Introduction
Colon cancer is a major cause of cancer-related
mortality in developed countries, and its incidence in developing
countries is increasing. In Saudi Arabia, colon cancer incidences
rank first among males and third among females after breast and
thyroid cancer (1). Approximately
80% of colon cancer patients are over 40 years of age, and the peak
of onset for most of the tumors is between 50 and 70 years of age
(2). Epidemiological studies have
revealed that environmental as well as genetic factors place
certain individuals at a higher risk of developing colon cancer. To
date, the numbers of genes linked to colon cancer are minuscule. To
identify new colon cancer genes, genetic variation analysis could
provide a window into the genetic landscape of human colon cancer.
The most frequent type of variation in the human genome and an
excellent genotypic marker for research are single nucleotide
polymorphisms (SNPs). Once the genes are identified, physicians
would be able to use these genetic markers to identify individuals
who are at high risk of the disease, and urge them to change their
lifestyle and undergo more frequent physical screening tests in
order to prevent colon cancer.
In the last 15 years, a number of investigators
around the world have focused on understanding the correlation
between the genetic role and biological regulation of
adipocytokines. As a unique member of the adipocytokine family,
adiponectin, which is an adipose-specific protein, appears to have
an anti-atherogenic, anti-inflammatory and anti-diabetic effect.
The adiponectin gene, ADIPOQ, is located on chromosome 3q27
and is comprised of three exons with two introns. It contains 244
amino acids, a signal peptide, a collagen-like domain at its
N-terminus and a globular domain at its C-terminus, which shares
sequence similarities with collagens X and VIII, as well as the
complement factor C1q (3–5). In human plasma, the circulating
adiponectin level ranges between 5 to 30 μg/ml (6) and is reduced in patients with insulin
resistance (7,8), type II diabetes (9–12),
obesity (13), cardiovascular
disease (14,15), gastric cancer (16) and colorectal adenomas and carcinoma
(17,18). Low plasma adiponectin levels in
these disease states are accompanied by reduced adiponectin gene
expression in adipose tissue (7,19) and
have been associated with ADIPOQ gene SNPs.
A number of SNPs have been identified in the
ADIPOQ gene. Although the function of most SNPs remains
unclear, it has been shown that two common SNPs, rs1501299 (G276T)
and rs2241766 (T45G), may affect disease susceptibility. They have
been associated with serum adiponectin (20–26),
obesity (21,25,27,28),
insulin sensitivity (18,21,29),
type II diabetes (17,20,30)
and coronary artery disease (24,31).
However, some of these associations have been unable to be
confirmed in other studies and the results have been controversial
(32–34).
Given the significance of obesity in colon cancer
development and the fundamental role of adiponectin in obesity, it
is reasonable to hypothesize that ADIPOQ gene variation may
play a role in colon cancer susceptibility. In this study, we
sought to evaluate the association between two SNPs, G276T and
T45G, in the ADIPOQ gene with the risk of colon cancer in
the Saudi population. The study was also designed to assess whether
the two SNPs contribute to circulating adiponectin levels.
Materials and methods
Subjects
This was a case-control study involving patients
(n=60) with a diagnosis of colon cancer and controls (n=60), who
were recruited from King Abdulaziz Hospital and Oncology Center in
Jeddah, Saudi Arabia. All cases (31 males and 29 females) had
positive colonoscopic results for malignancy, histologically
confirmed as colon cancer. Healthy, unrelated subjects (30 males
and 30 females) were selected from the family clinic of King
Abdulaziz Hospital, and they were judged to be in good health
according to their medical history and colonoscopy preventive
examination. None were taking any medication. Study subjects
provided information on their body mass index (BMI), diabetes and
family/personal history of cancer, using structured questionnaires.
All participants gave written informed consent.
Anthropometric measurement and
biochemical analyses
Anthropometric data including height, weight, BMI
and waist and hip circumferences, which were measured using a
standard technique: height without shoes by a stadiometer, weight
in light clothes by a beam balance, waist circumference over the
unclothed abdomen at the umbilicus at the end of a normal
expiration and hip circumference at a maximal diameter by a
non-stretchable standard tape. The BMI was calculated by dividing
the weight in kilograms by height in meters squared. Venous blood
was obtained following an overnight fast, and all serum samples
were analyzed together at the end of the study. Serum adiponectin
levels were measured by using an adiponectin (multimeric) EIA kit
that was purchased from Alpco Diagnostics (Salem, NH, USA), with a
measurement range of 0.075–4.8 ng/ml, with an observed value of
80–120%. All standards and unknown samples were analyzed in
duplicate.
