm) |
| ≤5.5 | 24 |
| >5.5 | 21 |
| Outside of
serosal |
| Yes | 5 |
| No | 40 |
| Node stage |
| N0-1 | 21 |
| N2-3 | 24 |
Cell culture
Four gastric cancer cell lines, AGS, MKN-45, NCL-N87
and SGC-7901, were maintained in Roswell Park Memorial Institute
(RPMI)-1640 medium supplemented with 10% heat-inactivated fetal
calf serum, 100 U/ml penicillin G and 100 mg/ml streptomycin.
RNA extraction and cDNA synthesis
Total RNA was extracted from freshly frozen gastric
tissues using the TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA). Total RNA was reverse-transcribed into
single-strand complementary DNA (cDNA) using Moloney-murine
leukemia (M-MLV) reverse transcriptase (Promega, Madison, WI, USA).
Briefly, the RNA was denatured by heating for 5 min at 70°C, cooled
on ice, and then used for reverse transcription (2 μg of total RNA,
25 U of RNAse inhibitor, 0.5 mM each of dNTPs, 1.5 μM reverse
primer and 200 U of M-MLV reverse transcriptase in a total volume
of 25 μl). For reverse transcription, tubes were incubated at 42°C
for 60 min, followed by rapid cooling.
Real-time quantitative PCR
Real-time RT-PCR analyses were performed with the
ABI Prism 7500 sequence detection system (Applied Biosystems,
Foster City, CA, USA). Reaction mixture (25 μl) containing 2 μl of
cDNA template, 1 μl each of sense and anti-sense primers and 1X
SYBR-Green Universal PCR Mix was amplified as follows: denaturation
at 95°C for 10 min and 40 cycles at 95°C for 30 sec, 60°C for 30
sec and 72°C for 40 sec. Real-time quantitative PCR was performed
in triplicate for each sample and a mean value of glyceraldehyde
3-phosphate dehydrogenase (GAPDH) was used to calculate mRNA
levels. Quantitative analysis was performed using the comparative
CT method (10,11). The ERα66 and ERα36 mRNA copy numbers
in normal and tumor tissues were normalized to mRNA copy numbers of
the housekeeping gene, GAPDH to give a value of ΔCT. This final
value was to determine changes in the expression of ERα66 and ERα36
in each sample. The primer sequences for ERα66 were: forward
5′-AAGAAAGAACAACATCAGCAGTAAAGCT-3′; and reverse
5′-GGGCTATGGCTTGGTTAAACAT-3′. The primer sequences for ERα36 were:
forward, 5′-CCAAGAATG TTCAACCACAACCT-3′; and reverse
5′-GCACGGTTCATT AACATCTTTCTG-3′. The primers for GAPDH were
obtained as previously described (12). Fluorescent data were converted i)
into RQ measurements, which represent relative expression, ii
)automatically by the SDS system software and iii) exported to
Microsoft Excel. Thermal dissociation plots were examined for
biphasic melting curves, indicative of whether primer dimers or
other non-specific products may be contributing to the
amplification signal.
Statistical analysis
Statistical analysis was conducted using the
statistical program SPSS 15.0 for Windows (SPSS, Chicago, IL, USA).
Pre-treatment characteristics were analyzed using the two-tailed
χ2 test. The two-tailed t-test was used to evaluate the
correlation between ERα36 expression and the clinicopathological
parameters.