DNA extraction and ADIPOQ genotyping
Genomic DNA was extracted from
ethylenediaminetetraacetic acid (EDTA)-whole blood samples using a
commercial kit (QIAamp DNA Blood Mini Kit; Hilden, Germany). DNA
samples were genotyped using polymerase chain reaction (PCR) and
restriction fragment length polymorphism (RFLP) assays. The
genotype assay was used for the analysis of the ADIPOQ gene,
G276T and T45G SNPs. Two sets of primers (5.0 nmol) from TIB (TIB
Molbiol Inc., Germany) were used. For exon 2 amplification, the
forward primer (5′-GAAGTAGACTCTGCTGAGATGG-3′) and the reverse
primer (5′-TATCAGTGTAGGAGGTCTGTGATG-3′) were used. For intron 2
region amplification, the forward primer
(5′-CTACACTGATATAAACTATATGGAG-3′) and the reverse primer
(5′-CCCCAAATCACTTCAGGTTG-3′) were used. The reactions were carried
out in a final volume of 25 μl, containing 1 μl genomic DNA (0.2
μg), 12.5 μl HotStarTaq Master Mix, 10.1 μl RNase free water and
0.2 μl of each primer (0.1 μM). Following the first denaturation
for 5 min at 96°C, PCR was carried out for 40 cycles with
denaturation at 96°C for 35 sec. The annealing temperature was at
63°C for G276T SNP and 53°C for T45G SNP, this was carried out for
35 sec and extension at 72°C for 45 sec, with a final extension for
4 min. PCR products that contain T45G and G276T SNPs were digested
at 37°C with HinfI and SmaI, respectively, and
electrophoresed on a 2% agarose gel.
Statistical analysis
All statistical analyses were performed with the
SPSS (v.16) for Windows software. Continuous variables were
expressed as the means ± standard deviation (SD). In addition,
one-way ANOVA was used. Genotype distributions, allele frequencies,
odds ratio (OR) and risk ratio between the study groups were
computed by 2×2 contingency table analysis. The Hardy-Weinberg
equilibrium (HWE) was tested for the genotypes. The differences in
frequencies of individual polymorphisms were assessed by frequent
analysis, Fisher’s exact tests and Chi-square (χ2)
tests. Results were considered statistically significant with a
P-value <0.05.
Results
Subjects characteristics
The clinical characteristics of the patient and the
control groups, including the serum concentration of adiponectin,
are shown in Table I. In comparing
the colon cancer patients to the controls, the results showed a
highly significant difference between the patient and control
groups regarding measurements of weight (P=0.0001), waist (P=0.01),
hip (P=0.0001), BMI (P=0.004) and the adiponectin concentration
(P=0.001).
 | Table IPhysical and biochemical comparison
between the patients and the control group. |
Table I
Physical and biochemical comparison
between the patients and the control group.
| Patients | Controls | |
|---|
|
|
| |
|---|
| Variables | Means ± SD | Means ± SD | P-value |
|---|
| Age (years) | | | |
| All | 54.28±12.32 | 50.77±12.26 | 0.09 |
| Females | 53.48±13.64 | 51.96±11.94 | |
| Males | 55.03±11.12 | 48.96±12.57 | |
| Height (cm) | | | |
| All | 160.79±9.14 | 163.50±9.70 | 0.12 |
| Females | 155.79±8.05 | 158.07±9.07 | |
| Males | 165.47±7.55 | 168.93±6.83 | |
| Weight (kg) | | | |
| All | 66.23±13.93 | 77.42±18.17 | 0.0001a |
| Females | 64.61±14.27 | 71.58±15.86 | |
| Males | 66.75±13.66 | 83.25±18.08 | |
| Waist (cm) | | | |
| All | 66.98±24.75 | 80.17±31.31 | 0.01b |
| Females | 61.02±28.05 | 84.63±30.78 | |
| Males | 72.55±20.11 | 75.69±31.70 | |
| Hip (cm) | | | |
| All | 66.04±30.18 | 88.56±35.66 | 0.0001a |
| Females | 66.81±32.11 | 93.80±34.66 | |
| Males | 65.32±28.77 | 83.32±36.44 | |
| WHR | | | |
| All | 1.09±0.43 | 0.98±0.47 | 0.15 |
| Females | 0.95±0.32 | 1.01±0.65 | |
| Males | 1.23±0.47 | 0.95±0.19 | |
| BMI | | | |
| All | 25.73±5.64 | 28.96±6.35 | 0.004a |
| Females | 26.77±6.36 | 28.82±6.70 | |
| Males | 24.75±4.77 | 29.10±6.08 | |
| Adiponectin
(ng/ml) | | | |
| All | 5.05±3.49 | 7.59±4.87 | 0.001a |
| Females | 4.47±3.45 | 8.89±5.64 | |
| Males | 5.59±3.50 | 6.38±3.69 | |
PCR-RFLP analysis
The PCR technique was used to amplify the regions
that contain G276T in intron 2 and T45G in exon 2 for all the
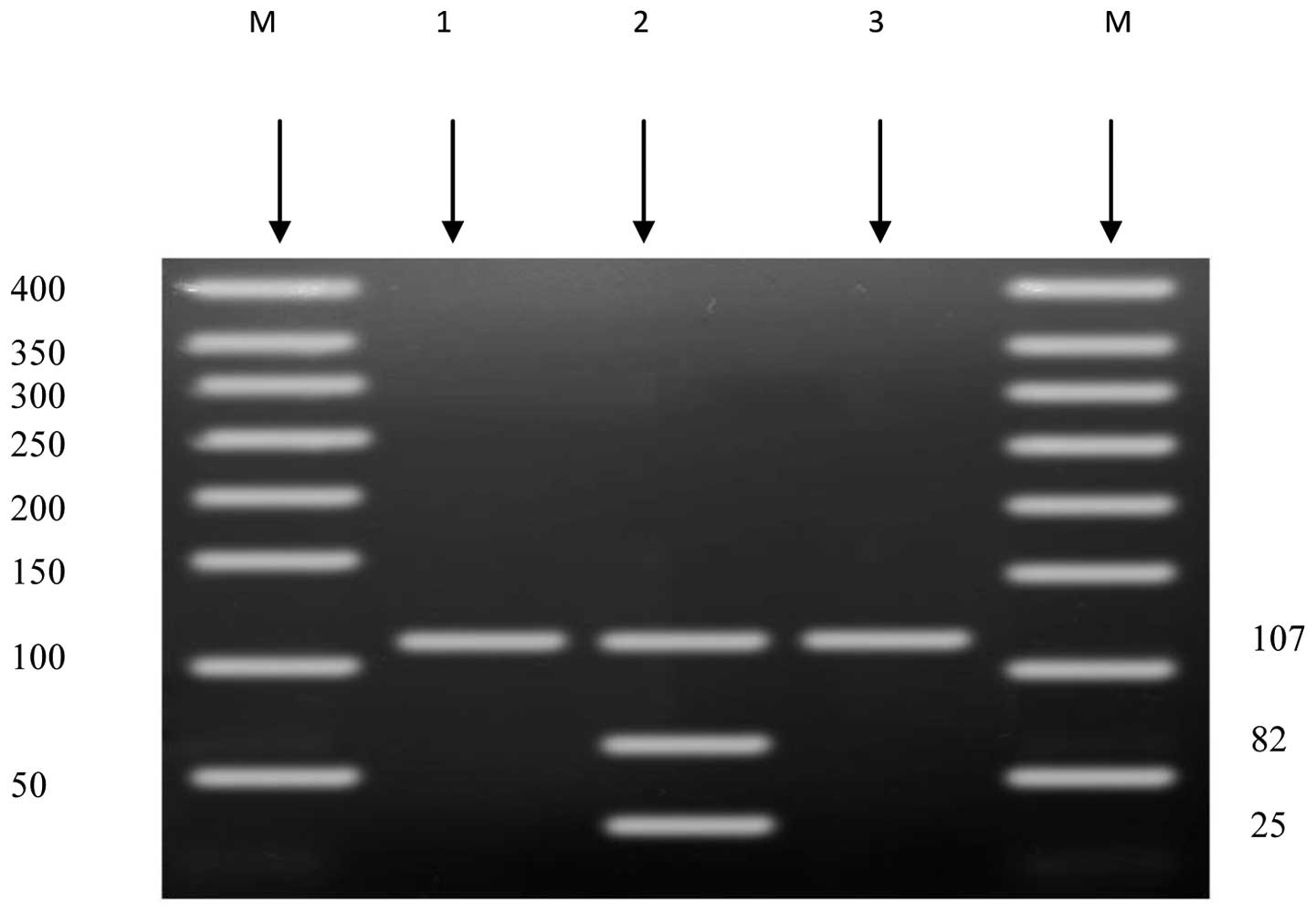
collected samples. The amplified fragment that contains G276T