Results
Real-time quantitative PCR of the
expression of ERα36 and ERα66 in gastric cancer cells
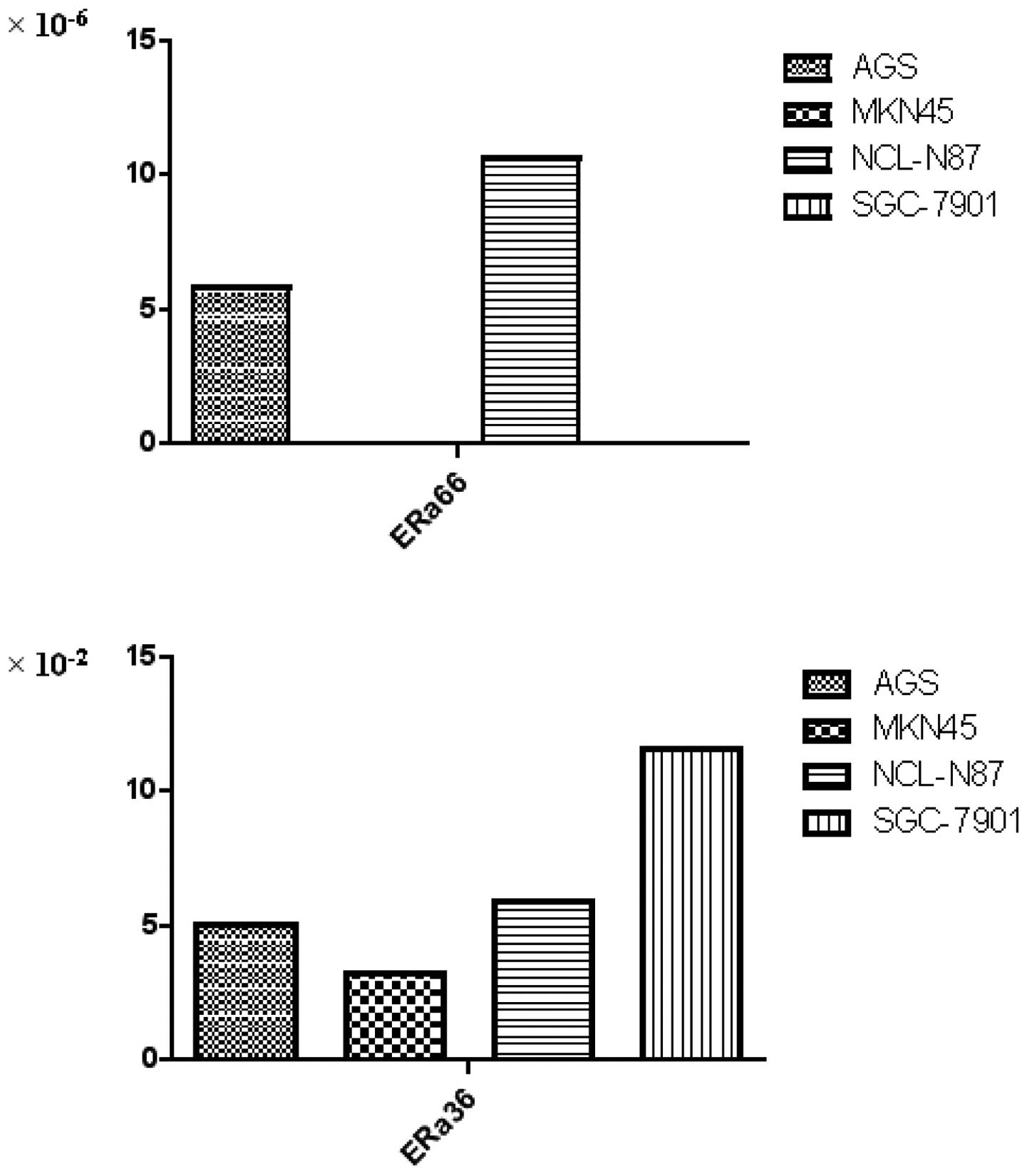
To evaluate mRNA expression of ERα66 and ERα36 in
cancer cells, we detected four gastric cancer cell lines. As shown
in Fig. 1, ERα66 mRNA was detected
in two cell lines, AGS and NCI-N87. By contrast, ERα36 mRNA was
detected in the four cell lines. Consistent with the clinical data,
the expression of ERα36 mRNA was more predominant than the ERα66
mRNA expression.
Expression of ERα36 and ERα66 mRNA in
gastric cancer tissues by real-time PCR
Among the 45 pairs of samples of gastric cancer
tissues and matched normal tissues adjacent to the tumor, the level
of ERα66 of the former was similar to that of the latter, and no
significant associations were found between ERα66 mRNA expression
in gastric cancer tissues and normal tissues (p=0.135).
As shown in Table
II, of the 45 samples of gastric cancer tissues and matched
normal tissues adjacent to the tumor, expression of ERα36 was
detected in the total samples. In normal tissues, the ERα36 mRNA
levels ranged from 0.029 to 157.696 with a median of 2.016. In
gastric cancer tissues, the ERα36 mRNA levels ranged from 0.004 to
39.233 with a median of 0.237. The ERα36 mRNA levels in normal
tissues were significantly higher than those observed in gastric
cancer tissues (p=0.040). Moreover, we found that the expression of
ERα36 mRNA was higher than that of ERα66 mRNA in gastric cancer
tissues and their matched normal tissues.
 | Table IIRelative quantity of ERα36 mRNA and
ERα66 mRNA in gastric cancer tissues and matched normal
tissues. |
Table II
Relative quantity of ERα36 mRNA and
ERα66 mRNA in gastric cancer tissues and matched normal
tissues.