showed a size of 107 bp. The normal (GG) genotype showed one band
of size 107 bp. The heterozygous (GT) genotype showed three
fragments of sizes 107, 82 and 25 bp. No homozygous (TT) genotype
was found in the selected group (Fig.
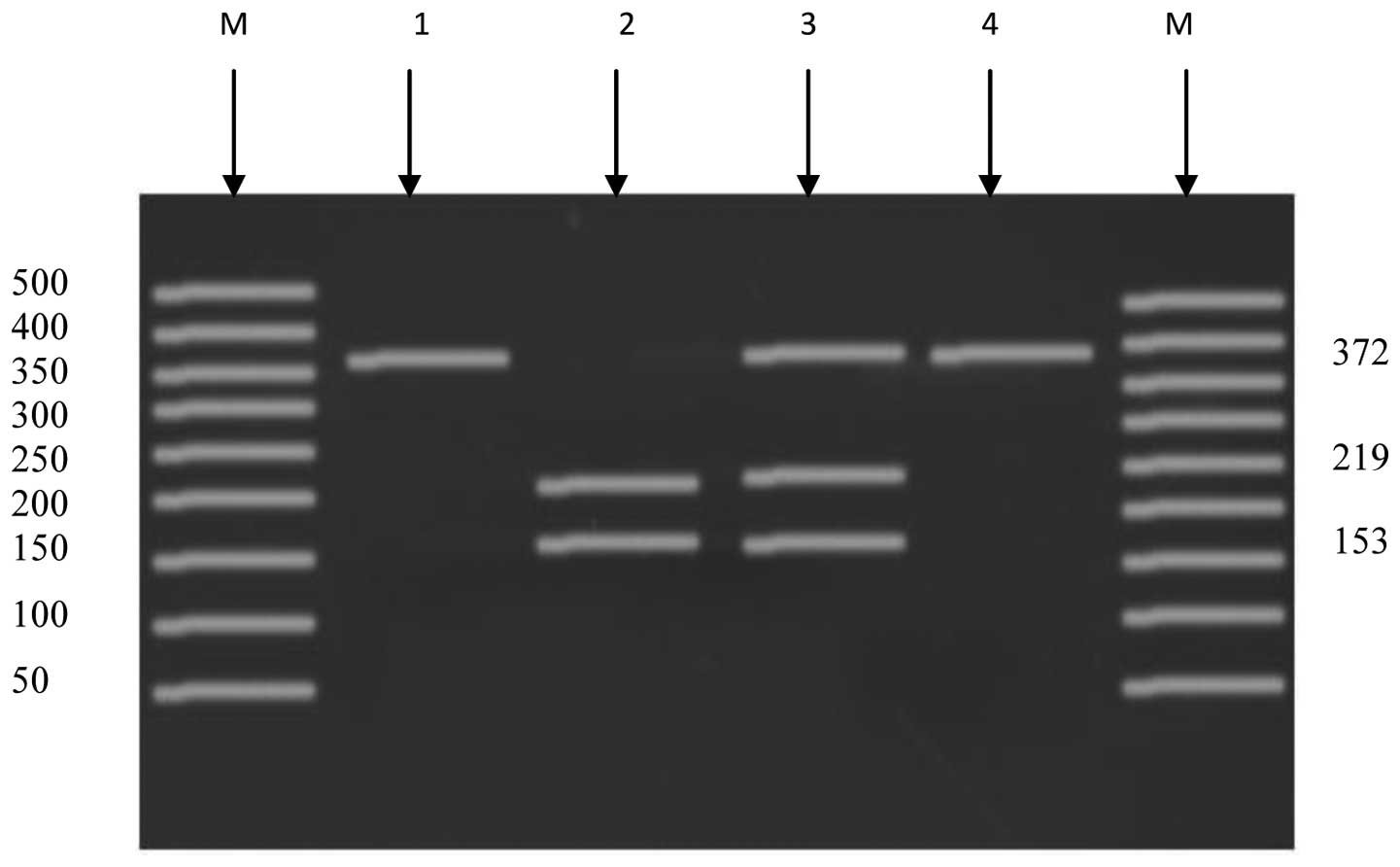
1). The other amplified fragment that contains T45G showed a
size of 372 bp. The genotype for the normal (TT) individuals
produced one band of size 372 bp. The heterozygous (TG) genotype
produced three bands of sizes 372, 219 and 153 bp. The homozygous
(GG) genotype produced two bands of sizes 219 and 153 bp (Fig. 2).
Genotype distributions and allele
frequencies of the ADIPOQ gene G276T SNP
The genotype and allele frequencies of the G276T
variant were examined (Table II).
The genotype frequencies of the patient group were 91.7% (n=55)
normal (GG) and 8.3% (n=5) heterozygous (GT). In the control group,
results revealed 96.7% (n=58) normal (GG) and 3.3% (n=2)
heterozygous (GT). The frequency of the G and T alleles for the
patient group were 95.9 and 4.1%, respectively. In the control
group, the frequency of the G and T alleles were 98.4 and 1.6%,
respectively. Genotype distributions for colon cancer patients
(goodness-of-fit χ2=0.12, df=1, P=0.73) and the controls
(goodness-of-fit χ2=0.014, df=1, P=0.91) were in HWE.
The T allele at the G276T polymorphism was associated with a higher
risk of colon cancer [OR=2.64; 95% confidence interval (CI),
0.49–14.6; P=0.44].
 | Table IIAllele distribution and genotype
frequency of the ADIPOQ gene G276T SNP. |
Table II
Allele distribution and genotype
frequency of the ADIPOQ gene G276T SNP.
| | Genotype
frequency | Allele
frequency |
|---|
| |
|
|
|---|
| Group | n | GG | GT+TT | G | T |
|---|
| Colon cancer | 60 | 55 (91.7%) | 5 (8.3%) | 115 (95.9%) | 5 (4.1%) |
| Control | 60 | 58 (96.7%) | 2 (3.3%) | 118 (98.4%) | 2 (1.6%) |
| | OR (95% CI), 2.64
(0.49–14.6) | OR (95% CI), 2.56
(0.49–13.94) |
| | RR (95% CI), 2.50
(0.5–12.39) | RR (95% CI), 2.50
(0.49–12.64) |
Genotype distributions and allele
frequencies of the ADIPOQ gene T45G SNP
The genotype and allele frequencies of the T45G
variant are shown in Table III.
The genotype frequencies of the patients were 63.3% (n=38) normal
(TT), 30% (n=18) heterozygous (TG) and 6.7% (n=4) homozygous (GG).
In the controls, results revealed 45.4% (n=27) normal (TT), 53%
(n=32) heterozygous (TG) and 1.6% (n=1) homozygous (GG). In the
patient subjects, the frequency of the T and G alleles were 78.3
and 21.7%, respectively. In the control subjects, the frequency of
the T and G alleles were 71.5 and 28.5%, respectively. Genotype
distributions for the colon cancer patients (goodness-of-fit
χ2=0.33, df=1, P=0.56) and the control group
(goodness-of-fit χ2=3.82, df=1, P=0.50) were in HWE. The
G allele at the T45G polymorphism was associated with a lower risk
of colon cancer (OR=0.41; 95% CI, 0.19–0.86; P=0.22).