| Tumor tissue | Normal tissues | P-value |
|---|
| Relative ERα36
expression | 1.73±5.85 | 10.54±2.70 | 0.040 |
| Relative ERα66
expression |
(7.87±15.66)×10−3 | (4.30±6.98)×
10−3 | 0.135 |
Correlation between ERα36 and
clinicopathological parameters
According to the median expression level of ERα36,
the 45 cases of gastric cancer were divided into two groups, the
high ERα36 expression group (ERα36 expression level >0.237) and
the low ERα36 expression group (ERα36 expression level ≤0.237). The
mean number of metastasis lymph nodes in the high ERα36 group was
lower than that in the low ERα36 expression group (11.4 vs. 7.3),
but the differences among them were not statistically significant
(p=0.150) (Table III). Moreover,
tumor size varied between the high ERα36 expression group versus
the low ERα36 expression group (6.4cm vs. 5.2 cm), but the
difference was also not statistically significant (p=0.099)
(Table III).
 | Table IIICorrelation between the expression of
ERα36 mRNA and the number of metastasis lymph nodes, tumor
size. |
Table III
Correlation between the expression of
ERα36 mRNA and the number of metastasis lymph nodes, tumor
size.
| ERα36 expression
level ≤0.237 | ERα36 expression
level >0.237 | P-value |
|---|
| Number of metastasis
lymph nodes | 11.4±11.3 | 7.3±7.1 | 0.150 |
| Tumor size | 6.4±2.4 | 5.2±2.6 | 0.100 |
Discussion
In the present study, we found the relative quantity
of ERα36 mRNA and ERα66 mRNA in 45 samples of gastric cancer
tissues as determined by real-time PCR. ERα36 mRNA was expressed
more predominantly than ERα66 mRNA in gastric cancer and normal
tissues adjacent to the tumor.
Recent studies have shown conflicting results of ERα
expression in gastric cancer (13,14).
Moreover, when using the immunohistochemical method, the expression
of ERα gastric cancer tissues showed marked variability (0–62.5%)
among a number of studies (15–17).
These data suggested that a more reliable and sensitive method was
required to evaluate the ERα expression in gastric cancer tissues,
particularly those with low expression levels. In the current
study, real-time quantitative PCR was used to compare the
expression of ERα66 and its splice variant ERα36 mRNA in 45 cases
of gastric cancer and their matched normal tissues, which allows
the detection of ERα expression in stomach tissues at a low level.
In our study, the expression of another ERα66 splice variant, ERα46
mRNA, was also detected; however, it was found in neither the
gastric cancer cells nor the gastric cancer tissues.
Estrogen not only modulates cell proliferation in
classic estrogen-sensitive tissues, but also in other tissues such
as the lungs (18), colon (19) and stomach (15,16).
An epidemiological study showed that tamoxifen, an anti-estrogen
agent, may increase the incidence of gastric cancer, which
suggested that estrogen may be involved in the pathogenesis of
gastric cancer (20). However, few
studies have reported the expression of ERα66 and its variant forms
in gastric cancer.
In the present study, we determined not only ERα66,
but also, for the first time, its splicing variant ERα36 mRNA in
gastric cancer samples and their matched normal tissues by
real-time quantitative PCR assay. Furthermore, we correlated these
findings with the clinicopathological parameters of the gastric
cancer samples.
The expression levels of ERα66, between gastric
cancer tissues and normal tissues did not exhibit a significant
difference, and the expression level was extremely low. ERα36 had a
differential expression level between normal and cancer tissues,
suggesting that ERα36 plays a more significant role in stomach
tumorigenesis, and the decrease in this variant was significantly
correlated with increased tumor size. This result suggests that
ERα36 is involved in gastric cancer proliferation.
Recently, it was reported that aromatase expression
in gastric cancer cells, and cancer cells in the presence of
testosterone, produced estradiol in a short incubation period,
suggesting estrogen is also localized in human gastric cancer
tissues (21). However, a
randomized, controlled study of adjuvant tamoxifen therapy in
gastric cancer found that estrogen receptor α expression is an
independent prognostic factor. By contrast, tamoxifen had no effect
on overall survival in gastric cancer patients; furthermore,
treatment with tamoxifen significantly decreased the survival time
of patients with estrogen receptor α-positive tumors (22).
It is notable that breast cancer patients with ERα66
expression-positive tumors that also express high levels of ERα36
are less likely to benefit from tamoxifen treatment (4). In our study, ERα36 mRNA was expressed
more predominantly than ERα66 mRNA in gastric cancer tissues, which
may be one of the factors impacting on the function of tamoxifen
treatment in gastric cancer patients.
The human ERα36 is known to mediate
membrane-initiated estrogen and antiestrogen signaling, such as the
mitogen-activated protein kinase (MAPK) signaling pathway, which
may provide an explanation for the antiestrogen resistance observed
in breast cancer patients. Similar results may present in gastric
cancer patients. Furthermore, elucidation of the roles of the
estrogen receptor and its variant in gastric cancer may contribute
to diagnosis and treatment.