 | Table IIIAllele distribution and genotype
frequency of the ADIPOQ gene T45G SNP. |
Table III
Allele distribution and genotype
frequency of the ADIPOQ gene T45G SNP.
| | Genotype
frequency | Allele
frequency |
|---|
| |
|
|
|---|
| Group | n | TT | TG+GG | T | G |
|---|
| Colon cancer | 60 | 40 (63.3%) | 20 (36.7%) | 94 (78.3%) | 26 (21.7%) |
| Control | 60 | 27 (45.4%) | 33 (54.6%) | 86 (71.5%) | 34 (28.5%) |
| | OR (95% CI), 0.41
(0.19–0.86) | OR (95% CI), 0.76
(0.39–1.26) |
| | RR (95% CI), 0.61
(0.39–0.93) | RR (95% CI), 0.69
(0.94–1.19) |
Association between the SNPs and
circulating adiponectin concentration
The colon cancer group had significantly lower
adiponectin concentrations than those in the control group
(P=0.001). Further analysis revealed that the level of adiponectin
of TG+GG genotype carriers was not significantly different from
that of the TT genotype in colon cancer patients (P=0.30) and in
the control group (P=0.37). The result of the association between
the G276T SNP and circulating adiponectin concentration showed that
the level of adiponectin of GT+TT genotype carriers was not
significantly different from that of the GG genotype in colon
cancer patients (P=0.32) and in the control group (P=0.84).
Discussion
The aim of this study was to evaluate the
association between two SNPs, rs1501299 (G276T) and rs2241766
(T45G), in the ADIPOQ gene with the risk of colon cancer in
Saudi patients. In addition, we focused on the effect of the two
SNPs on serum adiponectin levels. We examined the hypothesis that
the ADIPOQ gene polymorphisms may be one of the genetic
factors that affect colon cancer susceptibility. Our results showed
that carriers of the heterozygous (GT) genotype of G276T had more
than a two-fold (OR=2.64; 95% CI, 0.49–14.6; P=0.44) higher risk of
colon cancer than carriers of the normal (GG) genotype. By
contrast, we found that the G allele in position 45 of the
ADIPOQ gene had a lower risk (OR=0.41; 95% CI, 0.19–0.86;
P=0.22) of colon cancer than carriers of the normal (TT) genotype.
The results suggest that the G276T SNP of the ADIPOQ gene
contributes to the genetic risk of colon cancer and that the
presence of the G allele in position 45 of the ADIPOQ gene
appears to act as a protective factor against colon cancer. To the
best of our knowledge, this is the first study that has
investigated the association between G276T and T45G polymorphisms
of the ADIPOQ gene with colon cancer risk.
In the present study, although the patients with
colon cancer had significantly lower height, weight, waist and hip
circumferences than the controls, and differences of BMI were only
of borderline significance, adiponectin levels were significantly
(P=0.001) lower in the patient group than in the control group. Our
results are in agreement with those from other studies that have
shown an inverse association between adiponectin levels and risk of
cancer. Ishikawa et al observed lower adiponectin serum
levels in patients with gastric cancer in comparison to the control
group (16). Furthermore, they
observed that adiponectin serum concentration inversely correlated
with tumor size and cancer stage. Fukumoto et al observed in
patients with adenoma a slightly lower adiponectin serum
concentration than in healthy controls (17). Kumor et al observed in
patients with colorectal adenomas and carcinoma lower serum
adiponectin concentrations than in the control group (18). Those studies may give support to our
data regarding the significantly lower serum adiponectin levels in
colon cancer patients in comparison to the control group. We
speculate that decreased serum adiponectin levels may be a factor
involved in the development of colon cancer or a secondary effect
of the metabolic derangements in colon cancer.
To explore the molecular mechanism underlying the
role of adiponectin in colon cancer, we compared the levels of
adiponectin between the heterozygous, homozygous and normal
genotype carriers. Our results showed no significant difference in
the levels of adiponectin between heterozygous or homozygous
genotype carriers and the normal genotype carriers for the two SNPs
(data not shown). Our results suggest that the two SNPs are
non-functional and have no role in the expression of circulation
levels of adiponectin. By contrast, previous have studies found
that the T allele of SNP 276 is associated with higher adiponectin
levels (19–27). However, some studies have found no
association between the SNP 276 and circulating adiponectin levels
(28–30). Furthermore, Filippi et al
found significant associations between adiponectin levels and the
SNP G276T and they suggested that the adiponectin gene variant, or
a mutation in linkage with it, determines lower adiponectin gene
expression (31). Woo et al,
found that the haplotypes formed by SNPs 45, 276 and 349 were
associated with adiponectin levels in Caucasians but not in
African-Americans (32). These
results suggest that there may be multiple SNPs working in
conjunction with each other and with the environment to affect
adiponectin levels.
Despite the small number of colon cancer patients
tested, the study’s design was relatively strong owing to the fact
that the controls were recruited from the same cohort as the colon
cancer patients and were matched by age and gender.
In conclusion, our case-control study on the Saudi
population showed that the risk of developing colon cancer may be
partly explained by genetic polymorphisms in the ADIPOQ
gene. Our data needs to be replicated in other populations and the
mechanisms underlying the role of adiponectin in colon cancer
disease require further investigation.
Acknowledgements
This study was funded by grants (AT-16-129) from
King Abdul Aziz City for Science and Technology (Saudi Arabia). The
authors are most grateful to all the subjects who willingly
participated in this study.
References
|
1
|
Ibrahim M, Zeeneldin A, El-Khodary T,
Al-Gahmi A and Bin Sadiq B: Past present and future of colorectal
cancer in the Kingdom of Saudi Arabia. Saudi J Gastroenterol.
14:178–182. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aljebreen A: Clinico-pathological patterns
of colorectal cancer in Saudi Arabia: Younger with an advanced
stage presentation. Saudi J Gastroenterol. 13:84–87. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scherer P, Williams S, Fogliano M, Baldini
G and Lodish H: A novel serum protein similar to C1q, produced
exclusively in adipocytes. J Biol Chem. 270:26746–26749. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maeda K, Okubo K, Shimomura I, et al: cDNA
cloning and expression of a novel adipose specific collagen-like
factOR=apM1 (adipose most abundant gene transcript 1). Biochem
Biophys Res Commun. 221:286–289. 1996.
|
|
5
|
Kadowaki T and Yamauchi T: Adiponectin and
adiponectin receptors. Endocr Rev. 26:439–451. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hotta K, Funahashi T, Arita Y, et al:
Plasma concentrations of a novel, adipose-specific protein,
adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc
Biol. 20:1595–1599. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weyer C, Funahashi T, Tanaka S, et al:
Hypoadiponectinemia in obesity and type 2 diabetes: close
association with insulin resistance and hyperinsulinemia. J Clin
Endocrinol Metab. 86:1930–1935. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamauchi T, Kamon J, Ito Y, et al: Cloning
of adiponectin receptors that mediate antidiabetic metabolic
effects. Nature. 423:762–769. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kondo H, Shimomura I, Matsukawa Y, et al:
Association of adiponectin mutation with type 2 diabetes: a
candidate gene for the insulin resistance syndrome. Diabetes.
51:2325–2328. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chandran M, Ciaraldi T, Phillips SA and
Henry R: Adiponectin: more than just another fat cell hormone?
Diabetes Care. 26:2442–2450. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Diez J and Iglesias P: The role of the
novel adipocyte-derived hormone adiponectin in human disease. Eur J
Endocrinol. 148:293–300. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cruz M, García-Macedo R, García-Valerio Y,
et al: Low adiponectin levels predict type 2 diabetes in Mexican
children. Diabetes Care. 27:1451–1453. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Engeli S, Feldpausch M, Gorzelniak K, et
al: Association between adiponectin and mediators of inflammation
in obese women. Diabetes. 52:942–947. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kazumi T, Kawaguchi A, Sakai K, Hirano T
and Yoshino G: Young men with high-normal blood pressure have lower
serum adiponectin, smaller LDL size, and higher elevated heart rate
than those with optimal blood pressure. Diabetes Care. 25:971–976.
2002. View Article : Google Scholar
|
|
15
|
Ryo M, Nakamura T, Kihara S, et al:
Adiponectin as a biomarker of the metabolic syndrome. Circ J.