References
|
1
|
Freedman ND, Ahn J, Hou L, et al:
Polymorphisms in estrogen- and androgen-metabolizing genes and the
risk of gastric cancer. Carcinogenesis. 30:71–77. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu CY, Guo JL, Jiang ZN, et al: Prognostic
role of estrogen receptor alpha and estrogen receptor beta in
gastric cancer. Ann Surg Oncol. 17:2503–2509. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Z, Zhang X, Shen P, et al:
Identification, cloning, and expression of human estrogen
receptor-alpha36, a novel variant of human estrogen
receptor-alpha66. Biochem Biophys Res Commun. 336:1023–1027. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shi L, Dong B, Li Z, et al: Expression of
ER-{alpha}36, a novel variant of estrogen receptor {alpha}, and
resistance to tamoxifen treatment in breast cancer. J Clin Oncol.
20:3423–3429. 2009.
|
|
5
|
Lee LM, Cao J, Deng H, et al: ER-alpha36,
a novel variant of ER-alpha, is expressed in ER-positive and
-negative human breast carcinomas. Anticancer Res. 28:479–483.
2008.PubMed/NCBI
|
|
6
|
Jiang H, Teng R, Wang Q, et al:
Transcriptional analysis of estrogen receptor alpha variant mRNAs
in colorectal cancers and their matched normal colorectal tissues.
J Steroid Biochem Mol Biol. 112:20–24. 2008. View Article : Google Scholar
|
|
7
|
Lin SL, Yan LY, Liang XW, et al: A novel
variant of ER-alpha, ER-alpha36 mediates testosterone-stimulated
ERK and Akt activation in endometrial cancer Hec1A cells. Reprod
Biol Endocrinol. 24:1022009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Z, Zhang X, Shen P, et al: A variant
of estrogen receptor-{alpha}, hER-{alpha}36: transduction of
estrogen- and antiestrogen-dependent membrane-initiated mitogenic
signaling. Proc Natl Acad Sci USA. 103:9063–9068. 2006.
|
|
9
|
Sobin LH and Wittekind C: UICC: TNM
Classification of Malignant Tumours. 5. London: Wiley; 1997
|
|
10
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T). Method Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang HP, Teng RY, Wang Q, et al: Estrogen
receptor alpha variant ERalpha46 mediates growth inhibition and
apoptosis of human HT-29 colon adenocarcinoma cells in the presence
of 17beta-oestradiol. Chin Med J (Engl). 121:1025–1031. 2008.
|
|
13
|
Chandanos E, Rubio CA, Lindblad M, et al:
Endogenous estrogen exposure in relation to distribution of
histological type and estrogen receptors in gastric adenocarcinoma.
Gastric Cancer. 11:168–174. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kameda C, Nakamura M, Tanaka H, et al:
Oestrogen receptor-alpha contributes to the regulation of the
hedgehog signalling pathway in ERalpha-positive gastric cancer. Br
J Cancer. 16:738–747. 2010. View Article : Google Scholar
|
|
15
|
Zhao XH, Gu SZ, Liu SX, et al: Expression
of estrogen receptor and estrogen receptor messenger RNA in gastric
carcinoma tissues. World J Gastroenterol. 9:665–669.
2003.PubMed/NCBI
|
|
16
|
Wang M, Pan JY, Song GR, et al: Altered
expression of estrogen receptor alpha and beta in advanced gastric
adenocarcinoma: correlation with prothymosin alpha and
clinicopathological parameters. Eur J Surg Oncol. 33:195–201. 2007.
View Article : Google Scholar
|
|
17
|
Oshima CT, Wonraht DR, Catarino RM, Mattos
D and Forones NM: Estrogen and progesterone receptors in gastric
and colorectal cancer. Hepatogastroenterology. 46:3155–3158.
1999.PubMed/NCBI
|
|
18
|
Raso MG, Behrens C, Herynk MH, et al:
Immunohistochemical expression of estrogen and progesterone
receptors identifies a subset of NSCLCs and correlates with EGFR
mutation. Clin Cancer Res. 15:5359–5368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nüssler NC, Reinbacher K, Shanny N, et al:
Sex-specific differences in the expression levels of estrogen
receptor subtypes in colorectal cancer. Gend Med. 5:209–217.
2008.PubMed/NCBI
|
|
20
|
Chandanos E, Lindblad M, Rubio CA, et al:
Tamoxifen exposure in relation to gastric adenocarcinoma
development. Eur J Cancer. 44:1007–1014. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Izawa M, Inoue M, Osaki M, et al:
Cytochrome P450 aromatase gene (CYP19) expression in gastric
cancer. Gastric Cancer. 11:103–110. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Harrison JD, Morris DL, Ellis IO, Jones JA
and Jackson I: The effect of tamoxifen and estrogen receptor status
on survival in gastric carcinoma. Cancer. 64:1007–1010. 1989.
View Article : Google Scholar : PubMed/NCBI
|