68:975–981. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ishikawa M, Kitayama J, Yamauchi T, et al:
Adiponectin inhibits the growth and peritoneal metastasis of
gastric cancer through its specific membrane receptors AdipoR1 and
AdipoR2. Cancer Sci. 98:1120–1127. 2007. View Article : Google Scholar
|
|
17
|
Fukumoto J, Otake T, Tajima O, et al:
Adiponectin and colorectal adenomas: Self Defense Forces Health
Study. Cancer Sci. 99:781–786. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kumor A, Daniel P, Pietruczuk M and
Małecka-Panas E: Serum leptin, adiponectin, and resistin
concentration in colorectal adenoma and carcinoma (CC) patients.
Int J Colorectal Dis. 24:275–281. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hara K, Boutin P, Mori Y, et al: Genetic
variation in the gene encoding adiponectin is associated with an
increased risk of type 2 diabetes in the Japanese population.
Diabetes. 51:536–540. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Menzaghi C, Ercolino T, Di Paola R, et al:
A haplotype at the adiponectin locus is associated with obesity and
other features of the insulin resistance syndrome. Diabetes.
51:2306–2312. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Menzaghi C, Ercolino T, Salvemini L, et
al: Multigenic control of serum adiponectin levels: evidence for a
role of the ADIPOQ gene and a locus on 14q13. Physiol Genomics.
19:170–174. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Berthier MT, Houde A, Cote M, et al:
Impact of adiponectin gene polymorphisms on plasma lipoprotein and
adiponectin concentrations of viscerally obese men. J Lipid Res.
46:237–244. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pollin T, Tanner K, O’Connell J, et al:
Linkage of plasma adiponectin levels to 3q27 explained by
association with variation in the ADIPOQ gene. Diabetes.
54:268–274. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qi L, Li T, Rimm E, et al: The +276
polymorphism of the APM1 gene, plasma adiponectin concentration,
and cardiovascular risk in diabetic men. Diabetes. 54:1607–1610.
2005.
|
|
25
|
Melistas L, Mantzoros C, Kontogianni M, et
al: Association of the +45T>G and +276G>T polymorphisms in
the adiponectin gene with insulin resistance in non-diabetic Greek
women. Eur J Endocrinol. 161:845–852. 2009.
|
|
26
|
Siitonen N, Pulkkinen L, Lindström J, et
al: Association of ADIPOQ gene variants with body weight, type 2
diabetes and serum adiponectin concentrations: the Finnish Diabetes
Prevention Study. BMC Med Genet. 12:52011. View Article : Google Scholar
|
|
27
|
Jang Y, Lee J, Kim O, et al: The
SNP276G>T polymorphism in the adiponectin (ACDC) gene is more
strongly associated with insulin resistance and cardiovascular
disease risk than SNP45T>G in nonobese/nondiabetic Korean men
independent of abdominal adiposity and circulating plasma
adiponectin. Metabolism. 55:59–66. 2006.
|
|
28
|
Filippi E, Sentinelli F, Trischitta V, et
al: Association of the human adiponectin gene and insulin
resistance. Eur J Hum Genet. 12:199–205. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ohashi K, Ouchi N, Kihara S, et al:
Adiponectin I164 T mutation is associated with the metabolic
syndrome and coronary artery disease. J Am Coll Cardiol.
43:1195–1200. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vozarova de Courten B, Hanson R, et al:
Common polymorphisms in the adiponectin gene ACDC are not
associated with diabetes in Pima Indians. Diabetes. 54:284–289.
2005.PubMed/NCBI
|
|
31
|
Filippi E, Sentinelli F, Romeo S, et al:
The adiponectin gene SNP+276G>T associates with early-onset
coronary artery disease and with lower levels of adiponectin in
younger coronary artery disease patients (age <or=50 years). J
Mol Med (Berl). 83:711–719. 2005.
|
|
32
|
Woo J, Dolan L, Deka R, et al:
Interactions between noncontiguous haplotypes in the adiponectin
gene ACDC are associated with plasma adiponectin. Diabetes.
55:523–529. 2006. View Article : Google Scholar : PubMed/NCBI
|